Introduction
In recent years, exploration and use of natural bioactive compounds have gained considerable interest account for natural product is one of the most important sources in various fields such as cosmetics, food, and pharmaceutical industries [
1,
2]. Phenolic compounds are a heterogeneous group of secondary metabolites widely distributed in plants among the bioactive phytochemicals [
3]. They are acknowledged for their therapeutic properties and health benefits, including anti-oxidant, anti-bacterial, anti-obesity, anti-diabetic activities, and so on [
4]. As for their numerous biological attributes, the antioxidant characteristics of phenolic compounds are responsible for a significant portion of the protective effects and are related to their preventive role in various oxidative stress-related diseases like metabolic diseases, cancer, and other diseases [
5]. Phenolic-rich plant extracts have become a commercial ingredient in food and nutritional products [
6]. It is necessary to take them out of the raw material matrix to use them as medicinal agents or natural antioxidants. However, the solubility and separation properties of phenolics are affected by the differences in their nature and chemical structure and the presence of interfering compounds, thus phenolic compounds are often extracted from plant materials in crude forms [
7]. Therefore, there is a resurgence of interest in the development of efficient, green, and safe extraction processes of phenolic compounds, which will greatly improve their economic applications.
Extraction is the first and essential step in the processes (Isolation and Purification) of bioactive components from plant materials. The traditional extraction methods applied for phenols recovery from plants include maceration, reflux, and soxhlet extraction which are widely used today. Yet these techniques exhibit some specific drawbacks, for example, time-consuming, low extraction selectivity, and thermolabile compounds may decompose when high temperature is used [
8]. What’s more, the conventional organic extraction solvents commonly used in these methods are flammable, non-degradable, or/and toxic. Their use is recognized to be detrimental to the environment and human health, and the extracts must still be processed before subsequent use [
9,
10]. With the development of the "green chemistry" concept, it is necessary to replace traditional organic solvents with eco-friendly and safe solvents[
11,
12]. Although water is recognized as the greenest and most harmless solvent, its efficiency in extracting bioactive substances from plants is very limited. The large amount of solvent is difficult to evaporate and concentrate, the extremely high temperature destroys the activity, and the extract's complex composition necessitates further purification through columns [
13,
14]. Therefore, green solvents, such as ionic liquids (ILs), deep eutectic solvents (DESs), and supercritical fluids like CO2, have been gradually investigated for the extraction and separation of natural compounds [15-17]. However, each of these solvents has its advantages and disadvantages. As for ionic liquids, they are stand out due to their numerous composition options and capacity to modify the polarity of the target extract through changing solvent compositions, ranging from dipolar non-hydrogen-bonding solvents (DMF, DMSO, acetonitrile) to polar hydrogen-bonding solvents (primary alcohols, water). Regrettably, the similar toxicity to organic solvents and the low biodegradability makes the "green" character of ILs questionable [
18]. Subsequently, DESs has been pioneered as a possible alternative to ILs. They have a greater extraction efficiency for polar chemicals because of the creating intramolecular hydrogen bonds. Low preparation cost, low toxicity, and biodegradability also make DESs superior to conventional solvents [
19,
20]. The high viscosity of DESs at room temperature is actually one of their main drawbacks, hindering the transfer of solutes of interest and limiting their use in large-scale industry [
21]. In addition, they may carry other biochemicals (e.g., proteins, polar lipids, sugar residues, cellulose, etc.) during the extraction process [
22]. Supercritical fluids have a gas-like low viscosity and high diffusivity, and when used as solvents, can easily penetrate plant materials with fast mass transfer rates, but the main disadvantage of using CO2 as a solvent in the SCF state is that it is only applicable to pro-CO2 molecules, making it suitable only for small non-polar molecules since CO2 is inherently non-polar [
23].
Recently, the use of green and safe supramolecular solvents (SUPRAS) based on alkanols/alkanoic acids has been encouraged to reverse the drawbacks of the existing methods [
24]. Nanostructured liquids known as SUPRAS are formed automatically by colloidal suspensions of amphiphilic molecules through the processes of self-assembly and coalescence. Compared to molecular solvents, SUPRAS has unique properties that give it particular potential to replace organic solvents in the pre-treatment of analyses and the extraction of bioactive substances. At first, the molecules constituting the ordered structure in SUPRAS include both hydrophilic and hydrophobic parts with regions of different polarities, providing a wide variety of interactions for the analytes (e.g. π–π, dispersion, etc. in the nonpolar region and ionic, dipole-dipole, hydrogen bonding, etc. in the polar region) [
25]. Furthermore, the high concentration of amphiphiles in SUPRAS and the large surface area provides a stable extraction environment as well as rapid mass transfer capabilities in the SUPRAS-based extraction processes. In addition, the structure and properties of the SUPRAS can be modified by altering the type of amphiphilic molecules and the cohesive environment[
26]. Thus, various kinds of SUPRAS can be designed according to the different properties of the extracted target compounds and it can also exclude other components such as protein molecules and carbohydrates in the experiments [
25]. It is equally important that SUPRAS extracts are safe and can be conveniently used in nutraceuticals, cosmeceuticals, and other industries [
27].
SUPRAS has mainly been applied for sample preparation and detection of trace substances, such as cadmium or bisphenols, in food and environmental samples [
28,
29]. However, they also have promising applications in the extraction of bioactive compounds from plants, especially because their safe and green properties are compatible with green fields such as food[
30]. The structural characteristics and formation mechanisms of the SUPRAS have also been intensively studied, but the interaction-microstructure characterization of SUPRAS and the target compounds, and the molecular mechanism of extraction efficiency are still unclear and deserve further consideration [
31].
In this study, the prepared octanoic acid-ethanol-water-based SUPRAS was applied to extract the main phenolic acids (caffeic acid, salviaflaside, and rosmarinic acid) from Prunella vulgaris, a medicinal and dietary plant. Dependent on the yields of the target chemicals, a single factor was applied to optimize the conditions of SUPRAS extraction. Then, the extraction efficiency and antioxidant activity of SUPRAS extracts were compared with the conventional methods. In particular, to reveal the unique properties of SUPRAS, confocal laser microscopy was used to observe its microstructure and phenolic acid distribution after extraction, while molecular dynamics simulation was applied to analyze the intermolecular interactions between the extracted compounds and the extractants, which provided theoretical proof of its excellent extraction ability. Experiments and theories were combined to demonstrate the potential of SUPRAS for the application of effective and green extraction of active ingredients.
2. Material and Methods
2.1. Chemical Reagents
n-Octanol (AR, 99%), n-Octanoic acid (AR, 99.0%), n-Decanol (98%), and n-decanoic acid (AR, 99%) were purchased from Macklin Biochemical Co. Ltd, (Shanghai, China). Absolute ethanol was supplied by Sinopharm Chemical Reagent Co, Ltd (Shanghai, China). 2-aminoethyl diphenylborinate (2-APB, 97%) was from Sigma Aldrich, Merck, Germany. Reference standards including caffeic acid and rosmarinic acid (all purity≥98%) were obtained from Chengdu DesiTe Biological Technology Co. Ltd. (China), and the salviaflaside standard was prepared by the laboratory of Hunan University of Chinese Medicine. Methanol (chromatographic grade) was acquired from TEDIA, USA. Purified water was made by a Mili-Q water-purification system (Millipore, USA). All other reagents were of analytical quality and available commercially.
