Submitted:
12 March 2024
Posted:
13 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
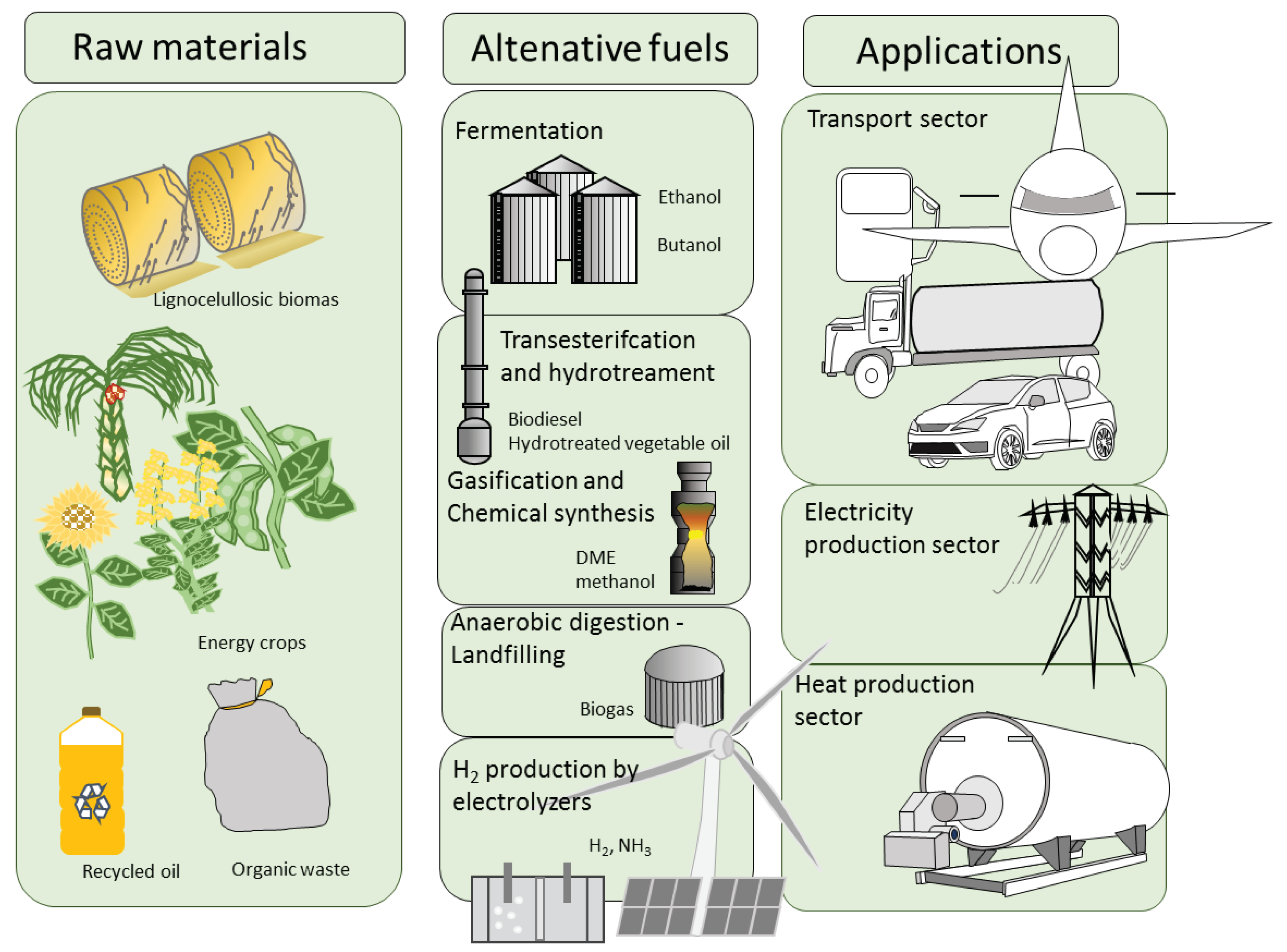
3. Alternative Fuels
4.1. Hydrogen
4.2. Biodiesel and Alcohols
4.3. Electrification of Transport Sector
4.4. Biogas, Syngas and Syngas Derivates
4.5. Return of Ammonia as Alternative Fuel
3. Ammonia Production
4. Use of Ammonia as Alternative Fuel: Ammonia Combustion
4.1. Gas Turbines
4.2. Spark Ignition Engines
4.3. Compression Ignition Engines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
References
- 2050 long-term strategy. Available online: https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2050-long-term-strategy_en (accessed on 12 December 2023).
- Total energy consumption. Available online: https://yearbook.enerdata.net/total-energy/world-consumption-statistics.html (accessed on 12 December 2023).
- Huang, Y.; Kuldasheva, Z.; Bobojanov, S.; Djalilov, B.; Salahodjaev, R.; Abbas, S. Exploring the links between fossil fuel energy consumption, industrial value-added, and carbon emissions in G20 countries. Environ. Sci. Pollut. Res. 2023, 30, 10854–10866. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, H.; Naghizadeh, R.A. Multi-criteria optimal sizing of hybrid renewable energy systems including wind, photovoltaic, battery, and hydrogen storage with ɛ-constraint method. IET Renew. Power Gener. 2018, 12, 883–892. [Google Scholar] [CrossRef]
- González, R.; Cabeza, I.O.; Casallas-Ojeda, M.; Gómez, X. Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy. Environments 2023, 10, 82. [Google Scholar] [CrossRef]
- Touati, F.A.; Al-Hitmi, M.A.; Bouchech, H.J. Study of the effects of dust, relative humidity, and temperature on solar PV performance in Doha: comparison between monocrystalline and amorphous PVS. Int. J. Green Energy 2013, 10, 680–689. [Google Scholar] [CrossRef]
- Salimi, H.; Mirabdolah Lavasani, A.; Ahmadi-Danesh-Ashtiani, H.; Fazaeli, R. Effect of dust concentration, wind speed, and relative humidity on the performance of photovoltaic panels in Tehran. Energ. Source Part A 2023, 45, 7867–7877. [Google Scholar] [CrossRef]
- Satkauskas, I.; Maack, J.; Reynolds, M.; Sigler, D.; Panda, K.; Jones, W. Simulating Impacts of Extreme Events on Grids with High Penetrations of Wind Power Resources. In IEEE/PES Transmission and Distribution Conference and Exposition (T&D), New Orleans, LA, USA, 25-28 April 2022, pp. 01–05. 28 April; 05. [CrossRef]
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- Alexander, S.; Floyd, J. The Political Economy of Deep Decarbonization: Tradable Energy Quotas for Energy Descent Futures. Energies 2020, 13, 4304. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Abánades, A. Comparative Analysis of Energy and Exergy Performance of Hydrogen Production Methods. Entropy 2020, 22, 1286. [Google Scholar] [CrossRef]
- Huo, J.; Wang, Z.; Oberschelp, C.; Guillén-Gosálbez, G.; Hellweg, S. Net-zero transition of the global chemical industry with CO2-feedstock by 2050: feasible yet challenging. Green Chem. 2023, 25, 415–430. [Google Scholar] [CrossRef]
- Bockris, J.O.; Nagy, Z. The Hydrogen Economy. In: Electrochemistry for Ecologists. (1974). Springer, Boston, MA. [CrossRef]
- Yap, J.; McLellan, B. A Historical Analysis of Hydrogen Economy Research, Development, and Expectations, 1972 to 2020. Environments 2023, 10, 11. [Google Scholar] [CrossRef]
- Ogden, J.M. Hydrogen: The Fuel of the Future? Phys. Today 2002, 55, 69–75. [Google Scholar] [CrossRef]
- Crabtree, G.W.; Dresselhaus, M.S.; Buchanan, M.V. The Hydrogen Economy. Phys. Today 2004, 57, 39–44. [Google Scholar] [CrossRef]
- Chanchetti, L.F.; Leiva, D.R.; Lopes de Faria, L.I.; Ishikawa, T.T. A scientometric review of research in hydrogen storage materials. Int. J. Hydrogen Energy 2020, 45, 5356–5366. [Google Scholar] [CrossRef]
- Hydrogen fueled gas turbines. Available online: https://www.gevernova.com/gas-power/future-of-energy/hydrogen-fueled-gas-turbines?utm_campaign=h2&utm_medium=cpc&utm_source=google&utm_content=rsa&utm_term=Ge%20gas%20turbine%20hydrogen&gad_source=1&gclid=CjwKCAiApuCrBhAuEiwA8VJ6JkR3-lQPrF-s1tt3rgZ46itzqPULaD2Sr6OxvQAyNnk3cZkRnRca4RoCoKUQAvD_BwE (accessed on 12 December 2023).
