Submitted:
08 March 2024
Posted:
12 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Alterations in Hemodynamic Indices and Cardiovascular Variability during AMI and Effects of Pyridostigmine
2.2. Alterations in Structural and Functional Echocardiographic Parameters during AMI and Effects of Pyridostigmine
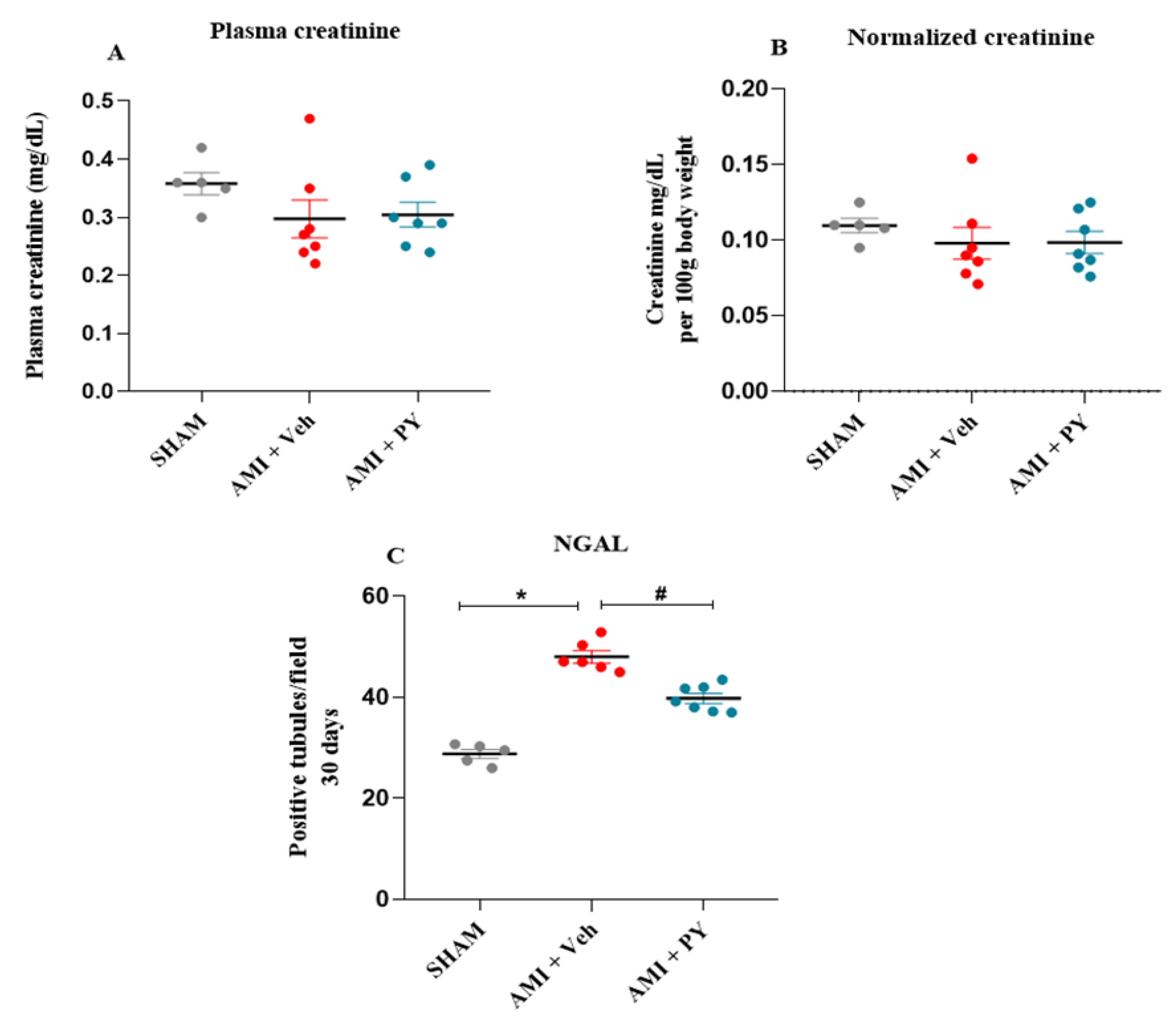
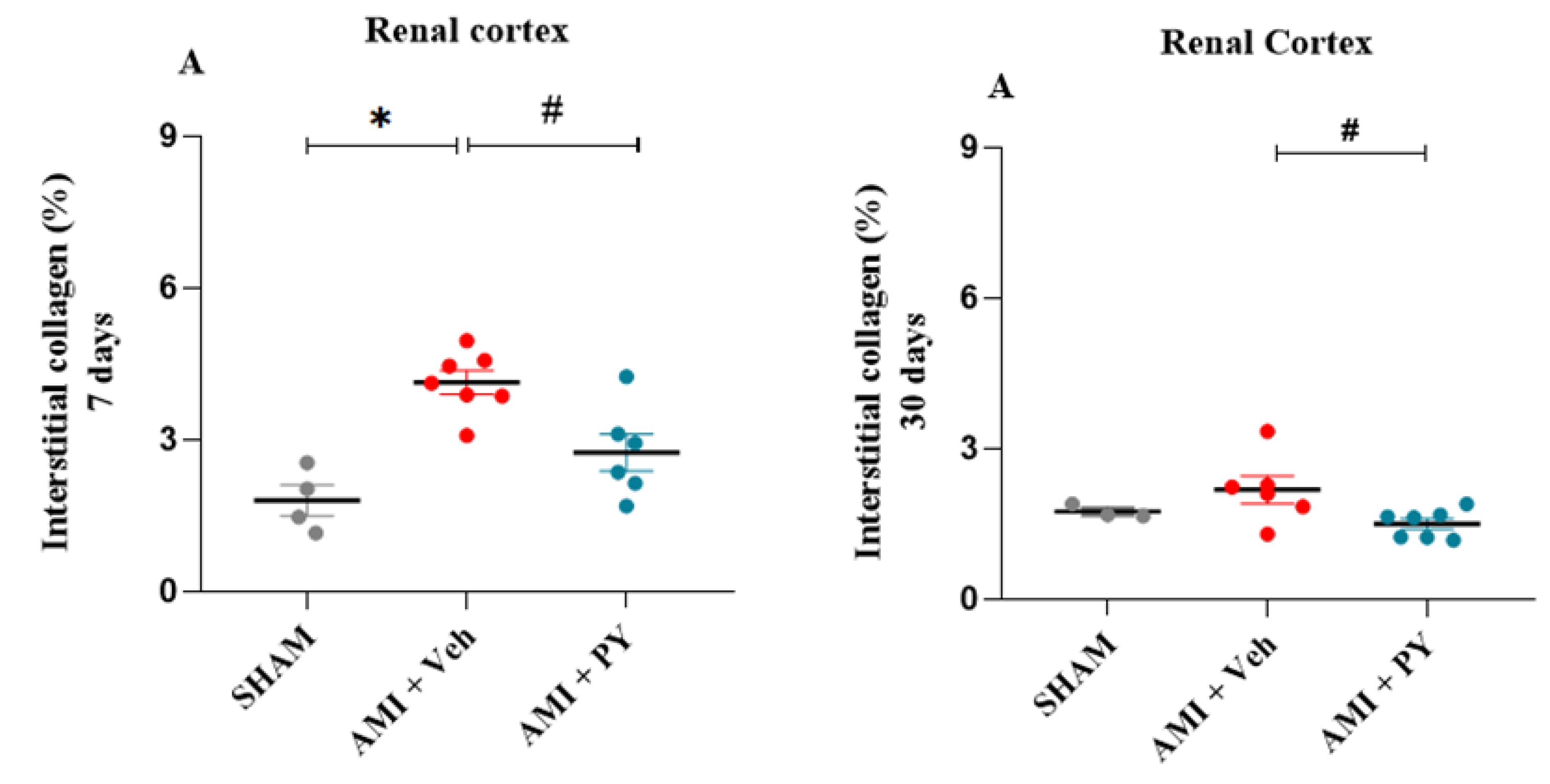
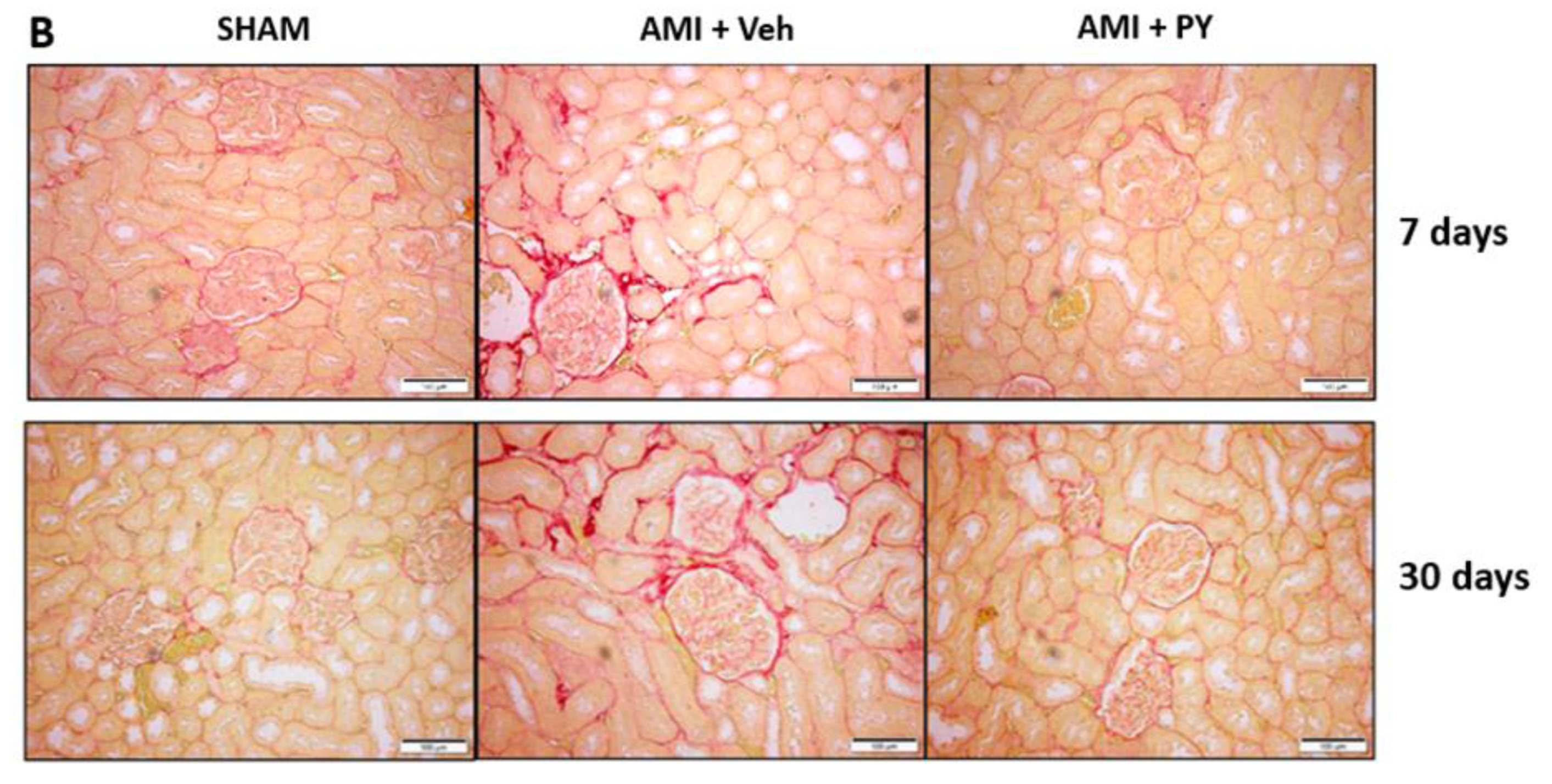
2.3. Lterations in Renal Function Markers during AMI and Effects of Pyridostigmine
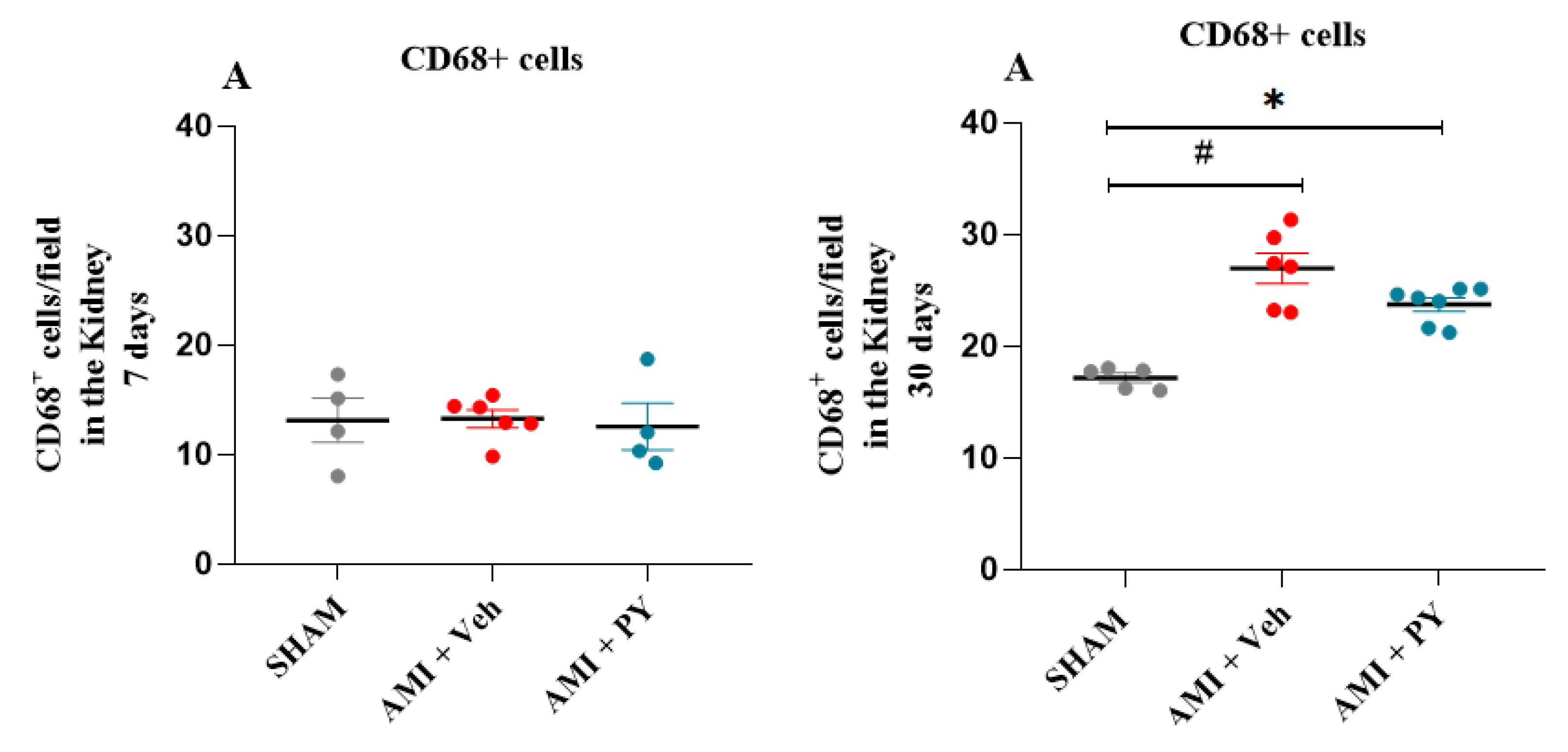
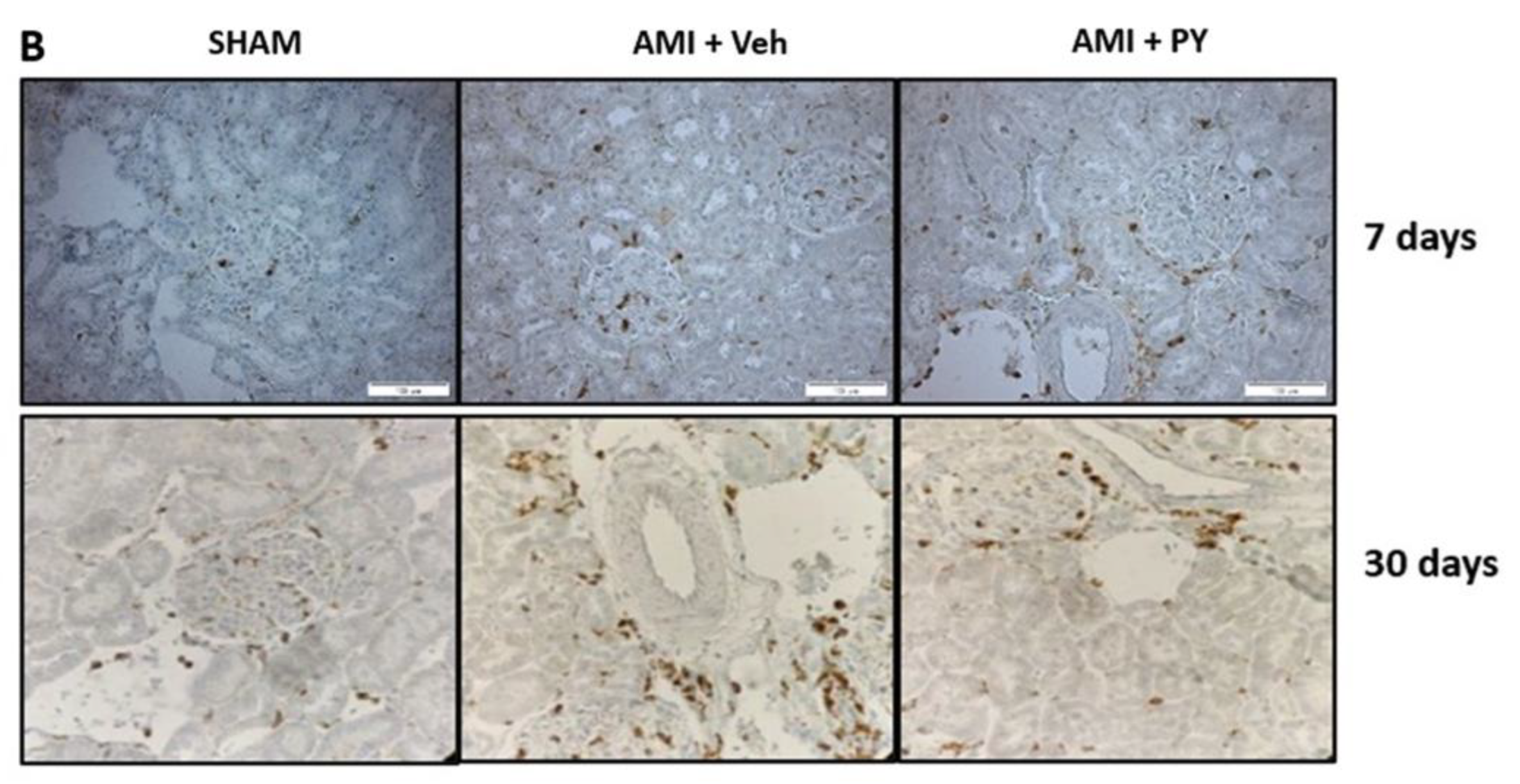
2.4. Changes in CD68+ Macrophages in Renal Tissue Following AMI and Effects of Pyridostigmine
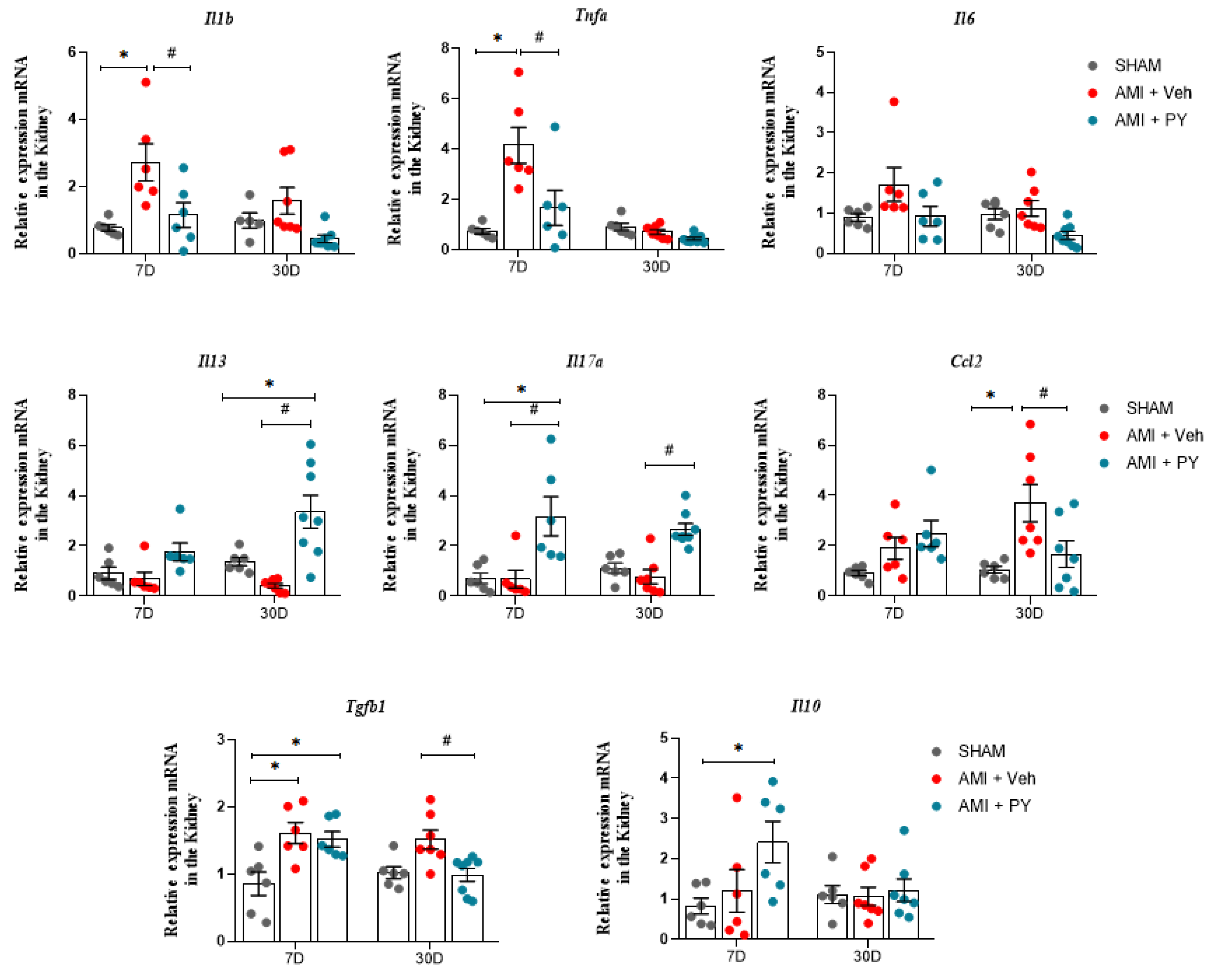
2.5. Changes in the Gene Expression of Pro-Inflammatory and Anti-Inflammatory Markers in Renal Tissue Post-ACUTE myocardial Infarction and Effects of Pyridostigmine
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Experimental Design
4.4. Myocardial Infarction
4.5. Arterial Catheterization and Cardiovascular Assessments
4.6. Echocardiographic Evaluation
4.7. Renal Function Marker Analysis
4.8. Histological Examination and Quantitative Collagen Analysis
4.9. Immunohistochemistry for Immune Cells and NGAL
4.10. Real-time Quantitative PCR
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salari N, Morddarvanjoghi F, Abdolmaleki A, Rasoulpoor S, Khaleghi AA, Hezarkhani LA, Shohaimi S., Mohammadi M. The global prevalence of myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2023 Apr 22;23(1):206. [CrossRef]
- Frangogiannis, N.G. The immune system and cardiac repair. Pharmacol Res. 2008 Aug;58(2):88-111. [CrossRef]
- Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008 May 12;168(9):987-95. [CrossRef]
