Submitted:
10 March 2024
Posted:
11 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
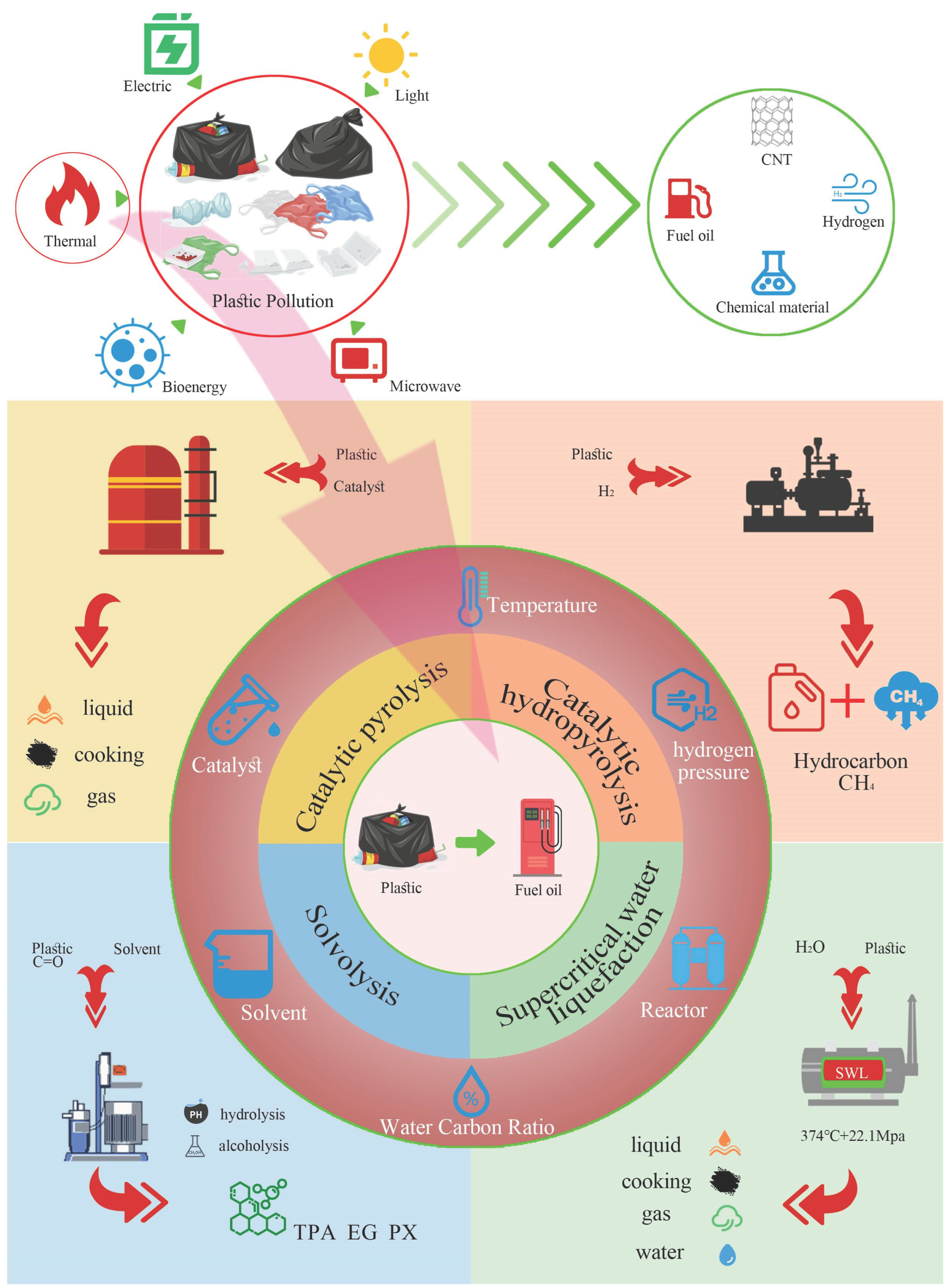
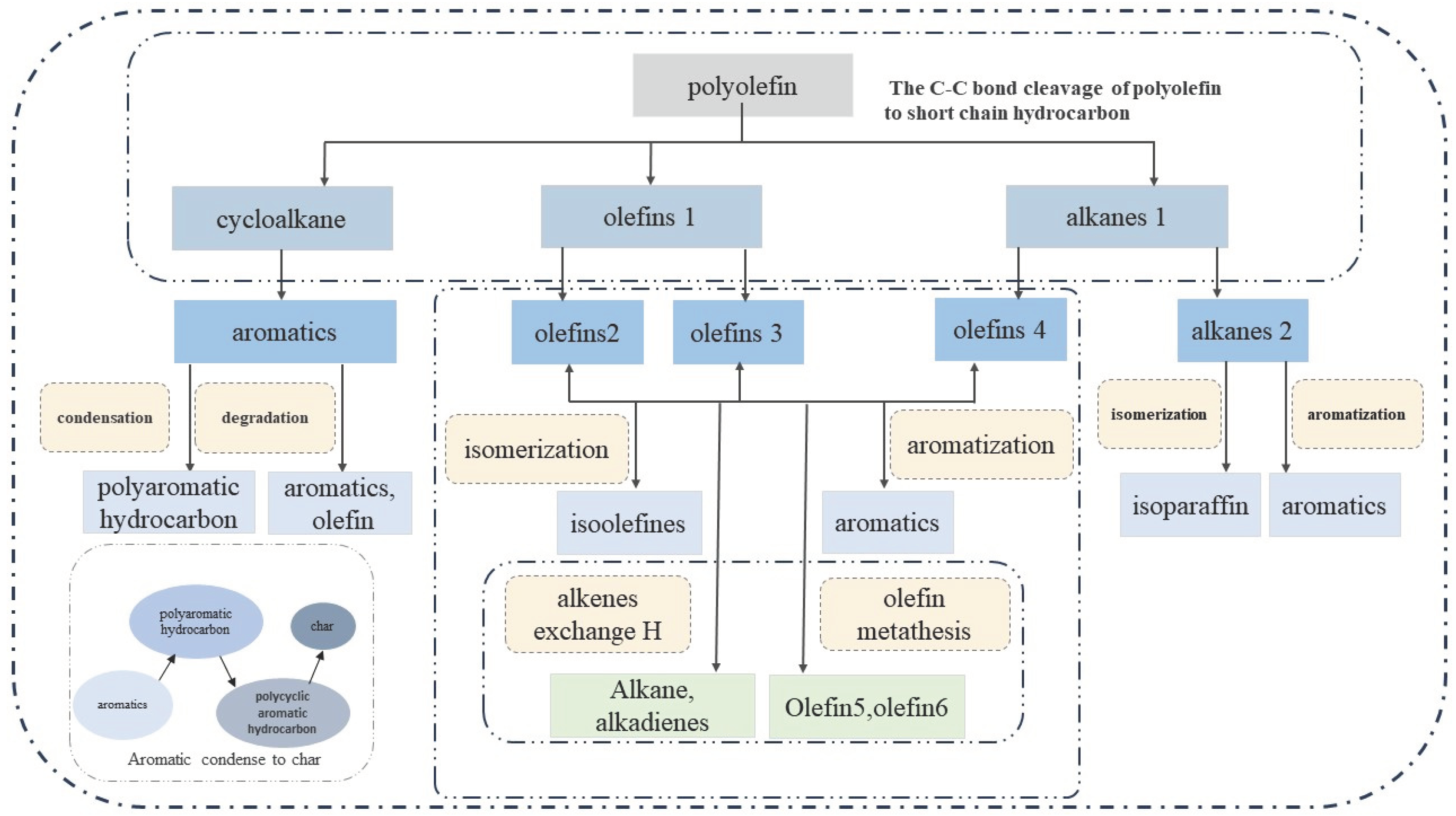
2. Plastic pyrolysis method for oil production
2.1. Thermal pyrolysis
2.1.1. Pyrolysis
2.1.2. Catalytic pyrolysis
2.1.3. Catalytic reforming
2.2. Catalytic Hydropyrolysis
2.3. Solvolysis
2.4. Supercritical Water Liquefaction
2.5. Tandem technology of degradation
3. Optimization paths of technological conditions for the production of fuel oil
3.1. Co-pyrolysis
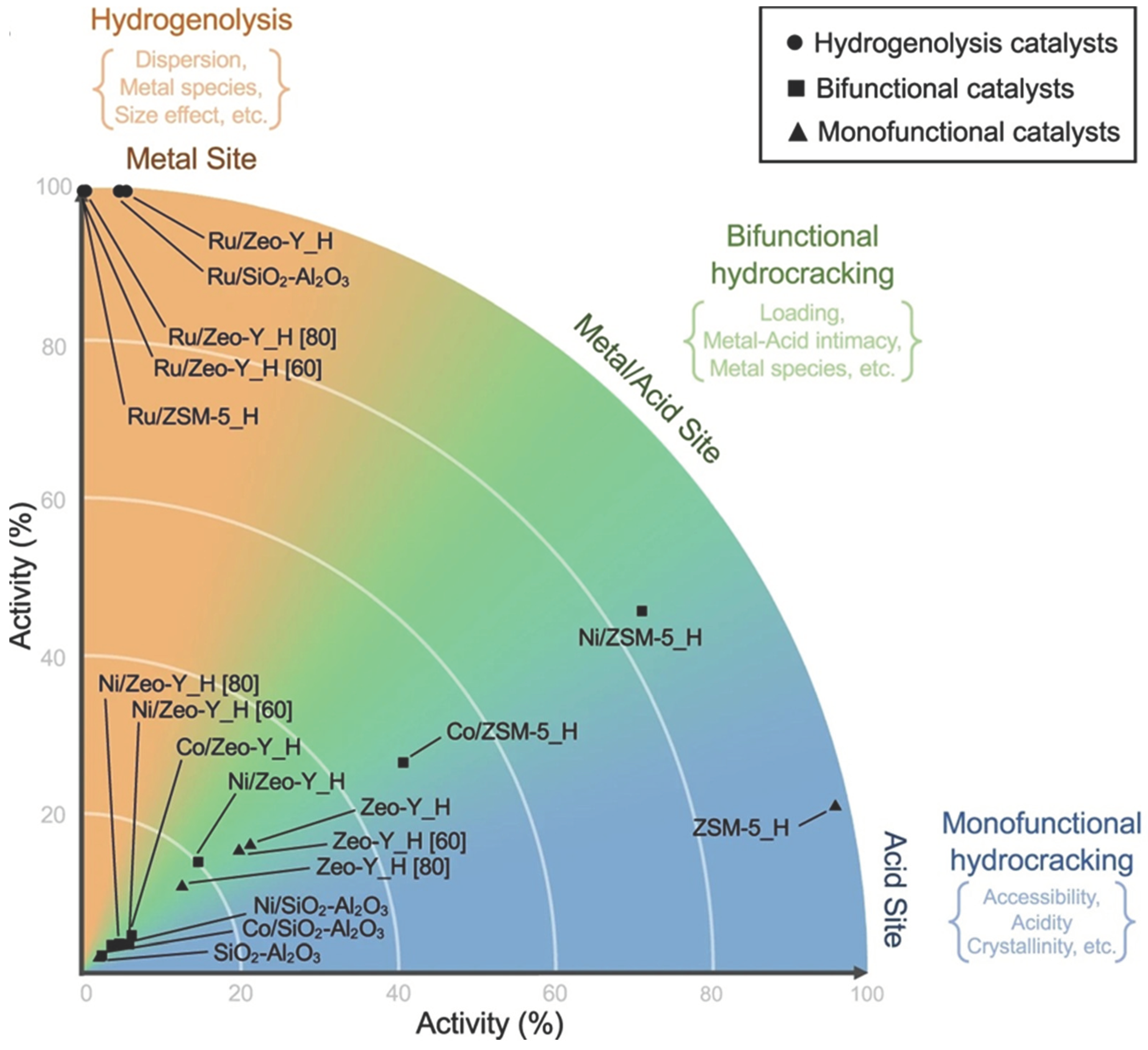
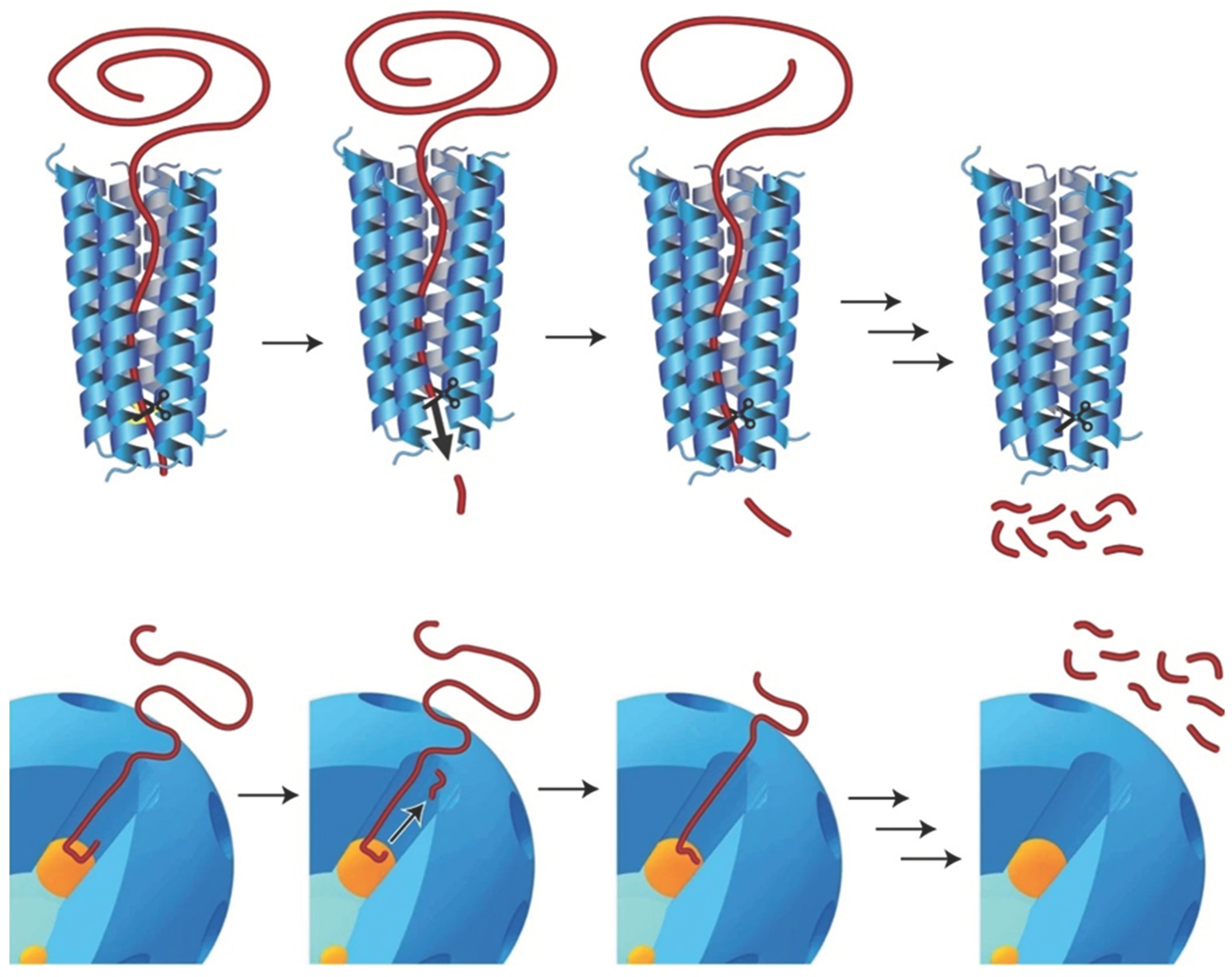
3.2. Catalysts
3.3. Reactors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Awasthi, A.K.; Wei, F.; et al. Single-use plastics: Production, usage, disposal, and adverse impacts[J]. Sci. Total Environ, 1: 752, 1417. [Google Scholar] [CrossRef]
- Baca, D.; Monroy, R.; Castillo, M.; Elkhazraji, A.; Farooq, A.; Ahmad, R. Dioxins and plastic waste: A scientometric analysis and systematic literature review of the detection methods. Environ. Adv. 2023, 13, 100439. [Google Scholar] [CrossRef]
- Kalali, E.N.; Lotfian, S.; Shabestari, M.E.; et al. A critical review of the current progress of plastic waste recycling technology in structural materials[J]. Curr. Opin. Green Sustainable Chem. 2023. [Google Scholar] [CrossRef]
