Submitted:
12 March 2024
Posted:
12 March 2024
You are already at the latest version
Abstract
Keywords:
1. Background
2. Randomized Clinical Phase III Trials Failed to Demonstrate Positive Effects of Ca4Mn(DPDP)5
3. Promising Clinical Findings with MnDPDP
4. Confusing Pharmacodynamic Difference between Ca4Mn(DPDP)5 and MnDPDP
5. DPDP as a Presumptive Chelation Drug
6. Do DPDP and PLED Fulfil Necessary Properties of a Platinum Chelation Drug?
Author Contributions
Funding
Conflicts of Interest
References
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, Veeder MH, Mailliard JA. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995 Mar 1;122(5):321-6. [CrossRef]
- O'Connell MJ, Laurie JA, Kahn M, Fitzgibbons RJ Jr, Erlichman C, Shepherd L, Moertel CG, Kocha WI, Pazdur R, Wieand HS, Rubin J, Vukov AM, Donohue JH, Krook JE, Figueredo A. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998 Jan;16(1):295-300. [CrossRef]
- de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000 Aug;18(16):2938-47. Corrected and republished in: J Clin Oncol. 2023 Nov 20;41(33):5080-5089. [CrossRef]
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004 Jun 3;350(23):2343-51. [CrossRef]
- O'Neil BH, Goldberg RM. Innovations in chemotherapy for metastatic colorectal cancer: an update of recent clinical trials. Oncologist. 2008 Oct;13(10):1074-83. Epub 2008 Oct 15. [CrossRef]
- Sørbye H, Glimelius B, Berglund A, Fokstuen T, Tveit KM, Braendengen M, Øgreid D, and Dahl O (2004). Multicenter phase II study of Nordic fluorouracil and folinic acid bolus schedule combined with oxaliplatin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 22, 31–38.
- Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004 Jan 1;22(1):23-30. Epub 2003 Dec 9. Corrected and republished in: J Clin Oncol. 2023 Jul 1;41(19):3461-3468. [CrossRef]
- Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, Adenis A, Faroux R, Rebischung C, Bergougnoux L, Kockler L, Douillard JY; GI Group of the French Anti-Cancer Centers. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer. 2011 Feb 1;128(3):682-90. [CrossRef]
- André T, Iveson T, Labianca R, Meyerhardt JA, Souglakos I, Yoshino T, Paul J, Sobrero A, Taieb J, Shields AF, Ohtsu A, Grothey A, Sargent DJ; for the IDEA Steering Committee. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) Collaboration: Prospective Combined Analysis of Phase III Trials Investigating Duration of Adjuvant Therapy with the FOLFOX (FOLFOX4 or Modified FOLFOX6) or XELOX (3 versus 6 months) Regimen for Patients with Stage III Colon Cancer: Trial Design and Current Status. Curr Colorectal Cancer Rep. 2013;9(3):261-269. [CrossRef]
- Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No.: CD005228. [CrossRef]
- Areti A, Yerra VG, Naidu V, Kumar A. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014 Jan 18;2:289-95. [CrossRef]
- Brouwers EE, Huitema AD, Beijnen JH, Schellens JH. Long-term platinum retention after treatment with cisplatin and oxaliplatin. BMC Clin Pharmacol. 2008 Sep 17;8:7. [CrossRef]
- Stehr JE, Lundström I, Karlsson JOG. Evidence that fodipir (DPDP) binds neurotoxic Pt2+ with a high affinity: An electron paramagnetic resonance study. Sci Rep. 2019 Nov 1;9(1):15813. [CrossRef]