- Goldberg A, Kogan E, Hammerman H, Markiewicz W, Aronson D. The impact of transient and persistent acute kidney injury on long-term outcomes after acute myocardial infarction. Kidney Int. 2009 Oct;76(8):900-6. [CrossRef]
- Ronco C, Haapio M, House AA, Anavekar N., Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008 Nov 4;52(19):1527-39. [CrossRef]
- Virzì G, Day S, de Cal M, Vescovo G., Ronco C. Heart-kidney crosstalk and role of humoral signaling in critical illness. Crit Care. 2014 Jan 6;18(1):201. [CrossRef]
- Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012 Sep 18;60(12):1031-42. [CrossRef]
- Lekawanvijit S, Kompa AR, Zhang Y, Wang BH, Kelly DJ, Krum H. Myocardial infarction impairs renal function, induces renal interstitial fibrosis, and increases renal KIM-1 expression: implications for cardiorenal syndrome. Am J Physiol Heart Circ Physiol. 2012 May 1;302(9):H1884-93. [CrossRef]
- Cho E, Kim M, Ko YS, Lee HY, Song M, Kim MG, Kim HK, Cho WY, Jo SK. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transplant. 2013 Nov;28(11):2766-78. [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature. 2002 Dec 19-26;420(6917):853-9. [CrossRef]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005 Nov;19(6):493-9. [CrossRef]
- Metz CN, Pavlov VA. Vagus nerve cholinergic circuitry to the liver and the gastrointestinal tract in the neuroimmune communicatome. Am J Physiol Gastrointest Liver Physiol. 2018 Nov 1;315(5):G651-G658. [CrossRef]
- Pavlov VA, Chavan SS, Tracey KJ. Molecular and Functional Neuroscience in Immunity. Annu Rev Immunol. 2018 Apr 26;36:783-812. [CrossRef]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003 Jan 23;421(6921):384-8. [CrossRef]
