Preprint
Review
Hydrocarbons Are the Backbone of Sustainable Civilization
Altmetrics
Downloads
125
Views
65
Comments
0
This version is not peer-reviewed
Submitted:
12 March 2024
Posted:
13 March 2024
You are already at the latest version
Alerts
Abstract
This article illustrates a comprehensive exploration of the multifaceted role of hydrocarbons in the field of material science, bridging the gap between theoretical understanding and practical applications, also providing acumens into future perceptions. Hydrocarbons, which consists of hydrogen and carbon atoms, play a critical role in numerous sectors due to their extensive presence in both natural and synthetic environments. The study categorizes hydrocarbons into diverse categories such as alkanes, alkenes, alkynes, and aromatic hydrocarbons, each owning unique features that influence their physical properties and applications. This inclusive review comprehends the fundamental aspects of hydrocarbons, their indispensable compounds and elements, and their substantial contributions to material science, including their utilization in coatings, composites, and polymers. Besides, the article investigates into case studies that demonstrate the theoretical advancements and practical applications of hydrocarbons, as well as their latent in addressing future energy and environmental encounters. By investigative the role of hydrocarbons in the expansion of innovative materials and their implications for sustainability and technological progress, the article accentuates the significance of hydrocarbons in evolving the field of material science and technology.
Keywords:
Subject: Engineering - Chemical Engineering
1. Introduction
Hydrocarbons are organic molecules that consist solely of two types of atoms: hydrogen and carbon. Hydrocarbons are typically colorless gases with weak odors. Hydrocarbons can be broadly divided into four subcategories: alkanes, alkenes, alkynes, and aromatic hydrocarbons. They can also have simple or very complex structures. Understanding the chemical composition and characteristics of various functional groups can be gained by studying hydrocarbons. These substances have the molecular formula CxHy. Hydrocarbons are present in both plants and trees. For instance, carrots and green leaves contain the organic pigment carotenes. Natural crude rubber is composed of 98% of these hydrocarbons. They are also very important because of their high internal energy. Additionally, Liquefied Petroleum Gas (LPG), a commercial fuel, is made from hydrocarbons like propane and butane. Several synthetic medications are made from benzene, one of the most basic aromatic hydrocarbons.
Hydrocarbons vary depending on whether they are cyclic (naphthene and aromatics), branched (isoparaffins), linear chains (paraffins), or double-bonded (olefins). A significant distinction between them is also represented by the quantity of carbons. All of these structural variations have an impact on characteristics like melting and boiling points as well as how they aggregate at room temperature either solid, liquid, or gas (Moreno-Montiel et al., 2019).
The following article examines the complex function that hydrocarbons play in the field of materials science, covering everything from theoretical foundations to practical uses and future expectations. It begins with a thorough overview of the importance of hydrocarbons and goes on to discuss the fundamental ingredients and key substances that propel material design innovation. The next section highlights the wide range of applications that hydrocarbons can be used for, including coatings, composites, polymers, and more. Further exploration of the topic reveals a variety of case studies that clarify theoretical developments, pilot-scale tests, and commercial applications, providing perspectives on how the field of hydrocarbon-based materials is changing. The article concludes by summarizing the principal discoveries and projecting the future trajectory of hydrocarbons as transformative agents for breakthroughs in materials science and technology.