- Airbus reveals hydrogen-powered zero-emission engine. Available online: https://www.airbus.com/en/newsroom/press-releases/2022-11-airbus-reveals-hydrogen-powered-zero-emission-engine (accessed on 17 December 2023).
- Airbus and CFM International to pioneer hydrogen combustion technology. Available online: https://www.airbus.com/en/newsroom/press-releases/2022-02-airbus-and-cfm-international-to-pioneer-hydrogen-combustion (accessed on 17 December 2023).
- Toyota. Available online: https://www.toyota.es/?gad_source=1&gclid=CjwKCAiApuCrBhAuEiwA8VJ6JvdD0b3ceOoBNrMc-iiOjzAn4NlXqK0WQOF8_CsaJ_IdrW6NkvsXdxoCWvQQAvD_BwE&gclsrc=aw.ds (accessed on 17 December 2023).
- Kurien, C.; Mittal, M. Review on the production and utilization of green ammonia as an alternate fuel in dual-fuel compression ignition engines. Energy Convers. Manag. 2022, 251, 114990. [Google Scholar] [CrossRef]
- Ershov, M.A.; Savelenko, V.D.; Makhova, U.A.; Makhmudova, A.E.; Zuikov, A.V.; Kapustin, V.M.; Abdellatief, T.M.M.; Burov, N.O.; Geng, T.; Abdelkareem, M.A. , Olabi, A.G. Current challenge and innovative progress for producing HVO and fame biodiesel fuels and their applications. Waste Biomass Valori. 2023, 14, 505–521. [Google Scholar] [CrossRef]
- Elía, M.F.; de la Torre, O. ; Larraz, R; Frontela, J. Cepsa: Towards The Integration of Vegetable Oils and Lignocellulosic Biomass into Conventional Petroleum Refinery Processing Units. In Industrial Biorenewables; P. Domínguez de María. Wiley, New Jersey, USA. 2016; pp. 141–174. [CrossRef]
- Power your future with Neste MY Renewable Diesel™ (HVO100). Available online: https://www.neste.com/products/all-products/renewable-road-transport/neste-my-renewable-diesel#1ff8d5dd (accessed on 19 December 2023).
- Dimitriadis, A.; Natsios, I.; Dimaratos, A.; Katsaounis, D.; Samaras, Z.; Bezergianni, S.; Lehto, K. Evaluation of a hydrotreated vegetable oil (HVO) and effects on emissions of a passenger car diesel engine. Front. Mech. Eng. 2018, 4, 7. [Google Scholar] [CrossRef]
- Padella, M.; O’Connell, A.; Prussi, M. What is still Limiting the Deployment of Cellulosic Ethanol? Analysis of the Current Status of the Sector. Appl. Sci. 2019, 9, 4523. [Google Scholar] [CrossRef]
- Tamburini, E.; Gaglio, M.; Castaldelli, G.; Fano, E.A. Is Bioenergy Truly Sustainable When Land-Use-Change (LUC) Emissions Are Accounted for? The Case-Study of Biogas from Agricultural Biomass in Emilia-Romagna Region, Italy. Sustainability 2020, 12, 3260. [Google Scholar] [CrossRef]
- Greenhouse gas emissions from transport in Europe. Available online: https://www.eea.europa.eu/en/analysis/indicators/greenhouse-gas-emissions-from-transport (accessed on 19 December 2023).
- Hu, K.; Chen, Y. Technological growth of fuel efficiency in European automobile market 1975–2015. Energy Policy 2016, 98, 142–148. [Google Scholar] [CrossRef]
- Dosier de prensa – Mazda motor Europe, Mazda e-SKYACTIV X. Available online: https://es.mazda-press.com/api/assets/download/167c8a2c-bb33-4dbc-ac38-4144d1de6d89_Pdf?isDownload=false (accessed on 19 December 2023).
- Average age of the EU vehicle fleet, by country. Available online: https://www.acea.auto/figure/average-age-of-eu-vehicle-fleet-by-country/#:~:text=Passenger%20cars%20are%20now%20on,of%20the%20EU%20member%20states (accessed on 19 December 2023).
- Global fleet the executive: Wikifleet. Available online: https://www.globalfleet.com/en/wikifleet (accessed on 19 December 2023).
- Sacchi, R.; Bauer, C.; Cox, B.; Mutel, C. When, where and how can the electrification of passenger cars reduce greenhouse gas emissions? Renew. Sustain. Energy Rev. 2022, 162, 112475. [Google Scholar] [CrossRef]
- Andersson, Ö.; Börjesson, P. The greenhouse gas emissions of an electrified vehicle combined with renewable fuels: Life cycle assessment and policy implications. Appl. Energy 2021, 289, 116621. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- European Biogas Association. EBA Statistical Report 2021; European Biogas Association: Brussels, Belgium. 2021; Available online: https://www.europeanbiogas.eu/wp-content/uploads/2021/11/EBA-STATISTICAL-REPORT-2021-SHORT-VERSION.pdf (accessed on 20 December 2023).
- Eurostats: Natural gas supply statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Natural_gas_supply_statistics#Consumption_trends (accessed on 19 December 2023).
- Sulewski, P.; Ignaciuk, W.; Szymańska, M.; Wąs, A. Development of the Biomethane Market in Europe. Energies 2023, 16, 2001. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; Gil, M.V.; Gómez, X. Syngas Fermentation: Cleaning of Syngas as a Critical Stage in Fermentation Performance. Fermentation 2023, 9, 898. [Google Scholar] [CrossRef]
- Maitlo, G.; Ali, I.; Mangi, K.H.; Ali, S.; Maitlo, H.A.; Unar, I.N.; Pirzada, A.M. Thermochemical Conversion of Biomass for Syngas Production: Current Status and Future Trends. Sustainability 2022, 14, 2596. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 Recycling to Dimethyl Ether: State-of-the-Art and Perspectives. Molecules 2018, 23, 31. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.; Freda, C.; Catizzone, E. Techno-Economic Assessment of Bio-Syngas Production for Methanol Synthesis: A Focus on the Water–Gas Shift and Carbon Capture Sections. Bioengineering 2020, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Kroch, E. Ammonia — a fuel for motor buses. https://clavertonenergy.com/cms4/wp-content/files/NH3_bus_1945_JInstPetrol31_Pg213.pdf. J. Inst. Pet. 1945, 31, 213–223. [Google Scholar]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Statista: Production of ammonia worldwide from 2010 to 2023. Available online: https://www.statista.com/statistics/1266378/global-ammonia-production/#:~:text=Ammonia production has remained fairly,approximately 64.6 million metric tons (accessed on 19 December 2023).
- Brown, A.E.; Hammerton, J.M.; Camargo-Valero, M.A.; Ross, A.B. Integration of Hydrothermal Carbonisation and Anaerobic Digestion for the Energy Valorisation of Grass. Energies 2022, 15, 3495. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Rousselle, C. Combustion characteristics of ammonia in a modern spark-ignition engine (No. 2019-24-0237). SAE Technical Paper, 2019. [Google Scholar] [CrossRef]
- Unlocking ammonia’s potential for shipping. Available online: https://www.man-es.com/discover/two-stroke-ammonia-engine?gad_source=1&gclid=CjwKCAiA-P-rBhBEEiwAQEXhHwmTLNnS5Oc5d-BzX3NU_aCBfD0ieFAmPFtUdECGAzLdvRQvM2qdGBoCpZQQAvD_BwE (accessed on 19 December 2023).
- Fumanelli, F. Toyota GT86-R Marangoni Eco Explorer: sport and technology in the new 2013 show car, fitted with Marangoni “M-Power EvoRed” tyres. 2013. Available online: https://marangonipress.com/wp-content/uploads/2019/07/Toyota_Marangoni_GT86-R_Eco_Explorer_EN.pdf (accessed on 19 December 2023).
- Michikawauchi, R., Ito, Y., Iwatani, K., Tanno, S. Washington, DC: U.S. Patent and Trademark Office. No. 8904994 B2. Ammonia burning internal combustion engine. 2014. https://ppubs.uspto.gov/dirsearch-public/print/downloadPdf/8904994,.