- Chen, H.; Wan, K.; Zhang, Y.; Wang, Y. Waste to Wealth: Chemical Recycling and Chemical Upcycling of Waste Plastics for a Great Future. ChemSusChem 2021, 14(19), 4123–4136. [Google Scholar] [CrossRef]
- Zhuo, C.; Levendis, Y.A. Upcycling waste plastics into carbon nanomaterials: A review[J]. J. Appl. Polym. Sci. 1001. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, Y.; Williams, P. T.; Yang, H.; Chen, H. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters. Appl. Catal., B. [CrossRef]
- Zhou, H.; Ren, Y.; Li, Z.; Xu, M.; Wang, Y.; Ge, R.; Duan, H. Electrocatalytic upcycling of polyethylene terephthalate to commodity chemicals and H2 fuel [J]. Nat. Commun. [CrossRef]
- Wei, R.; Tiso, T.; Bertling, J.; O’Connor, K.; Blank, L. M.; Bornscheuer, U. T. Possibilities and limitations of biotechnological plastic degradation and recycling. Nat. Catal. 2020, 3(11), 867–871. [Google Scholar] [CrossRef]
- Sullivan, K.P.; Werner, A.Z.; Ramirez, K.J. Ellis; Bussard, J.R.; Black, B.A.; Brandner, D.G.; et al. Mixed plastics waste valorization through tandem chemical oxidation and biological funneling[J]. Science, 6616. [Google Scholar]
- Huang, Z.; Shanmugam, M.; Liu, Z.; Brookfield, A.; Bennett, E. L.; et al. Chemical Recycling of Polystyrene to Valuable Chemicals via Selective Acid-Catalyzed Aerobic Oxidation under Visible Light. J. Am. Chem. Soc. 2022, 144(14), 6532–6542. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zhang, M.-Q.; Hu, C.; Xiao, D.; Wang, M.; Ma, D. Catalytic oxidation of polystyrene to aromatic oxygenates over a graphitic carbon nitride catalyst. Nat. Commun. [CrossRef]
