Submitted:
13 March 2024
Posted:
14 March 2024
You are already at the latest version
Abstract
Keywords:
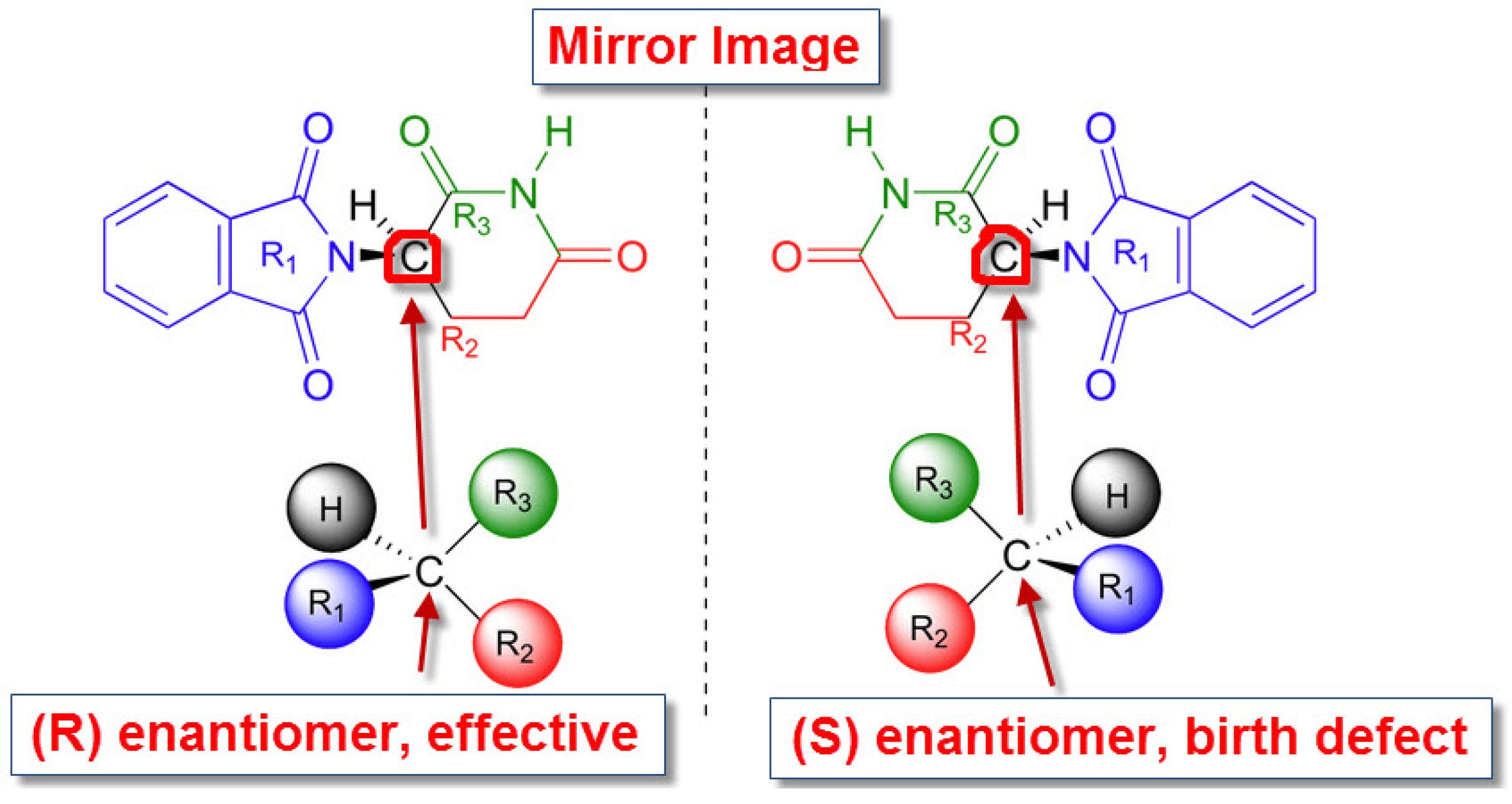
1. Introduction
2. Results and Discussion
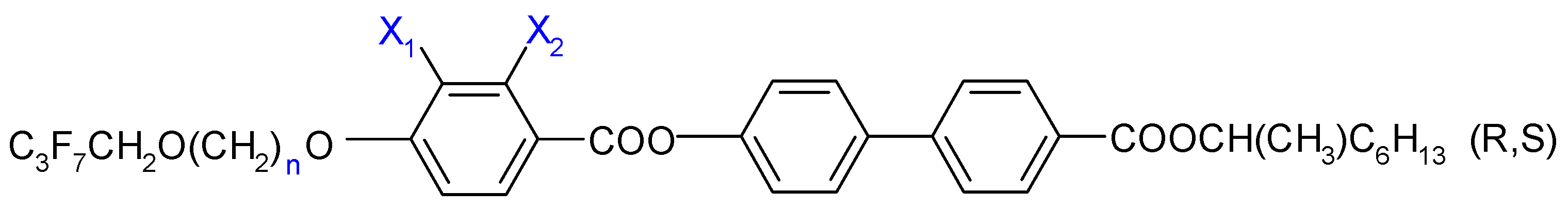
2.1. Racemic Mixtures
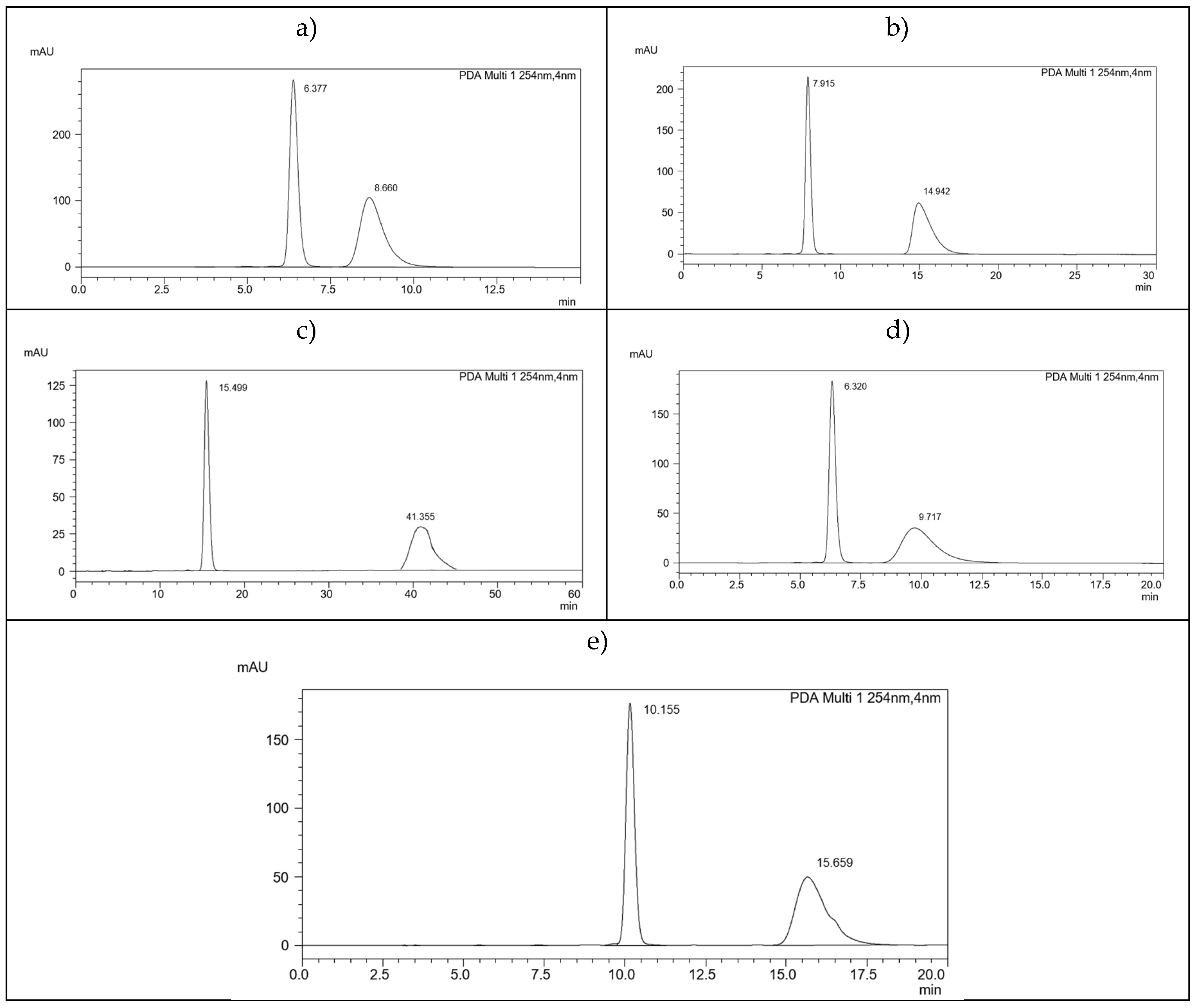
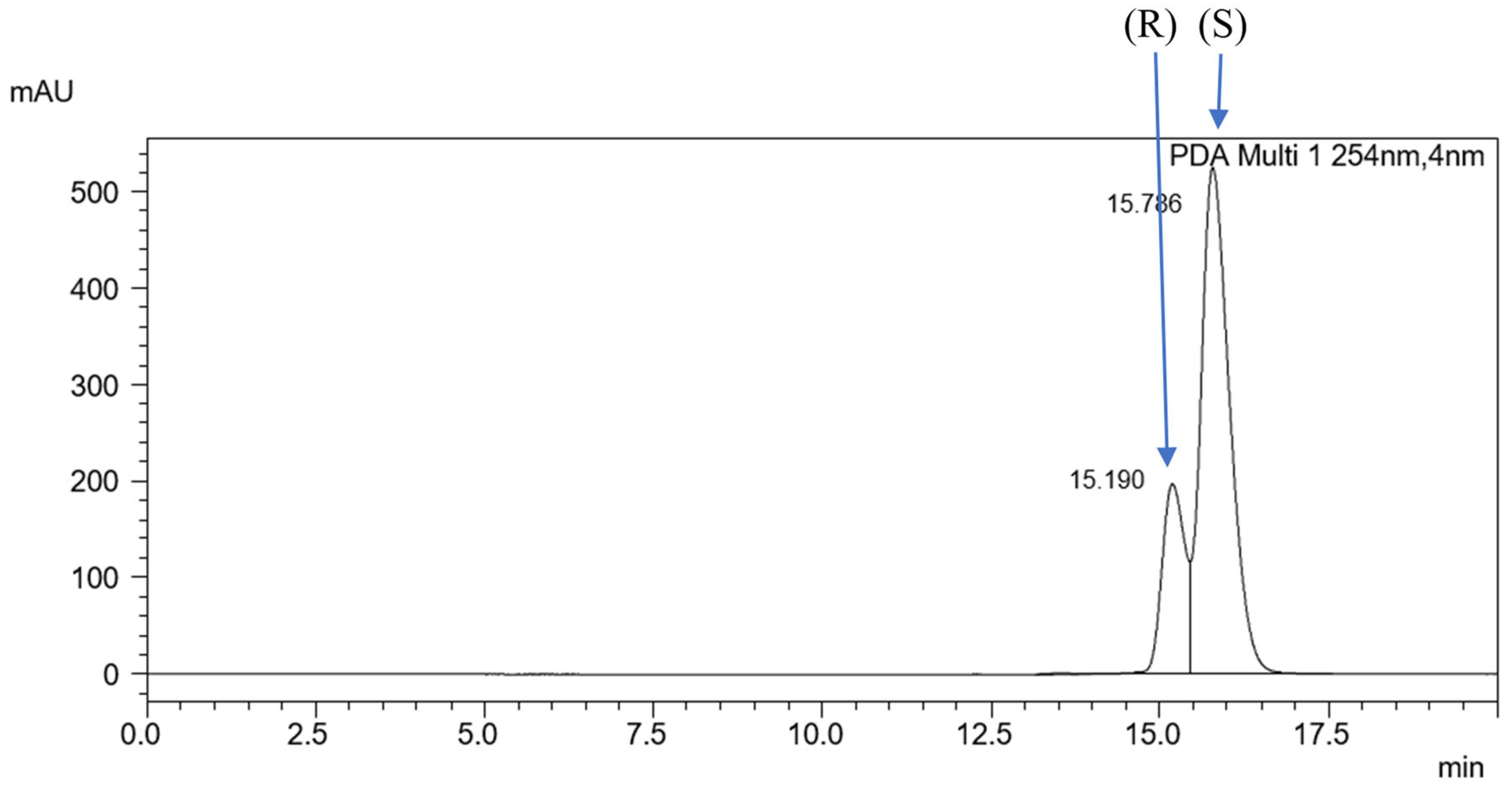
2.2. Chiral HPLC Separation
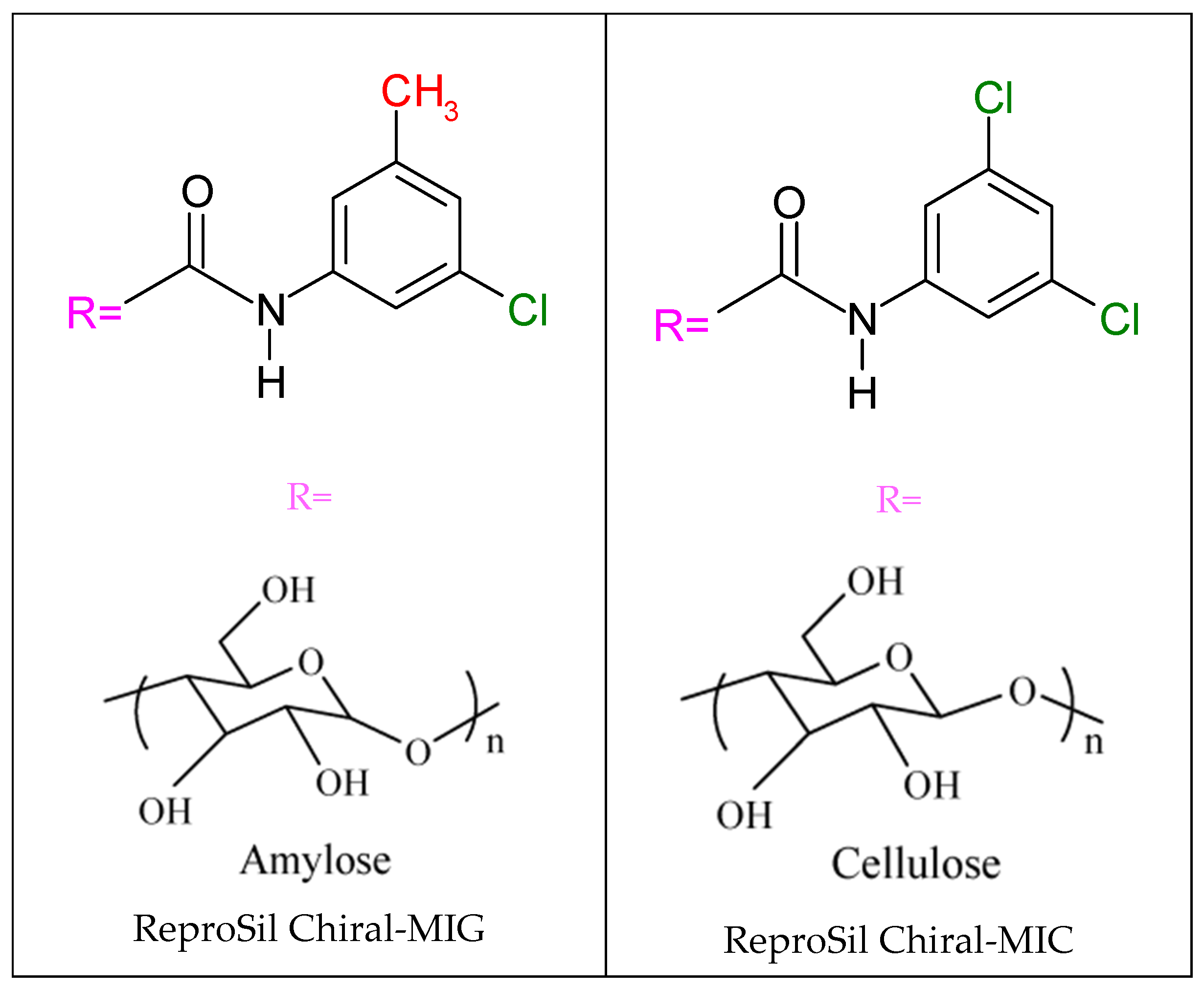
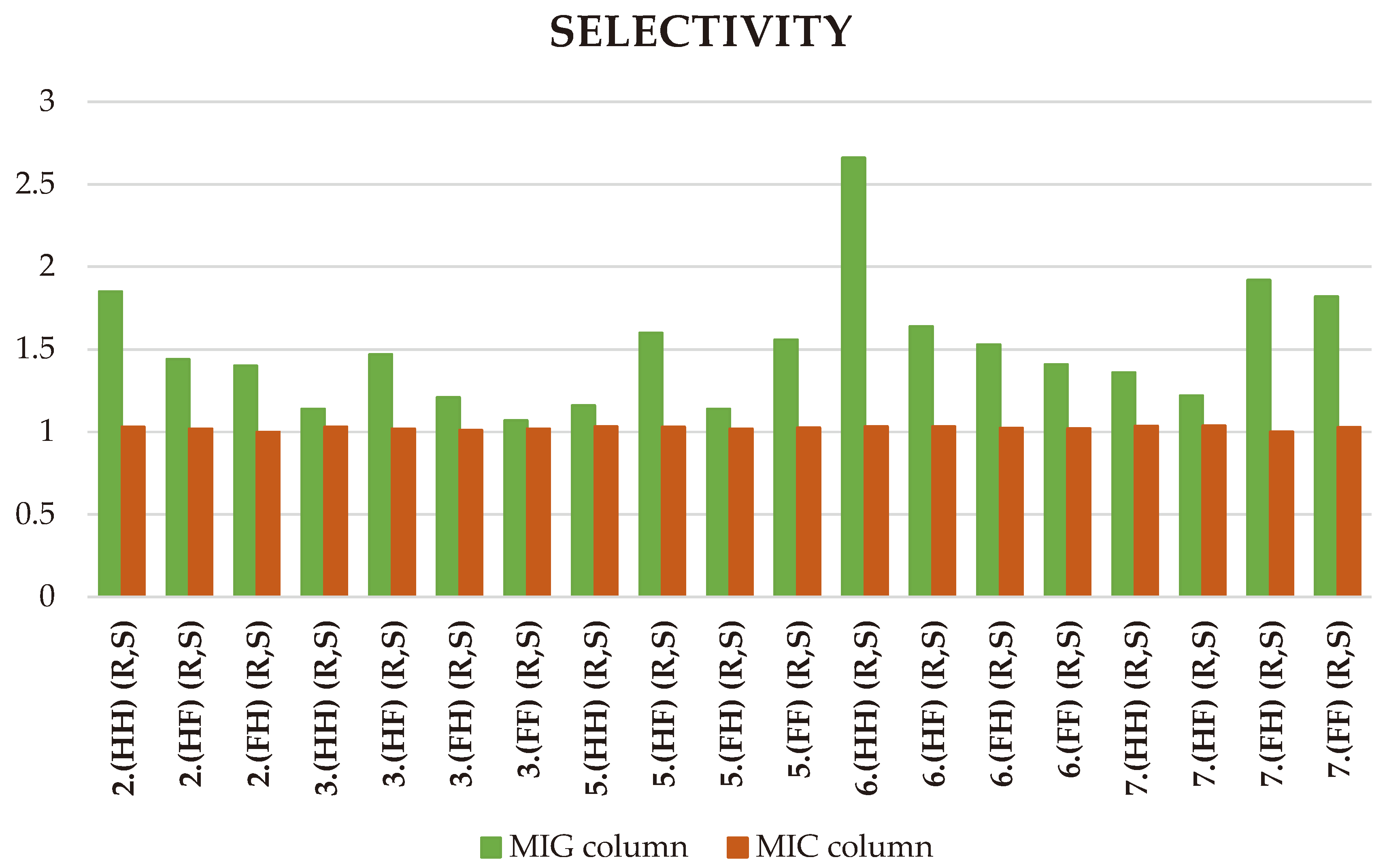
2.3. ReproSil Chiral-MIG Column
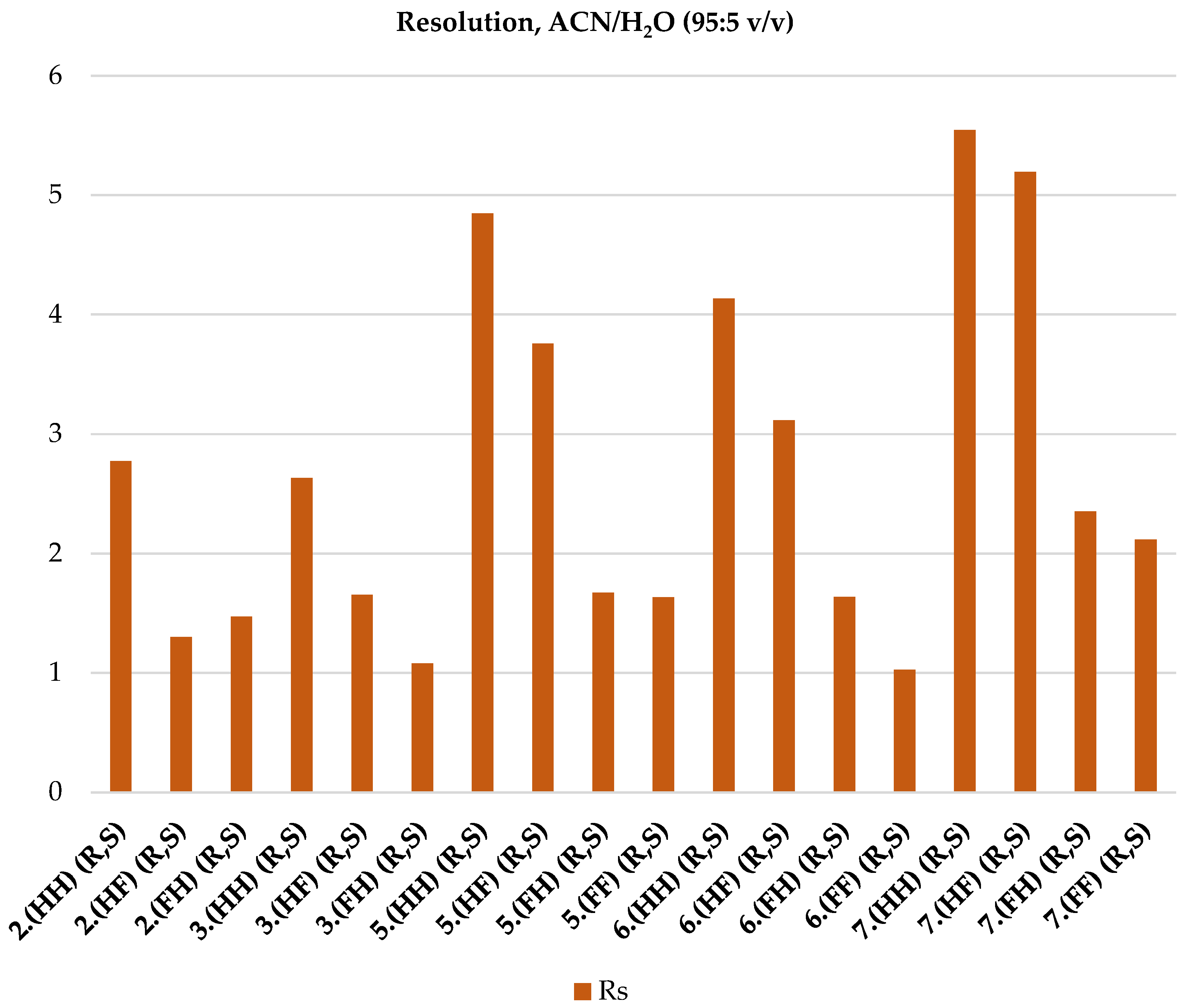
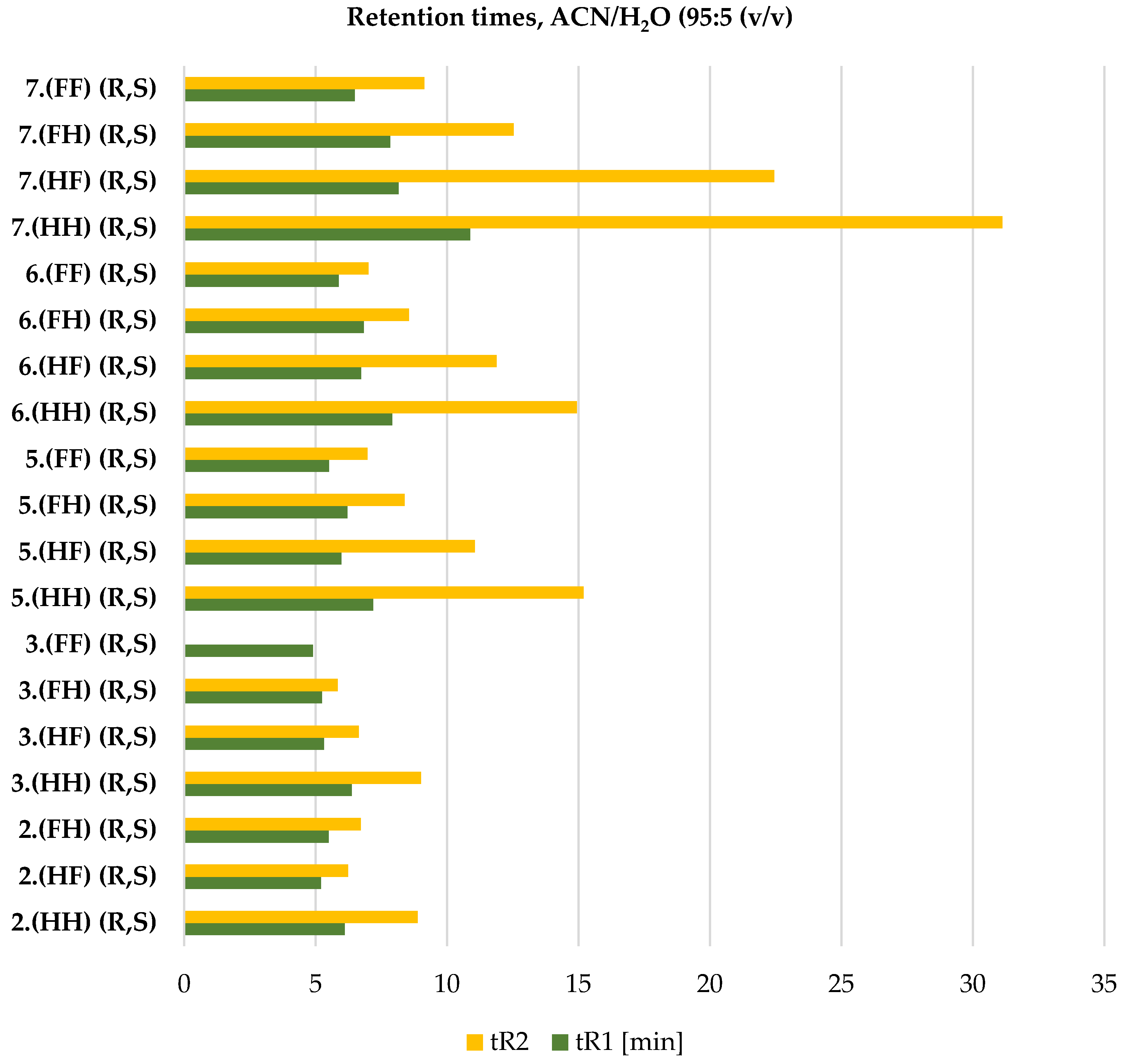
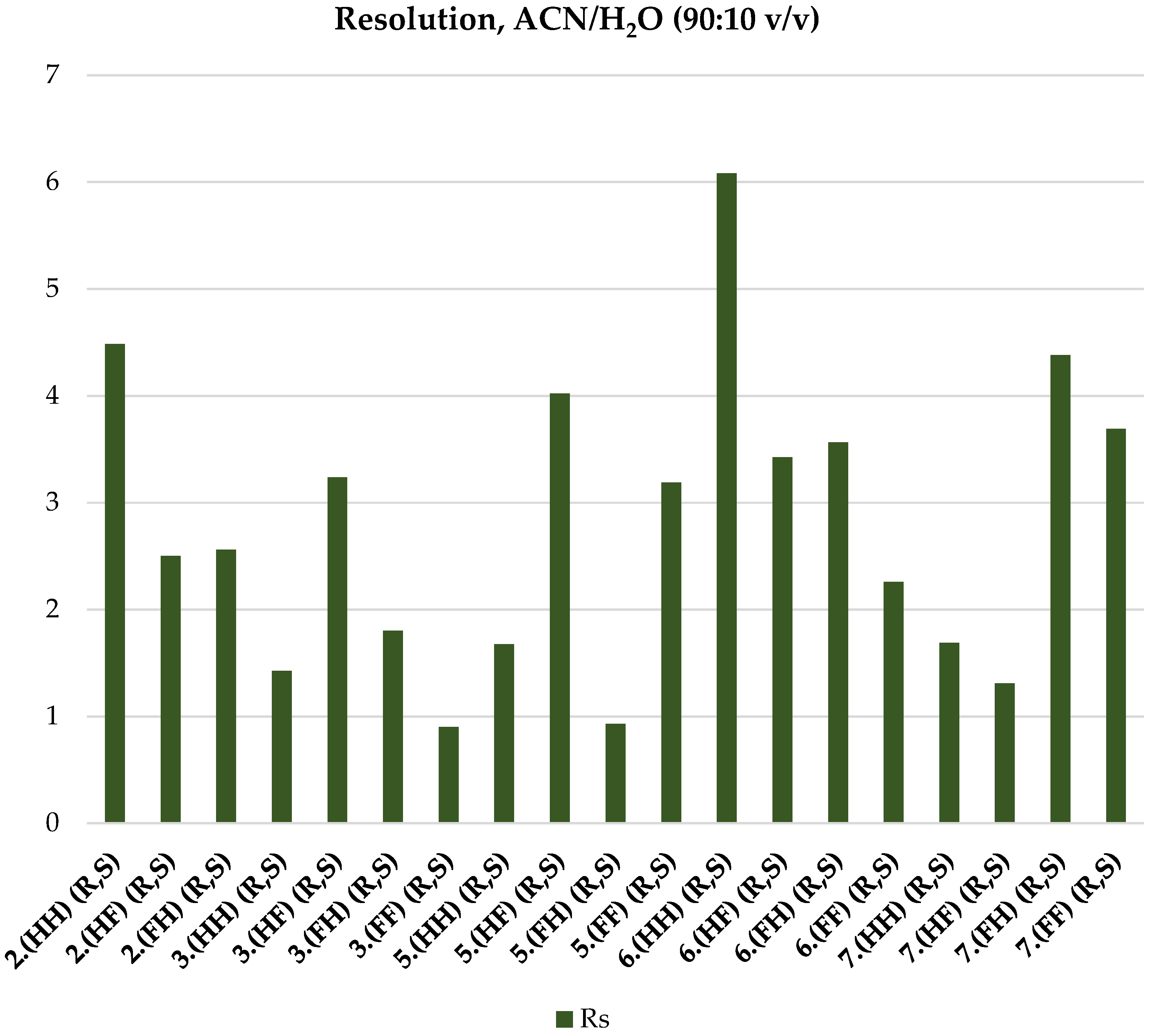
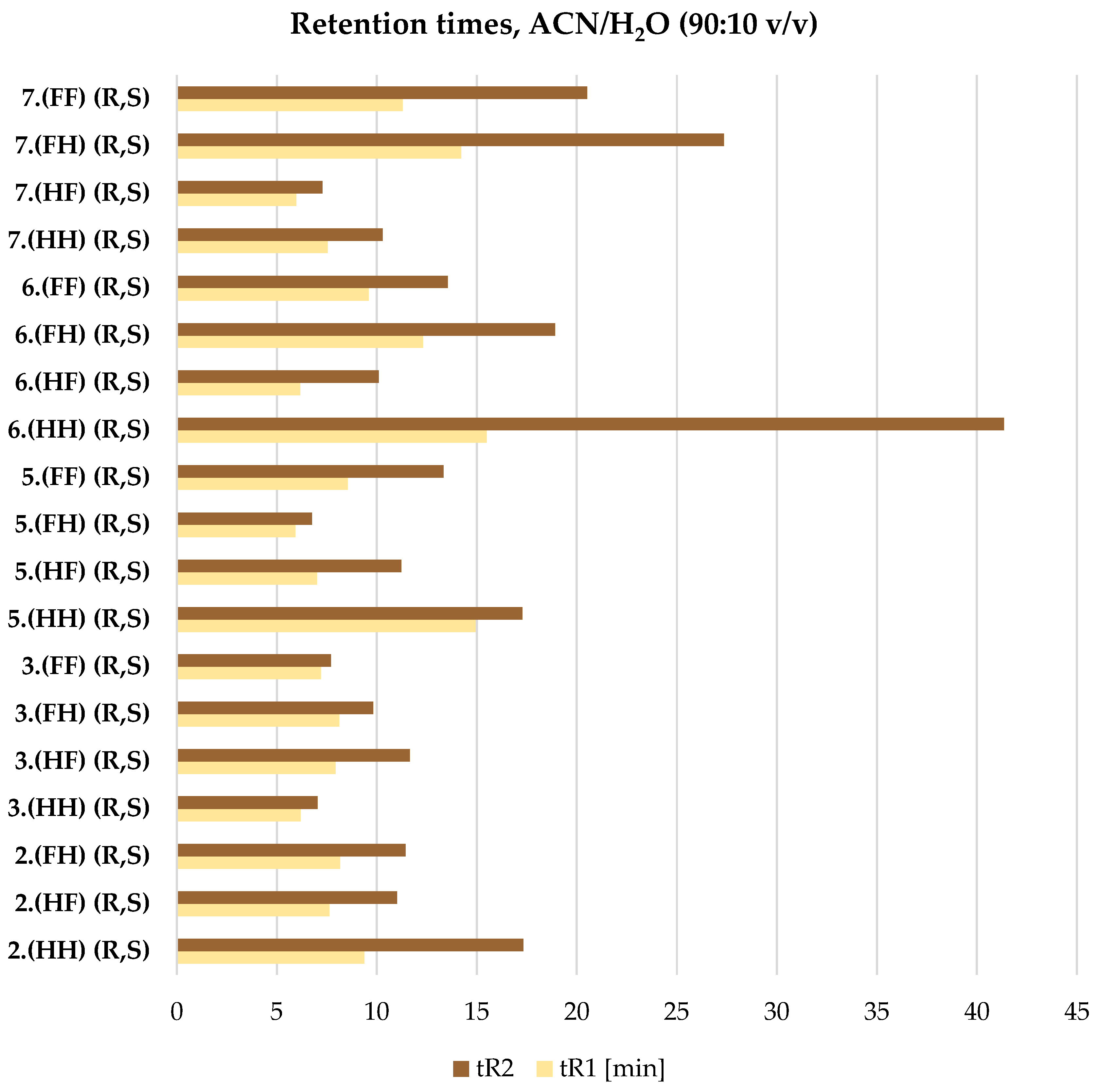
2.4. ReproSil Chiral-MIC Column
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clayden, J.; Greeves, N.; Warren, S.G. Organic chemistry; Oxford University Press: Oxford, 2012. [Google Scholar]
- Clark, A.; Kitson, R.R.A.; Mistry, N.; Taylor, P.; Taylor, M.; Lloyd, M.; Akamune, C. Introduction to stereochemistry; Cambridge, UK, 2021. [Google Scholar]
- Mislow, K.; Siegel, J. Stereoisomerism and local chirality. J. Am. Chem. Soc. 1984, 106, 3319–3328. [Google Scholar] [CrossRef]
- Smith, S.W. Chiral Toxicology: It’s the Same Thing...Only Different. Toxic. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef]
- Knoche, B.; Blaschke, G. Investigations on the in vitro racemization of thalidomide by high-performance liquid chromatography. J. Chrom. A. 1994, 666, 235–240. [Google Scholar] [CrossRef]
