1. Introduction
Lipodystrophies (LDs), best defined as lipodystrophic syndromes, are a group of extremely rare and heterogeneous diseases characterized by selective loss of subcutaneous adipose tissue (SAT) in the absence of nutritional deprivation or hyper-catabolic state [
1]. LDs display variable extent of fat loss with altered distribution of body fat [
1,
2]. Non-HIV LDs can be classified into 4 main categories: the congenital generalized lipodystrophy (CGL), the familial partial lipodystrophy (FPLD, predominantly type 1 and 2), the acquired generalized lipodystrophy (AGL) and the acquired partial lipodystrophy (APL). Additional subtypes include progeroid variants (PS) in which lipodystrophy is associated with premature aging [
3]. Subcutaneous fat loss and decreased SAT plasticity are accompanied by an ectopic accumulation of fat leading to metabolic derangements such as insulin resistance, type 2 diabetes, dyslipidemia (mostly hypertriglyceridemia) and fatty liver disease [
4]. With SAT being a dynamic hormone-secreting organ, LDs exhibit an altered adipokine profile. Indeed, leptin is extremely low in subjects with reduced amounts of SAT, such as patients with CGL and AGL [
5,
6], and severe hyperphagia occurs because of leptin deficiency. Serum concentrations of adiponectin are also reduced in LDs and correlate with the extension of fat loss [
6,
7].
Sirtuins (SIRTs) are class III NAD+-dependent histone-deacetylases expressed in relation to fat amount and energy variations linking protein acetylation to metabolism and response to oxidative stress [
8]. In mammals, seven nonredundant SIRTs have been described: SIRT1, SIRT6, and SIRT7 are expressed in the nucleus, SIRT3, SIRT4, and SIRT5 are imported into mitochondria, and SIRT2 is cytoplasmic [
9]. Their different cellular localizations allow the organisms to sense changes in energy status (assessed as NAD
+/NADH levels) in all cellular compartments. Nuclear SIRTs deacetylate histones, thereby modulating gene expression based on cellular metabolic state through epigenetic mechanisms: the main targets are transcription factors and metabolic enzymes that program cells for oxidative metabolism in mitochondria [
8]. In particular, SIRT1 plays a critical role in metabolic health by deacetylating target proteins in several tissues including liver, muscle, adipose tissue, heart and endothelium, and regulates effects on calorie restriction (CR), feeding behavior, body temperature and energy expenditure [
10,
11,
12,
13]. During fasting or energy depletion SIRT1 expression and activity increase, thus promoting SAT lipolysis, increase of glycolytic and gluconeogenic pathways, and muscle and liver fatty acid oxidation. Some of the metabolic actions of SIRT1 are also mediated through the hypothalamus [
14], and others by the adipose tissue; in fact, during fasting and CR, SIRT1 favors fat mobilization from adipose tissue to promote lipid oxidation in the liver and muscle [
15]. Consistently, genetic ablation of SIRT1 in adipose tissue leads to increased adiposity and insulin resistance [
16]. Conversely, excessive energy intake increases Caspase I activity, thus causing cleavage of SIRT1 in adipose tissue [
16].
The main source of serum SIRT1 and the mechanisms responsible for its regulation or secretion from its tissue origin are still unclear [
17]. Interestingly, serum SIRT1 levels were found to be reduced in obesity and increased in anorexia nervosa (AN) [
18] and in patients experiencing weight loss [
19,
20]. To further investigate the relationship between SIRT1 and variations of fat mass, in this study we evaluated the association between SIRT1 serum concentrations and fat amount percentage in patients with different subtypes of LDs compared to non-LDs subjects with different amounts of fat depots.
2. Results
As starting point of our research, we were interested in understanding if, in the whole spectrum of lipodystrophic syndromes encompassing various amounts of adiposity, SIRT1 serum concentrations reflected the amount of fat tissue, expressed as % of fat over the total body mass. No relationship could be documented between fat % and SIRT1 concentrations (
Figure 1), indicating that SIRT1 levels do not display significant differences attributable to varying amounts of fat in different LDs forms.