- Pohanka, M. Pharmacological Influencing of The Cholinergic Anti-inflammatory Pathway in Infectious Diseases and Inflammatory Pathologies. Mini Rev Med Chem. 2021;21(6):660-669. [CrossRef]
- Kelly MJ, Breathnach C, Tracey KJ, Donnelly SC. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep Med. 2022 Jul 19;3(7):100696. [CrossRef]
- Falvey A, Metz CN, Tracey KJ, Pavlov VA. Peripheral nerve stimulation and immunity: the expanding opportunities for providing mechanistic insight and therapeutic intervention. Int Immunol. 2022 Jan 22;34(2):107-118. [CrossRef]
- Metz CN, Pavlov VA. Treating disorders across the lifespan by modulating cholinergic signaling with galantamine. J Neurochem. 2021 Sep;158(6):1359-1380. [CrossRef]
- Chatterjee PK, Yeboah MM, Dowling O, Xue X, Powell SR, Al-Abed Y., Metz CN. Nicotinic acetylcholine receptor agonists attenuate septic acute kidney injury in mice by suppressing inflammation and proteasome activity. PLoS One. 2012;7(5):e35361. [CrossRef]
- Chatterjee PK, Yeboah MM, Solanki MH, Kumar G, Xue X, Pavlov VA, Al-Abed Y, Metz CN. Activation of the cholinergic anti-inflammatory pathway by GTS-21 attenuates cisplatin-induced acute kidney injury in mice. PLoS One. 2017 Nov 30;12(11):e0188797. [CrossRef]
- Nakamura Y, Inoue T. Neuroimmune Communication in the Kidney. JMA J. 2020 Jul 15;3(3):164-174. [CrossRef]
- Okusa MD, Rosin DL, Tracey KJ. Targeting neural reflex circuits in immunity to treat kidney disease. Nat Rev Nephrol. 2017 Nov;13(11):669-680. [CrossRef]
- Yeboah MM, Xue X, Duan B, Ochani M, Tracey KJ, Susin M, Metz CN. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008 Jul;74(1):62-9. [CrossRef]
