3.1. Vulcanization Process and Physical-Mechanical Properties of Sulfur Cured Rubber Compounds
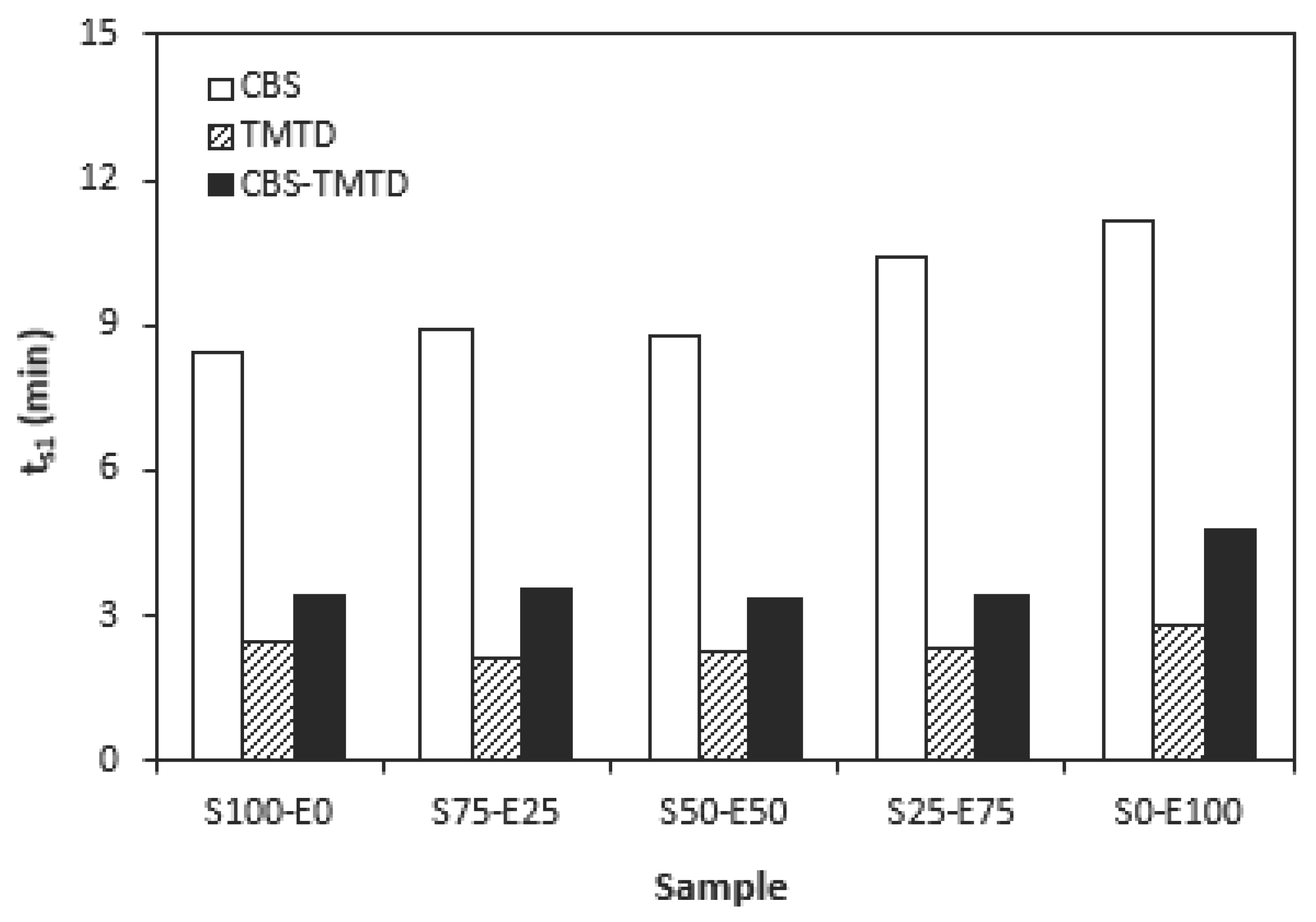
The influence of curing system compositions on vulcanization characteristics of rubber compounds was examined trough determination of curing characteristics, scorch time
ts1, optimum cure time
tc90, curing rate
R and the difference between the maximum and minimum torque
∆M. As seen in
Figure 1, the highest scorch time exhibited rubber compounds cured in the presence of CBS accelerator. On the other hand, the lowest
ts1 demonstrated the rubber formulations cured with TMTD. It can be stated that the scorch time showed slight increasing trend with increasing ratio of EPDM in rubbers combinations, and the highest scorch time exhibited rubber formulations based on EPDM (S0-E100). Looking at
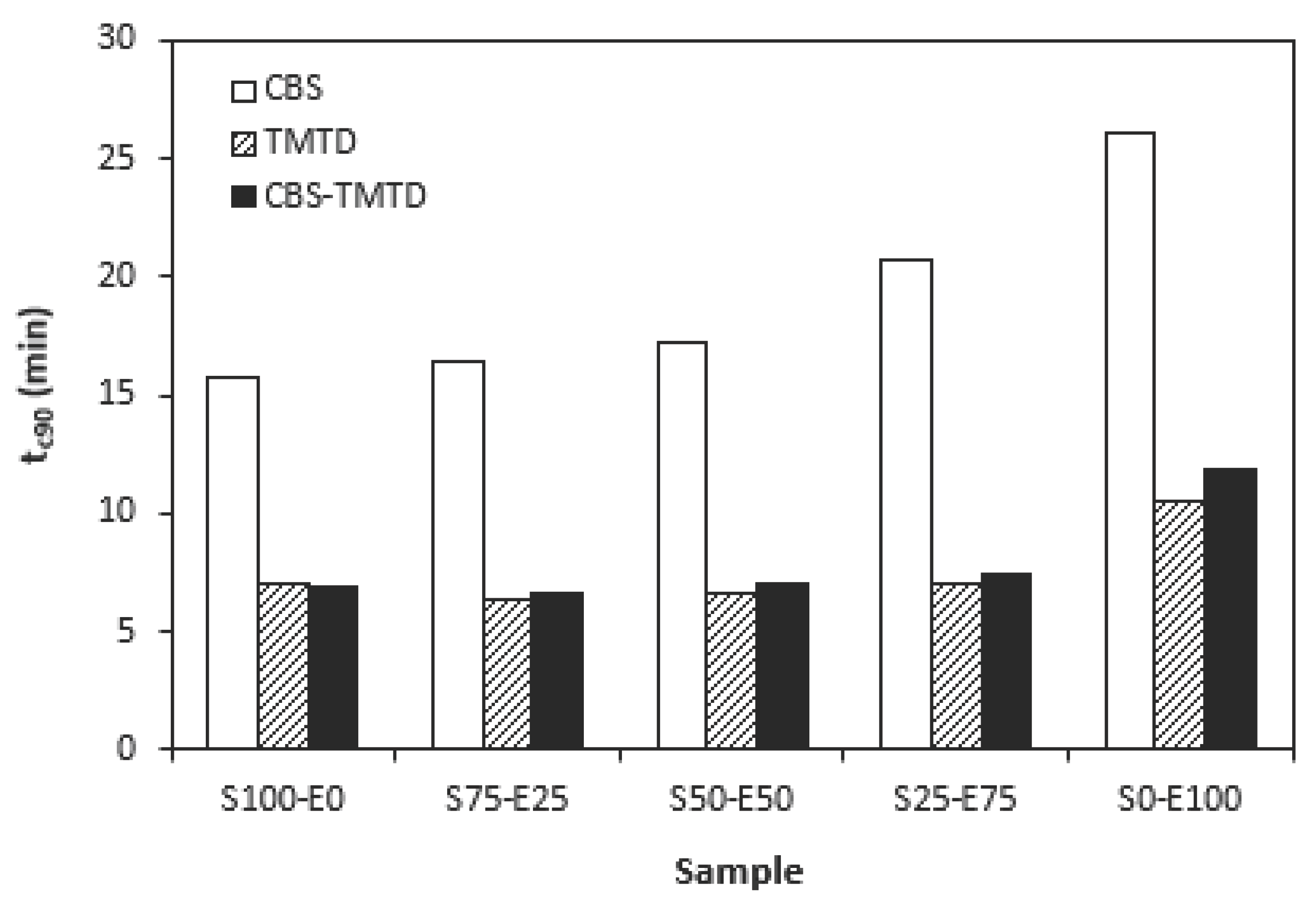
Figure 2, one can see very similar dependences of optimum cure time on the used accelerators. The highest optimum cure manifested rubber compounds cured in the presence of CBS. From
Figure 2 it also becomes apparent that the optimum cure time of CBS-based formulations increased with increasing ratio of EPDM. When compared to rubber compound based on SBR (S100-E0), the
tc90 of the compound based on EPDM (S0-E100) prolonged in more than 10 min (from 16 min to more than 26 min). The rubber compounds cured in the presence of TMTD or combination CBS-TMTD required almost identical time needed for their optimum cross-linking. Again, the highest
tc90 exhibited the rubber compounds with designation S0-E100.
The dependencies of the optimum cure time as well as scorch time on the type of accelerator used relate with the character and structure of the accelerators. CBS belongs to class of fast accelerators of sulfenamide type that are characterized by long induction period (delayed action accelerators). Thus, the compounds cured with CBS exhibited the longest scorch time. TMTD is from the group of very fast accelerators, which indicates that the curing process of rubber formulations proceeds faster. To that corresponds shorter scorch time as well as optimum cure time of the corresponding compounds. When combining both CBS and TMTD, the optimum cure time was not changed, while slight prolongation of scorch time was recorded in comparison with the equivalent TMTD cured rubber compounds. Although, the prolongation of scorch time is not very significant, it is a positive aspect regarding safe processability of rubber compounds into final cross-linked materials. The scorch time or induction period represents the time during which, the cross-linking does not occur yet. The mutual interactions among the additives of curing system occur and the materials must be heated uniformly in all volume. This is very important factor to achieve homogenous distribution of the formed cross-links within the rubber matrix and thus to form uniform spatial three-dimensional cross-linked structure. When reaching this, the following vulcanization can proceed fast, which brings time and economic benefits. Short optimum vulcanization time of rubber compounds with accelerators combination (CBS-TMTD) can be attributed to the presence of very fast accelerator (TMTD) on one hand, and to the increase of the overall accelerators content (2 phr). In general, the higher is the activity and amount of accelerators, the faster is the vulcanization process [
14].
It was recorded that the curing process of the materials based on EPDM was longer than that of the corresponding compounds based on SBR. Considering that SBR is highly unsaturated rubber, the concentration of double bonds in its structure is much higher when compared to EPDM (the double bonds are situated only in non-conjugated ENB monomer units, the concentration of which in EPDM is only 4.5 wt.%). In overall, the lower is the concentration of the double bonds in rubber structure, the slower is the vulcanization process.