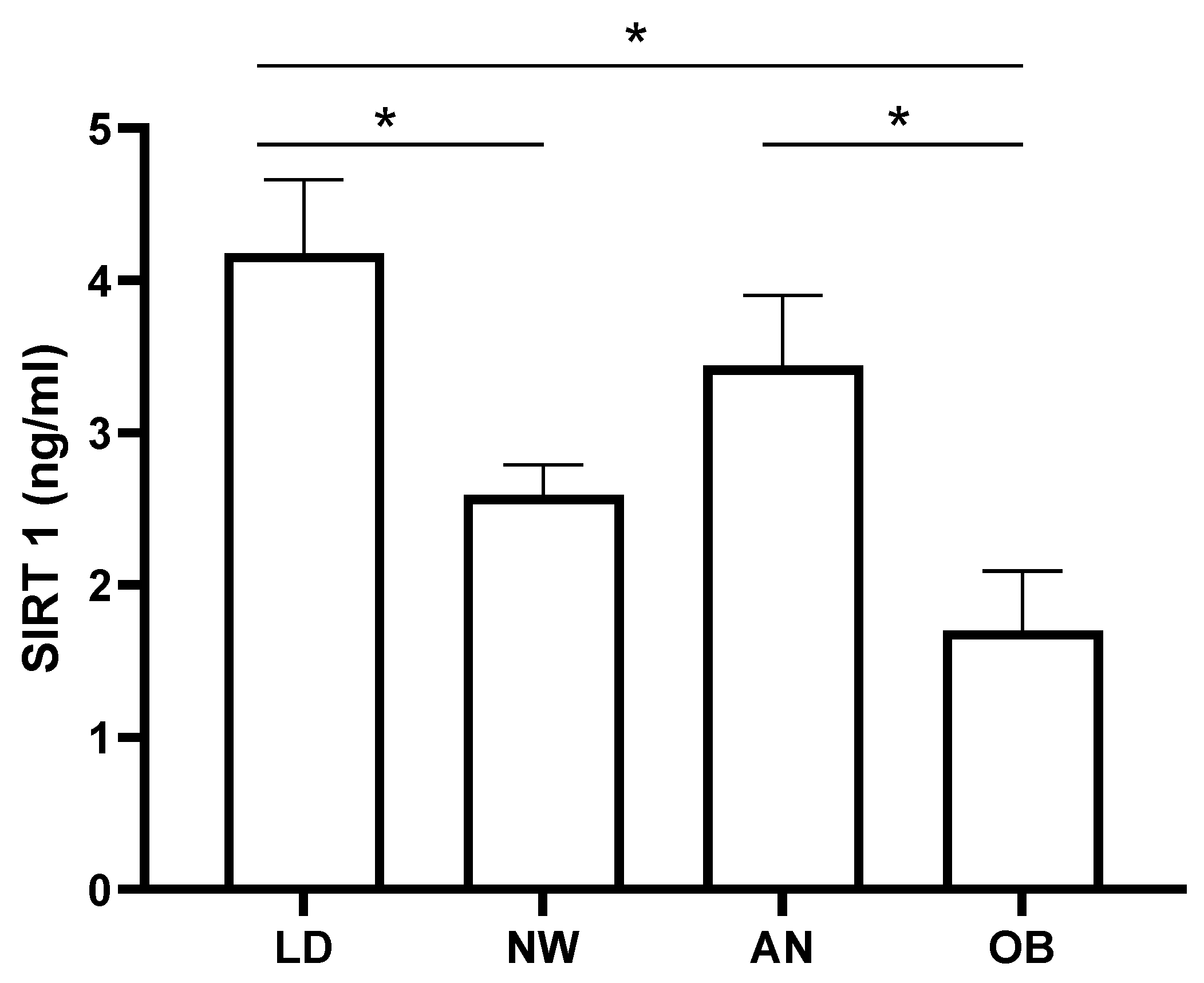
As expected, the comparison of SIRT1 levels in the non-LD study cohorts (
Figure 2) showed that obese patients’ levels were lower than those of the anorectic patients (p < 0.05) and normal weight subjects (p = 0.053), albeit not fully reaching statistical significance in the latter. Interestingly, SIRT1 levels in LDs were significantly higher than those measured in both normal weight and obesity (p < 0.05), while they were not different when compared to anorexia (p = 0.2) (
Figure 2).