2. Literature Review
2.1. Crucial Hydrocarbon Compounds and Elements
An organic compound with just two elements—hydrogen and carbon—is called a hydrocarbon. Derivatives of different hydrocarbons are made by substituting an atom or a set of other elements for the hydrogen in the molecule. Acknowledged as the father of hydrocarbon chemistry, German chemist Xiao Laima discovered hydrocarbons like butane. Hydrocarbons come in a wide variety of forms, with over 2000 recognized structures. It is separated into two categories: cyclic hydrocarbons and chain hydrocarbons, based on the carbon bond connection mechanism. A chain connecting the former carbon atoms has been formed. Hydrocarbons can be further divided into two groups based on the extent to which a hydrogen atom has saturated the valence bond: unsaturated hydrocarbons and saturated hydrocarbons. Alkanes like ethane and methane are examples of saturated hydrocarbons; olefins and alkynes, with ethylene and acetylene serving as common examples, are unsaturated hydrocarbons. Both aromatic and alicyclic hydrocarbons are members of the closed ring formed by the carbon bonds in cyclic hydrocarbon molecules. Chain hydrocarbons and alicyclic hydrocarbons have a lot in common. In general, cyclic alkenes and cycloalkynes are similar to olefins and alkynes, respectively, and cycloalkanes are similar to alkanes. A hydrocarbon containing a benzene ring structure is mostly referred to as an aromatic hydrocarbon. Since many of these open-chain compounds were present in the oils identified in the original investigation, chain hydrocarbons are also known as aliphatic hydrocarbons. Because of the similarities between their properties and those of aliphatic hydrocarbons, alicyclic hydrocarbons got their name.
Figure 1.
Classification of Hydrocarbons (HCs).
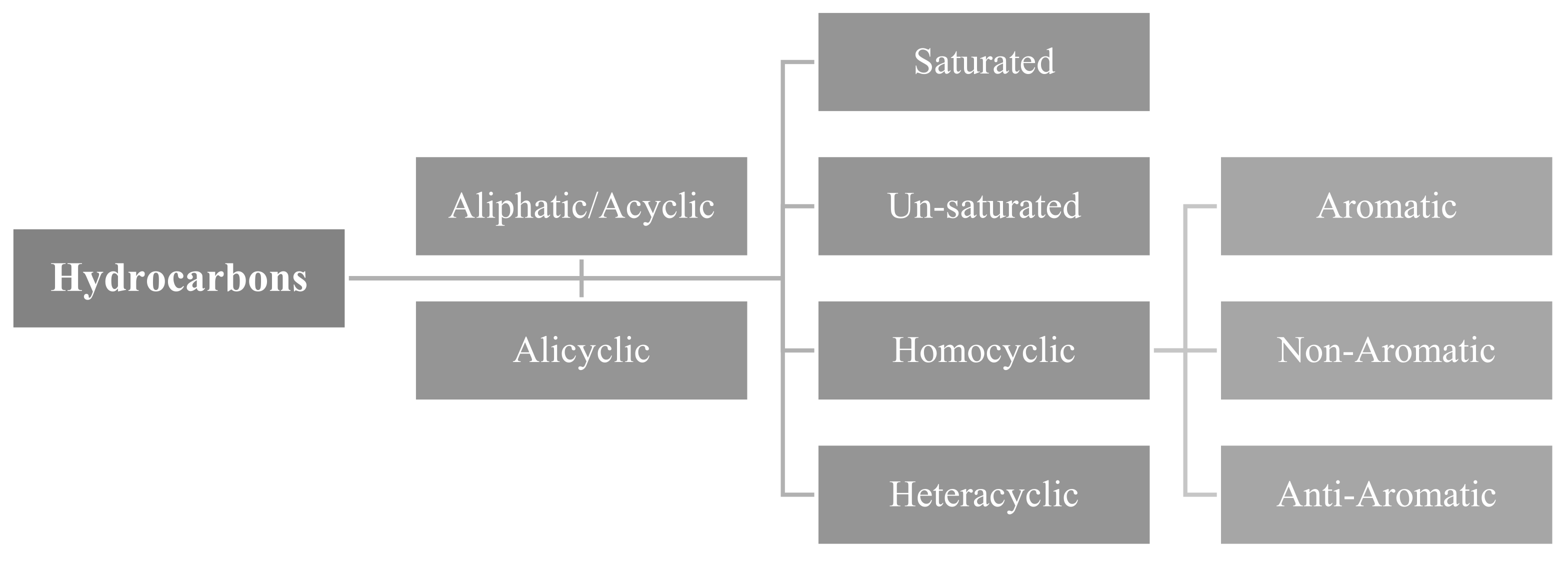
Since aromatic hydrocarbons have distinct features from other hydrocarbons and since some of the earliest chemicals discovered had scents, they were given names and are still in use today. Hydrocarbons are mostly found in coal, oil, and natural gas. Petroleum cracking and reforming can produce a variety of olefins, alicyclic hydrocarbons, and aromatic hydrocarbons; petroleum refining can provide a variety of alkane mixes, such as gasoline, kerosene, diesel, etc. Numerous aromatic compounds, including benzene and naphthalene, are found in coal tar.