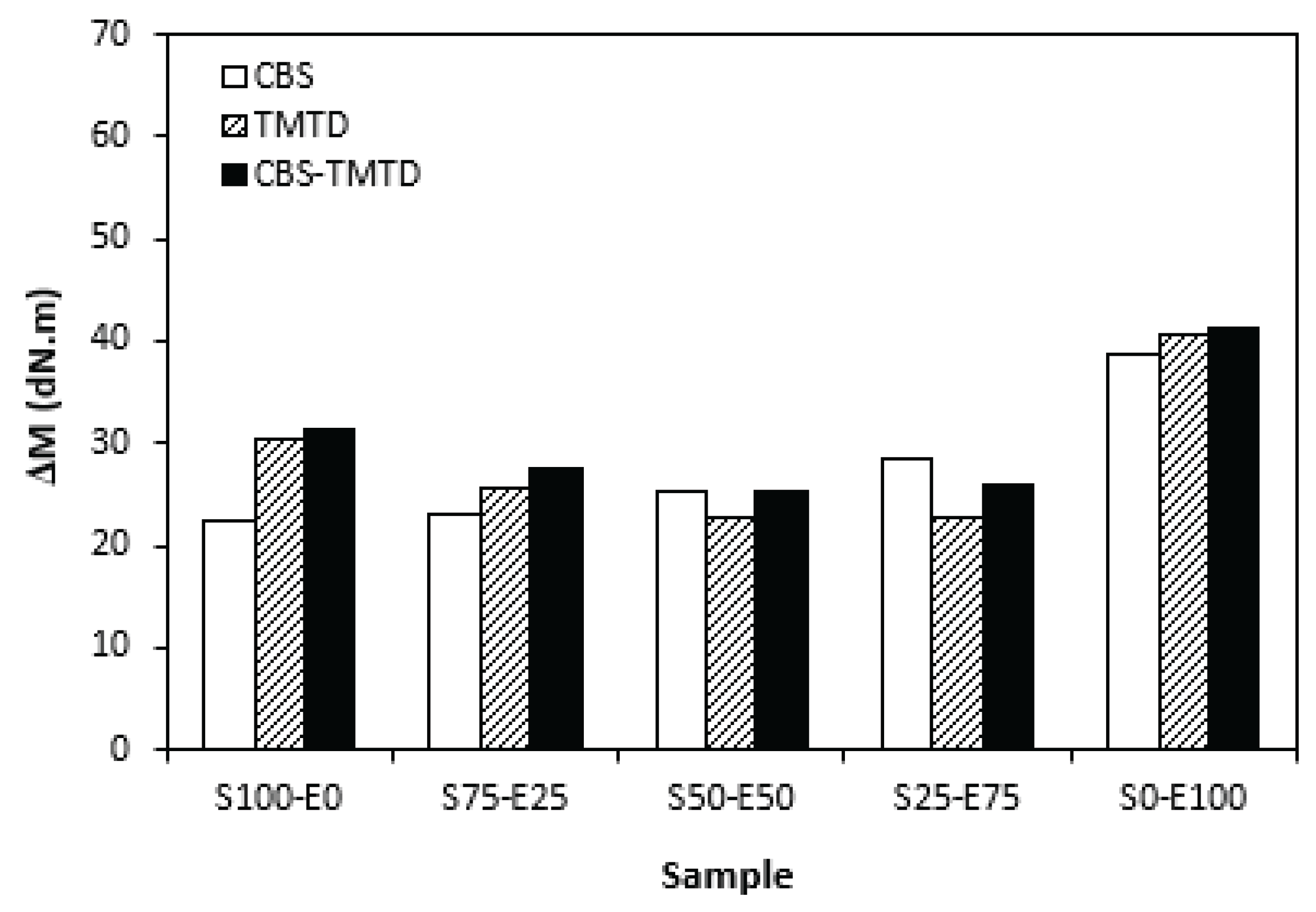
The difference between the maximum and minimum torque usually relates with the amount of the cross-links formed within the rubber matrix and expresses the transformation degree of uncured rubber compound into vulcanizate. Generally speaking, the higher is the torque difference, the higher is the cross-link density. Though, this is valid mainly for unfilled rubber compounds. As seen in
Figure 3, the biggest difference between the maximum and minimum torque exhibited rubber compounds based on EPDM (S0-E100). The differences in
ΔM values in dependence on the type of accelerator, or accelerators combinations were minimal. Looking at
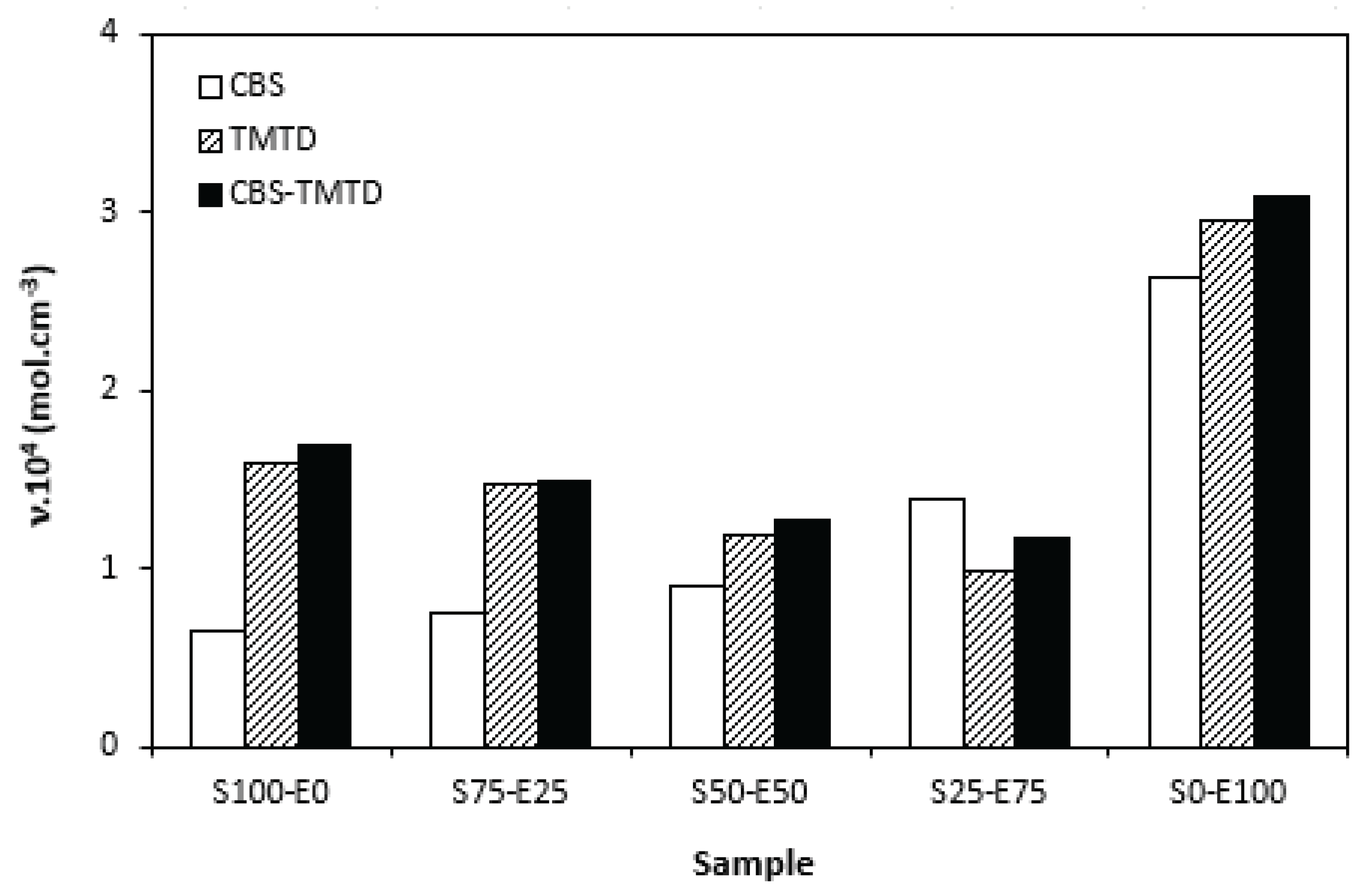
Figure 4, one can see certain correlation between the torque difference and cross-link density. That means, the highest torque difference of the compounds with designation S0-E100 was reflected in their highest cross-linking degree. It becomes interesting that the cross-link density of the materials with CBS showed increasing trend with increasing ratio of EPDM in rubbers combinations. On the other hand, the cross-link density of vulcanizates cured in the presence of TMTD and combination CBS-TMTD tended to decrease with increasing ratio of EPDM up to the composition S25-E75. Then, significant increase of cross-linking degree occurred for the vulcanizate S0-E100. When comparing the curing systems, the highest cross-link density exhibited rubber compounds cured with CBS-TMTD combination. TMTD acts not only as accelerator, but also as sulfur donor due to sulfur bridges in its structure and thus it contributes to the cross-links formation. The highest cross-link density of vulcanizates based on EPDM seems to be surprising, as EPDM has low value of unsaturation and thus the cross-link density was expected to be lower when compared to vulcanizates based on highly unsaturated SBR. The possible explanation could be the fact, the EPDM might have highly branched structure and the physical entanglements linked with chemical linkages can act as additional cross-links.
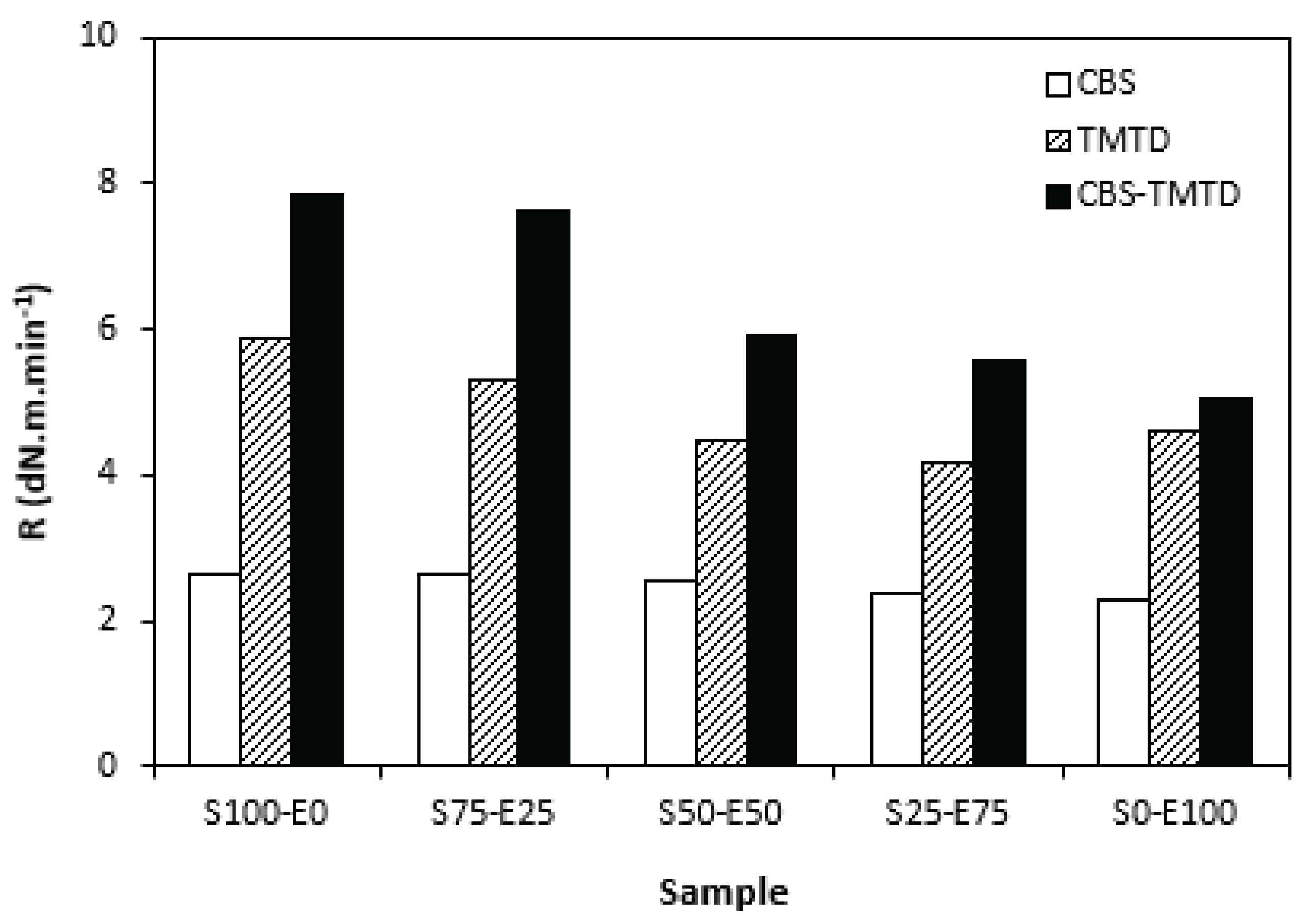
From
Figure 5 it becomes apparent that the lowest curing rate exhibited formulations cured with CBS with almost no dependence on the type of rubber or their combinations. The application of TMTD resulted in higher curing rate, which was found to slightly decrease with increasing ratio of EPDM in rubbers combinations. The highest curing rate demonstrated the materials cured with CBS-TMTD combination with the highest influence on the type of rubber or rubbers combinations. The cure rate accounts not only the differences between the optimum cure time and scorch time, but also the differences between torques at optimum cure time and scorch time thus it expresses the transformation degree of uncured rubber compound into vulcanizate. The highest curing rate of the compounds based on SBR (S100-E0) suggests that the curing process of equivalent rubber compounds proceeded the fastest.