- de La Fuente RN, Rodrigues B, Moraes-Silva IC, Souza LE, Sirvente R, Mostarda C, De Angelis K, Soares PP, Lacchini S, Consolim-Colombo F, Irigoyen MC. Cholinergic stimulation with pyridostigmine improves autonomic function in infarcted rats. Clin Exp Pharmacol Physiol. 2013 Sep;40(9):610-6. [CrossRef]
- Rocha JA, Ribeiro SP, França CM, Coelho O, Alves G, Lacchini S, Kallás EG, Irigoyen MC, Consolim-Colombo FM. Increase in cholinergic modulation with pyridostigmine induces anti-inflammatory cell recruitment soon after acute myocardial infarction in rats. Am J Physiol Regul Integr Comp Physiol. 2016 Apr 15;310(8):R697-706. [CrossRef]
- Bezerra OC, França CM, Rocha JA, Neves GA, Souza PRM, Teixeira Gomes M, Malfitano C, Loleiro TCA, Dourado PM, Llesuy S, de Angelis K, Irigoyen MCC, Ulloa L, Consolim-Colombo FM. Cholinergic Stimulation Improves Oxidative Stress and Inflammation in Experimental Myocardial Infarction. Sci Rep. 2017 Oct 20;7(1):13687. [CrossRef]
- Bandoni RL, Bricher Choque PN, Dellê H, de Moraes TL, Porter MHM, da Silva BD, Neves GA, Irigoyen MC, De Angelis K, Pavlov VA, Ulloa L, Consolim-Colombo FM. Cholinergic stimulation with pyridostigmine modulates a heart-spleen axis after acute myocardial infarction in spontaneous hypertensive rats. Sci Rep. 2021 May 5;11(1):9563. [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl. 2008;241:89-94. [CrossRef]
- Cooper TM, McKinley PS, Seeman TE, Choo TH, Lee S, Sloan RP. Heart rate variability predicts levels of inflammatory markers: Evidence for the vagal anti-inflammatory pathway. Brain Behav Immun. 2015 Oct;49:94-100. [CrossRef]
- Sangaleti CT, Katayama KY, De Angelis K, et al. The Cholinergic Drug Galantamine Alleviates Oxidative Stress Alongside Anti-inflammatory and Cardio-Metabolic Effects in Subjects With the Metabolic Syndrome in a Randomized Trial. Frontiers in Immunology. 2021;12:613979. [CrossRef]