3. Discussion
SIRT1 activity is known to regulate fat metabolism, and several SIRT1’s protein substrates are involved in maintaining homeostasis in adipogenesis. This prompted us to investigate the SIRT1 serum levels in lipodystrophic patients.
LDs are fatty tissue disorders characterized by the absence, reduction, or abnormal distribution of adipose tissue. LDs are classified based on their etiology into congenital (and familial) or acquired, and according to the distribution of fat loss, into generalized or partial. Due to its rarity, LDs pass often unrecognized, and a significant diagnostic delay has been reported [
30]. Appropriate LDs management and treatment are essential to improve life expectancy and quality of life: diet and specific pharmacological interventions, as well as follow-up by a multidisciplinary team, are necessary [
21]. Indeed, in LDs patients, lack of SAT is associated with metabolic abnormalities such as insulin resistance and type 2 diabetes mellitus, liver steatosis, hypertriglyceridemia, increased risk of pancreatitis, and coronary heart disease [
1,
31].
In LDs the inadequate subcutaneous fat storage is also associated with impaired adipokines secretion. The decrease of leptin and adiponectin has been extensively described in these disorders [
7,
32] and, depending on fat loss, lower levels of adipokines were found in patients with generalized LDs (CGL and AGL) compared to partial FPLD and APL [
6]. At variance, in this study, we found that SIRT1 levels were substantially homogeneous regardless the heterogeneity of fat amount in different LDs subtypes, and thus no correlation with the amount of fat mass was evident.
Interestingly, LDs and obesity exhibit several commonalities, sharing ectopic fat deposition and overlapping clinical complications [
33,
34]. Low concentrations of SIRT1 have been found in obesity, in both serum and tissues [
18,
35,
36]. The reduction of SIRT1 is also evident in visceral adipose-derived stem cells of obese patients [
37]. Intriguingly, although high levels of SIRT1 are generally indicative of good metabolic health [
38], it must be noted that this does not apply to patients with LDs who, as the ones of this cohort, are subject to metabolic derangements and severe lipotoxicity [
39].
We remark that our findings, instead of suggesting a relationship between metabolic derangements and SIRT1 levels, rather seem to point at a possible gradient of serum concentrations characterized by lower SIRT1 in obesity, intermediate in normal weight subjects, and higher in states of lower adiposity such as AN and LDs.
We have to acknowledge that our research has many limitations: 1) the low number of subjects enrolled and the heterogeneity of LDs patients; 2) the lack of in-depth metabolic characterization of our patient cohort; 3) the limited knowledge on the possible relationships between serum SIRT1 levels and their tissue expression, activity and origin.
In summary, serum SIRT1 levels were within a physiological range across all forms of LDs but didn’t reflect the heterogeneous amount of fat. The mechanisms responsible for secretion and regulation of SIRT1 from adipose tissue in LD need further studies, and disease-dedicated registries may offer this possibility [
40].
4. Materials and Methods
4.1. Participants
The study was conducted in 32 patients with LD, 24 patients affected by AN, 22 normal weight healthy subjects and 24 patients with obesity.
The diagnosis of LD was made based on accepted criteria [
21] and after exclusion of other causes of fat loss. Clinical data were obtained from the participants’ electronic medical records. Four patients were diagnosed with GL, including 3 patients affected by Berardinelli-Seip Syndrome confirmed by genetic testing documenting biallelic mutations of the 1-acylglycerol-3-phosphate O-acyltransferase 2 (
AGPAT2) gene, and 1 patient affected by acquired generalized LD (Lawrence Syndrome). Five patients were affected by PS: 4 with a genetic testing positive for a heterozygous mutation of the lamin A/C (
LMNA) gene - of which one with a p.R133L [
22] and three with a p.R349W mutation [
23] - the fifth patient with the heterozygous mutation p.R507C of the DNA polimerase delta 1 (
POLD1) gene [
24]. Six patients were affected by FPLD1 (Kobberling Syndrome) diagnosed based on accepted criteria [
25] and after testing negative for mutations involved in genetic forms of LD. Seven patients were affected by FPLD2 (Dunnigan Syndrome), with genetic testing confirming the pathogenic
LMNA missense variant p.R482W/Q. Ten patients were affected by APL: 6 of them had a Barraquer-Simons Syndrome [
26] and 4 had autoimmune-mediated subtypes of APL, one of whom underwent total body irradiation and hematopoietic stem cell transplant during childhood [
27,
28].