- Drayer, D.E. Pharmacodynamic and pharmacokinetic differences between drug enantiomers in humans: An overview. Clin. Pharm. Ther. 1986, 40, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Fabro, S.; Smith, R.L.; Williams, R.T. Toxicity and Teratogenicity of Optical Isomers of Thalidomide. Nature 1967, 215, 296. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, A. An extraordinary state of matter. Liquid crystals; Polish publishing house: Warsaw, 1979. [Google Scholar]
- Collings, P.J.; Hird, M. Introduction to Liquid Crystals: Chemistry and Physics; Taylor & Francis: London, UK, 1997. [Google Scholar]
- Żmija, J.; Zieliński, J.; Parka, J.; Nowinowski-Kruszelnicki, E. Liquid crystal displays. Physics, Technology and Applications; Polish scientific publishing house: Warsaw, 1993. [Google Scholar]
- Vojtylová, T.; Niezgoda, I.; Galewski, Z.; Hamplová, V.; Sykora, D. A new approach to the chiral separation of novel diazenes. J. Sep. Sci. 2015, 38, 4211–4215. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.A.; Mangelings, D.; Heyden, Y.V. Chiral separations in reversed-phase liquid chromatography: Evaluation of several polysaccharide-based chiral stationary phases for a separation strategy update. J. Chrom. A. 2012, 1269, 154–167. [Google Scholar] [CrossRef]
- Vojtylová, T.; Kašpar, M.; Hamplová, V.; Novotná, V.; Sýkora, D. Chiral HPLC for a study of the optical purity of new liquid crystalline materials derived from lactic acid. Phase Trans. 2014, 87, 758–769. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, G.; Han, Q.; Hu, X.; Zhang, Q.; He, Z. Methylamidation and normal phase chromatography with silica-hydride-based stationary phase for N-glycosylation characterization of monoclonal antibody drugs. Chinese J. Chrom. 2018, 36, 615–620. [Google Scholar] [CrossRef]