The physical-mechanical properties of sulfur cured vulcanizates are depicted in
Figure 6,
Figure 7,
Figure 8 and
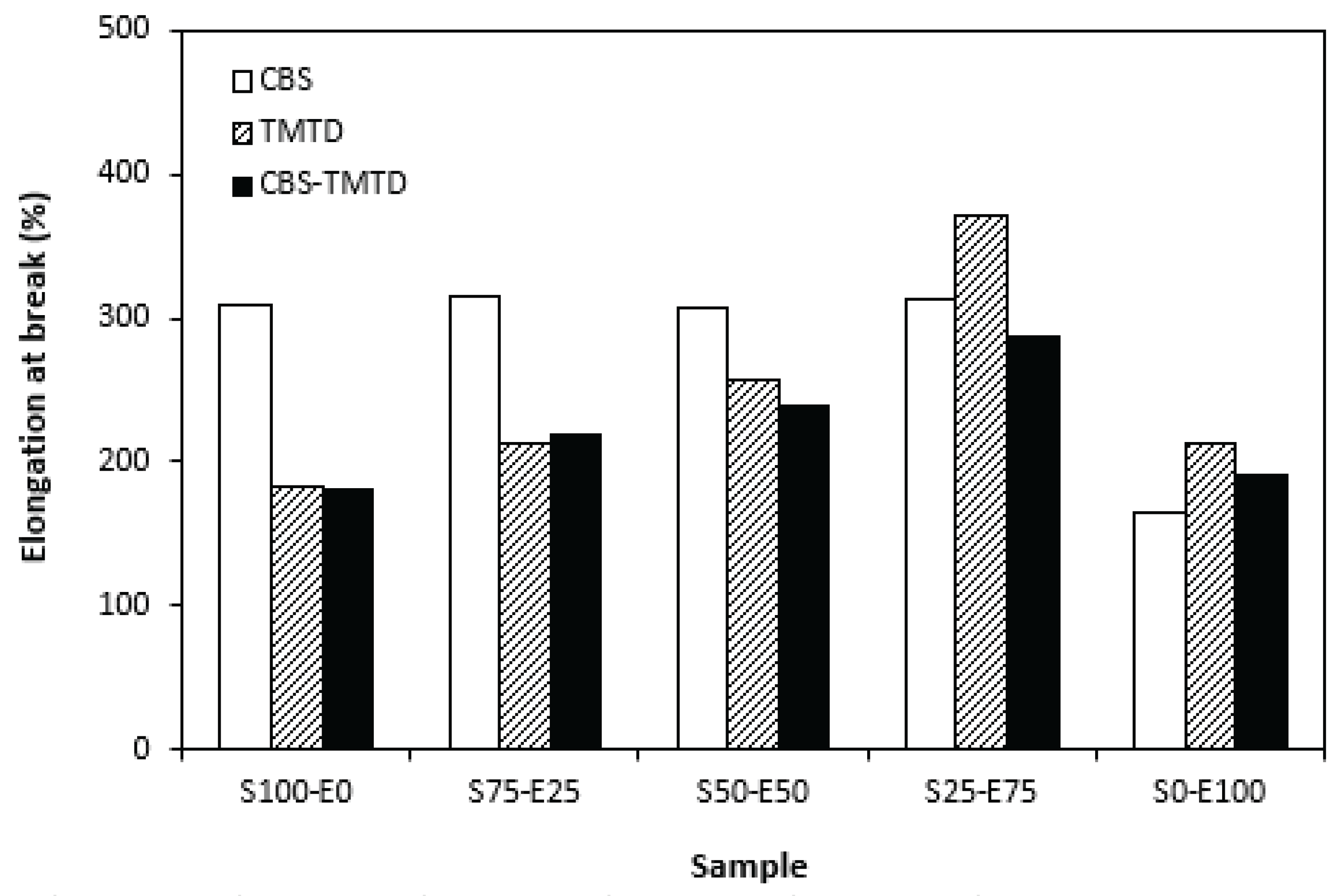
Figure 9. The elongation at break of vulcanizates is to certain extent in correlation with the cross-link density. As the rubber compounds cured in the presence of CBS (S100-E0, S75-E25, S50-E50) exhibited the lowest cross-link density, those vulcanizates were found to have the highest elongation at break (
Figure 6). Also, as the cross-link density of vulcanizates cured in the presence of TMTD and CBS-TMTD combination showed decreasing trend with increasing ratio of EPDM up to the composition S25-E75, the elongation at break increased in the same direction. The lowest elongation at break exhibited vulcanizates based on EPDM with the highest degree of cross-linking. The increasing degree of cross-linking leads to the restriction of elasticity and mobility of rubber chains, as a result of which, the elongation at break decreases.
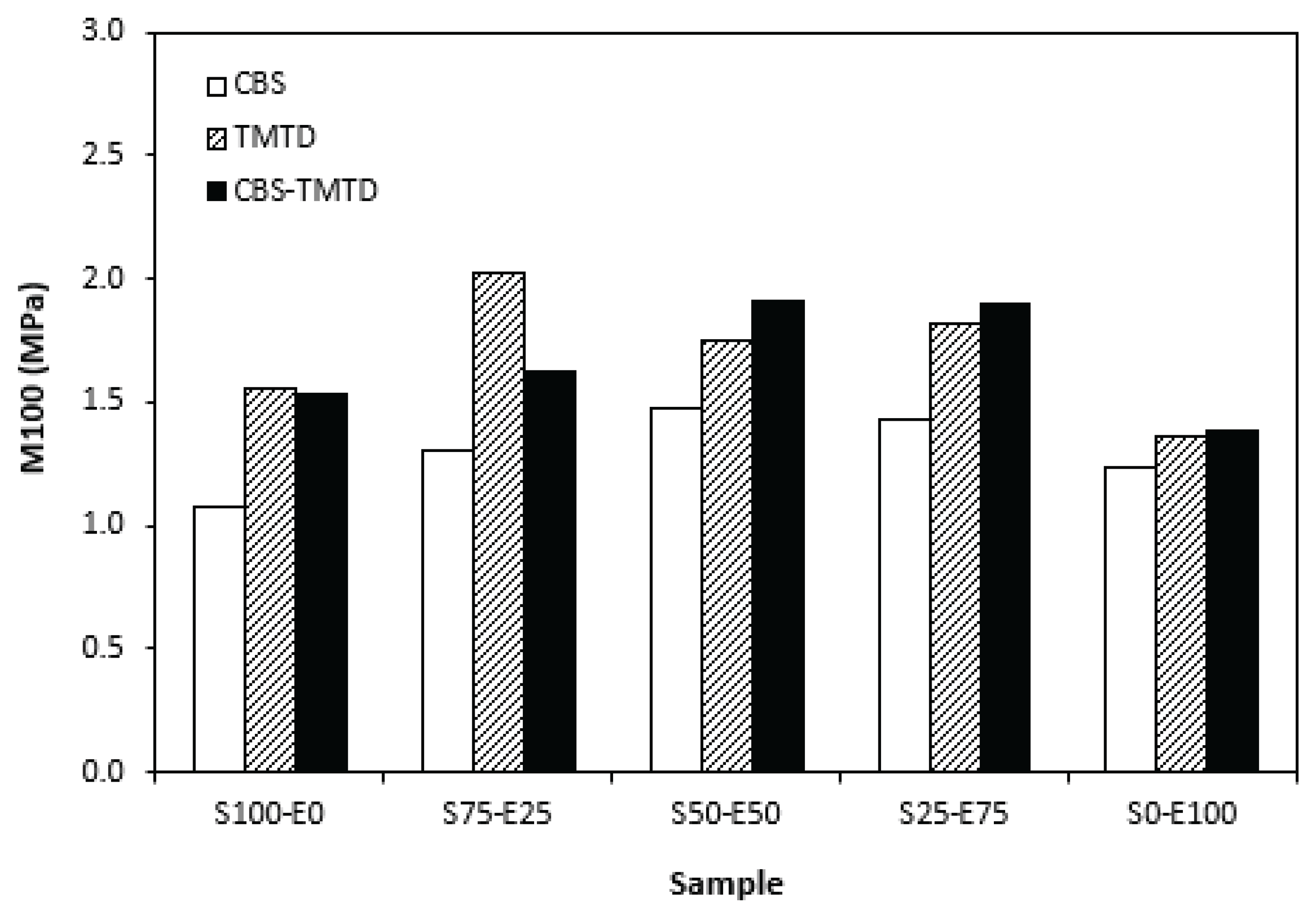
The lowest cross-link density of vulcanizates cured in the presence of CBS was reflected in their lowest modulus (
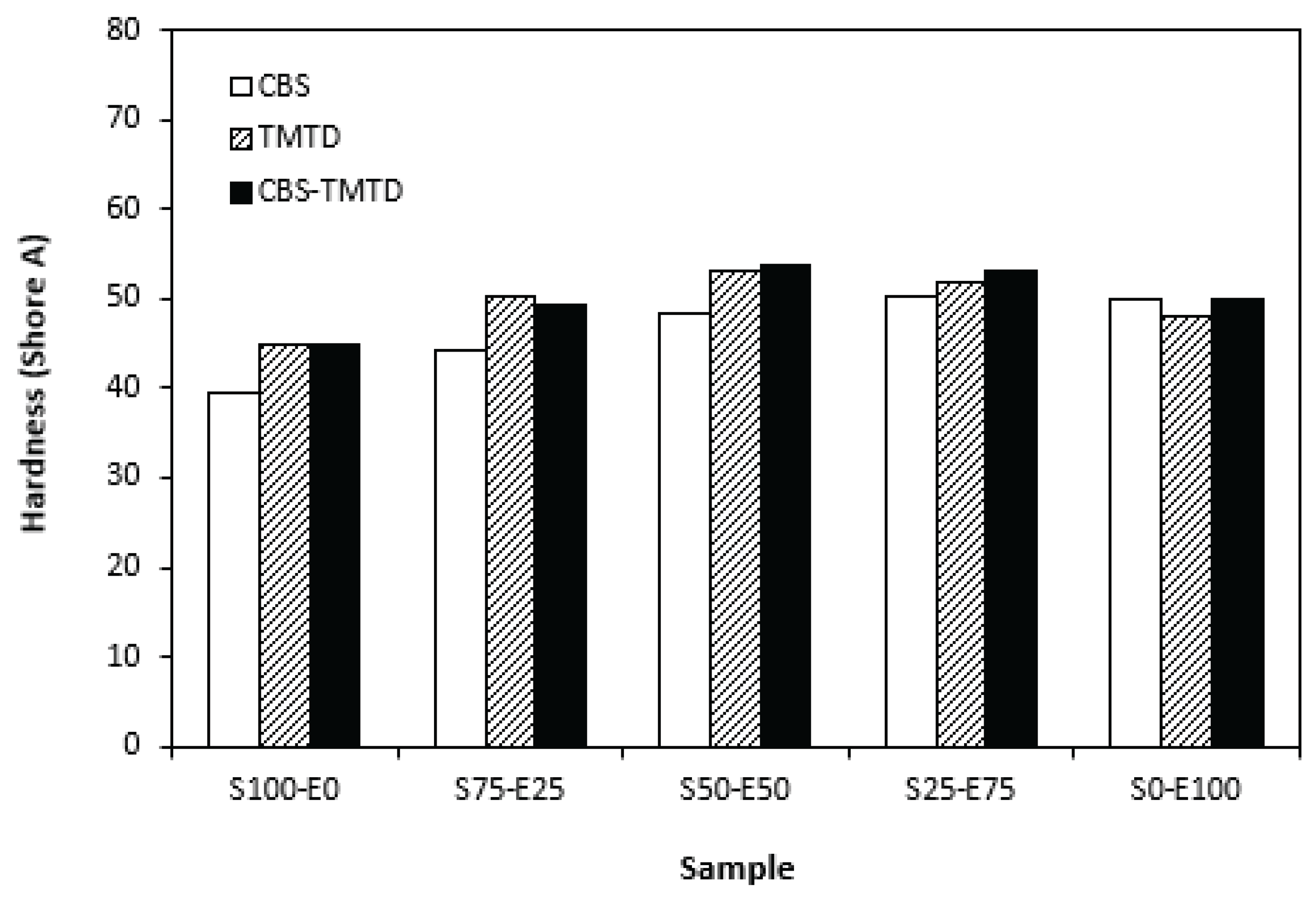
Figure 7). By application of TMTD and CBS-TMTD combination, the cross-link density increased, which resulted in the increase of modulus. From
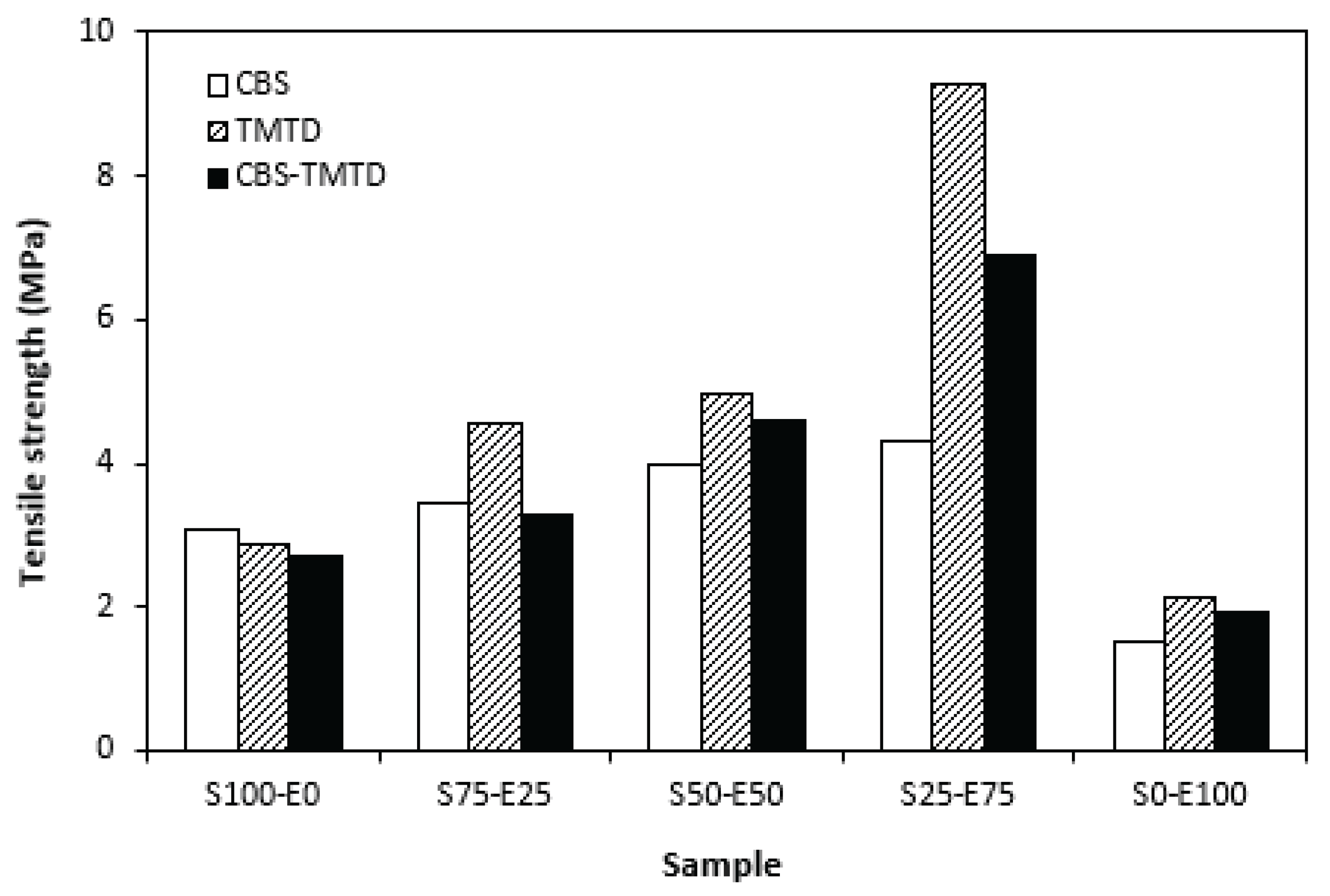
Figure 8 it is shown that the lowest hardness exhibited vulcanizates based on SBR (S100-E0), followed by the vulcanizates based on EPDM (S0-E100). The hardness of materials based on rubbers combinations (S75-E25, S50-E50, S25-E75) was slightly higher. Higher cross-link density of vulcanizates cured in the presence of TMTD and CBS-TMTD combination was responsible for higher hardness of equivalent vulcanizates. The lowest tensile strength exhibited vulcanizates based on EPDM (S0-E100) with no significant influence on the type of accelerator used. The tensile strength of vulcanizates with designation S100-E0 was higher. Again, no apparent influence of the accelerator composition on tensile strength was recorded. As shown in
Figure 9, in the case of vulcanizates based on rubbers combinations, the tensile strength showed increasing trend with increasing ratio of EPDM. Considering the type of the accelerator, the highest contribution to the tensile strength was recorded for TMTD. The highest tensile strength manifested vulcanizates with composition 25 phr SBR and 75 phr EPDM. The tensile strength of the sample S25-E75 cured with