1. Introduction
Global solutions to replace the use of petroleum-based chemicals and products are growing to solve environmental and ecological problems. Nanocelluloses are promising green materials which can be used in many fields such as packaging, paper, textiles, paint, aerospace, photonics and excipients (Li et al., 2015; Milwich et al., 2006; Thomas et al., 2018). The main characteristics of nanocelluloses are their renewability, abundance, biocompatibility, chemical inertness, high Young's modulus, low density, high tensile strength, large specific surface area, dimensional stability, low coefficient of thermal expansion and tunable surface chemistry (Fang et al., 2019; Phanthong et al., 2018; Rajinipriya et al., 2018; Vineeth et al., 2019). Nanocelluloses are a fibrous material with widths in the nanometer range (Klemm et al., 2011) mainly obtained from cellulose, the most abundant polymeric raw material on earth (Vazquez et al., 2015).
The preservation of historical and cultural heritage including artifacts, artworks, manuscripts, books, sculptures, paintings, historical buildings and archaeological finds, which are susceptible to degradation over time, is very important for the society. A large part is made of cellulose-based materials (wood, paper, archaeological fabrics and painting canvases) and requires an intervention due to: (i) chemical acidification processes caused by primers, paints and glues that decrease the mechanical properties; (ii) absorption of acid gases present in the atmosphere like sulfur dioxide and nitric oxides that increases the brittleness; (iii) biological attacks by microorganisms such as bacteria and fungi that induce thermal degradation (Antonelli et al., 2020; Böhme et al., 2020).
Nanocelluloses are one of the most recent novelties in the field of conservation and restoration of cultural heritage considering that many types of artistic substrates consist mainly of cellulose (Baglioni et al., 2015; Mateo et al., 2021). The use of paper for recording information is still very important, but the deterioration of this substrate can be a serious problem to world libraries, archives and museums (Buchanan, 1987; Sobucki & Drewniewska-Idziak, 2003; Zyska, 1996). Some illustrative examples are the acidic paper manufactured between the middle of the nineteenth and the twentieth centuries (Lee et al., 2010; Zyska, 1996) and paper documents written with iron-gall inks that have been used extensively from medieval times until the nineteenth century (Díaz Hidalgo et al., 2018; Kolar & Strlic, 2006).
2. Cellulose and Its Isolation
Cellulose is considered the most abundant renewable source on Earth with an estimated annual production of 1 × 1010 to 1 × 1011 tons. However, only a small amount around 6 × 109 tons is processed by the industry of paper, textile, chemical and other materials (Trache et al., 2020a). The sources of cellulose are innumerous namely wood, herbaceous plants, grass, agricultural crops and their byproducts, animal, algae, tunicates, fungi, bacteria and waste paper (Kumar et al., 2020; Nandi & Guha, 2018). Cellulose is a white biomacromolecule with no odor and no taste, it is insoluble in water and most organic solvents, and has a density around 1.5 g/cm3 independent of the source (Lavoine et al., 2012).
Chemically, cellulose is a linear homopolysaccharide produced from glucose monomers linked by β-1,4-glycosidic bonds. Depending on the source, the number of anhydroglucopyranoside units can reach values up to 10,000. Cellulose chains are held together by hydrogen bonds and van der Waals forces, presenting highly ordered (crystalline) regions and disordered (amorphous) regions. Hydrogen bonds arise between oxygens and hydroxyl groups positioned within the same cellulose molecule (intramolecular) and between neighboring cellulose chains (intermolecular) (Hon & Shiraishi, 1991). Intermolecular hydrogen bonds are responsible for the physical properties of cellulose such as good strength and flexibility. Crystalline regions are more resistant to chemical, mechanical and enzymatic treatments, have a higher resistance to degradation and a lower solubility in water and other solvents (Mishra et al., 2018; Rajinipriya et al., 2018). Amorphous regions are more prone to react with other molecular groups (Dufresne & Belgacem, 2013; Kargarzadeh et al., 2017; Wertz et al., 2010).
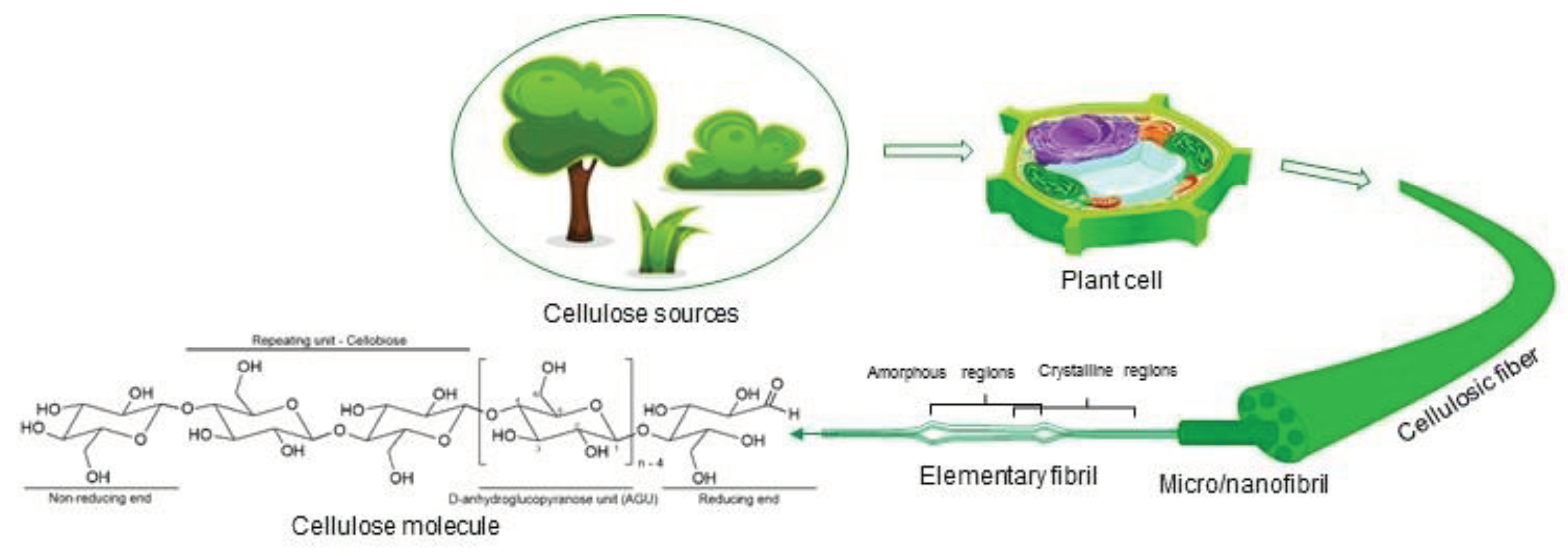
Native cellulose has a well-organized and aligned structure composed by cellulosic fibers, macrofibrils, microfibrils, nanofibrils and elementary fibrils (
Figure 1). The arrangement into distinct layers does not exist in regenerated cellulose because they are randomly positioned in the structure. Cellulosic fibers can be laterally disintegrated by mechanical processes to form cellulose nanofibrils or can be cleaved transversally at the less ordered regions to form cellulose nanocrystals (Habibi, 2014).
The isolation of cellulose from plant-based sources as wood, cotton, hemp and bamboo involves separating cellulose fibers from non-cellulosic components. The non-cellulosic components are essentially lignin and hemicelluloses that can typically be present in cells walls. Lignin is a complex aromatic polymer that surrounds cellulose and acts as a barrier conferring recalcitrance to the matrix and making it more difficult to remove. Hemicelluloses are branched carbohydrate polymers found alongside cellulose. The plant source material is prepared by removing bark or leaves and reducing the size which makes it easier to handle. Then the material is dried to decrease the moisture content, since a high moisture content can lead to microbial growth and degradation of cellulose. The prepared source material is subjected to pulping through mechanical or chemical methods which consist of breaking down the bonds between the cellulose fibers and the non-cellulosic components. Mechanical methods rely on physical forces to separate the cellulose fibers and chemical methods include the use of chemicals to dissolve or degrade the non-cellulosic components. The cellulose fibers are then washed to remove impurities, residual chemicals or other by-products and dried making it suitable to use. Methods of cellulose isolation depend on source, desired purity, properties and applications.
3. Nanocelluloses
Nanocelluloses are defined as the cellulose fibers with at least one dimension in nanoscale (1–100 nm). The versatility of nanocelluloses allows them to have a wide range of applications. Nanocelluloses properties are generally better compared to those of cellulose due to the reduced size that leads to structure and behavior changes (He et al., 2019; Suryanegara et al, 2010).
Three main types of nanocelluloses can be distinguished: (i) cellulose nanocrystals (CNCs) or nanocrystalline cellulose or cellulose whiskers, (ii) cellulose nanofibrils (CNFs) or nanofibrillated cellulose or cellulose nanofibers or microfibrillated cellulose; (iii) bacterial nanocellulose (BNC) or microbial cellulose (Thomas et al., 2021). CNCs are composed essentially of crystalline regions (crystallinity index of 54 – 88 %) possessing a well-ordered structure, CNFs consist of both crystalline and amorphous regions (crystallinity index < 50 %) having a more random structure and BNC is composed of almost pure cellulose with minimal impurities or amorphous regions (crystallinity index > 88 %) possessing an exceptionally well-organized structure (Ilyas et al., 2018). The choice between each type of nanocelluloses depends on the unique characteristics that can be used in specific applications. CNCs and CNFs are produced through plant-based cellulose sources requiring the isolation of cellulose from lignin and hemicelluloses, unlike BNC which is obtained through bacteria species from sugars and does not have unwanted polymers (Kaur et al., 2021; Mokhena & John, 2020; Nechyporchuk et al., 2016). The preparation processes of the three types of nanocelluloses are different because CNCs and CNFs are formed from largest to smallest dimensions called top-down process, and BNC is created from smallest to largest dimensions designed bottom-up process (Iguchi et al., 2000).
Table 1 presents the main differences between the three types of nanocelluloses.
3.1. Cellulose Nanocrystals
Cellulose nanocrystals (CNCs) production involves breaking down the fibers resulting in nanocrystals. The amorphous regions of cellulose are removed contributing to a high degree of crystallinity, excellent mechanical properties, high surface area, and good thermal stability (Oksman et al. 2016; Poulose et al., 2022; Sharma et al. 2019). CNCs consist of cylindrical, elongated and inflexible particles with a rod-like structure and dimensions of 50 – 500 nm in length and 3 – 50 nm in diameter. The most common sources are wood, cotton, hemp, flax, rice straw, bacterial cellulose, palm oil, kenaf fibers, corncob and grass (Ilyas et al., 2018).
3.1.1. Acid Hydrolysis
Acid hydrolysis is the most common method used to produce CNCs from cellulose fibers that involves a strong acid. Cellulose fibers are mixed with an aqueous acid solution at an appropriate concentration and temperature which breaks down the amorphous regions by cleaving the glycosidic bonds between the glucose units leading to the formation of nanocrystals. The acid hydrolysis is stopped by neutralizing the acid, typically by adding water or a basic solution (e.g. sodium hydroxide) resulting in a suspension that is washed by dialysis against deionized water to remove any remaining acid and neutralized salts. Nanocrystals are separated from the suspension through centrifugation or filtration and dried (freeze-drying or spray-drying). A mechanical treatment (sonication) is usually applied to prevent agglomeration and obtain a homogeneous dispersion (Brinchi et al., 2013).
The glycosidic bonds of the amorphous regions of cellulose fibers are easily hydrolyzed by acid while the crystalline regions are preserved since the acid has great difficulty in penetrating the well-ordered regions (Vanderfleet & Cranston, 2021). Various strong acids can be used successfully in the acid hydrolysis namely sulfuric, hydrochloric, phosphoric, hydrobromic and nitric acids (Liu et al., 2010). Sulfuric acid is the most used acid because it can introduce negatively charged sulphate groups on the cellulose fibers surface which leads to a high colloidal stability of the resultant CNC suspension (Punnadiyil et al., 2016). Hydrochloric acid, otherwise, tends to flocculate the CNC particles making a less stable aqueous suspension due to the absence of charged groups on the CNC surface (Gopi et al., 2019). The agglomeration of the crystals is prevented by the repulsion of surface groups with the same charge (Kumar et al., 2021).
Acid hydrolysis method is widely used because it is relatively simple, scalable and allows to obtain nanoparticles with a high crystallinity (amorphous regions of cellulose fibers are preferentially hydrolyzed), great surface reactivity (high density of hydroxyl groups on the surface) and high aspect ratio (elongated rod-like particles are formed). The main disadvantage is the use of strong acids which can lead to the degradation of the nanoparticles and raise environmental concerns.
3.1.2. Enzymatic Hydrolysis
Enzymatic hydrolysis is also used to obtain CNCs by a process that involves cellulolytic enzymes to cleave the cellulose fibers into nanocrystals. Cellulolytic enzymes (mainly cellulases) catalyze the hydrolysis of the glycosidic bonds between the glucose units. The mixture obtained is subjected to centrifugation to separate the nanocrystals from the remaining cellulose fibers and enzyme solution.
The glycosidic bonds of cellulose fibers can be the object of selective enzymatic hydrolysis depending on the cellulolytic enzymes used. Endoglucanases and cellobiohydrolases will attack preferentially the amorphous and the crystalline regions of cellulose fibers, respectively (Henriksson & Berglund, 2007).
The enzymatic hydrolysis method presents several advantages especially in terms of environmental problems (does not involve the use of strong acids or other hazardous chemicals), milder conditions (can help preserve the structural integrity of the nanoparticles) and reduced defects of the obtained CNCs (lead to nanoparticles with a more uniform size distribution). The principal challenges using this method are the enzyme’s activity and stability which depends on the temperature, incubation time, pH and inhibitors, the slower reaction rates due to the inherent nature of enzymatic reactions and the cost of cellulolytic enzymes.