- Vojtylová-Jurkovičová, T.; Vaňkátová, P.; Urbańska, M.; Hamplová, V.; Sýkora, D.; Bubnov, A. Effective control of optical purity by chiral HPLC separation for ester-based liquid crystalline materials forming anticlinic smectic phases. Liq. Cryst. 2021, 48, 43–53. [Google Scholar] [CrossRef]
- Vojtylová, T.; Hamplová, V.; Galewski, Z.; Korbecka, I.; Sýkora, D. Chiral separation of novel diazenes on a polysaccharide-based stationary phase in the reversed-phase mode. J. Sep. Sci. 2017, 40, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Poryvai, A.; Vojtylová-Jurkovičová, T.; Šmahel, M.; Kolderová, N.; Tomášková, P.; Sýkora, D.; Kohout, M. Determination of optical purity of lactic acid-based chiral liquid crystals and corresponding building blocks by chiral high-performance liquid chromatography and supercritical fluid chromatography. Molecules 2019, 24, 1099. [Google Scholar] [CrossRef] [PubMed]
- Vojtylová, T.; Żurowska, M.; Milewska, K.; Hamplová, V.; Sýkora, D. Chiral HPLC and physical characterisation of orthoconic antiferroelectric liquid crystals. Liq. Cryst. 2016, 43, 1244–1250. [Google Scholar] [CrossRef]
- Brown, C.L.; Dornan, L.M.; Muldoon, M.J.; Hembre, R.T.; Stevenson, P.J.; Manesiotis, P. Comparison of three stationary phases in the separation of polyphenyls by liquid chromatography. J. Chrom. A. 2022, 1671, 462992. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, M. Separation of liquid crystalline racemic mixtures obtained on the basis of (R,S)-2-hexanol on amylose tris(3-chloro-5-methylphenylcarbamate) covalently immobilised on silica in high-performance liquid chromatography. Liq. Cryst. 2023, 50, 1893–1901. [Google Scholar] [CrossRef]
