Submitted:
08 March 2024
Posted:
14 March 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Medicinal Use and Biological Activity of Mangrove Plants:
Inflammation:
NF-κB Pathways of Inflammation:
Materials and Methods:
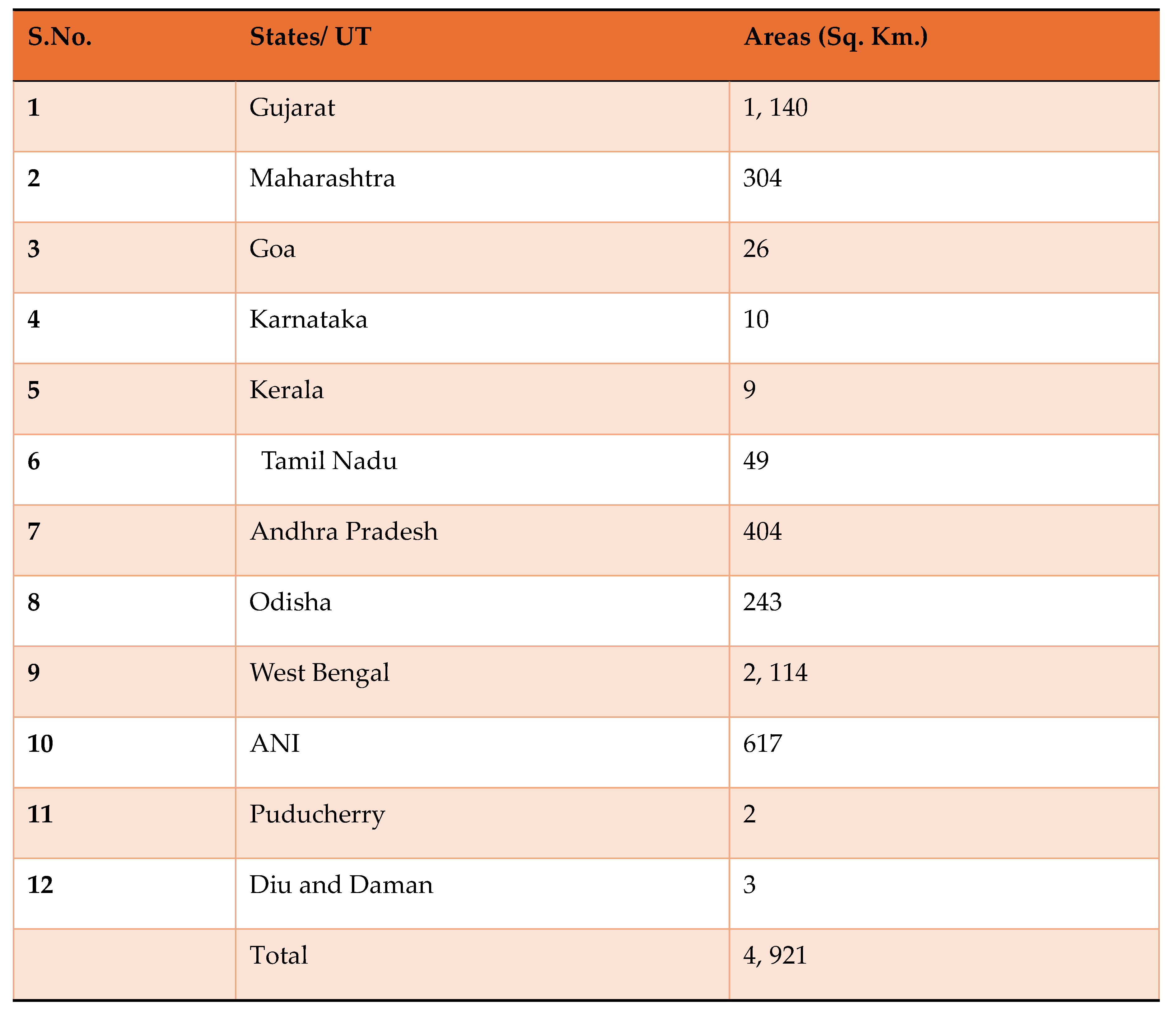
Special Focus on ANI Mangrove Resources:
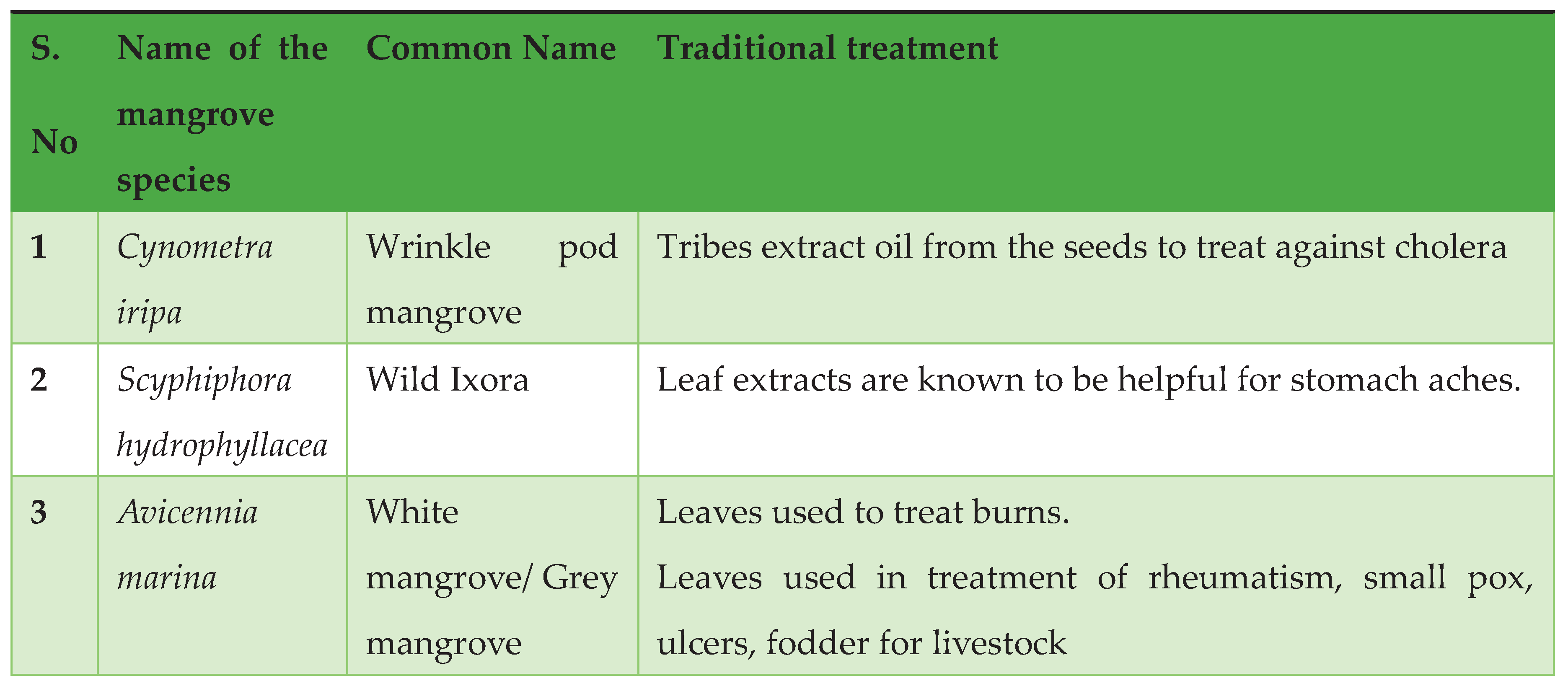
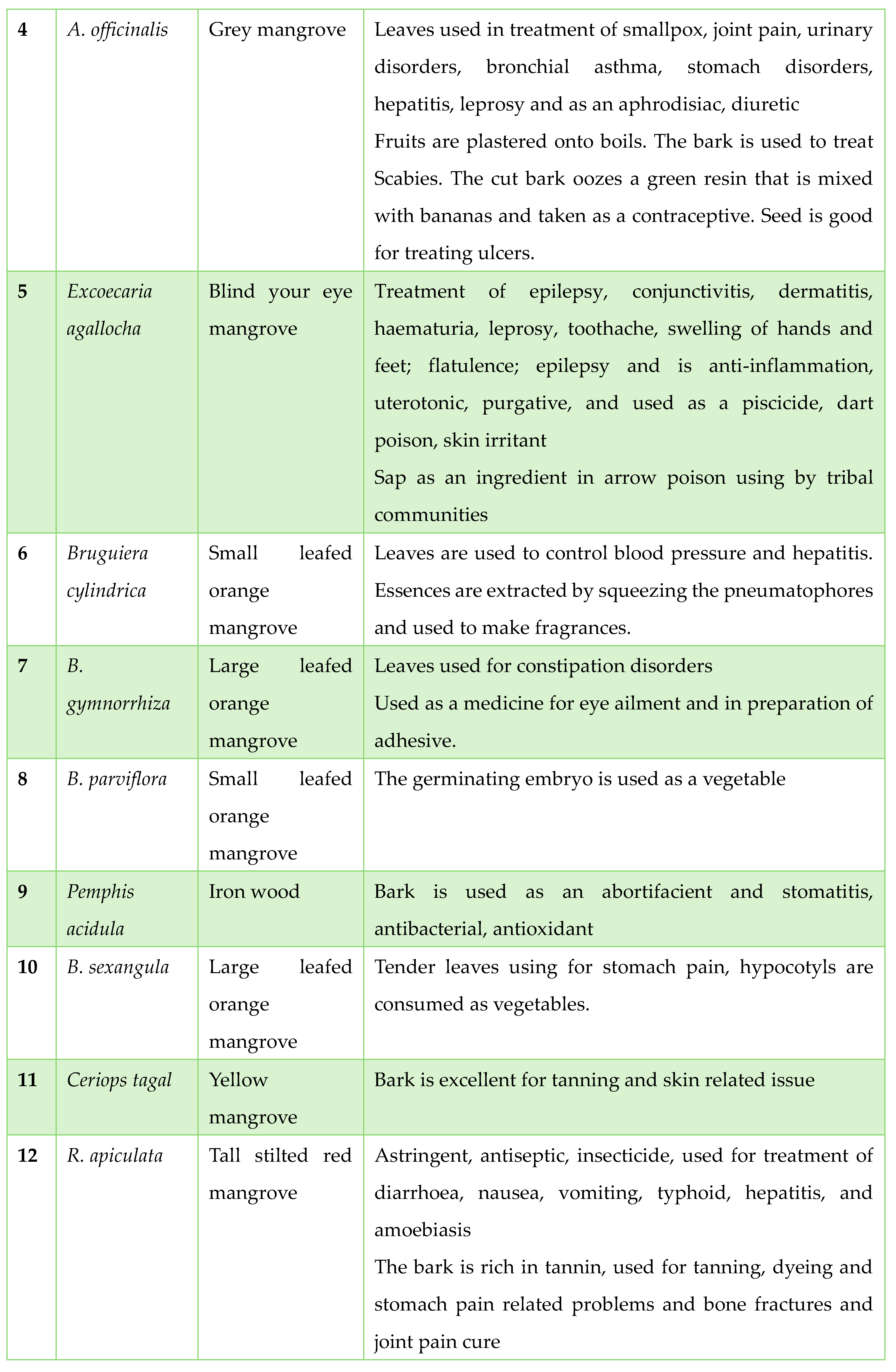
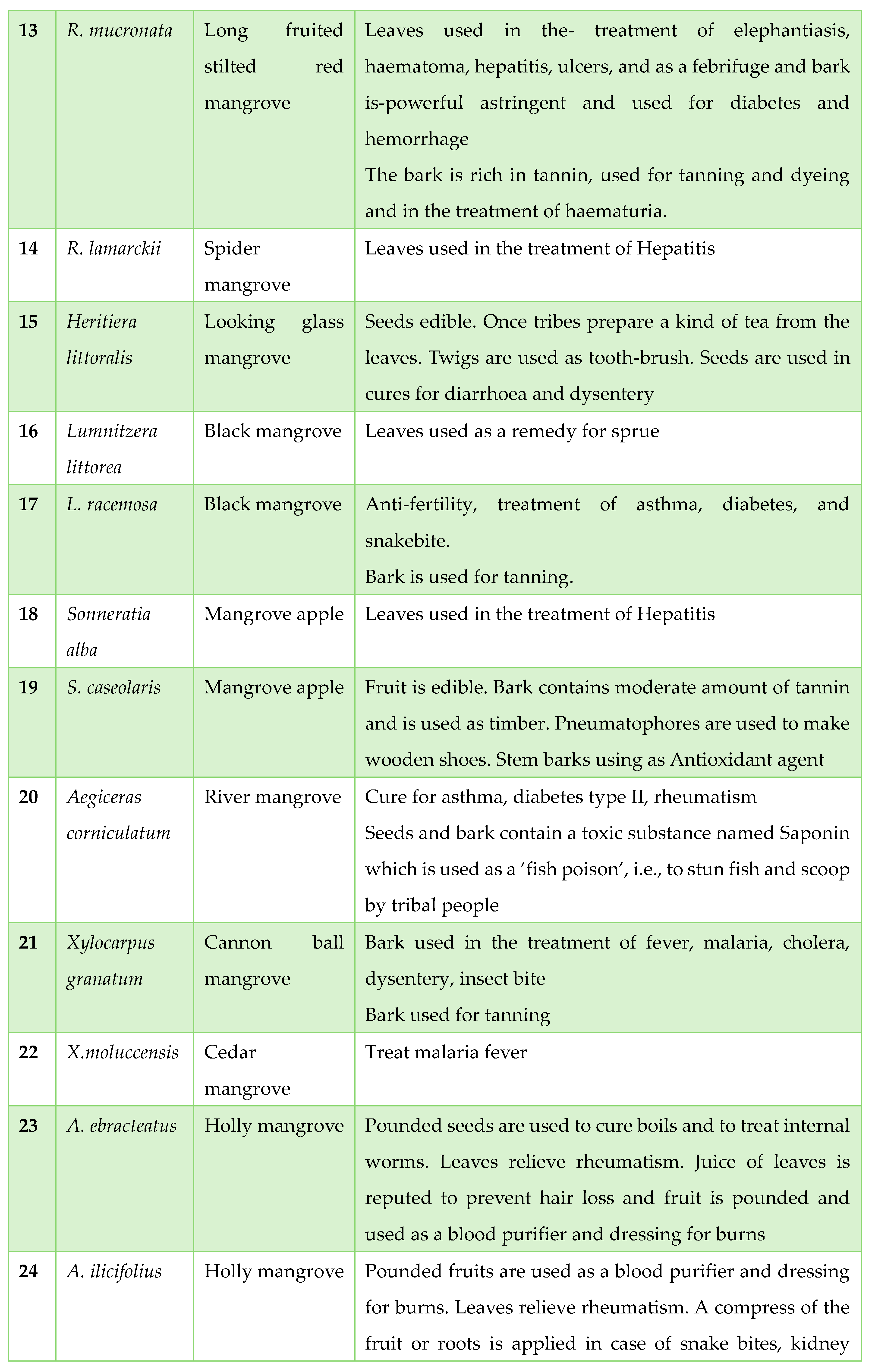
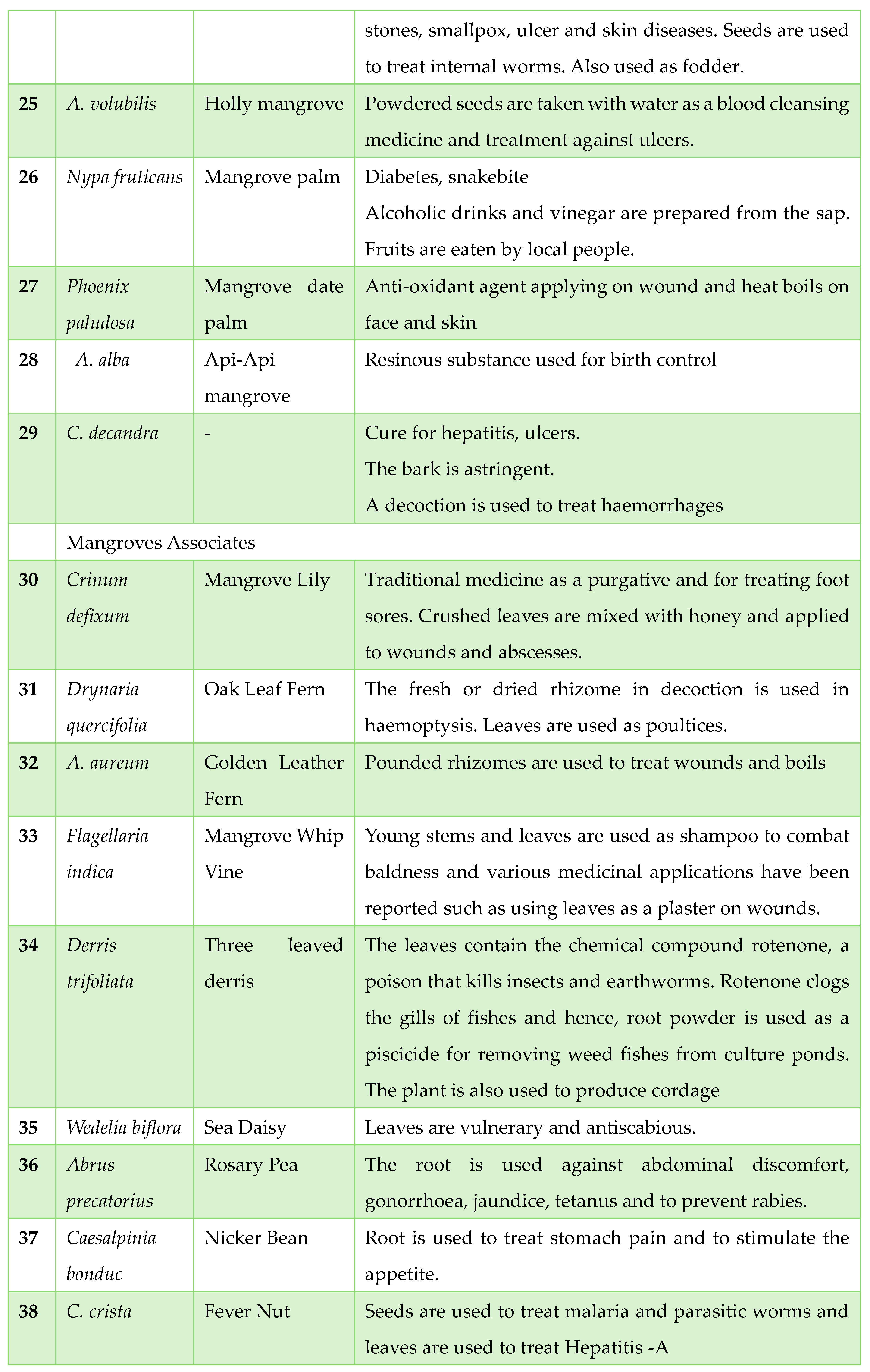
Traditionally Used Mangrove Plants in Tribal Areas:
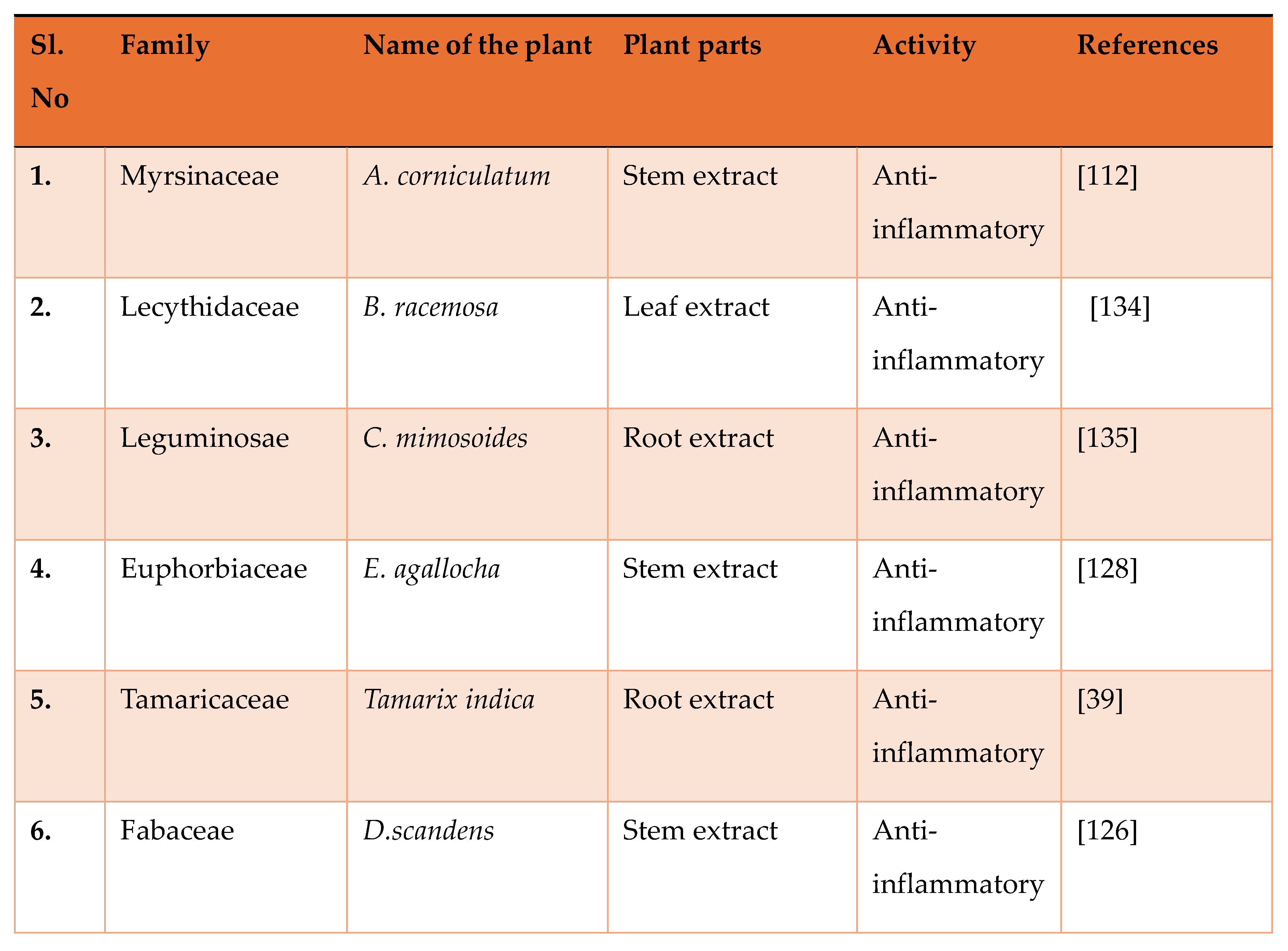