2.2. Pretreatment of Prunella vulgaris
Prunella vulgaris (PV) was purchased from GaoQiao Natural Herbal Special Market in Hunan Province, China, and identified as the dried fruit spikes of PV at Hunan University of Chinese Medicine. The herb samples were dried to constant weight, shattered, sieved through 40 mesh screens, and stored in the desiccator before subsequent experiments.
2.3. SUPRAS Synthesis and Characterization
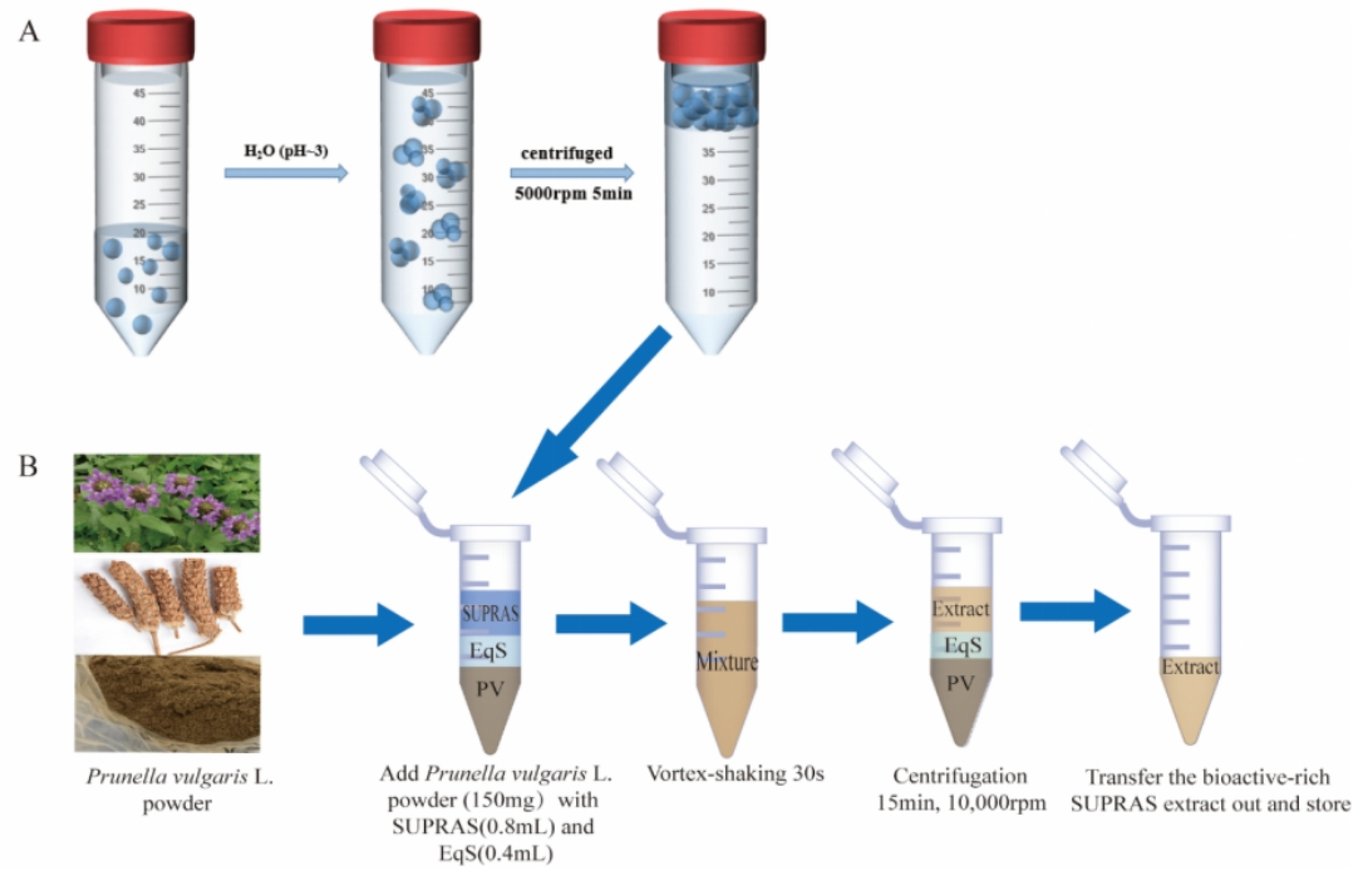
A range of SUPRAS was generated by dissolving long-chain alkanols/ alkanoic acids in different percentages of ethanol and then adding water (pH ~3) to induce the formation of the amphiphile aggregates. The total volume of the ternary mixture was kept at 50mL. The mixtures were shaken on a vortex and then centrifuged (TGL-20MB high-speed refrigerated centrifuge, Changsha Xiangzhi Centrifuge Instruments Co., Ltd.) for 10 min at 5000 rpm. After the centrifugation, a new liquid upper phase known as SUPRAS was separated in equilibrium with the bulk solution. Two phases (SUPRAS and equilibrium solution, EqS) were separately gathered and kept at room temperature in closed glass containers until use (~20-25℃, within one week). The production schematic for SUPRAS was shown in
Figure 1.
The composition of SUPRAS depended on the content of amphiphiles and the ratio of ethanol to water. To further understand the properties of SUPRAS, it was necessary to determine the composition of SUPRAS. The content of amphiphiles, ethanol, and water in the SUPRAS was measured with weight percentages (w/w, %). The alcohol meter was used to calculate the ethanol percentage. The amphiphiles content was weighed after ethanol and water were evaporated and the water content was determined by weight difference.
2.4. SUPRAS-Based Extraction and Optimization of Phenolic Compounds from PV
The entire extraction procedure of phenolic acid was shown in
Figure 1. Extractions were done in a 2.0 mL microfuge tube by mixing 150.00mg of crushed PV with 0.4 mL SUPRAS and corresponding EqS 0.8mL. The different types of SUPRAS were formerly produced as the method described in
Section 2.3. Next, the mixtures were vortexed at 3000 rpm for 30 s and ultrasonically extracted for 10 min. Then, the mixtures were centrifuged at 10,000 rpm for 10 min to make sure the residues were entirely removed from the solvents. Lastly, the liquid supernatants were collected and diluted with absolute methanol before HPLC analysis.
The extraction yields of caffeic acid, salviaflaside, and rosmarinic acid from PV were chosen as standards for valorization. Through one-factor-at-a-time experiments, variables of different solvent compositions and extraction conditions were optimized. Firstly, the impact of the amphiphiles (octanol, octanoic acid, decanol, or decanoic acid) was investigated and the SUPRAS with the highest yield of the target compounds was selected. Next, the ethanol concentration (from 20% to 35%, v/v) and the pH (from 1 to 5) for SUPRAS formation were studied. Furthermore, the influence of the ratios of SUPRAS: EqS (v/v, from 1:2 to 1), the volume of extraction solvent (from 0.6 to 1.8mL), and ultrasonic time (from 5 to 45min) were evaluated to obtain the best extraction yield. The whole experiments were conducted at room temperature (~25℃). Likewise, the ultrasonic-assisted traditional organic reagent (ethanol) extraction method, methanol reflux extraction method, and DES-based extraction method were also used for the extraction of PV under optimal conditions, which provide a reference and comparison for the SUPRAS extraction process.
