Introduction
Acute pancreatitis (AP) is an inflammatory disease, frequently caused by gallstones or excess alcohol consumption [
1]. Its course is highly variable and ranges from mild to severe. According to the revised Atlanta Classification (rAC) of 2012 [
2], there are three degrees of severity. Mild (MAP), which is self-limiting, is characterized by absence of organ failure and local complications and carries a low mortality and usually resolves spontaneously. Moderately severe acute pancreatitis (MSAP) is characterized by transient organ failure (less than 48 hours duration) and/or local or systemic complications. In about 20% of cases, patients suffer from severe acute pancreatitis (SAP), characterized by persistent organ failure (lasting more than 48 hours) often requiring intensive care and with an associated mortality of up to 30% if infected necrosis occurs [
3]. Multiorgan failure (MOF) and necrosis are the main causes of morbidity and mortality in AP [
4].
A variety of scores have been developed in the past to predict or assess the severity of AP, based on the shift in laboratory markers over 48 hours (e.g. Ranson score) [
5], on CT-images (e.g. Balthazar score) [
6], or on clinical and laboratory findings such as the acute physiology and chronic health evaluation score (APACHE II) [
7]. The current predictive models lack specificity and reliability, especially in the early course of disease [
8]. In contrast, the revised Atlanta classification (rAC) places more emphasis on organ dysfunction (as defined by the Modified Marshall score [
2]). Patients with SAP should be recognized early for monitoring and supportive treatment for example fluid resuscitation [
9]. These patients should also be considered for treatment in an intensive care unit [
10]. A large number of laboratory parameters have been examined to predict the severity of AP. C-reactive protein (CRP) remains the most widely used marker with a cut-off of 150mg/l at 48 hours [
11]. Amongst other parameters, our group was previously able to demonstrate that cortisol [
12] is a valuable predictor of development of organ failure and death [
13]. An ideal specific biomarker to reliably assess and predict the severity of AP has not yet been identified. In this study, we focus on the role of trypsinogen, trypsin and TAP as predictors of severity in AP. These markers have been studied previously, with conflicting results [
14,
15,
16,
17].
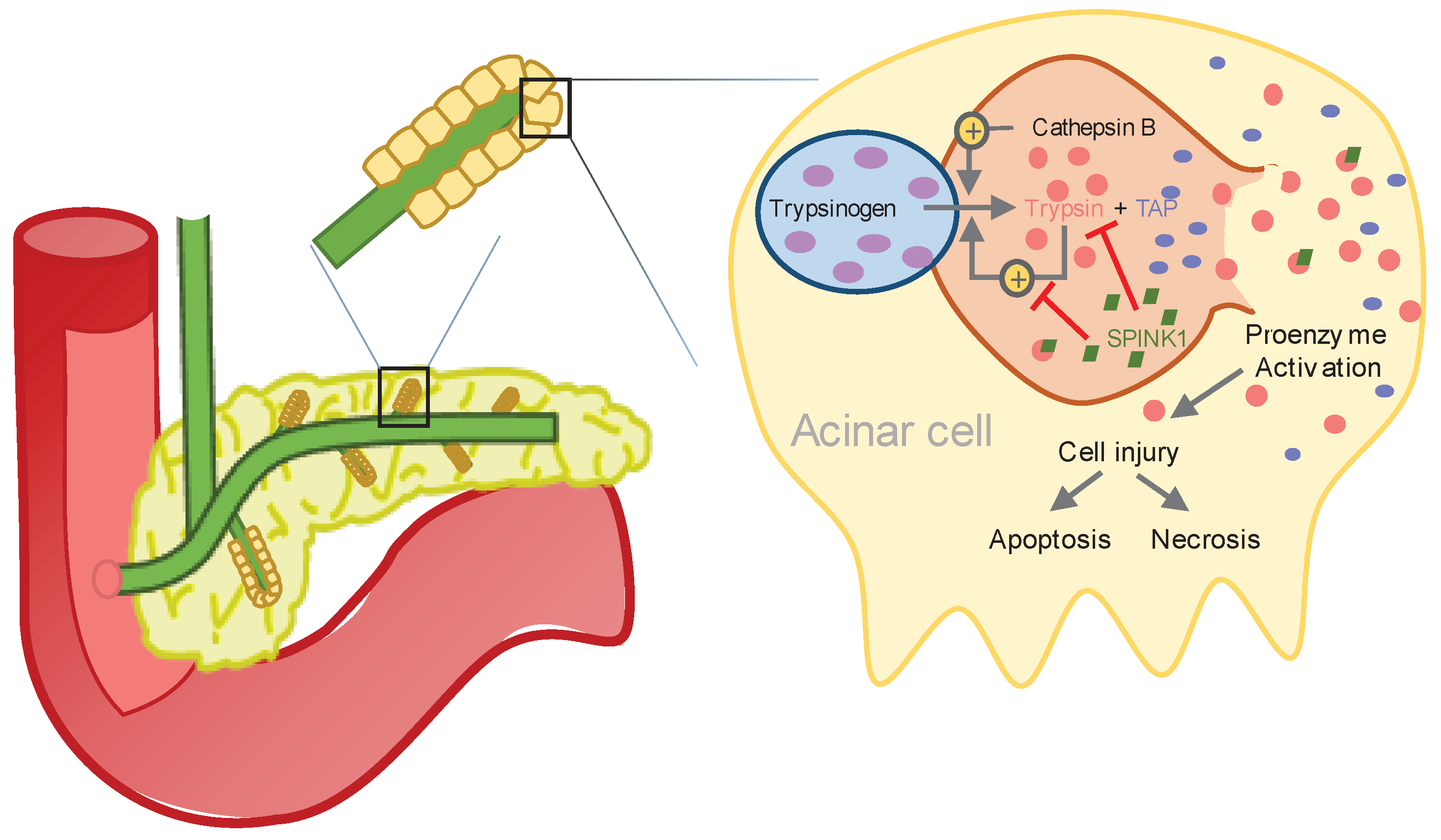
The initial phase of AP occurs within the acinar cells of the pancreas through activation of pancreatic proenzymes. The activated pancreatic enzymes leak into pancreatic tissue, which in turn leads to autodigestion, causing edema and apoptosis, hemorrhage and cellular lysis [
4], resulting in a complex sequence of inflammatory reactions, which determine the further course of the disease [
18].