TMTD reached more than 9 MPa, which represents more than threefold or fourfold increase of tensile strength in comparison with equivalent vulcanizates based on SBR or EPDM, respectively. The highest tensile strength of vulcanizates cured with TMTD can be attributed to the change of the sulfidic cross-links formed within the rubber matrices. As TMTD acts as sulfur donor it can be deduced that more disulfidic and polysulfidic cross-links are generated. In general, the vulcanizates with dominance of polysulfidic cross-links are characterized by higher tensile behavior in comparison with vulcanizates, in which rubber chain segments are linked with mono- and disulfidic cross-links. Longer and more flexible polysulfidic cross-links enable higher mobility and elasticity of rubber chain segments, which contributes to better redistribution of the deformation strains within the rubber matrix [
30]. To that corresponds better deformation behavior and higher tensile characteristics of vulcanizates.
3.2. Vulcanization Process and Physical-Mechanical Properties of Peroxide Cured Rubber Compounds
The influence of peroxide curing composition on optimum vulcanization time
tc90 and scorch time
ts1 of rubber compounds are graphically illustrated in
Figure 10 and
Figure 11. As shown in
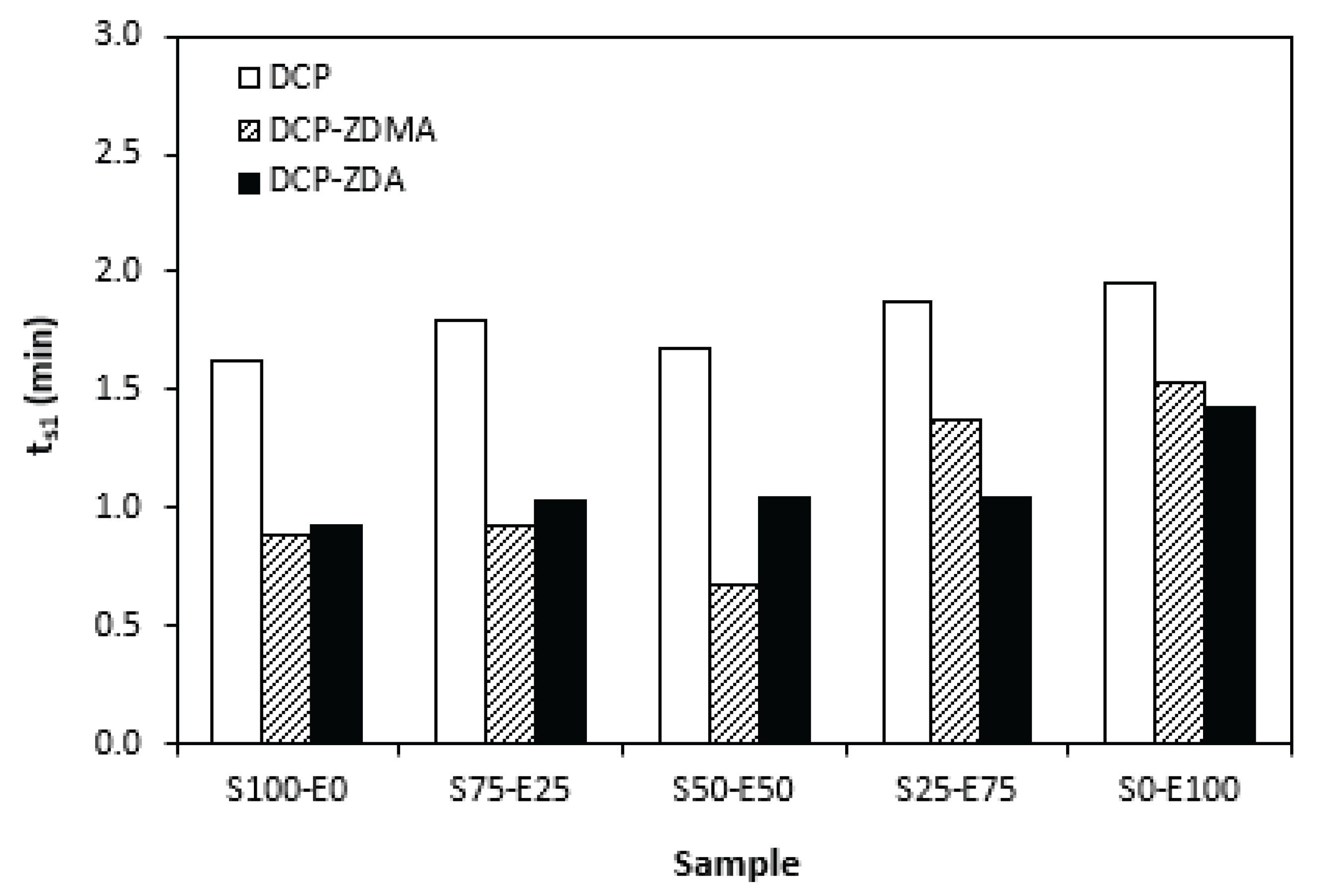
Figure 10, the highest scorch time exhibited the formulations cured with DCP. The application of co-agent resulted in the decrease of
ts1 values. Though, the decrease was only about half a minute, so it can be considered not to be significant. When comparing the scorch time of the compounds cured with sulfur systems (
Figure 1) and peroxide systems (
Figure 10), it becomes apparent that
ts1 of rubber compounds cured with peroxide systems is much shorter. Peroxide curing process of rubber compounds is relatively simple process, during which the organic peroxide first decomposes fast at a curing temperature with formation of peroxide radicals. The formed radicals then immediately react with rubber chains to form cross-links. To that corresponds very short scorch time. On the other hand, sulfur vulcanization is intricate process, generally running in several stages. The reaction between the accelerators and activators leads to the formation of salt that subsequently react with sulfur to form sulfur-rich transition complexes. This occurs in induction period and the length of this period is significantly influenced by the type of accelerator and sulfur to accelerator ratio.