2.5. Determination of Phenolic Compounds by High-Performance Liquid Chromatography (HPLC)
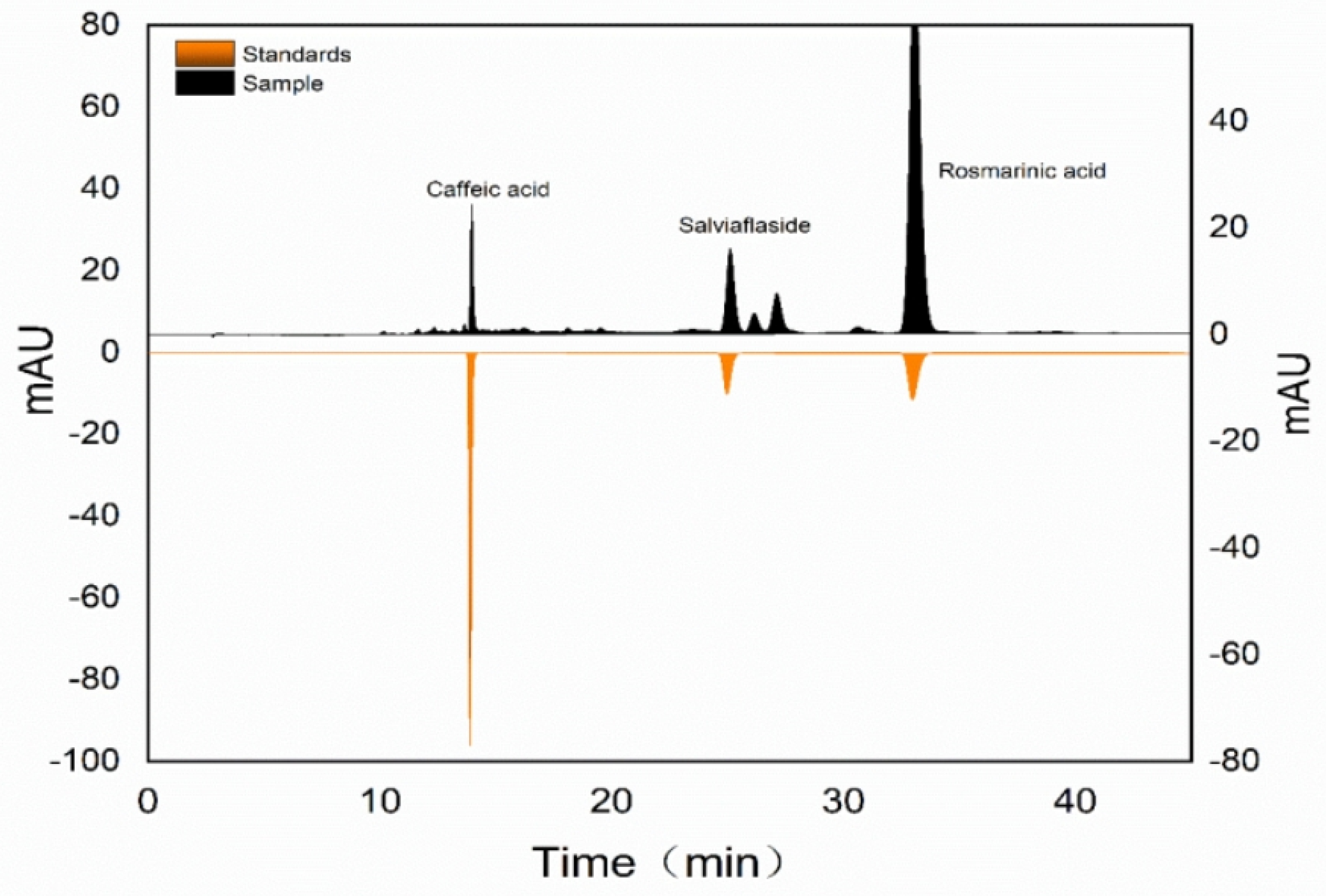
The qualitative and quantitative analysis of phenolic compounds was performed by an Agilent 1260 reversed-phase HPLC system with a ZORBAX SB-C18 column (4.6 × 250 mm, 5.0 μm). And the retention time of three standards was compared with the target compounds to identify them (
Figure 2.). The mobile phase A was 0.1% formic acid in water and B was methanol. The column temperature was kept at 25℃, the flow rate was set at 1.0 mL·min
-1 and the injection volume was 10μL. 330 nm was selected as the optimal detection wavelength for phenolic acids. The gradient elution conditions were as follows: 0-10min, 5-35% B; 10-40min, 35-37% B; 40-45min, 37% B. All samples were filtered with 0.22 μm before HPLC analysis.
Related studies have shown that the alkyl carboxylic acids used for the preparation of SUPRAS as extractants did not affect the quality detection of the analytes on the HPLC analysis [
32]. Therefore, the use of HPLC is reliable for the analysis of the phenolic compounds from PV in this study.
2.6. Antioxidant Activity Assays of Different Extraction Methods
The antioxidant activities of extracts obtained under the optimal SUPRAS conditions and other methods were evaluated by DPPH and ABTS•+ methods. The half-maximal inhibitory concentration (IC50) was calculated for comparison [
33].
2.6.1. DPPH Radical Scavenging Activity Assay
The DPPH radical-scavenging activity of the extracts was studied using the DPPH spectrophotometric method described in a previous study with some modifications (Chen, et al., 2018). Samples prepared by SUPRAS and other extraction methods were diluted to five concentrations. 1.0 mL of DPPH solution (0.09%, w/v, with methanol) was added to the sample solution (0.25mL). After incubating at 25℃ for 30min without light, the absorbance was tested at 517nm. The DPPH solutions were replaced by 1.0 mL of methanol as blank, and the control group did not contain any antioxidants. The formula for DPPH radical scavenging effect was as follows:
DPPH radical scavenging effect (%) = [Acontrol-(Asample-Ablank)/Acontrol] ×100%
2.6.2. ABTS•+ Radical Scavenging Activity Assay
ABTS•+ radical scavenging activity was measured according to the total antioxidant capacity test kit (ABTS•+ rapid method). Samples prepared by SUPRAS and other extraction methods were diluted into five concentrations and then measured according to the instructions. After the mixtures were gently shaken and reacted at 25℃ for 6 min, the absorbance values were measured at 414 nm using an enzyme-labeled instrument (BioTek Synergy Multifunctional Microplate Assay, Hunan Zidonglai Biotechnology Co., Ltd., Hunan China). The antioxidant capabilities of the extracts are represented as IC50. The ABTS•+ radical scavenging effects were evaluated with the formula:
ABTS•+ radical scavenging effect (%) = [(Ablank-Asample)/Ablank] ×100%
2.7. Interaction-Microstructure Characterization of SUPRAS Droplets and Target Compounds by Confocal Laser Scanning Microscopy (CLSM)
To further validate and observe the droplet microstructure specific to SUPRAS and the interaction with the target phenolic acid compounds, we performed the analysis with confocal laser excitation microscopy (CLSM). The extracts obtained by different extraction methods were diluted with anhydrous methanol to the same concentration as the extracts of SUPRAS. All samples were stained for 15 minutes with 0.25% (w/v) 2-aminoethyl diphenylborinate (2-APB, dissolved in anhydrous methanol) at the ratio of 1:1(v/v) and then centrifuged at 10,000 rpm for 10 minutes to remove impurities. The entire operation was kept out of the light. 5 μl of the 2-APB mixtures was dropped onto a microscope slide, covered with a 22×22 mm coverslip, and sealed. Fluorescence images of CLSM were acquired at excitation wavelengths of 405 and 488 nm and emission wavelengths of 450 and 525 nm, respectively, to observe the extraction conditions of phenolic acids and their positional distribution. Also, images under white light conditions were acquired to illustrate the microstructure of SUPRAS droplets. Identical settings and consistent parameters were used for all images and channels used for fluorescence. The fluorescence images were compared with the overlapping images of the SUPRAS, thus demonstrating that the droplet structure of SUPRAS could effectively extract phenolic acids.