Under physiological conditions, the proenzyme trypsinogen is activated by cleavage of trypsinogen activation peptide (TAP) through the duodenal brush-border enzyme enterokinase resulting in trypsin and TAP (see
Figure 1) [
19]. In AP, intra-acinar activation of trypsinogen leads to acinar cell injury. In addition, activated trypsin is able to cleave TAP from trypsinogen, leading to further autoactivation of trypsin [
18,
20].
There are two major human isoenzymes of trypsinogen - trypsinogen-1 (cationic trypsinogen) and trypsinogen-2 (anionic trypsinogen). In the bloodstream, trypsin is rapidly inactivated by α
2-macroglobulin and α
1-antitrypsin (AAT) [
21]. In humans, cleavage of trypsinogen-1 or trypsinogen-2 results in the same activation peptide (TAP), in equimolar quantity as trypsin [
19]. Trypsin is a member of the serine peptidase family, that cleaves peptide bonds [
22].
Part of early pancreatic damage during AP is due to excess trypsin activation and therefore serum trypsin levels, as well as serum TAP levels, may correlate earlier in the course of the disease with its severity than non-pancreatic specific markers. There are various publications examining the correlation between serum trypsinogen-2 and the severity of AP. Some showed promising results [
14,
23,
24], whereas others concluded no discriminatory value of trypsinogen-2 between mild and severe AP [
25]. The aim of this study is to present a systematic review of literature regarding the correlation of serum trypsinogen, trypsin and TAP with severity of AP, as well as to assess the utility of trypsin and TAP levels as predictors of severity of disease in serum of patients admitted for AP, by means of a post-hoc analysis of prospectively collected data of a single center cohort.
Methods
Systematic Review
Study Identification, Eligibility and Screening
A systematic review of articles published in English using Medline (PubMed), EMBASE and the Cochrane Central Register was conducted to identify eligible articles. The systematic review was conducted in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [
26]. The terms “acute pancreatitis”, “trypsinogen”, “trypsin”, “TAP”, “serum”, “plasma” and “severity” were used in different combinations for the search. The following search strings were used “acute pancreatitis trypsin serum severity, acute pancreatitis trypsinogen severity, acute pancreatitis TAP severity serum, acute pancreatitis TAP severity plasma, acute pancreatitis trypsin prospective study, acute pancreatitis trypsinogen serum, acute pancreatitis trypsinogen plasma severity”. Eligibility criteria consisted of (1) clinical studies published in peer-reviewed English-language journals with no restriction as to year of publication, (2) either serum levels of trypsinogen-1, trypinogen-2, TAP or trypsin were assessed in patients with AP, (3) the assessed serum levels of the aforementioned markers were related to the severity of AP. Studies using assays that were unable to differentiate between trypsinogen-2 and trypsin in complex with α
1-protease-inhibitor (immunoreactive trypsinogen-2) were excluded. Two searches were performed by two invesitgators (AA, SMS). Once the database search had been conducted and eligible publications had been identified, the reference lists of the selected publications were hand searched in addition.
Data Extraction and Quality Assessment
Regarding the data extraction, we placed emphasis on the area under the curve (AUC) values measured by the receiver-operating characteristics (ROC) curve analysis for the prognosis of AP (severe vs mild), ideally at admission or at within 72 hours of symptom onset. As AUC values were not available for every study, we also listed the absolute median concentrations of the respective markers.
The systematic review provides a qualitative analysis of whether serum levels of trypsin, trypsinogen-1, trypsinogen-2 and TAP on admission are useful in clinical practice as prognostic markers of the severity of AP. The eligible studies included consecutive patients presenting with AP in a defined time period. Demographic and etiologic information are presented. Studies focusing on post-ERCP induced AP only were excluded. Patients with abdominal pain of extra-pancreatic origin and no history of AP, drawn from the same patient population usually served as controls. Patients were classified prospectively according to the AC or rAC. In one publication, patients were classified according to a grading system of AP that was not further specified [
27].