Plants contain many higher hydrocarbons as well; hydrocarbons are found in colors like tomatoes and carrots. Higher alkanes are also present in a large number of animal and plant waxes. For example, beeswax contains C27H56 and C31H64, spinach leaves include C33H68, C35H72, and C37H76, while cabbage leaves contain C29H60. Polyisoprene, the primary constituent of natural rubber, is likewise a hydrocarbon. Hydrocarbons are used extensively as chemical raw materials and as fuel. Basic organic industrial raw materials like ethylene, propylene, butadiene, benzene, toluene, xylene, and naphthalene can be obtained from the secondary processing of petroleum. These raw materials can then be used to prepare additional chemicals like styrene, ethanol, and acetone. These basic ingredients can be processed to create a range of polymers, fine chemical compounds, synthetic rubber, and synthetic textiles. Certain bacteria can also eat hydrocarbons, and they can use the petroleum (protein) these bacteria excrete as nourishment.
A country’s degree of economic and technological progress can be inferred from the scope and intensity of its hydrocarbon compound processing. A chemical that is more complicated than a hydrocarbon molecule when one or more hydrogen atoms are replaced with other atoms or groups of atoms is referred to as a hydrocarbon derivative. A halogenated hydrocarbon is a compound that has been generated through substitution; an alcohol or phenol is a substance that has been substituted with a hydroxyl group; and a carboxylic acid is a compound that has been replaced with a carboxyl group. Compounds formed from the substitution of a corresponding atomic group for a hydrogen atom in a hydrocarbon molecule include ester, acid halide, acid anhydride, amide, aldehyde, ketone, amine, nitrile, and the like.
The chemistry of hydrocarbons and their derivatives was initially established by the German chemist Xiao Laima at the beginning of the 19th century. This definition was the result of years of experimental study and theoretical debate. The foundation of this definition is the idea of atomic combination theory. Many chemists at the time accepted it since it was more progressive and logical than all previous definitions. It does not, however, demonstrate the distinction between organic and inorganic. The theory of organic chemical structure has advanced significantly since the definition of Xiao Laima was established. As stated above, he was the first to create a scientific system and scientifically classify organic substances. He separated the organic materials first into aromatic and aliphatic hydrocarbons, then further separated the aliphatic molecules into acids, esters, alcohols, ethers, halogen hydrocarbons, ketones, aldehydes, and other similar substances (ChemSrc, 2019).
2.2. Hydrocarbons Usage in Materials
The main application for hydrocarbons is as flammable fuel. Methane is the main component of natural gas. The main constituents of jet fuel, gasoline, naphtha, and specialized industrial solvent blends are isomeric cycloalkanes, alkenes, and C6–C10 alkanes.
The following table describes the most popular applications for propane among end users. One fuel that can be used to make chemicals is propane. For powering gas fireplaces, barbecue grills, and backup electrical generators in homes, as well as for cooking, drying clothes, and heating spaces and water. It powers irrigation pumps and agricultural machinery, dries crops, manages weeds and pests, and heats greenhouses and animal housing on farms. to supply electricity for electric welders, forklifts, and other machinery in businesses and industries.
Table 1.