3.2. Cellulose Nanofibrils
Cellulose nanofibrils (CNFs) are typically produced through mechanical treatment which involves the disintegration of the cellulose fibers preserving some of the amorphous regions, thus contributing to form a network structure with nano-sized fibers and high specific surface area. Other interesting CNF characteristics are easy surface modification and functionalization, excellent mechanical properties and good thermal stability (Fornari et al., 2022; Kumar et al., 2021). CNFs are typically composed of long and flexible entangled particles with dimensions of up to more than 1,000 nm in length and 5 – 100 nm in diameter. The best classical sources to produce CNFs are hardwood and softwood pulps, cotton, hemp, wheat straw, bacterial cellulose, kenaf, and recycled pulp (Ilyas et al., 2018).
3.2.1. Mechanical Methods
Mechanical methods such as high-pressure homogenization, microfluidization, grinding and high-intensity ultrasonication are among the most common methods used to produce CNFs from cellulose fibers. The main achievement of mechanical methods is the non-use of organic solvents, which is very attractive in terms of environmental impacts (Keerati-U-Rai & Corredig, 2009). Mechanical methods have, however, a great challenge to overcome, related with the high energy consumption (Klemm et al., 2011).
High-pressure homogenization involves forcing a cellulose fibers-water suspension through a narrow gap at high pressures. The shear forces generated by the rapid flow through the gap cause cellulose fibers to break down into nanofibrils. The fibrillation degree will depend on the number of times the suspension passes through the homogenizer and the applied pressure (Kargarzadeh et al., 2018). High-pressure homogenization has an important limitation which is the clogging of the system when using long fibers (Petroudy et al., 2021; Spence et al., 2011).
Microfluidization uses an intensifier pump to enhance pressure and is similar to high-pressure homogenization. The cellulose fibers-water suspension is passed through a thin chamber with a specific geometry at high pressures and high velocities. Intense turbulence flow and shear forces are achieved leading to cellulose fibrillation. The number of the passes through the microfluidizer and the chamber sizes will define the extent of fibrillation (Missoum et al., 2013). Microfluidization has the same drawback of high-pressure homogenization, that is, the clogging situation (Spence et al., 2011).
Grinding has been used for centuries in the papermaking industry and must be used carefully to ensure that it does not cause excessive damage to the nanofibrils resulting in poor physical strength, crystallinity and thermal stability (Rambabu et al., 2016; Ghasemi et al., 2016). The cellulose fibers pulp is passed through a couple of stones where one is fixed and the other is rotating. Shear forces created by the mechanical stress are responsible for the cellulose fibers break down into nanofibrils. The fibrillation degree will depend on the distance between the stones and the number of pulp passes (Kargarzadeh et al., 2018). The clogging of the system found in high-pressure homogenization and microfluidization can be minimized by adjusting the distance between the stones (Nechyporchuk et al., 2016).
High-intensity ultrasonication involves subjecting a cellulose fibers-liquid suspension to high-intensity ultrasonic waves (> 20 kHz). Tiny bubbles in the liquid are formed, which rapidly grow and implode generating intense shear forces which leads to cellulose fibrillation. Cellulose fibers suspension consistency, sonication time, temperature and power will define the extent of fibrillation (Mokhena & John, 2020; Wang & Cheng, 2009).
3.2.2. Combination of Methods
Combination of mechanical methods with enzymatic or chemical pre-treatments can be an alternative to obtain CNFs from cellulose fibers with lower energy consumption. The enzymatic pre-treatments break down the glycosidic bonds in cellulose chains helping to improve the formation of nanofibrils and the chemical pre-treatments introduce charged functional groups (positive or negative) on the cellulose fibers surface generating repulsive forces and improving the fibrillation degree. Repulsive forces are responsible for turning the hydrogen bonds in cellulose fibers weaker making it easier to separate the nanofibrils (fibrillation). Most common pre-treatments are enzymatic hydrolysis, 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) mediated oxidation, carboxymethylation, cationization and ionic liquid/deep eutectic solvent treatments.
Enzymatic hydrolysis is a pre-treatment similar to the method used to produce CNCs and involves the use of enzymes (specifically cellulases). The cellulases are applied to cellulose fibers suspensions being attached to their surface and initiate the hydrolysis of the amorphous regions increasing the accessibility to the mechanical cellulose fibrillation. Lignin must be removed prior to enzymatic hydrolysis due to its recalcitrance and interaction with cellulases (Berlin et al., 2006). The non-toxic nature of the enzymes and the non-production of hazardous by-products make enzymatic hydrolysis a more environmentally friendly pre-treatment compared to chemical pre-treatments (Arantes et al., 2020).
TEMPO mediated oxidation is a well-known chemical pre-treatment and one of the most common methods for the cellulose fibers surface modification. The cellulose fibers are soaked in a solution containing TEMPO (catalyst), NaClO (oxidant) and NaBr under alkaline conditions. Negative charges are introduced on the cellulose fibers surface through the selective oxidation of primary hydroxyl groups to carboxylate groups (COO⁻) via aldehyde groups leading to repulsion forces which facilitate the mechanical cellulose fibrillation. The formation of carboxylate groups is directly proportional to the amount of NaClO used and also to the oxidation time, up to a certain degree of substitution (Saito et al., 2007).
Carboxymethylation is an alternative chemical pre-treatment to TEMPO mediated oxidation. The process involves the treatment of cellulose fibers with an alkali solution (typically NaOH) to activate the hydroxyl groups which will react with monochloroacetic acid or sodium chloroacetate. Carboxymethyl groups (CH2COO⁻) are introduced on the cellulose fibers surface generating repulsion forces that enhance the mechanical cellulose fibrillation.
Cationization is a common chemical pre-treatment and another method for the cellulose fibers surface modification. The hydroxyl groups, after activation through treatment of cellulose fibers with an alkali solution (NaOH), react with cationic reagents such as 2,3-epoxypropyl trimethylammonium chloride (EPTMAC) and chlorocholine chloride. The cationic groups introduced on the cellulose fibers surface promote the mechanical cellulose fibrillation.
3.3. Bacterial Nanocellulose
Bacterial nanocellulose (BNC) is obtained through certain bacteria species mainly Gluconacetobacter xylinum. The bacteria ferment low molecular weight sugars to synthesize a well-organized, dense and coherent structural network of nanocellulose. BNC is exceptionally pure and has a high degree of crystallinity because it is composed of pure cellulose with minimal impurities or amorphous regions. The main properties of BNC are their excellent mechanical strength, good thermal stability, excellent water retention and biocompatibility (Klemm et al., 2006). In terms of morphology, BNC consist of twisted ribbon particles with dimensions of 1,000 – 5,000 nm in length and less than 100 nm in diameter. The most usual sources are low molecular weight sugars such as glucose, fructose, sucrose, arabitol and mannitol (Ilyas et al., 2018).
Bacterial fermentation is a process where bacteria are inoculated in an aqueous medium to grow through consumption of the carbon source (often glucose) and other essential nutrients as nitrogen sources and mineral salts. The synthesis of nanocellulose occurs at the same time during the bacterial fermentation, by self-assembling in a pellicle at the air-liquid interface of the culture medium. The pellicle is composed of a complex entangled network and can be carefully harvested through gently lifting or using filtration. The harvested pellicle is washed to remove any remaining bacterial cells, nutrients and by-products. Nanocelluloses may be then dried (air drying or freeze-drying) to remove excess water and to make easier to handle.
3.4. Properties and Applications
Nanocelluloses exhibit remarkable properties including biodegradability (can replace non-biodegradable materials), renewability (produced from cellulose which is abundant in nature), biocompatibility (can be used in a biological system without causing any harm to it), high aspect ratio and surface area (which may improve interactions with other materials), transparency (afford transparent films and suspensions), surface functionalization ability (can be modified by the incorporation of new functional groups), barrier properties (which enhance the shield against gases and liquids), hydrophilicity (improving the dispersion in water-based systems), excellent mechanical properties (high tensile strength and Young´s modulus), tunable electrical conductivity (allow the creation of materials from insulating to conductive) (Kaur et al., 2021; Mondal, 2017; Siqueira et al, 2010).
Nanocelluloses are very versatile and valuable materials with a wide range of applications such as in electronic devices (transparent and flexible films), construction (reinforcing agents), automotive industry (lightweight and sustainable materials), biomedical and pharmaceutical area (drug delivery systems), cosmetics (texture and stability of lotions), packaging (biodegradable materials), coatings (water repellency), textiles for clothing (breathable and moisture-wicking sportswear), and environmental (water purification processes) (Kaur et al., 2021; Nasir et al., 2017; Poulose et al., 2022; Thomas et al., 2018).
4. Conservation and Restoration of Historical Documents
Paper was originally produced from rags of linen, hemp and cotton (Sánchez Hernampérez, 1999) which consisted of crystalline cellulose of high quality with strong structure and excellent chemical stability. The demand for paper in the mid-nineteenth century, the invention of the printing press and the scarcity of rag sources made it necessary to search for other raw materials. Paper has played a crucial role for centuries and continues to be an important medium for recording and communicating information in the form of books, scientific journals, magazines, newspapers, historical documents and archives. The deterioration of paper is still a problem, caused by many factors such as the acid and alkaline hydrolysis, oxidation processes, inks and pigments, insects and microorganisms, humidity and light absorption, air pollution or storage conditions (Zhang et al., 2003). Mechanical damages like tears, cuts and deformations can also deteriorate paper. The most common degradation process is the acid hydrolysis contributing to a significant decrease in the mechanical properties of cellulose (Liu et al., 2021; Völkel et al., 2017; Wang et al., 2021). Acid hydrolysis can be caused by acids formed from cellulose and hemicelluloses with aging (formic, lactic and oxalic acids), the presence of non-removed lignin and of inks, and by the absorption of air pollutants (mostly sulfur and nitrogen oxides) (Jablonsky et al., 2020).
The conservation and restoration of cultural heritage is a promising and innovative application for nanocelluloses. Nanocelluloses can be used as: (i) consolidating agent, to prevent further deterioration of fragile materials; (ii) adhesive, to repair and restore damaged or detached components; (iii) ink and pigment stabilizer, to prevent bleeding or fading of inks and pigments; (iv) anti-aging agent, by creating a protective barrier against oxidative and environmental damages; (v) humidity controller, to maintain stable environmental conditions; (vi) ultraviolet radiation protector, to prevent fading and deterioration of the artifacts and artworks; (vii) antibacterial agent, by inhibiting growth of microorganisms.
4.1. Iron Gall Ink
Documents in paper written with iron gall ink often require careful conservation and restoration efforts. The acidity of the iron gall ink can lead to degradation of paper including weakening, embrittlement and discoloration, resulting in the so-called iron gall ink corrosion. Conservators use specialized techniques to stabilize and repair documents that may have been damaged by the iron gall ink.
Iron gall ink was widely used for centuries from the Roman Empire to the Renaissance and is the primary ink used for writing and drawing in Europe and the Middle East during the medieval and early modern periods. The documents written with iron gall ink present a blue-black color. However, over time the iron gall ink can darken and turn blacker due to the oxidation of iron ions. Iron gall ink was commonly used for legal documents, manuscripts, religious texts, letters and other important written records due to the dark color, resistance to fading and excellent adhesion to paper. The permanent character of iron gall ink on the respective cellulosic support provided high protection against forgery of documents contributing to its popularity (Banik, 2009).
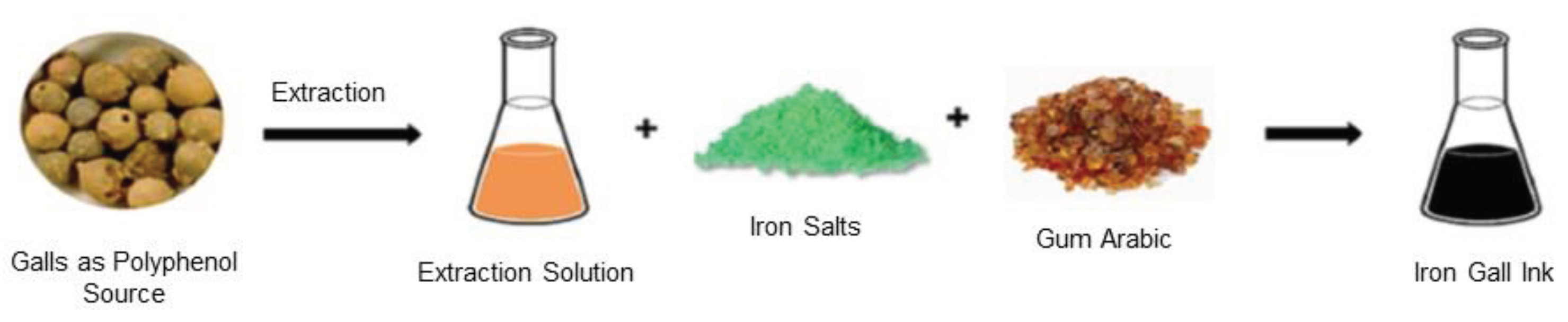
Formation of iron gall ink results from the combination of iron salt (ferrous sulfate, also called green vitriol), tannic acids often derived from galls, bark, leaves, roots or other plant materials (gallic acid), binding agent (gum Arabic) and solvent (water, beer or wine) (
Figure 2). The tannin-rich sources are crushed and soaked in the solvent to extract gallic acid that will react with ferrous (II) sulfate to produce a blue-black iron (III)-tannin complex, known as ferrogallotannate or ferrotannate, which is insoluble in water. Gum Arabic, soluble in water, is added to increase the viscosity, to keep the pigment particles in suspension and to bind the ink to the writing surface. The mechanism consists in the formation of an iron (III)-tannin complex from the air oxidation of iron (II)-tannin complex which is colorless and soluble in water. Low pH of iron gall ink between 2 and 3 confers a high acidity to the paper requiring proper storage in controlled environments (light, temperature and humidity) (da Costa et al., 2016).