Single Center Observational Study
Study Design and Population
This study was designed as a single-center, observational cohort study at the University Hospital of Basel, Switzerland. The cohort had been studied previously by our group [
12]. The study protocol was approved by the local ethics committee (Ethikkommission beider Basel, EKBB 281/10) and registered on ClinicalTrials.gov (NCT01293318). The methodology was performed according to all relevant guidelines and regulations. All information about the patient’s identity and clinical data remained on the hospital’s password-protected server. All patient data were anonymized. From April 2011 to January 2015, all patients diagnosed with AP admitted to the University Hospital Basel, Switzerland were eligible for the observational study, following diagnosis of AP either in the emergency department or on the ward.
The inclusion criteria were informed written consent, age older than 18 years, and less than 96 hours between the onset of abdominal pain and study inclusion. Pregnancy was not an exclusion criterion. Initial treatment followed local emergency standards of care (
www.emergencystandards.com) [
12].
Clinical Assessment
On study inclusion, medical personnel recorded the vital parameters and filled a short questionnaire assessing baseline characteristics. Routine blood samples, as well as 2 additional tubes (1x serum and 1x EDTA) of 7.5mL each, intended for this study, were drawn at admission and at day 2. The required parameters of the rAC of 2012, as well as the Ranson, APACHE II, and the modified Marshall scores were collected from electronic health records and noted on case report forms. Patients were observed during the first 4 days after the diagnosis of AP. Mortality and computed tomography scans were evaluated in patients throughout the duration of hospitalization. Patients discharged within 4 days of admission remained in the study irrespective of incomplete follow-up data. If blood oxygen saturation was higher than 90%, arterial blood gas analysis was omitted and a partial pressure for oxygen of 90mmHg was assumed and used for further calculation of scores. The severity of pancreatitis was classified according to the rAC, over the 4-day observation period. Local complications were defined as peripancreatic fluid collection, pancreatic and peripancreatic necrosis, pseudocyst and walled-off necrosis. Organ failure was defined according to the modified Marshall score and the rAC criteria [
12].
Assay
The blood samples drawn for the study were centrifuged and immediately frozen at -80°C. EDTA plasma levels of trypsin were determined using the commercially available human Trypsin ELISA kit (SEA230Hu, Cloud-Clone Corp, TX, USA). Plasma levels of TAP were determined using the commercially available human TAP ELISA kit (CEA634Hu, Cloud-Clone Corp, TX, USA) assay. These kits fulfilled the required coefficients of variation. The limits of detection of the mentioned assays are 15.6-1,000pg/mL and 123.5-10000pg/mL, respectively [
12].
Sample Size
A formal sample size calculation was performed on the basis of the primary research question of our previous study [
12]. The present study is exploratory in nature and is based on the available data for the cohort of patients with AP.
Statistical Analysis
We used linear regression models to assess the associations of trypsin and TAP with disease severity according to the rAC criteria. Biomarker levels were analyzed by logarithmic transformation. The results are presented as estimated ratios of geometric means (with their 95% confidence intervals).
Development and Assessment of Prognostic Models
To assess the prognostic accuracy of trypsin, TAP and the APACHE II score as measured on admission (day 0) in predicting the combined endpoint of organ failure or death within the first four days after study inclusion, we used logistic regression models and calculated the area under the receiver operating curve (AUC) separately for each prognostic variable. In addition, the results are presented as estimated odds ratios (with their 95% confidence intervals) per standard deviation increase in the log-transformed prognostic variables [
12].
Statistical Software
For the analyses and graphics we used R 3.2.1 (R Foundation for Statistical Computing Vienna, Austria) and for the ROC analysis and reclassification methods we used the ROCR and PredictABEL add-on packages [
28].