2.8. Molecular Dynamic Simulation Analysis
The molecular dynamic simulation analysis was conducted utilizing the GROMACS 2020.6 package employed with the Amber (99SB-ildn) force field [
34]. The structure of molecules in extraction, including ethanol, octanoic acid, water, and rosmarinic acid, were drawn in Gauss View 05 and optimized by the density functional theory of B3LYP/6-311G** level. The ANTECHAMBER module of AmberTools was used to produce the topology information of the compounds. Restrained electrostatic potential (RESP) charge was adopted for the Multiwfn-calculated partial charges [
35].
Three extraction systems were all generated in the cube box of 10 nm × 10 nm × 10 nm by Packmol. Specifically, the SUPRAS box contained 700 octanoic acid molecules, 1500 ethanol molecules, 3250 water molecules, and 100 molecules of rosmarinic acid. The ethanol (70%, v/v) box was made up of 3600 ethanol molecules, 4701 water molecules, and 100 molecules of rosmarinic acid. The water cartridge contained 15274 molecules of water and 100 molecules of rosmarinic acid. The cut-off method with a cutoff distance of 1.0 nm was adopted for both van der Waals and short-range Coulomb interaction. The system pressure was kept constant at 1 atm using the Parrinello-Rahman approach throughout the producing phase and the Berendsen method during the equilibrium phase. The temperature was maintained at 298K throughout the whole process. Periodic boundary conditions were applied in all directions, and the generating phase last for 100 ns with a step value of 2 fs. Lastly, the simulated processes were analyzed for the hydrogen bonding interaction and the average noncovalent interaction, etc.
2.9. Statistic Analysis
Each experiment was carried out in triplicate and the results were expressed as mean ± standard deviation (SD). The one-way analysis of variance (ANOVA) and Tukey’s tests was obtained by SPSS statistics 17.0 to analyze the significant differences in the experimental data. p < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. SUPRAS Production and Composition
SUPRAS are nanostructured liquids generated in amphiphile colloidal suspensions through self-assembly coacervation [
25]. The use of SUPRAS is aimed to emphasize their characters in the formation process, taking into account the non-covalent interactions that hold the molecules together and the self-assembly processes of their synthesis [
26]. The synthesis of SUPPAS usually involves two stages as shown in
Figure 1. At first, amphiphiles were dissolved in protonic or dipolar non-protonic solvents, and the formed colloidal system self-aggregated to form vesicles or reverse micelles when beyond the critical aggregation concentration. Next, the addition of water (the “poor solvent” of the amphiphilic compounds) causes the aggregates to self-assembly into the SUPRAS, a new highly packed phase with an inverted hexagonal arrangement. In this structure, the hydrocarbon chains are distributed in the organic solvent, while the carboxylic groups encircle the aqueous holes.
In this study, ethanol was selected for the production of SUPRAS because of its lower toxicity. Indeed, octanoic acid, octanol, decanol, decanoic acid, and ethanol used in the synthesis were Generally Recognized as Safe (GRAS). The proportion of amphiphiles like octanoic acid was set at 5% v/v because previous studies demonstrated that the SUPRAS volume increased linearly with the percentage of amphiphiles at constant organic solvent ratios and that higher ratios of octanoic acid require more ethanol, so a suitable ratio of 5% was chosen for the test [
36,
37].
Moreover, SUPRAS was prepared by centrifugation at 5000 rpm for 10 min in our experiments. Available experiments showed that the equilibrium phase separation conditions were reached after centrifugation at 3000 rpm for 5 min. In contrast, sonication at 40 kHz could not completely separate the two phases after 2 h [
32]. These findings led to the recommendation of centrifugation for the production of SUPRAS.
Based on all the preliminary research above, SUPRAS will be better used to extract bioactive in PV and it provides suitable conditions for further process optimizations.
3.2. Optimized Results of SUPRAS-Based Extraction of Phenolic Acid
The optimization procedure has been illustrated in
Section 2.4 and the extraction efficiency of the phenolic acids was evaluated.
3.2.1. Different Amphiphilic Organic Solvents
The choice of appropriate amphiphiles was the first aspect to take into account while optimizing the SUPRAS-based extraction procedure. The structure and physicochemical properties of the amphiphiles are crucial for the extraction efficiency of the target bioactivities [
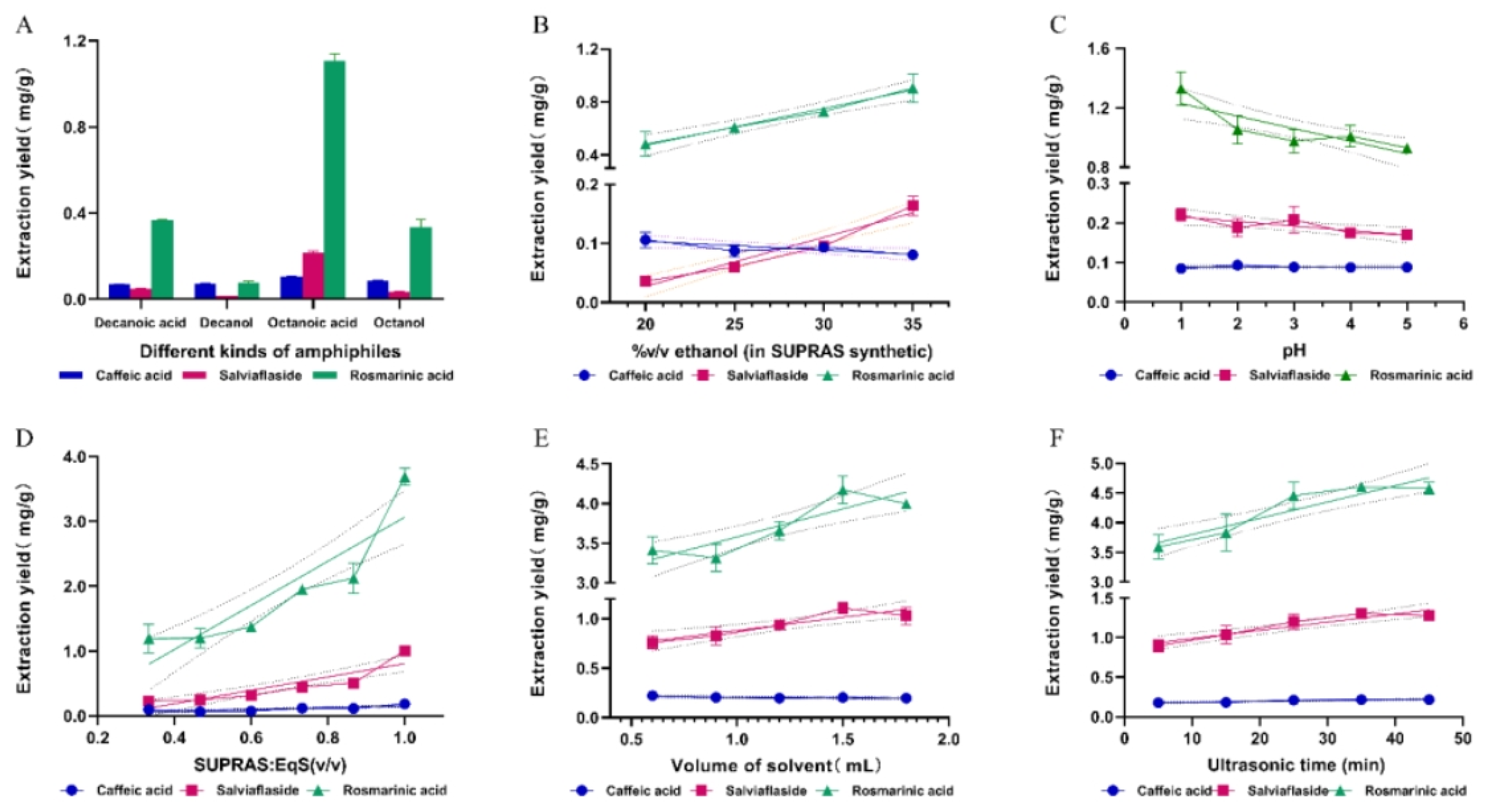
31]. By carefully choosing the amphiphiles and the coacervation environment, amphiphiles self-assemble spontaneously to produce nanostructured liquids where the shape and characteristics of the supramolecular aggregates may be customized. For this purpose, octanoic acid, octanol, decanoic acid, and decanol were selected to prepare different SUPRAS. Other variables were fixed at 35%(v/v) ethanol, water (pH~3), SURAS: EqS of 1:2, and ultrasound for 15min at 25℃. As shown in
Figure 3A, octanoic acid-based SUPRAS extracted more 14.40-fold salviaflaside (0.216mg·g
-1) than decanol (0.015mg·g
-1) and 6.17-fold more than octanol (6.94mg·g
-1). The extraction yield of rosmarinic acid reached 1.126 mg·g