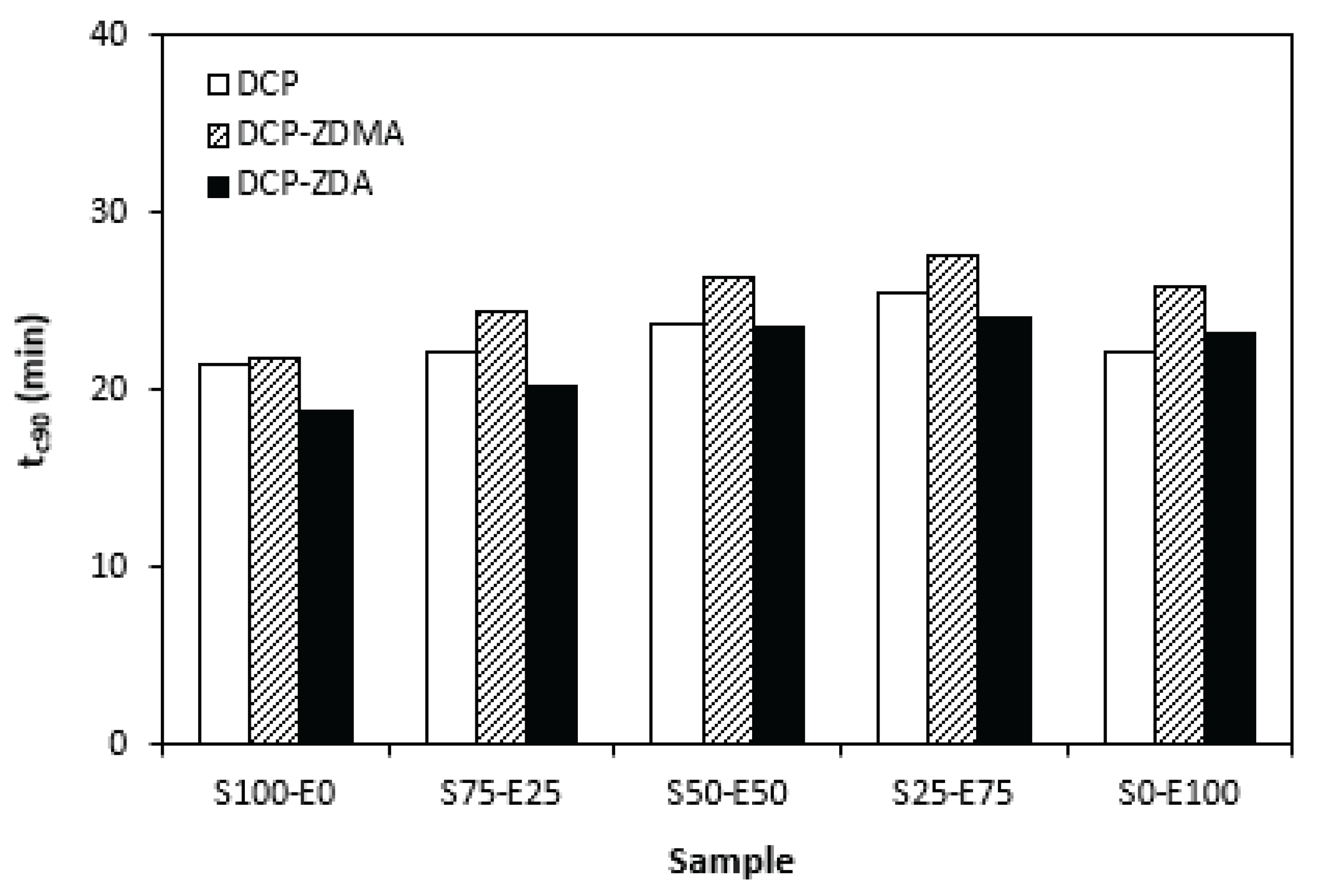
From
Figure 11 it is seen that there is no significant difference of the optimum cure time in dependence on peroxide curing composition. Though, the longest time needed for optimum cross-linking exhibited materials cured with DCP and ZDMA. As also shown, the optimum cure time showed slight increasing tendency with increasing ratio of EPDM in rubbers combinations.
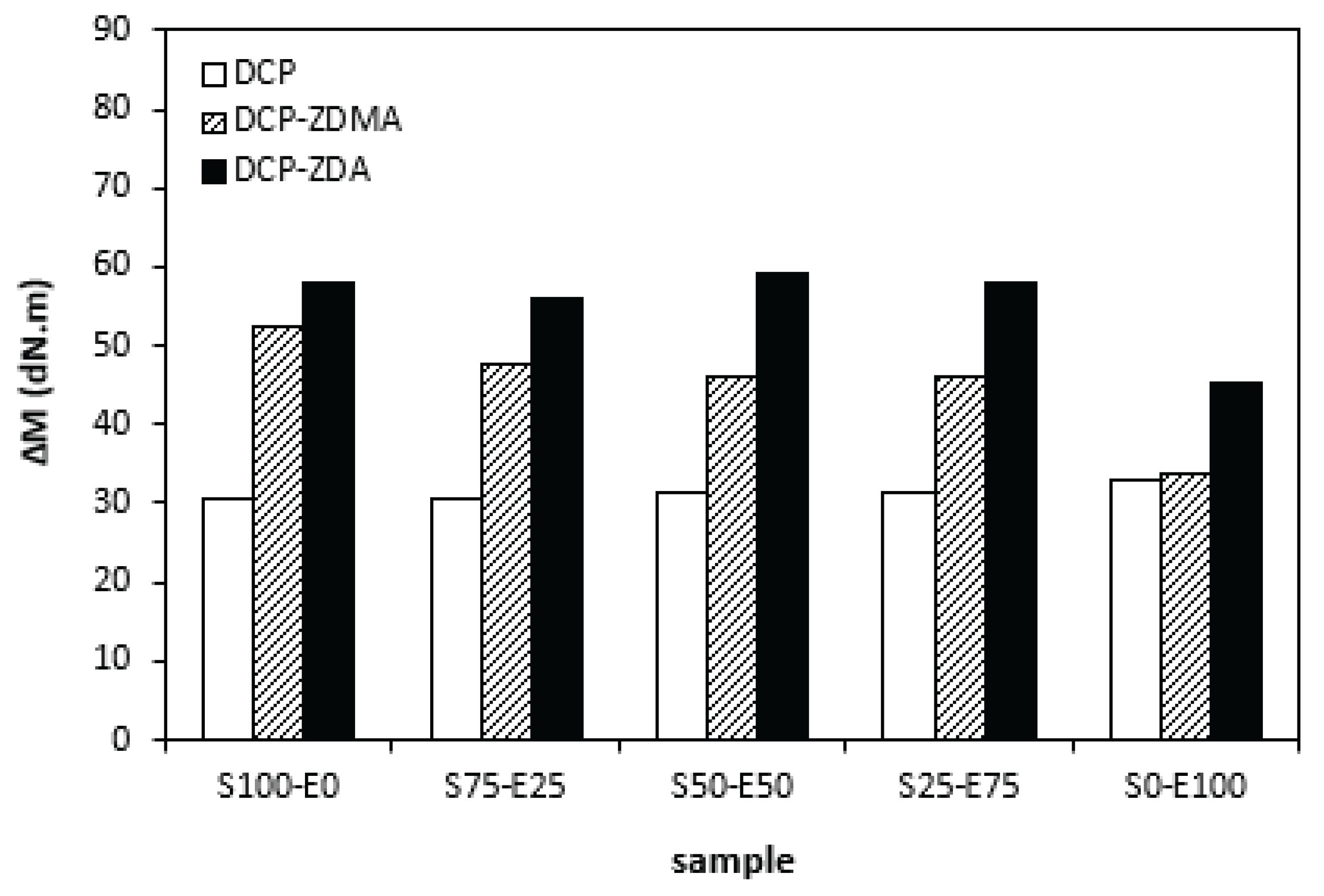
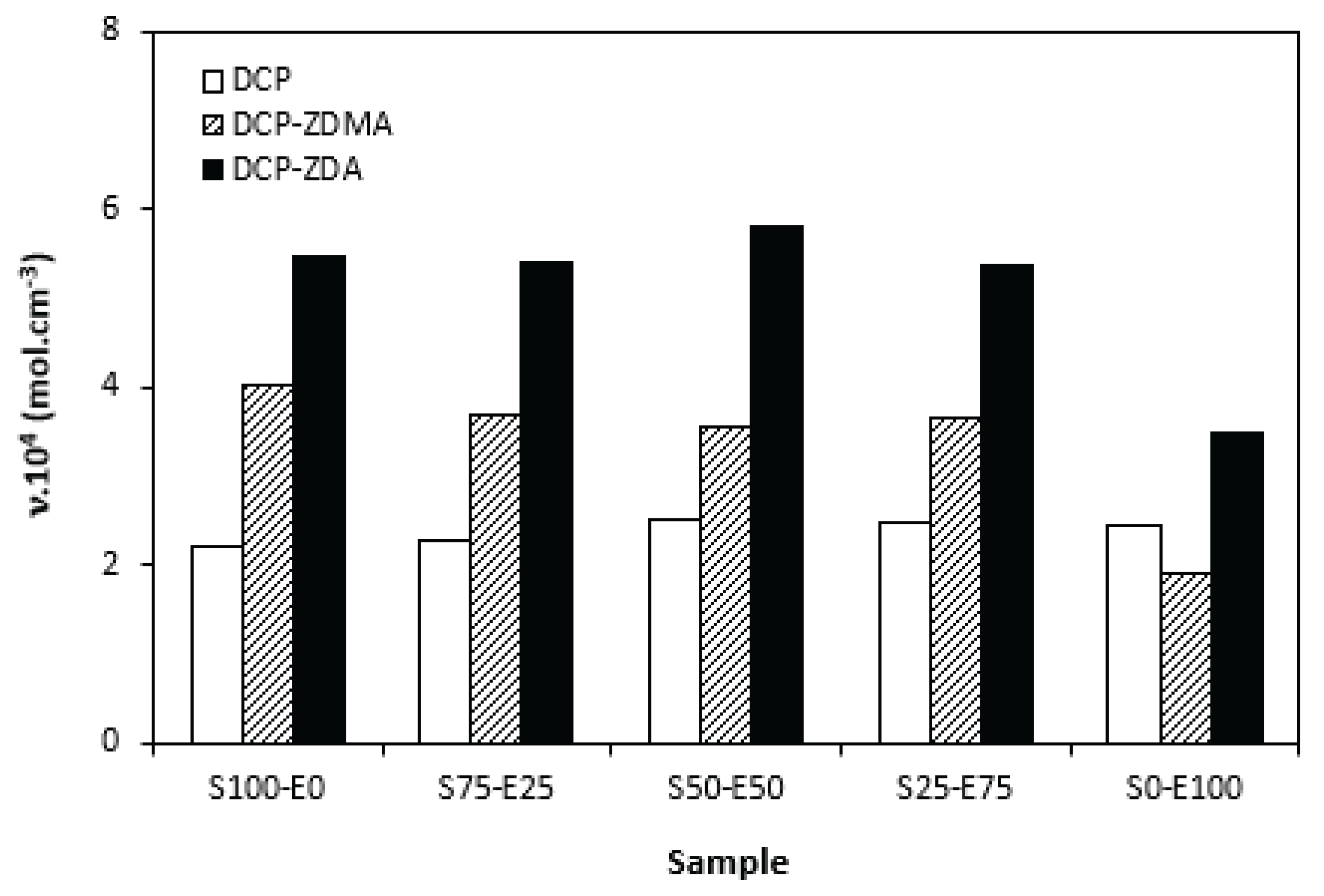
When comparing the difference between the maximum and minimum torque
∆M (
Figure 12) and cross-link density (
Figure 13) one can see very similar dependences on curing system composition and type of rubber or rubbers combinations. It again points out to close relation between both characteristics. The lowest
∆M values and cross-link density demonstrated materials cured with DCP. As seen, there was almost no difference in
∆M values as well as cross-link density in dependence on the type of rubber of rubbers combinations. The application of co-agents resulted in the increase of cross-link density, while more significant increase was recorded for vulcanizates cured with DCP and ZDA. Co-agents contribute to the increase in peroxide curing efficiency by formation of multifunctional cross-links within the rubber matrix. Several reaction mechanisms have been suggested for rubber compounds cured with peroxide in the presence of co-agents. In overall, the co-agents can link the rubber chains by formation of chemical cross-links between the chain segments, they can also homopolymerize to form interpenetrating networks with rubber chains or high modulus filler-like domains [
31,
32,
33]. In addition, ion pairs in ZDMA and ZDA can form ion clusters via static electronic interactions. These clusters serve as ionic or physical cross-linking with the ability of stress relaxation upon external deformation of the sample, which contributes to the improved physical-mechanical properties [
34,
35]. The achieved results suggest that both co-agents engage in cross-link network formation within the rubber matrix. More significant impact on cross-link density was recorded for zinc diacrylate. Thus, it can be deduced that the dominant reaction mechanism of ZDA is coupling of co-agent molecules onto rubber chains. On the other hand, lower cross-link density of rubber compounds cured with DCP and ZDMA suggests that dominant reaction pathway for ZDMA is homopolymerization due to the presence of side methyl groups, that can sterically hinder the coupling of co-agent molecules onto rubber chains.