Results
Systematic Review
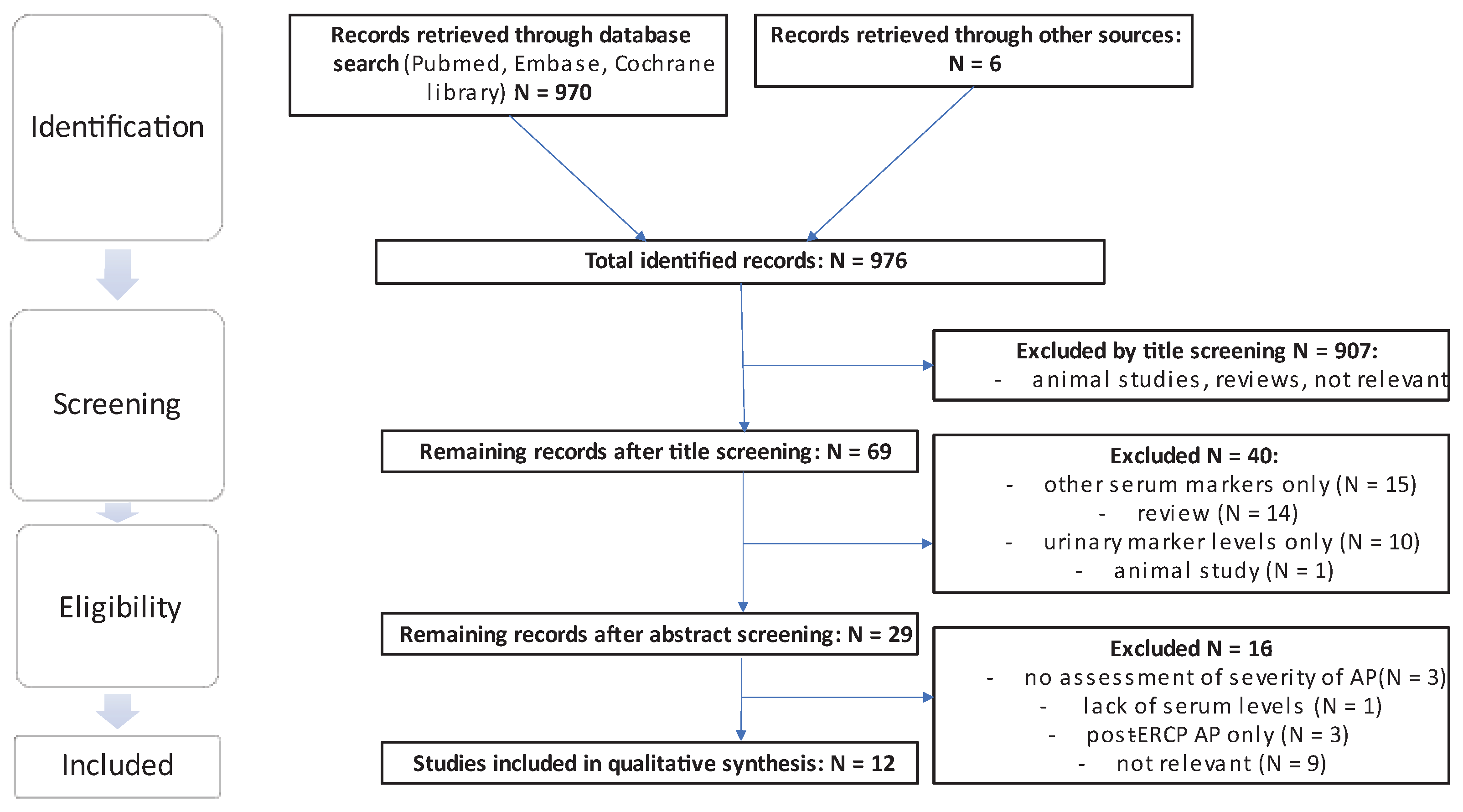
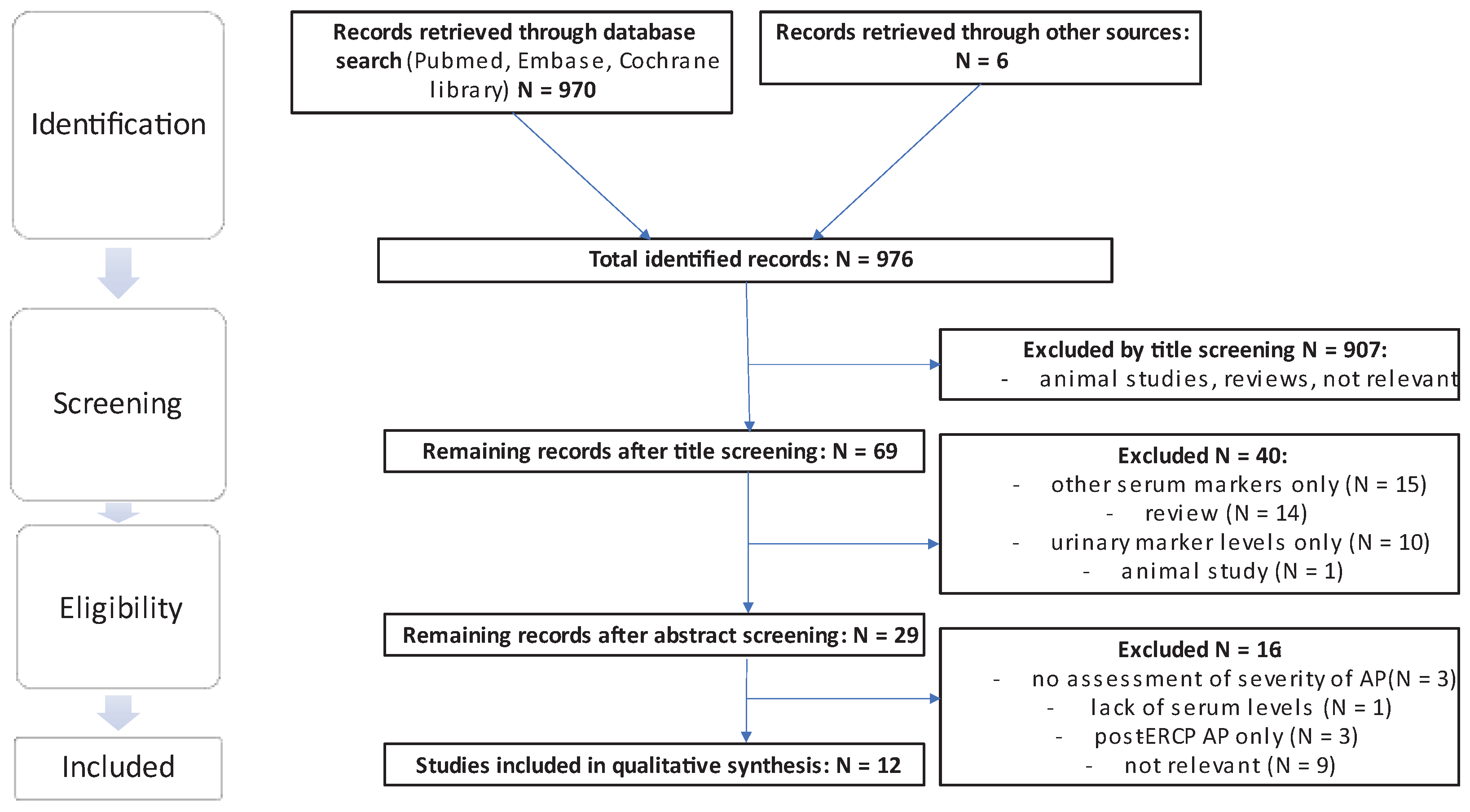
The literature search yielded a total of 970 results, excluding duplicates in different search strings, and an additional search of the reference lists yielded a further 6 eligible publications. From the total sum of publications, 907 were excluded by title screening using the previously mentioned criteria. Of the remaining 69 publications, 40 were excluded by abstract screening. Of the remaining 29 publications 17 were excluded following assessment of the full text and thus 12 studies were included in the final analysis. Of the 6 publications extracted from the references, 1 relevant publication was included [
17] (see
Figure 2).
Trypsinogen
7 publications examining the correlation between serum levels of trypsinogen with severity of AP, with a total of 828 patients, were included (
Table 1).
The most recent publication, also the one with most AP patients included (N = 239), showed that serum levels of trypsinogen-2 on admission (AUC 0.726) outperformed trypsinogen-1 (AUC 0.656) [
29] in predicting the development of organ dysfunction and a severe course of disease.
Three publications by Hedström
et al. examined serum levels of trypsinogen-2 in connection with the diagnosis and prediction of severity of AP. In these studies, an AUC of 0.744 for trypsinogen-2, for the differentiation between a mild and severe course of disease 24h after admission, was reported [
15]. A publication comparing levels of urinary and serological trypsinogen-2 found an AUC of 0.721 for the differentiation of mild vs severe AP on admission for serum trypsinogen-2, performing similarly to levels of urinary trypsinogen-2 [
30]. Another study by the same group examining levels of trypsinogen-2 in the serum in AP found an AUC of 0.792 for the differentiation of a mild vs severe course of disease, performing significantly better than lipase and CRP levels [
14]. Lempinen
et al. found that an AUC of 0.745 at 24h after admission for serum levels of trypsinogen-2 could differentiate between a mild and severe course of AP, performing similarly to trypsinogen-1 (AUC of 0.768), but significantly poorer than urinary levels of trypsinogen-2 with an AUC of 0.925 [
24]. A study by Regnér