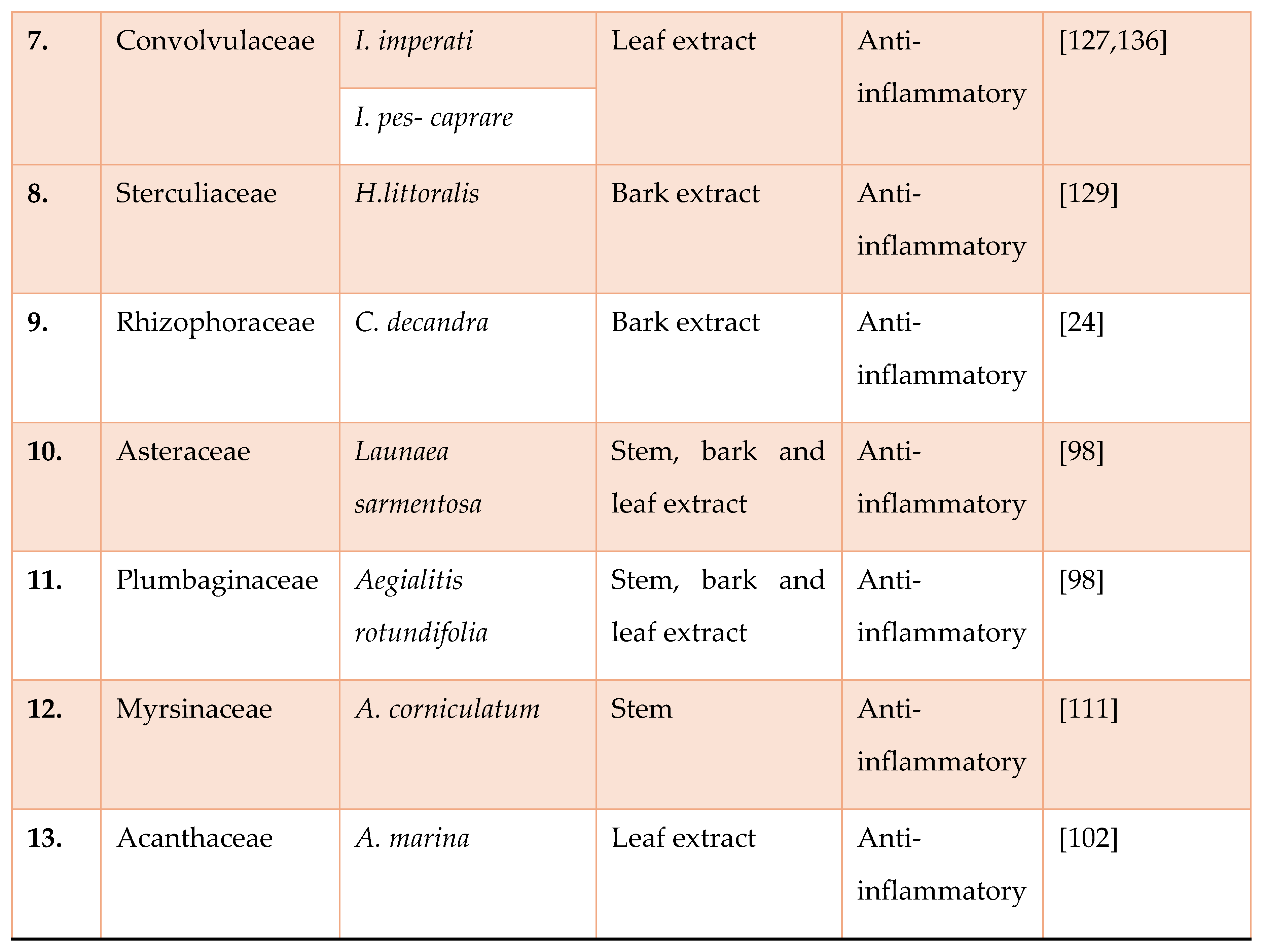
Study on Anti-Inflammatory Activity of Mangrove Plants in India:

Status of Biological Study Using Marine Flora in ANI:
Research Gap:
Conclusions
References
- Ansari J, Siraj A, Inamdar N. Pharmacotherapeutic approaches of Parkinson’s disease. Int. J.Pharmacol. 2010; 6(5): 584–90. [CrossRef]
- Bandaranayake, W. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes 1998, 2, 133– 148. [CrossRef]
- Composition in an antibacterial extract from Rhizophora mangle L.’s bark. J. Herba lPharmacother. 7: 107-128. Choudhari, A.S., et al., Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Frontiers in Pharmacology, 2020. 10(1614).
- Haq, M.; Sani, W.; Hossain, A.; Taha, R.M.; Monneruzzaman, K. Total phenolic contents, antioxidant and antimicrobial activities of Bruguiera gymnorrhiza. J. Med. Plants Res. 2011, 5, 4112–4118.
- Hossain, M.A.; Panthi, S.; Asadujjaman, M.; Khan, S.A.; Ferdous, F.; Sadhu, S.K. Phytochemical and pharmacological assessment of the ethanol leaves extract of Heritiera fomes buch. Ham. (familysterculiaceae). J. Porphyr. Phthalocyanines. 2013, 2, 95–101.