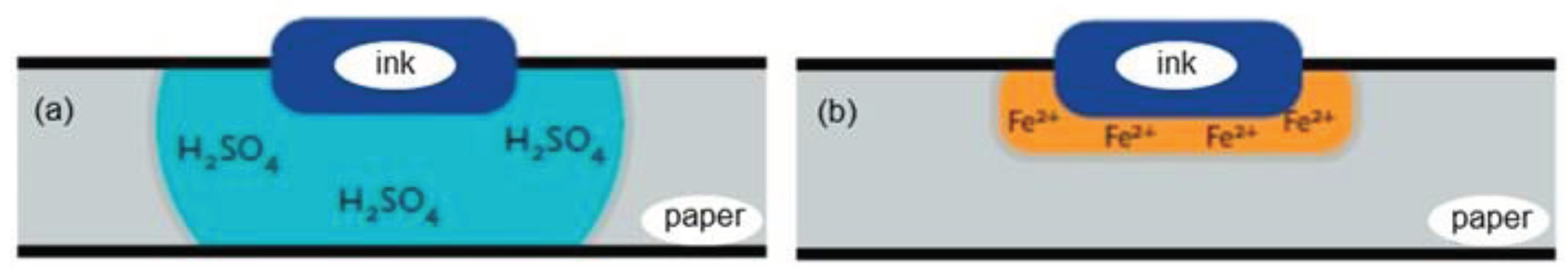
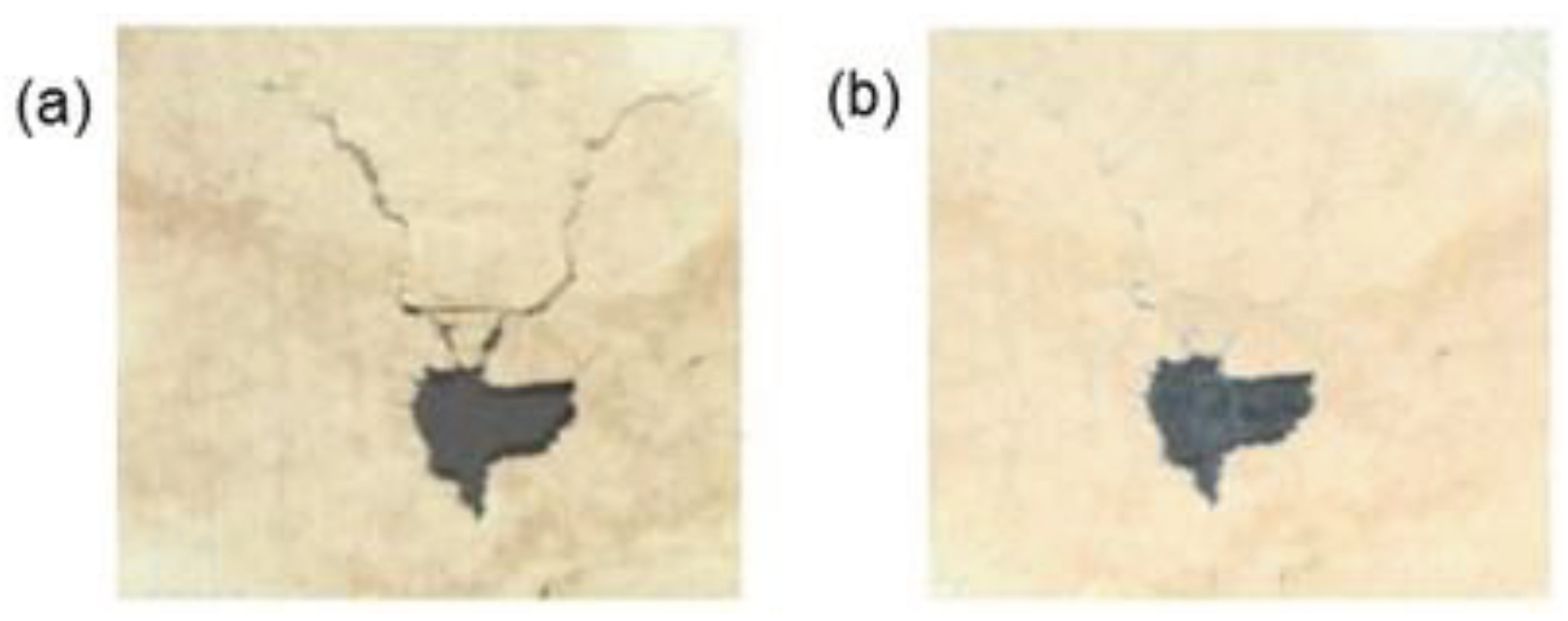
Iron gall ink was replaced by modern commercial inks because it is corrosive and can damage paper putting in risk a significant part of the cultural heritage. For instance, the complete works of Victor Hugo almost disappeared and 60 – 70 % of Leonardo da Vinci’s legacy presents signs of deterioration (Scholten, 1997). The main reasons for the corrosion caused by iron gall ink are acid hydrolysis and oxidation of cellulose which contribute to the weakening and discoloration (Reissland, 2000; Reissland, 2001; Van Gulik & Kersten, 1994). Acid hydrolysis results from the acidity of the iron-gall ink components and the sulfuric acid produced during the preparation of the iron-gall ink, where both act as catalysts leading to the chain scission of cellulose (Costa et al., 2015). The process can continue over the centuries, except if the acid is neutralized, contributing to a loss of the paper mechanical strength (Neevel & Mensch, 1999). Oxidation process is caused through the excess of iron (II) ions, used to produce the iron-gall ink, which catalyze the formation of very reactive hydroxyl radicals from hydrogen peroxide by Fenton reaction (Botti et al. 2005). The hydrogen peroxide is formed during the reduction of atmospheric oxygen by iron (II) ions. Hydroxyl radicals start a sequence of radical reactions resulting in the scission and cross-linking of cellulose, thus contributing to a loss of mechanical strength and a decrease in water absorption of the paper structure (Reißland & Graaff, 2001). The localization of the paper degradation can be surrounding the ink through the long migration of sulfuric acid in the acid hydrolysis or can be close to the ink through the short migration of iron (II) ions in the oxidation process (
Figure 3) (Reißland & Graaff, 2001).
4.2. Conservation and Restoration Methods
Conservation and restoration methods for documents in paper are essential to preserve and protect cultural heritage. The main goals are to prevent the degradation, repair the damage, improve the legibility, maintain the integrity and extend the lifetime of the documents. Conservation and restoration methods involve several main steps namely: (i) assessment and documentation to identify physical damage or deterioration; (ii) surface cleaning to remove dirt, dust and surface contaminants; (iii) testing and analysis to determine paper composition, ink type and chemical issues, in order to choose the best method to apply; (iv) deacidification to neutralize acidic compounds that can lead to degradation; (v) consolidation, repair and mend using an appropriate method to reinforce weakened areas and to treat tears and losses (includes the use of Japanese Paper and the calcium phytate method); (vi) final checks to ensure that the work has been successfully completed and the document is stable, preserved and ready for storage or display. Some of the most common methods used in the conservation and restoration of paper documents are described below.
4.2.1. Surface Cleaning
Surface cleaning can include disinfestation (elimination of rodents and insects) and disinfection/sterilization (extermination of bacteria, viruses and fungi) to protect the artworks from biological agents (Zervos & Alexopoulou, 2015). The most common methods for disinfestation are: (i) fumigation with chemicals like methyl bromide or sulfuryl fluoride to kill or repel pests; (ii) anoxia, involving the creation of an oxygen-free environment to suffocate pests; (iii) cold storage, consisting on subjecting the paper to very low temperatures slowing down or stopping the activity of pests. The disinfection/sterilization processes include: (i) ultraviolet light treatment consisting in the exposition of paper to an ultraviolet light to kill surface microorganisms (Cappitelli et al., 2020); (ii) autoclaving by subjecting the paper to a high-temperature steam under pressure to kill all microorganisms; (iii) gamma radiation treatment involving the irradiation of paper with gamma radiation to sterilize paper (Choi et al., 2012).
Mechanical cleaning is another method of surface cleaning used to remove dust, dirt and contaminants from the paper surface for aesthetic reasons (Zervos & Alexopoulou, 2015). The most typical techniques include: (i) soft brushes made of natural hair or synthetic materials to gently brush away dust and dirt; (ii) special sponges designed to attract and absorb dirt and contaminants; (iii) rubber erasers such as vinyl or gum erasers, used for gentle rubbing to lift off surface dirt. Mechanical cleaning relies on the application of mechanical energy without the use of solvents or liquids. However, this method can be a disruptive process which may cause abrasion of the paper surface, forcing contaminants into the paper structure and causing severe damage.
4.2.2. Deacidification
Deacidification of paper is a chemical process which involves the introduction of alkaline substances to neutralize the acids and to create an alkaline reserve to neutralize any acidic substance that might be formed (Baty et al., 2010; Cedzova et al., 2006; Hubbe et al., 2017). High acidity levels contribute to the deterioration of paper causing brittleness and instability. Deacidification helps to stabilize, prevent further deterioration and prolong the lifetime of paper document (Zhang et al., 2023); however, it cannot restore the loss of mechanical strength (Zervos and Alexopoulou, 2015). Liquid (aqueous and organic solvents) and gaseous processes can be used. Aqueous deacidification involves the use of a liquid solution containing alkaline agents (calcium bicarbonate or magnesium bicarbonate) where the paper is immersed or sprayed. This process causes fiber swelling and color bleeding, and cannot be used on documents that are sensitive to water or contain water-soluble inks. Organic solvents deacidification uses volatile organic solvents with dissolved alkaline agents (magnesium carbonate in methanol or barium hydroxide in methanol) and the paper is soaked or sprayed. This process wets the paper more rapidly, provokes less fiber swelling and can be employed on books and other bound documents. The main drawback is the flammability and toxicity of the organic solvents. Gaseous deacidification involves exposing the paper to alkaline gases (ammonia or amines) within a sealed chamber or enclosure. This process may not produce the amount of alkaline reserve necessary and some deacidification agents may introduce the risk of burning and explosion. The principal advantage is its suitability to be employed on books and other bound documents. The documents can be treated individually as single paper sheets (aqueous deacidification) or in groups as books and bound documents (organic solvents deacidification and gaseous deacidification). Mass deacidification process has been developed to rescue books and bound documents being very useful for libraries, archives and institutions with extensive collections (Cunha, 1987; Malešič et al., 2022).
4.2.3. Japanese Paper
Japanese Paper, often referred as washi, is a traditional handmade paper that has been used for centuries in Japan and is among the best materials for the conservation and restoration methods (Tkalčec et al., 2016). The Japanese Paper can be obtained from three different types of fibers as kozo bush (longest fiber that makes the strongest paper), mitsumata shrub (softer and shorter fiber with a warm tone) or gampi tree (noble fiber with an exquisite natural sheen). The most commonly used material is kozo which can be found in 90 % of all Japanese Paper used for conservation and restoration purposes (Tkalčec et al., 2016). The kozo fibers are cooked with a mild alkaline solution of wood ash (K₂CO₃), soda ash (Na₂CO₃) or lime (Ca(OH)₂) in traditional methods or a strong alkaline solution of caustic soda (NaOH) in modern methods to help break down the fibers and to remove lignin and other impurities. Japanese Paper presents unique properties namely: (i) longevity (is made from fibers that are naturally resistant to deterioration); (ii) acid-free, and alkaline pH (prevents acid migration and degradation of the repaired area over time); (iii) strength and durability (reinforces weakened or torn areas); (iv) semi-transparency (allows to see and/or read the printed or handwritten text); (v) flexibility (adapts to the contours of the repaired area); (vi) adhesion ability (creates strong bonds that are reversible and can be easily removed if necessary) and (vii) reversibility in the adhesion (allows to remove or adjust repairs without causing harm to the original document). The basis to repair and mend a document with this material is to apply a thin layer of adhesive to Japanese Paper or to original document, place one on top of the other and press down gently to ensure that they are glued together. The paper surface must be cleaned and free from dust, dirt and contaminants before Japanese Paper is applied. The used adhesives need to have sufficient bonding strength, resistance to aging, color stability, chemical inactivity and be easily removed without using severe conditions, toxic solvents and complicated procedures (Zervos & Alexopoulou, 2015). Wheat starch paste is the most common adhesive and can be simply removed by adding moisture. Other adhesives are gelatin, chitosan, hydroxypropylcellulose, methylcellulose, polyvinyl alcohol and polyethylene (Jacobi et al., 2011; Zervos & Alexopoulou, 2015). Japanese Paper is available in a wide range of types and thicknesses that can be used in machine made papers, handmade papers, brittle papers, manuscripts and drawings, where the focus is to stabilize and reinforce the damaged paper-based materials.
4.2.4. Calcium Phytate Method
The calcium phytate method is, presently, the most effective method used for the conservation and restoration of paper documents written with iron gall ink and was proposed for the first time by Neevel in 1995. The phytic acid is a natural antioxidant present in the seeds of plants, which can block the oxidation process of unsatured fatty acids catalysed by iron. Phytate is the anion of the phytic acid, myo-inositol hexakisphosphate or inositol polyphosphate, a strong chelating or complexing agent of important metal ions. The “free” iron ions (not coordinated to tannins) responsible for the cellulose oxidation process will be inactivated by phytates (Neevel, 1995). The reaction between the iron ions and phytates leads to the formation of high-affinity complexes preventing their participation in Fenton oxidation reactions (Rouchon et al., 2011). Various salts of phytic acid such as sodium, magnesium and calcium salts were tested and all are equally effective with some side effects (Botti et al., 2005). The sodium phytate is very soluble and can migrate to the borders of the paper during drying causing brownish discoloration. The treatment with magnesium phytate tends to produce a brown ink and a yellow paper. The calcium phytate allows the migration of the ink changing the color. Treatments with phytates must always be followed by deacidification to neutralize the acidity of paper and prevent cellulose acid hydrolysis (Zervos & Alexopoulou, 2015). Calcium phytate method involves the immersion of paper in a calcium phytate solution with a pH between 5.0 – 6.0 (adjusted using ammonia) to complex iron (II) and iron (III) ions where the calcium ions can exchange with the iron (II) and iron (III) ions forming iron-phytate complexes. Paper is also immersed in a gelatin solution bath to add a protective film between the atmosphere and the surface of the ink increasing mechanical strength and flexibility of paper (Melo et al., 2022). The gelatin is used as a resizing agent for paper documents written with iron gall ink, since it possesses a better blocking effect towards ink corrosion compared to other adhesives commonly used for paper conservation (Kolbe, 2004). Calcium phytate method was developed specifically for paper documents written with iron gall ink and several studies have proven that it is very effective (Henniges & Potthast, 2008; Henniges et al., 2008).