- Tange, K., Nakamura, N., Nakanishi, H., Arikawa, H.Washington, DC: U.S. Patent and Trademark Office. Patent No. 9506400. Hydrogen generator, ammonia-burning internal combustion engine, and fuel cell. 2016. https://ppubs.uspto.gov/dirsearch-public/print/downloadPdf/9506400. and.
- Kable, G. GAC and Toyota develop ammonia engine for 90% CO2 reduction. Available online: https://www.autocar.co.uk/car-news/new-cars/gac-and-toyota-develop-ammonia-engine-90-co2-reduction (accessed on 20 December 2023).
- Gubbi, S.; Cole, R.; Emerson, B.; Noble, D.; Steele, R.; Sun, W.; Lieuwen, T. Air Quality Implications of Using Ammonia as a Renewable Fuel: How Low Can NO x Emissions Go? ACS Energy Lett. 2023, 8, 4421–4426. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, D.; Dincer, I. A perspective on the use of ammonia as a clean fuel: Challenges and solutions. Int. J. Energy Res. 2021, 45, 4827–4834. [Google Scholar] [CrossRef]
- Hasan, M.H.; Mahlia, T.M.; Mofijur, M.; Rizwanul Fattah, I.M.; Handayani, F.; Ong, H.C.; Silitonga, A.S. A Comprehensive Review on the Recent Development of Ammonia as a Renewable Energy Carrier. Energies 2021, 14, 3732. [Google Scholar] [CrossRef]
- Statista Research department: Production capacity of ammonia worldwide from 2018 to 2022, with a forecast for 2026 and 2030. Available online: https://www.statista.com/statistics/1065865/ammonia-production-capacity-globally/#:~:text=The global production capacity of,million metric tons by 2030 (accessed on 22 December 2023).
- Basf: BASF hystory we create chemistry 1865-2015. Available online: https://www.basf.com/global/images/about-us/history/BASF_Chronik_Gesamt_en.pdf.assetdownload.pdf (accessed on 22 December 2023).
- Travis, A.S. Luigi Casale’s enterprise: Pioneer of global catalytic high-pressure industrial chemistry. Catal. Today 2022, 387, 4–8. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I.; Zamfirescu, C.; Vezina, G.; Raso, F. Comparative life cycle assessment of various ammonia production methods. J. Clean. Prod. 2016, 135, 1379–1395. [Google Scholar] [CrossRef]
- López-Fernández, E.; Sacedón, C.G.; Gil-Rostra, J.; Yubero, F.; González-Elipe, A.R.; de Lucas-Consuegra, A. Recent Advances in Alkaline Exchange Membrane Water Electrolysis and Electrode Manufacturing. Molecules 2021, 26, 6326. [Google Scholar] [CrossRef]
- Partidário, P.; Aguiar, R.; Martins, P.; Rangel, C.M.; Cabrita, I. The hydrogen roadmap in the Portuguese energy system–developing the P2G case. Int. J. Hydrog. Energy 2020, 45, 25646–25657. [Google Scholar] [CrossRef]
- NEL hydrogen electrolizers. Available online: https://nelhydrogen.com/water-electrolysers-hydrogen-generators/?ppc_keyword=water electrolyzer&gclid=Cj0KCQiAm4WsBhCiARIsAEJIEzV6AsnlJMZVeXF022ipll_r21bThveR82DVWTJf4v2YeETcE9vQNwgaAtInEALw_wcB (accessed on 10 January 2023).
- Mcphy Electrolyzers. Available online: https://mcphy.com/en/ (accessed on 10 January 2023).
- Xia, Y.; Cheng, H.; He, H.; Wei, W. Efficiency and consistency enhancement for alkaline electrolyzers driven by renewable energy sources. Commun. Eng. 2023, 2, 22. [Google Scholar] [CrossRef]
- Haleem, A.A.; Huyan, J.; Nagasawa, K.; Kuroda, Y.; Nishiki, Y.; Kato, A.; Nakai, T.; Araki, T.; Mitsushima, S. Effects of operation and shutdown parameters and electrode materials on the reverse current phenomenon in alkaline water analyzers. J. Power Sources 2022, 535, 231454. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, S.M.; Kim, K.S.; Kim, H.Y.; Kwon, J.; Lee, J.; Cho, H.-S.; Kim, Y.T. Cathodic protection system against a reverse-current after shut-down in zero-gap alkaline water electrolysis. JACS Au 2022, 2, 2491–2500. [Google Scholar] [CrossRef]
- Chau, K.; Djire, A.; Khan, F. Review and analysis of the hydrogen production technologies from a safety perspective. Int. J. Hydrogen Energy 2022, 47, 13990–14007. [Google Scholar] [CrossRef]
- Fertiberia: Planta de hidrógeno, amoniaco y fertilizantes verdes. Available online: https://www.fertiberia.com/amoniacoverde/puertollano-proyecto-h2f-planta-de-hidrogeno-amoniaco-y-fertilizantes-verdes/ (accessed on 10 January 2023).
- Harrou, F.; Sun, Y.; Taghezouit, B.; Dairi, A. Artificial Intelligence Techniques for Solar Irradiance and PV Modeling and Forecasting. Energies 2023, 16, 6731. [Google Scholar] [CrossRef]
- Lehner, B.; Döll, P.; Alcamo, J.; Henrichs, T.; Kaspar, F. Estimating the impact of global change on flood and drought risks in Europe: a continental, integrated analysis. Clim. Change 2006, 75, 273–299. [Google Scholar] [CrossRef]
- Barbeta, A.; Mejía-Chang, M.; Ogaya, R.; Voltas, J.; Dawson, T.E.; Peñuelas, J. The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Glob. Chang. Biol. 2015, 21, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: a review. Results Eng. 2023, 101426. [Google Scholar] [CrossRef]
- Dresp, S.; Thanh, T.N.; Klingenhof, M.; Brückner, S.; Hauke, P.; Strasser, P. Efficient direct seawater electrolysers using selective alkaline NiFe-LDH as OER catalyst in asymmetric electrolyte feeds. Energy Environ. Sci. 2020, 13, 1725–1729. [Google Scholar] [CrossRef]
- Rao, P.; Morrow III, W.R.; Aghajanzadeh, A.; Sheaffer, P.; Dollinger, C.; Brueske, S.; Cresko, J. Energy considerations associated with increased adoption of seawater desalination in the United States. Desalination 2018, 445, 213–224. [Google Scholar] [CrossRef]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.; Styring, P. Sustainable ammonia production processes. Front. Energy Res. 2021, 9, 34. [Google Scholar] [CrossRef]
- Rezaei, M.; Mostafaeipour, A.; Qolipour, M.; Arabnia, H.R. Hydrogen production using wind energy from sea water: A case study on Southern and Northern coasts of Iran. Energy Environ. 2018, 29, 333–357. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Attas, T.; Roy, S.; Rahman, M.M.; Ghaffour, N.; Thangadurai, V.; Larter, S.; Hu, J.; Ajayan, P.M.; Kibria, M.G. Seawater electrolysis for hydrogen production: a solution looking for a problem? Energy Environ. Sci. 2021, 14, 4831–4839. [Google Scholar] [CrossRef]
- Calado, G.; Castro, R. Hydrogen Production from Offshore Wind Parks: Current Situation and Future Perspectives. Appl. Sci. 2021, 11, 5561. [Google Scholar] [CrossRef]
- Saygin, D.; Blanco, H.; Boshell, F.; Cordonnier, J.; Rouwenhorst, K.; Lathwal, P.; Gielen, D. Ammonia Production from Clean Hydrogen and the Implications for Global Natural Gas Demand. Sustainability 2023, 15, 1623. [Google Scholar] [CrossRef]
- Hamulczuk, M.; Pawlak, K.; Stefańczyk, J.; Gołębiewski, J. Agri-Food Supply and Retail Food Prices during the Russia–Ukraine Conflict’s Early Stage: Implications for Food Security. Agriculture 2023, 13, 2154. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Maréchal, F.; Desideri, U. Techno-economic comparison of green ammonia production processes. Appl. Energy 2020, 259, 114135. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Travis, A.S.; Lefferts, L. 1921–2021: A Century of Renewable Ammonia Synthesis. Sustain. Chem. 2022, 3, 149–171. [Google Scholar] [CrossRef]
- Enaex. Available online: https://www.enaex.com/pe/us/enaex-peru-history/ (accessed on 10 January 2023).