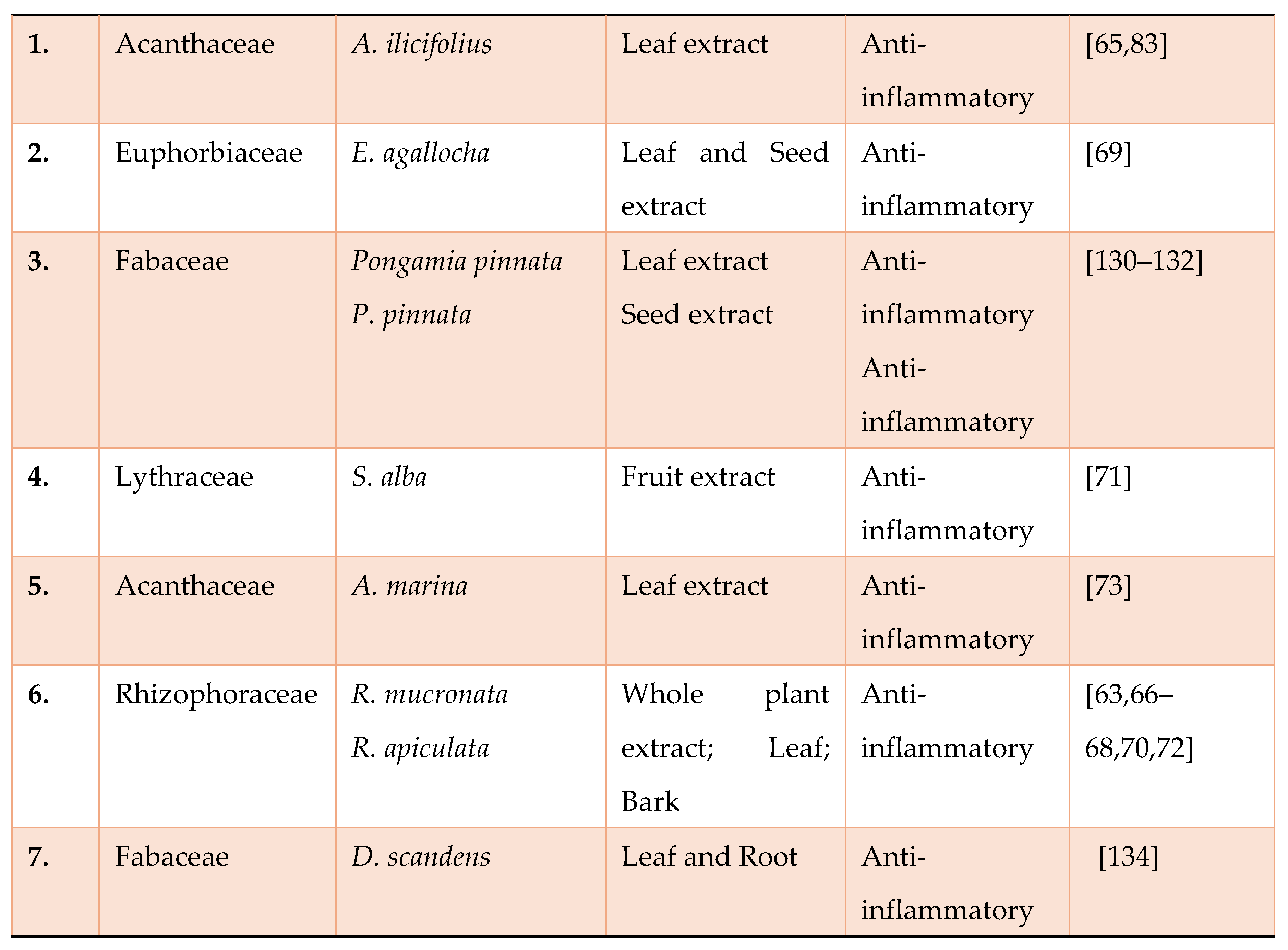
- Islam, M.A.; Saifuzzaman, M.; Ahmed, F.; Rahman, M.M.; Sultana, N.A.; Naher, K. Antinociceptive activity of methanolic extract of Acanthus ilicifolius linn. Leaves. Turk. J. Pharm. Sci. 2012, 9, 51–60.
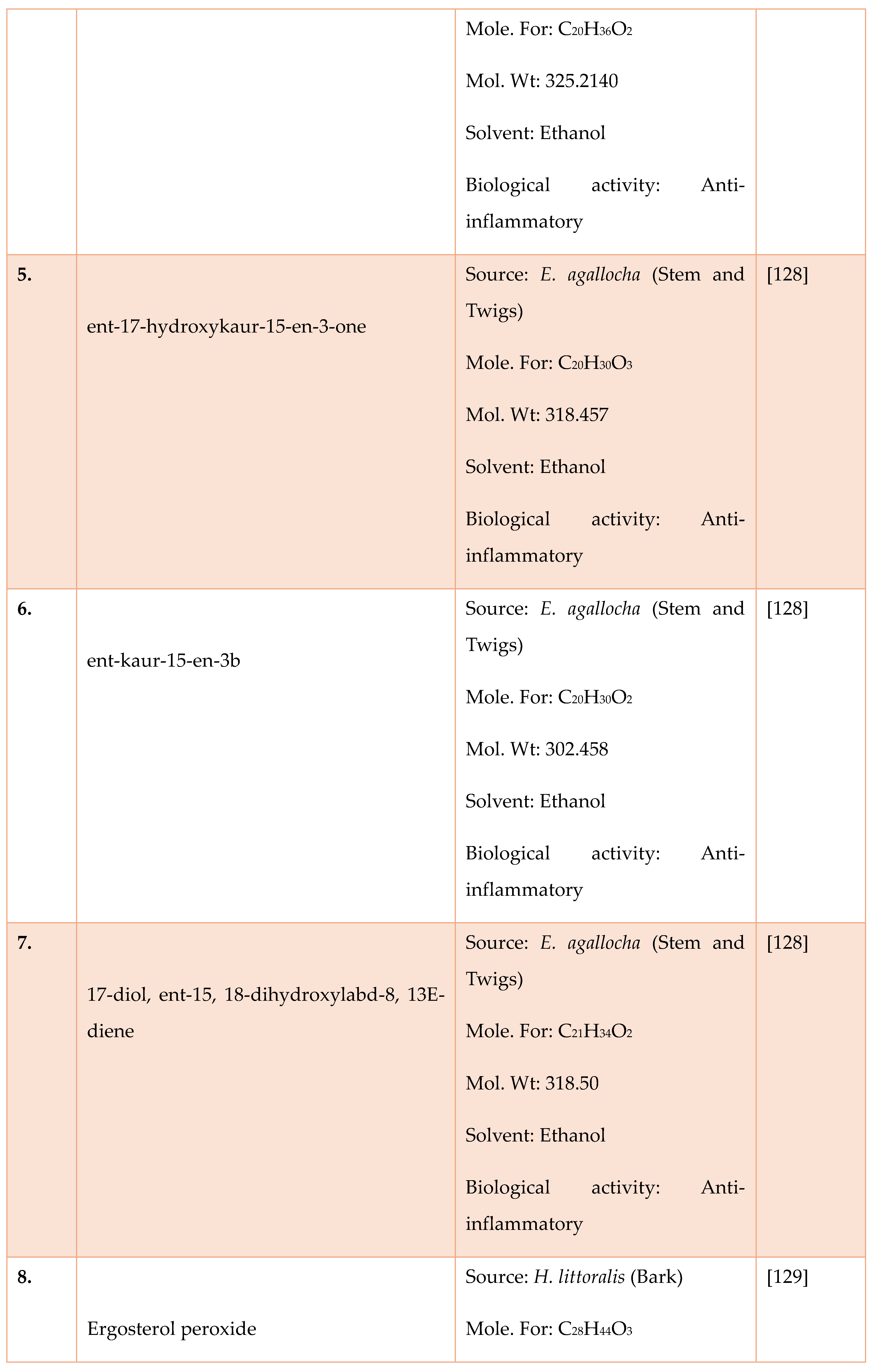
- Mondal, S.; Ghosh, D.; Ramakrishna, K. A complete profile on blind-your-eye mangrove Excoecaria agallochal.(euphorbiaceae): Ethnobotany, phytochemistry, and pharmacological aspects. Pharmacogn. Rev. 2016, 10, 123.
- Premanathan, M.; Arakaki, R.; Izumi, H.; Kathiresan, K.; Nakano, M.; Yamamoto, N.; Nakashima, H. Antiviral properties of a mangrove plant, Rhizophora apiculata blume, against human immunodeficiency virus. Antiviral Res. 1999, 44, 113–122. [CrossRef]
- Sánchez PLM, Melchor G, Alvarez S, Bulnes C (1998). Chemical and toxicological characterization of one wound healing formulation form Rhizophora mangle L. Rev. Salud Animal 20:69-72.
- Shu, Y.Z., Recent natural products based drug development: a pharmaceutical industry prespective. Journal of Natural Products, 1998. 61(8): p. 1053-71. Smuckler EA. Alcoholic Drink: Its Production And Effects.Fed Proe 1975; 34:2038-44.
- Sumithra, M.; Anbu, J.; Nithya, S.; Ravichandiran, V. Anticancer activity of methanolic leaves extract of Avicennia officinalis on ehrlich ascitis carcinoma cell lines in rodents. Int. J. Pharm. Tech. Res. 2011, 3, 1290–1292. [Google Scholar].
- Supriya Roy, Himani Awasthi (2017)Herbal Medicines As Neuroprotective Agent: A Mechanistic Approach.Int Journal of Pharmacy and Pharmaceutical science. Vol 9, Issue 11, 1-7Review Article.
- Teltumbde AK, Wahurwagh AK, Lonare MK, Nesari TM. Effect of yashtimadhu (Glycyrrhiza glabra) on intelligence and memory function in male adolescents. Scholars J Appl Med Sci 2013;1:90-5.
- Thatoi, H.; Samantaray, D.; Das, S.K. The genus Avicennia, a pioneer group of dominant mangrove plant species with potential medicinal values: A review. Front. Life Sci. 2016, 9, 267–291. [CrossRef]
- Banerjee D, Chakrabarti S, Hazra A K, Banerjee S, Ray J, and Mukherjee B. Antioxidant activity and total phenolics of some mangroves in Sundarbans. Afr J Biotechnol 2008; 7:805–810.
- Bandaranayake WM. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl Ecol Manag. 2002; 10:421–452. [CrossRef]
- Kothari MJ and Rao KM. Mangroves of Goa, Botanical Survey of India, Kolkatta, 2002.
- Wealth of India (Raw Materials) Vol. I. 1985. Acacia ferruginea, CSIR, New Delhi, 20003; 32-33.
- Naskar K and Mandal R. Ecology and Biodiversity of Indian Mangroves. Daya Publishing House, New Delhi, 1999.
- Hajra PK and Sanjappa M (eds.): Fascicle 22, Fascicles of Flora of India. Botanical Survey of India, Calcutta, 1996.