et al. showed significant discriminatory ability between mild and severe disease by determination of serum trypsinogen-2 levels, and the time course analysis of trypsinogen-2 levels showed significantly higher levels on the first 3 days of hospitalization in severe vs mild disease [
31]. Another time course analysis of serum levels of trypsinogen-2 by Kemppainen
et al showed a significant discrimination between mild and severe disease, most pronounced on admission and gradually declining thereafter.
TAP
We found four publications examining the correlation of serum levels of TAP with severity of AP, including a total of 295 patients (
Table 2). A study by Mayer
et al. with 25 patients, 16 of whom presented with SAP according to rAC, found serum levels of TAP at admission could significantly differentiate between a mild and a severe course of disease [
32]. Similarly, Kemppainen
et al showed a statistically significant difference in serum levels of TAP at admission between patients with a mild vs a severe course of disease. Thereafter serum TAP levels declined rapidly to below the limit of detection [
17]. Lempinen
et al only found elevated serum TAP levels in severe AP on admission followed by a rapid decrease to undetectable levels, whereas in mild AP the concentrations remained mostly under the detection level of the assay. On admission, the AUC for differentiating between a mild and a severe course of disease was high at 0.823 [
24]. Pezzilli
et al. found no significant predictive value of serum TAP levels, which were under the limit of detection in 70.6% of patients with AP. In a time-course analysis the serum concentrations of TAP were slightly higher in patients with MAP than SAP, although this was not statistically significant. No significant difference in serum TAP levels was found in patients with AP compared to patients with acute abdominal pain of extra pancreatic origin [
16].
Trypsin
We found one publication examining the correlation between serum levels of trypsin with severity of AP, with a total of 140 patients (
Table 3). Hu
et al. studied serum trypsin levels in acute pancreatitis, showing increased levels in patients with SAP (grade III-IV) vs MAP (grade I-II) according to the Determinant Based Classification of AP [
27].