Patients affected by LDs were recruited at the Obesity and Lipodystrophy Center of the University Hospital of Pisa, Italy. Participants with AN, healthy normal weight and obesity were recruited at the Day Hospital of the Department of Experimental Medicine, Policlinico Umberto I, Sapienza University of Rome, Italy. The diagnosis of AN was made based on the Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5) and after exclusion of other causes of fat loss [
29].
The reduction of SAT and its altered distribution in patients with LDs, responsible for the onset and development of metabolic complications, led to disorders of varying severity requiring pharmacological intervention for most of the patients. Indeed, 65% of LDs patients (21/32) had diabetes or impaired glucose tolerance and used glucose lowering agents (metformin, thiazolidinediones, glinides and/or liraglutide based on specific patient characteristics), and 5 of them were also on insulin treatment. Further, 81% of patients (26/32) had dyslipidemia, 15 of whom (58%) had isolated hypertriglyceridemia, 3 (11%) isolated hypercholesterolemia, and 8 (31%) the combined hyperlipidemia. Dyslipidemic patients used specific lipid-lowering agents (statins and/or ezetimibe or cholestyramine; omega-3 polyunsaturated fatty acids and/or fibrates). Three of the LD patients were receiving pharmacological treatment with metreleptin. Hepatic steatosis was documented in 30 out of 32 patients (93%).
The study was conducted according to the Declaration of Helsinki and all participants provided written informed consent for participation in the study and for the publication of their clinical and biochemical information.
4.2. Anthropometric Measurements
Height and body weight were measured by standard procedures to calculate BMI (kg/m2) as the ratio between weight (kg) and square of height (m2). Obesity was defined following WHO criteria when BMI was > 30. Total and segmental body fat in the trunk and upper and lower extremities was evaluated by whole-body DEXA (Hologic, Discovery A, S/N 84551) in 29 lipodystrophic patients. The adiposity was expressed as the percent of body fat mass (%) over the total mass.
4.3. Biochemistry
Circulating SIRT1 was determined by a commercially available human ELISA kit (MyBioSource, San Diego, CA, USA) after a 12 h fasting.
4.4. Statistical Analysis
Data are expressed as the mean ± standard error (SEM) values. Simple linear regression analysis and unpaired t-tests were used. All the graphs, calculations, and statistical analyses were performed using GraphPad Prism version 8.4.3. A two-sided p value of 0.05 was the criterion for statistical significance.
5. Conclusions
SIRT1 is a nuclear master regulator of energy homeostasis and its concentrations, measured in serum, are reduced in obesity and increased in AN. SIRT1 serum levels in LDs did not reflect the amount of body fat and were higher than in normal weight subjects and obesity, and comparable to those measured in AN. Further investigation into the possible involvement of SIRT1 in the pathophysiology of LDs is valuable.
Author Contributions
Conceptualization, S.M. (Stefania Mariani); investigation, S.M. (Silvia Magno), R.T., S.C., C.P.; formal analysis, L.S., G.C., S.M. (Silvia Magno), R.T., S.M. (Stefania Mariani); writing—original draft preparation, L.S., S.M. (Silvia Magno), S.M. (Stefania Mariani); writing—review and editing, L.S., G.C., S.M. (Silvia Magno), F.S., L.G., S.M. (Stefania Mariani); funding acquisition, G.C., S.M. (Stefania Mariani). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of University and Research Grants PRIN 2020NCKXBR (to G.C. and L.G.), entitled ‘Suscettibilità alle malattie infettive nell’obesità: una valutazione endocrina, traslazionale e sociologica (SIDERALE)’, and by the Sapienza University of Rome, Ricerca di Ateneo 2022 n. RM122181681F2246 (to S.M., Stefania Mariani), entitled ‘A translational pilot study evaluating the acute effects of different macronutrient compositions on circulating Sirtuin-1, adiposity indices, incretin responses and brown adipose tissue activation: a possible role for ketone bodies’.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of “Sapienza” University of Rome, Policlinico Umberto I (ref. EC 5475).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All datasets generated and/or analyzed during this study are not publicly available but are available from the corresponding author.