- Kokpol U, Miles D, Payne A, Chittawong V. Chemical constituents and bioactive compounds from mangrove plants. In: Atta-ur-Rahman (ed) Studies in natural products chemistry, vol 7. Elsevier, Amsterdam, 1990; 175-195.
- Uddin SJ et al. Evaluation of cytotoxic activity of patriscabratine, tetracosane and various flavonoids isolated from the Bangladeshi medicinal plant Acrostichum aureum. Pharma Biol 2012; 50:1276-80.
- Dhar JD et al., Post-coital antifertility activity of marine plant Acrostichum aureum L in rats. Indian J Med Res 1992; 96:150-152.
- Hossain H, Moniruzzaman S, Nimmi I, Kawsar H, Hossain A, Islam A, and Jahan IA. Anti-inflammatory and antioxidant activities of the ethanolic extract of Ceriops decandra (Griff.) Ding Hou bark. Orient Pharm Exp Med 2011; 11:215-220. [CrossRef]
- Hossain H et al. Anti-inflammatory activity of the ethanolic extract of Acrostichum aureum (Linn.) root. Bangladesh J Pharmacol 2011; 14:107-109.
- Choudhary N, et al. An overview of advances in the standardization of herbal drugs. J Pharm Res 2011; 2:55-70.
- Khan SA et al. Assessment of antioxidant and analgesic activity of Acrostichum aureum Linn. (Family- Pteridaceae). Pharmacologyonline, 2013; 1:166-171.
- Uddin SJ et al. (2S, 3S)-sulfated pterosin C, a cytotoxic sesquiterpene from the Bangladeshi mangrove fern Acrostichum aureum. J Nat Prod 2011; 74:2010-2013.
- Mouafi F, Abdel-Aziz SM, Bashir A, Amal AF. Phytochemical analysis and antimicrobial activity of c (Avicenna marina and Rhizophora stylosa) against some pathogens. World Appl Sci J 2014; 29:547-554.
- Neelam Garg, Shadia Mohammad abdel-aziz, Abhinav Aeron. Microbes in Food and Health. 1st Edition, 2016.
- Devi P, Solimabi W, D’Souza L, Kamat, SY. Toxic effects of coastal and marine plant extracts on mosquito larvae. Botanica Marina 1997; 40:533-535. [CrossRef]
- Harborne JB. Introduction to ecological biochemistry. Academic, London, 1982.
- Shahbudin S, Taher M, Deny S, Haitham Q, Anis. In vitro antimicrobial activity of antimicrobial plant Sonneratia alba. Asian Pac J Trop Biomed 2012; 2:427–429.
- Feller IC, Lovelock CE, Berger U, McKee KL, Joye SB, Ball MC. Biocomplexity in mangrove ecosystems. Annu Rev Mar Sci 2010; 2:395-417. [CrossRef]
- Singh CR, and Kathiresan K. Effect of cigarette smoking on human health and promising remedy by mangroves. Asian Pac J Trop Biomed 2015; 5:162-167. [CrossRef]
- Chinnappan R, Kandasamy K. Cigarette smoking effect on human health and promising remedy by mangroves. Asian Pac J Trop Biomed 2012; 1:1-6.
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 2017; 9:7204-7218. [CrossRef]
- Rahman MA, Haque E, Hasanuzzaman M, and Shahid IZ. Antinociceptive, antiinflammatory and antibacterial properties of Tamarix indica roots. Int J Pharmacol 2011; 7:527–531. [CrossRef]
- Srivastav S, Singh P, Mishra G, Jha KK, and Khosa RL. Achyranthes aspera-An important medicinal plant: A review. J Nat Prod Plant Resour 2011; 1:1-14.
- Kaushik U Lachake P Shreedhara CS and Ram HA. In-Vitro Antioxidant Activity of Extracts of Avipattikar Churna. Pharmacology online 2009; 3:581-589.
- Habib MA, Khatun F, khatun Ruma M, Kabir AH, Chowdhury AR S, Rahman A, and Hossain MI. A Review On Phytochemical Constituents of Pharmaceutically Important Mangrove Plants, Their Medicinal Uses and Pharmacological Activities. VRI Phytomedicine 2018; 6:1-6.
- Biswas R, Dutta PK, Achari B, Bandyopadhyay D, Mishra M, Pramanik KC, Chatterjee TK. Isolation of pure compound R/J/3 from Pluchea indica (L.) Less. and its anti-amoebic activities against Entamoeba histolytica. Phytom 2007; 14:534-537. [CrossRef]
- Sagnia B, Fedeli D, Casetti R, Montesano C, Falcioni G, Colizzi V. Antioxidant and Anti-Inflammatory Activities of Extracts from Cassia alata, Eleusine indica, Eremomastax speciosa, Carica papaya and Polyscias fulva Medicinal Plants Collected in Cameroon. PLOS ONE. 9: e103999. 2014. [CrossRef]
- Liu T, Zhang L, Joo D, and Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther, 2017; 2:1-9.
- Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 2012; 4:1-19. [CrossRef]
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009; 22:40-273. [CrossRef]
- Sun SC, Chang JH, Jin, J. Regulation of nuclear factor-kappaB in autoimmunity. Trends Immunol 2013; 34:282-289.
- Ghosh S and Karin M. Missing pieces in the NF-kappaB puzzle. Cell 2002; 109: S81-S96.
- Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Res 2011; 21:223-244.
- Huang Z, Cai X, Shao C, She Z, Xia X, Chen Y, Yan J., Zhou S, Lin Y. Chemistry and weak antimicrobial activities of phomopsins produced by mangrove endophytic fungus Phomopsis sp. ZSU-H76. Phytochem 2008; 69:1604-1608. [CrossRef]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 2008; 42:145-151. [CrossRef]
- Gohda J, Matsumura T, Inoue J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J Immunol 2004; 173: 2913-2917.
- Murphy KM. Janeway’s Immunobiology, 8th edn. Garland, 2010.
- O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 2010; 327:1098-1102. [CrossRef]
- Wang N, Liang H, Zen K (2014) Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol 2014; 5:614.
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122:787-795. [CrossRef]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol 2003; 73:209-212. [CrossRef]
- Balakrishnan A, and Graves SC. A composite algorithm for a concave-cost network flow problem. Networks 1989; 19:175-202. [CrossRef]
- Andrews HV and Sankaran V. Sustainable management of protected areas in the Andaman and Nicobar Islands, Andaman and Nicobar Islands environmental team. Indian Institute of Public Administration, and Fauna and Flora International, New Delhi, 2002.
- Sachithanandam V, Mageswaran T, Ragavan P, Mahapatra M, Sridhar R, Ramesh R and Mohan PM. Mangrove regeneration in tsunami affected area of north and south Andaman using insitu and remote sensing techniques. IJMS 2014; 43:1061-1067.
- Dam Roy S. A Compendium on mangrove biodiversity of Andaman and Nicobar Islands. Shri Vignesh Prints, Chennai 196, 2003.
- Jain K, Afidah AR, Azman IM. Anti-corrosive performance of wash primer based on mangrove tannin. Proceedings of the 15th Symposium of Malaysian Chemical Engineering, September 11-12, UTM, Malaysia, 2002; 323-327.
- Prabhu V, and Guruvayoorappan C. Anti-inflammatory and antitumor activity of the marine mangrove Rhizophora apiculata, J Immunotoxicol 2012; 9:341-352. [CrossRef]
- Krishnamoorthy M, Saikumar JM, Shama R, Pandiaraja C, Sofia P, Nagarajan. Antioxidant activities of bark extract from mangroves, Bruguiera cylindrica (L.) Blume and Ceriops decandra Perr. Indian J Pharmacol 2010; 43:557-582. [CrossRef]
- Kumar MS, Gorain B, Roy DK, et al., Anti-inflammatory activity of Acanthus ilicifolius, J Ethnopharmacol; 2008; 120:7-12. [CrossRef]
- Chakraborty K, Krishna Raola V. Two rare antioxidant and anti-inflammatory oleanenes from loop root Asiatic mangrove Rhizophora mucronata. Phytochem 2017; 135:160-165. [CrossRef]
- Raola VK, and Chakraborty K. Two rare antioxidative prenylated terpenoids from loop-root Asiatic mangrove Rhizophora mucronata (Family Rhizophoraceae) and their activity against pro-inflammatory cyclooxygenases and lipoxidase. Nat Prod Res 2017; 31:1-10. [CrossRef]
- Rohini RM and Amit Kumar Das. A comparative evaluation of a algesic a d anti- flammatory activities of Rhizophora mucroata bark extracts. Pharmacologyonline 2009; 1:780-791.
- Babuselvam M, Ravikumar S, Mohamed Farook KA, Abideen S, Peer Mohamed M, and Uthiraselvam M. Evaluation of anti-inflammatory and analgesic effects on the extracts of different parts of Excoecaria agallocha L. J Appl Pharm Sci 2012; 2:108-112.
- Kumari SC, Yasmin N, Raffiq Hussain M and Babuselvam M. Invitro anti-inflammatory and anti-arthritic property of Rhizopora mucronata leaves. Intern J Pharma Scie Res 2015; 6:482-485.
- Gawali P, Jadhav BL, and Ramteke L. Comparative studies on antioxidant and anti-inflammatory activities of ethanolic extracts of true mangrove and mangrove associate located in bhatye beach areas of maharashtra, india. Int. J Pharm Sci Rev Res 2017; 8:124-130. [CrossRef]
- Ray M, Adhikari A, Tapas K. Sur, Besra, SE, Biswas S, Das AK. Evaluation of anti-inflammatory potential of ethanolic extract of the leaves of Rhizophora mucronata, a sunderban mangrove. Inter J Res Develop Pharm Life Scien (2016); 6:2506-2516.
- Vijayaraj R, Dinesh Kumar G, Sri Kumaran, N. In vitro anti-inflammatory activity of silver nanoparticle synthesized Avicennia marina (Forssk.) Vierh.: A green synthetic approach. Intern J G Pharm 2018; 12: S528.