Single Center Observational Study
Patients
This post-hoc analysis of a prospective, single center, observational cohort-study included 142 patients. 7 patients had two episodes of AP during the study period and in these cases, only the first episode was considered. The clinical and demographic characteristics are shown in
Table 4. The median age was 57 years, 43% were female, 61% presented with a biliary etiology of AP, and 18% had a history of AP without criteria for chronic pancreatitis. 40% of patients underwent a CT scan.
Severity of AP and Biomarker Serum Levels
According to the rAC, 9 patients were classified as having severe AP, 35 as having moderate AP, and 81 patients as having mild AP. In 17 patients, missing covariate information precluded the classification of disease severity. Baseline characteristics and biomarker levels are shown in
Table 4. Overall, patients with severe disease had lower serum TAP levels at study inclusion than patients with mild disease. The same tendency was observed with serum trypsin levels in patients with severe vs mild disease (see
Figure 3).
In a sensitivity analysis, we excluded a few outliers with unusual and potentially confounding values, but generally, the results were compatible with our main analysis as shown on
Table 5.
Prediction of Organ Failure and Death
Twenty percent of all patients had organ failure according to the modified Marshall score upon study inclusion or within four days after admission. Five patients died during hospitalization. Assuming the ten patients discharged before the end of the four-day observation period neither died nor developed organ failure within four days following study inclusion, a total of 30 patients died or developed organ failure within four days of admission.
Trypsin, as well as TAP serum levels, showed an inverse correlation with the APACHE II score and presence of organ failure or death: In SAP, trypsin and TAP levels tend to be lower than in patients with mild AP. The predictive ability of the biomarkers was weak and inferior to the APACHE II score (odds ratio (OR) of 2.76 and AUC of 0.73 (p < 0.001)). The OR for trypsin is 0.64 with an AUC of 0.66 (p < 0.036). The OR for TAP is 0.88 with an AUC of 0.55 (p < 0.575) (
Table 6).
Discussion
Our study suggests that trypsin and TAP are inferior to the APACHE II score in predicting the severity of AP. Our literature review showed promising results for predicting the severity of AP by assessing serum levels of trypsinogen-2 on admission. Studies investigating the predictive value of trypsinogen-2 for the assessment of severity of AP often showed a two-fold increase in median serum concentrations exceeding in SAP vs MAP - a finding that translates into promising AUC values in most of the publications. In our own cohort however, we could not confirm these results. Both serum parameters, trypsin and TAP were outperformed by the APACHE II score as measured by AUC (0.66, 0.55 and 0.73 respectively).
The strength of the study lies in the high number of enrolled patients, in the use of the latest classification system (rAC), and in the prospectively collected mostly complete outcome data regarding a population of AP patients with all etiologies. One possible weakness of our clinical study is the low ratio of cases with SAP vs MAP, compared to other publications. One possible explanation might be the rather short observation period of 4 days, as sometimes SAP occurs later in the course of disease. This is not a prospective clinical trial, but a post-hoc analysis of prospectively collected data. Patients excluded were not included in a separate intention to diagnose analysis. As patients with an onset of symptoms of up to 96 hours before admission was included and peak levels of markers that decrease over time, e.g. TAP, might have been missed or underestimated. Ten patients were discharged due to an uneventful recovery before completion of the 4day observation period. Trypsinogen-2 is the most promising marker according to our systematic review, as well as the one with the most clinical data available. In our clinical study, we did not assess the predictive value of trypsinogen-2 for the severity of AP. Clinical studies on serum TAP and trypsin for the prediction of AP are sparse, and the number of patients enrolled are often low and present conflicting results. Another factor is the consideration of the references from the selected publications literature and subsequent review and inclusion of one publication not detected through our database searches.
Our clinical study shows an inverse correlation between serum levels of TAP and trypsin and the severity of AP. Patients with a severe course of AP show lower levels of trypsin and TAP at study inclusion than patients with a mild course of disease. Other publications show higher serum levels of TAP and trypsin in patients with SAP than MAP. The studies examining serum TAP levels in AP describe low serum levels of TAP at admission, sometimes even below the limit of detection limit – and, if detectable, rapidly declining thereafter [
16,
17,
24] limiting its usefulness in clinical practice. As TAP is a relatively small molecule of 7-10 amino acids, it is rapidly eliminated from the circulation by renal excretion, with a half-life of about 8 minutes [
33]. Therefore the publications by Tenner
et al. [
34] and Neoptolemos
et al. [
35] focus on urinary TAP concentrations.
Results pertaining to the serum levels of trypsin are sparse, but Hu
et al. found a correlation between serum trypsin levels and the severity of AP. Patients with a more severe course of disease show higher levels of serum trypsin [
27]. In contrast, our clinical trial showed an inverse correlation of serum trypsin concentration with severity of AP. One possible explanation might be that blood samples were collected on admission, whereas Hu
et al. collected blood samples at different, undisclosed timepoints. Another potential cause for the difference in trypsin levels might be the difference in etiology of AP between the two studies; 33% of the patients with AP included in our publication, but none in the publication by Hu
et al. presented with AP due to alcohol consumption.