- de Moraes TL, Costa FO, Cabral DG, et al. Brief periods of transcutaneous auricular vagus nerve stimulation improve autonomic balance and alter circulating monocytes and endothelial cells in patients with metabolic syndrome: a pilot study. Bioelectronic Medicine. 2023 Mar;9(1):7. [CrossRef]
- Pavlov VA, Tracey KJ. Bioelectronic medicine: Preclinical insights and clinical advances. Neuron. 2022 Nov 2;110(21):3627-3644. [CrossRef]
- Okamoto, K. & Aoki, K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 27, 282–293 (1963). [CrossRef]
- Harwani, S.C. , Chapleau, M.W., Legge, K.L., Ballas, Z.K. & Abboud, F.M. Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ. Res. 111, 1190–1197 (2012). [CrossRef]
- Li DJ, Evans RG, Yang ZW, Song SW, Wang P, Ma XJ, Liu C, Xi T, Su DF, Shen FM. Dysfunction of the cholinergic anti-inflammatory pathway mediates organ damage in hypertension. Hypertension. 2011 Feb;57(2):298-307. [CrossRef]
- La Rovere, M.T. , Pinna, G.D. & Raczak, G. Baroreflex sensitivity: measurement and clinical implications. Ann. Noninvasive Electrocardiol. 13, 191–207 (2008). [CrossRef]
- La Rovere MT, Pinna GD, Maestri R, Robbi E, Caporotondi A, Guazzotti G, Sleight P, Febo O. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J Am Coll Cardiol. 2009 Jan 13;53(2):193-9. [CrossRef]
- Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, Ye H, Rosin DL, Guyenet PG, Okusa MD. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J Clin Invest. 2016 May 2;126(5):1939-52. [CrossRef]
- Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Comprehensive Physiology. 2011 Apr;1(2):731-767. [CrossRef]