- Baskaran R and Mohan PM. In vitro antibacterial activity of leaf extracts of Rhizophora mucronata L. against multi drug resistant Vibrio spp. isolated from marine water Lobster’s larvae hatcheries. IJMS 2012; 41:218-222.
- Chander MP, Sachithanandam V and Vijayachari P. Antimicrobial and Hemolytic activity of seaweed Padina gymnospora from South Andaman, Andaman and Nicobar Islands of India. Int J Curr Microbiol App Sci 2014; 3:364-369.
- Geetha I and Manonmani AM. Surfactin: a novel mosquitocidal biosurfactant produced by Bacillus subtilis sp. (VCRC B471) and influence of abiotic factors on its pupicidal efficacy. Letter Appl Micro 2010; 51:406-412.
- Suman T, Kaleeswaran B, Immanuel T, Dam Roy S and Krishnan P. Antibacterial Activity of Pseudoceratina purpurea- A Marine Sponge from Andaman. Research J Pharm Tech 2013; 6:544-549.
- Meena B, Rajan LA, Vinithkumar NV and Kirubagaran. Novel marine actinobacteria from emerald Andaman and Nicobar Islands: a prospective sourcefor industrial and pharmaceutical by products. BMC Microbiol 2013; 13:145-162.
- Krishnan P, Veeramani S, Damroy S, et al. Antimicrobial Activity of Ircinia sp; a Marine Sponge and Its Associated Bacteria from Andaman Coast. Adv Animal Vet Sci 2014; 2: 37-41.
- Thirunavukkarasu P, Ramanathan T, Ramkumar L, Shanmugapriya R, and Renugadevi G. The antioxidant and free radical scavenging effect of Avicennia officinalis. J Med Plants Res 2011; 5:4754-4758.
- Bakshi M and Chaudhuri P. Antimicrobial potential of leaf extracts of ten mangrove species from Indian Sundarban. Int J Pharm Bio Sci 2014; 5:294-304.
- Sura S, Anbu J, Sultan MAD, and Uma BM. Antiulcer effect of ethanolic leaf extract of Avicennia officinalis. Pharmacologyonline 2011; 3:12-19.
- Sumithra M, Anbu J, Nithya S, and Ravichandiran V. Anticancer activity of methanolic leaves extract of Avicennia officinalis on ehrlich ascitis carcinoma cell lines in rodents. Int J Pharm Tech Res 2011; 3:1290–292.
- A Rege A, Y Ambaye R, and A Deshmukh R. In-vitro testing of anti-HIV activity of some medicinal plants. Indian J Nat Prod Resour 2010; 1:193-199.
- Sharief MN, and Umamaheswararao V. Antibacterial activity of stem and root extracts of Avicennia officinalis L. Int J Pharm App 2011; 2:231-236.
- Hossain ML. Medicinal activity of Avicennia officinalis: Evaluation of phytochemical and pharmacological properties. Saudi J Med Pharm Sci 2016; 2:250-255.
- Joel EL and Bhimba V. Isolation and characterization of secondary metabolites from the mangrove plant Rhizophora mucronata. Asian Pac J Trop Med 2010; 3:602–604.
- Kusuma S, Anil Kumar P, and Boopalan K. Potent antimicrobial activity of Rhizophora mucronata. J Ecobiotechnol 2011; 3:40-41.
- Sur TK, Hazra A, Hazra AK, and Bhattacharyya D. Antioxidant and hepatoprotective properties of Indian Sunderban mangrove Bruguiera gymnorrhiza L. leave. J Basic Clin Pharm 2016; 7:75.
- Ramanathan TAH. In Studies on Analgesic Activity of a Mangrove Species—Rhizophora mucronata Poir. In Proceedings of the Annual International Conference on Advances in Biotechnology (Biotech 2011), Bozen, Italy, 12-13, 2011.
- Nurdiani R, Firdaus M, and Prihanto AA. Phytochemical screening and antibacterial activity of methanol extract of mangrove plant (Rhizophora mucronata) from Porong River Estuary. J Basic Science Techn 2012; 1:27-29.
- Premanathan M, Nokashima H, Kathiresan K, Rajendran N, and Yamamoto N. In vitro anti human immunodeficiency virus activity of mangrove plants. Indian J Med Res 1996; 103:278-281.
- Suganthy N, and Pandima Devi K. In vitro antioxidant and anti-cholinesterase activities of Rhizophora mucronata. Pharm Biol 2016; 54:118-129.
- Jadhav RN and Jadhav BL. Evaluation of Antimicrobial principles of Rhizophora species along Mumbai Coast. J Adv Sci Res 2012; 3:30-33.
- Wahyuni WT, Darusman, LK and Surya NK. Potency of Rhizopora sp. Extracts as Antioxidant and Inhibitor of Acetylcholinesterase. Procedia Chem 2015; 16:681-686.
- Hardoko ES, Puspitasari YE, and Amalia R. Study of ripe Rhizophora mucronata fruit flour as functional food for antidiabetic. Int Food Res J 2015; 22:953-959.
- Pandey AK, Gupta PP, and Lal VK. Hypoglycemic effect of Rhizophora mucronata in streptozotocin induced diabetic rats. J Complement Integr Med 2014; 11:179–183. [CrossRef]
- Raju G S, RahmanMoghal MM, Hossain MS, Hassan MM, Billah MM, Ahamed SK and Rana SM. Assessment of pharmacological activities of two medicinal plant of Bangladesh: Launaea sarmentosa and Aegialitis rotundifolia roxb in the management of pain, pyrexia and inflammation. Biolog Res 2014; 47:1-11.
- Reddy ARK and Grace JR. Anticancer activity of methanolic extracts of selected mangrove plants. Int J Pharm Sci Res 2016; 7: 3852.
- Hasan I, Hussain MS, Millat MS, Sen N, Rahman MA, Rahman MA., and Moghal MM. Ascertainment of pharmacological activities of Allamanda neriifolia hook and Aegialitis rotundifolia Roxb used in Bangladesh: an in vitro study. J Tradit Complement Med 2018; 8:107-112.
- Premanathan M, Arakaki R, Izumi H, Kathiresan K, Nakano M, Yamamoto N, and Nakashima H. Antiviral properties of a mangrove plant, Rhizophora apiculata Blume, against human immunodeficiency virus. Antiviral Res 1999; 44:113–122. [CrossRef]
- Shafie, M., Forghani, A., and Moshtaghiyan J. Anti-inflammatory effects of hydro-alcoholic extracts of mangrove (Avicennia marina) and vitamin C on arthritic rats. Bull Environ Pharmacol Life Sci, 203; 2:32-37.
- Rout P, and Basak UC. Antioxidant Properties in Leaf and Root Extracts of Some Medicinally Important Mangrove Species of Odisha Coast. American J PharmTech Res 2012; 4:1-13.
- Afidah AR, Emmanuel R, Jein, S, Jain MK, Sani MI, Hasnah O. Antioxidant activities of mangrove Rhizophora apiculata bark extracts. Food Chem 2008; 107:200-207.
- Karami L, Majd A, Mehrabian S, Nabiuni M, Salehi M, and Irian S. Antimutagenic and anticancer effects of Avicennia marina leaf extract on Salmonella typhimurium TA100 bacterium and human promyelocytic leukaemia HL-60 cells. Sci Asia 2012; 38:349-355. [CrossRef]
- Al Maqtari Maher A. Screening of Salt-stress, antioxidant enzyme, and antimicrobial activity of leave extracts of mangroves Avicennia marina L. from Hodaidah, Yemen. J Stress Physiol Biochem 2014; 10:190-199.
- Gawali poonam and Jadhav BL. Antioxidant activity and antioxidant phytochemical analysis of mangrove species Sonneratia alba and Bruguiera cylindrica. Asian J Microbiol Biotechnol Environ Sci 2011; 13:257-261.
- Rahmatullah M, Jahan N, Chowdhury MH, Jahan R, and Nasrin, D. Brine shrimp toxicity study of different Bangladeshi medicinal plants. Adv Nat Appl Scien, 2010; 4:163–173.
- Hossain MA, Panthi S, Asadujjaman M, Khan SR, Ferdous F, and Sadhu SK. Phytochemical and pharmacological assessment of the ethanol leaves extract of Heritiera fomes Buch. Ham. (Family- Sterculiaceae)”. J Porphyrins Phthalocyanines 2013; 2:95–101.
- Mahmud I, Islam MK, Saha S, Barman AK, Rahman MM, Anisuzzman, M., and Rahmatullah, M. Pharmacological and ethnomedicinal overview of Heritiera fomes: future prospects. Int Sch Res Notices 2014; 1-12. [CrossRef]
- Roome T, Dar A, Naqvi S, Ali S, and Choudhary MI. Aegiceras corniculatum extract suppresses initial and late phases of inflammation in rat paw and attenuates the production of eicosanoids in rat neutrophils and human platelets. J Ethnopharmacol 2008; 120:248-254. [CrossRef]
- Roome T, Dar A, Naqvi S, and Choudhary MI. Evaluation of antinociceptive effect of Aegiceras corniculatum stems extracts and its possible mechanism of action in rodents. J Ethnopharmacol, 2011; 135:351-358. [CrossRef]
- Janmanchi H, Raju A, Degani MS, Ray MK, and Rajan, MGR (2017) Antituberculosis, antibacterial and antioxidant activities of Aegiceras corniculatum, a mangrove plant and effect of various extraction processes on its phytoconstituents and bioactivity. S Afr J Bot 2017; 113:421-427. [CrossRef]
- Mondal S, Ghosh D, and Ramakrishna K. A complete profile on blind-your-eye mangrove Excoecaria agallocha L.(Euphorbiaceae): Ethnobotany, phytochemistry, and pharmacological aspects. Pharmacogn Rev 2016; 10:123. [CrossRef]
- Simlai A and Roy A. Biological activities and chemical constituents of some mangrove species from Sundarban estuary: An overview. Pharmacognosy reviews 2013; 7:170.