Fact Sheet for Hydrocarbon Utilization (The University of Western Australia 2015.
| Product | Derived from various hydrocarbon forms |
|---|---|
| Aviation fuel | Aviation fuel can be divided into two main categories: jet fuel and aviation gasoline, or “avgas.” Similar to kerosene, jet fuel is usually a mixture of C8 to C16 hydrocarbons that is intended to keep its viscosity at low temperatures. Avgas is a refined gasoline that has been given several additives. |
| Biodiesel | Diesel that is made from vegetable or animal fats is known as biodiesel. Regular diesel and biodiesel are both made of long-chain hydrocarbons, usually C15. But at one end of the chain, biodiesel has a methyl- or ethyl-ester group (a mono-alkyl ester). |
| Bitumen | Bitumen, sometimes referred to as asphalt, is a semi-solid or sticky, black liquid. While bitumen naturally occurs in oil sands, most bitumen is made through crude oil distillation. It is the leftover material that has been removed from materials with a lower boiling point (less than 500 °C). Chemically speaking, highly condensed polycyclic aromatic hydrocarbons make up the majority of bitumen. Bitumen is mostly used to build roads. |
| Coal | Carbonization is the process by which heat and pressure are applied to decomposing plant matter to generate coal. As the proportion of carbon rises and the fraction of volatile material falls over time, a range of products are created. • Coal is preceded by meat. The main application of brown coal, also known as lignite, is in the production of electricity. Black coal is further classified into various grades, such as bituminous coal, steam coal, and anthracite. Example structure of coal http://commons.wikimedia.org/wiki/File:Struktura_chemiczna_węgla_kamiennego.svg |
| Coal seam gas | Natural gas derived from subterranean coal reserves is known as coal seam gas. Much like regular natural gas, its primary component is methane. Fracking, often known as hydraulic fracturing, is one technique for obtaining coal seam gas from deep underground. |
| Condensate | Certain components condense when natural gas is harvested from gas fields because of a drop in pressure and temperature. We refer to these as “condensate.” One such ingredient is pentane (C5H12), which has a boiling point of 36 °C. |
| Crude oil | The process of forming crude oil, commonly referred to as petroleum, involves applying pressure and heat to microorganisms, usually plankton and algae, that are buried in sedimentary rocks. A variety of unsaturated cyclic hydrocarbons, cycloalkanes, and alkanes in the C5–C40 range make up this complicated mixture of hydrocarbons. The composition of crude oil varies: rich in short-chain alkanes, light crude oil has a low specific gravity; heavy crude oil has a high specific gravity because it contains more cyclic compounds; sweet crude oil has a low sulfur content, which is an unwanted contaminant; and sour crude oil has a high sulfur concentration. For example, benchmark prices are established for crude oil from the following locations: Tapis (Malaysia); Brent (North Sea); and West Texas Intermediate (USA). Fractional distillation is a process used to separate different components of crude oil according to their boiling points. These fractions undergo additional processing that involves cracking (splitting chains), unification (connecting chains), and alteration (changing chains) to change their properties. |
| Diesel | Diesel is a fuel used in diesel engines, often known as diesel fuel or diesel oil. Crude oil is the source of conventional diesel, sometimes known as petrol diesel. It is the portion with a composition ranging from C8 to C21 that separates between 200 and 350 degrees Celsius. Biodiesel is a fuel replacement for petroleum that is derived from plant or animal sources. Blended diesel (B20 diesel) has 20% biodiesel and petroleum diesel (Petro diesel). |
| Gas oil | See fuel oil. |
| Heating oil | One byproduct of distilling crude oil is heating oil. It usually contains hydrocarbons with a boiling point between 250 and 350 °C, ranging from C14 to C20. Its primary function is heating homes and businesses, as its name implies. |
| Fuel oil | Heating oil and fuel oil are comparable, although fuel oil may be heavier due to its longer hydrocarbon chains. In addition to powering vehicles and ships, it is utilized for heating. |
| Gasoline | See petrol. |