- Śliwka-Kaszyńska, M.; Momotko, M.; Szarmańska, J.; Boczkaj, G.; Kamiński, M. Types of chiral stationary phases and its applications to liquid chromatography (LC)—A mini review. Cam. Sep. 2015, 7, 1–30. [Google Scholar]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Pol. Env. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: mechanisms and modern chiral stationary phases. J. Chrom. A. 2010, 1217, 814–856. [Google Scholar] [CrossRef]
- Xiaoming, C.; Yamamoto, C.; Okamoto, Y. Polysaccharide derivatives as useful chiral stationary phases in high-performance liquid chromatography. Pure Appl. Chem. 2007, 79, 1561–1573. [Google Scholar] [CrossRef]
- Ikai, T.; Yamamoto, C.; Kamigaito, M.; Okamoto, Y. Immobilized Polysaccharide-Based Chiral Stationary Phases for HPLC. Polym. J. 2006, 38, 91–108. [Google Scholar] [CrossRef]
- Yashima, E. Polysaccharide-based chiral stationary phases for high-performance liquid chromatographic enantioseparation. J. Chrom. A. 2001, 906, 105–125. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yashima, E. Polysaccharide Derivatives for Chromatographic Separation of Enantiomers. Angew. Chem. Int. Ed. 1998, 37, 1020–1043. [Google Scholar] [CrossRef]
- Tachibana, K.; Ohnishi, A. Reversed-phase liquid chromatographic separation of enantiomers on polysaccharide type chiral stationary phases. J. Chrom. A. 2001, 906, 127–154. [Google Scholar] [CrossRef]
- Okamoto, Y. Chiral Polymers for Resolution of Enantiomers. J. Polym. Sci. Part A: Polym. Chem. 2009, 47, 1731–1970. [Google Scholar] [CrossRef]