5. Nanocelluloses in Historical Documents
One of the methods used by conservators to restore damaged paper is the application of a reinforcing layer on the paper surface, known as lining, which allows the loss of mechanical resistance to be recovered (Bansa and Ishii, 1997). The material typically used for this purpose is Japanese Paper which provides a high resistance, but it is not entirely satisfactory for all documents because the sheets are not very homogeneous (Gómez et al., 2017). Nanocelluloses have emerged as a novel and innovative approach in the conservation and restoration of paper documents because they can solve many challenges such as structural weaknesses, pH instability, moisture exposure and damage repair. The natural compatibility to the paper matrix (cellulose) and the inherent characteristics of nanocelluloses allows them to form strong, transparent and fibrillar networks which is very important to paper stabilization (Völkel et al., 2017). Nanocelluloses act as a coating layer for protecting diverse cellulose-based materials leading to consolidation, strengthening, and improvement of barrier properties (Ferrer et al., 2017; Mondal, 2017; Wang et al., 2019). The protective coating should not change the physical, chemical and optical properties of the paper substrate including shine or color saturation (Xu et al., 2020). Nanocelluloses are adequate because they are transparent, act as a nano-lining and tend to accumulate on the surface of the paper with limited penetration (Bridarolli et al., 2018; Nechyporchuk et al., 2018; Santos et al., 2016a; Völkel et al., 2017). The use of nanocelluloses also meets the requirement of sustainable materials based on a renewable and biodegradable resource (Dufresne, 2013).
Nanocelluloses have been employed in several studies in the field of conservation and restoration which will be reported by chronological order.
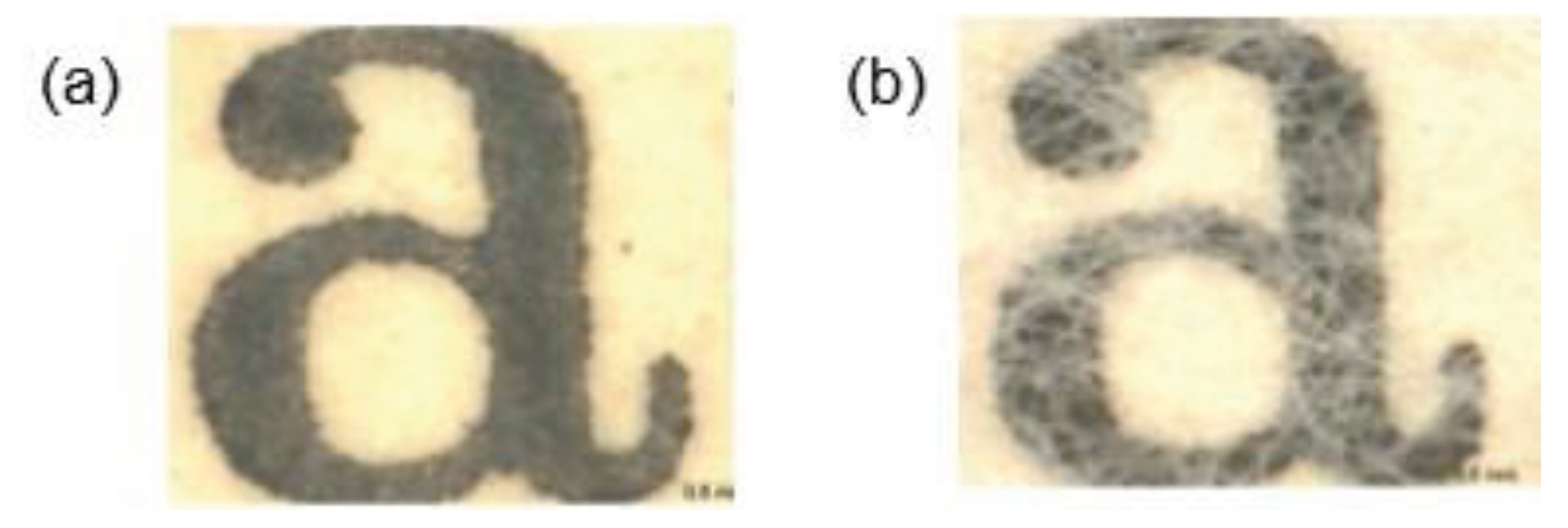
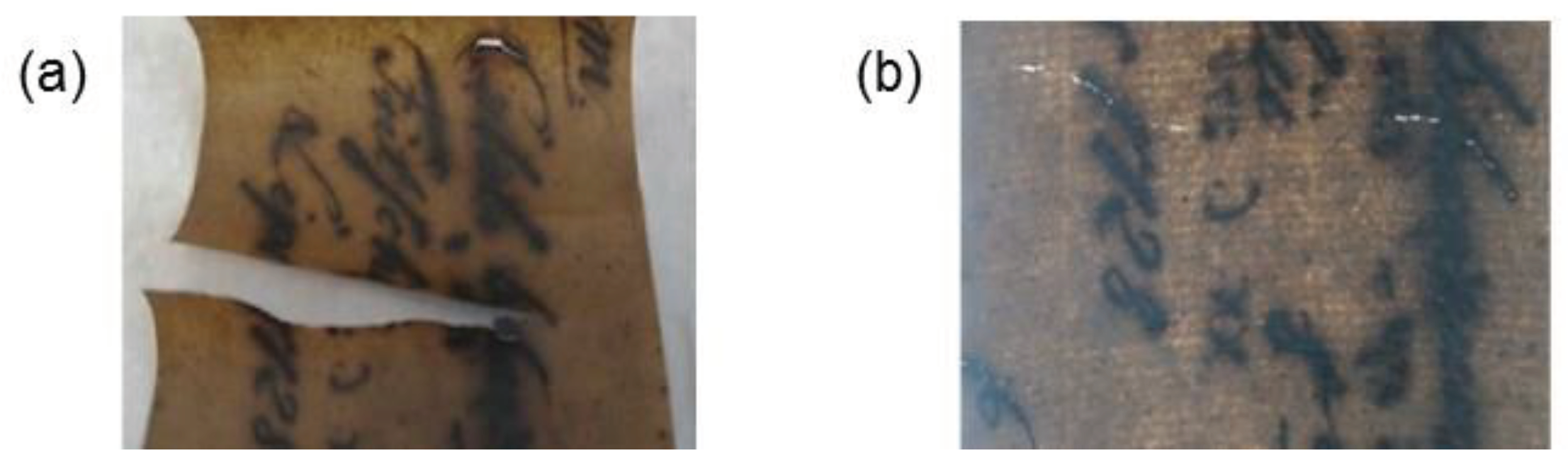
Santos et al. (2016b) compared the application of bacterial cellulose with Japanese Paper to reinforce books from 1940 to 1960. Similar mechanical properties were found with an improvement in the optical properties (better legibility of letters) when using bacterial cellulose compared to Japanese Paper (
Figure 4). Paper reinforced with bacterial cellulose exhibited Gurley air resistance higher than 900 s/100 mL, while Japanese Paper showed values between 20 and 40 s/100 mL. Bacterial cellulose provided high stability and enhanced the quality of deteriorated paper over time. The results indicated that the closed structure of bacterial cellulose offers adequate protection against humidity and atmospheric pollutants.
Völkel et al. (2017) tested bacterial cellulose suspensions and a mechanically nanofibrillated cellulose suspension based on wood pulp to stabilize rag papers from the nineteenth century and a book paper from the twentieth century. The mechanically damaged areas were treated and the weakened areas were consolidated without an additional adhesive and negative side effects in long term (
Figure 5).
Jia et al. (2017) prepared a coating based on cellulose nanocrystals and zinc oxide suspensions using Klucel as adhesive to protect a school newspaper from 1960 belonging to the Renmin University of China. An improvement in mechanical properties (tensile strength from 2.7 to 3.2 kNm-1), stronger antibacterial and antifungal activity (Aspergillus niger colonial area of 75.6 % and 7.8 %) and higher protection against UV light (color difference of 3.7 and 1.6) were revealed for treated paper in comparison with untreated paper. The microbiological tests were made with two bacteria found in everyday life (Staphylococcus aureus and Escherichia coli) and five fungi often detected in archives or museums (Aspergillus niger, Aspergillus versicolor, Rhizopus nigricans, Saccharomycetes and Mucor).
Camargos et al. (2017) studied an aqueous dispersion of cellulose nanocrystals, calcium carbonate, propylene glycol and methylcellulose as an alternative material to fill lacunae of paper sheets from a book printed in the twentieth century. Calcium carbonate is the filler, propylene glycol is the plasticizer, and methylcellulose is the sizing agent. The properties of the cellulose nanocrystal-based composite were compared with the properties of conventional paper. The crystallinity index of the cellulose nanocrystal-based composite (80.8 %) was around three times higher than that of the conventional paper (27.2 %). The cellulose nanocrystal-based composite presented mild basic character (pH of 7.5) which is advantageous over the acidity of the conventional paper (pH of 6.0). Similar mechanical properties were found in cellulose nanocrystal-based composite (Young’s modulus of 310 MPa) and conventional paper (Young modulus of 306 MPa).
Dreyfuss-Deseigne (2017a, 2017b, 2017c) developed microfibrillated cellulose films to mend paper viewing slides from the mid-nineteenth century belonging to the French Museum of Cinema. The remarkable transparency and the very good stability to light, temperature and humidity aging allowed treatment of structural problems in the slides such as weaknesses, tears and losses (
Figure 6). The microfibrillated cellulose film and different types of Japanese Paper (gampi paper, hemp paper, kozo paper and tengujo kozo paper) were compared for a few physical properties (Dreyfuss-Deseigne, 2017a). Among the Japanese Papers, the gampi paper and the tengujo kozo paper showed the best performance. However, almost all the results were worse than those obtained for the microfibrillated cellulose film such as luminance (40 % for tengujo kozo paper and 38 % for microfibrillated cellulose film), color changes during light aging (0.8 for tengujo kozo paper and 0.3 for microfibrillated cellulose film) and thickness (270 μm for gampi paper and 110 μm for microfibrillated cellulose film). The microfibrillated cellulose film and the Japanese Papers were also combined with four adhesives generally utilized in paper conservation (wheat starch paste, methylhydroxyethylcellulose, hydroxypropylcellulose (Klucel G) and Culminal MC2000). The Klucel G exhibited the best results compared to the other types of adhesives in terms of tension, planar distortion and adhesion. This adhesive was the only one that could be directly used with the microfibrillated cellulose film because it is ethanol-based and does not cause shrinkage unlike the others which are water-based adhesives.
Xu et al. (2020) studied a self-healing coating based on a composite of modified nanocellulose, calcium carbonate, fluorineacrylamide styrene and acrylic copolymer to protect paper cultural relics. The calcium carbonate microcapsules are responsible for the properties of self-healing and could release the inside healing agents to repair the film cracks when necessary. A comparison between the composite without and with calcium carbonate was made demonstrating an increase in hardness (from 87.7 to 94.3 shore A), tensile strength (from 2.3 to 5.4 MPa) and water resistance (contact angle of 68 and 98°). Another comparison between untreated paper relics and relics treated with the composite containing calcium carbonate was conducted revealing an improvement in thickness (from 73 to 82 μm), tensile strength (from 358 to 2068 Nm-1) and antiaging properties (mass loss rate of 13.3 and 2.4 %).
Völkel et al. (2020) tested a calcium phytate/calcium hydrogen carbonate treatment combined with the application of nanofibrillated cellulose suspensions to stabilize chemically and mechanically rag papers written with iron gall ink from a collection of sermons from 1839 and 1840. The viability of the procedure was confirmed with the benefits of chemical stabilization (deacidification) of iron gall ink with calcium phytate/calcium hydrogen carbonate treatment and an additional significant mechanical stabilization of iron gall ink paper with the nanofibrillated cellulose application. Damaged samples, very sensitive to be handled with the risk of material loss, could be chemically and mechanically stabilized. Fractures, cracks and imperfections were closed and sealed making the samples manageable with a minimum risk of future damage (
Figure 7). Nanofibrillated celluloses acted as a protective layer and had a minimum influence on the optical and haptic properties of the manuscripts. The integration of nanofibrillated celluloses into the calcium phytate/calcium hydrogen carbonate treatment is important because an additional stabilization and drying step is avoided.
Operamolla et al. (2021) employed sulfated and neutral cellulose nanocrystal suspensions to restore and reinforce a book entitled “Breviarium Romanum ad usum Fratrum Minorum” from the eighteen century. The papers treated with neutral cellulose nanocrystal exhibited an improvement in optical quality and mechanical properties such as maximum stress (7.8 MPa for untreated papers, 7.3 MPa for papers treated with sulfated cellulose nanocrystals and 12 MPa for papers treated with neutral cellulose nanocrystals) and maximum strain (1.4 % for untreated papers, 1.6 % for papers treated with sulfated cellulose nanocrystals and 2.2 % for papers treated with neutral cellulose nanocrystals). This study also showed that the presence of surface sulfation may have a negative influence on the conservation of a paper artifact compromising its pH and mechanical properties with aging unlike neutral cellulose nanocrystals.