- Hong, J.; Prawer, S.; Murphy, A.B. Plasma catalysis as an alternative route for ammonia production: status, mechanisms, and prospects for progress. ACS Sustain. Chem. Eng. 2018, 6, 15–31. [Google Scholar] [CrossRef]
- Meloni, E.; Cafiero, L.; Martino, M.; Palma, V. Structured Catalysts for Non-Thermal Plasma-Assisted Ammonia Synthesis. Energies 2023, 16, 3218. [Google Scholar] [CrossRef]
- Veselovskaya, J.V.; Parunin, P.D.; Okunev, A.G. Catalytic process for methane production from atmospheric carbon dioxide utilizing renewable energy. Catal. Today 2017, 298, 117–123. [Google Scholar] [CrossRef]
- Navarro, J.C.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Policies and Motivations for the CO2 Valorization through the Sabatier Reaction Using Structured Catalysts. A Review of the Most Recent Advances. Catalysts 2018, 8, 578. [Google Scholar] [CrossRef]
- Korosec, K.; Audi E-Gas Plant Uses CO2, Renewables to Make Fuel. Environment + Energy Leader 2012. Available online: https://www.environmentenergyleader.com/2012/12/audi-e-gas-plant-uses-co2-renewables-to-make-fuel/ (accessed on 12 January 2023).
- Hy2Gen acquires e-Gas plant and project pipeline of kiwi AG in Germany; formerly Audi e-Gas plant. Available online: https://www.greencarcongress.com/2023/12/20231212-hy2gen.html#:~:text=In%202013%2C%20the%20plant%20in,%2Dto%2DeMethane%20plant%20today (accessed on 12 January 2023).
- Cai, T.; Zhao, D. Overview of Autoignition and Flame Propagation Properties for Ammonia Combustion. AIAA Journal 2023, 1–25. [Google Scholar] [CrossRef]
- Chiong, M.; Chong, C.T.; Ng, J.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of combustion technologies in the ammonia-fuelled engines. Energy Convers. Manag. 2021, 244, 114460. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Wang, S.; Guiberti, T.F.; Roberts, W.L. Review on the recent advances on ammonia combustion from the fundamentals to the applications. Fuel Communications 2022, 10, 100053. [Google Scholar] [CrossRef]
- Liang, X.; Zheng, Z.; Zhang, H.; Wang, Y.; Yu, H. A Review of Early Injection Strategy in Premixed Combustion Engines. Appl. Sciences 2018, 9, 3737. [Google Scholar] [CrossRef]
- Alger, T.; Gingrich, J.; Roberts, C.; Mangold, B. Cooled exhaust-gas recirculation for fuel economy and emissions improvement in gasoline engines. Int. J. Engine Res. 2011, 12, 252–264. [Google Scholar] [CrossRef]
- Piqueras, P.; Morena, J.D.; Sanchis, E.J.; Pitarch, R. Impact of Exhaust Gas Recirculation on Gaseous Emissions of Turbocharged Spark-Ignition Engines. Appl. Sciences 2020, 10, 7634. [Google Scholar] [CrossRef]
- Verkamp, F.; Hardin, M.; Williams, J. Ammonia combustion properties and performance in gas-turbine burners. Symp. (Int.) Combust. 1967, 11, 985–992. [Google Scholar] [CrossRef]
- Haputhanthri, S.O.; Maxwell, T.T.; Fleming, J.; Austin, C. Ammonia and gasoline fuel blends for spark ignited internal combustion engines. J. Energy Resour. Technol. 2015, 137, 062201. [Google Scholar] [CrossRef]
- Rodríguez, C.G.; Lamas, M.I.; Rodríguez, J.d.D.; Abbas, A. Multi-Criteria Analysis to Determine the Most Appropriate Fuel Composition in an Ammonia/Diesel Oil Dual Fuel Engine. J. Mar. Sci. Eng. 2023, 11, 689. [Google Scholar] [CrossRef]
- Elumalai, R.; Ravi, K. Optimization of ideal engine parameters to adopt ammonia and algae biodiesel for reactivity controlled compression ignition engine using response surface methodology. Proc. Inst. Mech. Eng., Part C. 2023. [Google Scholar] [CrossRef]
- Ma, F.; Guo, L.; Li, Z.; Zeng, X.; Zheng, Z.; Li, W.; Zhao, F.; Yu, W. A Review of Current Advances in Ammonia Combustion from the Fundamentals to Applications in Internal Combustion Engines. Energies 2023, 16, 6304. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Hayakawa, A.; Kobayashi, H. Success of ammonia-fired, regenerator-heated, diffusion combustion gas turbine power generation and prospect of low NOx combustion with high combustion efficiency (Vol. 57601, p. V001T04A026). In ASME Power Conference, Charlotte, North Carolina, USA, (26-30 June 2017) American Society of Mechanical Engineers. [CrossRef]
- Gubbi, S.; Cole, R.; Emerson, B.; Noble, D.; Steele, R.; Sun, W.; Lieuwen, T. Evaluation of Minimum NOx Emission From Ammonia Combustion. J. Eng. Gas Turbines Power 2024, 146, 031023. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kim, H.; Kim, H.; Kang, S.; Ryu, J.; Shim, S. Reduction of NOx Emission from the Cement Industry in South Korea: A Review. Atmosphere 2021, 13, 121. [Google Scholar] [CrossRef]
- Miller, J.A.; Smooke, M.D.; Green, R.M.; Kee, R.J. Kinetic modeling of the oxidation of ammonia in flames. Combust. Sci. Technol. 1983, 34, 149–176. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Gutmark, E. Overview of fundamental kinetic mechanisms and emission mitigation in ammonia combustion. Chem. Eng. J. 2023, 458, 141391. [Google Scholar] [CrossRef]
- Shu, B.; Vallabhuni, S.; He, X.; Issayev, G.; Moshammer, K.; Farooq, A.; Fernandes, R. A shock tube and modeling study on the autoignition properties of ammonia at intermediate temperatures. Proc. Combust. Inst. 2018, 37, 205–211. [Google Scholar] [CrossRef]
- Karimkashi, S.; Tamadonfar, P.; Kaario, O.; Vuorinen, V. A Numerical Investigation on Effects of Hydrogen Enrichment and Turbulence on NO Formation Pathways in Premixed Ammonia/Air Flames. Combust. Sci. Technol. 2023, 1–30. [Google Scholar] [CrossRef]
- Rocha, R.C.; Zhong, S.; Xu, L.; Bai, X.S.; Costa, M.; Cai, X.; Kim, H.; Brackmann, C.; Li, Z.; Alden, M. Structure and laminar flame speed of an ammonia/methane/air premixed flame under varying pressure and equivalence ratio. Energy Fuels 2021, 35, 7179–7192. [Google Scholar] [CrossRef]
- Khalil, A.T.; Manias, D.M.; Kyritsis, D.C.; Goussis, D.A. NO formation and autoignition dynamics during combustion of H2O-diluted NH3/H2O2 mixtures with air. Energies 2021, 14, 84. [Google Scholar] [CrossRef]
- Kim, N.; Lee, M.; Park, J.; Park, J.; Lee, T. A Comparative Study of NOx Emission Characteristics in a Fuel Staging and Air Staging Combustor Fueled with Partially Cracked Ammonia. Energies 2022, 15, 9617. [Google Scholar] [CrossRef]
- Woo, M.; Choi, B.C. Numerical study on fuel-NO formation characteristics of ammonia-added methane fuel in laminar non-premixed flames with oxygen/carbon dioxide oxidizer. Energy 2021, 226, 120365. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Nozari, H.; Karabeyoğlu, A. Numerical study of combustion characteristics of ammonia as a renewable fuel and establishment of reduced reaction mechanisms. Fuel 2015, 159, 223–233. [Google Scholar] [CrossRef]
- Westlye, F.R.; Ivarsson, A.; Schramm, J. Experimental investigation of nitrogen based emissions from an ammonia fueled SI-engine. Fuel 2013, 111, 239–247. [Google Scholar] [CrossRef]
- Pedersen, K.A.; Lewandowski, M.T.; Schulze-Netzer, C.; Pasternak, M.; Løvås, T. Ammonia in Dual-Fueled Internal Combustion Engines: Impact on NOx, N2O, and Soot Formation. Energy Fuels 2023, 37, 17585–17604. [Google Scholar] [CrossRef]
- Glarborg, P. The NH3/NO2/O2 system: Constraining key steps in ammonia ignition and N2O formation. Combust. Flame 2023, 257, 112311. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Syred, N.; Griffiths, A. Visualisation of isothermal large coherent structures in a swirl burner. Combust. Flame 2009, 156, 1723–1734. [Google Scholar] [CrossRef]