- Kazusaki, M.; Kawabata, H.; Matsukura, H. Comparative study of amylose and cellulose derivatized chiral stationary phases in the reversed-phase mode. J. Liq. Chrom. & Rel. Techn. 2000, 23, 2819–2828. [Google Scholar] [CrossRef]
- Fan, X.; Cao, L.; Geng, L.; Ma, L.; Wei, Y.; Wang, Y. Polysaccharides as separation media for the separation of proteins, peptides and stereoisomers of amino acids. Inter. J. Biol. Macromol. 2021, 186, 616–638. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Ali, I. Optimization strategies for HPLC enantioseparation of racemic drugs using polysaccharides and macrocyclic glycopeptide antibiotic chiral stationary phases. Farmaco. 2002, 57, 513–529. [Google Scholar] [CrossRef]
- Ellington, J.J.; Evans, J.J.; Prickett, K.B.; Champion, W.L., Jr. High-performance liquid chromatographic separation of the enantiomers of organophosphorus pesticides on polysaccharide chiral stationary phases. J. Chrom. A. 2001, 928, 145–154. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development; John Wiley & Sons, Inc., 1997. [Google Scholar]
- Skoog, D.A.; Holler, F.J.; Nieman, T.A. Principles of Instrumental Analysis. Sensor Rev. 1999, 19. [Google Scholar] [CrossRef]
- Swadesh, J.K. HPLC Practical and Industrial Applications, Second Edition; 2000. [Google Scholar] [CrossRef]
- https://dr-maisch.com/dr-maisch-phases/reprosil-chiral.
- Urbańska, M.; Dziaduszek, J.; Strzeżysz, O.; Szala, M. Synclinic and anticlinic properties of (R,S) 4′-(1-methylheptyloxycarbonyl)biphenyl-4-yl 4-[7-(2,2,3,3,4,4,4-heptafluorobutoxy)heptyl-1-oxy]benzoates. Phase Trans. 2019, 92, 657–666. [Google Scholar] [CrossRef]