- Das SK, Samantaray D, and Thatoi H. Ethnomedicinal, antimicrobial and antidiarrhoeal studies on the mangrove plants of the genus Xylocarpus: a mini review. J Bioanal Biomed 2014; 12:1-7. [CrossRef]
- Paul T and Ramasubbu S. The antioxidant, anticancer and anticoagulant activities of Acanthus ilicifolius L. roots and Lumnitzera racemosa Willd. leaves, from southeast coast of India. J Appl Pharm Sci 2017; 7:081-087.
- Gao M, and Xiao H. Activity-guided isolation of antioxidant compounds from Rhizophora apiculata. Molecule 2012; 17:10675-10682. [CrossRef]
- Lim SH, Darah I, and Jain K. Antimicrobial activities of tannins extracted from Rhizophora apiculata barks. J Trop For Sci 2006; 18:59-65.
- Thomas T. In vitro evaluation of antibacterial activity of Acrostichum aureum Linn Indian J Nat Prod Resour 2012; 3:135-138.
- Abeysinghe PD. Antibacterial activity of some medicinal mangroves against antibiotic resistant pathogenic bacteria. Indian J Pharm Sci, 2010; 72:167. [CrossRef]
- Wang Y, Zhu H, and Tam NFY. Polyphenols, tannins and antioxidant activities of eight true mangrove plant species in South China. Plant and soil 2014; 374:549-563. [CrossRef]
- Wei SD, Zhou HC, and Lin YM. Antioxidant activities of extract and fractions from the hypocotyls of the mangrove plant Kandelia candel. Int J Mol Sci 2010; 11:4080-4093. [CrossRef]
- Bunyapraphatsara N, Jutiviboonsuk A, Sornlek P, Therathanathorn W, Aksornkaew S, Fong H H., Kosmeder, J. Pharmacological studies of plants in the mangrove forest. Thai J Pharmacol 2003; 10:1-12.
- Angalabiri-Owei BE and Isirima, JC. Evaluation of the lethal dose of the methanol extract of Rhizophora racemosa leaf using Karbers method. Afr J Cell Path 2014; 2:65–68.
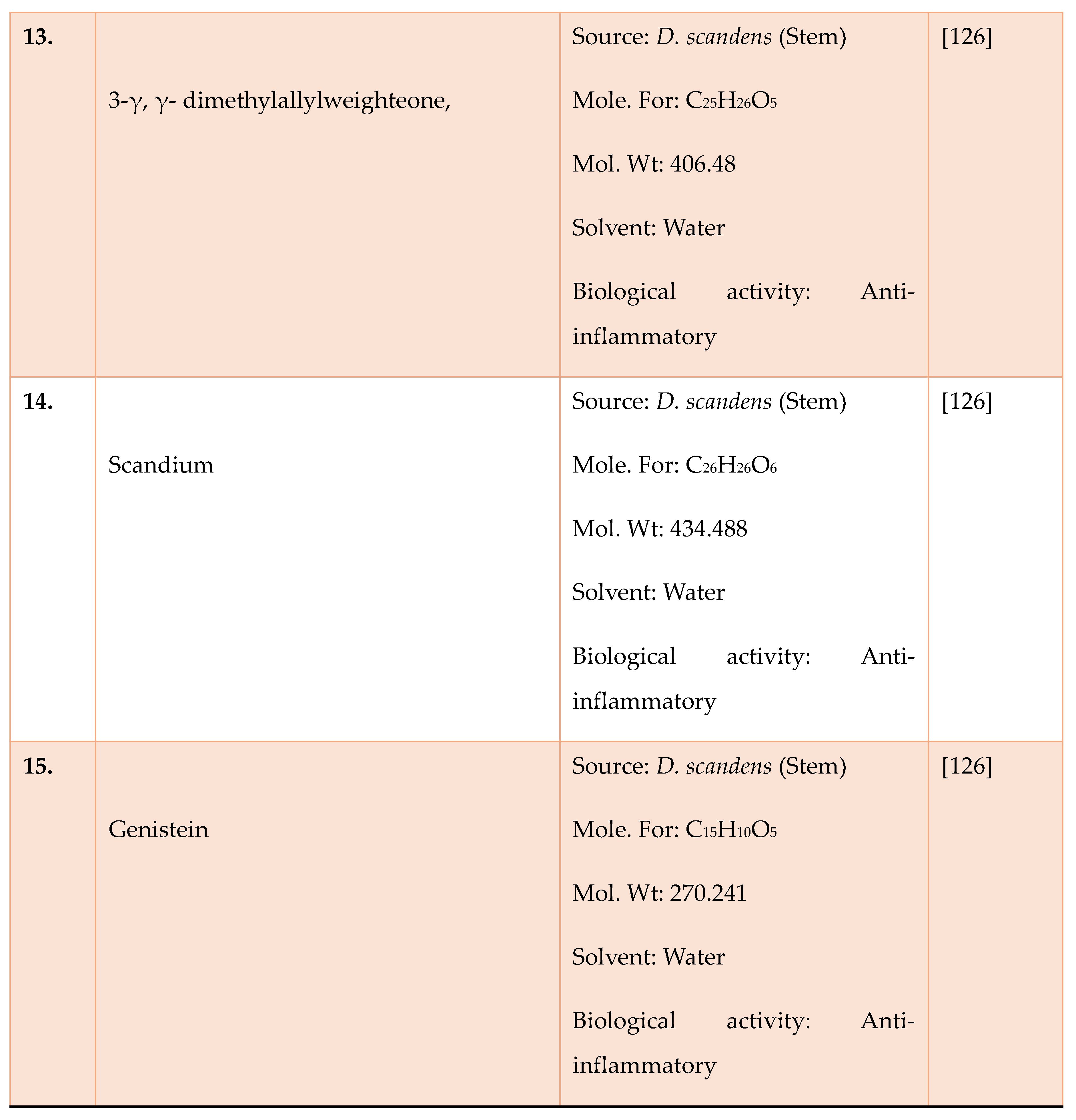
- Laupattarakasem P, Houghton PJ and Hoult JRS. Anti-inflammatory isoflavonoids from the stems of Derris scandens. Planta medica 2004; 70:496-501. [CrossRef]
- Pongprayoon U, Bohlin L, Soonthornsaratune P, and Wasuwat, S. Anti-inflammatory activity of Ipomoea pes-caprae (L.) R. Br. Phytother Res 1991; 5:63-66.
- Li Y, Liu J, Yu S, Proksch P, Gu J, and Lin W. TNF-α inhibitory diterpenoids from the Chinese mangrove plant Excoecaria agallocha L. Phytochem 2010; 71:2124-2131. [CrossRef]
- Tewtrakul S, Tansakul P, Daengrot C, Ponglimanon C, and Karalai C. Anti-inflammatory principles from Heritiera littoralis bark. Phytomedicine 2010; 17:851-855. [CrossRef]
- Singh RK, Joshi VK, Goel RV, Gambhir SS, and Achaiya SB. Pharmacological actions of Pongamia pinnata seeds—a preliminary study, Indian J Exp Biol 1996; 34:1204-1207.
- Muruganandan S, Srinivasan K, Tandan SK, Lal J, Chandra S, and Raviprakash, V “Anti-inflammatory and antinociceptive activities of some medicinal plants, ” J Med Plants Res 2000; 22:56-58.
- Srinivasan K, Muruganandan S, Lal J, et al., Antinociceptive and antipyretic activities of Pongamia pinnata leaves. Phytother Res 2003; 17:259-264. [CrossRef]
- Ganapaty S, Josaphine JS and Thomas PS. Anti-Inflammatory activity of Derris scandens. J Nat Remedies 2006; 6:73-76.