- Choi ME, Ding Y, Kim SI. TGF-β signaling via TAK1 pathway: role in kidney fibrosis. Semin Nephrol. 2012 May;32(3):244-52. [CrossRef]
- Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X, Patel N, Johnson SM, Chavan S, Goldstein RS, Czura CJ, Miller EJ, Al-Abed Y, Tracey KJ, Pavlov VA. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008 Sep-Oct;14(9-10):567-74. [CrossRef]
- Wu SJ, Shi ZW, Wang X, Ren FF, Xie ZY, Lei L, Chen P. Activation of the Cholinergic Anti-inflammatory Pathway Attenuated Angiotension II-Dependent Hypertension and Renal Injury. Front Pharmacol. 2021 Mar 17;12:593682. [CrossRef]
- Rudemiller NP, Crowley SD. The role of chemokines in hypertension and consequent target organ damage. Pharmacol Res. 2017 May;119:404-411. [CrossRef]
- Liu Y, Xu K, Xiang Y, Ma B, Li H, Li Y, Shi Y, Li S, Bai Y. Role of MCP-1 as an inflammatory biomarker in nephropathy. Front Immunol. 2024 Jan 4;14:1303076. [CrossRef]
- Uni R, Inoue T, Nakamura Y, Fukaya D, Hasegawa S, Wu CH, Fujii R, Surattichaiyakul B, Peerapanyasut W, Ozeki A, Akimitsu N, Wada Y, Nangaku M, Inagi R. Vagus nerve stimulation even after injury ameliorates cisplatin-induced nephropathy via reducing macrophage infiltration. Sci Rep. 2020 Jun 11;10(1):9472. [CrossRef]
- Cantero-Navarro E, Rayego-Mateos S, Orejudo M, Tejedor-Santamaria L, Tejera-Muñoz A, Sanz AB, Marquez-Exposito L, Marchant V, Santos-Sanchez L, Egido J, Ortiz A, Bellon T, Rodrigues-Diez RR, Ruiz-Ortega M. Role of Macrophages and Related Cytokines in Kidney Disease. Front Med (Lausanne). 2021 Jul 8;8:688060. [CrossRef]
- Zhang MZ, Wang X, Wang Y, Niu A, Wang S, Zou C, Harris RC. IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int. 2017 Feb;91(2):375-386. [CrossRef]