- Brock PR, Maibach R, Childs M, Rajput K, Roebuck D, Sullivan MJ, Laithier V, Ronghe M, Dall'Igna P, Hiyama E, Brichard B, Skeen J, Mateos ME, Capra M, Rangaswami AA, Ansari M, Rechnitzer C, Veal GJ, Covezzoli A, Brugières L, Perilongo G, Czauderna P, Morland B, Neuwelt EA. Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. N Engl J Med. 2018 Jun 21;378(25):2376-2385. [CrossRef]
- Freyer DR, Chen L, Krailo MD, Knight K, Villaluna D, Bliss B, Pollock BH, Ramdas J, Lange B, Van Hoff D, VanSoelen ML, Wiernikowski J, Neuwelt EA, Sung L. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017 Jan;18(1):63-74. Epub 2016 Dec 1. Erratum in: Lancet Oncol. 2017 Jun;18(6):e301. PMID: 27914822; PMCID: PMC5520988. [CrossRef]
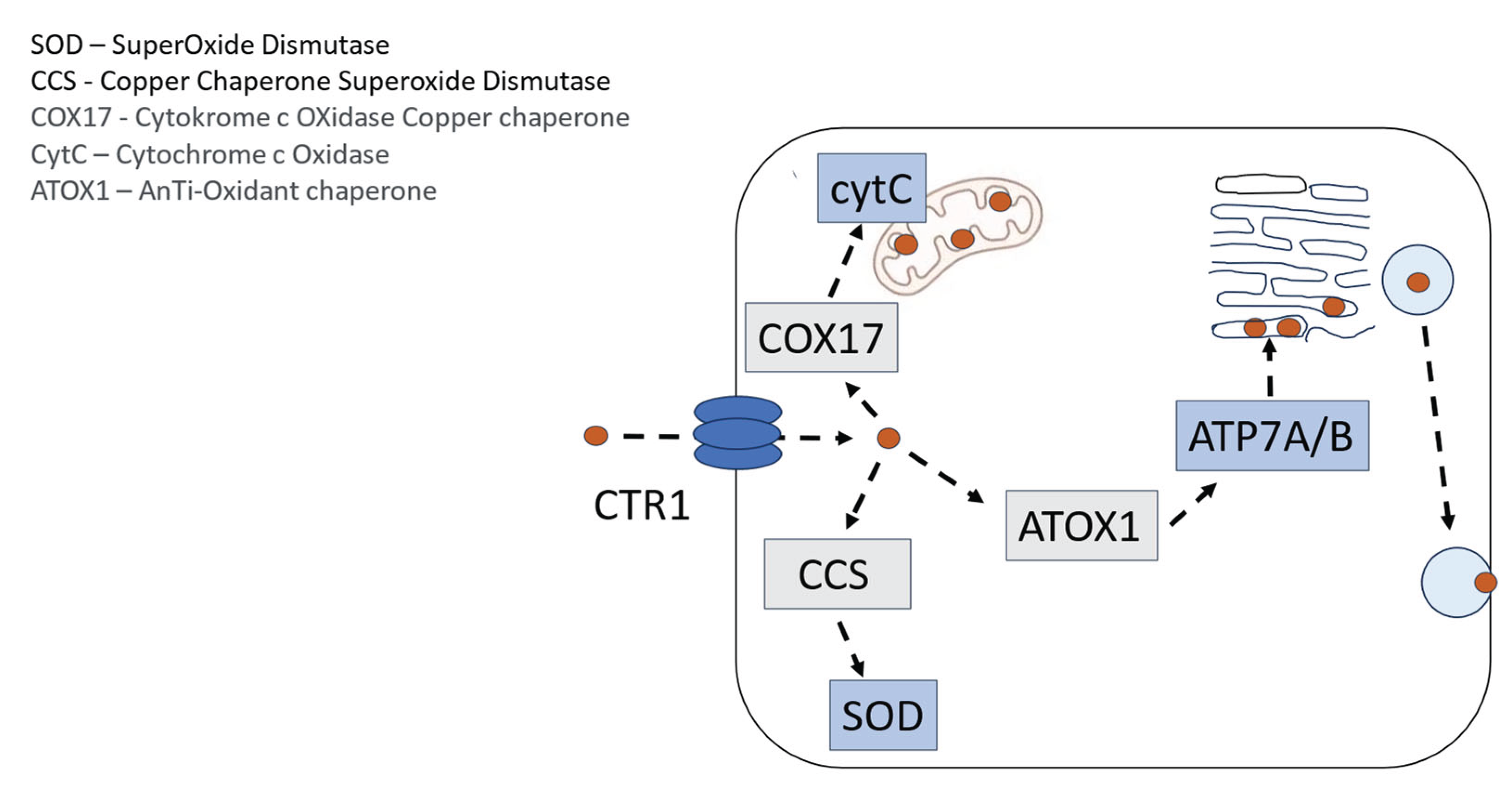
- Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol. 2010 Jun;77(6):887-94. . Epub 2010 Feb 16. [CrossRef]
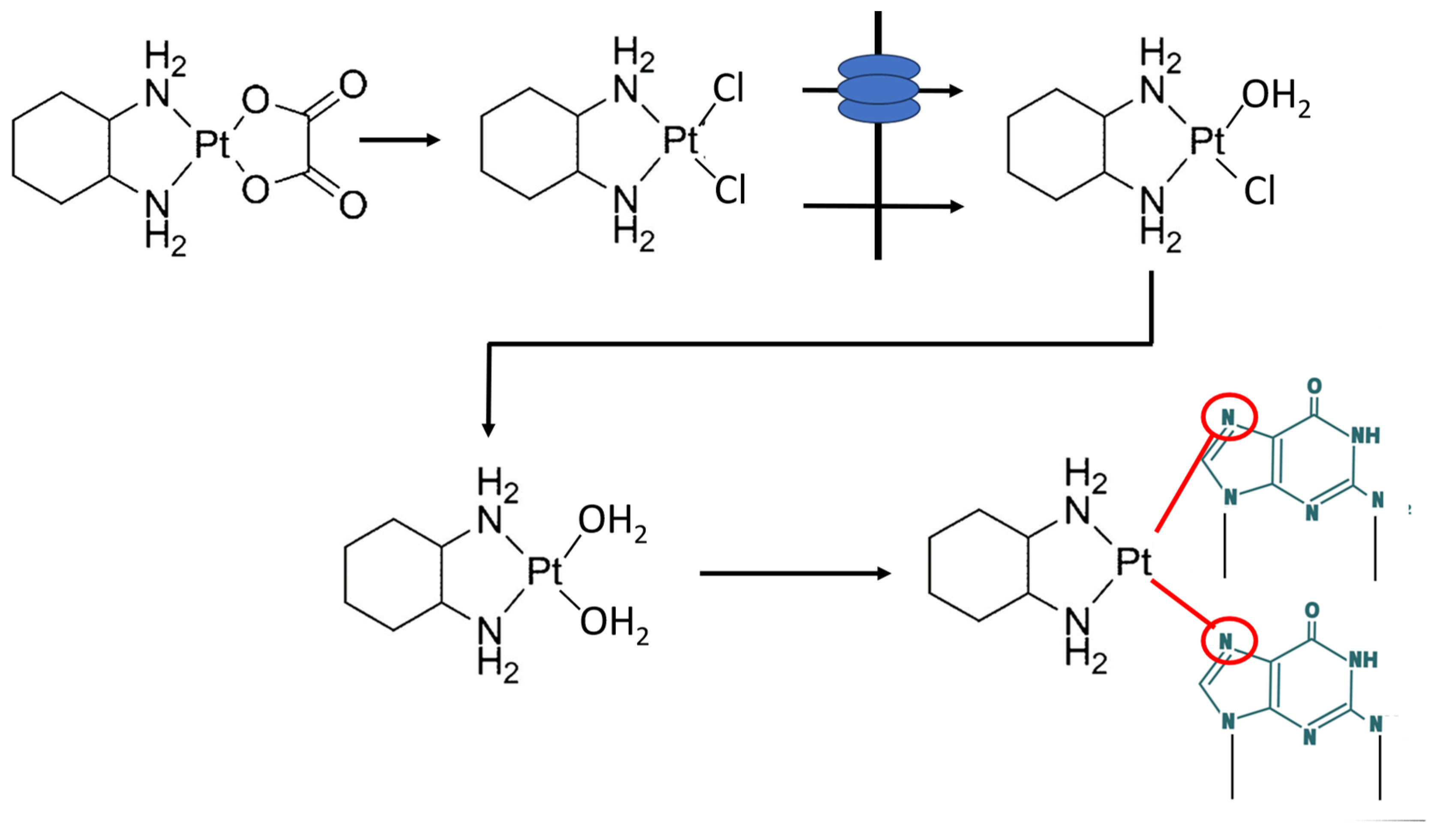
- Reedijk J. New clues for platinum antitumor chemistry: kinetically controlled metal binding to DNA. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3611-6. Epub 2003 Mar 24. [CrossRef]
- Taylor SW, Laughlin RS, Kumar N, Goodman B, Klein CJ, Dyck PJ, Dyck PJB. Clinical, physiological and pathological characterisation of the sensory predominant peripheral neuropathy in copper deficiency. J Neurol Neurosurg Psychiatry. 2017 Oct;88(10):839-845. Epub 2017 Aug 5. [CrossRef]
- Pfeiffer P, Lustberg M, Näsström J, Carlsson S, Persson A, Nagahama F, Cavaletti G, Glimelius B, Muro K. Calmangafodipir for Prevention of Oxaliplatin-Induced Peripheral Neuropathy: Two Placebo-Controlled, Randomized Phase 3 Studies (POLAR-A/POLAR-M). JNCI Cancer Spectr. 2022 Nov 1;6(6):pkac075. [CrossRef]