- Behbahani M, Shanehsazzadeh M, and Hessami MJ. Optimization of callus and cell suspension cultures of Barringtonia racemosa (Lecythidaceae family) for lycopene production. Scientia Agricola, 2011; 68:69-76. [CrossRef]
- Yodsaoue O, Karalai C, Ponglimanont C, Tewtrakul S, and Chantrapromma, S. Potential anti-inflammatory diterpenoids from the roots of Caesalpinia mimosoides Lamk. Phytochem 2010; 71:1756-1764. [CrossRef]
- Paula ACB, Hayashi LSS, and Freitas JCD. Anti-inflammatory and antispasmodic activity of Ipomoea imperati (Vahl) Griseb (Convolvulaceae). Braz J Med Biol Res, 2003; 36:105-112. [CrossRef]
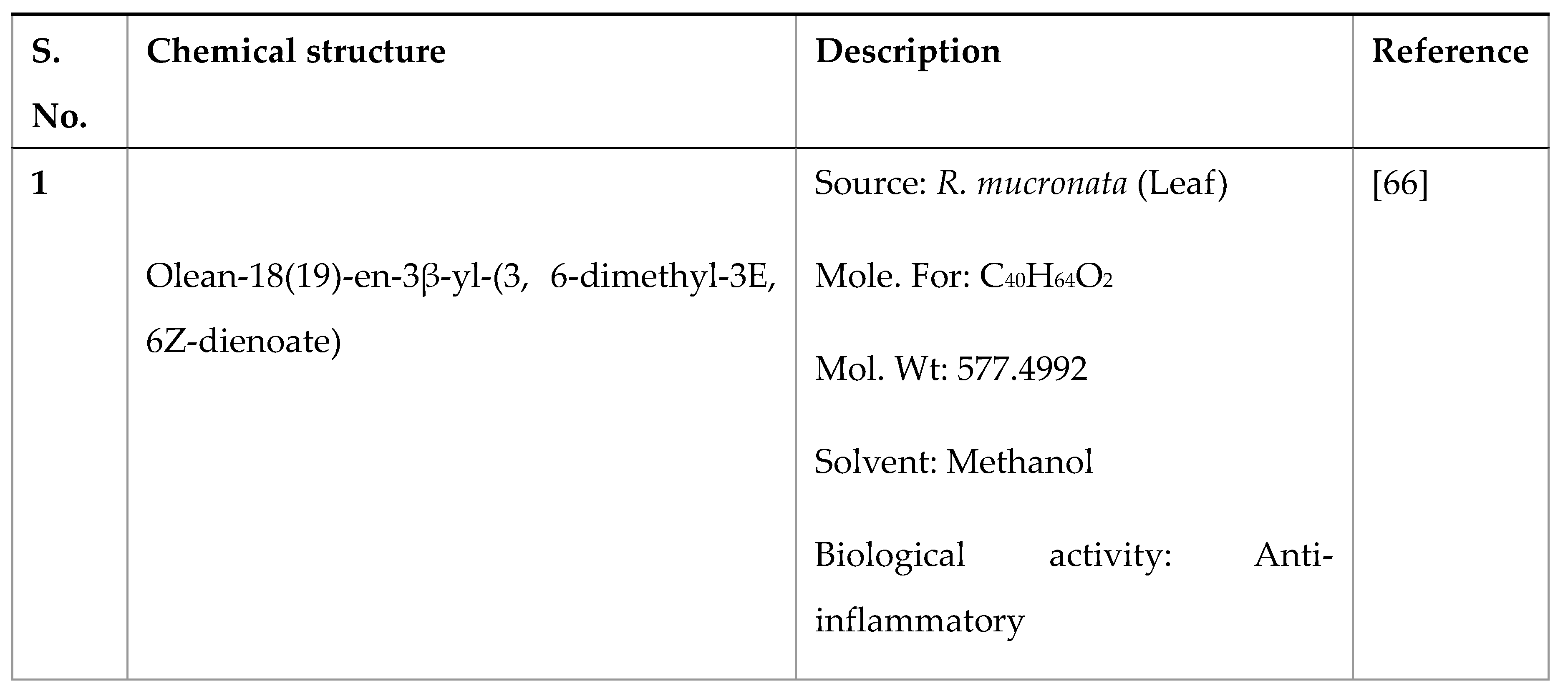
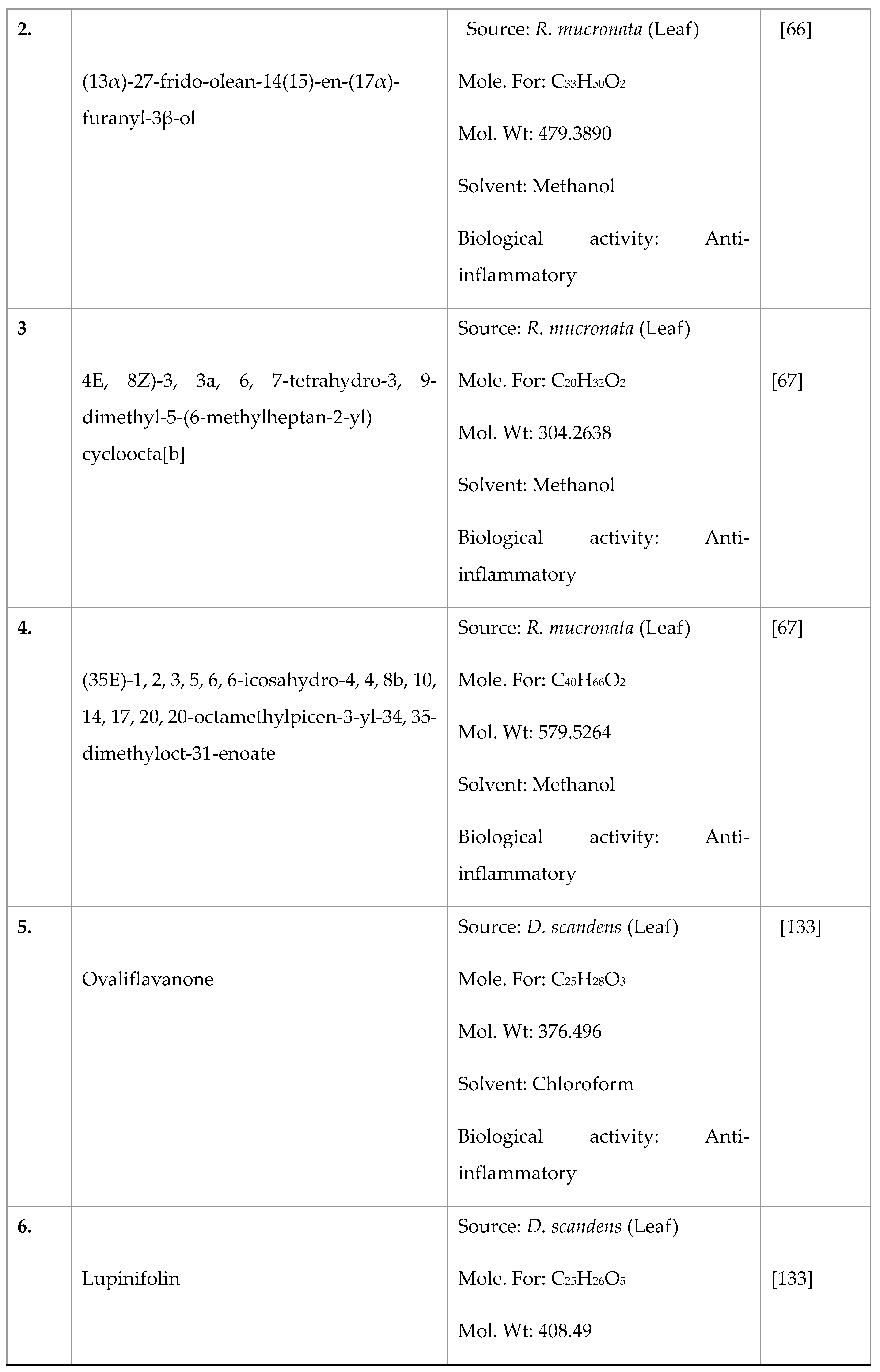
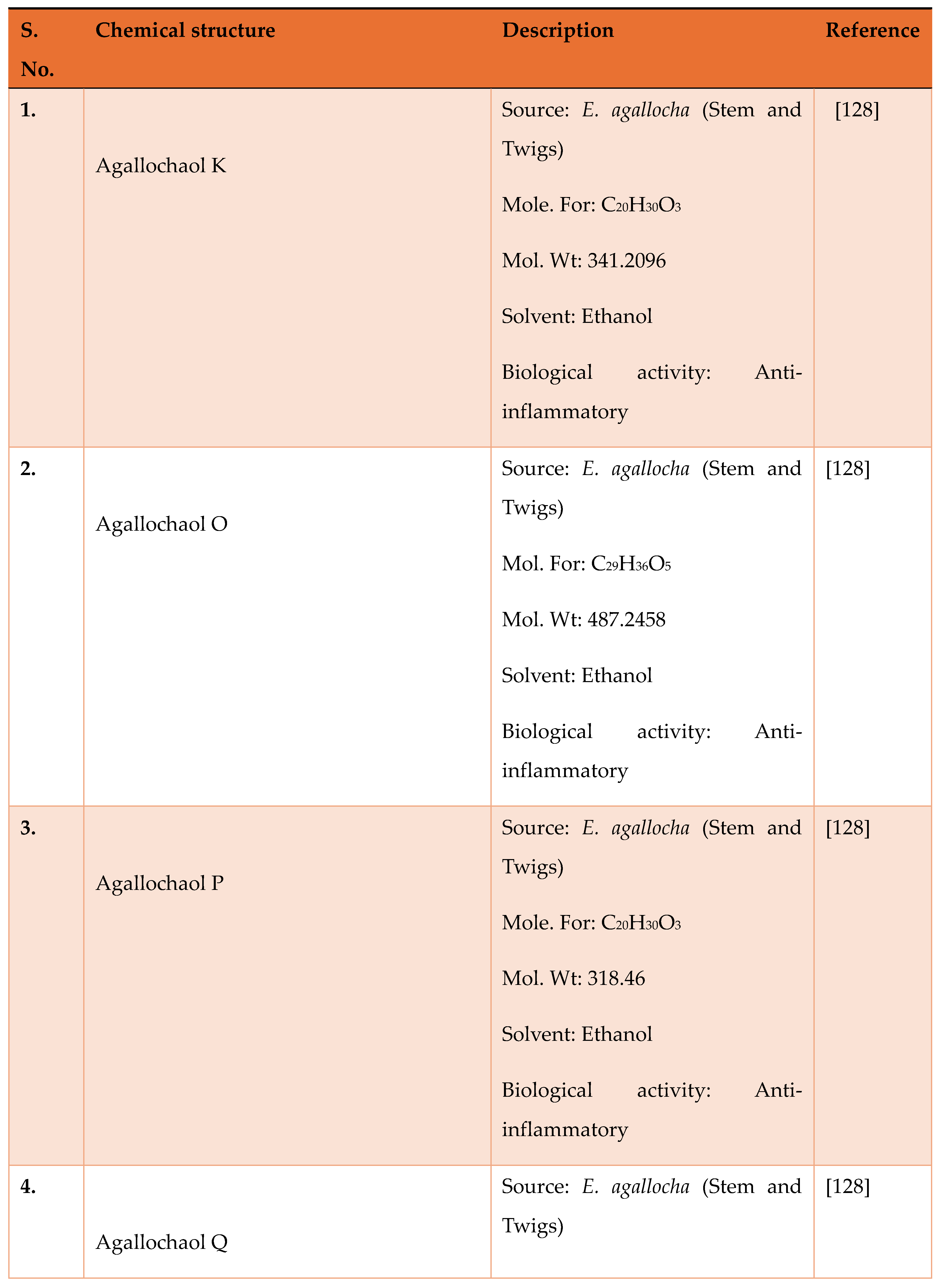

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