- Uekert, T.; Kuehnel, M. F.; Wakerley, D. W.; Reisner, E. Plastic waste as a feedstock for solar-driven H2 generation. Energy Environ. Sci. 2018, 11(10), 2853–2857. [Google Scholar] [CrossRef]
- Ding, K.; Liu, S.; Huang, Y.; Liu, S.; Zhou, N.; Peng, P.; Ruan, R. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers. Manage. 2019, 196, 1316–1325. [Google Scholar] [CrossRef]
- Ali, S. S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E. A.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, X.; Miller, J. B.; Huber, G. W. The Chemistry and Kinetics of Polyethylene Pyrolysis: A Process to Produce Fuels and Chemicals. ChemSusChem 2020, 13(7), 1764–1774. [Google Scholar] [CrossRef]
- Wong, S. L.; Ngadi, N.; Abdullah, T. A. T.; Inuwa, I. M. Conversion of low density polyethylene (LDPE) over ZSM-5 zeolite to liquid fuel. Fuel 2017, 192, 71–82. [Google Scholar] [CrossRef]
- Elordi, G.; Olazar, M.; Lopez, G.; Amutio, M.; Artetxe, M.; Aguado, R.; Bilbao, J. Catalytic pyrolysis of HDPE in continuous mode over zeolite catalysts in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis. [CrossRef]
- Ding, W.; Liang, J.; Anderson, L. L. Thermal and catalytic degradation of high density polyethylene and commingled post-consumer plastic waste. Fuel Process. Technol.