-1, which was 3.06-fold more than that of decanoic acid, 14.25 -fold more than decanol, and 3.42-fold more than octanol. The SUPRAS prepared with octanoic acid was most suitable for the extraction of phenolic acids.
This result might be explained as follows: hydrogen bonding and dispersion were the main binding forces influencing the extraction of target components by SUPRAS. As the hydrocarbon chain lengthened, hydrogen bonding decreased while the dispersion increased. For polar compounds, hydrogen bonding was an extremely effective retention mechanism, thus the shorten-chain alcohols/alkyl carboxylic acids were preferred because they were better proton donors than longer ones [
31]. In the comparison of acids and alcohols, alkyl carboxylic acids are better proton donors than alkyl alcohols [
32]. Considering the high polarity and the number of hydrogen bond acceptors of the target bioactivities, it was reliable to assume that extraction would be preferred with SUPRAS constituted of octanoic acid since it could establish stronger hydrogen bonds.
3.2.2. The Percentage of Ethanol
Secondly, the impact of the different ethanol content in the synthesis of SUPRAS was investigated. As it was determined that when the proportion of ethanol in the solution incremented, both the volume of SUPRAS and the size of the spherical droplets forming them increased [
31,
38]. Thereby, the extraction efficiency can be affected and optimized. SUPRAS with different compositions and vesicle sizes used in the following experiments were generated by varying the relative ratios of water and ethanol [
24]. The influence of ethanol content was investigated within 20% to 35% v/v, where the SUPRAS was stratified, and the results were shown in
Figure 3B.
As the percentage of ethanol increased, the extraction rate of caffeic acid did not change much, but the extraction rate of salviaflaside and rosmarinic acid increased significantly. The highest extraction yields of phenolic acids were obtained at 35%(v/v). Phenolic acids, high polar compounds, were estimated to have higher extraction rates in SUPRAS that had larger water cavities made from higher ethanol percentages [
27].
3.2.3. pH
pH was also a key factor that greatly affected extraction efficiency because of its influence on analyte solubilization and charge density of extracts and solvents [
39]. The
pKa values of caffeic acid and rosmarinic acid are 4.58 and 2.78, respectively. Both two phenolic compounds have low
pKa values and are more acidic. Therefore, to analyze the impact of
pH on the extraction performance, the
pH of the water used in the synthesis of SUPRAS was adjusted with HCl over the range of 1 to 5. According to the results in
Figure 3C, it is clear that the change in
pH had a large effect on the extraction rate of phenolic acids. When the pH was 1, the extraction yield of rosmarinic acid reached 1.33mg·g
-1, ~1.43-fold higher than other values. This phenomenon was account for the fact that phenolic compounds tend to take on molecular forms under acidic conditions (
pH<
pKa), which enabled the target compounds easily extracted from the sample phase into the extraction phase [
40].
3.2.4. The ratio SUPRAS: EqS
Next, how the SUPRAS: EqS ratio affected the extraction yields was studied. Two strategies have been typically used to apply SUPRAS in the extraction of target compounds from solid materials. The first one involved adding the SUPRAS directly to the solid samples [
8], which was suggested for the extraction of compounds encompassing a wide polar range. The second strategy is that volumes of both SUPRAS and EqS are used [
41]. The EqS was commonly used with SUPRAS to wet the samples.
In this optimization, the total volume of solvents (SUPRAS+EqS) used for extraction was kept constant at 1.2 mL and the SUPRAS phase content ranged from 33% to 100%. The results in
Figure 3D demonstrated that when the SUPRAS phase increased, the extraction of three phenolic acids increased considerably. It was possibly caused by the highly polar target compounds’ dispersion distribution between the SUPRAS and EqS phases [
42]. The best option for maximizing extraction efficiency was 100% SUPRAS.
3.2.5. The Sample: Extractant Phase Ratios
Then we studied the samples: extractant phase ratios (g: mL) ranging from 1:4 to 1:12 (
Figure 3E). For this purpose, the amount of PV powder maintained at 150.00mg and the volume of the SUPRAS varied from 0.6 to 1.8mL. For phenolic acids, the extraction rates grew gradually as the sample volume increased, reaching saturation at a ratio of 1:10. (g: mL). The extraction yields did not continue to improve with increasing solvent volume and even decreased slightly at 1:12 (g: mL) due to the decrease in mass transfer efficiency. The solid-liquid ratio of 1:10 (g: mL) was chosen as the best ratio.
3.2.6. Ultrasonic Time
Ultrasonic time was also a significant factor that affected the extraction yields of target analytes. In the ultrasonic-assisted extraction procedure, the cell wall of the PV was broken by the ultrasonic wave and the vesicle solution could infiltrate into the cell and interact with the active substances [
43]. To gain the best extraction yields, different extraction time (5-45min) was assessed under the same extraction conditions. As
Figure 3F revealed, the extraction of phenolic acids was not impacted by the extraction time. And it was completed at an extraction time of 25min. It can be seen that SUPRASs had high extraction efficiency for bioactive substances from solid samples and did not require higher temperature and longer heating time compared with the traditional organic solvent reflux extraction method.