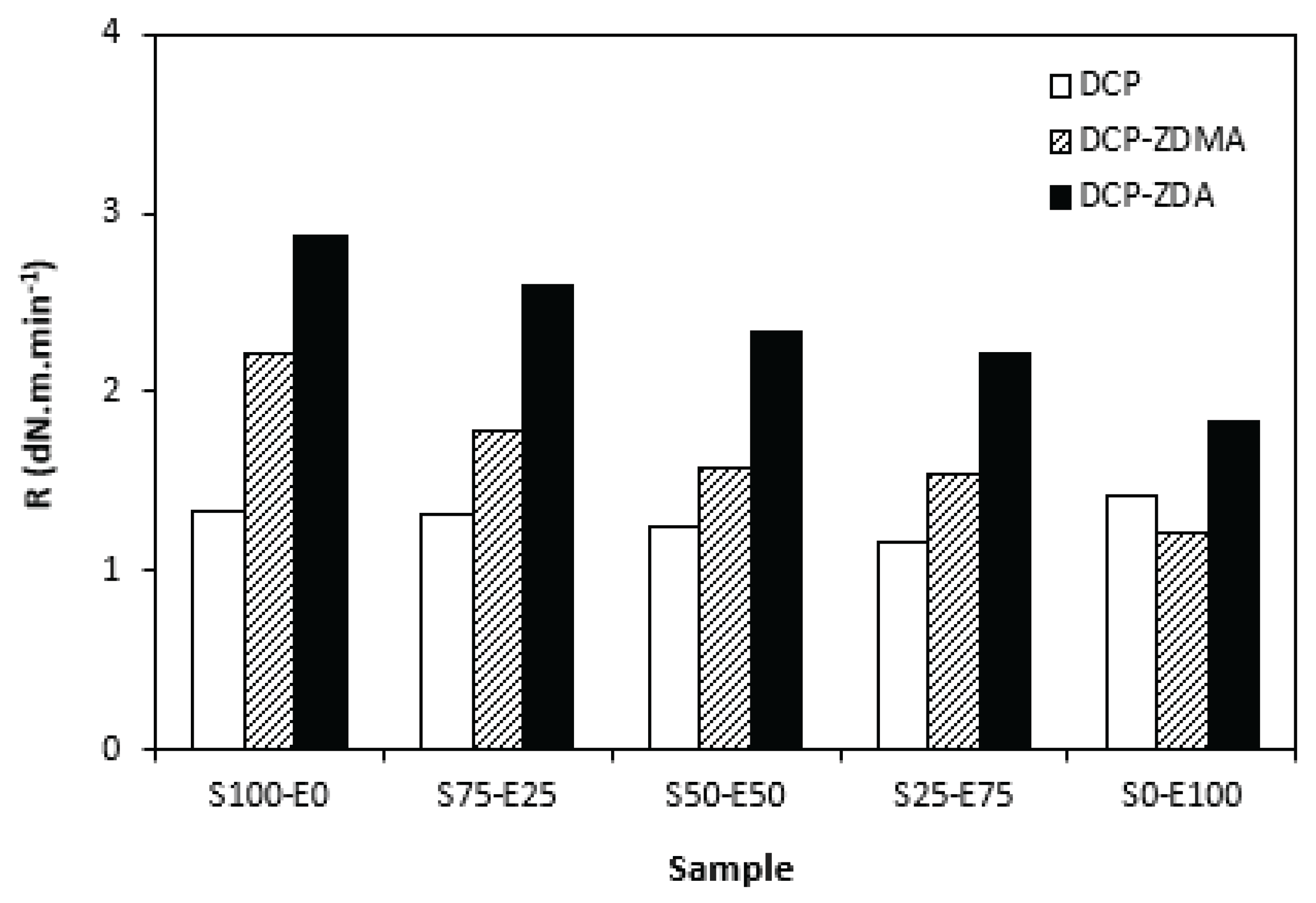
As shown in
Figure 14, the curing rate was the lowest for rubber compounds cured with DCP and was found not be dependent on the rubbers compositions. The curing rate of rubber compounds with applied ZDA and ZDMA was higher and showed decreasing trend with increasing amount of EPDM in rubbers combinations. It again suggests that the curing process with peroxide systems proceeds faster for SBR.
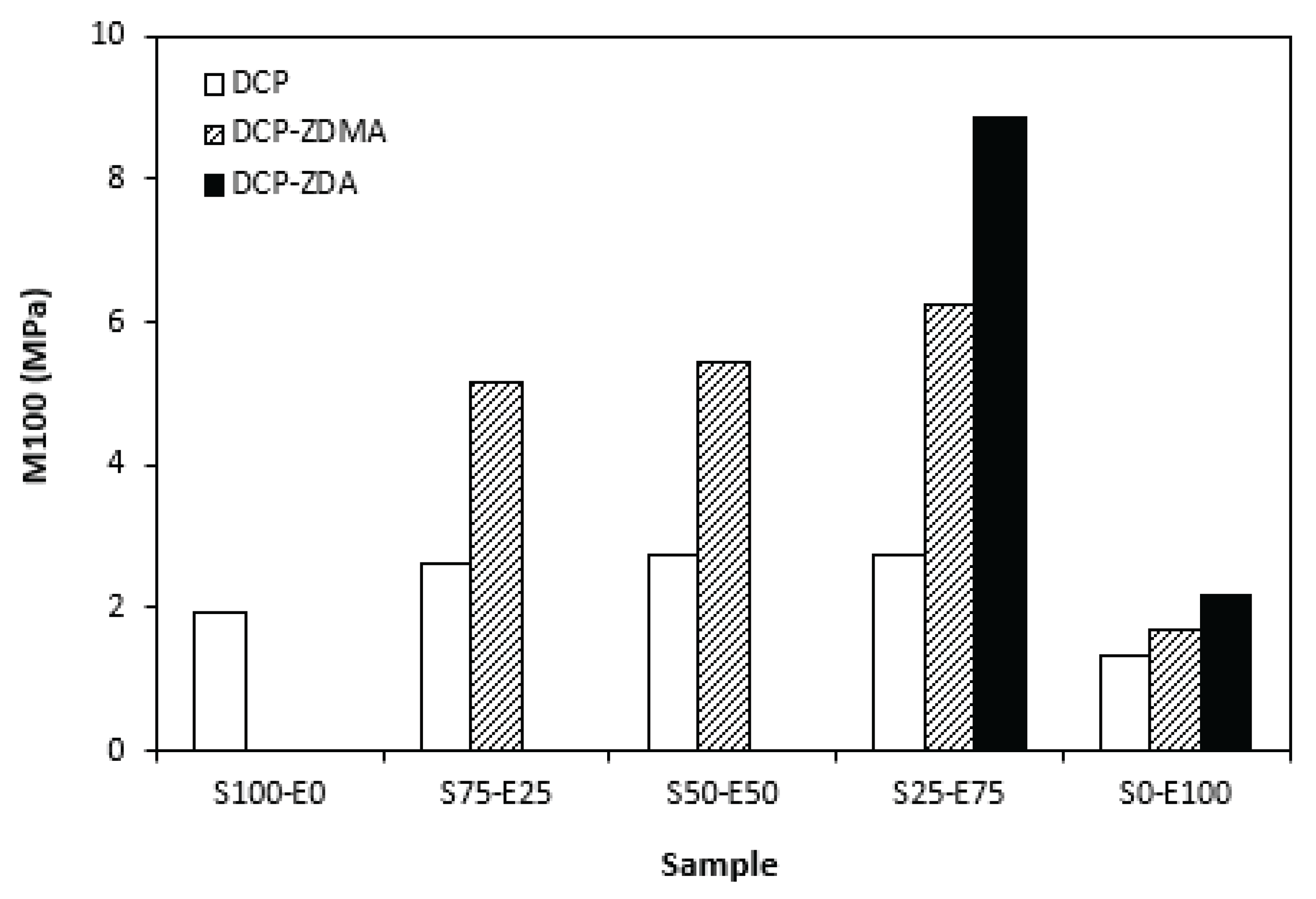
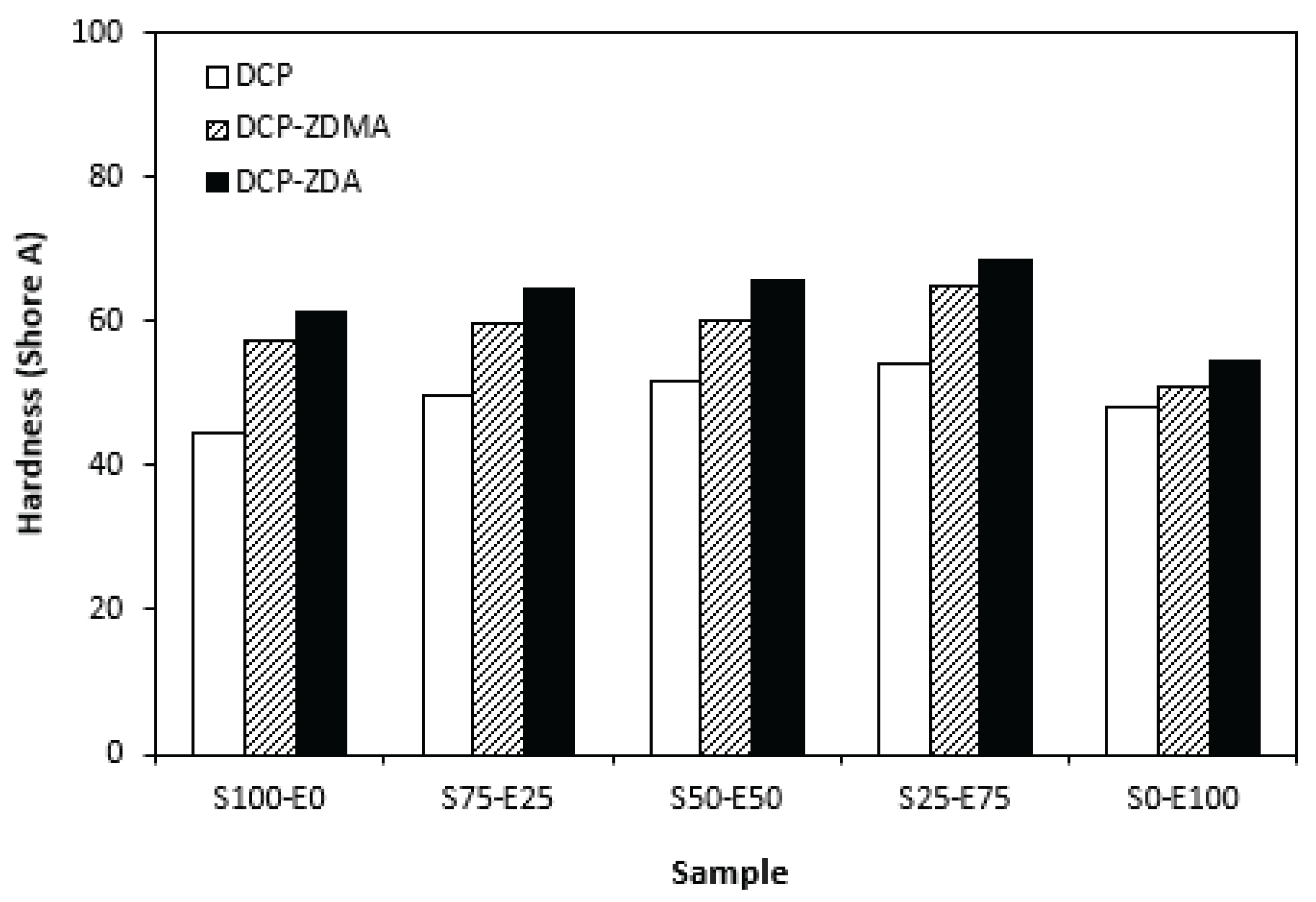
From graphical dependence of modulus M100 on the rubbers and curing system composition one can see that the lowest modulus exhibited vulcanizates cured with DCP (
Figure 15). It is a consequence of their lowest cross-link density. The application of co-agents resulted in the increase of cross-link density, which was reflected in the increase of modulus. The modulus was not possible to determine for several samples, mainly those cured with DCP and ZDA, as due to high cross-link density, they did not reach 100% elongation. It also becomes interesting that M100 of vulcanizates based on rubber combinations cured with DCP and co-agents was significantly higher when compared to equivalent vulcanizates based on EPDM (S0-E100). The dependences of hardness also followed the trend of cross-link density, suggesting that the highest cross-link density of vulcanizates cured with DCP and ZDA resulted in the highest hardness of the equivalent vulcanizates (
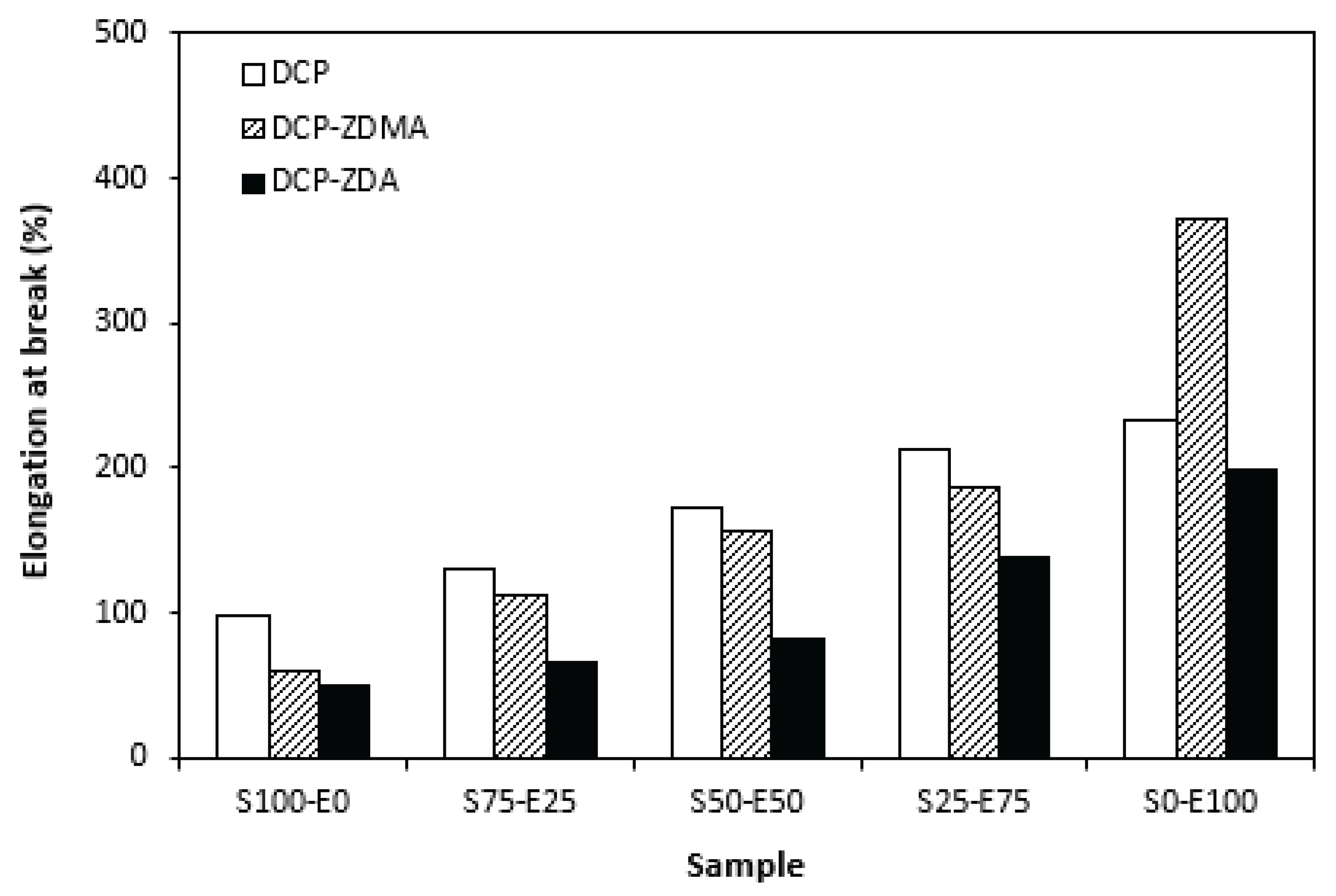
Figure 16). The lowest modulus and hardness were found to have vulcanizates based on EPDM (S0-E100) with the lowest cross-linking degree. The elongation at break of vulcanizates showed increasing trend with increasing ratio of EPDM in rubbers combinations (
Figure 17). The highest cross-link density of vulcanizates cured with DCP and ZDA caused the highest restriction of rubber chains mobility, leading to the lowest elongation at break. The highest elongation at break manifested vulcanizates based on EPDM (S0-E100) with the lowest cross-link density.
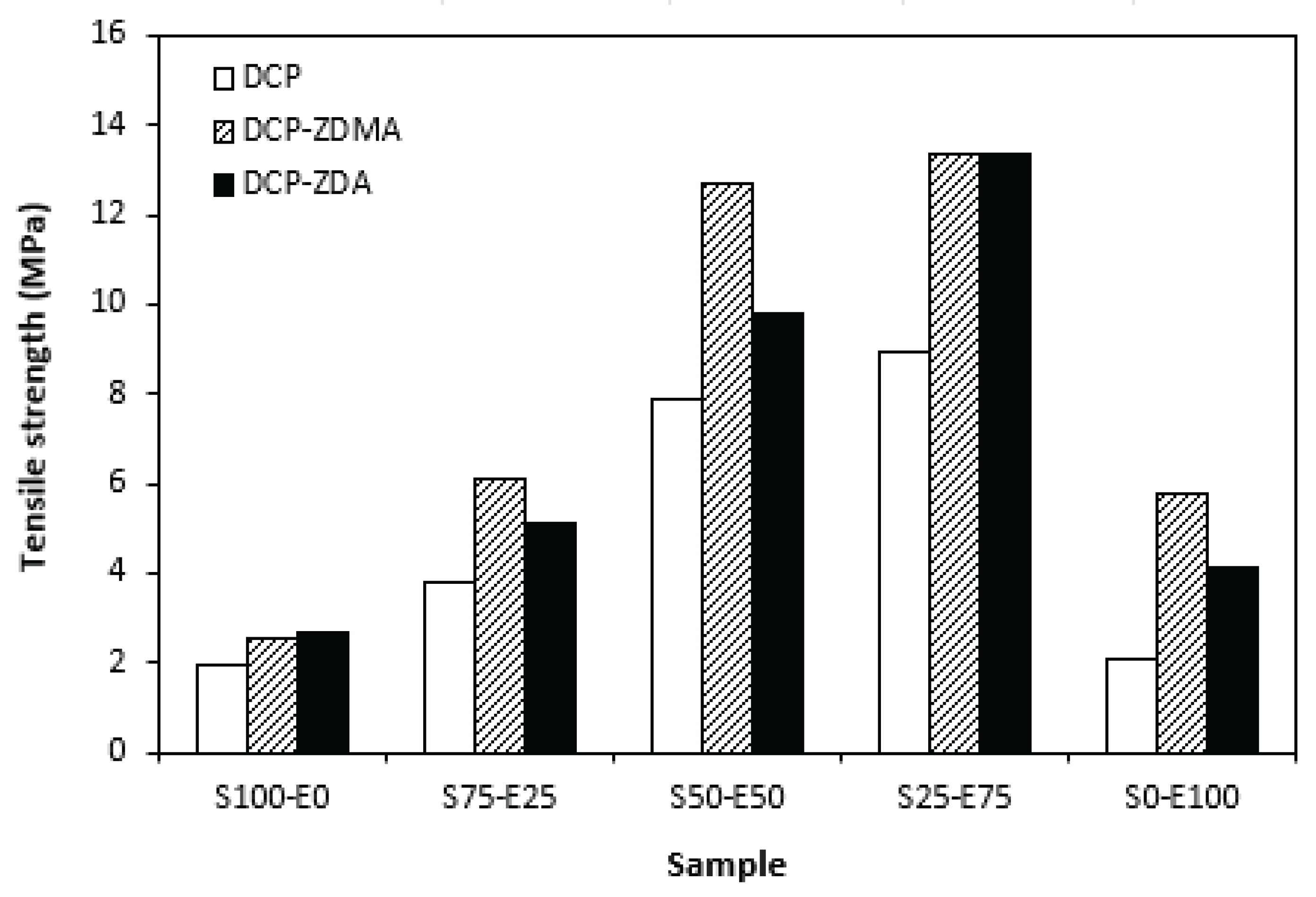
The type and structure of rubber as well as the structure of the formed cross-links are key factors influencing the tensile strength of unfilled vulcanizates. From