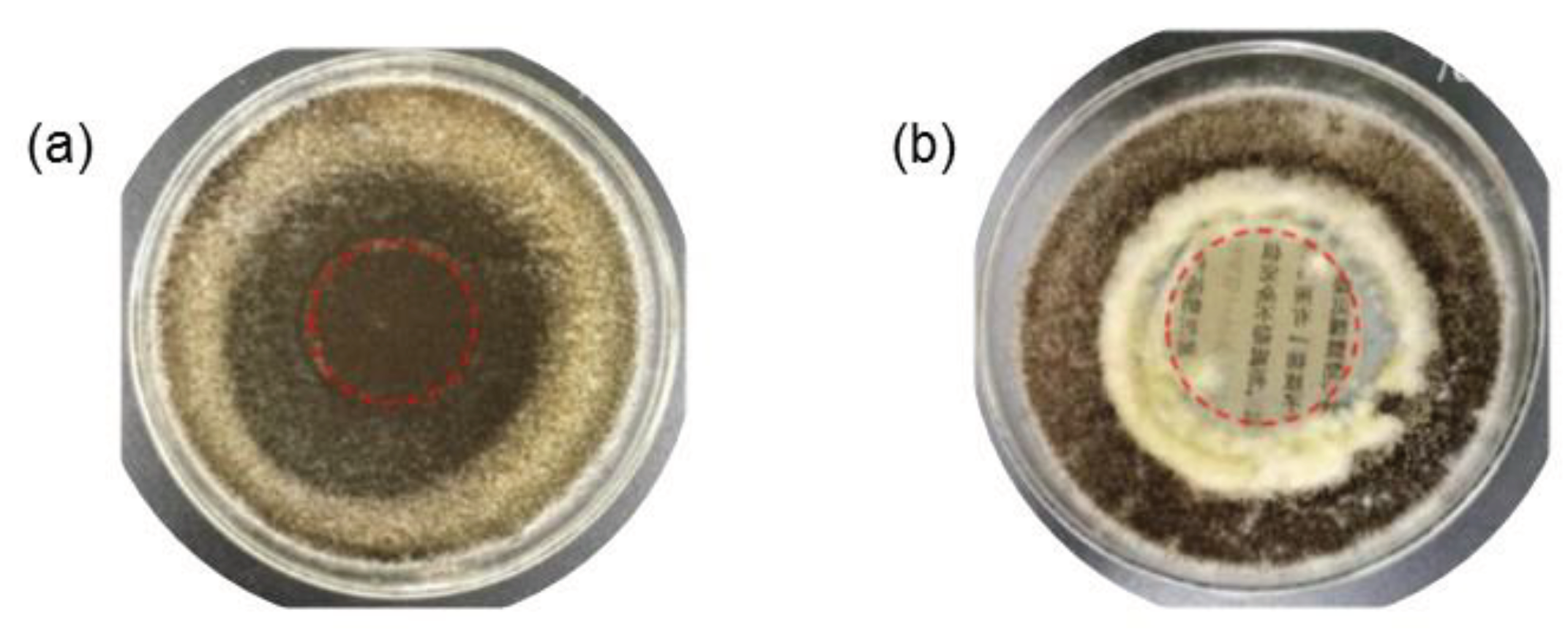
Ma et al. (2021) applied suspensions of cellulose nanocrystals with polyhexamethylene guanidine to reinforce ancient books. An excellent biocidal activity against
Aspergillus Niger and mixed mold (
Aspergillus Niger,
Trichoderma Viride,
Penicillium Funiculosum,
Chaetomium Globosum) was demonstrated with a growth trace of less than 10% for treated paper (
Figure 8). An improvement in mechanical properties (tearing strength of 3.6 and 4.7 mNm
2g
-1; tensile strain of 1.4 and 2.1 %) and outstanding performance in aging tests were also observed for treated paper in comparison with untreated paper.
Henniges et al. (2022) tested microfibrillated cellulose films, prepared with and without methylcellulose as an “internal adhesive”, to repair historic papers from the collection of The National Archives in UK. The addition of methylcellulose to pure microfibrillated cellulose films provided an increase in transparency and mechanical strength of the films. The two microfibrillated cellulose films were compared with two commercial nanocellulose films (Innovatech Nanopaper Art Paper and Innovatech Nanopaper Art Paper Pro) and one Japanese Paper (tengujo). The microfibrillated cellulose films and the Innovatech Nanopaper Art Paper exhibited similar properties, unlike the Innovatech Nanopaper Art Paper Pro possibly due to differences in the production process. Compared with the Japanese Paper, the microfibrillated cellulose films showed an increased tendency of curling when moistened and lower permeability. The different films and Japanese Paper were combined with two adhesives (Klucel G and Isinglass) commonly utilized for mending tears by the conservators of The National Archives in UK. Klucel G used in tracing papers exhibited the best results for planar deformation, but the worst results for adhesive strength compared with Isinglass. The latter adhesive generated the most significant distortion when applied to smooth films such as Innovatech Nanopaper Art Paper Pro. Tracing papers repaired with microfibrillated cellulose films containing methylcellulose presented a higher tensile strength than when repaired with the other types of films. This property was measured by the mass load required to break the tear mend and the results with Klucel G were 1600 g for microfibrillated cellulose films without methylcellulose, 1900 g for microfibrillated cellulose films with methylcellulose, 1700 g for Innovatech Nanopaper Art Paper, 1200 g for Innovatech Nanopaper Art Paper Pro and 700 g for Japanese Paper. The results with Isinglass were 800 g for microfibrillated cellulose films without methylcellulose, 2200 g for microfibrillated cellulose films with methylcellulose, 1300 g for Innovatech Nanopaper Art Paper, 1600 g for Innovatech Nanopaper Art Paper Pro and 600 g for Japanese Paper. The best combination to repair historic tracing papers was the microfibrillated cellulose film containing methylcellulose with Klucel G, affording satisfactory transparency, high strength after adhesion, stable aging properties and potential for customization.
Völkel et al. (2022) prepared suspensions of cellulose nanofibers to stabilize historical papers damaged during the fire of the Duchess Anna Amalia Library in Germany in 2004. The artworks were from the eighteenth and early nineteenth century made of rag paper and a large part belonged to musical literature. The application of the cellulose nanofiber suspensions did not show a negative visual impact and provided mechanical stabilization in long-term reducing paper fragility.
Camargos et al. (2022) studied multifunctional coatings based on cellulose nanocrystals, cellulose nanofibrils and lignin nanoparticles in aqueous dispersions to protect paper-based artifacts. The coatings revealed to be effective against UV radiation and moist-heat aging (color difference of 6.5 for untreated paper and 1.9 for treated paper) protecting or attenuating the paper degradation. Surface morphology, roughness and vapor permeability of paper were mainly maintained after the coating application. A decrease in wettability (enhancement of water resistance) with contact angle of < 20° for untreated paper and 60° for treated paper was also observed (
Figure 9).
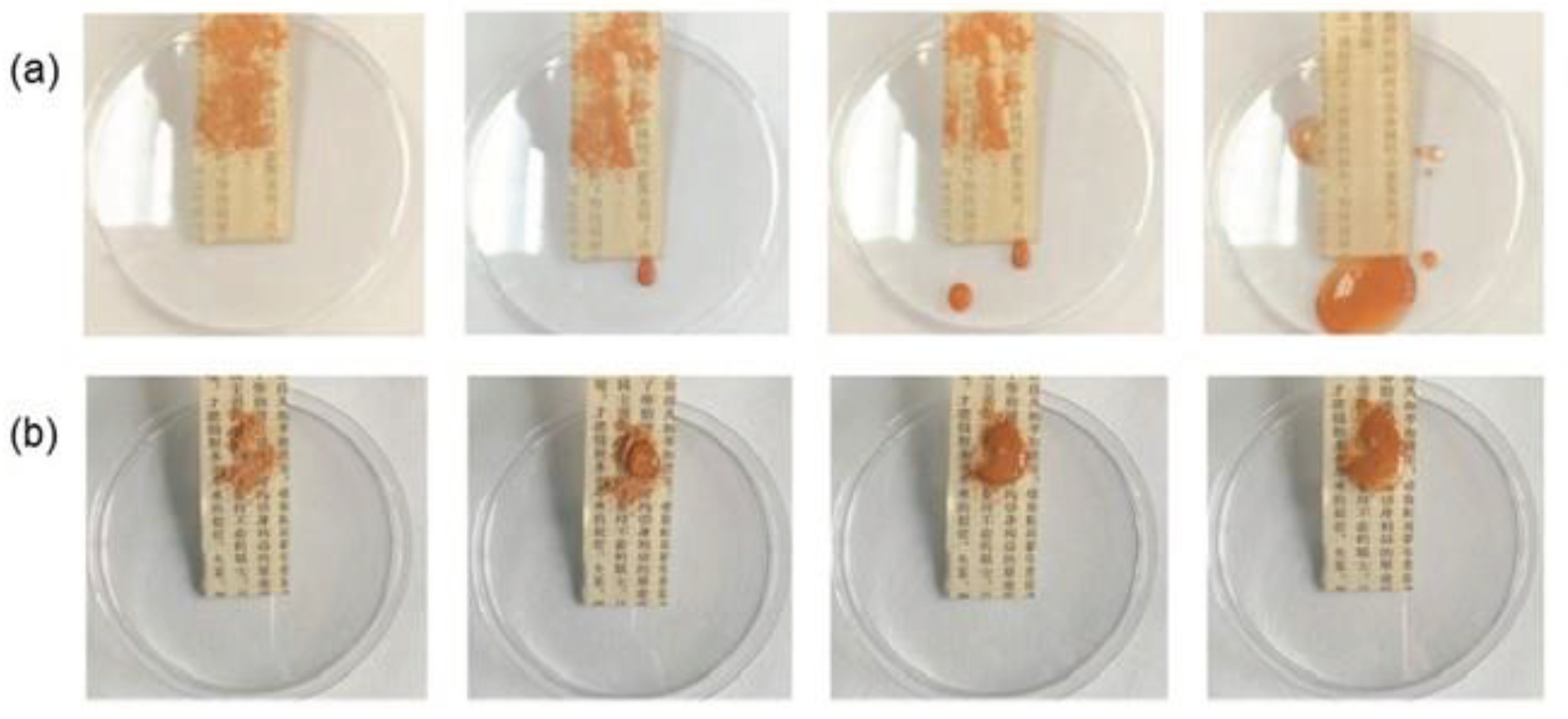
Ma et al. (2022) used superhydrophobic self-cleaning coatings based on cellulose nanocrystals, calcium carbonate and polydimethylsiloxane suspensions to reinforce and re-repair historical books. The cellulose nanocrystals were responsible for the high mechanical strength achieved and the calcium carbonate nanoparticles for the excellent properties of self-cleaning and deacidification effect (removal of the acidic substances). The coated historical paper was hydrophobically modified using methyltrimethoxysilane which is a low surface energy modifier. An increase in thickness (75 to 92 μm), water contact angle (87.5 to 152.5°) and roughness (3.8 to 4.3 μm) was demonstrated for treated paper. The self-cleaning property was confirmed by placing garden soil on the paper surface and then washing it. Untreated paper quickly became wet and contaminated unlike treated paper where water droplets rolled down from the paper surface (
Figure 10). The pH value of treated paper (7.5–7.8) was appropriate for the purposes of paper preservation compared to untreated paper (5.3), since the recommended pH value is between 7.0 and 8.5. A general improvement in the mechanical properties was observed for treated paper in terms of tensile strength (28.6 to 33.9 Nmg
-1) and tearing strength (3.8 to 4.3 mNm
2g
-1).
Elmetwaly et al. (2022) applied multifunctional protective coatings based on inorganic nanotubes dispersed in cellulose nanocrystals to reinforce historical papers. An improvement in the tensile strength (from 12.4 to 15.8 N), elongation at break (from 0.3 to 0.8 %), thermal stability and UV protection (from 39.0 to 49.7) without altering the optical properties of the paper substrate was revealed.
All the studies showed that bacterial cellulose, cellulose nanofibrils and cellulose nanocrystals with or without other particles such as zinc oxide, calcium carbonate, polyhexamethylene guanidine, lignin nanoparticles and inorganic nanotubes in suspensions or films are promising and innovative materials for reinforcing, mending, filling, protecting and stabilizing historical documents.
Table 2 presents a summary of the main results obtained from the applications of nanocelluloses in historical documents.
The most important application of nanocelluloses in the field of conservation and restoration of paper documents is as a reinforcing agent due to the formation of strong hydrogen bonds with the different hydroxyl groups among the cellulosic fibers. The inherent compatibility of nanocelluloses with paper results from the fact that cellulose is the primary component of paper and nanocelluloses are derived from cellulose. Paper strength depends on paper fibers strength, strength of bond between fibers and tendency of entanglement of fibers in the paper (Li et al., 2021). The introduction of cellulose particles with nanoscale dimensions will contribute to fill the voids in the fiber-to-fiber contact area, increasing the number of hydrogen bonds formed, the bonded area, and thus improving the strength of paper without compromising the appearance or texture (Perdoch et al., 2022).
The use of Japanese Paper is the approach most employed in the conservation and restoration of paper documents. The long-standing traditional Japanese Paper offers a fibrous structure, strength and flexibility to make it a reliable choice for repairing tears, reinforcing fragile areas and infilling losses in historical documents. However, Japanese Paper has limitations regarding the fiber size and potential variability in quality, limited mechanical strength and barrier properties especially at low grammages, low transparency, and visually structures the paper surface (Dreyfuss-Deseigne, 2017a; Rouchon et al., 2012; Völkel et al., 2017). On the other hand, nanocelluloses are innovative materials with high transparency and remarkable mechanical properties providing a significant reinforcing effect on paper, enhancing durability and document longevity. However, being a relatively recent alternative, the use of nanocelluloses requires in-depth study for long-term effects and presents some additional drawbacks related to methodologies standardization, cost considerations and large-scale production. Both approaches have advantages and the choice depends on the specific conservation and restoration goals.