- Zhao, D.; Wang, X.; Miller, J. B.; Huber, G. W. The Chemistry and Kinetics of Polyethylene Pyrolysis: A Process to Produce Fuels and Chemicals. ChemSusChem 2020, 13(7), 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pakdel, H.; Mihai, B.; Onu, P.; Darie, H. Thermal and catalytic decomposition of mixed plastics[J]. J. Anal. Appl. Pyrolysis, 2: 57(2), 2001. [Google Scholar]
- Lerici, L. C.; Renzini, M. S.; Pierella, L. B. Chemical Catalyzed Recycling of Polymers: Catalytic Conversion of PE, PP and PS into Fuels and Chemicals over H-Y. Procedia Mater. Sci. 2015, 8, 297–303. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Elordi, G.; Bilbao, J.; Olazar, M. Cracking of High Density Polyethylene Pyrolysis Waxes on HZSM-5 Catalysts of Different Acidity. Ind. Eng. Chem. Res. 2013, 52(31), 10637–10645. [Google Scholar] [CrossRef]
- Neves, I. C.; Botelho, G.; Machado, A. V.; Rebelo, P. The effect of acidity behaviour of Y zeolites on the catalytic degradation of polyethylene. Eur. Polym. J. 2006, 42(7), 1541–1547. [Google Scholar] [CrossRef]
- Dwivedi, U.; Naik, S. N.; Pant, K. K. High quality liquid fuel production from waste plastics via two-step cracking route in a bottom-up approach using bi-functional Fe/HZSM-5 catalyst. Waste Manage. 2021, 132, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Choi, D. H.; Park, J. W.; Kim, J.-H.; Sugi, Y.; Seo, G. Liquid-phase degradation of HDPE over alkali-treated MFI zeolites with mesopores. Polym. Degrad. Stab. 2006, 91(12), 2860–2866. [Google Scholar] [CrossRef]
- Lee, H.; Park, Y.-K. Catalytic Pyrolysis of Polyethylene and Polypropylene over Desilicated Beta and Al-MSU-F. Catalysts 2018, 8(11), 501. [Google Scholar] [CrossRef]
- Santos, B. P. S.; Almeida, D.; Marques, M. d. F. V.; Henriques, C. A. Petrochemical feedstock from pyrolysis of waste polyethylene and polypropylene using different catalysts. Fuel 2018, 215, 515–521. [Google Scholar] [CrossRef]
- Lee, J. Y.; Park, S. M.; Saha, S. K.; Cho, S. J.; Seo, G. Liquid-phase degradation of polyethylene (PE) over MFI zeolites with mesopores: Effects of the structure of PE and the characteristics of mesopores. Appl. Catal., B. [CrossRef]
- Zhou, Q.; Zheng, L.; Wang, Y.-Z.; Zhao, G.-M.; Wang, B. Catalytic degradation of low-density polyethylene and polypropylene using modified ZSM-5 zeolites. Polym. Degrad. Stab. 2004, 84(3), 493–497. [Google Scholar] [CrossRef]
- Murata, K.; Brebu, M.; Sakata, Y. The effect of silica–alumina catalysts on degradation of polyolefins by a continuous flow reactor. J. Anal. Appl. Pyrolysis 2010, 89(1), 30–38. [Google Scholar] [CrossRef]
- Nwankwor, P. E.; Onuigbo, I. O.; Chukwuneke, C. E.; Yahaya, M. F.; Agboola, B. O.; Jahng, W. J. Synthesis of gasoline range fuels by the catalytic cracking of waste plastics using titanium dioxide and zeolite. Int. J. Energy Environ. Eng. 2020, 12(1), 77–86. [Google Scholar] [CrossRef]
- Singh, M. V. Waste and virgin high-density poly(ethylene) into renewable hydrocarbons fuel by pyrolysis-catalytic cracking with a CoCO3 catalyst. J. Anal. Appl. Pyrolysis 2018, 134, 150–161. [Google Scholar] [CrossRef]
- Singh, M. V.; Kumar, S.; Sarker, M. Waste HD-PE plastic, deformation into liquid hydrocarbon fuel using pyrolysis-catalytic cracking with a CuCO3 catalyst. Sustainable Energy Fuels 2018, 2(5), 1057–1068. [Google Scholar] [CrossRef]
- Habyarimana, J. B.; Njiemon, M.; Abdulnasir, R.; Neksumi, M.; Yahaya, M.; Sylvester, O. D.; Jahng, W. J. Synthesis of Hydrocarbon Fuel by Thermal Catalytic Cracking of Polypropylene. International Journal of Scientific & Engineering Research, 1193. [Google Scholar] [CrossRef]
- Sun, K.; Huang, Q.; Ali, M.; Chi, Y.; Yan, J. Producing Aromatic-Enriched Oil from Mixed Plastics Using Activated Biochar as Catalyst. Energy Fuels 2018, 32(4), 5471–5479. [Google Scholar] [CrossRef]
- Lovás, P.; Hudec, P.; Jambor, B.; Hájeková, E.; Horňáček, M. Catalytic cracking of heavy fractions from the pyrolysis of waste HDPE and PP. Fuel 2017, 203, 244–252. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Elordi, G.; Amutio, M.; Bilbao, J.; Olazar, M. Production of Light Olefins from Polyethylene in a Two-Step Process: Pyrolysis in a Conical Spouted Bed and Downstream High-Temperature Thermal Cracking. Ind. Eng. Chem. Res. 2012, 51(43), 13915–13923. [Google Scholar] [CrossRef]
- Park, K.-B.; Jeong, Y.-S.; Kim, J.-S. Activator-assisted pyrolysis of polypropylene. Appl Energy 2019, 253, 113558. [Google Scholar] [CrossRef]
- Miandad, R.; Nizami, A. S.; Rehan, M.; Barakat, M. A.; Khan, M. I.; Mustafa, A.; Murphy, J. D. Influence of temperature and reaction time on the conversion of polystyrene waste to pyrolysis liquid oil. Waste Manage. 2016, 58, 250–259. [Google Scholar] [CrossRef]
- Nisar, J.; Ali, G.; Shah, A.; Iqbal, M.; Khan, R. A.; Akhter, M. S. Fuel production from waste polystyrene via pyrolysis: Kinetics and products distribution. Waste Manage. 2019, 88, 236–247. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M. I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis Study of Polypropylene and Polyethylene Into Premium Oil Products. Int. J. Green Energy 2014, 12(7), 663–671. [Google Scholar] [CrossRef]
- Budsaereechai, S.; Hunt, A. J.; Ngernyen, Y. Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Adv. 2019, 9(10), 5844–5857. [Google Scholar] [CrossRef] [PubMed]
- Auxilio, A. R.; Choo, W.-L.; Kohli, I.; Chakravartula Srivatsa, S.; Bhattacharya, S. An experimental study on thermo-catalytic pyrolysis of plastic waste using a continuous pyrolyser. Waste Manage. 2017, 67, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Rehan, M.; Barakat, M. A.; Aburiazaiza, A. S.; Khan, H.; Ismail, I. M. I.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front Energy Res. 2019, 7. [Google Scholar] [CrossRef]
- Chaianansutcharit, S.; Katsutath, R.; Chaisuwan, A.; Bhaskar, T.; Nigo, A.; Muto, A.; Sakata, Y. Catalytic degradation of polyolefins over hexagonal mesoporous silica: Effect of aluminum addition. J. Anal. Appl. Pyrolysis 2007, 80(2), 360–368. [Google Scholar] [CrossRef]
- Almustapha M., N.; Andrésen J., M. Recovery of valuable chemicals from high density polyethylene (HDPE) polymer: a catalytic approach for plastic waste recycling[J]. Int. J. Environ. Sci. Dev.