| Kerosene | Although far less flammable than gasoline, kerosene, often known as paraffin, is comparable. Its boiling point is between 150 and 200 °C, and its carbon chains range from C10 to C18. Kerosene is utilized as a starting material for various products and as fuel for tractors and jet engines. |
| LNG | LNG, or liquefied natural gas, is simply methane natural gas that has been cooled to a temperature of roughly -162 °C to turn it into a liquid. In places where pipelines would not be practical, this is done to deliver gas by ship. Approximately 1.6 L of LNG are comparable to one cubic meter of natural gas (a factor of x600 volume reduction). |
| Lubricant | Crude oil is used to make a variety of lubricants. Friction between surfaces is decreased by lubricants. Heavy crude oil fractions, such as long-chain alkanes (C20 to C50), cycloalkanes, and aromatics with boiling points between 300 and 370 °C, are used to make motor oils, grease, and lubricants. |
| LPG | Liquid petroleum gas, or LPG, is a propane and butane blend that is frequently used as fuel for cars and cooking. Refining oil produces LPG. When gas and crude oil are collected from natural sources, they are also separated from it. LPG cylinders for domestic use http://commons.wikimedia.org/wiki/File:LPG_cylinders.JPG |
| Naphtha | Naphtha is the term used to describe the lighter fractions with lower boiling points in crude oil fractional distillation. Typically, light naphtha is a blend of alkanes having boiling points between 60 and 100 °C, ranging from C5 to C9. It is the main ingredient in gasoline. With a boiling point between 80 and 180 degrees Celsius, heavy naphtha (C5 to C12) is also utilized as motor fuel. |
| Natural gas | When buried plant and animal remains are heated and compressed for millions of years, natural gas is created. With trace amounts of other gases, methane (CH4) makes up the majority of its composition. |
| Petrol | Petroleum is a fuel used in internal combustion engines; it is referred to as gasoline in North America. It is an alkane-alkene-cycloalkane combination with typical chain lengths ranging from C4 to C12. At roughly 250 °C, gasoline will spontaneously ignite; this is known as its autoignition point. The resistance of gasoline to autoignition is indicated by its octane rating. Fuel with an “E” rating includes mixed ethanol. For instance, E10 is 90% gasoline and 10% ethanol. |
| Petroleum | See crude oil. |
| Sales gas | Sales gas is just natural gas that has been purified—that is, stripped of impurities like water—for use in residential settings. |
2.3. Use of Hydrocarbon-Propane in the United States
All end-use sectors, including residential, commercial, industrial (industry and agriculture), transportation, and electric power, employ hydrocarbon gas liquids (HGLs), which are multipurpose products. Although the applications of HGL purity products (HGL streams containing at least 90% of one type of HGL) differ, their chemical compositions are comparable.
Table 2.
Uses, Products and Consumers of Hydrocarbons.
| Hydrocarbon gas liquids, uses, products, and consumers | |||
|---|---|---|---|
| HGL | Uses | End-use products | End-use sectors |
| Ethane | Petrochemical feedstock for ethylene production; power generation | Plastics, anti-freeze, detergents | Industrial |
| Propane | Fuel for space heating, water heating, cooking, drying, and transportation; petrochemical feedstock | Fuel for heating, cooking, and drying; plastics | Industrial (includes manufacturing and agriculture), residential, commercial, and transportation |
| Butanes: normal butane and isobutane | Petrochemical and petroleum refinery feedstock, motor gasoline blending | Motor gasoline, plastics, synthetic rubber, lighter fuel | Industrial and transportation |
| Natural gasoline (pentanes plus) | Petrochemical feedstock, additive to motor gasoline, diluent for heavy crude oil | Motor gasoline, ethanol denaturant, solvents | Industrial and transportation |
| Refinery olefins (ethylene, propylene, normal butylene, and isobutylene) | Intermediate feedstocks in the petrochemical industry | Plastics, artificial rubber, paints and solvents, resins | Industrial |
2.3.1. Propane Is Used as a Fuel and to Make Chemicals
The majority of propane utilized in the US is used as fuel, usually in places where natural gas is scarce or nonexistent. Utilizing propane is very seasonal. Fall and winter are the seasons with the highest consumption. HD-5, or minimum 90% propane by volume with trace amounts of other hydrocarbon gases, is the usual definition of propane sold for consumer use. In California, the approved propane standard is HD-10, which includes up to 10% propylene.