6. Conclusions
The most popular medium for recording and communicating information through the times is paper which can deteriorate with aging due to inks and pigments, insects and microorganisms, humidity and light absorption, air pollution and storage conditions. Nanocelluloses are environmentally friendly and sustainable representing a class of materials with an extraordinary potential in the area of conservation and restoration of paper documents allowing to improve the mechanical strength and maintain the authenticity and integrity of the artworks. Nanocelluloses can increase the resistance to tears, wrinkles and losses which is particularly valuable for handling and exhibition of historical documents. Their barrier properties against environmental factors slow down and/or inhibit the degradation enhancing the longevity of paper substrates. Nanocelluloses can be incorporated without causing harm to the artworks and their application can be reverted which is perfectly aligned with the principles of the conservation and restoration field. Additionally, it should be noted that the optical and mechanical properties of nanocelluloses are better compared with Japanese Paper giving more transparent and stronger films even if thinner.
The efficacy of nanocelluloses in strengthening, reinforcing and stabilizing historical documents could be particularly crucial in texts written with iron gall ink that have been weakened or compromised through the problem of iron gall ink corrosion. Nanocelluloses may prevent the migration and fading of iron gall ink helping to increase the legibility and longevity of the artworks. Unfortunately, only one report was found on this matter. Systematic studies, asserting the effects of nanocelluloses in conservation and restoration of paper documents damaged by the iron gall ink should be developed.
Overall, nanocelluloses represent a promising opportunity to revolutionize the conservation and restoration field, particularly concerning paper documents, offering versatile and effective techniques for safeguarding cultural heritage. In-depth research of nanocelluloses is required involving an interdisciplinary collaboration among scientists, conservators and historians to solve some challenges such as easy availability, long-term stability, standardization of methodologies, cost-effectiveness and ethical considerations.
Funding
This research was funded by Foundation for Science and Technology – FCT (Portugal) through project “NANOCELIRONGAL – Nanocellulose films for the repair of old documents containing iron gall ink” (2022.01621.PTDC) and grant of Ricardo O. Almeida (2022.11471.BD).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Almeida, R. O., Maloney, T. C., & Gamelas, J. A. F. (2023). Production of functionalized nanocelluloses from different sources using deep eutectic solvents and their applications. Industrial Crops & Products, 199, 1−30. [CrossRef]
- Antonelli, F., Galotta, G., Sidoti, G., Zikeli, F., Nisi, R., Davidde Petriaggi, B., & Romagnoli, M. (2020). Cellulose and lignin nano-scale consolidants for waterlogged archaeological wood. Frontiers in Chemistry, 8, 1−12. [CrossRef]
- Arantes, V., Dias, I. K. R., Berto, G. L., Pereira, B., Marotti, B. S.,& Nogueira, C. F. O. (2020). The current status of the enzyme-mediated isolation and functionalization of nanocelluloses: production, properties, techno-economics, and opportunities. Cellulose, 27, 10571–10630. [CrossRef]
- Aulin, C. (2009). Novel oil resistant cellulosic materials (pulp and paper technology). Sweden: KTH Chemical Science and Engineering.
- Baglioni, P., Chelazzi, D., & Giorgi, R. (2015). Nanotechnologies in the conservation of cultural heritage. Springer: Dordrecht. [CrossRef]
- Banik, G. (2009). Scientific conservation: transfer of scientific research on ink corrosion to conservation practice – does it take place? Restaurator, 131–146. [CrossRef]
- Bansa, H., & Ishii, R. (1997). The effect of different strengthening methods on different kinds of paper. Restaurator, 18, 51–72. [CrossRef]
- Baty, J. W., Maitland, C. L., Minter, W., Hubbe, M. A., & Jordan-Mowery, S. K. (2010). Deacidification for the conservation and preservation of paper-based works: a review. Bioresources, 5, 1955–2023. [CrossRef]
- Berlin, A., Balakshin, M., Gilkes, N., Kadla, J., Maximenko, V., Kubo, S., & Saddler, J. (2006). Inhibition of cellulase, xylanase and -glucosidase activities by softwood lignin preparations. Journal of Biotechnology, 125, 198–209. [CrossRef]
- Böhme, N., Anders, M., Reichelt, T., Schuhmann, K., Bridarolli, A., & Chevalier (2020). A. new treatments for canvas consolidation and conservation. Heritage Science, 8, 1−10. [CrossRef]
- Botti, L., Mantovani, O., & Ruggiero, D. (2005). Calcium phytate in the treatment of corrosion caused by iron gall inks: effects on paper. Restaurator, 44–62. [CrossRef]
- Bridarolli, A., Nechyporchuk, O., Odlyha, M., Oriola, M., Bordes, R., Holmberg, K., Anders, M., Chevalier, A., & Bozec, L. (2018). Nanocellulose-based materials for the reinforcement of modern canvas-supported paintings. Studies in Conservation. 63, 332–334. [CrossRef]
- Brinchi, L., Cotana, F., Fortunati, E., & Kenny, J. M. (2013). Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications. Carbohydrate Polymers, 94, 154–169. [CrossRef]
- Buchanan, S. A. (1987). The brittle book problem: approaches by research libraries in the United States. The Paper Conservator, 11, 69–72. [CrossRef]
- Camargos, C. H. M., Figueiredo Junior, J. C. D., & Pereira, F. V. (2017). Cellulose nanocrystal-based composite for restoration of lacunae on damaged documents and artworks on paper. Journal of Cultural Heritage, 23, 170–175. [CrossRef]
- Camargos, C. H. M., Poggi, G., Chelazzi, D., Baglioni, P., & Rezende, C. A. (2022). Protective coatings based on cellulose nanofibrils, cellulose nanocrystals, and lignin nanoparticles for the conservation of cellulosic artifacts. ACS Applied Nano Materials, 5, 13245−13259. [CrossRef]
- Cappitelli, F., Catto, C., & Villa, F. (2020) The control of cultural heritage microbial deterioration. Microorganisms, 8, 1542. [CrossRef]
- Cedzova, M., Gallova, I., & Katuscak, S. (2006). Patents for paper deacidification. Restaurator, 27, 35–45. [CrossRef]
- Choi, J.-I., Chung, Y. J., Kang, D. I., Lee, K. S., & Lee, J.-W. (2012). Effect of radiation on disinfection and mechanical properties of Korean traditional paper, Hanji. Radiation Physics and Chemistry, 81, 1051–1054. [CrossRef]
- Cunha, G. M. (1987). Mass deacidification for libraries. Library Technology Reports, 361–472.
- da Costa, A. C .A., Corrêa, F. N., Sant’Anna, G. S., Tonietto, G. B., Godoy, J. M. O., Gonçalves, R. A., & Lutterbach, M. T. S. (2016). Kinetic study of non-reactive iron removal from iron-gall inks. Chemical Papers, 70, 602–609. [CrossRef]
- de Amorim, J. D. P., de Souza, K. C., Duarte, C. R., Duarte, I. S., Ribeiro, F. A. S., Silva, G. S., de Farias, P. M. A., Stingl, A., Costa, A. F. S., Vinhas, G. M., & Sarubbo, L. A. (2020). Plant and bacterial nanocellulose: production, properties and applications in medicine, food, cosmetics, electronics and engineering: a review. Environmental Chemistry Letters, 18, 851-869. [CrossRef]
- Díaz Hidalgo, R. J., Cordoba, R., Nabais, P., Silva, V., Melo, M. J., Pina, F., Teixeira, N., & Freitas, V. (2018). New insights into iron-gall inks through the use of historically accurate reconstructions. Heritage Science, 6, 1–15. [CrossRef]
- Dreyfuss-Deseigne, R. (2017a). Nanocellulose films in art conservation: a new and promising mending material for translucent paper objects. Journal of Paper Conservation, 18, 18–29. [CrossRef]
- Dreyfuss-Deseigne, R. (2017b). A new mending material: nanocellulose film. Journal of Paper Conservation, 18, 36–37. [CrossRef]
- Dreyfuss-Deseigne, R. (2017c). Nanocellulose films: properties, development, and new applications for translucent and transparent artworks and documents in The Book and Paper Group Annual, 36, 108–114.
- Dufresne, A. (2013). Nanocellulose: a new ageless bionanomaterial. Materials Today, 16, 220–227. [CrossRef]
- Dufresne, A., & Belgacem, M. N. (2013). Cellulose-Reinforced Composites: from Micro-to Nanoscale. Polímeros, 23, 277–286. [CrossRef]
- Elmetwaly, T. E., Darwish, S. S, Attia, N. F, Hassan, R. R. A., El Ebissy, A. A., Eltaweil, A. S., Omer, A. M., El-Seedi, H. R., & Elashery, S. E. A. (2022). Cellulose nanocrystals and its hybrid composite with inorganic nanotubes as green tool for historical paper conservation. Progress in Organic Coatings, 168, 106890. [CrossRef]
- Fang, Z., Hou, G., Chen, C., & Hu, L. (2019). Nanocellulose-based films and their emerging applications. Current Opinion in Solid State & Materials Science, 23, 100764. [CrossRef]
- Ferrer, A., Pal, L., & Hubbe, M. (2017). Nanocellulose in packaging: advances in barrier layer technologies. Industrial Crops and Products, 95, 574–582. [CrossRef]
- Fornari, A., Rossi, M., Rocco, D., & Mattiello, L. (2022). A review of applications of nanocellulose to preserve and protect cultural heritage wood, paintings, and historical papers. Applied Sciences, 12, 1−31. [CrossRef]
- Ghasemi, S., Behrooz, R., & Ghasemi, I. (2016). Extraction and characterization of nanocellulose structures from linter dissolving pulp using ultrafine grinder. Journal of Nanoscience and Nanotechnology, 16, 5791–5797. [CrossRef]
- Gómez, N., Santos, S. M., Carbajo, J. M., & Villar, J. C. (2017). Use of bacterial cellulose in degraded paper restoration: effect on visual appearance of printed paper. BioResources, 12, 9130–9142. [CrossRef]
- Gopi, S., Balakrishnan, P., Chandradhara, D., Poovathankandy, D., & Thomas, S. (2019). General Scenarios of cellulose and its use in the biomedical field. Materials Today Chemistry, 13, 59–78. [CrossRef]
- Habibi, Y. (2014). Key advances in the chemical modification of nanocelluloses. Chemical Society Reviews, 43, 1519–1542. [CrossRef]
- He, X., Deng, H., & Hwang, H. (2019). The current application of nanotechnology in food and agriculture. Journal of Food and Drug Analysis, 27, 1–21. [CrossRef]
- Henniges, U., Angelova, L., Schwoll, S., Smith, H., & Brückle, I. (2022). Microfibrillated cellulose films for mending translucent paper: an assessment of film preparation and treatment application options. Journal of the Institute of Conservation, 45, 36–51. [CrossRef]
- Henniges, U., & Potthast, A. (2008). Phytate treatment of metallo-gallate inks: investigation of its effectiveness on model and historic paper samples. Restaurator, 29, 219–234. [CrossRef]
- Henniges, U., Reibke, R., Banik, G., Huhsmann, E., Hähner, U., Prohaska, T., & Potthast, A. (2008). Iron gall ink-induced corrosion of cellulose: aging, degradation and stabilization. Part 2: application on historic sample material. Cellulose, 15, 861–870. [CrossRef]
- Henriksson, M., & Berglund, L. A. (2007). Structure and properties of cellulose nanocomposite films containing melamine formaldehyde. Journal of Applied Polymer Science, 106, 2817–2824. [CrossRef]
- Hon, D. N.-S., & Shiraishi, N. (1991). Wood and Cellulosic Chemistry. New York: Marcel Dekker, Inc.
- Hubbe, M. A., Smith, R. D., Zou, X. J., Katuscak, S., Potthast, A., & Ahn, K. (2017). Deacidification of acidic books and paper by means of non-aqueous dispersions of alkaline particles: a review focusing on completeness of the reaction. Bioresources, 12, 4410–4477. [CrossRef]
- Ilyas, R. A., Sapuan, S. M., Ishak, M. R., Zainudin, E. S., & Atikah, M. S. N. (2018). Nanocellulose reinforced starch polymer composite: a review of preparation, properties and application in Proceedings of the 5th International Conference on Applied Sciences and Engineering Application (ICASEA, 2018). Capthorne Hotel, Cameron Highlands, Malaysia, 17.
- Iguchi, M., Yamanaka, S., & Budhiono, A. (2000). Bacterial cellulose a masterpiece of nature’s arts. Journal of Materials Science, 35, 261–270. [CrossRef]
- Jablonsky, M., Šima, J., & Lelovsky, M. (2000). Considerations on factors influencing the degradation of cellulose in alumrosin sized paper. Carbohydrate Polymers, 245, 116534. [CrossRef]
- Jacobi, E., Reissland, B., Luu, C. P. T., van Velzen, B., & Ligterink, F. (2011). Rendering the invisible visible: preventing solvent-induced migration during local repairs on iron gall ink. Journal of Paper Conservation, 12, 25–34.
- Jia, M., Zhanga, X., Wenga, J., Zhanga, J., & Zhang, M. (2017). Protective coating of paper works: ZnO/cellulose nanocrystal composites and analytical characterization. Journal of Cultural Heritage, 38, 64–74. [CrossRef]
- Kargarzadeh, H., Ahmad, I., Thomas, S., & Dufresne, A. (2017). Handbook of nanocellulose and cellulose nanocomposites. Weinheim: John Wiley & Sons.