- Reddy, V.M.; Katoch, A.; Roberts, W.L.; Kumar, S. Experimental and numerical analysis for high intensity swirl based ultra-low emission flameless combustor operating with liquid fuels. Proc. Combust. Inst. 2015, 35, 3581–3589. [Google Scholar] [CrossRef]
- Faqih, M.; Omar, M.B.; Ibrahim, R.; Omar, B.A. Dry-Low Emission Gas Turbine Technology: Recent Trends and Challenges. Appl. Sciences 2021, 12, 10922. [Google Scholar] [CrossRef]
- Albin, T.; da Franca, A.A.; Varea, E.; Kruse, S.; Pitsch, H.; Abel, D. Potential and challenges of MILD combustion control for gas turbine applications. In: Active Flow and Combustion Control 2014, King, R. Eds.; Springer International Publishing, Manhattan, USA, 2015; pp. 181-195). Notes on Numerical Fluid Mechanics and Multidisciplinary Design, Volume 127. Springer, Cham. [CrossRef]
- Iki, N.; Kurata, O.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Koboyashi, H.; Hayakawa, A.; Okafor, E. NOx reduction in a swirl combustor firing ammonia for a micro gas turbine, (Vol. 51173, p. V008T26A009). In Turbo Expo: Power for Land, Sea, and Air, Oslo, Norway (11–15 June 2018). American Society of Mechanical Engineers. [CrossRef]
- Okafor, E.C.; Somarathne, K.K.A. , Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Kobayashi, H. Towards the development of an efficient low-NOx ammonia combustor for a micro gas turbine. Proc. Combust. Inst. 2019, 37, 4597–4606. [Google Scholar] [CrossRef]
- Tumanovskii, A.G.; Bulysova, L.A.; Vasil’ev, V.D.; Gutnik, M.N.; Gutnik, M.M. Development of Low-Emission Combustors for Power-Generating GTUs. Therm. Eng. 2021, 68, 473–480. [Google Scholar] [CrossRef]
- Jin, T.; Dong, W.; Qiu, B.; Xu, C.; Liu, Y.; Chu, H. Effect of Ammonia on Laminar Combustion Characteristics of Methane–Air Flames at Elevated Pressures. ACS omega 2022, 7, 15326–15337. [Google Scholar] [CrossRef]
- Rocha, R. C.; Costa, M.; Bai, X.S. Combustion and emission characteristics of ammonia under conditions relevant to modern gas turbines. Combust. Sci. Technol. 2021, 193, 2514–2533. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Gutesa, M.; Xiao, H.; Pugh, D.; Giles, A.; Goktepe, B.; Marsh, R.; Bowen, P. Premixed ammonia/hydrogen swirl combustion under rich fuel conditions for gas turbines operation. Int. J. Hydrogen Energy 2019, 44, 8615–8626. [Google Scholar] [CrossRef]
- Mashruk, S.; Xiao, H.; Pugh, D.; Chiong, M.C.; Runyon, J.; Goktepe, B.; Giles, A.; Valera-Medina, A. Numerical analysis on the evolution of NH2 in ammonia/hydrogen swirling flames and detailed sensitivity analysis under elevated conditions. Combust. Sci. Technol. 2023, 195, 1251–1278. [Google Scholar] [CrossRef]
- Zhu, D.; Ruwe, L.; Schmitt, S.; Shu, B.; Kohse-Höinghaus, K.; Lucassen, A. Interactions in Ammonia and Hydrogen Oxidation Examined in a Flow Reactor and a Shock Tube. J. Phys. Chem. A 2023, 127, 2351–2366. [Google Scholar] [CrossRef]
- Stefanizzi, M.; Capurso, T.; Filomeno, G.; Torresi, M.; Pascazio, G. Recent Combustion Strategies in Gas Turbines for Propulsion and Power Generation toward a Zero-Emissions Future: Fuels, Burners, and Combustion Techniques. Energies 2021, 14, 6694. [Google Scholar] [CrossRef]
- Colson, S.; Hirano, Y.; Hayakawa, A.; Kudo, T.; Kobayashi, H.; Galizzi, C.; Escudie, D. Experimental and numerical study of NH3/CH4 counterflow premixed and non-premixed flames for various NH3 mixing ratios. Combust. Sci. Technol. 2021, 193, 2872–2889. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia–methane combustion in tangential swirl burners for gas turbine power generation. Appl. Energy 2017, 185, 1362–1371. [Google Scholar] [CrossRef]
- Khateeb, A.A.; Guiberti, T.F.; Zhu, X.; Younes, M.; Jamal, A.; Roberts, W.L. Stability limits and exhaust NO performances of ammonia-methane-air swirl flames. Exp. Therm. Fluid Sci. 2020, 114, 110058. [Google Scholar] [CrossRef]
- Henshaw, P.F.; D'andrea, T.I.N.A.; Mann, K.R.; Ting, D.S.K. Premixed ammonia-methane-air combustion. Combust. Sci. Technol. 2005, 177, 2151–2170. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Wang, P.; Mao, C.; Cheng, K.; Sun, Y.; Yin, Z. Combustion Performance of the Premixed Ammonia-Hydrogen-Air Flame in Porous Burner. Combust. Sci. Technol. 2023, 1–18. [Google Scholar] [CrossRef]
- Iki, N.; Kurata, O.; Matsunuma, T.; Inoue, T.; Suzuki, M.; Tsujimura, T.; Furutani, H. Micro gas turbine firing kerosene and ammonia (Vol. 56796, p. V008T23A023). In Turbo Expo: Power for Land, Sea, and Air. ASME Turbo Expo 2015: Turbine Technical Conference and Exposition, Montreal, Quebec, Canada, (15–19 June 2015). American Society of Mechanical Engineers. [CrossRef]
- Iki, N.; Kurata, O.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Hayakawa, A. Operation and flame observation of micro gas turbine firing ammonia (Vol. 50954, p. V008T26A018). In Turbo Expo: Power for Land, Sea, and Air. ASME Turbo Expo 2017: Turbomachinery Technical Conference and Exposition. Charlotte, North Carolina, USA, (26–30 June 2017). American Society of Mechanical. Engineers. [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3–air and NH3CH4–air combustion gas-turbine power generations. Proc. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Ávila, C.D.; Cardona, S.; Abdullah, M.; Younes, M.; Jamal, A.; Guiberti, T.F.; Roberts, W.L. Experimental assessment of the performance of a commercial micro gas turbine fueled by ammonia-methane blends. Appl. Energy Combust. Sci. 2023, 13, 100104. [Google Scholar] [CrossRef]
- Somarathne, K.D.K.A.; Colson, S.; Hayakawa, A.; Kobayashi, H. Modelling of ammonia/air non-premixed turbulent swirling flames in a gas turbine-like combustor at various pressures. Combust. Theory Model. 2018, 22, 973–997. [Google Scholar] [CrossRef]
- Bonasio, V.; Ravelli, S. Performance Analysis of an Ammonia-Fueled Micro Gas Turbine. Energies 2022, 15, 3874. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and other emissions in micro gas turbine combustors fuelled with mixtures of methane and ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]
- Ditaranto, M.; Saanum, I.; Larfeldt, J. Experimental study on high pressure combustion of decomposed ammonia: How can ammonia be best used in a gas turbine? (Vol. 84959, p. V03BT04A027). In Turbo Expo: Power for Land, Sea, and Air. ASME Turbo Expo 2021: Turbomachinery Technical Conference and Exposition, Virtual, Online (7–11 June 2021). American Society of Mechanical Engineers. [CrossRef]
- Matriculaciones de turismos en España. Available online: https://www.epdata.es/datos/matriculaciones-turimos-espana-carburante/206/espana/106 (accessed on 17 January 2023).
- Dobslaw, D.; Engesser, K.; Störk, H.; Gerl, T. Low-cost process for emission abatement of biogas internal combustion engines. J. Clean. Prod. 2019, 227, 1079–1092. [Google Scholar] [CrossRef]
- Benato, A.; Macor, A.; Rossetti, A. Biogas Engine Emissions: Standards and On-Site Measurements. Energy Procedia 2017, 126, 398–405. [Google Scholar] [CrossRef]
- INNIO, Jenbacher. Available online: https://www.jenbacher.com/en/energy-solutions/applications/cogeneration-combined-heat-power (accessed on 17 January 2023).