Figure 18 it becomes apparent that the lowest tensile strength exhibited vulcanizates based on reference SBR and EPDM rubbers (S100-E0 and S0-E100) cured with DCP. While there was almost no influence of co-agents on tensile strength of the vulcanizate S100-E0, the application of ZDA and ZDMA caused the enhancement in tensile behavior of EPDM based vulcanizate (S0-E100). The tensile strength of vulcanizates based on rubbers combinations (S75-E25, S50-E50, S25-E75) was higher and, as in the case of vulcanizates cured with sulfur system, showed increasing trend with increasing ratio of EPDM. When combining SBR and EPDM in 25 phr to 75 phr, the tensile strength of DCP cured vulcanizate achieved almost 9 MPa, which was more than 7 MPa higher than that of the vulcanizates S100-E0 and S0-E100 (both vulcanizates exhibited the tensile strength equivalent to only 2 MPa). The utilization of co-agents contributed to the formation of more complex cross-linked structure within the rubber matrices by formation of multifunctional cross-links, which contributed to the improvement of tensile behavior of vulcanizates. Again, the highest tensile strength (remarkable 13 MPa) manifested vulcanizates with designation S25-E75 cured with DCP and both co-agent types.
When comparing tensile strength of sulfur cured vulcanizates (including all accelerator systems) and vulcanizates cured with DCP, it is shown that higher tensile strength exhibited vulcanizates cured with sulfur system. It is in line with general knowledge demonstrating that sulfur cured vulcanizates are characterized by higher tensile behavior compared to peroxide cured vulcanizates. This can be attributed to the structure of the formed cross-links. Longer and more flexible sulfidic cross-links make rubber chain segments to be more flexible and elastic. Higher elasticity and mobility of rubber chains leads to better redistribution of applied deformation strains onto the rubber matrix more uniformly, which can bear more strain load without negative impact on tensile behavior. On the other side, short and rigid carbon-carbon bonds restrict the mobility and elasticity of rubber chains. The highly cross-linked rubber sites can act as stress concentrators upon applied deformation strains, which can more easily lead to cracks growing and their propagation. To that correspond lower tensile strength of peroxide cured vulcanizates. Though, by application of both co-agent types, the tensile strength increased and overcame the tensile strength of sulfur cured vulcanizates. So, it can be stated that by proper selection of co-agents, it is possible to fabricate peroxide cured materials with applicable physical-mechanical properties.