- Zahler D, Merdler I, Banai A, Shusterman E, Feder O, Itach T, Robb L, Banai S, Shacham Y. Predictive Value of Elevated Neutrophil Gelatinase-Associated Lipocalin (NGAL) Levels for Assessment of Cardio-Renal Interactions among ST-Segment Elevation Myocardial Infarction Patients. J Clin Med. 2022 Apr 13;11(8):2162. [CrossRef]
|
SHAM 7 days (n=10) |
AMI+Veh 7 days (n=10) |
AMI + PY 7 days (n=10) |
SHAM 30 days (n=10) |
AMI+Veh 30 days (n=10) |
AMI + PY 30 days (n=10) |
|
|---|---|---|---|---|---|---|
|
SBP mmHg mean (SD) |
203.4 (6.9) |
174.8* (17.5) |
163.3* (9.2) |
212.5 (8.2) |
207.2 (12.5) |
189.3*# (13.4) |
|
DBP mmHg mean (SD) |
143.7 (7.2) |
129.0* (8.3) |
119.6*# (7.9) |
148.5 (6.0) |
144.4 (6.3) |
134.2 (8.2) |
|
MBP mmHg mean (SD) |
171.1 (5.6) |
148.8* (13.9) |
142.4* (8.7) |
176.3 (7.0) |
174.8 (9.5) |
161.8*# (10.0) |
|
HR bpm mean (SD) |
373.5 (26.1) |
393.0 (22.1) |
375.7 (26.7) |
374.4 (26.3) |
384.3 (22.1) |
369.0 (11.9) |
|
SHAM 7 d (n=10) |
AMI+Veh 7 d (n=8) |
AMI + PY 7 d (n=10) |
SHAM 30 d (n=10) |
AMI+Veh 30 d (n=10) |
AMI + PY 30 d (n=10) |
|
|---|---|---|---|---|---|---|
| HRV | ||||||
|
RMSSD (ms) SD |
6.2 (2.0) |
5.8 (2.2) |
9.1*# (2.1) |
8.1 (2.9) |
9.8 (2.7) |
10.1 (2.0) |
|
VARPI (ms2) SD |
28.5 (13.0) |
15.7 (6.0) |
60.1*# (27.9) |
51.5 (18.7) |
69.6 (30.6) |
91.8* (49.0) |
|
LF ab (ms²) SD |
2.5 (1.9) |
2.5 (1.0) |
7.2*# (4.6) |
2.2 (1.4) |
2.6 (1.4) |
3.9 (2.0) |
|
HF ab (ms²) SD |
9.6 (3.5) |
5.9 (2.9) |
22.7*# (9.0) |
25.6 (17.5) |
26.3 (12.8) |
30.1 (11.0) |
|
LF (nu) SD |
19.3 (7.3) |
31.7* (9.8) |
22.9 (6.9) |
10.1 (3.1) |
11.1 (4.0) |
12.0 (2.9) |
|
HF (nu) SD |
80.6 (7.3) |
68.3* (9.8) |
77.1 (6.9) |
89.5 (2.7) |
88.5 (4.0) |
87.5 (3.8) |
|
LF/HF SD |
0.2 (0.1) |