- MWM Gas Engines and Gensets – Output. Reliability. Economy. For Your Success. Available online: https://www.mwm.net/en/gas-engines-gensets/ (accessed on 19 January 2023).
- Discover The Latest Gas-Powered Generator Sets. Available online: https://www.cat.com/en_GB/campaigns/npi/cg170b-gas-generator-sets.html (accessed on 19 January 2023).
- GAS GENERATOR SETS. Available online: https://www.mtu-solutions.com/eu/en/applications/power-generation/power-generation-products/gas-generator-sets.html (accessed on 20 January 2023).
- Powering your reliable, responsible energy future. Available online: https://www.waukeshaengine.com/en (accessed on 19 January 2023).
- MAN Energy Solutions. A cleaner future with gas engines. Available online: https://www.man-es.com/energy-storage/products/gas-fuel-engines?utm_medium=sea&utm_source=google&utm_campaign=always_ao_sp_brand-energy-and-storage-products-all_230101&utm_term=sp&gad_source=1&gclid=CjwKCAiArfauBhApEiwAeoB7qJYQnAMEpJYX67n-whaDwvggV52Y3MwULQW-FY9PamNRH3MDEXz-cRoCtugQAvD_BwE&gclsrc=aw.ds (accessed on 19 January 2023).
- Cornelius, W.; Huellmantel, L.W.; Mitchell, H.R. Ammonia as an engine fuel. SAE Transactions 1966, 300–326. https://www.jstor.org/stable/44460524. /.
- Starkman, E.S.; Newhall, H.K.; Sutton, R.; Maguire, T.; Farbar, L. Ammonia as a spark ignition engine fuel: theory and application. SAE Transactions 1967, 765–784. https://www.jstor.org/stable/44563674. /.
- Frigo, S.; Gentili, R.; Doveri, N. Ammonia Plus Hydrogen as Fuel in a S.I. Engine: Experimental Results. SAE Technical Paper 2012, 2012-32-0019. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Z.; Liu, J. An evaluation of the conversion of gasoline and natural gas spark ignition engines to ammonia/hydrogen operation from the perspective of laminar flame speed. J. Energy Resour. Technol. 2022, 145, 012302. [Google Scholar] [CrossRef]
- Park, C.; Jang, Y.; Kim, S.; Kim, Y.; Choi, Y. Influence of Hydrogen on the Performance and Emissions Characteristics of a Spark Ignition Ammonia Direct Injection Engine. Gases 2023, 3, 144–157. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Experimental study on ammonia/hydrogen/air combustion in spark ignition engine conditions. Fuel 2020, 269, 117448. [Google Scholar] [CrossRef]
- Pyrc, M.; Gruca, M.; Tutak, W.; Jamrozik, A. Assessment of the co-combustion process of ammonia with hydrogen in a research VCR piston engine. Int. J. Hydrogen Energy 2023, 48, 2821–2834. [Google Scholar] [CrossRef]
- Ge, H.; Bakir, A.H.; Zhao, P. Knock Mitigation and Power Enhancement of Hydrogen Spark-Ignition Engine through Ammonia Blending. Machines 2023, 11, 651. [Google Scholar] [CrossRef]
- Grannell, S.M.; Assanis, D.N.; Bohac, S.V.; Gillespie, D.E. The fuel mix limits and efficiency of a stoichiometric, ammonia, and gasoline dual fueled spark ignition engine. J. Eng. Gas Turbines Power. 2008, 130, 042802. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S. Effects of gaseous ammonia direct injection on performance characteristics of a spark-ignition engine. Appl. Energy 2014, 116, 206–215. [Google Scholar] [CrossRef]
- Lanni, D.; Galloni, E.; Fontana, G. Assessment of the Operation of an SI Engine Fueled with Ammonia. Energies 2022, 15, 8583. [Google Scholar] [CrossRef]
- Polanski, J.; Bartczak, P.; Ambrozkiewicz, W.; Sitko, R.; Siudyga, T.; Mianowski, A.; Szade, J.; Balin, K.; Lelątko, J. Ni-Supported Pd Nanoparticles with Ca Promoter: A New Catalyst for Low-Temperature Ammonia Cracking. PLOS ONE 2015, 10, e0136805. [Google Scholar] [CrossRef]
- Lee, Y. J.; Cha, J.; Kwak, Y.; Park, Y.; Jo, Y.S.; Jeong, H.; Sohn, H.; Chang, W.Y.; Kim, Y.; Kim, K.-B.; Nam, S.W. Top-down syntheses of nickel-based structured catalysts for hydrogen production from ammonia. ACS Appl. Mater. Interfaces 2021, 13, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Cechetto, V.; Agnolin, S.; Di Felice, L.; Pacheco Tanaka, A.; Llosa Tanco, M.; Gallucci, F. Metallic Supported Pd-Ag Membranes for Simultaneous Ammonia Decomposition and H2 Separation in a Membrane Reactor: Experimental Proof of Concept. Catalysts 2023, 13, 920. [Google Scholar] [CrossRef]
- Khan, W.U.; Alasiri, H.S.; Ali, S.A.; Hossain, M.M. Recent Advances in Bimetallic Catalysts for Hydrogen Production from Ammonia. Chem. Rec. 2022, 22, e202200030. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lei, K.; Mi, Y.; Fang, W.; Li, X. Recent Progress on Hydrogen Production from Ammonia Decomposition: Technical Roadmap and Catalytic Mechanism. Molecules 2023, 28, 5245. [Google Scholar] [CrossRef] [PubMed]
- Spatolisano, E.; Pellegrini, L.A.; de Angelis, A.R.; Cattaneo, S.; Roccaro, E. Ammonia as a carbon-free energy carrier: NH3 cracking to H2. Ind. Eng. Chem. Res. 2023, 62, 10813–10827. [Google Scholar] [CrossRef]
- Mashhadimoslem, H.; Safarzadeh Khosrowshahi, M.; Delpisheh, M.; Convery, C.; Rezakazemi, M.; Aminabhavi, T.M.; Kamkar, M.; Elkamel, A. Green ammonia to Hydrogen: Reduction and oxidation catalytic processes. Chem. Eng. J. 2023, 474, 145661. [Google Scholar] [CrossRef]
- Comotti, M.; Frigo, S. Hydrogen generation system for ammonia–hydrogen fuelled internal combustion engines. Int. J. Hydrogen Energy 2015, 40, 10673–10686. [Google Scholar] [CrossRef]
- Bréquigny, P.; Dumand, C.; Houillé, S. Operating Limits for Ammonia Fuel Spark-Ignition Engine. Energies 2021, 14, 4141. [Google Scholar] [CrossRef]
- Tutak, W.; Pyrc, M.; Gruca, M.; Jamrozik, A. Ammonia Combustion in a Spark-Ignition Engine Supported with Dimethyl Ether. Energies 2023, 16, 7283. [Google Scholar] [CrossRef]
- Oh, S.; Park, C.; Kim, S.; Kim, Y.; Choi, Y.; Kim, C. Natural gas–ammonia dual-fuel combustion in spark-ignited engine with various air–fuel ratios and split ratios of ammonia under part load condition. Fuel 2021, 290, 120095. [Google Scholar] [CrossRef]
- Mørch, C.; Bjerre, A.; Gøttrup, M.; Sorenson, S.; Schramm, J. Ammonia/hydrogen mixtures in an SI-engine: Engine performance and analysis of a proposed fuel system. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S. Performance characteristics of compression-ignition engine using high concentration of ammonia mixed with dimethyl ether. Appl. Energy 2014, 113, 488–499. [Google Scholar] [CrossRef]
- Ambalakatte, A.; Geng, S.; Cairns, A.; Harrington, A.; Hall, J.; Bassett, M. Evaluation of ammonia-gasoline co-combustion in a modern spark ignition research engine. Carbon Neutrality 2023, 2, 35. [Google Scholar] [CrossRef]
- Abd El Fattah, S.F.; Ezzat, M.F.; Mourad, M.A.; Elkelawy, M.; Youssef, I.M. Experimental Investigation of the Performance and Exhaust Emissions of a Spark-Ignition Engine Operating with Different Proportional Blends of Gasoline and Water Ammonia Solution. J. Eng. Res. 2022, 5, 38–45. [Google Scholar]
- Ciatti, S.A. Compression Ignition Engines – Revolutionary Technology That has Civilized Frontiers all Over the Globe from the Industrial Revolution into the Twenty-First Century. Front. Mech. Eng. 2015, 1, 145182. [Google Scholar] [CrossRef]
- Reşitoğlu, İ.A.; Altinişik, K.; Keskin, A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef]
- Ayodhya, A.S.; Narayanappa, K.G. An overview of after-treatment systems for diesel engines. Environ. Sci. Pollut. Res. 2018, 25, 35034–35047. [Google Scholar] [CrossRef]
- Staffel, I. The Energy and Fuel Data Sheet. 2011. https://www.claverton-energy.com/wordpress/wp-content/uploads/2012/08/the_energy_and_fuel_data_sheet1.pdf.