- López, A.; de Marco, I.; Caballero, B. M.; Laresgoiti, M. F.; Adrados, A.; Aranzabal, A. Catalytic pyrolysis of plastic wastes with two different types of catalysts: ZSM-5 zeolite and Red Mud. Appl. Catal., B. [CrossRef]
- Li, K.; Lei, J.; Yuan, G.; Weerachanchai, P.; Wang, J.-Y.; Zhao, J.; Yang, Y. Fe-, Ti-, Zr- and Al-pillared clays for efficient catalytic pyrolysis of mixed plastics. Chem Eng J. 2017, 317, 800–809. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gutiérrez, A.; Palos, R.; Vela, F. J.; Arandes, J. M.; Bilbao, J. Fuel production by cracking of polyolefins pyrolysis waxes under fluid catalytic cracking (FCC) operating conditions. Waste Manage. 2019, 93, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Munir, D.; Irfan, M. F.; Usman, M. R. Hydrocracking of virgin and waste plastics: A detailed review. Renew Sust Energ Rev. 2018, 90, 490–515. [Google Scholar] [CrossRef]
- Lee, W.-T.; van Muyden, A.; Bobbink, F. D.; Mensi, M. D.; Carullo, J. R.; Dyson, P. J. Mechanistic classification and benchmarking of polyolefin depolymerization over silica-alumina-based catalysts. Nature Commun. [CrossRef]
- Kots, P. A.; Liu, S.; Vance, B. C.; Wang, C.; Sheehan, J. D.; Vlachos, D. G. Polypropylene Plastic Waste Conversion to Lubricants over Ru/TiO2 Catalysts. ACS Catal. 2021, 11(13), 8104–8115. [Google Scholar] [CrossRef]
- Kots, P. A.; Xie, T.; Vance, B. C.; Quinn, C. M.; de Mello, M. D.; Boscoboinik, J. A.; Vlachos, D. G. Electronic modulation of metal-support interactions improves polypropylene hydrogenolysis over ruthenium catalysts. Nature Commun. [CrossRef]
- Rorrer, J. E.; Beckham, G. T.; Román-Leshkov, Y. Conversion of Polyolefin Waste to Liquid Alkanes with Ru-Based Catalysts under Mild Conditions. JACS Au 2020, 1(1), 8–12. [Google Scholar] [CrossRef]
- Celik, G.; Kennedy, R. M.; Hackler, R. A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; Delferro, M. Upcycling Single-Use Polyethylene into High-Quality Liquid Products. ACS Cent. Sci. 2019, 5(11), 1795–1803. [Google Scholar] [CrossRef]
- Tennakoon, A.; Wu, X.; Paterson, A. L.; Patnaik, S.; Pei, Y.; LaPointe, A. M.; Perras, F. A. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 2020, 3(11), 893–901. [Google Scholar] [CrossRef]
- Bin Jumah, A.; Anbumuthu, V.; Tedstone, A. A.; Garforth, A. A. Catalyzing the Hydrocracking of Low Density Polyethylene. Ind. Eng. Chem. Res. 2019, 58(45), 20601–20609. [Google Scholar] [CrossRef]
- Liu, S.; Kots, P. A.; Vance, B. C.; Danielson, A.; Vlachos, D.G. Plastic waste to fuels by hydrocracking at mild conditions[J]. Sci Adv. 8283. [Google Scholar]
- Escola, J. M.; Aguado, J.; Serrano, D. P.; Briones, L. Transportation fuel production by combination of LDPE thermal cracking and catalytic hydroreforming. Waste Manage. 2014, 34(11), 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Mangesh, V. L.; Perumal, T.; Subramanian, S.; Padmanabhan, S. Clean Energy from Plastic: Production of Hydroprocessed Waste Polypropylene Pyrolysis Oil Utilizing a Ni–Mo/Laponite Catalyst. Energy Fuels 2020, 34(7), 8824–8836. [Google Scholar] [CrossRef]