- Glimelius B, Manojlovic N, Pfeiffer P, Mosidze B, Kurteva G, Karlberg M, Mahalingam D, Buhl Jensen P, Kowalski J, Bengtson M, Nittve M, Näsström J. Persistent prevention of oxaliplatin-induced peripheral neuropathy using calmangafodipir (PledOx®): a placebo-controlled randomised phase II study (PLIANT). Acta Oncol. 2018 Mar;57(3):393-402. Epub 2017 Nov 15. [CrossRef] [PubMed]
- Karlsson JOG, Jynge P. Is it possible to draw firm conclusions from the PLIANT trial? Acta Oncol. 2018 Jun;57(6):862-864. Epub 2017 Dec 15. [CrossRef]
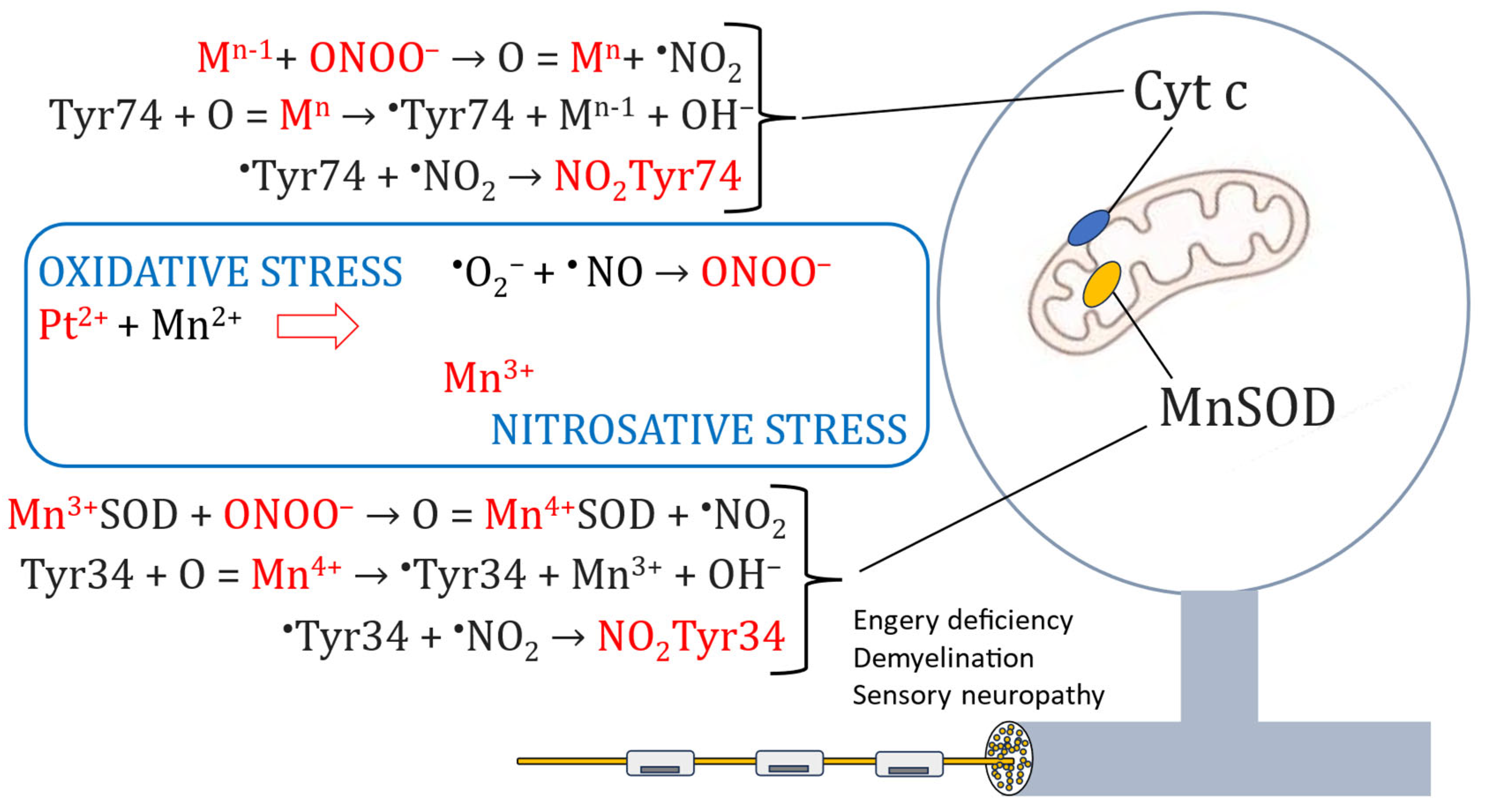
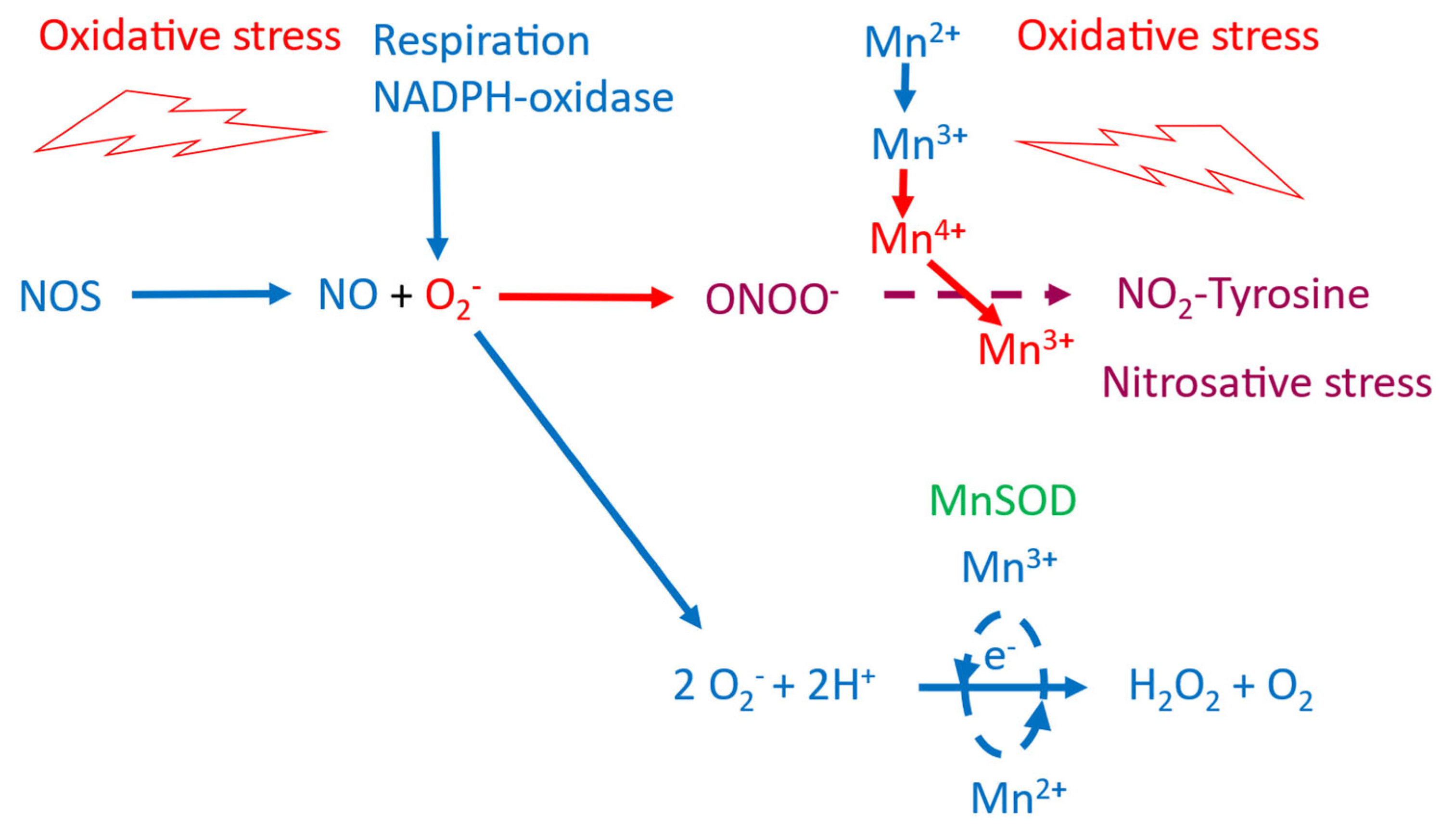
- Karlsson JOG, Jynge P, Ignarro LJ. Exacerbated Neuropathy in POLAR A and M Trials Due to Redox Interaction of PledOx-Associated Mn2+ and Oxaliplatin-Associated Pt2+. Antioxidants (Basel). 2023 Mar 1;12(3):608. [CrossRef]
- Yri OE, Vig J, Hegstad E, Hovde O, Pignon I, Jynge P. Mangafodipir as a cytoprotective adjunct to chemotherapy--a case report. Acta Oncol. 2009;48(4):633-5. [CrossRef]
- Coriat R, Alexandre J, Nicco C, Quinquis L, Benoit E, Chéreau C, Lemaréchal H, Mir O, Borderie D, Tréluyer JM, Weill B, Coste J, Goldwasser F, Batteux F. Treatment of oxaliplatin-induced peripheral neuropathy by intravenous mangafodipir. J Clin Invest. 2014 Jan;124(1):262-72. Epub 2013 Dec 20. [CrossRef]
- Laurent A, Nicco C, Chéreau C, Goulvestre C, Alexandre J, Alves A, Lévy E, Goldwasser F, Panis Y, Soubrane O, Weill B, Batteux F. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005 Feb 1;65(3):948-56.