- Kargarzadeh, H., Mariano, M., Gopakumar, D., Ahmad, I., Thomas, S., Dufresne, A., Huang, J., & Lin, N. (2018). Advances in cellulose nanomaterials. Cellulose, 25, 2151–2189. [CrossRef]
- Kaur, P., Sharma, N., Munagala, M., Rajkhowa, R., Aallardyce, B., Shastri, Y., & Agrawal, R. (2021). Nanocellulose: resources, physio-chemical properties, current uses and future applications. Frontiers in Nanotechnology, 3, 1−17. [CrossRef]
- Keerati-U-Rai, M., & Corredig, M. (2009). Effect of dynamic high Pressure homogenization on the aggregation state of soy protein. Journal of Agricultural and Food Chemistry, 57, 3556–3562. [CrossRef]
- Klemm, D., Kramer, F., Moritz, S., Lindström, T., Ankerfors, M., Gray, D., & Dorris, A. (2011). Nanocelluloses: a new family of nature-based materials. Angewandte Chemie International Edition, 50, 5438−5466. [CrossRef]
- Klemm, D., Schumann, D., Kramer, F., Hessler, N., Hornung, M., Schmauder, H. P., & Marsch, S. (2006). Nanocelluloses as innovative polymers in research and application. Polysaccharides, 205, 49−96. [CrossRef]
- Kolar, J., & Strlic, M. (2006). Iron gall inks: on manufacture, characterisation, degradation and stabilisation. Ljubljana: National and University Library of Slovenia.
- Kolbe G. (2004). Gelatine in historical paper production and as inhibiting agent for iron-gall ink corrosion on paper. Restaurator, 25, 26–39. [CrossRef]
- Kumar, V., Pathak, P., & Bhardwaj, N. K. (2020). Waste paper: an underutilized but promising source for nanocellulose mining. Waste Management, 102, 281–303. [CrossRef]
- Kumar, R., Rai, B., Gahlyan, S., & Kumar, G. (2021). A comprehensive review on production, surface modification and characterization of nanocellulose derived from biomass and its commercial applications. Express Polymer Letters, 15, 104–120. [CrossRef]
- Lavoine, N., Desloges, I., Dufresne, A., & Bras, J. (2012). Microfibrillated cellulose – its barrier properties and applications in cellulosic materials: a review. Carbohydrate Polymers, 90, 735–764. [CrossRef]
- Lee, S. Y., Baty, J., & Minter, W. (2010). Study of the aging behavior of rosin-alum sized papers by analysis of mechanical strength, optical properties, and chemical composition following accelerated aging. The Book and Paper Group Annual, 29, 127–128.
- Li, A., Xu, D., Luo, L., Zhou, Y., Yan, W., Leng, X., Dai, D., Zhou, Y., Ahmad, H., Rao, J., & Fan, M. (2021). Overview of nanocellulose as additives in paper processing and paper products. Nanotechnology Reviews, 10, 264–281. [CrossRef]
- Li, F., Mascheroni, E., & Piergiovanni, L. (2015). The potential of nanocellulose in the packaging field: a review. Packaging Technology and Science, 28, 475−508. [CrossRef]
- Liu, W., Du, H., Zhang, M., Liu, K., Liu, H., Xie, H., Zhang, X., & Chuanling, S. (2020). Bacterial cellulose-based composite scaffolds for biomedical applications: a review. ACS Sustainable Chemistry & Engineering, 8, 7536–7562. [CrossRef]
- Liu, W., Liu, K., Du, H., Zheng, T., Zhang, N., Xu, T., Pang, B., Zhang, X., Si, C., & Zhang, K. (2022). Cellulose nanopaper: fabrication, functionalization, and applications. Nano-Micro Letters, 14, 104. [CrossRef]
- Liu, J., Xing, H., Wang, J., Cao, J., Chao, X., Jia, Z., & Li, Y. (2021). A new reinforcement method for the conservation of fragile, double-sided, printed paper cultural relics. Heritage Science, 9, 123. [CrossRef]
- Liu, D., Zhong, T., Chang, P. R., Li, K., & Wu, Q. (2010). Starch composites reinforced by bamboo cellulosic crystals. Bioresource Technology, 101, 2529–2536. [CrossRef]
- Ma, X., Tian, S., Li, X., Fan, H., & Fu, S. (2021). Combined polyhexamethylene guanidine and nanocellulose for the conservation and enhancement of ancient paper. Cellulose, 28, 8027–8042. [CrossRef]
- Ma, X., Zhu, Z., Zhang, H., Tian, S., Li, X., Fan, H., & Fu, S. (2022). Superhydrophobic and deacidified cellulose/CaCO3-derived granular coating toward historic paper preservation. International Journal of Biological Macromolecules, 207, 232–241. [CrossRef]
- Malešič, J., Marinsek, M., & Cigic, I. K. (2022). Evaluation of bookkeeper mass deacidification based on historical book papers. Cellulose, 29, 6889–6905. [CrossRef]
- Mateo, S., Peinado, S., Morillas-Gutiérrez, F., La Rubia, M. D., & Moya, A. J. (2021). Nanocellulose from agricultural wastes: products and applications – a review. Processes, 9, 1594. [CrossRef]
- Melo, M. J., Otero, V., Nabais, P., Teixeira, N., Pina, F., Casanova, C., Fragoso, S., & Sequeira, S. O. (2022). Iron-gall inks: a review of their degradation mechanisms and conservation treatments. Heritage Science, 10, 145. [CrossRef]
- Milwich, M., Speck, T., Speck, O., Stegmaier, T., & Planck, H. (2006). Biomimetics and technical textiles: solving engineering problems with the help of nature’s wisdom. American Journal of Botany, 93, 1455−1465. [CrossRef]
- Mishra, R. K., Sabu, A., & Tiwari, S. K. (2018). Materials chemistry and the futurist eco-friendly applications of nanocellulose: status and prospect. Journal of Saudi Chemical Society, 22, 949–978. [CrossRef]
- Missoum, K., Belgacem, M., & Bras, J. (2013). Nanofibrillated cellulose surface modification: a review. Materials, 6, 1745–1766. [CrossRef]
- Mokhena, T. C., & John, M. J. (2020). Cellulose nanomaterials: new generation materials for solving global issues. Cellulose, 27, 1149–1194. [CrossRef]
- Mondal, S. (2017). Preparation, properties and applications of nanocellulosic materials. Carbohydrate Polymers, 163, 301–316. [CrossRef]
- Nandi, S., & Guha, P. (2018). A review on preparation and properties of cellulose nanocrystal-incorporated natural biopolymer. Journal of Packaging Technology and Research, 2, 149–166. [CrossRef]
- Nasir, M., Hashim, R., Sulaiman, O., & Asim, M. (2017). Nanocellulose: preparation methods and applications in Cellulose-Reinforced Nanofibre Composites. Elsevier. [CrossRef]
- Nechyporchuk, O., Belgacem, M. N., & Bras, J. (2016). Production of cellulose nanofibrils: a review of recent advances. Industrial Crops and Products, 93, 2–25. [CrossRef]
- Nechyporchuk, O., Kolman, K., Bridarolli, A., Odlyha, M., Bozec, L., Oriola, M., Campo-Francés, G., Persson, M., Holmberg, K., & Bordes, R. (2018). On the potential of using nanocellulose for consolidation of painting canvases. Carbohydrate Polymers, 194, 161–169. [CrossRef]
- Neevel J. (1995). The development of a new conservation treatment for ink corrosion, based on the natural anti-oxidant phytate in Conference Papers, 8th Congress of, IADA. Tubingen: IADA.
- Neevel, J. G., & Mensch, C. T. J. (1999). The behaviour of iron and sulphuric acid during iron-gall ink corrosion in COM Committee for Conservation 12th Trienn Meet Lyon.
- Oksman, K., Aitomäki, Y., Mathew, A. P., Siqueira, G., Zhou, Q., Butylina, S., Tanpichai, S., Zhou, X., & Hooshmand, S. (2016). Review of the recent developments in cellulose nanocomposite processing. Composites Part A: Applied Science and Manufacturing, 83, 2–18. [CrossRef]
- Operamolla, A., Mazzuca, C., Capodieci, L., Di Benedetto, F., Severini, L., Titubante, M., Martinelli, A., Castelvetro, V., & Micheli, L. (2021). Toward a reversible consolidation of paper materials using cellulose nanocrystals. ACS Applied Materials & Interfaces, 13, 44972–44982. [CrossRef]
- Pecoraro, É., Manzani, D., Messaddeq, Y., & Ribeiro, S. J. L. (2008). Bacterial cellulose from Glucanacetobacter xylinus: preparation, properties and applications in Monomers, Polymers and Composites from Renewable Resources. Elsevier.
- Perdoch, W., Cao, Z., Florczak. P., Markiewicz, R., Jarek, M., Olejnik, K., & Mazela. B. (2022). Influence of nanocellulose structure on paper reinforcement. Molecules, 27, 4696. [CrossRef]
- Petroudy, S. R. D., Chabot, B., Loranger, E., Naebe, M., Shojaeiarani, J., Gharehkhani, S., Ahvazi, B., Hu, J., & Thomas, S. (2021). Recent advances in cellulose nanofibers preparation through energy-efficient approaches: a review. Energies, 14, 6792. [CrossRef]
- Phanthong, P., Reubroycharoen, P., Hao, X., Xu, G., Abudula, A., & Guan, G. (2018). Nanocellulose: extraction and application. Carbon Resources Conversion, 1, 32–43. [CrossRef]
- Poulose, A., Parameswaranpillai, J., George, J. J., Gopi, J. A., Krishnasamy, S., Dominic C. D. M., Hameed, N., Salim, N. V., Radoor, S., & Sienkiewicz, N. (2022). Nanocellulose: a fundamental material for science and technology applications. Molecules, 27, 1-27. [CrossRef]
- Punnadiyil, R. K., Sreejith, M., & Purushothaman, E. (2016). Isolation of microcrystalline and nano cellulose from peanut shells. Journal of Chemical and Pharmaceutical Sciences, 974, 12–16.
- Rajinipriya, M., Nagalakshmaiah, M., Robert, M., & Elkoun, S. (2018). Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: a review. ACS Sustainable Chemistry & Engineering, 6, 2807–2828. [CrossRef]
- Rambabu, N., Panthapulakkal, S., Sain, M., Dalai, A., & Dalai, K. (2016). Production of nanocellulose fibers from pinecone biomass: evaluation and optimization of chemical and mechanical treatment conditions on mechanical properties of nanocellulose films. Industrial Crops and Products, 83, 746–754. [CrossRef]
- Reißland, B., & Graaff, J. H. (2001). Condition rating for paper objects with iron gall ink in ICN-Information Instituut Collectie Nederland. Amsterdam: Netherlands Institute for Cultural Heritage.
- Reissland, B. (2000). Visible progress of paper degradation caused by iron gall inks in The Iron Gall Ink Meeting. Newcastle upon Tyne: University of Northumbria.
- Reissland, B. (2001). Ink corrosion: side-effects caused by aqueous treatments for paper objects in The Iron Gall Ink Meeting. Newcastle upon Tyne: University of Northumbria.
- Rouchon, V., Desroches, M., Duplat, V., Letouzey, M., & Stordiau-Pallot, J. (2012). Methods of aqueous treatments: the last resort for badly damaged iron gall ink manuscripts. Journal of Paper Conservation, 13, 7–13.
- Rouchon, V., Pellizzi, E., Duranton, M., Vanmeert, F., & Janssens, K. (2011). Combining XANES, ICP-AES and SEM/EDS for the study of phytate chelating treatments used on iron gall ink damaged manuscripts. Journal of Analytical Atomic Spectrometry, 26, 2434–2441. [CrossRef]
- Saito, T., Kimura, S., Nishiyama, Y., & Isogai, A. (2007). Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules, 8, 2485–2491. [CrossRef]
- Sánchez Hernampérez, A. (1999). Políticas de conservación en bibliotecas. Arco Libros, Madrid.
- Santos, S. M., Carbajo, J. M., Gómez, N., Quintana, E., Ladero, M., Sánchez, A., Chinga-Carrasco, G., & Villar, J. C. (2016a). Use of bacterial cellulose in degraded paper restoration. Part I: application on model papers. Journal of Materials Science, 51, 1541–1552. [CrossRef]
- Santos, S. M., Carbajo, J. M., Gómez, N., Quintana, E., Ladero, M., Sánchez, A., Chinga-Carrasco, G., & Villar, J. C. (2016b). Use of bacterial cellulose in degraded paper restoration. Part II: application on real samples. Journal of Materials Science, 51, 1553–1561. [CrossRef]
- Scholten, S. (1997) in Proceedings of the European Workshop on Iron-Gall Ink Corrosion. Instituut Collectie Nederland, Amsterdam.
- Sharma, A., Thakur, M., Bhattacharya, M., & Mandal, T. (2019). Commercial application of cellulose nano-composites – a review. Biotechnology Reports, 21, 1-15. [CrossRef]
- Siqueira, G., Bras, J., & Dufresne, A. (2010). Cellulosic bionanocomposites: a review of preparation, properties and applications. Polymer, 2, 728–765. [CrossRef]
- Sobucki, W., & Drewniewska-Idziak, B. (2003). Survey of the preservation status of the 19th and 20th century collections at the national library in Warsaw. Restaurator, 24, 189–201. [CrossRef]
- Spence, K. L., Venditti, R. A., Rojas, O. J., Habibi, Y., & Pawlak, J. J.(2011). A comparative study of energy consumption and physical properties of microfibrillatedcellulose produced by different processing methods. Cellulose, 18, 1097–1111. [CrossRef]
- Suryanegara, L., Nakagaito, A. N., & Yano, H. (2010). Thermo-mechanical properties of microfibrillated cellulose-reinforced partially crystallized PLA composites. Cellulose, 17, 771–778. [CrossRef]
- Thomas, B., Raj, M. C., Athira, K. B., Rubiyah, M. H., Joy, J., Moores, A., Drisko, G. L., & Sanchez, C. (2018). Nanocellulose, a versatile green platform: from biosources to materials and their applications. Chemical Reviews, 118, 11575−11625. [CrossRef]
- Thomas, S. K., Begum, S., Midhun Dominic, C. D., Salim, N. V.; Hameed, N., Rangappa, S. M., Siengchin, S., & Parameswaranpillai, J. (2021). Isolation and characterization of cellulose nanowhiskers from Acacia caesia plant. Journal of Applied Polymer Science, 138, 50213. [CrossRef]
- Tkalčec, M. M., Bistričić, L., & Leskovac, M. (2016). Influence of adhesive layer on the stability of kozo paper. Cellulose, 23, 853–872. [CrossRef]
- Trache, D., Tarchoun, A. F., Derradji, M., Mehelli, O., Hussin, M. H., & Bessa, W. (2020a). Cellulose fibers and nanocrystals: preparation, characterization and surface modification in Functionalized Nanomaterials I: Fabrication. Taylor & Francis.