- Escola, J. M.; Aguado, J.; Serrano, D. P.; García, A.; Peral, A.; Briones, L.; Fernandez, E. Catalytic hydroreforming of the polyethylene thermal cracking oil over Ni supported hierarchical zeolites and mesostructured aluminosilicates. Appl Catal B. [CrossRef]
- Chen, H.; Wan, K.; Zhang, Y.; Wang, Y. Waste to Wealth: Chemical Recycling and Chemical Upcycling of Waste Plastics for a Great Future. ChemSusChem 2021, 14(19), 4123–4136. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A. B.; Noor, Z. Z.; Hassan, A.; Abd Hamid, M. K.; Samsudin, S. A.; Sabeen, A. H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Cleaner Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
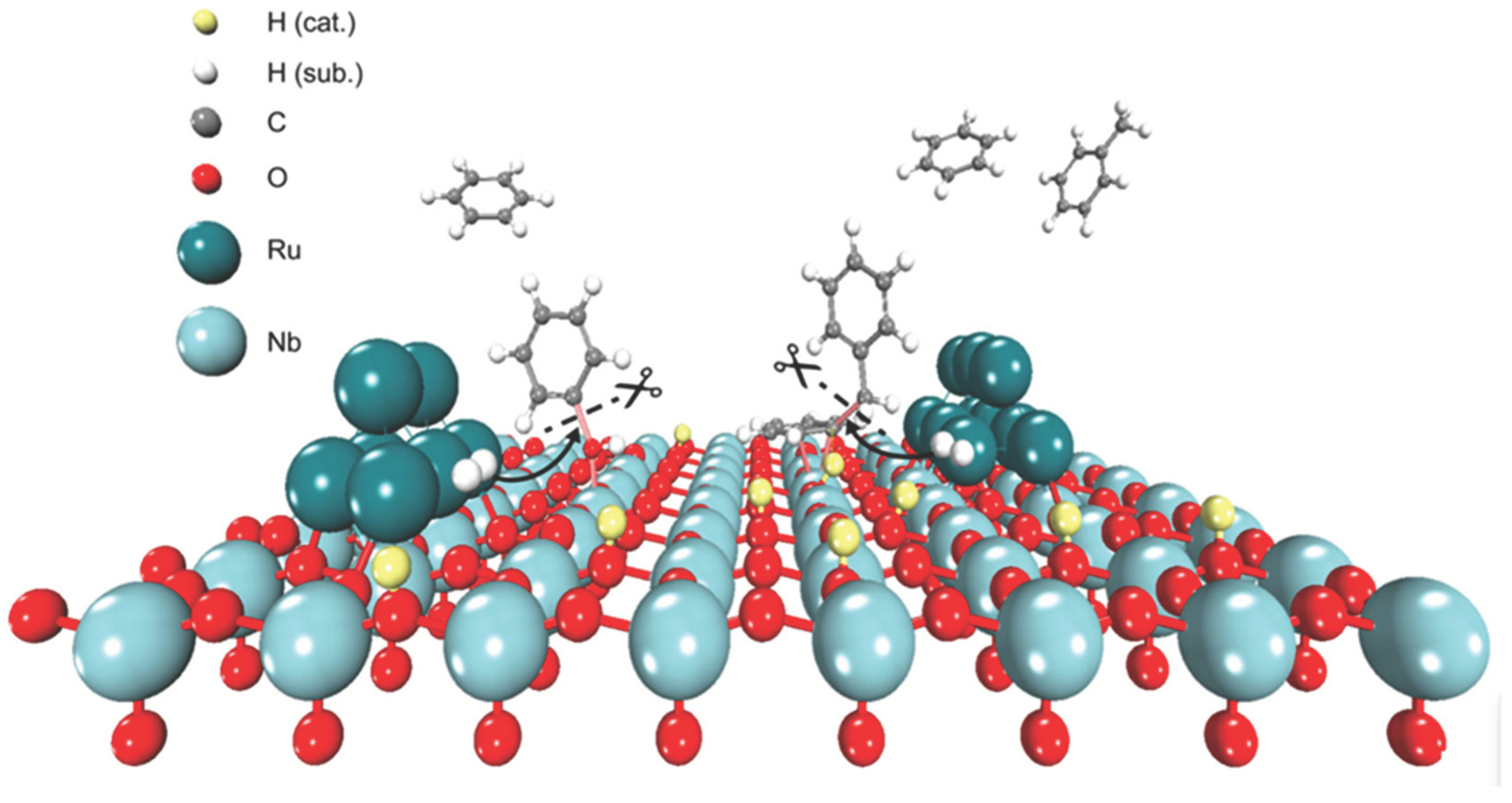
- Jing, Y.; Wang, Y.; Furukawa, S.; Xia, J.; Sun, C.; Hülsey, M. J.; Yan, N. Towards the Circular Economy: Converting Aromatic Plastic Waste Back to Arenes over a Ru/Nb2O5 Catalyst. Angew. Chem., Int. Ed. 5527. [Google Scholar] [CrossRef]
- Lu, S.; Feng, B.; Guo, Y.; Liu, X.; Wang, Y. H2-free Plastic Conversion: Converting PET back to BTX by Unlocking Hidden Hydrogen[J]. ChemSusChem 2021, 14(19): 4242-4250.
- Kang, M. J.; Yu, H. J.; Jegal, J.; Kim, H. S.; Cha, H. G. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem Eng J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, B.; Chen, S.; Tian, J.; Zhao, C. Converting waste PET plastics into automobile fuels and antifreeze components. Nature Commun. [CrossRef]
- Tang, H.; Li, N.; Li, G.; Wang, A.; Cong, Y.; Xu, G.; Wang, X.; Zhang, T. Synthesis of gasoline and jet fuel range cycloalkanes and aromatics from poly (ethylene terephthalate) waste[J]. Green Chemi. 2709. [Google Scholar]
- Zhang, H.-f.; Su, X.-l.; Sun, D.-k.; Zhang, R.; Bi, J.-c. Investigation on degradation of polyethylene to oil in a continuous supercritical water reactor. Journal of Fuel Chemistry and Technology 2007, 35(4), 487–491. [Google Scholar] [CrossRef]
- Jie, H.; Ke, H.; Qing, Z.; Lei, C.; Yongqiang, W.; Zibin, Z. Study on depolymerization of polycarbonate in supercritical ethanol. Polym. Degrad. Stab. 2006, 91(10), 2307–2314. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, C.; Bai, B.; Jin, H.; Wei, W. Study on the polystyrene plastic degradation in supercritical water/CO2 mixed environment and carbon fixation of polystyrene plastic in CO2 environment. J. Hazard. Mater. 2022, 421, 126763. [Google Scholar] [CrossRef]
- Bai, B.; Wang, W.; Jin, H. Experimental study on gasification performance of polypropylene (PP) plastics in supercritical water. Energy 2020, 191, 116527. [Google Scholar] [CrossRef]
- Arregi, A.; Seifali Abbas-Abadi, M.; Lopez, G.; Santamaria, L.; Artetxe, M.; Bilbao, J.; Olazar, M. CeO2 and La2O3 Promoters in the Steam Reforming of Polyolefinic Waste Plastic Pyrolysis Volatiles on Ni-Based Catalysts. ACS Sustainable Chem. Eng. 2020, 8(46), 17307–17321. [Google Scholar] [CrossRef]