- Żurowska, M.; Dąbrowski, R.; Dziaduszek, J.; Rejmer, W.; Czupryński, K.; Raszewski, Z.; Piecek, W. Comparison of racemic and enantiomeric 4′-(1-Methylheptyloxycarbonyl)Biphenyl-4-yl 4-[3-(2,2,3,3,4,4,4-Heptafluorobutoxy)Prop-1-Oxy]benzoates. Mol. Cryst. Liq. Cryst. 2010, 525, 219–225. [Google Scholar] [CrossRef]
- Drzewiński, W.; Dąbrowski, R.; Czupryński, K. Orthoconic antiferroelectrics. Synthesis and mesomorphic properties of optically active (S)-(+)-4-(1-methylheptyloxycarbonyl)phenyl 4′-(fluoroalkanoyloxyalkoxy)biphenyl-4-carboxylates and 4′-(alkanoyloxyalkoxy)biphenyl-4-carboxylates. Polish J. Chem. 2002, 76, 273–284. [Google Scholar] [CrossRef]
- Ravisankar, P.; Anusha, S.; Supriya, K.; Kumar, U.A. Fundamental Chromatographic Parameters. Inter. J. Pharm. Sci. Rev. Res. 2019, 55, 46–50. [Google Scholar]
- Urbańska, M.; Vaňkátová, P.; Kubíčková, A.; Kalíková, K. Synthesis, characterisation and supercritical fluid chromatography enantioseparation of new liquid crystalline materials. Liq. Cryst. 2020, 47, 1832–1843. [Google Scholar] [CrossRef]
- Vaňkátová, P.; Kalíkova, K.; Kubíckova, A. Ultra-performance supercritical fluid chromatography: A powerful tool for the enantioseparation of thermotropic fluorinated liquid crystals. Analyt. Chim. Act. 2018, 1038, 191–197. [Google Scholar] [CrossRef]
- Vaňkátová, P.; Folprechtová, V.D.; Kalíková, K.; Kubícková, A.; Armstrong, D.W.; Tesarová, E. Enantiorecognition ability of different chiral selectors for separation of liquid crystals in supercritical fluid chromatography; critical evaluation. J. Chrom. A. 2020, 1622, 461138. [Google Scholar] [CrossRef]