It can also be stated that for both, sulfur as well as peroxide cured vulcanizates, the materials based on rubbers combinations exhibited higher tensile strength when compared to the equivalent vulcanizates based on SBR or EPDM. The highest tensile strength demonstrated vulcanizates with composition 25 phr SBR and 75 phr EPDM. This is a very positive aspect suggesting that by combination of rubbers, not only the advantages of both rubbers can be combined, but also improved tensile behavior can be achieved.
3.3. Dynamical-Mechanical Analysis
The dynamic-mechanical analysis of vulcanizates was performed to investigate the influence of rubbers combinations and curing system composition on visco-elastic properties of vulcanizates. For this purpose, vulcanizates cured with sulfur system in the presence of TMTD and vulcanizates cured with combination of DCP and ZDMA were chosen. Those vulcanizates were selected due to their highest tensile characteristics.
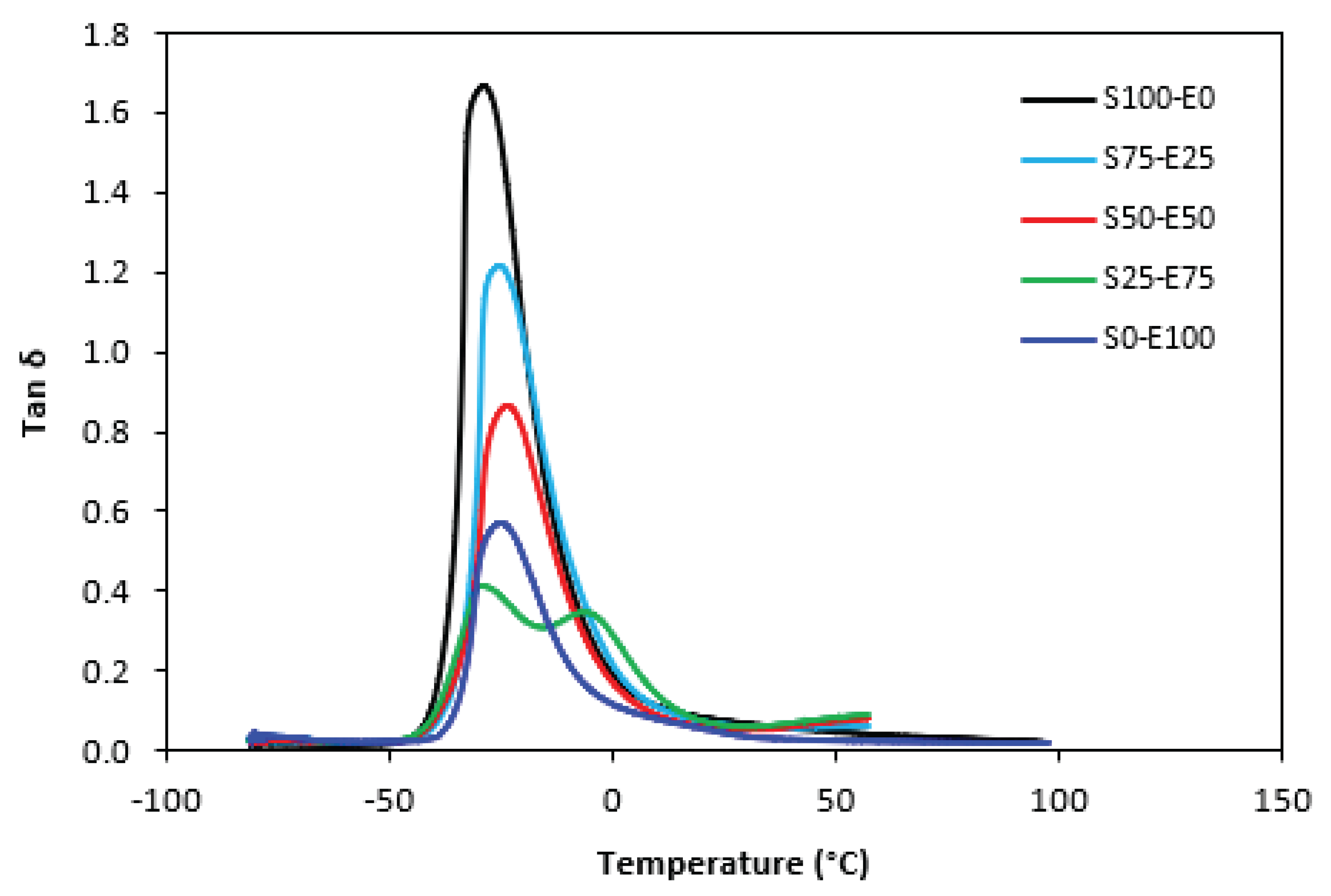
The temperature dependences of loss factor
tan δ for both types of tested vulcanizates are graphically illustrated in
Figure 19 and
Figure 20. The peak maximum in
tan δ temperature dependences corresponds to glass transition temperature
Tg of vulcanizates and the values are summarized in Tab. 5. In addition to
Tg of vulcanizates,
Tg of virgin SBR and EPDM rubbers are also listed in Tab. 5, too. As seen, virgin SBR has lower
Tg when compared to EPDM. The cross-linking of rubber matrices leads to the increase of glass transition temperature in comparison with virgin rubbers. The vulcanizate based on SBR cured with sulfur system (S100-E0) exhibited lower
Tg when compared to equivalent vulcanizate based on EPDM (S0-E100). On the other hand, vulcanizates with designation S100-E0 and S0-E100 cured with peroxide system demonstrated practically the same glass transition temperature. This can be explained by almost twofold higher cross-link density of SBR-based vulcanizate compared to that based on EPDM. The higher is the cross-link density, the more is restricted the rubber chains mobility and elasticity resulting in the increase of
Tg. It can be stated for both vulcanizate types that the highest
Tg exhibited vulcanizates based on equivalent ratio of both rubbers (S50-E50). On the other side, the lowest and almost the same
Tg (around -29 °C) were found to have both types of vulcanizates with designation S25-E75. It becomes obvious from
Figure 19 that sulfur cured vulcanizate S25-E75 showed two peaks on loss factor temperature dependences. The second peak occurred at – 6°C. Since the two transition peaks are typical for block copolymers, it might be stated that the formation of some block copolymers might occur just at this composition of both rubbers. From
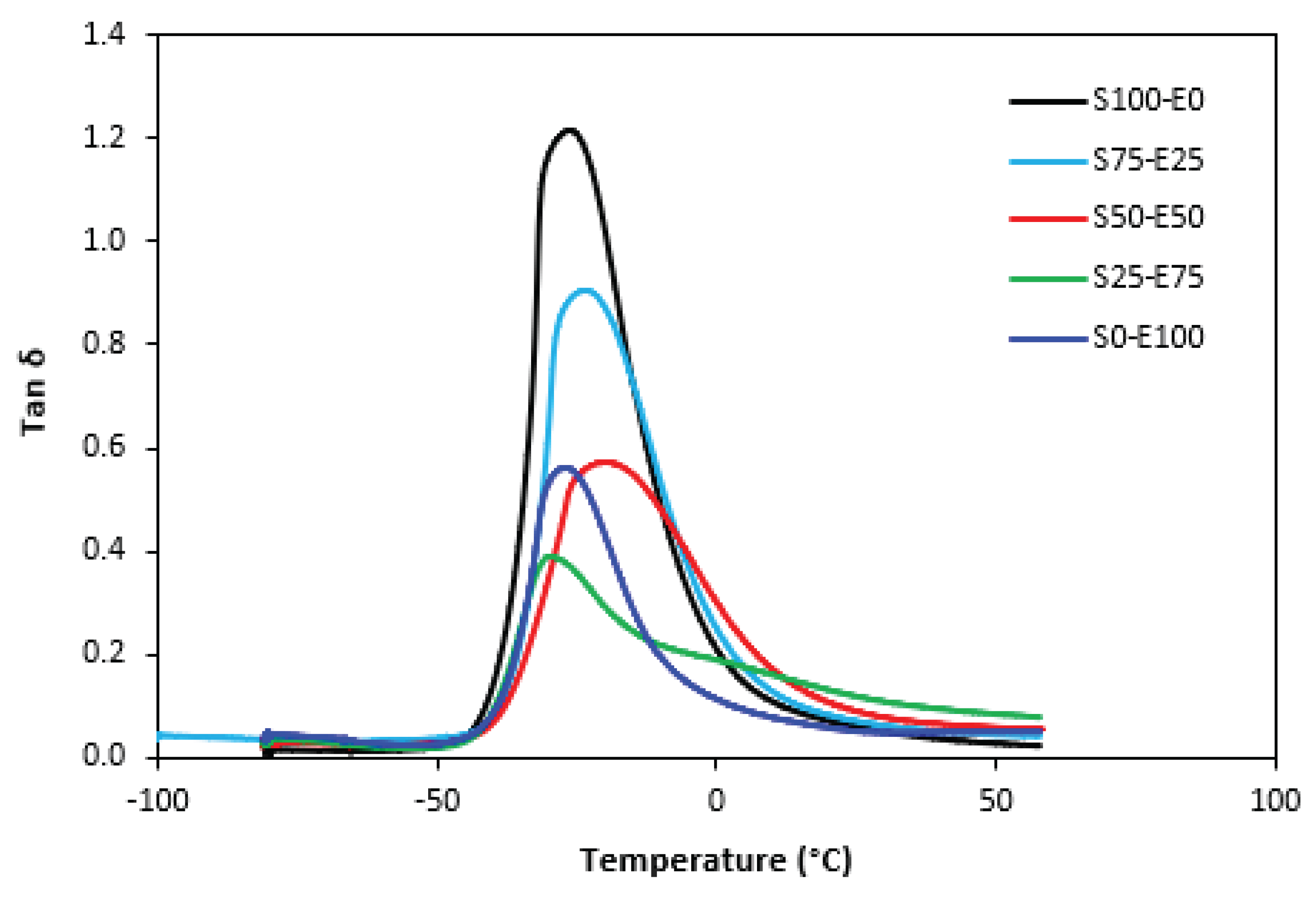
Figure 20 it can also be seen that the second peak on
tan δ temperature dependences of the vulcanizate S25-E75 cured with peroxide system starts to appear, though it is not as visible as for the equivalent vulcanizate cured with sulfur system.
When comparing vulcanizates based on SBR or EPDM (S100-E0, S0-E100), vulcanizate S100-E0 cured with sulfur system was found to have lower cross-link density compared to equivalent SBR based vulcanizate cured with peroxide system and thus it exhibited lower Tg. The opposite statement can be applied on vulcanizates based on EPDM. The vulcanizate S0-E100 cured with sulfur system demonstrated higher cross-link density and thus higher Tg, when compared to corresponding vulcanizate cured with peroxide system.
In the case of vulcanizates based on rubbers combinations is the trend of Tg on cross-link density unambiguous, which can be attributed to mutual compounding of both rubbers, distribution of curing system additives in rubber matrices as well as the structure of the formed cross-links.
Table 5.
Glass transition temperature Tg and cross-link density ν of vulcanizates cured with sulfur and peroxide systems.
Table 5.
Glass transition temperature Tg and cross-link density ν of vulcanizates cured with sulfur and peroxide systems.
| |
S100–E0 |
S75–E25 |
S50–E50 |
S25–E75 |
S0–E100 |
|
Tg of virgin rubbers (°C) |
-34.7 |
|
|
|
-29.6 |
|
Tg of vulcanizaes with sulfur system (°C) |
-28.5 |
-25.4 |
-23.3 |
-29.2 (-6.2) |
-24.9 |
|
Tg of vulcanizates with peroxide system (°C) |
-26.4 |
-23.5 |
-19.8 |
-29.9 |
-27 |
| Cross-link density of vulcanizates with sulfur system ν.104 (mol.cm-3) |
1.59 |
1.47 |
1.20 |
0.98 |
2.95 |
| Cross-link density of vulcanizates with peroxide system ν.104 (mol.cm-3) |
4.02 |
3.68 |
3.56 |
3.66 |
1.90 |