0.5* (0.2) |
0.3 (0.1) |
0.11 (0.03) |
0.12 (0.04) |
0.14 (0.05) |
| SBPV | ||||||
|
VAR-SAP(mmHg²) SD |
57.8 (18.5) |
28.6* (12.1) |
30.4* (14.2) |
41.2 (8.1) |
43.0 (18.4) |
47.6 (19.3) |
|
LF-SAP (mmHg²) SD |
14.7 (8.2) |
11.1 (8.0) |
8.7 (4.5) |
11.1 (3.0) |
12.1 (4.5) |
7.8 # (2.8) |
|
ALFA INDEX ms²/ mmHg² SD |
0.49 (0.34) |
0.55 (0.19) |
0.98*# (0.48) |
0.44 (0.15) |
0.57 (0.17) |
0.70* (0.26) |
| SHAM 7 days (n=10) |
AMI+Veh 7 days (n=10) |
AMI + PY 7days (n=10) |
SHAM 30 days (n=10) |
AMI+Veh 30 days (n=10) |
AMI + PY 30 days (n=10) |
|
|---|---|---|---|---|---|---|
| PARAMETER | ||||||
|
LAD (mm) SD |
4.25 (0.95) |
4.58 (1.19) |
4.08 (0.70) |
5.11 (0.67) |
5.52 (0.66) |
5.18 (0.50) |
|
LVEF (%) SD |
51.48 (8.54) |
33.62* (13.17) |
37.19* (10.59) |
53.69 (6.76) |
39.54* (6.49) |
29.21*# (6.50) |
|
LVFAC (%) SD |
43.9 (5.9) |
28.02* (7.11) |
32.04* (9.17) |
47.91 (6.72) |
28.11* (6.27) |
25.26* (3.27) |
|
LVSD (mm) SD |
5.64 (0.76) |
7.12* (0.60) |
6.06# (1.21) |
6.30 (0.80) |
7.54* (0.64) |
8.18* (0.57) |
|
LVDD (mm) SD |
7.77 (0.61) |
8.53* (0.92) |
7.44# (1.02) |
8.76 (0.38) |
9.70* (0.63) |
9.44* (0.26) |
|
LV M (mg) SD |
460.2 (59.52) |
522.7 (123.1) |
447.6 (142.7) |
591.2 (112.7) |
550.1 (82.42) |
531.9 (68.57) |
|
IVRT (ms) SD |
18.25 (3.11) |
17.59 (3.71) |
20.53 (4.06) |
17.24 (3.95) |
19.58 (2.12) |
24.62* (6.64) |
|
E/A Ratio SD |
1.42 (0.12) |
2.12* (0.62) |
1.47# (0.18) |
1.91 (0.67) |
2.15 (0.76) |
1.72 (0.30) |
|
E’/’A Ratio SD |
0.69 (0.16) |
0.98 (0.37) |
1.28* (0.53) |
1.04 (0.39) |
0.87 (0.26) |
1.05 (0.28) |
|
E/E’ Ratio SD |
24.89 (5.84) |
30.09 (9.59) |
18.15# (3.44) |
23.87 (8.72) |
31.84 (8.69) |
31.86 (8.19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