Our systematic review as well as our clinical trial show the clinical APACHE II score to be superior to the serum markers assessed. The routine use of trypsinogen-2, trypsinogen-1, TAP or trypsin for predicting the severity of AP cannot be recommended. As some studies showed higher concentrations of TAP and trypsin in patients with MAP, and other in patients with SAP, further research in the form of a large controlled randomized prospective trial is needed.
Author Contributions
CN and SS conceptualized and designed the study. AA and SS carried out data collection, analysis and interpretation, as well as drafting the article. CN provided critical revision and final approval of the article to be published.
Funding
This study was financially supported by the Gottfried and Julia Bangerter-Rhyner Foundation.
Data Availability Statement
The data of the single center observational study as well as the literature review will be made available upon reasonable request.
Acknowledgments
We thank Susanne Rogers for English editing.
Conflicts of Interest
All authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Approved by the Ethikkommission beider Basel, EKBB 281/10.
References
- Forsmark, C.E.; Vege, S.S.; Wilcox, C.M. Acute pancreatitis. N. Engl. J. Med. 2016, 375, 1972–1981. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Windsor, J.A.; Horvath, K.D.; et al. Classification of acute pancreatitis - 2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Yadav, D.; Lowenfels, A.B. Trends in the Epidemiology of the First Attack of Acute Pancreatitis. Pancreas 2006, 33, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Fei, L.W.; Cao, Y.; Hon, Y.L.; Huang, J.; Puneet, P.; Chevali, L. Pathophysiology of acute pancreatitis. Pancreatology 2005, 5, 132–144. [Google Scholar] [CrossRef]
- Ranson, J.; Rifkind, K.; Roses, D.; Fink, S.; Eng, K.; Spencer, F. Prognostic signs and the role of operative management in acute pancreatitis. Surg. Gynecol. Obstet. 1974, 139, 69–81. [Google Scholar]
- Balthazar, E.; Robinson, D.; Megibow, A.; Ranson, J. Acute Pancreatitis : Value of CT in Establishing Prognosis. Radiology 1990, 174, 331–336. [Google Scholar] [CrossRef]
- Thandassery, R.B.; Yadav, T.D.; Dutta, U.; Appasani, S.; Singh, K.; Kochhar, R. Hypotension in the First Week of Acute Pancreatitis and APACHE II Score Predict Development of Infected Pancreatic Necrosis. Dig. Dis. Sci. 2015, 60, 537–542. [Google Scholar] [CrossRef]
- Schütte, K.; Malfertheiner, P. Markers for predicting severity and progression of acute pancreatitis. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 75–90. [Google Scholar] [CrossRef]
- Fisher, J.M.; Gardner, T.B. The golden hours of management in acute pancreatitis. Am. J. Gastroenterol. 2012, 107, 1146–1150. [Google Scholar] [CrossRef]
- Tenner, S.; Baillie, J.; Dewitt, J.; Vege, S.S. American college of gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415. [Google Scholar] [CrossRef]
- Baillie, J. AGA Institute Medical Position Statement on Acute Pancreatitis. Gastroenterology 2007, 132, 2019–2021. [Google Scholar] [CrossRef]
- Nebiker, C.A.; Staubli, S.; Schäfer, J.; Bingisser, R.; Christ-Crain, M.; Dell-Kuster, S.; Mueller, C.; Scamardi, K.; Viehl, C.T.; Kolleth, D.; et al. Cortisol Outperforms Novel Cardiovascular, Inflammatory, and Neurohumoral Biomarkers in the Prediction of Outcome in Acute Pancreatitis. Pancreas 2018, 47, 55–64. [Google Scholar] [CrossRef]
- Staubli, S.M.; Oertli, D.; Nebiker, C.A. Laboratory markers predicting severity of acute pancreatitis. Crit. Rev. Clin. Lab. Sci. 2015, 52, 273–283. [Google Scholar] [CrossRef]
- Hedstrom, J.; Kemppainen, E.; Andersen, J.; Jokela, H.; Puolakkainen, P.; Stenman, U.H. A comparison of serum trypsinogen-2 and trypsin-2-alpha1-antitrypsin complex with lipase and amylase in the diagnosis and assessment of severity in the early phase of acute pancreatitis. Am. J. Gastroenterol. 2001, 96, 424–430. [Google Scholar] [CrossRef]
- Hedstrom, J.; Sainio, V.; Kemppainen, E.; Haapiainen, R.; Kivilaakso, E.; Schroder, T.; Leinonen, J.; Stenman, U.H. Serum complex of trypsin 2 and alpha-1-antitrypsin as diagnostic and prognostic marker of acute pancreatitis: Clinical study in consecutive patients. Br. Med. J. 1996, 313, 333–337. [Google Scholar] [CrossRef]