- Alexandre J, Nicco C, Chéreau C, Laurent A, Weill B, Goldwasser F, Batteux F. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J Natl Cancer Inst. 2006 Feb 15;98(4):236-44. [CrossRef]
- Doroshow, JH. Redox modulation of chemotherapy-induced tumor cell killing and normal tissue toxicity. J Natl Cancer Inst. 2006 Feb 15;98(4):223-5. [CrossRef]
- Karlsson JO, Kurz T, Flechsig S, Näsström J, Andersson RG. Superior therapeutic index of calmangafodipir in comparison to mangafodipir as a chemotherapy adjunct. Transl Oncol. 2012 Dec;5(6):492-502. Epub 2012 Dec 1. [CrossRef]
- Toft KG, Hustvedt SO, Grant D, Martinsen I, Gordon PB, Friisk GA, Korsmo AJ, Skotland T. Metabolism and pharmacokinetics of MnDPDP in man. Acta Radiol. 1997 Jul;38(4 Pt 2):677-89. [CrossRef]
- Doyle T, Chen Z, Muscoli C, Bryant L, Esposito E, Cuzzocrea S, Dagostino C, Ryerse J, Rausaria S, Kamadulski A, Neumann WL, Salvemini D. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J Neurosci. 2012 May 2;32(18):6149-60. [CrossRef]
- Janes K, Doyle T, Bryant L, Esposito E, Cuzzocrea S, Ryerse J, Bennett GJ, Salvemini D. Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain. 2013 Nov;154(11):2432-2440. Epub 2013 Jul 25. [CrossRef]
- Radi, R. Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc Chem Res. 2013 Feb 19;46(2):550-9. Epub 2012 Nov 16. [CrossRef]
- Bartesaghi S, Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018 Apr;14:618-625. Epub 2017 Sep 19. [CrossRef]
- Bratosin D, Estaquier J, Petit F, Arnoult D, Quatannens B, Tissier JP, Slomianny C, Sartiaux C, Alonso C, Huart JJ, Montreuil J, Ameisen JC. Programmed cell death in mature erythrocytes: a model for investigating death effector pathways operating in the absence of mitochondria. Cell Death Differ. 2001 Dec;8(12):1143-56. [CrossRef]
- Schmidt PP, Toft KG, Skotland T, Andersson K. Stability and transmetallation of the magnetic resonance contrast agent MnDPDP measured by EPR. J Biol Inorg Chem. 2002 Mar;7(3):241-8. Epub 2001 Oct 19. [CrossRef]
- Rocklage SM, Cacheris WP, Quay SC, Hahn FE, Raymond KN. Manganese(II) N,N′-dipyridoxylethylenediamine-N,N′diacetate 5, 5-bis(phosphate). Synthesis and characterization of a paramagnetic chelate for magnetic resonance imaging enhancement. Inorg Chem. 1989;28:477–485. [CrossRef]
- Rocklage SM, et al. Structural and Thermodynamic Characterization of Manganese (II) N,N′.
- -Dipyridoxylethylenediamine-N, N′-diacetate. A Novel Manganese (II) Chelate. Inorg. Chem. 1988;27:3530–3534. [CrossRef]
- Shannon, RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976;A32:751–767. [CrossRef]
- Karlsson JO, Ignarro LJ, Lundström I, Jynge P, Almén T. Calmangafodipir [Ca4Mn(DPDP)5], mangafodipir (MnDPDP) and MnPLED with special reference to their SOD mimetic and therapeutic properties. Drug Discov Today. 2015 Apr;20(4):411-21. Epub 2014 Nov 20. [CrossRef]
- Kurz T, Grant D, Andersson RG, Towart R, De Cesare M, Karlsson JO. Effects of MnDPDP and ICRF-187 on Doxorubicin-Induced Cardiotoxicity and Anticancer Activity. Transl Oncol. 2012 Aug;5(4):252-9. Epub 2012 Aug 1. PMID: 22937177; PMCID: PMC3431035. [CrossRef]
- Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM, Dick RD, Brewer GJ, Merajver SD. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002 Sep 1;62(17):4854-9.
- Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, Dou QP, Franz KJ, Gohil VM, Gupta S, Kaler SG, Lutsenko S, Mittal V, Petris MJ, Polishchuk R, Ralle M, Schilsky ML, Tonks NK, Vahdat LT, Van Aelst L, Xi D, Yuan P, Brady DC, Chang CJ. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer. 2022 Feb;22(2):102-113. Epub 2021 Nov 11. [CrossRef]
- Graham MA, Lockwood GF, Greenslade D, Brienza S, Bayssas M, Gamelin E. Clinical pharmacokinetics of oxaliplatin: a critical review. Clin Cancer Res. 2000 Apr;6(4):1205-18.
- McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009 Jan;8(1):10-6. PMID: 19139108; PMCID: PMC2651829. [CrossRef]
- Shord SS, Bernard SA, Lindley C, Blodgett A, Mehta V, Churchel MA, Poole M, Pescatore SL, Luo FR, Chaney SG. Oxaliplatin biotransformation and pharmacokinetics: a pilot study to determine the possible relationship to neurotoxicity. Anticancer Res. 2002 Jul-Aug;22(4):2301-9.
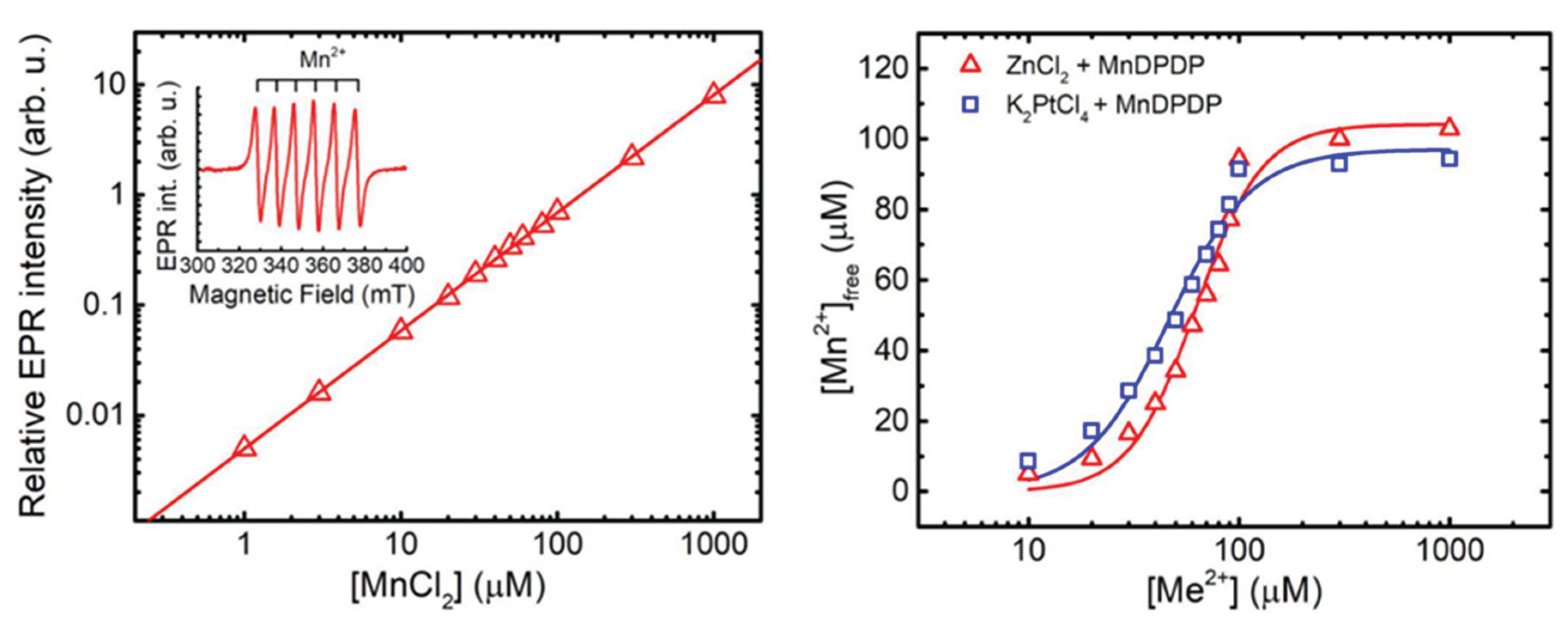
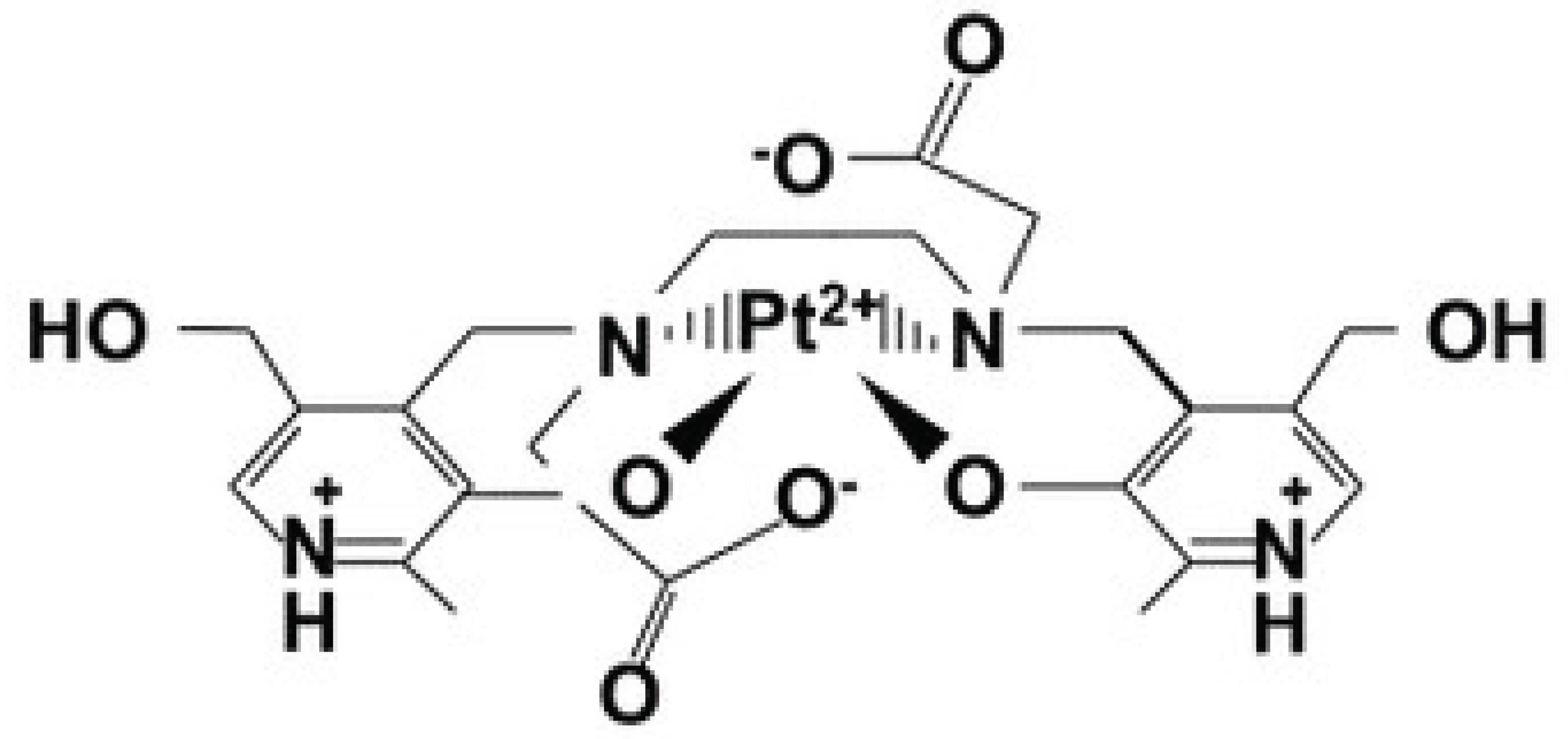
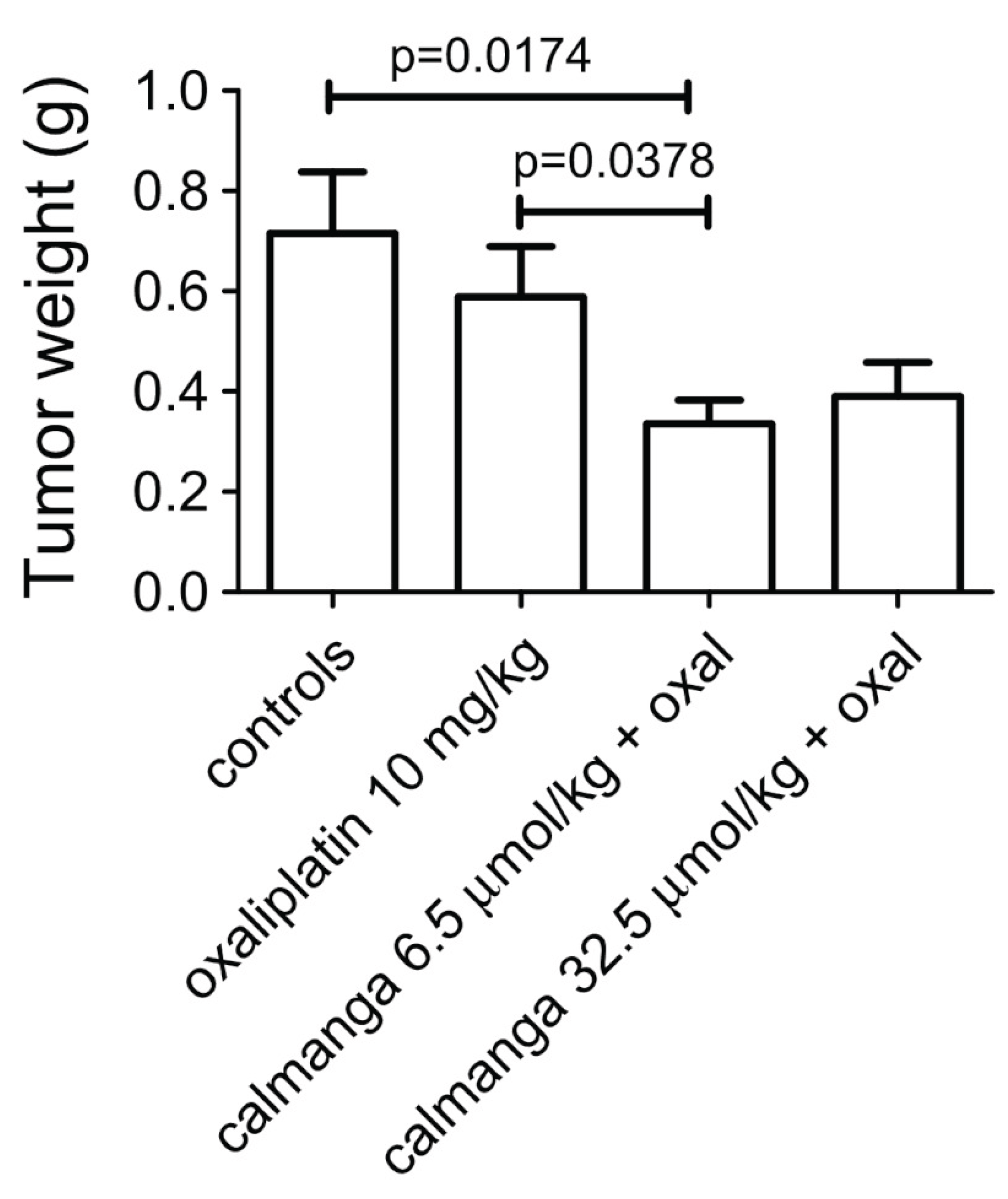
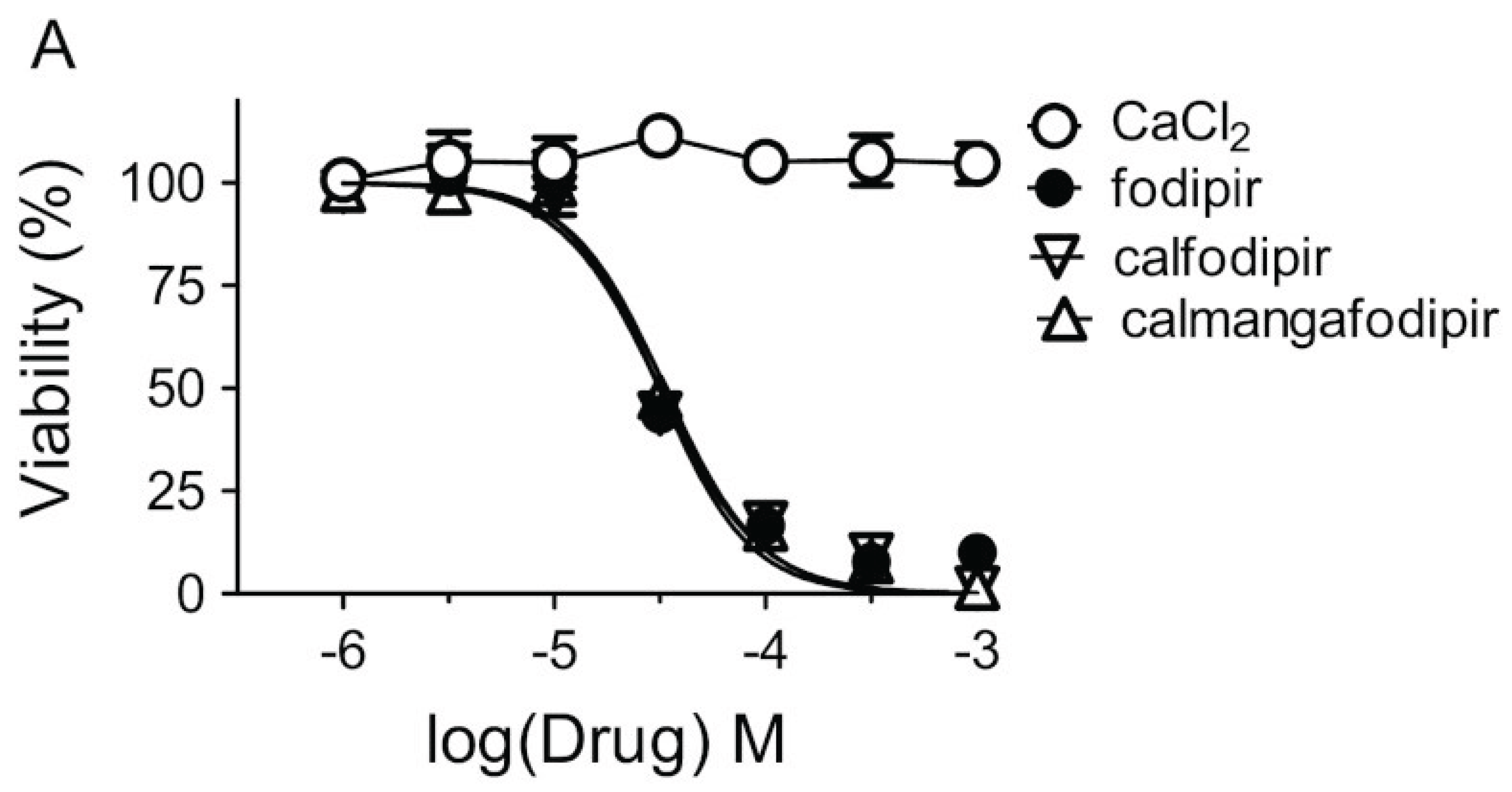
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