- Helmer Pedersen, T.; Conti, F. Improving the circular economy via hydrothermal processing of high-density waste plastics. Waste Manage. 2017, 68, 24–31. [Google Scholar] [CrossRef]
- Onwudili, J. A.; Williams, P. T. Catalytic supercritical water gasification of plastics with supported RuO2: A potential solution to hydrocarbons–water pollution problem. Process Saf. Environ. Prot. 2016, 102, 140–149. [Google Scholar] [CrossRef]
- Chen, W.-T.; Jin, K.; Linda Wang, N.-H. Use of Supercritical Water for the Liquefaction of Polypropylene into Oil. ACS Sustainable Chem. Eng. 2019, 7(4), 3749–3758. [Google Scholar] [CrossRef]
- Xu, Z. R.; Zhu, W.; Gong, M.; Zhang, H. W. Direct gasification of dewatered sewage sludge in supercritical water. Part 1: Effects of alkali salts. Int. J. Hydrogen Energy, 3963. [Google Scholar] [CrossRef]
- Seshasayee, M. S.; Savage, P. E. Oil from plastic via hydrothermal liquefaction: Production and characterization. Appl. Energy 2020, 278, 115673. [Google Scholar] [CrossRef]
- Su, X.; Zhao, Y.; Zhang, R.; Bi, J. Investigation on degradation of polyethylene to oils in supercritical water. Fuel Process. Technol. 1249. [Google Scholar] [CrossRef]
- Bai, B.; Jin, H.; Fan, C.; Cao, C.; Wei, W.; Cao, W. Experimental investigation on liquefaction of plastic waste to oil in supercritical water. Waste Manage. 2019, 89, 247–253. [Google Scholar] [CrossRef]
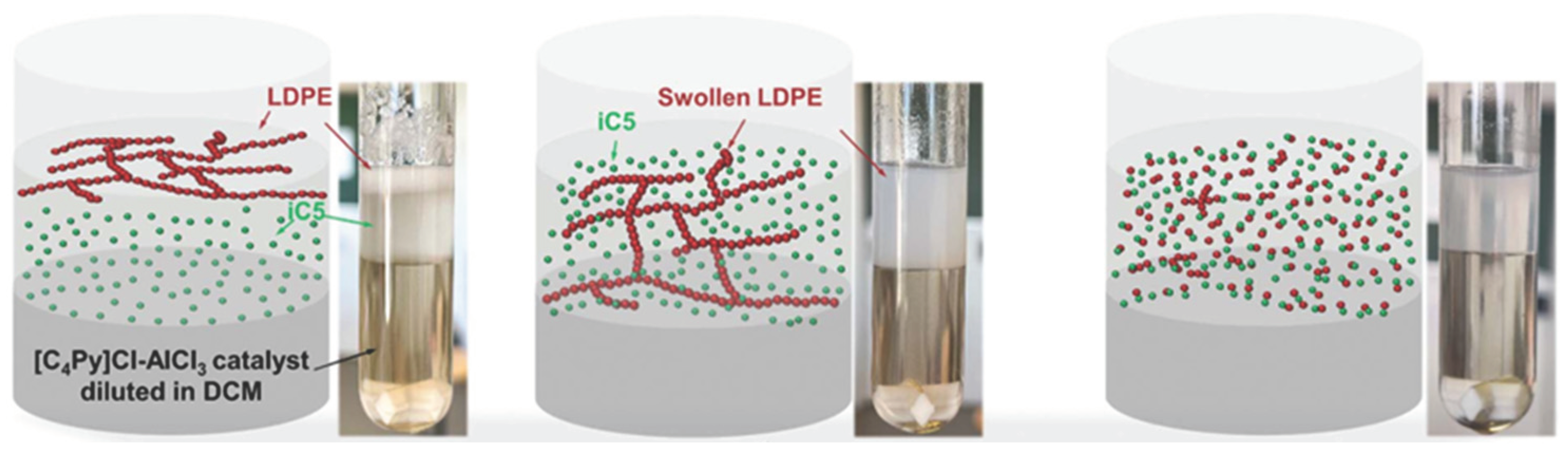
- Zhang, W.; Kim, S.; Wahl, L.; Khare, R.; Hale, L.; Hu, J.; Liu, Y.; et al. Low-temperature upcycling of polyolefins into liquid alkanes via tandem cracking-alkylation[J]. Science, 6634. [Google Scholar]
- Jia, X.; Qin, C.; Friedberger, T.; Guan, Z.; Huang, Z. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions[J]. Sci. adv. 1501. [Google Scholar]
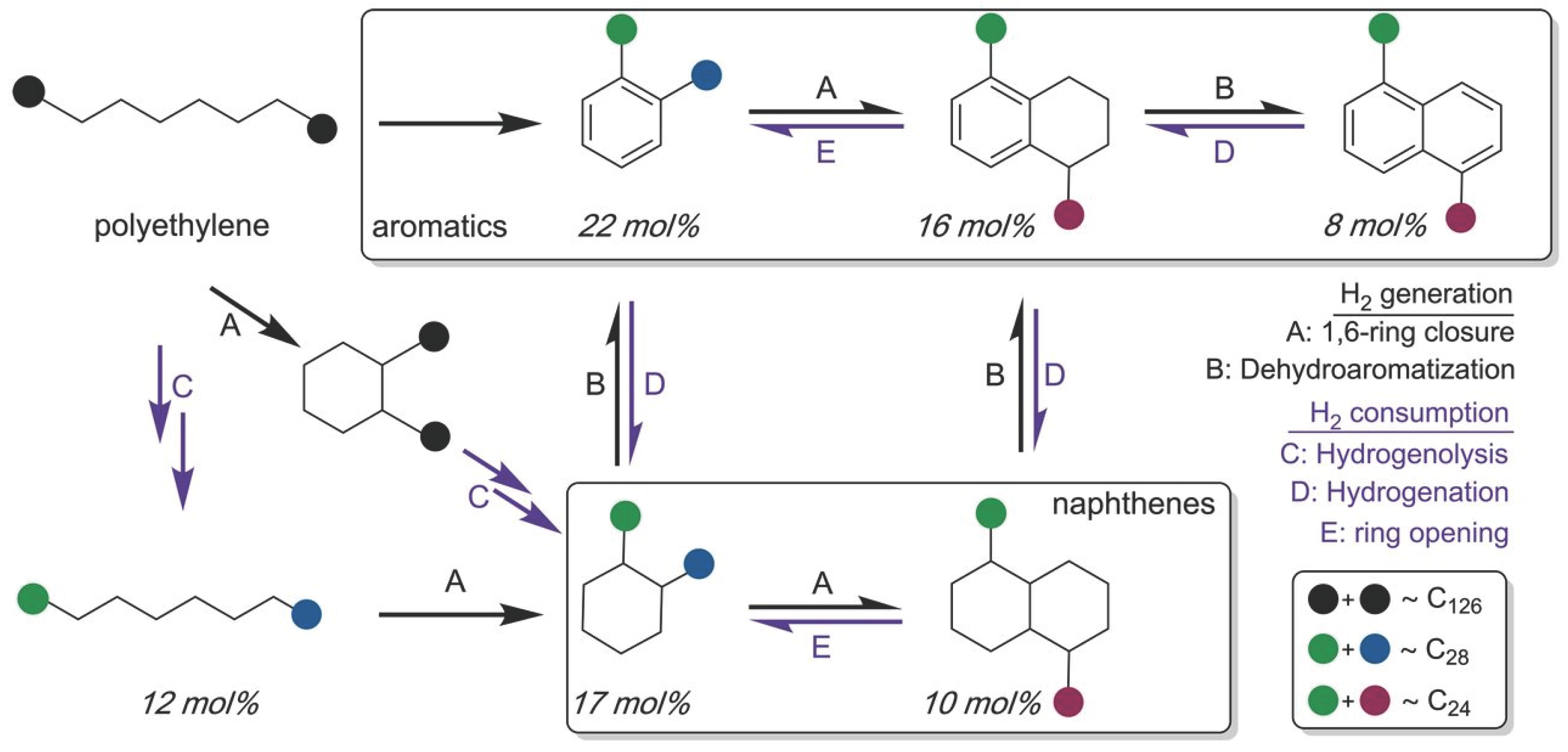
- Zhang, F.; Zeng, M.; Yappert, R. D.; Sun, J.; Lee, Y.H.; LaPointe, A.M.; Peters, B.; et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization[J]. Science, 6515. [Google Scholar]
- Jing, X.; Zhao, Y.; Wen, H.; Xu, Z. Interactions between Low-Density Polyethylene (LDPE) and Polypropylene (PP) during the Mild Cracking of Polyolefin Mixtures in a Closed-Batch Reactor. Energy Fuels 2013, 27(10), 5841–5851. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Y.; Zhang, J. Hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water for oil: Product distribution. J. Anal. Appl. Pyrolysis 2014, 110, 382–389. [Google Scholar] [CrossRef]
- Park, Y.-K.; Lee, B.; Watanabe, A.; Lee, H. W.; Lee, J. Y.; Kim, S.; Kim, Y.-M. Catalytic Copyrolysis of Cork Oak and Waste Plastic Films over HBeta. Catalysts 2018, 8(8), 318. [Google Scholar] [CrossRef]
- Serrano, D. P.; Aguado, J.; Escola, J. M. Developing Advanced Catalysts for the Conversion of Polyolefinic Waste Plastics into Fuels and Chemicals. ACS Catal. 2012, 2(9), 1924–1941. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Elordi, G.; Lopez, G.; Borsella, E.; Bilbao, J.; Olazar, M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2012, 94, 230–237. [Google Scholar] [CrossRef]