2.3.2. Ethane Is Mainly Used to Produce Ethylene, a Feedstock to Make Plastics
The petrochemical industry uses ethylene, which is mostly made from ethane, to create a variety of intermediate goods, the majority of which are turned into plastics. Due to its increased supply and cheaper price when compared to other petrochemical feedstocks like propane and naphtha, ethane usage in the US has surged during the past several years. Ethane can also be used straight, either alone or in combination with natural gas, as a fuel for the production of electricity.
2.3.3. Butanes: Normal Butane and Isobutane Are Mostly Used as Blending Stocks for Gasoline
While some regular butane finds its way into lighters, most of it finds its way into gasoline, particularly in the winter months. Normal butane is also isomerized into isobutane because the demand for isobutane exceeds the supply. Petrochemical companies can also use regular butane as a feedstock. Among the compounds produced by petrochemical cracking using regular butane is butadiene, a precursor to synthetic rubber.
2.3.4. Natural Gasoline Is Used in Fuel and Transportation
It is possible to blend natural gasoline, commonly referred to as pentanes plus, with the fuels used in internal combustion engines, especially motor gasoline. As mandated by law, natural gasoline may be added to fuel ethanol in the US as a denaturant to render it unfit for human consumption. To create E85, some ethanol manufacturers use natural gasoline.
3. Case Studies: Ranging from Theory to Practical and Future Projections
3.1. Theoretical Advancements
One of the most crucial but energy-intensive processes in the petrochemical industry is the separation and purification of light hydrocarbon (LHs) mixtures, according to a study by Wen-Gang Cui on Metal–Metal–Organicwork Materials for the Separation and Purification of Light Hydrocarbons. Adsorptive separation employing specific solid adsorbents presents a promising substitute for energy-intensive conventional separation techniques including extraction, absorption, distillation, and so on. This technology has the potential to reduce energy costs while enhancing efficiency. Therefore, it is crucial and urgent to create solid materials for the effectively selective adsorption of LH molecules in moderate settings. Because of their unique characteristics, metal–organic frameworks (MOFs), a relatively novel family of porous organic–inorganic hybrid materials, have shown promise for tackling this difficult challenge. This paper provides an overview of the latest developments in the use of MOFs as separating agents for LH separation and purification, including the purification of CH4, alkynes/alkenes, alkanes/alkenes, C5–C6–C7 normal/isoalkanes, and C8 alkyl aromatics. The connections between the newly synthesized MOF materials’ structural and compositional characteristics and their separation methods and properties are emphasized. Lastly, the current obstacles and potential avenues for further investigation into the discovery of porous MOFs in this rapidly developing sector are also covered.
3.2. Pilot-Scale Applications
Modern engines using fuel as coolant frequently incorporate regenerative cooling technologies. Fuel flow instability during the cooling process should not occur as it compromises the engine’s capacity to operate safely. In this study by Zhou et al. (2014), the instability of supercritical endothermic hydrocarbon fuel flow during cooling is experimentally investigated. The interpretation of the instability mechanism is based on the simulation outcomes of a homogenous zero-dimension model. In the realm of automatic control, the stability criterion is created, and affecting factors are examined through the use of the stability analysis method and small deviation linearization theory. The experimental findings show that the critical and cracking temperature regions are where supercritical hydrocarbon fuel flow instability occurs. Fuel flow instability is primarily caused by an acute fall in fuel density in the vicinity of the critical temperature area and the cracking temperature region. Additionally, by lowering the mass flow rate, choosing a fuel whose density changes smoothly with temperature, and increasing tube heat capacity and compressible volume stiffness coefficient, the instability can be lessened. The analysis of the instability mechanism and contributing variables provides recommendations for inhibitory techniques that can guarantee the cooling system’s stable fuel flow.
3.3. Potential Future Projections in Hydro-Carbon Based Materials
Hydrogen production will depend on fossil fuels in the short- to medium-term future, which means it could continue to be a major source of CO2 emissions into the environment. Traditional CO2 sequestration techniques provide answers that are both environmentally questionable and highly costly. This research aims to investigate new methods for resolving energy and environmental issues related to hydrogen production from fossil fuels. The technological, environmental, and financial aspects of producing hydrogen and carbon on a large scale through the catalytic dissociation of natural gas (NG) are covered in this study. The authors present a scenario of fossil fuel-based “hydrogen–carbon” infrastructure, in which the carbon component is used in a variety of applications, including soil amendment, structural materials, power generation, and environmental remediation, and the hydrogen component of NG is used as a clean energy carrier (e.g., in transportation). A seamless shift from the existing hydrocarbon-based economy to a hydrogen-carbon economy will be possible under this scenario, serving as a halfway point towards the ultimate hydrogen-from-renewables economy of the future.