- Gray Jr., J.T., Dimitroff, E.; Meckel, N.T.; Quillian Jr., R.D. Ammonia fuel—engine compatibility and combustion. SAE Trans. 1967, 785–807. https://www.jstor.org/stable/44563675. /.
- Starkman, E.S.; James, G.E.; Newhall, H.K. Ammonia as a diesel engine fuel: theory and application. SAE Trans. 1968, 3193–3212. https://www.jstor.org/stable/44562852. /.
- Pearsall, T.J.; Garabedian, C.G. Combustion of anhydrous ammonia in diesel engines. SAE Trans. 1968, 3213–3221. https://www.jstor.org/stable/44562853. /.
- Reiter, A.J.; Kong, S.C. Demonstration of compression-ignition engine combustion using ammonia in reducing greenhouse gas emissions. Energy Fuels 2008, 22, 2963–2971. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef]
- Voniati, G.; Dimaratos, A.; Koltsakis, G.; Ntziachristos, L. Ammonia as a Marine Fuel towards Decarbonization: Emission Control Challenges. Sustainability 2023, 15, 15565. [Google Scholar] [CrossRef]
- Yousefi, A.; Guo, H.; Dev, S.; Lafrance, S.; Liko, B. A study on split diesel injection on thermal efficiency and emissions of an ammonia/diesel dual-fuel engine. Fuel 2022, 316, 123412. [Google Scholar] [CrossRef]
- Imhoff, T.B.; Gkantonas, S.; Mastorakos, E. Analysing the Performance of Ammonia Powertrains in the Marine Environment. Energies 2021, 14, 7447. [Google Scholar] [CrossRef]
- Schönborn, A. Aqueous solution of ammonia as marine fuel. Proc. Inst. Mech. Eng M 2020, 235, 142–151. [Google Scholar] [CrossRef]
- Nadimi, E.; Przybyła, G.; Lewandowski, M.T.; Adamczyk, W. Effects of ammonia on combustion, emissions, and performance of the ammonia/diesel dual-fuel compression ignition engine. J. Energy Inst. 2023, 107, 101158. [Google Scholar] [CrossRef]
- Nadimi, E.; Przybyła, G.; Emberson, D.; Løvås, T.; Ziółkowski, Ł.; Adamczyk, W. Effects of using ammonia as a primary fuel on engine performance and emissions in an ammonia/biodiesel dual-fuel CI engine. Int. J. Energy Res. 2022, 46, 15347–15361. [Google Scholar] [CrossRef]
- Gross, C.W.; Kong, S. Performance characteristics of a compression-ignition engine using direct-injection ammonia–DME mixtures. Fuel 2013, 103, 1069–1079. [Google Scholar] [CrossRef]
- Frost, J.; Tall, A.; Sheriff, A.M.; Schönborn, A.; Hellier, P. An experimental and modelling study of dual fuel aqueous ammonia and diesel combustion in a single cylinder compression ignition engine. Int. J. Hydrogen Energy 2021, 46, 35495–35510. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Wang, Y.; Wu, J.; Wang, X. Comparison of the effect of diesel and hydrogen addition on ammonia combustion characteristics in a marine engine. SAE Technical Paper No. 2023-32-0065. 2023. [Google Scholar] [CrossRef]
- Huang, Z.; Lyu, Z.; Luo, P.; Zhang, G.; Ying, W.; Chen, A.; Xiao, H. Effects of Methanol–Ammonia Blending Ratio on Performance and Emission Characteristics of a Compression Ignition Engine. J. Mar. Sci. Eng. 2023, 11, 2388. [Google Scholar] [CrossRef]
- Liu, L.; Tan, F.; Wu, Z.; Wang, Y.; Liu, H. Comparison of the combustion and emission characteristics of NH3/NH4NO2 and NH3/H2 in a two-stroke low speed marine engine. Int. J. Hydrogen Energy 2022, 47, 17778–17787. [Google Scholar] [CrossRef]
- Xu, L.; Bai, X.S. Numerical investigation of engine performance and emission characteristics of an ammonia/hydrogen/n-heptane engine under RCCI operating conditions. Flow Turbul. Combust. 2023, 1–18. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, X.; Cui, L.; Wang, K.; Wang, X.; Zhu, S. Simulation Research on the Injection Strategy of a Diesel-Ammonia Dual-Fuel Marine Engine. Energy Fuels 2023. [Google Scholar] [CrossRef]
- Rehbein, M.C.; Meier, C.; Eilts, P.; Scholl, S. Mixtures of ammonia and organic solvents as alternative fuel for internal combustion engines. Energy Fuels 2019, 33, 10331–10342. [Google Scholar] [CrossRef]
- Mounaïm-Rousselle, C.; Mercier, A.; Brequigny, P.; Dumand, C.; Bouriot, J.; Houillé, S. Performance of ammonia fuel in a spark assisted compression Ignition engine. Int. J. Eng. Res. 2021, 23, 781–792. [Google Scholar] [CrossRef]
- Lee, D.; Song, H.H. Development of combustion strategy for the internal combustion engine fueled by ammonia and its operating characteristics. J. Mech. Sci. Technol. 2018, 32, 1905–1925. [Google Scholar] [CrossRef]
- Scharl, V.; Sattelmayer, T. Ignition and combustion characteristics of diesel piloted ammonia injections. Fuel Communications 2022, 11, 100068. [Google Scholar] [CrossRef]
| Fuel | Experimental characteristics | Main results | Reference |
|---|---|---|---|
| Ammonia/methane mixtures | Measurement of burning velocity and combustion products using an adiabatic flat flame burner. | Addition of ammonia from 0% to 5% by volume in ammonia-methane mixture. The addition of 4% ammonia resulted in a 10% to 20% decrease in burning velocities. Adding ammonia increased NO concentration. | [137] |
| Ammonia/methane mixtures | Premixed and non-premixed counterflow flames: evaluating extinction stretch rate. | Premixed flames under lean conditions were not well predicted for any of the mechanisms studied. However, Okafor’s mechanism accurately predicted the extinction stretch rate of non-premixed flame. | [134] |
| Ammonia/H2 mixtures | Flame stability and pollutant emissions using a porous burner. | Addition of ammonia in the fuel blend reduced the flame stability limits and thermal flame thickness. NO emissions decreased under rich conditions. |
[138] |
| Ammonia | Burning liquid and gaseous ammonia. | Partial dissociation of ammonia was considered necessary for attaining stable combustion in conventional gas turbine burners. | [100] |
| Ammonia/methane; Ammonia/kerosene mixtures |
Microgas turbine firing ammonia (50 kW). | NO emission increased with ammonia addition until a certain limit. | [139,140] |
| Ammonia/methane mixtures | Use of tangential swirl burner. Atmospheric and medium pressure conditions. | Fully premixed injection strategy is not appropriate for optimized ammonia combustion. High flame instabilities can be produced at medium swirl numbers. | [135] |
| Ammonia/methane mixtures | Microgas Turbine (50 kW). | The combustion efficiencies of the NH3–air combustors ranged from 89% to 96%, increasing the efficiency with the increase in power. Addition of ammonia to a methane-based mixture leads to an increase in NO emission. |
[141] |
| Ammonia/methane mixtures | Laboratory-scale swirl burner. | Ammonia addition reduces flame flash back propensity. Good performance in terms of NO emissions is attained only under rich conditions. | [136] |
| Ammonia/methane mixtures | T100 micro gas turbine (reverse burner). Pilot injector (non-premixed swirl flame). Main injector (premixed flame). |
Ammonia addition decreased combustion efficiency. High NOx emissions cancelled any benefit of decarbonization. | [142] |
| Ammonia | Simulation study: bluff body stabilized non-premixed turbulent ammonia (NH3)/air flames using swirling flows. Evaluating the effect of pressure (swirling flames). |
Increasing pressure reduces the availability of OH radicals and therefore, limits NO generation. | [143] |
| Ammonia | Microgas turbine (100 kW) Load range: 40 – 100%. |
Full replacement of natural gas with ammonia was found to reduce electric efficiency by about 0.5 percentage points | [144] |
| Ammonia | Gas turbine with heat regenerator. | The addition of H2 is effective for low NOx combustion with high combustion efficiency. A system of selective catalytic reduction needs to be coupled to the gas turbine system to comply with pollutant emission limits. | [105] |
| Ammonia | Microgas turbine. | Inclined fuel injection and use of swirler. Two-stage (rich-lean) combustion resulted in high efficiency and low NO emission (42 ppmv) NO emission decreased with pressure increase. |