- Trache, D., Tarchoun, A. F., Derradji, M., Hamidon, T. S., Masruchin, N., Brosse, N., & Hussin, M. H. (2020b). Nanocellulose: from fundamentals to advanced applications. Frontiers in Chemistry, 8, 1–33. [CrossRef]
- Thomas, B., Raj, M. C., Athira K. B., Rubiyah M. H., Joy, J., Moores A., Drisko, G. L. & Sanchez, C. (2018). Nanocellulose, a versatile green platform: from biosources to materials and their applications. Chemical Reviews, 118, 11575−11625.
- Van Gulik, R., & Kersten, P. (1994). Closer look at iron gall ink burn. Restaurator, 15, 173–178.
- Vanderfleet, O. M. & Cranston, E. D. (2021). Production routes to tailor the performance of cellulose nanocrystals. Nature Reviews Materials, 6, 124–144. [CrossRef]
- Vazquez, A., Foresti, M. L., Moran, J. I., & Cyras, V. P. (2015). Extraction and production of cellulose nanofibers in Handbook of Polymer Nanocomposites. Processing, Performance and Application. Springer: Heidelberg.
- Vineeth, S., Gadhave, R. V., & Gadekar, P. T. (2019). Chemical modification of nanocellulose in wood adhesive. Open Journal of Polymer Chemistry, 9, 86–99. [CrossRef]
- Völkel, L., Ahn, K., Hähner, U., Gindl-Altmutter, W., & Potthast, A. (2017). Nano meets the sheet: adhesive-free application of nanocellulosic suspensions in paper conservation. Heritage Science, 5, 23. [CrossRef]
- Völkel, L., Prohaska, T., & Potthast, A. (2020). Combining phytate treatment and nanocellulose stabilization for mitigating iron gall ink damage in historic papers. Heritage Science, 8, 86. [CrossRef]
- Völkel, L., Beaumont, M., Johansson, L.-S., Czibula, C., Rusakov, D., Mautner, A., Teichert, C., Kontturi, E., Rosenau, T, &; Potthast, A. (2022). Assessing fire-damage in historical papers and alleviating damage with soft cellulose nanofibers. Small, 18, 2105420. [CrossRef]
- Wang, S., & Cheng, Q. (2009). A novel process to isolate fibrils from cellulose fibers by high-intensity ultrasonication. Part 1: process optimization. Journal of Applied Polymer Science, 113, 1270–1275. [CrossRef]
- Wang, Z., Cheng, C., Cheng, Y., Zheng, L., & Hu, D. (2021). Quaternary alloy quantum dots as fluorescence probes for total acidity detection of paper-based relics. Nanomaterials, 11, 1726. [CrossRef]
- Wang, Q., Yao, Q., Liu, J., Sun, J., Zhu, Q., & Chen, H. (2019). Processing nanocellulose to bulk materials: a review. Cellulose, 26, 7585–7617. [CrossRef]
- Wertz, J. L., Mercier, J. P., & Bédué, O. (2010). Cellulose Science and Technology. Lausanne: CRC Press.
- Xu, J., Zhang, T., Jiang, Y., Yang, D., Qiu, F., Chen, Q., & Yu, Z. (2020). Preparation of self-healing acrylic copolymer composite coatings for application in protection of paper cultural relics. Polymer Engineering & Science, 60, 288–296. [CrossRef]
- Zervos, S., & Alexopoulou, I. (2015). Paper conservation methods: a literature review. Cellulose, 22, 2859–2897. [CrossRef]
- Zhang, X, Yan, Y., Yao, J., Jin, S., & Tang, Y. (2003). Chemistry directs the conservation of paper cultural relics. Polymer Degradation and Stability, 207, 110228. [CrossRef]
- Zyska, B. (1996). Permanence of paper in Polish books of the period 1900-1994. Restaurator, 17, 214–228. [CrossRef]
Figure 1.
Hierarchical structure of cellulose (Reproduced with permission from Almeida et al. Copyright © 2023, Elsevier).
Figure 1.
Hierarchical structure of cellulose (Reproduced with permission from Almeida et al. Copyright © 2023, Elsevier).
Figure 2.
Main steps involved in the preparation of iron gall ink (Reproduced with permission from Díaz Hidalgo et al. Copyright © 2018, Springer).
Figure 2.
Main steps involved in the preparation of iron gall ink (Reproduced with permission from Díaz Hidalgo et al. Copyright © 2018, Springer).
Figure 3.
Location of the cellulose degradation: in (a) acid hydrolysis and (b) oxidation process (Reproduced with permission from Reißland & Graaff. Copyright © 2001).
Figure 3.
Location of the cellulose degradation: in (a) acid hydrolysis and (b) oxidation process (Reproduced with permission from Reißland & Graaff. Copyright © 2001).
Figure 4.
Microphotographs from a book from 1940 to 1960 lined with: (a) bacterial cellulose and (b) Japanese Paper (Reproduced with permission from Santos et al. Copyright © 2016, Springer).
Figure 4.
Microphotographs from a book from 1940 to 1960 lined with: (a) bacterial cellulose and (b) Japanese Paper (Reproduced with permission from Santos et al. Copyright © 2016, Springer).
Figure 5.
Mechanical damages such as cracks and losses: (a) before and (b) after a direct application of nanocellulose (Reproduced with permission from Völkel et al. Copyright © 2017, Springer).
Figure 5.
Mechanical damages such as cracks and losses: (a) before and (b) after a direct application of nanocellulose (Reproduced with permission from Völkel et al. Copyright © 2017, Springer).
Figure 6.
One viewing slide from the French Museum of Cinema under transmitted light: (a) before and (b) after treatment with microfibrillated cellulose film (Reproduced with permission from Dreyfuss-Deseigne. Copyright © 2017, Taylor & Francis).
Figure 6.
One viewing slide from the French Museum of Cinema under transmitted light: (a) before and (b) after treatment with microfibrillated cellulose film (Reproduced with permission from Dreyfuss-Deseigne. Copyright © 2017, Taylor & Francis).
Figure 7.
Damaged sample: (a) before and (b) after mechanical stabilization with nanofibrillated cellulose and chemical stabilization with calcium phytate/calcium hydrogen carbonate (Reproduced with permission from Völkel et al. Copyright © 2020, Springer).
Figure 7.
Damaged sample: (a) before and (b) after mechanical stabilization with nanofibrillated cellulose and chemical stabilization with calcium phytate/calcium hydrogen carbonate (Reproduced with permission from Völkel et al. Copyright © 2020, Springer).
Figure 8.
Effect of Aspergillus Niger in ancient paper: (a) before and (b) after treatment with cellulose nanocrystals (Reproduced with permission from Ma et al. Copyright © 2021, Springer).
Figure 8.
Effect of Aspergillus Niger in ancient paper: (a) before and (b) after treatment with cellulose nanocrystals (Reproduced with permission from Ma et al. Copyright © 2021, Springer).
Figure 9.
Effect of wettability: (a) before and (b) after treatment with a nanocomposite of cellulose nanocrystals, cellulose nanofibrils and lignin nanoparticles (Reproduced with permission from Camargos et al. Copyright © 2022, American Chemical Society).
Figure 9.
Effect of wettability: (a) before and (b) after treatment with a nanocomposite of cellulose nanocrystals, cellulose nanofibrils and lignin nanoparticles (Reproduced with permission from Camargos et al. Copyright © 2022, American Chemical Society).
Figure 10.
Effect of self-cleaning in historic paper: (a) before and (b) after treatment with a coating of cellulose nanocrystals, calcium carbonate and polydimethylsiloxane (Reproduced with permission from Ma et al. Copyright © 2022, Elsevier).
Figure 10.
Effect of self-cleaning in historic paper: (a) before and (b) after treatment with a coating of cellulose nanocrystals, calcium carbonate and polydimethylsiloxane (Reproduced with permission from Ma et al. Copyright © 2022, Elsevier).
Table 1.
Comparison of different types of nanocelluloses.
Table 1.
Comparison of different types of nanocelluloses.
| Properties/Applications |
Cellulose Nanocrystals |
Cellulose Nanofibrils |
Bacterial Nanocellulose |
Morphology
(Liu et al., 2022) |
 |
 |
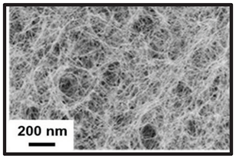 |
| Preparation Process (Iguchi et al., 2000) |
top-down |
top-down |
bottom-up |
Size
(Thomas et al., 2018) |
Length (nm) |
50 – 500 |
> 1,000 |
1,000 – 5,000 |
| Diameter (nm) |
3 – 50 |
5 – 100 |
< 100 |
| Crystallinity Index (%) (Ilyas et al., 2018) |
54 – 88 |
< 50 |
> 88 |
| Young’s Modulus (GPa) (de Amorim et al., 2020) |
50 – 100 |
39 – 78 |
15 – 30 |
| Purity (Pecoraro et al. 2008) |
low |
low |
high |
| Cost (Thomas et al., 2018) |
low |
low |
high |
Main Applications
(Thomas et al., 2018) |
optical devices, composite materials and coatings |
packaging, energy storage and flexible electronics |
antimicrobial products and flexible supercapacitors |
Table 2.
Principal results of nanocelluloses applications in historical documents.
Table 2.
Principal results of nanocelluloses applications in historical documents.
| Nanocellulose-Based Material |
Document Type |
Application Methodology |
General Results |
Reference |
| Bacterial cellulose |
Books from 1940 to 1960 |
Layer
(wheat starch as adhesive) |
High stability over time
Lower air permeability
Similar mechanical properties
Better legibility of the letters
Improvement in deteriorated paper quality
Adequate protection against humidity and atmospheric pollutants |
Santos et al., 2016b |
| Bacterial cellulose and a mechanically nanofibrillated cellulose |
Rag papers from the nineteenth century
Book paper from the twentieth century
|
Suspension
(no adhesive) |
Treatment of mechanical damage areas
Consolidation of weakened areas
No negative side effects in long term |
Völkel et al., 2017 |
| Cellulose nanocrystals/zinc oxide |
School newspaper from 1960 |
Suspension
(Klucel as adhesive) |
Improvement in mechanical properties
Stronger antibacterial and antifungal activity
High protection against UV light |
Jia et al., 2017 |
| Cellulose nanocrystals/propylene glycol/methylcellulose/calcium carbonate |
Two paper sheets from a bookprinted in twentieth century |
Sheet
(methylcellulose asinternal sizing agent) |
High crystallinity index
Mild basic character
Similar mechanical properties |
Camargos et al., 2017 |
| Microfibrillated cellulose |
Paper viewing slides from the mid nineteenth century |
Film
(wheat starch paste, methylhydroxyethylcellulose MH300P®, Klucel G® and Culminal MC2000® as adhesives) |
Very thinner
Remarkable properties of transparency
Very good stability to light, temperature and humidity aging
Good results with Klucel G® |
Dreyfuss-Deseigne, 2017a, 2017b, 2017c |
| Calcium phytate/calcium hydrogen carbonate treatment combined with nanofibrillated cellulose |
Rag papers written with iron gall ink from a collection of sermonsbelonging to 1839 and 1840 |
Suspension
(no adhesive) |
Viable combined procedure of chemical and mechanical stabilization
Closed and sealed of fractures, cracks and imperfections
Minimal influence on the optical and haptic properties
Additional step avoided (stabilization and drying) |
Völkel et al., 2020 |
| Sulfated and neutral cellulose nanocrystals |
Book from the eighteen century |
Suspension
(no adhesive) |
Improvement in optical and mechanical properties
Safer neutral cellulose nanocrystals |
Operamolla et al., 2021 |
| Cellulose nanocrystals/polyhexamethylene guanidine |
Book published in 1954 |
Suspension
(no adhesive) |
Excellent biocidal activity against mold
Improvement in mechanical properties
Outstanding performance in aging tests |
Ma et al., 2021 |
| Microfibrillated cellulose/methycellulose |
Volume with designs dating from 1859 to 1882 |
Film
(methycellulose as internal adhesive and Klucel® G and IG Isinglass as adhesives) |
Best combination with Klucel G®
Potential for customization
Satisfactory transparency
High strength after adhesion
Stable aging properties |
Henniges et al., 2022 |
| Cellulose nanofiber |
Fire-damaged historical papers from eighteenth and early nineteenth century |
Suspension
(no adhesive) |
No negative visual impact
Mechanical stabilization in long-term to reduce paper fragility |
Völkel et al., 2022 |
| Cellulose nanocrystals/calcium carbonate/polydimethylsiloxane (superhydrophobic self-cleaning coating |
Book published in 1954 |
Suspension
(no adhesive) |
Increase in thickness, water contact angle and roughness
Improvement in mechanical strength
Achievement superhydrophobic protection
Excellent properties of self-cleaning and deacidification effect |
Ma et al., 2022 |
| Cellulose nanocrystals/inorganic nanotubes (multifunctional protective coatings) |
Historical paper produced in 1943 |
Colloidal solution
(no adhesive) |
Improvement in tensile strength, elongation at break, thermal stability and UV protection without altering optical properties |
Elmetwaly et al., 2022 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).