3.4. Potential Case Studies: Utilizing Hydrocarbons for Non-Combustion Purposes
A study on Climate Policy Needs Non-combustion uses for Hydrocarbons in 2021 demonstrates that the primary topic of discussion in climate policy is the concern of fossil fuel stock owners that their resources would become stranded assets due to high CO2 taxes and prohibitions on burning hydrocarbons. In a hurry to burn, they may respond by taking out and selling their reserves today. They present ways to either strongly mitigate or prevent the stranded-asset issue. They examine a basic intertemporal equilibrium for a specific fossil hydrocarbon stock. The following characteristics apply to this framework: In the future, they provide examples of climate-neutral products that can be produced from fossil hydrocarbons in order to preserve current markets and create new ones in order to address the rush-to-burn problem in a climate-neutral manner. The rush-to-burn issue is lessened by subsidies for these goods (or for their innovation). The market for items derived from fossil hydrocarbons is diminished, however, when alternatives to climate-neutral products based on fossil hydrocarbons are developed or when these products receive subsidies. This may worsen the issue of stranded assets and perhaps cause climate damage (Konrad et al., 2021).
According to an article by U.S. Energy Information Administration (EIA) in 2018, Although the majority of fossil fuels used in the US are burned, or combusted, to generate electricity and heat, the EIA projects that in 2017, the US used 5.5 quadrillion British thermal units of fossil fuels for non-combustion applications. In the last ten years, the United States’ non-combustion fossil fuel consumption has generally made up 7% of the country’s overall fossil fuel consumption and 6% of its total energy consumption. When fossil fuels are utilized directly in the production of lubricants, solvents, waxes, building materials, and other items, they can be consumed but not burned. Typical instances include natural gas used in fertilizers, petroleum products used in plastics, and coal tars used in skin care products. In 2017, non-combustion applications accounted for around 13% of all petroleum products consumed. Coal made up less than 1% of all-natural gas, but natural gas used for non-combustion purposes made up approximately 3%.
If non-combustion fuel had been burned, 2017 carbon dioxide (CO2) emissions would have increased by 196 million metric tons, or almost 4%. To determine the overall carbon dioxide emissions from the United States, the amount of fossil fuels used for non-combustion consumption must be estimated. Some (but not all) of the carbon in these fuels is sequestered during non-combustion use, and this stored carbon is not factored into fuel consumption numbers used in emissions calculations. When building pavement and roofs, asphalt and road lubricants are utilized. Various industrial processes, machinery, and automobiles all require lubricants, which include greases and motor oil. For chemical catalysts, petroleum coke is utilized, and for paints with a petroleum base, specific naphthas are used. In addition to polishes and waxes, other petroleum products include distillate and leftover fuel oils used as chemical feedstocks.
Throughout the industrial sector, relatively little natural gas is used for purposes other than combustion. Nitrogenous fertilizers and various chemical products such as methanol, hydrogen, and ammonia are produced using natural gas as a feedstock.
In the industrial sector, very limited volumes of coal are used for non-combustion applications. Coal tars, which are rich in aromatic hydrocarbons like benzene and utilized as feedstocks in the chemical industry to generate paints, synthetic colors, and sealcoats for pavement, are among the byproducts of the process used to produce metallurgical coke. Coal tars are an ingredient in several anti-dandruff shampoos and other medical skin care products (eia.gov, 2018).
In order to tackle the issue of fossil fuel assets hypothetically becoming stranded as a result of environmental policies, it is critical to prioritize the exploration of non-combustion applications of hydrocarbons that do not add to CO2 emissions. By readdressing the utilization of hydrocarbons away from energy generation and towards novel and inventive purposes, we cannister effectively minimize environmental repercussions while instantaneously unlocking fresh avenues for economic growth.