- Vaňkátová, P.; Kubícková, A.; Cigl, M.; Kalíková, K. Ultra-performance chromatographic methods for enantioseparation of liquid crystals based on lactic acid. J. Supercrit. Fluid. 2019, 146, 217–225. [Google Scholar] [CrossRef]
- Si-Hung, L.; Bamba, T. Current state and future perspectives of supercritical fluid chromatography. TrAC Tren. Analyt. Chem. 2022, 149, 116550. [Google Scholar] [CrossRef]
- Taylor, L.T. Supercritical fluid chromatography for the 21st century. J. Supercrit. Fluid. 2009, 47, 566–573. [Google Scholar] [CrossRef]
- Peyrin, E.; Lipka, E. Preparative supercritical fluid chromatography as green purification methodology. TrAC Tren. Analyt. Chem. 2024, 171, 117505. [Google Scholar] [CrossRef]
- Kalíková, K.; Šlechtová, T.; Vozka, J.; Tesařová, E. Supercritical Fluid Chromatography as a Tool for Enantioselective Separation; A Review. Analyt. Chim. Act. 2014, 821, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Nai, E.A.; Thurbide, K.B. A novel switchable water stationary phase for supercritical fluid chromatography. Analyt. Chim. Act. 2023, 1278, 341686. [Google Scholar] [CrossRef] [PubMed]
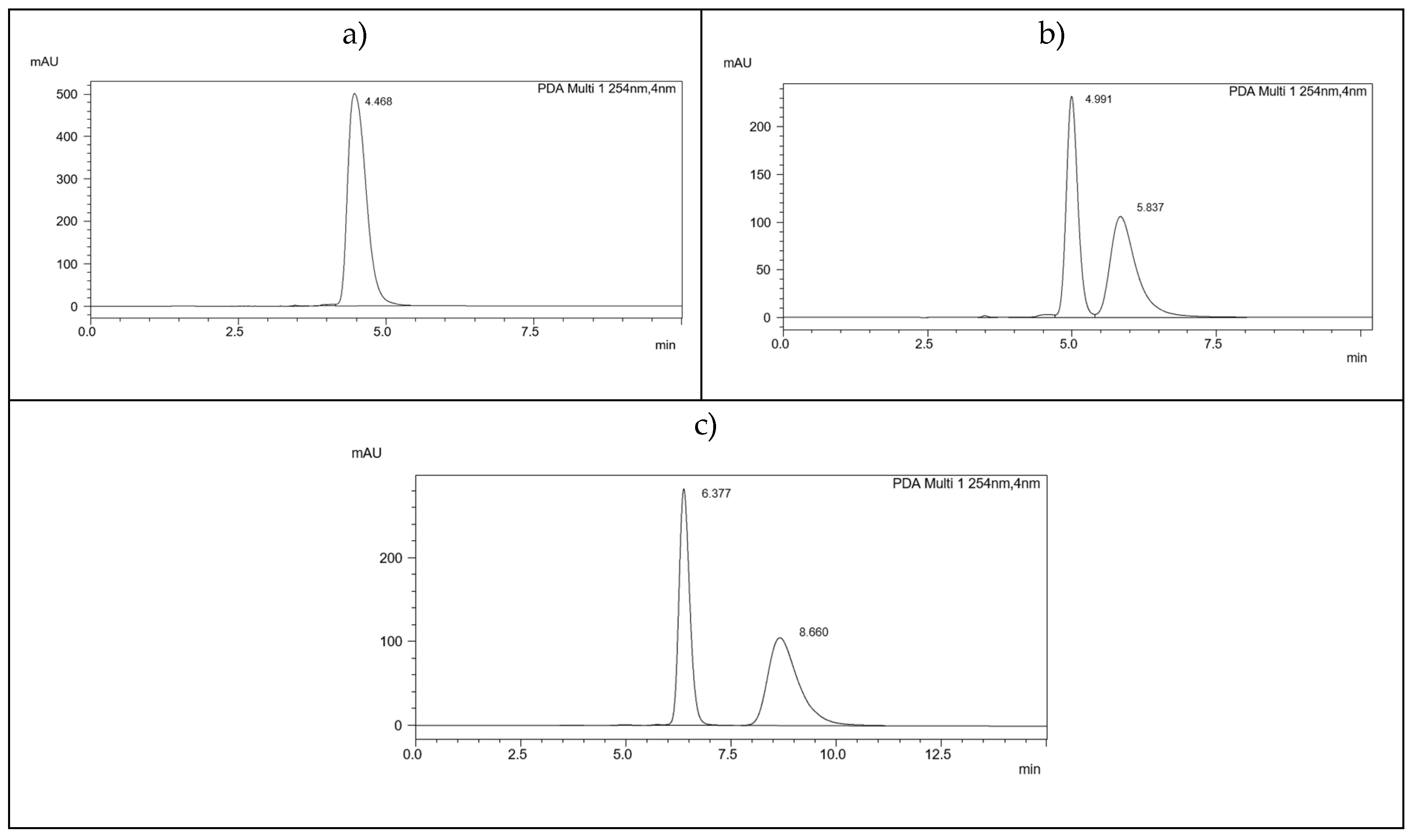
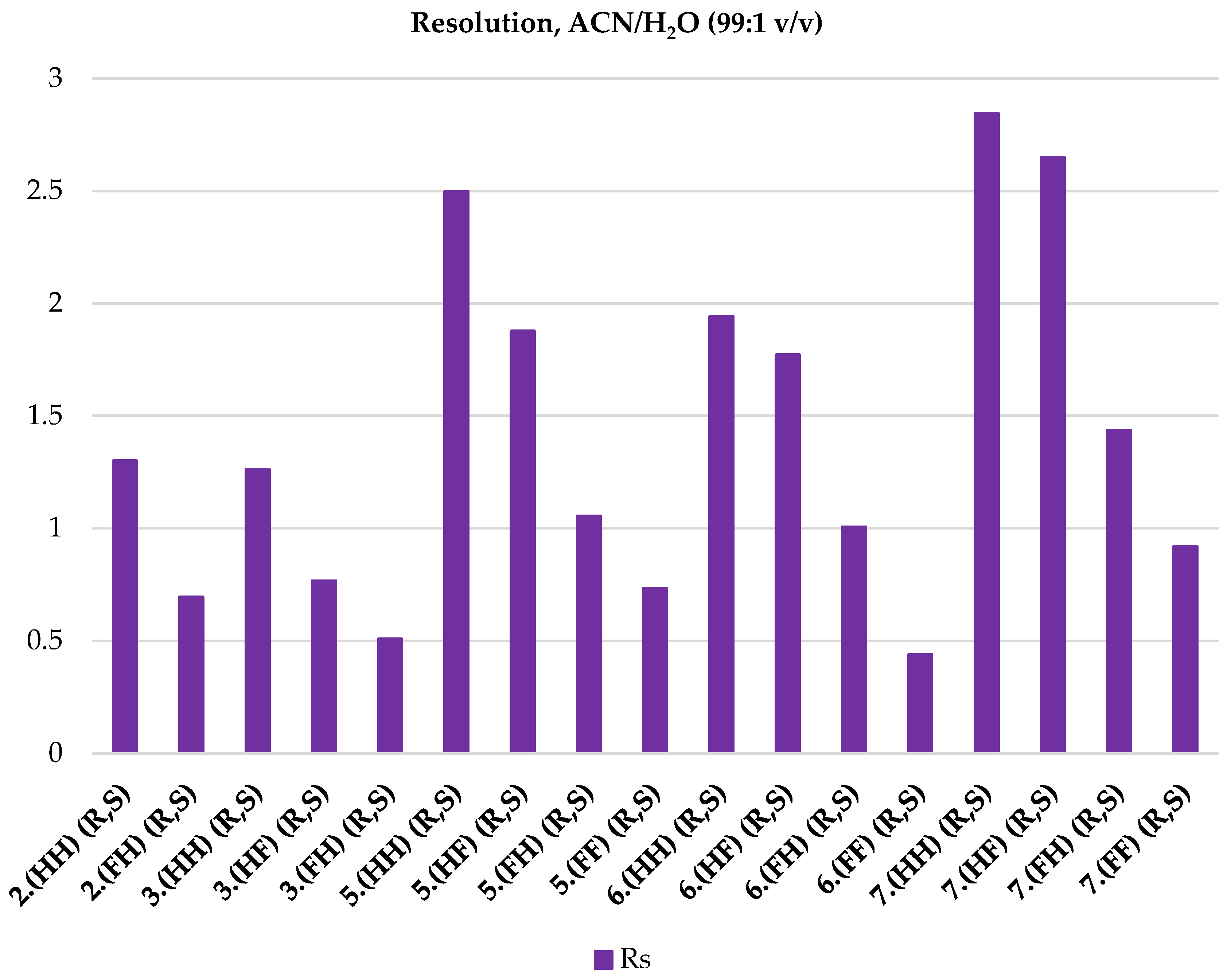
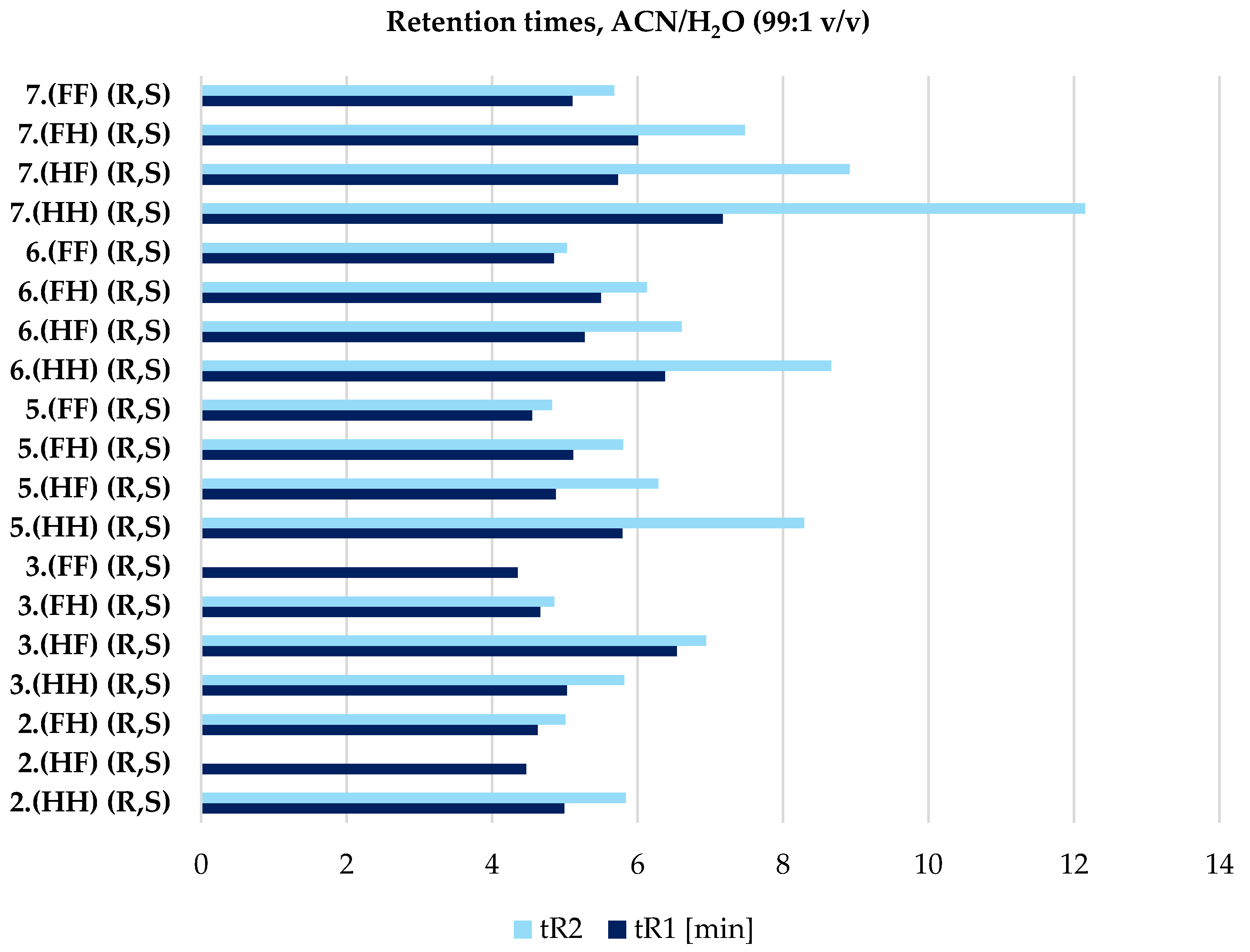
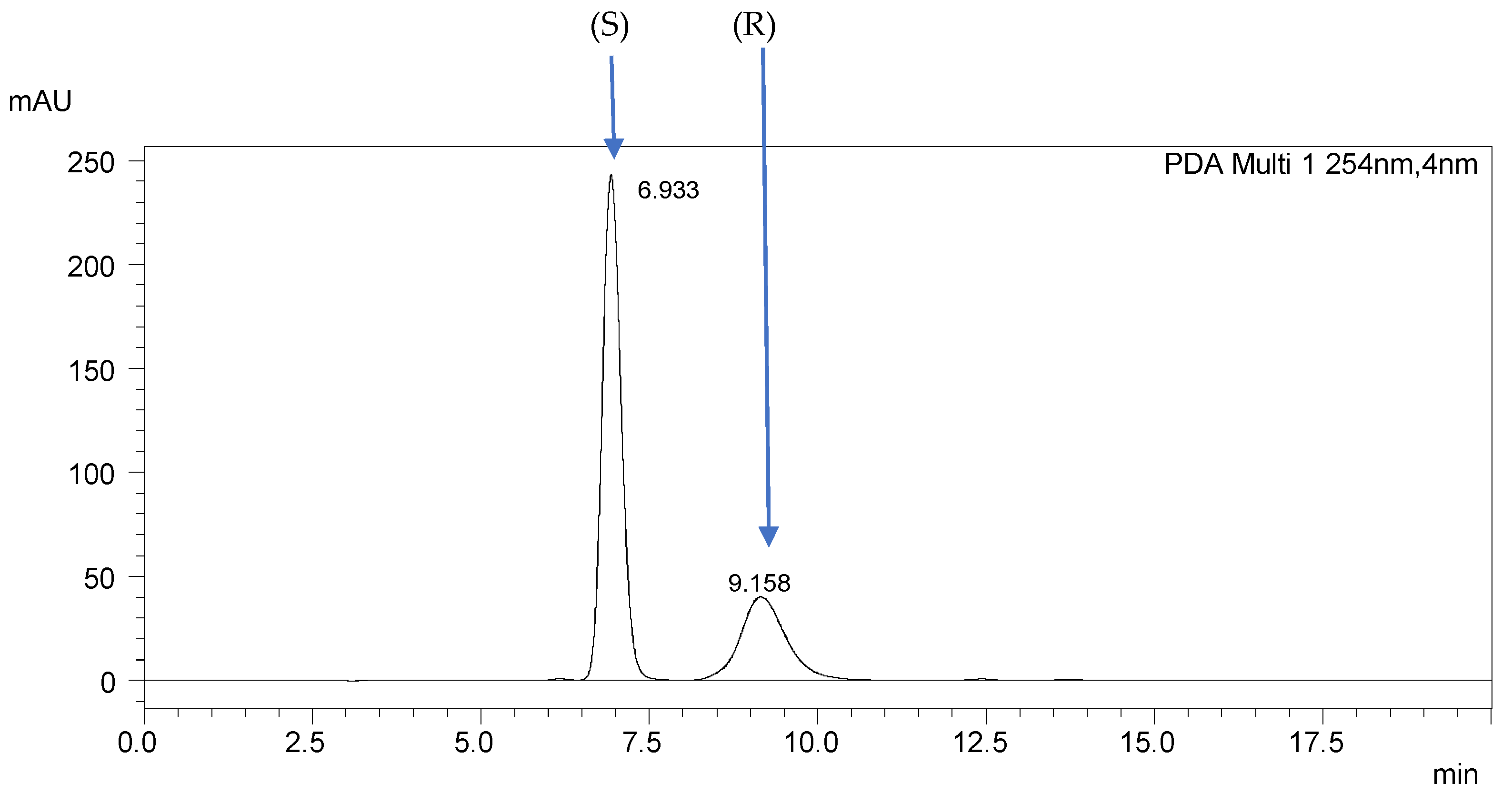
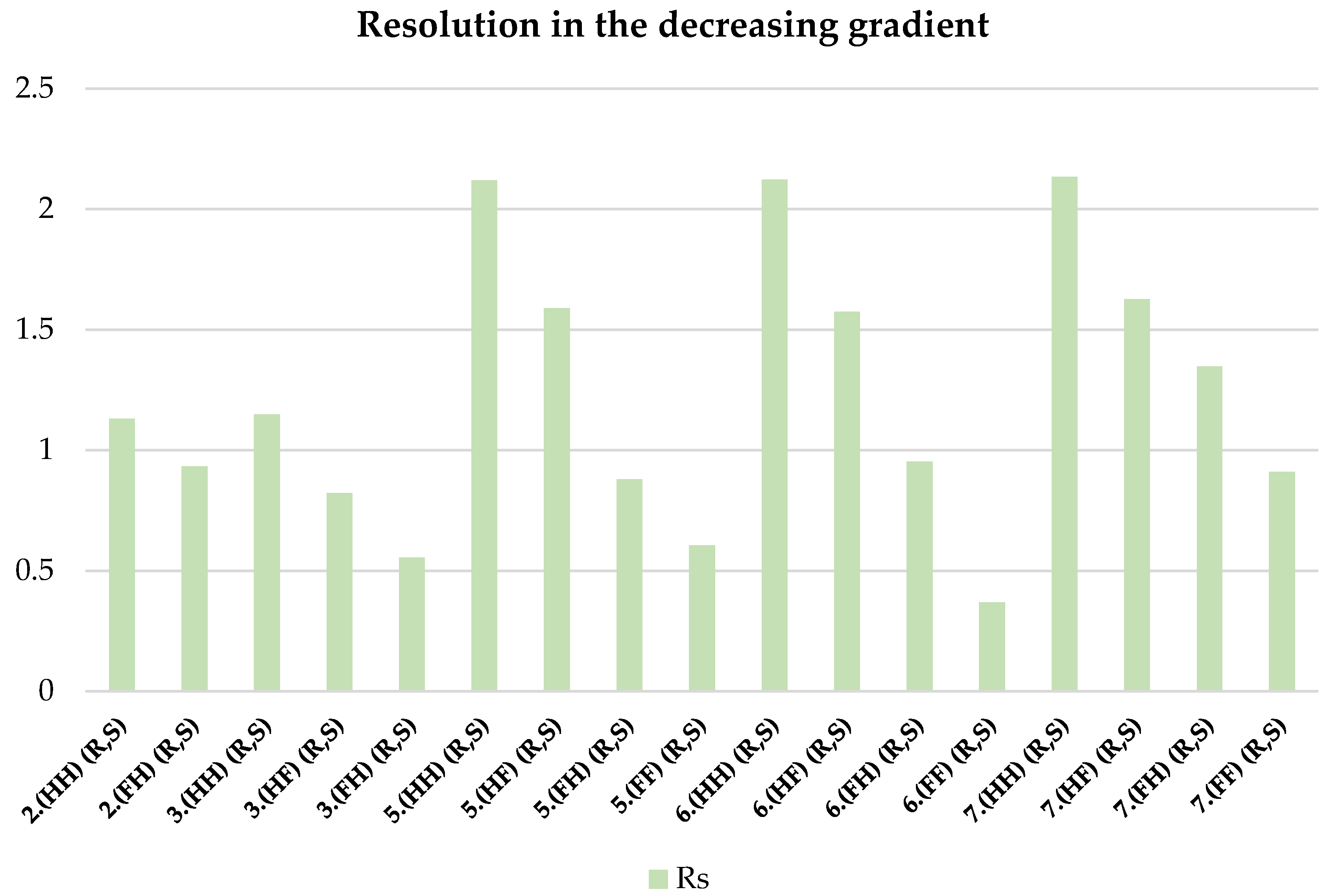
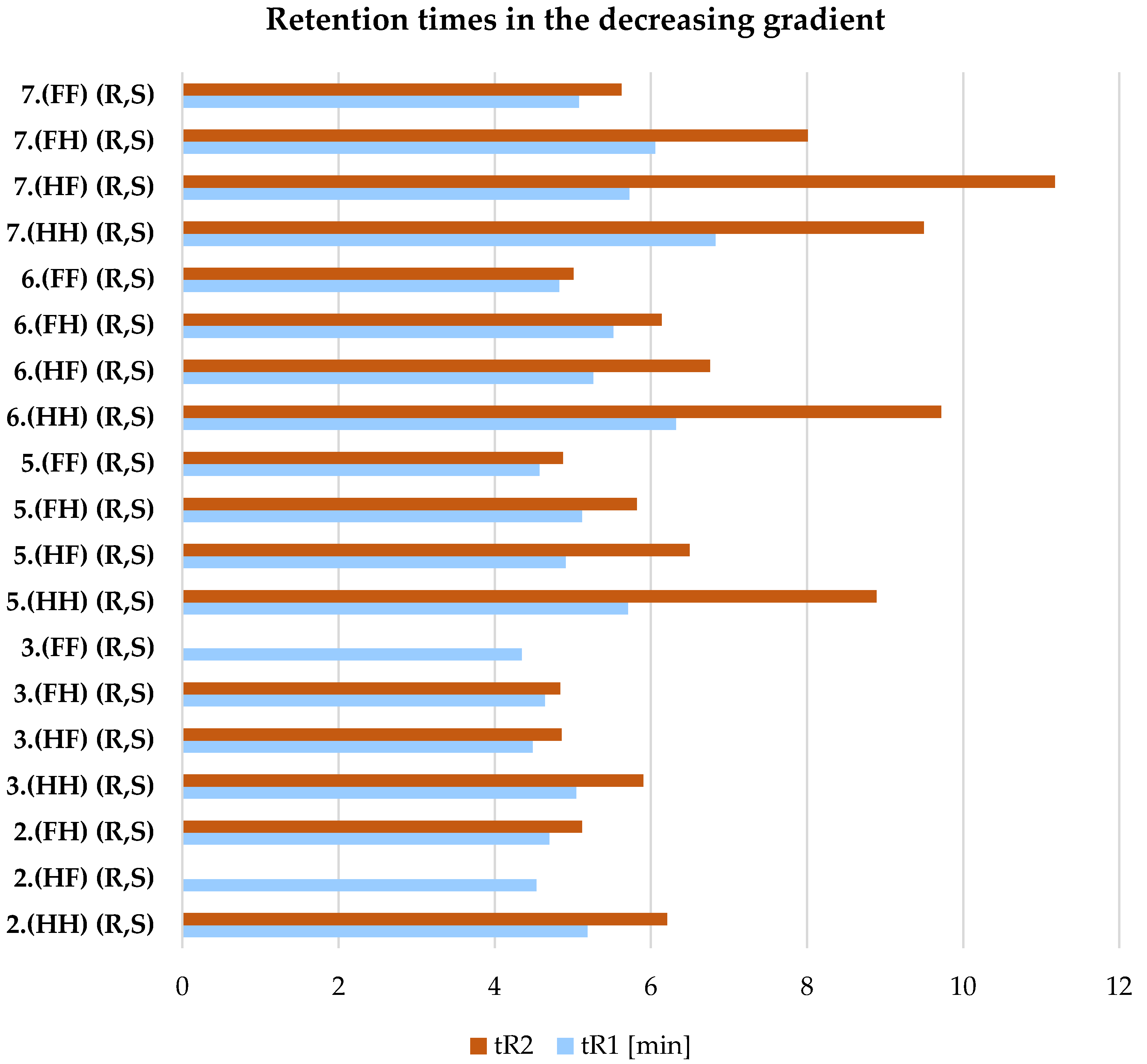
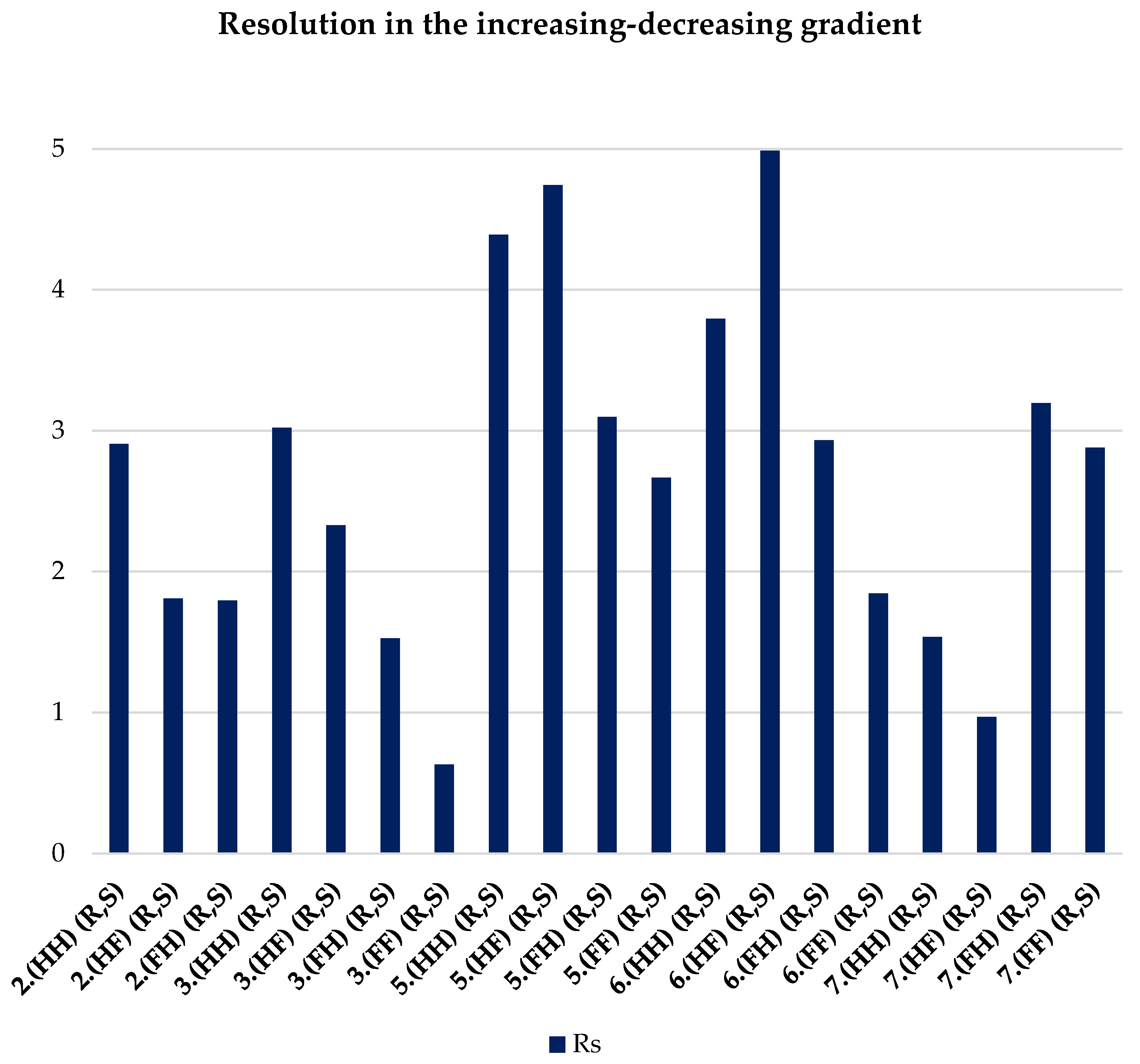
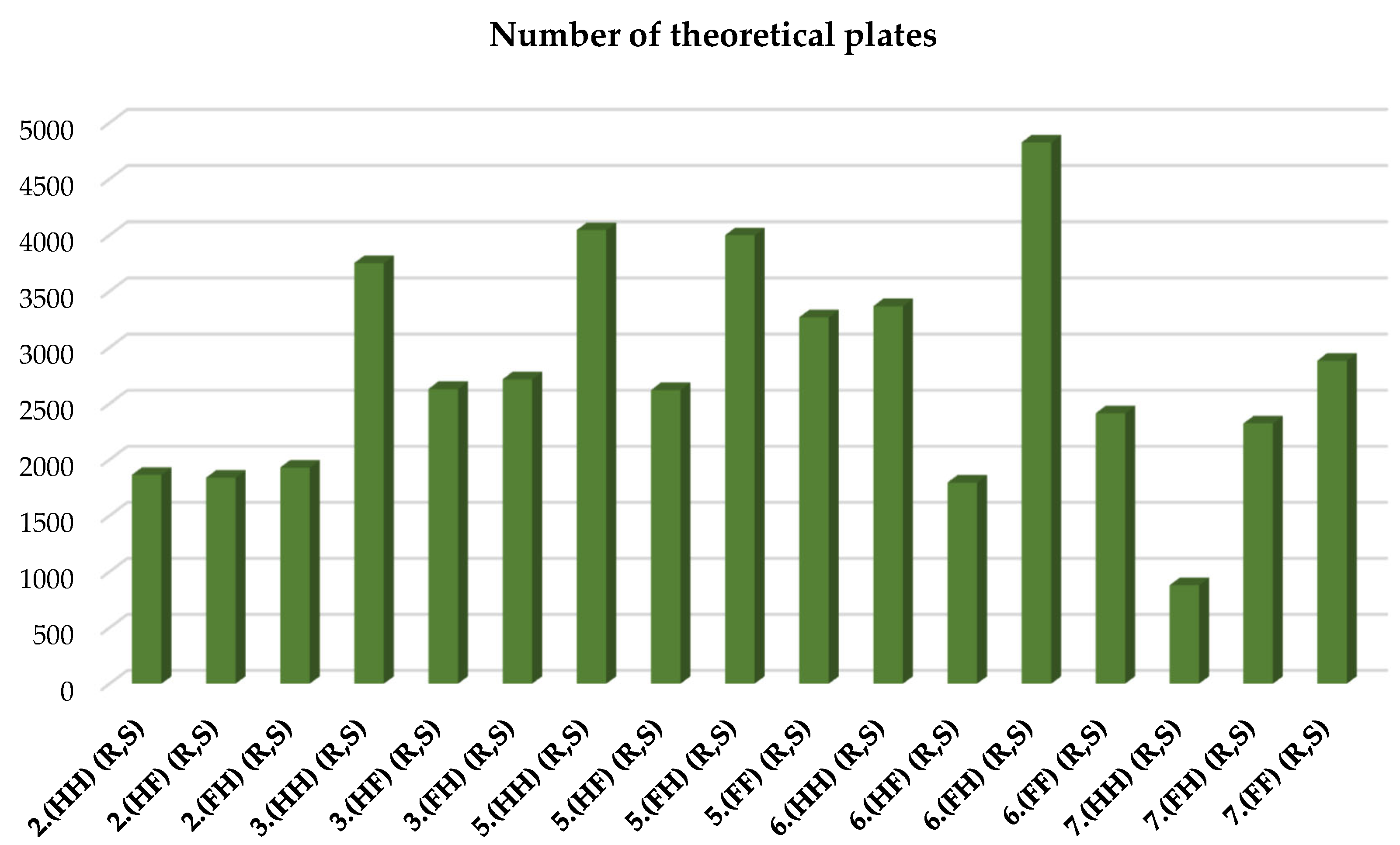
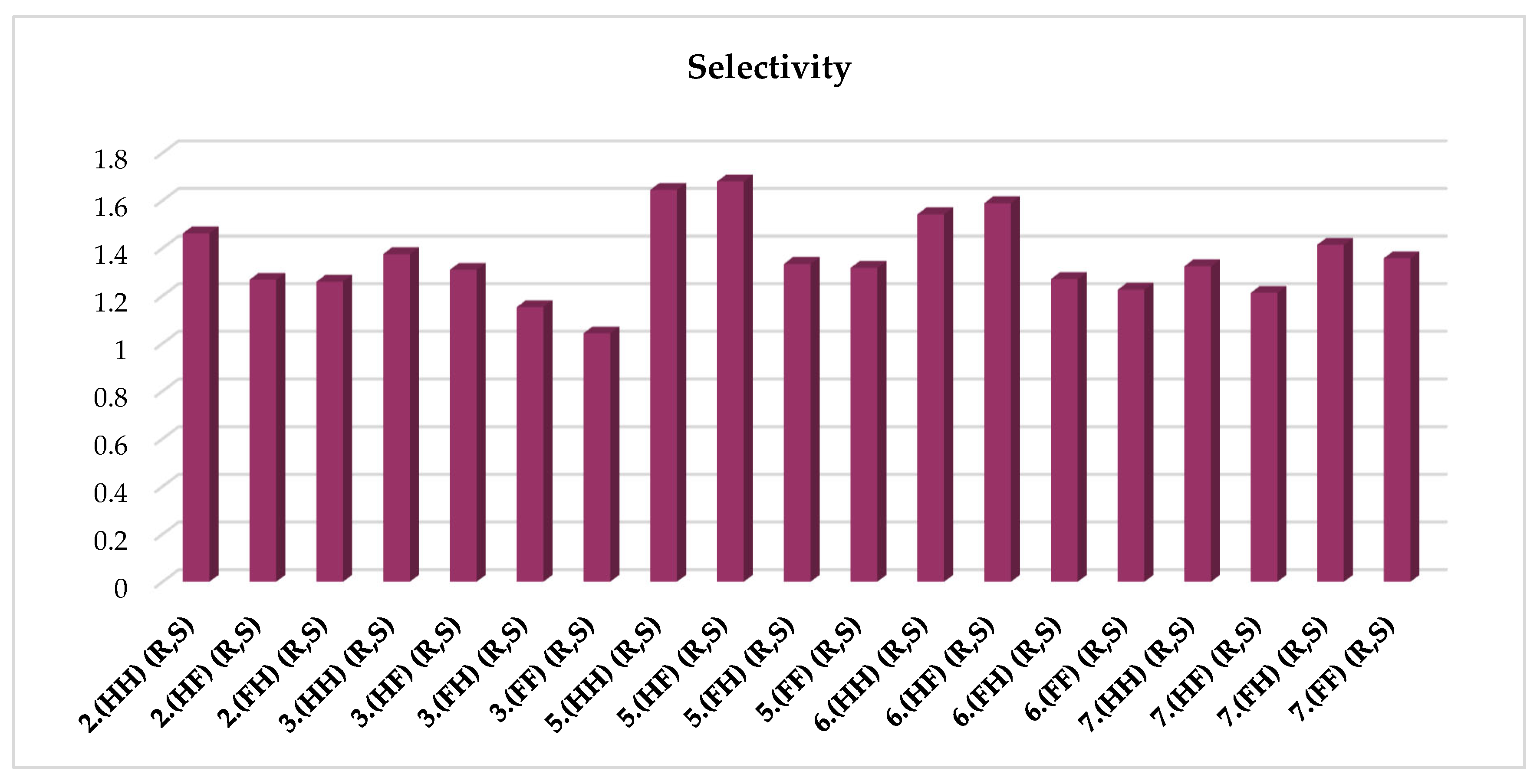
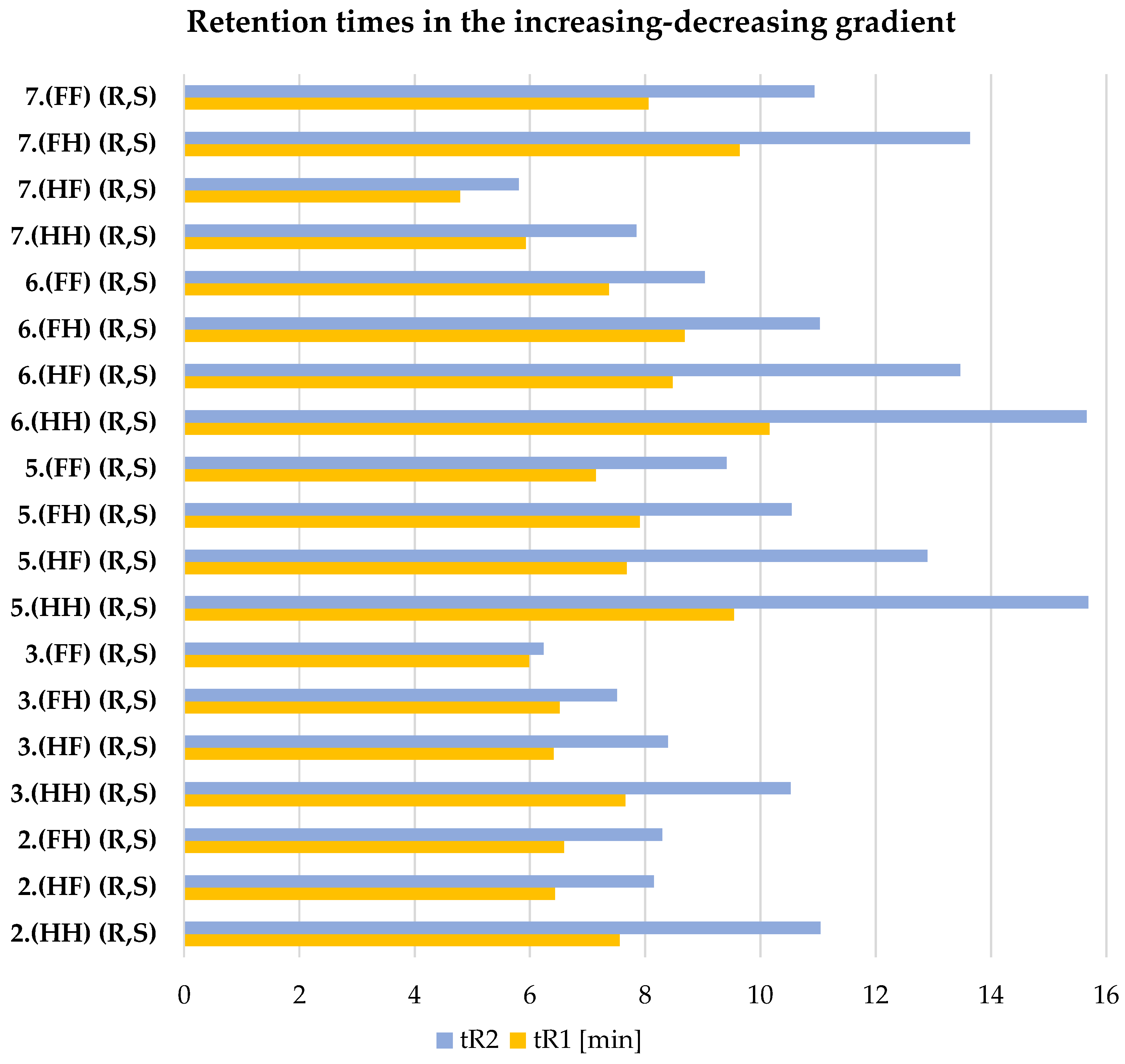
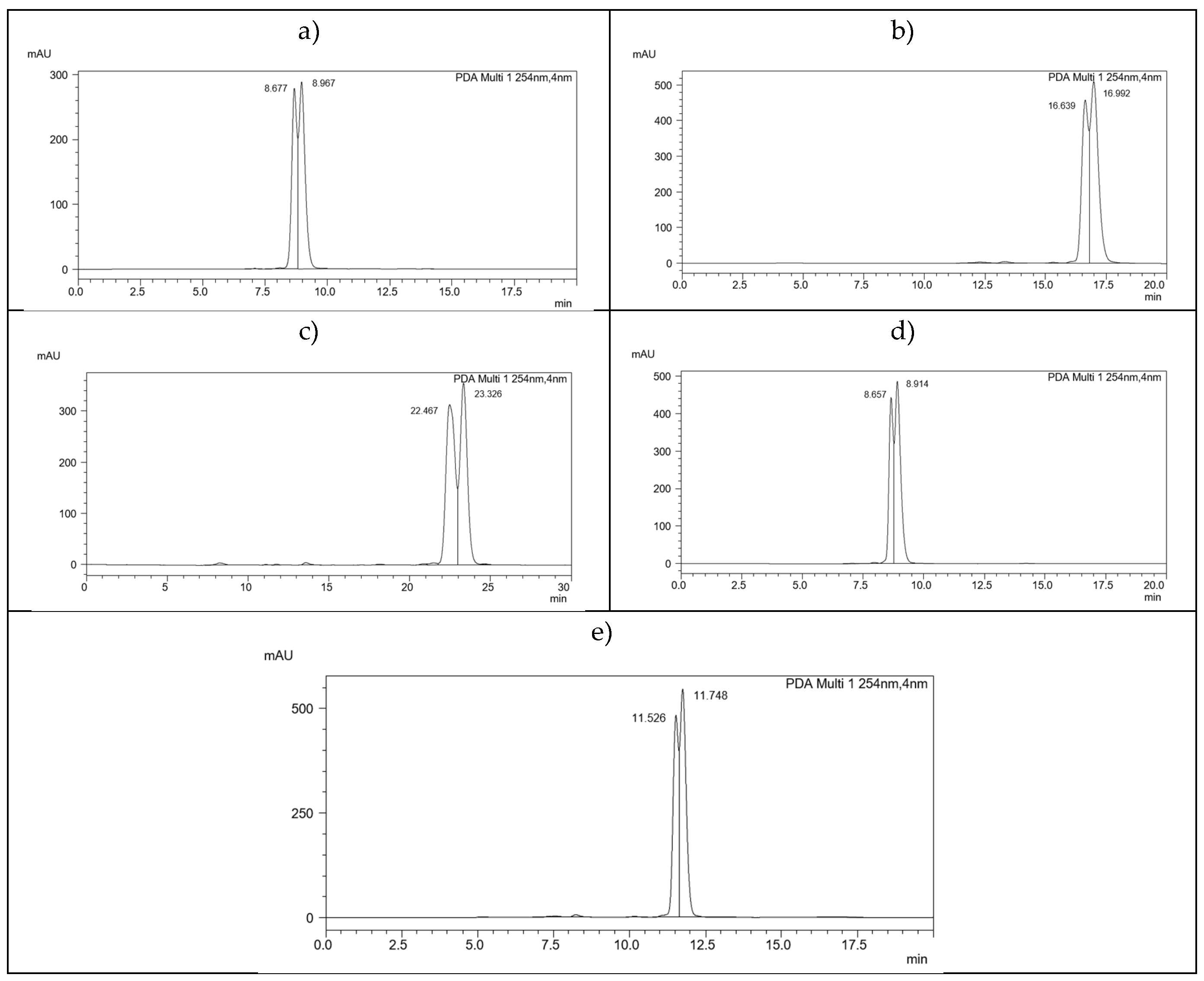
| 2.(HH) (R,S) | 3.(HH) (R,S) | 5.(HH) (R,S) | 6.(HH) (R,S) | 7.(HH) (R,S) |