Single-factor optimization is one of the most commonly used strategies in experimental studies to obtain preliminary optimal protocols. However, we also believed it was these factors that would interact with each other. In the next further study, we will envisage a multiple regression analysis of the data to determine the optimal extraction conditions and analyze the relationship between responses and variables by means of a quadratic ANOVA model, while RSM is also used to illustrate well the interaction of the factors affecting the extraction process.
3.3. Method Validation
The method validation included the standard curve, correlation coefficient (R
2), relative standard deviation (RSD), stability, spiked recovery, LODs, and LOQs (as shown in
Table 1).
In this experiment, the standard curves were established for the qualitative and quantitative determination by HPLC. The correlation coefficients of the three phenolic acids were above 0.9996, confirming the excellent linearity. Then, six extractions were parallelly performed under optimal conditions to determine the precision of the method, and the RSD of caffeic acid, salviaflaside, and rosmarinic acid was 3.31%, 3.14%, and 1.15%, respectively. The limits of determination (LODs) of three phenolic acids ranged from 0.08-0.16 μg mL-1 and the limit of Quantitation (LOQs) were within 0.29-0.53 μg mL-1, which were calculated by the signal-to-noise (S/N) ratio of 3 and 10, respectively. To further confirm the reliability of the method, the spiked recoveries were tested by adding different concentrations of standards to the sample solutions, and the results ranged from 100.81% to 101.98% with the RSD of 1.02-1.39%. Based on the above results, the extraction method established in this experiment was sufficiently accurate and reliable. It had potential application in the determination of other pharmaceutical products.
3.4. Comparison of the Extraction Efficiency of Phenolic Acids by Different Extraction Methods
The choice of appropriate solvents was essential in the field of extraction chemistry The extraction efficiencies of caffeic acid, salviaflaside, and rosmarinic acid obtained using SUPRAS were compared to those extracted by other different solvents previously applied. As shown in
Table 2, the SUPRAS extracts of caffeic acid (0.240mg·g
-1) and salviaflaside (1.443mg·g
-1) was higher than 30% ethanol method (0.146mg·g
-1 and 0.800mg·g
-1) and the DES method (0.213mg·g
-1 and 1.199mg·g
-1).
Similarly, SUPRAS showed the highest extraction yield of rosmarinic acid which was ~1.78-fold more efficiently (5.254mg·g-1) than 30% methanol (2.957mg·g-1). And it was higher than both the anhydrous methanol reflux method (4.667mg·g-1) and DES method (3.706mg·g-1). ANOVA analysis of the data further demonstrated that the phenolic acid yields extracted using SUPRAS were statistically significantly different (p < 0.05) compared to the other methods.
In addition, pretreatment, high temperature, and large solvent volumes have been reported to obtain higher extraction rates. For example, the conventional solvent extraction method based on aqueous methanol had a solid-to-liquid ratio of 20mL·g
-1 and required twice continuous reflux for 2h, which consumed a lot of time and energy [
15]. Among the extraction methods assisted by ultrasound, the method of Huafu Wang et al. (2004) had a solid-to-liquid ratio as high as 500mL·g
-1 [
44]. The method using DES although reported a lower solvent consumption of 10 mL·g
-1, required 80℃ heating and stirring for 46min [
45]. Hence, considering the solvent dosage, extraction time, and energy consumption, the SUPRAS showed great promise for extracting bioactive ingredients.
3.5. Comparison of Antioxidant Activity by Different Extraction Methods and Solvents In Vitro
Phenolic compounds, including caffeic acid, salviafaside, and rosmarinic acid, are the main contributors to the antioxidant properties of PV[
46]. Previous studies indicated that rosmarinic acid and caffeic acid possessed excellent high antioxidant capabilities [
47,
48]. So, it is worth determining the antioxidant activity of different solvents extracts when studying extraction methods. In this work, the DPPH and ABTS•
+ assays were used to evaluate the antioxidant activity of plant extracts.
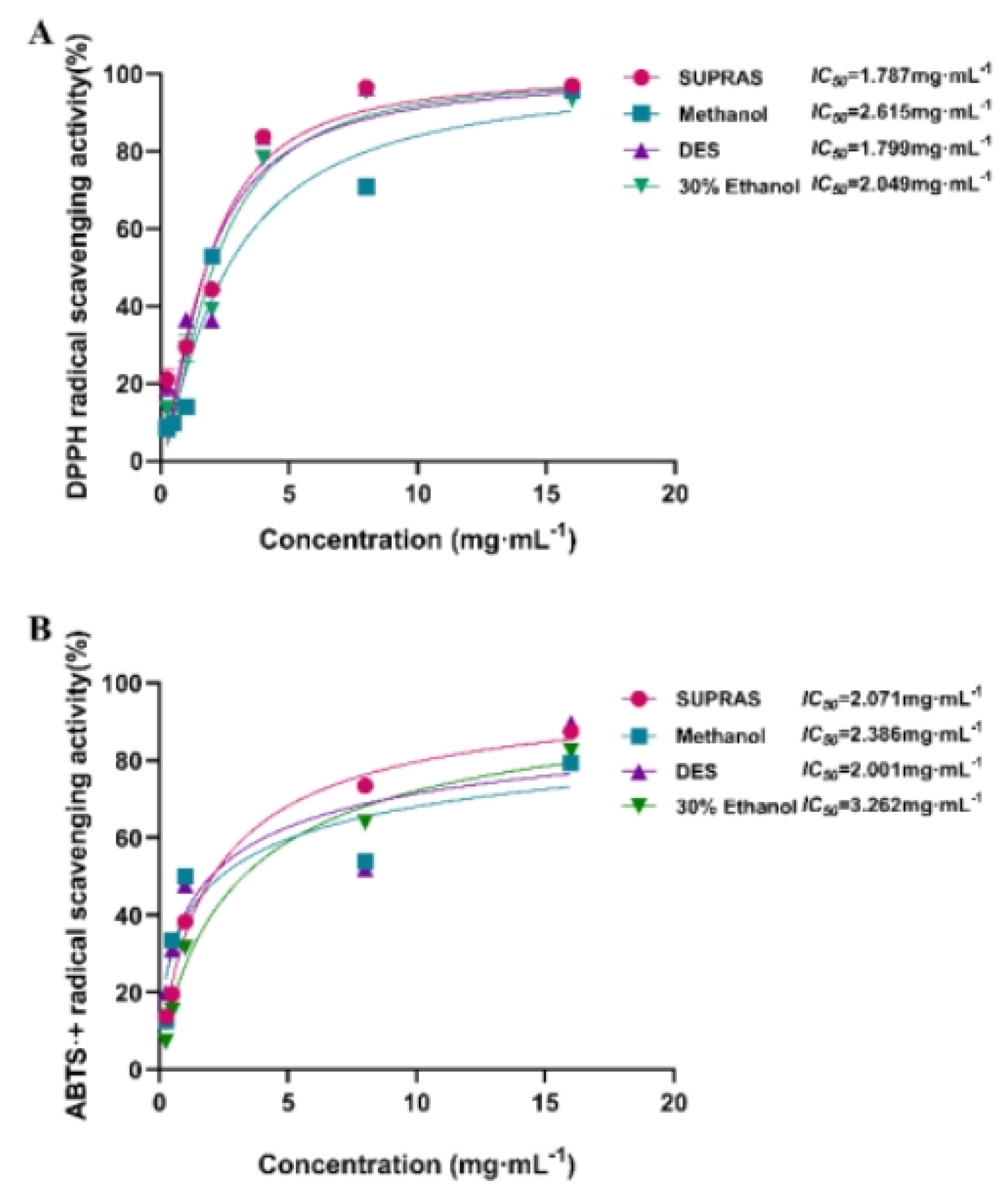
According to the method in section 3.4, extraction using SUPRAS was compared for antioxidant properties with 30% ethanol, anhydrous methanol, and DES (36% vol water in ChCl/ethylene glycol). The results (
Figure 4.) showed that the extraction solvents also had a significant influence on the antioxidant properties of extracts. According to ABTS•
+ radical scavenging assays, the extracts prepared with innovative green solvents like SUPRAS (
IC50=2.061 mg·mL
-1) and DES (
IC50=2.001 mg·mL
-1) had significantly higher antioxidant capacities than those prepared with conventional organic solvents (
IC50=2.386 mg·mL
-1 for 30% ethanol, and 3.262 mg·mL
-1 for methanol). Although the
IC50 value of DES extracts was slightly lower than SUPRAS extracts, the inhibition rate of SUPRAS extracts was higher than those of DES when the concentration exceeded 1mg·mL
-1. As for the DPPH assays, the results showed that the DPPH• generation was inhibited by all the extracts with the following degree of inhibition: SUPRAS >DES > 30% ethanol > methanol (
IC50 = 1.787, 1.997, 2.049, and 2.615 mg·mL
-1, respectively). The results suggested that SUPRAS extracts exerted the highest free radical scavenging capacity. Therefore, the SUPRASs could be used as reliable and effective extraction solvents, which also exhibited good antioxidant potential.
3.6. CLSM Results for Efficient Extraction Performance of SUPRAS
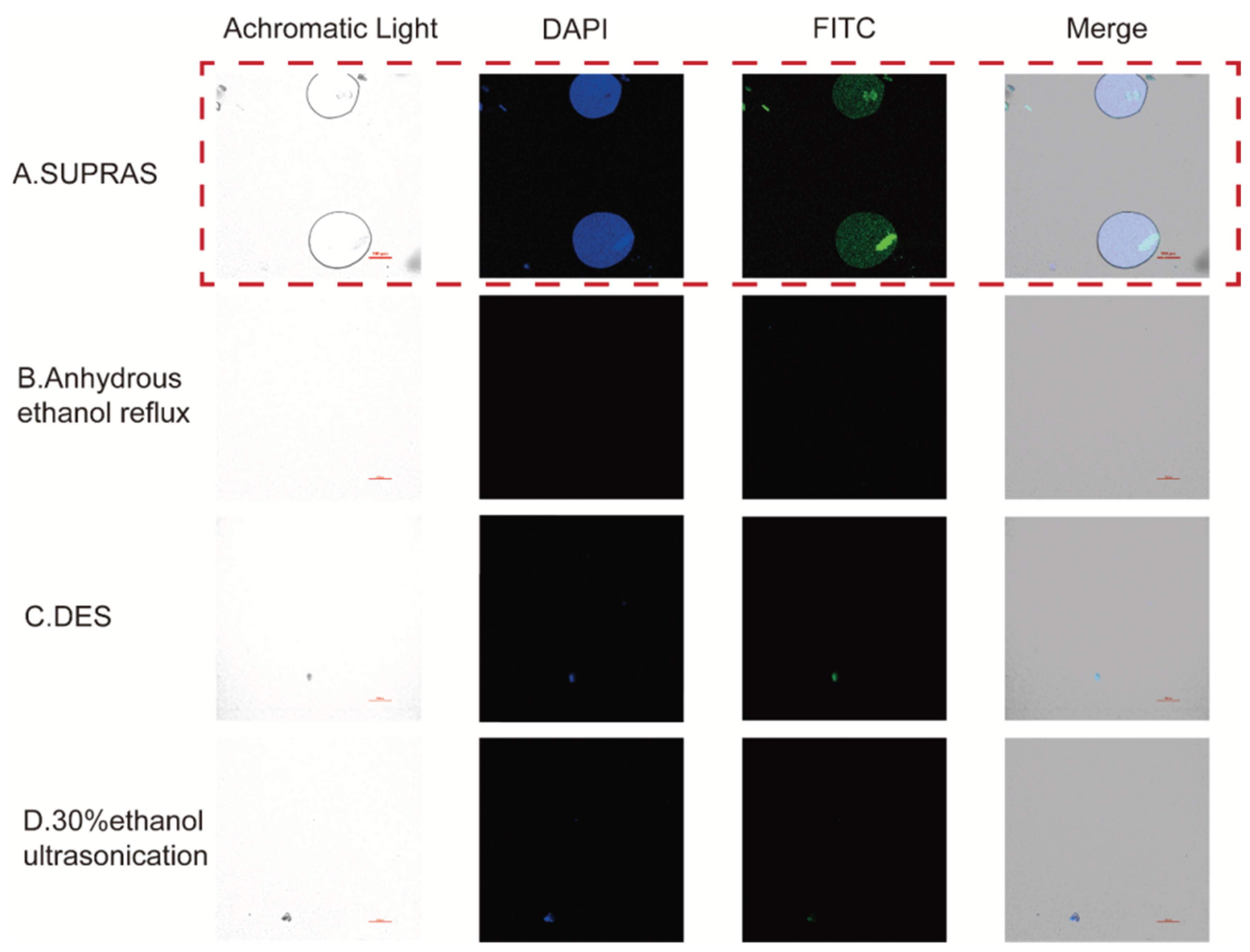
Previous studies reported that SUPRAS consisted of spherical droplets dispersed in a continuous phase, which was also seen as a characteristic feature of SUPRAS that distinguished it from ordinary solvents and was thought to be related to its superior extraction ability [
32]. Under achromatic irradiation conditions of CLSM, we observed the spherical droplet structure of SUPRAS, while similar structures were completely absent in other solvents (
Figure 5).
However, the droplet structure of SUPRAS itself has been studied several times, but its interaction with the extracts had not been mentioned explicitly. To better understand the extraction process and investigate the extraction mechanism, CLSM was used in this experiment to concurrently capture pictures of the solvent microstructures and the distribution of phenolic acid after the extraction.
For determining the location and morphology at the CLSM interface, the target compound (e.g., phenolic compounds) must be fluorescent or treated with a fluorescent dye so that a clear signal is available [
49]. In this experiment, 2-APB was applied to amplify the fluorescence signal to visualize the phenolic acid compounds. As shown in
Figure 5, phenolic acids fluoresced blue at an excitation of 450 nm and emission of 405 nm and showed green fluorescence at an excitation wavelength of 488 nm and emission of 525 nm. The CLSM images presented the fluorescence position of phenolic acids, which coincided with the position of the SUPRAS droplets, and the phenolic acids were encapsulated in the vesicles by the SUPRAS droplets. In addition, the fluorescence images of the SUPRAS extracts (
Figure 5A) were more concentrated and intense compared to the other solvents, while the other solvent extracts (
Figure 5B-D) had only weak and dispersed fluorescence. Thus, CLSM provided direct evidence that SUPRAS was able to extract phenolic acids into its droplets. It was reasonable to assume that SUPRAS droplets had enrichment effects and efficient extraction capacities for the target compounds. The specifics of the droplets and extracts after SUPRAS extraction were visualized to facilitate a more direct study of the extraction mechanism of SUPRAS.