- Kodera, Y.; Ishihara, Y.; Kuroki, T. Novel process for recycling waste plastics to fuel gas using a moving-bed reactor[J]. Energy Fuels. [CrossRef]
| plastic | method | catalyst | condition | reactor | liquid yield | references |
|---|---|---|---|---|---|---|
| PS | pyrolysis | — | 450 oC, 75 min | batch reactor | 81 % | [39] |
| PS | pyrolysis | — | 410 oC, 70 min | fixed bed reactor | 85 % | [40] |
| PP | pyrolysis | — | 300 oC | fixed bed reactor | 69 % | [41] |
| PE | pyrolysis | — | 500 oC, | fluidized bed reactor |
81 % | [11] |
| PS | catalytic reforming |
pelletized bentonite | 700 oC /500 oC | fixed-bed pyrolysis stainless steel batch reactor | 88 % | [42] |
| PP | catalytic reforming |
pelletized bentonite | 700 oC /500 oC | fixed-bed pyrolysis stainless steel batch reactor | 91 % | [38] |
| HDPE | catalytic reforming |
CAT-1 | 425 oC /425 oC | continuous stirred tank reactor, reactive distillation column |
80 % | [43] |
| mixed plastic | catalytic reforming |
Fe/HZSM-5 | 350 oC /350 oC | batch reactor, fixed bed reactor | 76 % | [20] |
| PS | catalytic pyrolysis |
NZ zeolite | 450 oC, 75 min | batch reactor | 70 % | [44] |
| PS | catalytic pyrolysis |
HY | 450 oC, 44 min | batch reactor | 71 % | [17] |
| PP | catalytic pyrolysis |
HY | 500 oC, 44 min | batch reactor | 44 % | [17] |
| PP | catalytic pyrolysis |
hexagonal mesoporous silica | 380 oC, 1:10 | fixed bed reactor | 65 % | [45] |
| PE | catalytic pyrolysis |
hexagonal mesoporous silica | 430 oC, 1:10 | fixed bed reactor | 75 % | [41] |
| HDPE | catalytic pyrolysis |
7% SO3 | 400 oC | fixed bed reactor | 32 % | [46] |
| HDPE | catalytic pyrolysis |
HZSM-5 | 550 oC, 6 h | conical spouted bed reactor | 20 % | [13] |
| mixed plastic | catalytic pyrolysis |
ZSM-5 zeolite and Red Mud |
440 oC, 1:10 | batch reactor | 57 % | [47] |
| mixed plastic | catalytic pyrolysis |
Al-pillared clays | 500 oC, 30 min | tube reactor | 79.3% | [48] |
| plastic | catalyst | condition | yield | references |
|---|---|---|---|---|
| LDPEO | Ni/beta | 310 oC, 20 bar, 45 min | 81% gasoline fuel | [59] |
| PPO | Ni-Mo/Lap | 350 oC, 70 bar | 95% diesel range of n-alkanes, isoalkanes, aromatics |
[60] |
| LDPEO | Ni/beta | 310 oC, 20 bar, 45 min | 54% gasoline, 40% diesel | [61] |
| LDPE | Pt/WO3/ZrO2 and HY | 225 oC, 30 bar, 2 h |
85% diesel and jet fuel oils, gasoline range hydrocarbons |
[54] |
| LDPE | Pt/USY and beta | 330 oC, 20bar, 15 min |
95% liquid hydrocarbon | [53] |
| PP | Ru/TiO2 | 250 oC, 30 bar, | 66% liquid hydrocarbon, 25%C1-C2,4% C3-C6 |
[49] |
| PE | Pt/SrTiO3 | 300 oC, 11.7 bar, 96 h | 100% lubricants, waxes | [51] |
| PE | Ru/C | 200 oC, 20 bar, 16 h | 45% liquid n-alkanes, C1-C6 | [50] |
| PP | Ru/TiO2 | 250 oC, 40 bar, 6 h |
74% liquid hydrocarbon | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