| 2.(HF) (R,S) | 3.(HF) (R,S) | 5.(HF) (R,S) | 6.(HF) (R,S) | 7.(HF) (R,S) |
| 2.(FH) (R,S) | 3.(FH) (R,S) | 5.(FH) (R,S) | 6.(FH) (R,S) | 7.(FH) (R,S) |
| - | 3.(FF) (R,S) | 5.(FF) (R,S) | 6.(FF) (R,S) | 7.(FF) (R,S) |
| No. | v/v | Flow rate [mL·min-1] |
Injection volume [µl] | |
|---|---|---|---|---|
| CAN | H2O | |||
| 1. | 99 | 1 | 1 | 15 |
| 2. | 95 | 5 | 1 | 15 |
| 3. | 90 | 10 | 1 | 15 |
| Decreasing Gradient | Increasing-Decreasing Gradient | ||||
|---|---|---|---|---|---|
| Time [min] |
ACN/H2O (v/v) | Injection volume [µl] | Time [min] |
ACN/H2O (v/v) | Injection volume [µl] |
| 0.01 | 99/1 | 10 | 0.01 | 92/8 | 10 |
| 5 | 96/4 | 3 | 94/6 | ||
| 10 | 93/7 | 6 | 96/4 | ||
| 15 | 90/10 | 9 | 98/2 | ||
| 20 | STOP | 12 | 95/5 | ||
| - | - | 15 | 92/8 | ||
| - | - | 20 | STOP | ||
| Resolution | ||
|---|---|---|
| Acronym of the racemic mixtures | MIG column | MIC column |
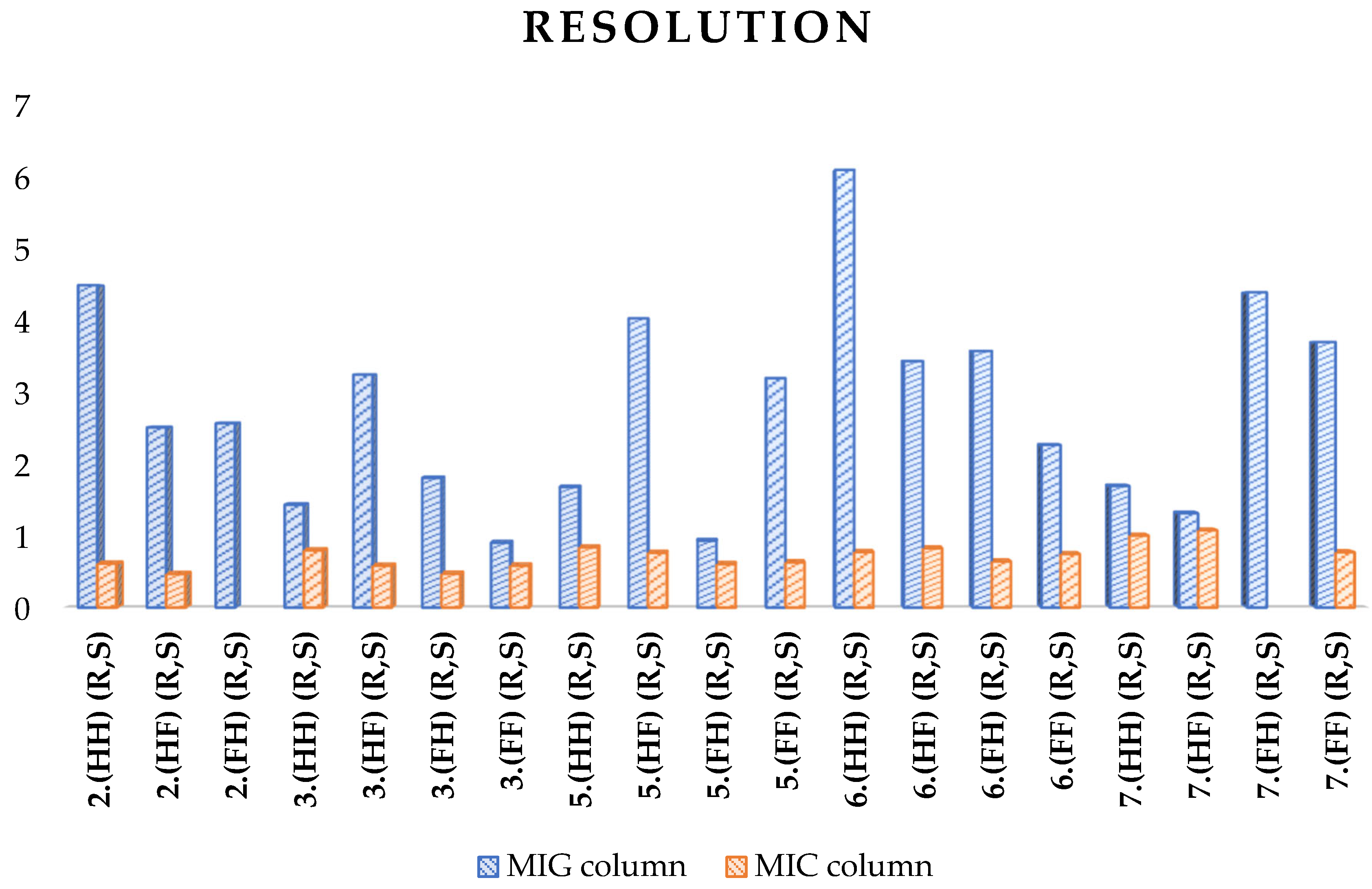
| 2.(HH) (R,S) | 4.482 | 0.611 |
| 2.(HF) (R,S) | 2.503 | 0.466 |
| 2.(FH) (R,S) | 2.561 | - |
| 3.(HH) (R,S) | 1.428 | 0.796 |
| 3.(HF) (R,S) | 3.237 | 0.582 |
| 3.(FH) (R,S) | 1.802 | 0.469 |
| 3.(FF) (R,S) | 0.900 | 0.583 |
| 5.(HH) (R,S) | 1.675 | 0.836 |
| 5.(HF) (R,S) | 4.021 | 0.761 |
| 5.(FH) (R,S) | 0.928 | 0.604 |
| 5.(FF) (R,S) | 3.188 | 0.628 |
| 6.(HH) (R,S) | 6.083 | 0.772 |
| 6.(HF) (R,S) | 3.425 | 0.824 |
| 6.(FH) (R,S) | 3.565 | 0.640 |
| 6.(FF) (R,S) | 2.260 | 0.743 |
| 7.(HH) (R,S) | 1.689 | 1.000 |
| 7.(HF) (R,S) | 1.310 | 1.073 |
| 7.(FH) (R,S) | 4.382 | - |
| 7.(FF) (R,S) | 3.690 | 0.768 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