[145] |
| Ammonia | Small-scale Siemens burner used in the SGT-750 gas turbine. Testing ammonia decomposition degree. | Full and partial ammonia decomposition were tested. When applying partial decomposition, rich conditions are required in the primary zone to avoid the presence of O2 close to the burner (minimum NOx obtained at φ of 1.1). Increase in pressure reduces NOx formation. When total ammonia decomposition is attained, low NOx levels were measured but in this case pressure increase favor NOx emissions. |
[146] |
| Fuel | Experimental characteristics | Main results | References |
|---|---|---|---|
| Ammonia: Storage using metal complex and partial ammonia reforming for H2 production | CFR-engine1. Compression ratio: 6.23-13.58 Ammonia/H2 5-100% vol. |
Best performance at a fuel mixture of 10 vol.% H2 with respect to efficiency and power. | [178] |
| Ammonia-H2 mixture | 4-cylinder 4-stroke SI engine, retrofitted to a single-cylinder by fueling only one cylinder (Volume displacement 399.5 cm3). Compression ratio: 10.5:1 1500 rpm |
%H2 addition from 0-60%. Stable operation was achieved for NH3/air stoichiometric condition (0.1-0.12 intake pressure), needing a small amount of H2 to ensure ignitability and stability. Only mixtures with high H2 fraction were suitable for lean operation where efficiency was found to be improved. Mitigation strategies for both NH3 and NOx are required if ammonia is to be considered an acceptable fuel. | [161] |
| Ammonia-H2 mixture | 4-cylinder 4-stroke Gasoline Direct Injection-SI EP6 PSA engine. Engine modified to become indirect injection, single cylinder. | 10% H2 addition was required for operation. The highest levels of NO and N2O emissions were found on the lean side. NH3 emissions were present under rich conditions. EGR2 reduces NOx. | [175] |
| Ammonia-H2 mixture | 2.5 L supercharged LPG3 engine with direct-fuel injection (fuel injection pressures up to 15 MPa). Compression ratio of 10.5 | Stable combustion is impossible under low-load or low-speed operating conditions due to the cooling of the mixture by latent heat and slow combustion. 10% H2 addition improved performance. However, NOx emissions increased as torque increased with a peak at 200 Nm, whereas ammonia showed minimum values at 160 Nm. |
[160] |
| Ammonia-H2 mixture | Port fuel injection (PFI) turbo-charged spark-ignition engine, 1.37 L 4-cylinder with compression ratio of 9.8 | 15% H2 (vol./vol.). The engine operated properly between 1500 and 3000 rpm but could not reach 6000 rpm. Maximum engine torque (about 240 Nm) was delivered at 1500 rpm. |
[166] |
| Ammonia-H2 mixture | Single-cylinder internal combustion engine with a variable compression ratio UIT-85, Volume displacement 652.57 cm3. Compression ratio: 8:1 and 10:1. |
Increasing hydrogen energy content up to 12% in the fuel mixture eliminates ignition instability. | [162] |
| Ammonia-methane mixture | 11 L 6-cylinder heavy-duty turbocharged spark-ignited engine, originally used in city buses using compressed natural gas (CNG) as fuel | Ammonia addition caused a moderate deterioration in the generation of brake work compared with the case of pure natural gas. Combustion efficiency showed a steep deterioration with the increase in ammonia in the fuel mixture at a lambda of 1.5 because the laminar flame speed is extremely slow at this condition, and the flame temperature is also extremely low; thus, quenching will occur earlier. | [177] |
| Ammonia-DME mixture | Single-cylinder spark-ignition research engine with a compression ratio of 10 and a constant rotational speed of 600 rpm. |
DME was added as a component with higher reactivity to attain stable performance, reducing ignition delay time and combustion duration | [176] |
| Ammonia-gasoline mixture | CFR engine: compression ratio of 10:1 and constant speed of 1800 rpm. |
The brake-specific energy consumption (BSEC) with gasoline–ammonia was very similar to that with gasoline alone. |
[179] |
| Ammonia-gasoline (E10) mixture | Externally boosted SI research engine (single cylinder) derivative of the MAHLE Powertrain “DI3” demonstrator engine. Volume displacement 400 cm3 Maximum speed: 6000 rpm Compression ratio: 11.33 and 12.39 φ: 1 |
Increasing the compression ratio from 11.2 to 12.4 allowed the engine to operate with neat ammonia once a warmed-up state was reached. Under all conditions, the indicated thermal efficiency of the engine was either equivalent to or slightly higher than that obtained using gasoline-only due to the favorable anti-knock rating of NH3. (40% efficiency at 1800 rpm/16 bar IMEPn4, a 14% improvement over pure E10) |
[180] |
| Water ammonia solution(WAS)-gasoline mixture | Single cylinder four-stroke air cooled engine (Volume displacement 212 cm3). Maximum output power of 2.5 kW at 3000 rpm. Compression ratio: 8.5:1 |
WAS at 25% ammonia. Mixtures of WAS-gasoline were tested at a range of 5-25%. The engine’s thermal efficiency was improved but emissions performance (CO, NOx, HC5) was not. | [181] |
| Fuel | Experimental characteristics | Main results | References |
|---|---|---|---|
| Ammonia-diesel mixture | John Deere (Model 4045) multi-cylinder turbocharged diesel engine Displacement volume: 4.5 L Compression ratio: 17.0:1 Engine speed: 1000 rpm. Performance tested under constant engine power and variable engine power operation |
NOx emissions are reduced if ammonia accounts for less than 40% of the total fuel energy. CO and HC1 emissions were generally higher with the dual-fuel configuration than those of single diesel fuel use for the same power output. NH3 emission reached 1000-3000 ppmv. Under variable power operation, fuel efficiency was poor. |
[190] |
| Ammonia-diesel mixture | Lifan engine 4 stroke single cylinder. Compression ratio: 16.5:1 Operating conditions: 1200 rpm and full load |
A maximum of 84.2% of input energy can be provided by ammonia. Ammonia reduced CO2 emissions but also produced N2O, which has a 298 times greater GHG2 effect. | [195] |
| Ammonia-biodiesel mixture | Lifan engine 4 stroke single cylinder. Compression ratio: 16.5:1 Operating conditions: 1500 rpm |
Ammonia-/biodiesel-fueled engine had lower BTE3 than pure biodiesel at the same operating point. Increasing ammonia in the mixture changes combustion from diffusion to premixed mode. | [196] |
| Ammonia-DME mixture | Yanmar L70 V single-cylinder, direct-injection diesel engine. Natural aspiration. Displacement volume: 320 cm3 Compression ratio: 20.0:1.0 Engine rated speed: 3600 rpm |
The operating range of the engine is reduced when using ammonia. Adding ammonia to the mixture decreases combustion temperature, producing higher CO and HC emissions but much lower soot emissions. NOx emissions increased due to fuel bond nitrogen. Increasing injection pressure allows the use of mixtures with higher ammonia content. |
[197] |
| Ammonia-DME mixture | Modified Caterpillar 3401 heavy-duty, single-cylinder, four-stroke Compression ratio: 16.25:1 Displacement volume: 2.44 L Engine speed: 910 rpm |
The authors proposed split diesel injection as a strategy for improving combustion performance. Lower GHG emissions were achieved than with diesel-only combustion mode. | [192] |
| Aqueous solution of ammonium hydroxide (NH4OH) solution (28%ammonia)-diesel | Ford Duratorq CD132 130 PS 4-stroke single cylinder Displacement volume: 499.56 cm3 |
Aqueous ammonia contributed a maximum of 25% of the total engine load. Increasing this proportion caused higher CO emissions and cycle-to-cycle variability. | [198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