3.7. Molecular Dynamics Mechanism for Efficient Extraction Performance of SUPRAS
The simulation parameters chosen for the molecular dynamics simulations were based on the actual content of the solvents and extracts used in the experiments. To further ensure the realism and reliability of the simulations, the composition of the supramolecular solvents was determined to facilitate the determination of the number of solvent molecules in the simulations. The final optimized SUPRAS was determined to contain 43.96% w/w of octanoic acid, 30.33% w/w of ethanol, and 25.17% w/w of water. The high content of amphoteric organic solvents gave SUPRAS a good extraction efficiency.
In general, the hydrogen bonds developed between the solvent and phenolic compounds hydrogen bonds were extremely effective retention mechanisms of extraction and promote the dissolution of the extractive [
24]. The molecular dynamics simulations were performed to understand the extraction mechanism of SUPRAS which had high extraction efficiency compared to other solvents. In this study, concerning the existing extraction methods, three typical solvents—the octanoic acid-ethanol-water SUPRAS, ethanol (30%, v/v), and water were selected for comparison. And rosmarinic acid which had the highest extraction amount was chosen as the typical phenolic compound from PV for subsequent simulations.
The distribution state of rosmarinic acid molecules in three different solvents was firstly simulated in Figure 6. It could be seen that rosmarinic acid molecules formed a very tight cluster in water, while they were the most dispersed in the ethanol system. The solvent-accessible surface area (SASA) was a critical indicator of the degree of contact between rosmarinic acid and solvent. A larger SASA can enhance solubilization and facilitate extraction [
50]. Within the first 20 ns, the SASA value of rosmarinic acid molecules in water decreased rapidly, while it decreased less and remained relatively stable in ethanol or SUPRAS. The average SASA values of three different solvents shown in Fig.7B corresponded to the stable dissolving state of rosmarinic acid molecules in SUPRAS. The SASA of the rosmarinic acid molecule in SUPRAS is 348.79 nm
2 and in ethanol is 367.91 nm
2, both of which are much higher than that of water at 167.37 nm
2.
Combining the simulation results of the distribution state and the values of SASA, we found an interesting phenomenon worthy to be considered — the SASA values of rosmarinic acid molecules in the SUPRAS solvent system and the ethanol system do not differ much, but there is a significant and obvious aggregation of rosmarinic acid molecules in SUPRAS. This suggested that SUPRAS may have an enrichment effect on bioactive substances with the same extraction efficiency, and SUPRAS is worthy of further development and research in the application of rapid, efficient, and micro-extraction.
In addition, the average hydrogen bonding lifetime between reactants and solvents could indicate the stability of hydrogen bonds between hydrogen donors and acceptors. It was used as one of the more important parameters in molecular dynamics simulations to quantify the strength of solvent-reactant binding [
51]. In general, stronger interactions between solvent and reactants could translate into lower transition state free energies, thus increasing the reaction rate and extraction efficiency. As shown in Fig.7E, the average lifetime of the hydrogen bond of rosmarinic acid in SUPRAS was 2.11 ps, which was higher than that in the ethanol system (1.92 ps) or water (1.52 ps). This suggested the hydrogen bond formed of rosmarinic acid in the SUPRAS system possessed higher stability than in the other two solvent systems, which accounted for higher extraction efficiency and solubility of phenolic acids using SUPRAS.
The averaged non-covalent interaction (aNCI) was used to deeply analyze the weak interactions in the fluctuating circumstances [
52]. The types and distribution of non-covalent interaction sites in SUPRAS systems could be demonstrated intuitively [
53]. For the aNCI study, the dark blue region around the hydroxyl groups implied the stable hydrogen bond, the green region surrounding the rosmarinic acid molecules reflected the van der Waals interaction, and the red region indicated the steric influence. As seen in Fig.8A, there are more dark blue areas of hydrogen bonding between the solvent and rosmarinic acid molecules in the SUPRAS system. Compared to water or ethanol systems, the SUPRAS solvent system contained more solvent-target molecule interactions (reflected in the red dashed line). However, in the ethanol system, there is more solvent-solvent hydrogen bonding (reflected by the blue dashed line). Thus, even though the water system contained somewhat more total hydrogen bonds, the SUPRAS were more exclusive and efficient in extracting rosmarinic acid.
The thermal fluctuation index (TFI) could indicate the stability of the interaction mentioned in the aNCI analysis. The interactions, which ranged from being highly stable to being very weak, were reflected in the color calibration from blue to red. From the TFI plots, the red area of the rosmarinic acid molecules in the SUPRAS system was much smaller than in the other two systems. So, the hydrogen bonds formed between phenolic acids and solvent molecules in SUPRAS were more stable than those in other solvents, which facilitates the extraction.
Recently, molecular dynamics analysis combined with quantum chemical calculations was applied to verify and quantify the interaction between extractants (e.g., NADES) and components [
54,
55]. In this study, an attempt was made to further reveal the extraction of SUPRAS through molecular dynamics simulations, hoping to provide an insight into the efficient extraction using SUPRAS. Also, it was theoretically demonstrated that SUPRAS had an efficient extraction capacity.
3.8. Existing Problems and Prospects
However, there still Exist some drawbacks to SUPRAS. For example, additional purification processes for the separation of bioactivities, as well as the recovery and reuse of SUPRAS, still need to be studied. On the other hand, the industrial application of SUPRAS is still limited due to factors such as lack of application information and research on different types of SUPRAS. In the future, more specific and suitable SUPRAS will be better designed, investigated, and used. More sophisticated characterization tools will be applied to elucidate the structure formation and extraction mechanisms of various SUPRAS. These will improve the use of SUPRAS in various fields and give it a superior potential to take the place of conventional organic reagents for the extraction and enrichment of target compounds.
Conclusion
In this work, octanoic acid-ethanol-water-based SUPRAS provided a novel, green, and efficient method for the extraction of phenolic compounds from PV. The solvents used in this method are green and safe combined with the outstanding antioxidant capacity of phenols, which makes it more suitable for extracting them from plants as active ingredients in food or nutritional products Under the optimal extraction conditions, the extraction yields of caffeic acid, salviaflaside, and rosmarinic acid reached 0.240 mg·g-1, 1.443 mg·g-1, and 5.254 mg·g-1, respectively. Compared with other reported extraction methods, PV extracts with SUPRAS had the highest extraction yields and antioxidant activity. And the HPLC analysis showed that SUPRAS extract has good specificity with fewer other impurities. Additionally, CLSM characterization and molecular dynamics simulations were used to investigate the extraction mechanism and the specific interactions between the target molecules and SUPRAS. Compared with other solvents, phenolic compounds can be extracted into the droplet structure of SUPRAS. The more stable hydrogen bonding and larger solvent-accessible surface area of SUPRAS could explain the efficiency difference between different solvents and contribute to a further understanding of the extraction mechanism of SUPRAS. Thus, the designed SUPRAS could be used as an efficient green solvent for the extraction of natural products required in the food, pharmaceutical, and chemical industries.