Acknowledgments
The authors thank patients for their availability to participate in this report. The Obesity and Lipodystrophy Center at the University Hospital of Pisa is part of the European Consortium of Lipodystrophies, the European Reference Network for Rare Endocrine Conditions (EndoERN Project ID No 739572) and European Reference Network for Rare Hereditary Metabolic Disorders (MetabERN-Project ID No 739543). EndoERN is co-funded by the European Union within the framework of the 3rd Health Program. EndoERN is supported by the European Society of Endocrinology and the European Society for Pediatric Endocrinology.
Conflicts of Interest
GC has received fees for consulting and/or received travel funds or participated in studies from the following companies, which are involved with lipodystrophy and/or diabetes: Aegerion/Amryt Pharmaceuticals, Novo-Nordisk, and Rhythm Pharmaceuticals. SM (Silvia Magno) and CP received travel funds from the following company, which was involved with lipodystrophy: Amryt Pharmaceuticals. FS has worked as a consultant, participated in studies, and/or received travel funds from the following companies, which are involved with lipodystrophy and/or diabetes: Aegerion/Amryt, Novo Nordisk, Lilly, Bruno Pharma, and Pfizer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Araújo-Vilar, D.; Santini, F. Diagnosis and treatment of lipodystrophy: A step-by-step approach. J. Endocrinol. Invest. 2019, 42, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Garg, A. Lipodystrophies. Am. J. Med. 2000, 108, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Vilar, D.; Fernández-Pombo, A.; Cobelo-Gómez, S.; Castro, AI.; Sánchez-Iglesias, S. Lipodystrophy-associated progeroid syndromes. Hormones 2022, 21, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Akinci, G.; Celik, M.; Akinci, B. Complications of lipodystrophy syndromes. Presse. Med. 2021, 50, 104085. [Google Scholar] [CrossRef] [PubMed]
- Pardini, V.C.; Victória, I.M.; Rocha, S.M.; Andrade, D.G.; Rocha, A.M.; Pieroni, F.B.; Milagres, G.; Purisch, S.; Velho, G. Leptin levels, beta-cell function, and insulin sensitivity in families with congenital and acquired generalized lipoatropic diabetes. J. Clin. Endocrinol. Metab. 1998, 83, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Haque, W.A.; Shimomura, I.; Matsuzawa, Y.; Garg, A. Serum adiponectin and leptin levels in patients with lipodystrophies. J. Clin. Endocrinol. Metab. 2002, 87, 2395. [Google Scholar] [CrossRef]
- Ceccarini, G.; Pelosini, C.; Paoli, M.; Tyutyusheva, N.; Magno, S.; Gilio, D.; Palladino, L.; Sessa, M.R.; Bertelloni, S.; Santini, F. Serum Levels of Adiponectin Differentiate Generalized Lipodystrophies from Anorexia Nervosa. J. Endocrinol. Invest. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Guarente, L. Sirtuins, aging, and metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 81–90. [Google Scholar] [CrossRef]
- Finkel, T.; Deng, C.X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591. [Google Scholar] [CrossRef]
- Guarente, L. Calorie restriction and sirtuins revisited. Genes Dev. 2013, 27, 2072–2085. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, Y. The Essential Role of SIRT1 in Hypothalamic-Pituitary Axis. Front. Endocrinol. 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Guarente, L. Sirtuins at a glance. J. Cell. Sci. 2011, 124, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, R.; Cipriani, F.; Masi, D.; Basciani, S.; Watanabe, M.; Lubrano, C.; Gnessi, L.; Mariani, S. Ketone Bodies and SIRT1, Synergic Epigenetic Regulators for Metabolic Health: A Narrative Review. Nutrients 2022, 14, 3145. [Google Scholar] [CrossRef]
- Cakir, I.; Perello, M.; Lansari, O.; Messier, N.J.; Vaslet, C.A.; Nillni, E.A. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS ONE. 2009, 4, e8322. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; Machado De Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing ppargamma. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; and Guarente, L. “High-Fat Diet Triggers Inflammation-Induced Cleavage of SIRT1 in Adipose Tissue to Promote Metabolic Dysfunction. ” Cell Metab. 2012, 16, 180–188. [Google Scholar] [CrossRef]
- Boutant, M.; Cantó, C. SIRT1 metabolic actions: Integrating recent advances from mouse models. Mol. Metab. 2013, 3, 5–18. [Google Scholar] [CrossRef]
- Mariani, S.; di Giorgio, M.R.; Martini, P.; Persichetti, A.; Barbaro, G.; Basciani, S.; Contini, S.; Poggiogalle, E.; Sarnicola, A.; Genco, A.; Lubrano, C.; Rosano, A.; Donini, L.M.; Lenzi, A.; Gnessi, L. Inverse Association of Circulating SIRT1 and Adiposity: A Study on Underweight, Normal Weight, and Obese Patients. Front. Endocrinol. 2018, 9, 449. [Google Scholar] [CrossRef]
- Mansur, A.P.; Roggerio, A.; Goes, M.F.S.; Avakian, S.D.; Leal, D.P.; Maranhão, R.C.; Strunz, C.M.C. Serum concentrations and gene expression of sirtuin 1 in healthy and slightly overweight subjects after caloric restriction or resveratrol supplementation: A randomized trial. Int. J. Cardiol. 2017, 227, 788–794. [Google Scholar] [CrossRef]
- Mariani, S.; Fiore, D.; Persichetti, A.; Basciani, S.; Lubrano, C.; Poggiogalle, E.; Genco, A.; Donini, L.M.; Gnessi, L. Circulating SIRT1 Increases After Intragastric Balloon Fat Loss in Obese Patients. Obes. Surg. 2016, 26, 1215–1220. [Google Scholar] [CrossRef]
- Brown, R.J.; Araujo-Vilar, D.; Cheung, P.T.; Dunger, D.; Garg, A.; Jack, M.; Mungai, L.; Oral, E.A.; Patni, N.; Rother, K.I.; von Schnurbein, J.; Sorkina, E.; Stanley, T.; Vigouroux, C.; Wabitsch, M.; Williams, R.; Yorifuji, T. The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 4500–4511. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lee, L.; Kudlow, B.A.; Dos Santos, H.G.; Sletvold, O.; Shafeghati, Y.; Botha, E.G.; Garg, A.; Hanson, N.B.; Martin, G.M.; Mian, I.S.; Kennedy, B.K.; Oshima, J. LMNA mutations in atypical Werner's syndrome. Lancet 2003, 362, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Magno, S.; Ceccarini, G.; Pelosini, C.; Ferrari, F.; Prodam, F.; Gilio, D.; Maffei, M.; Sessa, M.R.; Barison, A.; Ciccarone, A.; Emdin, M.; Aimaretti, G.; Santini, F. Atypical Progeroid Syndrome and Partial Lipodystrophy Due to LMNA Gene p.R349W Mutation. J. Endocr. Soc. 2020, 4, bvaa108. [Google Scholar] [CrossRef] [PubMed]
- Pelosini, C.; Martinelli, S.; Ceccarini, G.; Magno, S.; Barone, I.; Basolo, A.; Fierabracci, P.; Vitti, P.; Maffei, M.; Santini, F. Identification of a novel mutation in the polymerase delta 1 (POLD1) gene in a lipodystrophic patient affected by mandibular hypoplasia, deafness, progeroid features (MDPL) syndrome. Metabolism 2014, 63, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Guillín-Amarelle, C.; Sánchez-Iglesias, S.; Castro-Pais, A.; Rodriguez-Cañete, L.; Ordóñez-Mayán, L.; Pazos, M.; González-Méndez, B.; Rodríguez-García, S.; Casanueva, F.F.; Fernández-Marmiesse, A.; Araújo-Vilar, D. Type 1 familial partial lipodystrophy: Understanding the Köbberling syndrome. Endocrine 2016, 54, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Magno, S.; Ceccarini, G.; Corvillo, F.; Pelosini, C.; Gilio, D.; Paoli, M.; Fornaciari, S.; Pandolfo, G.; Sanchez-Iglesias, S.; Nozal, P.; Curcio, M.; Sessa, M.R.; López-Trascasa, M.; Araújo-Vilar, D.; Santini, F. Clinical characteristics of patients with acquired partial lipodystrophy: A multicenter retrospective study. J. Clin. Endocrinol. Metab. 2023, dgad700. [Google Scholar] [CrossRef]
- Ceccarini, G.; Ferrari, F.; Santini, F. Acquired partial lipodystrophy after bone marrow transplant during childhood: A novel syndrome to be added to the disease classification list. J. Endocrinol. Invest. 2017, 40, 1273–1274. [Google Scholar] [CrossRef]
- Tews, D.; Schulz, A.; Denzer, C.; von Schnurbein, J.; Ceccarini, G.; Debatin, K.M.; Wabitsch, M. Lipodystrophy as a Late Effect after Stem Cell Transplantation. J. Clin. Med. 2021, 10, 1559. [Google Scholar] [CrossRef]
- Lindvall Dahlgren, C.; Wisting, L.; Rø, Ø. Feeding and eating disorders in the DSM-5 era: A systematic review of prevalence rates in non-clinical male and female samples. J. Eat. Disord. 2017, 5, 56. [Google Scholar] [CrossRef]
- Akinci, B.; Oral, E.A.; Neidert, A.; Rus, D.; Cheng, W.Y.; Thompson-Leduc, P.; Cheung, H.C.; Bradt, P.; Foss de Freitas, M.C.; Montenegro, R.M.; Fernandes, V.O.; Cochran, E.; Brown, R.J. ; Comorbidities and Survival in Patients with Lipodystrophy: An International Chart Review Study. J. Clin. Endocrinol. Metab. 2019, 104, 5120–5135. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lun, M.; Wang, M.; Senyo, S.E.; Guillermier, C.; Patwari, P.; and Steinhauser, M.L. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014, 20, 1049–1058. [Google Scholar] [CrossRef]
- Akinci, B.; Meral, R.; Oral, E.A. Phenotypic and Genetic Characteristics of Lipodystrophy: Pathophysiology, Metabolic Abnormalities, and Comorbidities. Curr. Diab. Rep. 2018, 18, 143. [Google Scholar] [CrossRef]
- Chehab, F.F. Obesity and lipodystrophy--where do the circles intersect? Endocrinology 2008, 149, 925–934. [Google Scholar] [CrossRef]
- Lim, K.; Haider, A.; Adams, C.; Sleigh, A.; Savage, D.B. Lipodistrophy: A paradigm for understanding the consequences of "overloading" adipose tissue. Physiol. Rev. 2021, 101, 907–993. [Google Scholar] [CrossRef]
- Pardo, P.S.; Boriek, A.M. SIRT1 Regulation in Ageing and Obesity. Mech. Ageing Dev. 2020, 188, 111249. [Google Scholar] [CrossRef] [PubMed]
- Perrini, S.; Porro, S.; Nigro, P.; Cignarelli, A.; Caccioppoli, C.; Genchi, V.A.; Martines, G.; De Fazio, M.; Capuano, P.; Natalicchio, A.; Laviola, L.; Giorgino, F. Reduced SIRT1 and SIRT2 expression promotes adipogenesis of human visceral adipose stem cells and associates with accumulation of visceral fat in human obesity. Int. J. Obes. 2020, 44, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Di Rocco, G.; Toietta, G.; Russo, M.A.; Petrangeli, E.; Salvatori, L. Sirtuins 1-7 expression in human adipose-derived stem cells from subcutaneous and visceral fat depots: Influence of obesity and hypoxia. Endocrine. 2017, 57, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Li, X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013, 45, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Vigouroux, C.; Caron-Debarle, M.; Le Dour, C.; Magré, J.; Capeau, J. Molecular mechanisms of human lipodystrophies: From adipocyte lipid droplet to oxidative stress and lipotoxicity. Int J Biochem Cell Biol. 2011, 43, 862–876. [Google Scholar] [CrossRef] [PubMed]
- von Schnurbein, J.; Adams, C.; Akinci, B.; Ceccarini, G.; D'Apice, M.R.; Gambineri, A.; Hennekam, R.C.M.; Jeru, I.; Lattanzi, G.; Miehle, K.; Nagel, G.; Novelli, G.; Santini, F.; Santos Silva, E.; Savage, D.B.; Sbraccia, P.; Schaaf, J.; Sorkina, E.; Tanteles, G.; Vantyghem, M.C.; Vatier, C.; Vigouroux, C.; Vorona, E.; Araújo-Vilar, D.; Wabitsch, M. European lipodystrophy registry: Background and structure. Orphanet, J. Rare Dis. 2020, 15, 17. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).