- Pezzilli, R.; Venturi, M.; Morselli-Labate, A.M.; Ceciliato, R.; Lamparelli, M.G.; Rossi, A.; Moneta, D.; Piscitelli, L.; Corinaldesi, R. Serum trypsinogen activation peptide in the assessment of the diagnosis and severity of acute pancreatic damage: A pilot study using a new determination technique. Pancreas 2004, 29, 298–305. [Google Scholar] [CrossRef]
- Kemppainen, E.; Mayer, J.; Puolakkainen, P.; Raraty, M.; Slavin, J.; Neoptolemos, J.P. Plasma trypsinogen activation peptide in patients with acute pancreatitis. Br. J. Surg. 2001, 88, 679–680. [Google Scholar] [CrossRef]
- Steer, M.L. Frank Brooks Memorial Lecture: The early intraacinar cell events which occur during acute pancreatitis. Pancreas 1998, 17, 31–37. [Google Scholar] [CrossRef]
- Kassell, B.; Kay, J. Zymogens of Proteolytic Enzymes. Sci. New Ser. 1973, 180, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Hietaranta, A.J.; Saluja, A.K.; Bhagat, L.; Singh, V.P.; Song, A.M.; Steer, M.L. Relationship between NF-κB and trypsinogen activation in rat pancreas after supramaximal caerulein stimulation. Biochem. Biophys. Res. Commun. 2001, 280, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Hedstrom, J.; Leinonen, J.; Sainio, V. Time-Resolved Immu nofluorometric Assay of Trypsi n-2 Complexed with a1-Antitrypsin in Serum. Clin. Chem. 1994, 40, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Kukor, Z.; Le Maréchal, C.; Tóth, M.; Tsakiris, L.; Raguénès, O.; Férec, C.; Sahin-Tóth, M. Evolution of Trypsinogen Activation Peptides. Mol. Biol. Evol. 2003, 20, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Sainio, V.; Puolakkainen, P.; Kemppainen, E.; Hedstrom, J.; Haapiainen, R.; Kivisaari, L.; Stenman, U.H.; Schroder, T.; Kivilaakso, E. Serum trypsinogen-2 in the prediction of outcome in acute necrotizing pancreatitis. Scand. J. Gastroenterol. 1996, 31, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Lempinen, M.; Stenman, U.H.; Puolakkainen, P.; Hietaranta, A.; Haapiainen, R.; Kemppainen, E. Sequential changes in pancreatic markers in acute pancreatitis. Scand. J. Gastroenterol. 2003, 38, 666–675. [Google Scholar] [CrossRef]
- Appelros, S.; Thim, L.; Borgström, A. Activation peptide of carboxypeptidase B in serum and urine in acute pancreatitis. Gut 1998, 42, 97–102. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions : Explanation and elaboration; 2009; Volume 62, ISBN 2006062298. [Google Scholar]
- Hu, J.; Lin, W.; Zhao, C.; Chen, J.; Lin, W. The Relationship between Trypsin / Calcitonin Gene Related Peptide ( CGRP ) in Serum and Acute Pancreatitis ( AP ). Clin. Lab. 2018, 64, 93–97. [Google Scholar] [CrossRef]
- Pencina, M.J. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Rainio, M.; Lindström, O.; Penttilä, A.; Itkonen, O. Serum Serine Peptidase Inhibitor Kazal-Type 1, Trypsinogens 1 to 3, and Complex of Trypsin 2 and α1-Antitrypsin in the Diagnosis of Severe Acute Pancreatitis. Pancreas 2019, 48, 374–380. [Google Scholar] [CrossRef]
- Hedström, J.; Sainio, V.; Kemppainen, E.; Puolakkainen, P.; Haapiainen, R.; Kivilaakso, E.; Schauman, K.O.; Stenman, U.H. Urine trypsinogen-2 as marker of acute pancreatitis. Clin. Chem. 1996, 42, 685–690. [Google Scholar] [CrossRef]
- Regnér, S.; Manjer, J.; Appelros, S.; Hjalmarsson, C.; Sadic, J.; Borgström, A. Protease activation, pancreatic leakage, and inflammation in acute pancreatitis: Differences between mild and severe cases and changes over the first three days. Pancreatology 2008, 8, 600–607. [Google Scholar] [CrossRef]
- Mayer, J.M.; Rau, B.; Siech, M.; Beger, H.G. Local and systemic zymogen activation in human acute pancreatitis. Digestion 2000, 62, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.W.; Beechey-Newman, N.; Lamb, C.R.; Smyth, J.B.A.; Hughes, G.; Coombe, K.; Sumar, N.; Hermon-Taylor, J. Cholecystokinin-8 induces edematous pancreatitis in dogs associated with short burst of trypsinogen activation. Dig. Dis. Sci. 1995, 40, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
- Tenner, S.; FernandezdelCastillo, C.; Warshaw, A.; Steinberg, W.; HermonTaylor, J.; Valenzuela, J.E.; Hariri, M.; Hughes, M.; Banks, P.A.; Fernandez-del Castillo, C.; et al. Urinary trypsinogen activation peptide (TAP) predicts severity in patients with acute pancreatitis. Int. J. Pancreatol. 1997, 21, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Kemppainen, E.A.; Mayer, J.M.; Fitzpatrick, J.M.; Raraty, M.G.T.; Slavin, J.; Beger, H.G.; Hietaranta, A.J.; Puolakkainen, P.A. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: A multicentre study. Lancet 2000, 355, 1955–1960. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).