Cutting-edge materials, for instance, carbon-based nanomaterials like graphene, carbon nanotubes, and fullerenes, grasp immense potential in numerous fields. These ingredients novelty applications in electronics, energy storage, and the invention of composite materials for the automotive and aerospace sectors. Particularly, their manufacturing processes do not essentially emit CO2, showcasing the ability to repurpose hydrocarbons into sustainable, high-value products.
The integration of Carbon Capture and Utilization (CCU) technologies with hydrocarbon dispensation industries represents a circular economy model where CO2 emissions are captured and rehabilitated into useful products like synthetic fuels and chemicals. This not only diminishes emissions but also creates value from waste CO2.
Furthermore, hydrocarbons can ease the storage and transportation of renewable energy. Processes such as hydrogenation can convert excess renewable energy into synthetic hydrocarbons, which can be elated using existing infrastructure, thus ornamental the efficiency and reliability of renewable energy systems (eia.gov, 2018).
4. Conclusions
To sum up, the diverse applications of hydrocarbons in literature highlight their significance in multiple areas of contemporary existence. Hydrocarbons are used extensively in both our everyday and contemporary lifestyles. It has a major impact on the medical industry as well. The bulk of natural gas and petroleum is composed of hydrocarbons. They serve as raw materials for industrial chemicals, textiles, rubbers, solvents, explosives, and fuels and lubricants. With their extensive use in adhesives and coatings, as well as their major contribution to the synthesis of polymers and composites, hydrocarbons have completely changed the fields of information science and engineering. The variety and development of hydrocarbon products—from theoretical research to commercial success—were demonstrated through case studies. Nonetheless, it is important to acknowledge and take into account the environmental factors related to their use. Carbon emissions, pollution, and resource depletion are just a few of the major environmental issues brought on by the extraction, processing, and disposal of hydrocarbons. Because of this, it’s critical to balance the advantages of hydrocarbons for a range of uses with the importance of research and innovation in applications and other materials. One can maximize the advantages of hydrocarbons, lessen their negative effects on the environment, and create a more equitable and safe future by looking at the environmental implications of their usage and encouraging prudent management.
References
- Moreno-Montiel, Noemi & Moreno-Montiel, Benjamin & Montiel, Carlos & MacKinney-Romero, René. Data Mining on Data of Catalytic Cracking. Int. J. Environ. Sci. Dev. 2019, 10, 380–388. [CrossRef]
- Available online: https://www.chem.fsu.edu/chemlab/chm1046course/hydrocarbons.html.
- Available online: https://www.eia.gov/energyexplained/hydrocarbon-gas-liquids/uses-of-hydrocarbon-gas-liquids.php.
- Cui, W.G., Hu, T.L., Bu, X.H. Metal–organic framework materials for the separation and purification of light hydrocarbons. Adv. Mater. 2020; 32, 1806445. [CrossRef]
- Schiffmann, J., Favrat. Design, experimental investigation, and multi-objective optimization of a small-scale radial compressor for heat pump applications. Energy 2010, 35, 436–450. [Google Scholar] [CrossRef]
- Zhou, W., Yu. Mechanism and influencing factors analysis of flowing instability of supercritical endothermic hydrocarbon fuel within a small-scale channel. Appl. Therm. Eng. 2014, 71, 34–42. [Google Scholar] [CrossRef]
- Ionescu, R., Zilberman, Y., Haick, H. Development of an extremely selective e-nose employing a single polycyclic aromatic hydrocarbon-based chemFET. IEEE SENSORS Proceedings 2011. [CrossRef]
- Konrad, K.A.; Lommerud, K.E. Effective climate policy needs non-combustion uses for hydrocarbons. Energy Policy 2021, 157, 112446. [Google Scholar] [CrossRef]
- Available online: https://www.eia.gov/todayinenergy/detail.php?id=35672.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated





