Submitted:
13 March 2024
Posted:
14 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
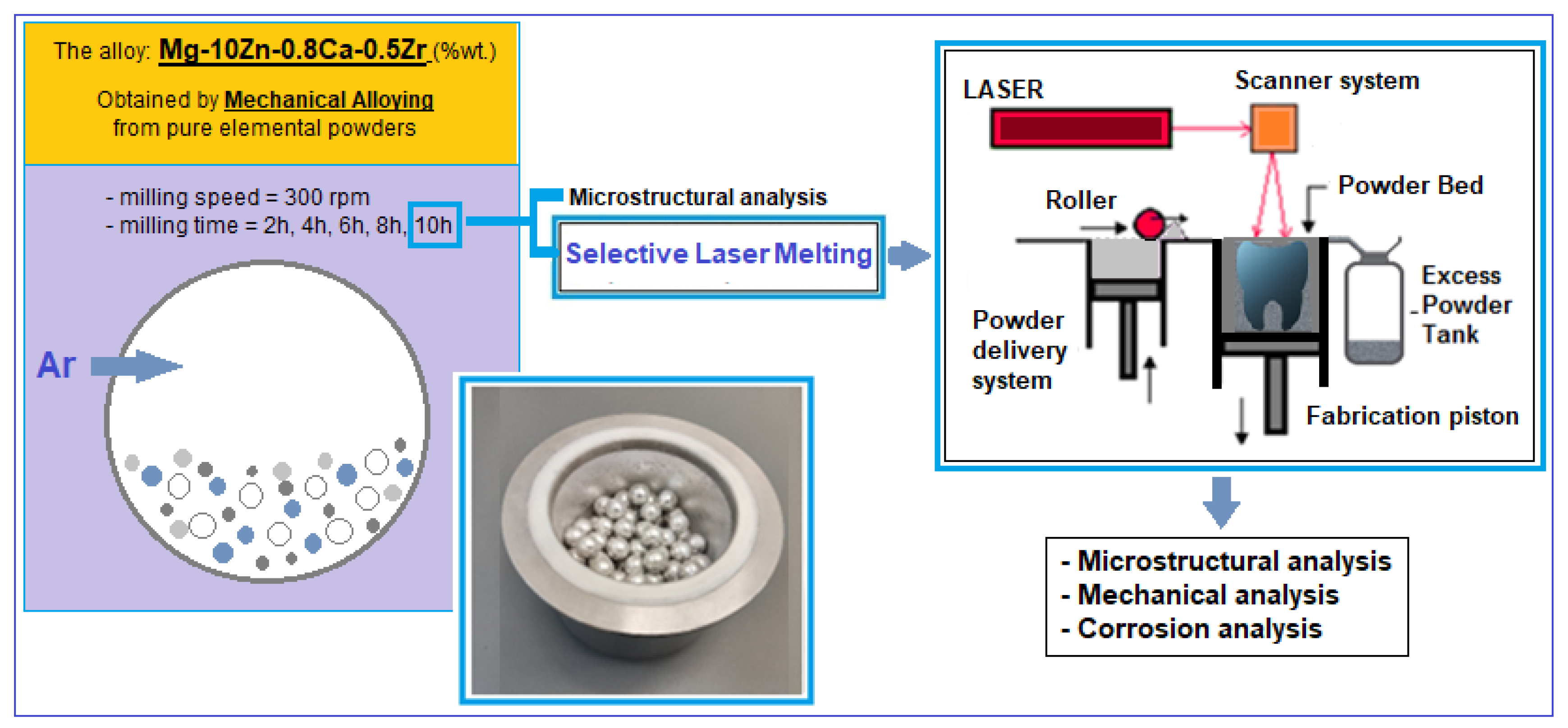
2. Materials and Methods
3. Results and Discussions
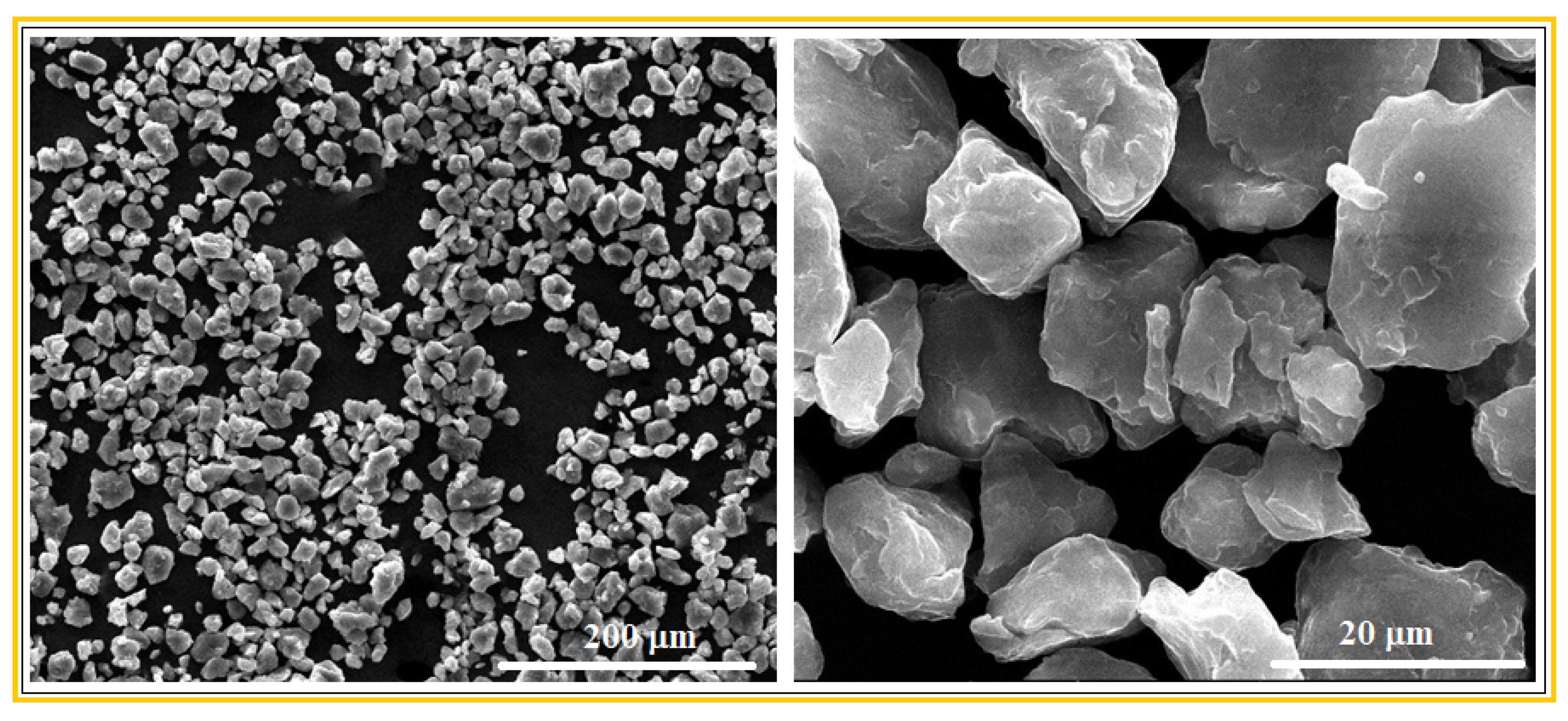
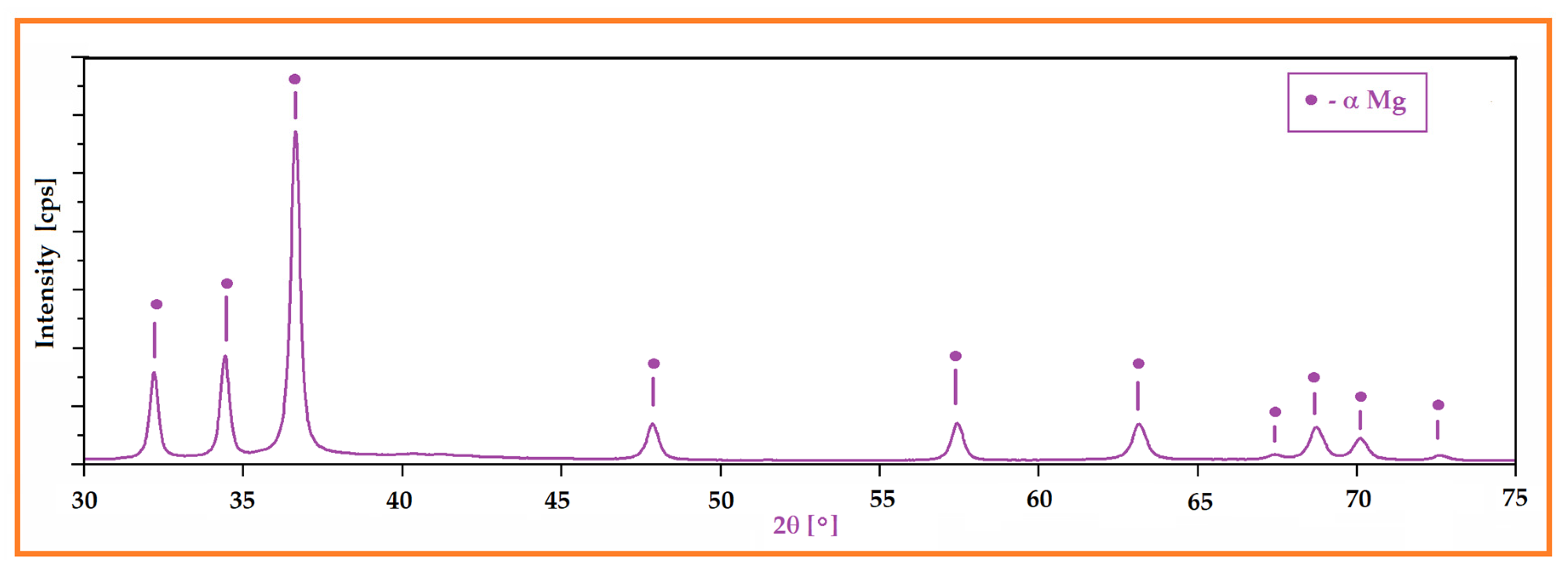
3.1. Microstructural Characterization of the Mg-Zn-Ca-Zr Alloy in Powder State and after SLM Processing
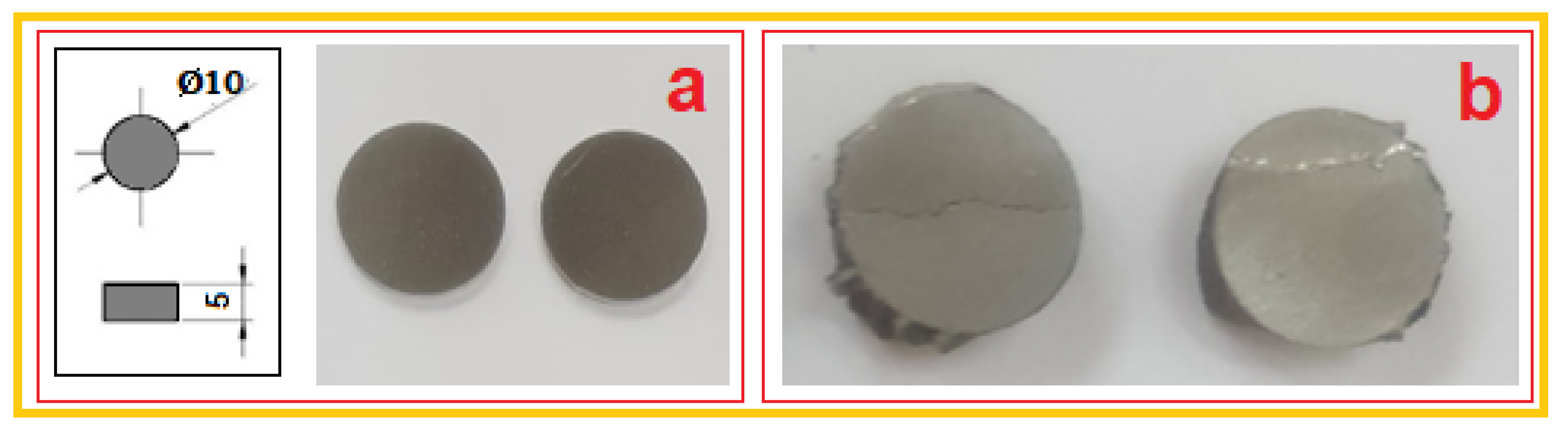
3.1. Mechanical Characterization of the Mg-Zn-Ca-Zr Alloy in Powder State and after SLM Processing
3.1. Corrosion Analysis of the SLM Processed Mg-Based Alloy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esmaily M., Svensson J.E., Fajardo S., Birbilis N., Frankel G.S., Virtanen S., Arrabal R., Thomas S., Johansson L.G., Fundamentals and advances in magnesium alloy corrosion, Prog. Mater. Sci., 2017, 89, 92-193. [CrossRef]
- Song J., She J., Chen D., Pan F., Latest research advances on magnesium and magnesium alloys worldwide, J. Magnes. Alloy, 2020, 8(1), 1-41. [CrossRef]
- Lovašiová, P.; Lovaši, T.; Kubásek, J.; Jablonská, E.; Msallamová, Š.; Michalcová, A.; Vojtˇech, D.; Suchý, J.; Koutný, D.; Ghassan Hamed Alzubi, E. Biodegradable WE43 Magnesium Alloy Produced by Selective Laser Melting: Mechanical Properties, Corrosion Behavior, and In-Vitro Cytotoxicity. Metals 2022, 12, 469. [CrossRef]
- Chen X., Ning S., Wang A., Le Q., Liao Q., Jia Y., Cheng C., Li X., Atrens A., Yu F., Microstructure, mechanical properties and corrosion behavior of quasicrystal-reinforced Mg-Zn-Y alloy subjected to dual-frequency ultrasonic field, Corros. Sci., 2020, 163, Article 108289. [CrossRef]
- Zhang J., Zhang B., Zhang J., Lin W., Zhang S., Magnesium promotes the regeneration of the peripheral nerve; Front. Cell Dev. Biol., 2021, 2169, 717854. [CrossRef]
- Dong J., Lin T., Shao H., Wang H., Wang X., Song K., et al., Advances in degradation behavior of biomedical magnesium alloys: A review. J. Alloys Compd. 2022, 908, 164600. [CrossRef]
- Cao F., Xiao B., Wang Z., Ying T., Zheng D., Atrens A., Song G.-L., A Mg alloy with no hydrogen evolution during dissolution, J. Magnes. Alloys, 2023, 11(6), 2084-2095. [CrossRef]
- Li W., Qiao W., Liu X., Bian D., Shen D., Zheng Y., et al., Biomimicking bone-implant interface facilitates the bioadaption of a new degradable magnesium alloy to the bone tissue microenvironement. Adv. Sci. 2021, 8, 2102035. [CrossRef]
- Amukarimi S., Mozafari M., Biodegradable magnesium-based biomaterials: An overview of challenges and opportunities. MedComm, 2021, 2, 123-144. [CrossRef]
- Banerjee P., Saadi S., Choudhary L., Harandi S.E., Singh R., Magnesium implants: Prospects and challenges. Materials, 2019, 12, 136. [CrossRef]
- O’Neill E., Awale G., Daneshmandi L., Umerah O., Lo K.W.H., The roles of ions on bone regeneration, Drug Discov. Today, 2018, 23, 879-890. [CrossRef]
- Qin Y., Wen P., Guo H., Xia D., Zheng Y., Jauer L., et al., Additive manufacturing of biodegradable metals: Current research status and future perspectives. Acta Biomater. 2019, 98, 3-22. [CrossRef]
- Zhou H., Liang B., Jiang H., Deng Z., Yu K., Magnesium based biomaterials as emerging agents for bone repair and regeneration: From mechanism to application. J. Magnes. Alloys, 2021, 9, 779-804. [CrossRef]
- Ting Zhang, Wen Wang, Jia Liu, Liqiang Wang, Yujin Tang, Kuaishe Wang, A review on magnesium alloys for biomedical applications, Front. Bioeng. Biotechnol., 16 August 2022, Sec. Biomaterials, Vol. 10-2022. [CrossRef]
- Wang J.L., Xu J.K., Hopkins C., Chow D.H.K., Qin L., Biodegradable magnesium based implants in orthopedics – A general review and perspectives. Adv. Sci. 2020, 7, 1902443. [CrossRef]
- Wang W., Han P., Peng P., Zhang T., Liu Q., Yuan S.-N., et al., Friction stir processing of magnesium alloys: A review, Acta Metall. Sinica (Eng Letters), 2020, 33(1), 43-57. [CrossRef]
- Jung O., Porchetta D., Schroeder M.-L., Klein M., Wegner N., Walther F., et al., In vivo simulation of magnesium degradability using a new fluid dynamic bench testing approach. Int. J. Mol. Sci. 2019, 20, 4859. [CrossRef]
- Kumar K., Gill R., Batra U., Challenges and opportunities for biodegradable magnesium alloy implants. Mat. Technol. (N.Y.N.Y.) 2018, 33, 153-172. [CrossRef]
- Chin, P., Cheok, Q., Glowacz, A., and Caesarendra, W., A review of in-vivo and in-vitro real-time corrosion monitoring systems of biodegradable metal implants, Appl. Sci. (Basel), 2020, 10(9), 3141. [CrossRef]
- Kim Y.K.; Lee K.B.; Kim S.Y.; Bode K.; Jang Y.S.; Kwon T.Y.; Jeon M.H.; Lee M.H.; Gas formation and biological effects of biodegradable magnesium in a preclinical and clinical observation, Sci. Technol. Adv. Mater., 2018, 19:1, 324 – 335. [CrossRef]
- Kuhlmann J., Bartsch, Willbold E.; Schuchardt S.; Holz O.; Hort N.; Höche D.; Heineman W.R.; Witte F.; Fast escape of hydrogen from gas cavities around corroding magnesium implants, Acta Biomaterialia, 2013, 9(10), 8714-8721. [CrossRef]
- Huan, Z.G.; Leeflang, M.A.; Zhou, J.; Fratila-Apachitei, L.E.; Duszczyk, J. In vitro degradation behavior and cytocompatibility of Mg–Zn–Zr alloys. J. Mater. Sci.Mater. Med. 2010, 21, 2623–2635. [CrossRef]
- Gu, X.; Xie, X.; Li, N.; Zheng, Y. In vitro and in vivo studies on a Mg–Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [CrossRef]
- Seifen, S.; Hock, M. Development of magnesium implants by application of conjoint-based quality function deployment. J. Biomed. Mater. Res. 2019, 107, 2814–2834. [CrossRef]
- Shuai, C.; Zhou, Y.; Yang, Y.; Gao, C.; Peng, S.; Wang, G. Ag-Introduced Antibacterial Ability and Corrosion Resistance for Bio-Mg Alloys. BioMed Res. Int. 2018, 6023460. [CrossRef]
- Seetharaman, S.; Sankaranarayanan, D.; Gupta, M. Magnesium-Based Temporary Implants: Potential, Current Status, Applications, and Challenges. J. Funct. Biomater. 2023, 14, 324. [CrossRef]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koça, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloys 2018, 6, 23–43. [CrossRef]
- Global Personalized 3D Printed Orthopedic Implants Market, Data Bridge Market Research. Available online: https://www. databridgemarketresearch.com/reports/global-personalized-3d-printed-orthopedic-implants-market (accessed on 8 December 2023).
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. Available online: https://www.researchgate.net/publication/27120142 (accessed on 16 December 2023).
- Zhang, M.; Chen, C.; Liu, C.; Wang, S. Study on Porous Mg-Zn-Zr ZK61 Alloys Produced by Laser Additive Manufacturing. Metals 2018, 8(8), 635. [CrossRef]
- Liu, C.; Zhang, M.; Chen, C. Effect of laser processing parameters on porosity, microstructure and mechanical properties of porous Mg-Ca alloys produced by laser additive manufacturing. Mater. Sci. Eng. A 2017, 703, 359–371. [CrossRef]
- Bita T., Corneschi I., Ungureanu E., Bita A.I., Ignat N.D., Dura H., Carstoc I.D., Nicolcescu P., Influence of heat treatment on microstructure and corrosion behavior of biodegradable Mg-Ca alloy, U.P.B. Sci. Bull., Series B, 2023, 85(4), https://www.scientificbulletin.upb.ro/rev_docs_arhiva/full9d8_327223.pdf.
- N. Ikeo, M. Nishioka, T. Mukai, Fabrication of biodegradable materials with high strength by grain refinement of Mg–0.3 %at. Ca alloys, Materials Letters, 2018, 223, 65-68. [CrossRef]
- M. Deng, L. Wang, D. Snihirova, J. Bohlen, G. Kurz, S.V. Lamaka, D. Höche, M.L. Zheludkevich, Micro-alloyed Mg-Ca: Corrosion susceptibility to heating history and a plain problem-solving approach, J. Magnes. Alloys, 2023, 11(4), 1193-1205. [CrossRef]
- J. Wang, J. Dou, Z. Wang, C. Hu, H. Yu, C. Chen, Research progress of biodegradable magnesium-based biomedical materials: A review, Journal of Alloys and Compounds, Vol. 923, 2022. [CrossRef]
- H.L. Ding, P. Zhang, G.P. Cheng, S. Kamado, Effect of Ca addition on the microstructure and creep behaviour of AZ91 Mg alloy, Materials Today: Proceedings, online 16 March 2023. [CrossRef]
- L. Shi, S. Chen, F. Zheng, M. Liu, H. Yang, B. Zhang, Corrosion resistance evaluation of biodegradable magnesium alloy vascular stents optimized by mechanical adapted polymer coating strategy, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 658, 5 February 2023, 130664. [CrossRef]
- Nowosielski, R.; Gawlas-Mucha, A.; Borowski, A.; Guwer, A.; Fabrication and properties of magnesium based alloys Mg-Ca, JAMME, 2013 (21), 61(2):367-374.
- Shuai, C.; Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Zhou, Y.; Feng, P.; Liu, X.; Peng, S. Laser rapid solidification improves corrosion behavior of Mg-Zn-Zr alloy. J. Alloys Compd. 2017, 691, 961–969. [CrossRef]
- Nayeb-Hashemi, A.A., Clark, J.B. The Mg−Zr (Magnesium-Zirconium) system. Bulletin of Alloy Phase Diagrams, 1985, 6, 246–250; [CrossRef]
- Manic, M., Vitković, N., Mitic, J. (2022). Design and Manufacturing of the Personalized Plate Implants. In: Canciglieri Junior, O., Trajanovic, M.D. (eds) Personalized Orthopedics. Springer, Cham. [CrossRef]
- Jariwala, S.H.; Lewis, G.S.; Bushman, Z.J.; Adair, J.H.; Donahue, H.J. 3D printing of personalized artificial bone scaffolds. 3D Print. Addit. Manuf. 2015, 2, 56–64. [CrossRef]
- Nopová, K.; Jaroš, J.; Cervinek, O.; Pantˇelejev, L.; Gneiger, S.; Senck, S.; Koutný, D. Processing of AZ91D Magnesium Alloy by Laser Powder Bed Fusion. Appl. Sci. 2023, 13, 1377. [CrossRef]
- Yang, Y.; Ping, W.; Xin, L.; Yong, L.; Hong, B.; Zhou, Y.; Gao, C.; Shuai, C. System development, formability quality and microstructure evolution of selective laser-melted magnesium. Virtual Phys. Prototyp. 2016, 11, 173–181.
- Chen, L.; Lin, Z.; Wang, M.; Huang, W.; Ke, J.; Zhao, D.; Yin, Q.; Zhang, Y. Treatment of trauma-induced femoral head necrosis with biodegradable pure Mg screw-fixed pedicle iliac bone flap. J. Orthop. Transl. 2019, 17, 133–137. [CrossRef]
- Karunakaran ROrtgies, S.; Tamayol, A.; Bobaru, F.; Sealy, M.P. Additive manufacturing of magnesium alloys. Bioact. Mater. 2020, 5, 44–54. [CrossRef]
- Hassan, S.F.; Islam, M.T.; Saheb, N.; Baig, M.M.A. Magnesium for Implants: A Review on the Effect of Alloying Elements on Biocompatibility and Properties. Materials 2022, 15, 5669. [CrossRef]
- Chen, J.; Xu, Y.; Kolawole, S.K.; Wang, J.; Su, X.; Tan, L.; Yang, K. Systems, Properties, Surface Modification and Applications of Biodegradable Magnesium-Based Alloys: A Review. Materials 2022, 15, 5031. [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [CrossRef]
- Lesz, S.; Hrapkowicz, B.; Karolus, M.; Gołombek, K. Characteristics of the Mg-Zn-Ca-Gd Alloy after Mechanical Alloying. Materials 2021, 14, 226. [CrossRef]
- Salleh, E.M.; Ramakrishnan, S.; Hussain, Z. Synthesis of Biodegradable Mg-Zn Alloy by Mechanical Alloying: Effect of Milling Time. Procedia Chem. 2016, 19, 525–530. [CrossRef]
- Abbasi, M.; Sajjadi, S.A.; Azadbeh, M. An investigation on the variations occurring during Ni3Al powder formation by mechanical alloying technique. J. Alloys Compd. 2010, 497, 171–175. [CrossRef]
- Raducanu, D.; Cojocaru, V.D.; Nocivin, A.; Hendea, R.E.; Ivanescu, S.; Stanciu, D.; Trisca-Rusu, C.; Serban, N.; Drob, S.I.; Campian, R.S. Microstructure Evolution during Mechanical Alloying of a Biodegradable Magnesium Alloy. Crystals 2022, 12(11), 1641. [CrossRef]
- Hendea R.E., Raducanu D., Nocivin A., Ivanescu S., Stanciu D., Trisca-Rusu C., Campian R.S., Drob S.I., Cojocaru V.D., Gălbinasu B.M., Laser Powder Bed Fusion Applied to a New Biodegradable Mg-Zn-Zr-Ca Alloy, Materials 2022, 15(7), 2561. [CrossRef]
- K. Park, B.Y. Chang, and S. Hwang, Correlation between Tafel Analysis and Electrochemical Impedance Spectroscopy by Prediction of Amperometric Response from EIS, ACS Omega 2019, 4(21), 19307-19313; [CrossRef]
- Suryanarayana C., Mechanical Alloying: A Novel Technique to Synthesize Advanced Materials, Research 2019, Article ID 4219812, 17 p. [CrossRef]
- Lesz, S.; Hrapkowicz, B.; Karolus, M.; Gołombek, K. Characteristics of the Mg-Zn-Ca-Gd Alloy after Mechanical Alloying. Materials 2021, 14, 226. [CrossRef]
- M. Lichtenauer, S. Nickl, K. Hoetzenecker, A. Mangold, B. Moser, M. Zimmermann, S. Hacker, T. Niederpold, A. Mitterbauer, H. J. Ankersmit, Phosphate Buffered Saline Containing Calcium and Magnesium Elicits Increased Secretion of Interleukin-1 Receptor Antagonist, Laboratory Medicine, 2009, 40(5), 290–293; [CrossRef]
- Ouyang, Y.; Chen, Z.; Jiang, C.; Yang, W.; Chen, Y.; Yin, X.; Liu, Y. Design of the double-layer biocompatible coating on AZ31 magnesium alloy for highly effective corrosion resistance. Surf. Coat. Technol. 2021, 428, 127897. [CrossRef]
- Ma, E. Alloys created between immiscible metals. Prog. Mater. Sci. 2005, 50, 413–509. [CrossRef]
- Suryanarayana, C.; Liu, J. Processing and characterization of mechanically alloyed immiscible metals. Int. J. Mater. Res. 2012, 103, 1125–1129. [CrossRef]
- Fu, W.; Yang, H.; Li, T.; Sun, J.; Guo, S.; Fang, D.; Qin, W.; Ding, X.; Gao, Y.; Sun, J. Enhancing corrosion resistance of ZK60 magnesium alloys via Ca microalloying: The impact of nanoscale precipitates. J. Magnes. Alloys 2023, 11(9), 3214-3230. [CrossRef]
- Biancardi, O.V.; Luccas Rosa, V.; de Abreu, L.B.; Tavares, A.P.R.; Corrêa, R.G.; Cavalcanti, P.H.; Napoleão, B.I.; da Silva, E.P. Corrosion behavior of as-cast ZK60 alloy modified with rare earth addition in sodium sulfate medium. Corros. Sci. 2019, 158, 108092. [CrossRef]
- Mandal, M.; Mondal, K. Effect of Micro-alloying and Microstructure on the Corrosion Behavior of As-Cast Mg-6.2 wt.% Zn Alloy. J. Mater. Eng. Perform. 2020, 29, 6691–6700. [CrossRef]
- Xue, Y.; Pang, X.; Karparvarfard, S.M.H.; Jahed, H.; Luo, S.; Shen, Y. Corrosion Protection of ZK60 Wrought Magnesium Alloys by Micro-Arc Oxidation. Metals 2022, 12, 449. [CrossRef]
- Merson, D.; Vasiliev, E.; Markushev, M.; Vinogradov, A. On the corrosion of ZK60 magnesium alloy after severe plastic deformation. Lett. Mater. 2017, 7, 421–427. [CrossRef]
- Merson, E.; Poluyanov, V.; Myagkikh, P.; Merson, D.; Vinogradov, A. Fractographic features of technically pure magnesium, AZ31 and ZK60 alloys subjected to stress corrosion cracking. Mater. Sci. Eng. A, 2020, 772, 138744. [CrossRef]
- Hassan, H.W.; Rahmati, M.; Barrantes, A.; Haugen, H.J.; Mirtaheri, P. In Vitro Monitoring of Magnesium-Based Implants Degradation by Surface Analysis and Optical Spectroscopy; Int. J. Mol. Sci. 2022, 23, 6099; [CrossRef]

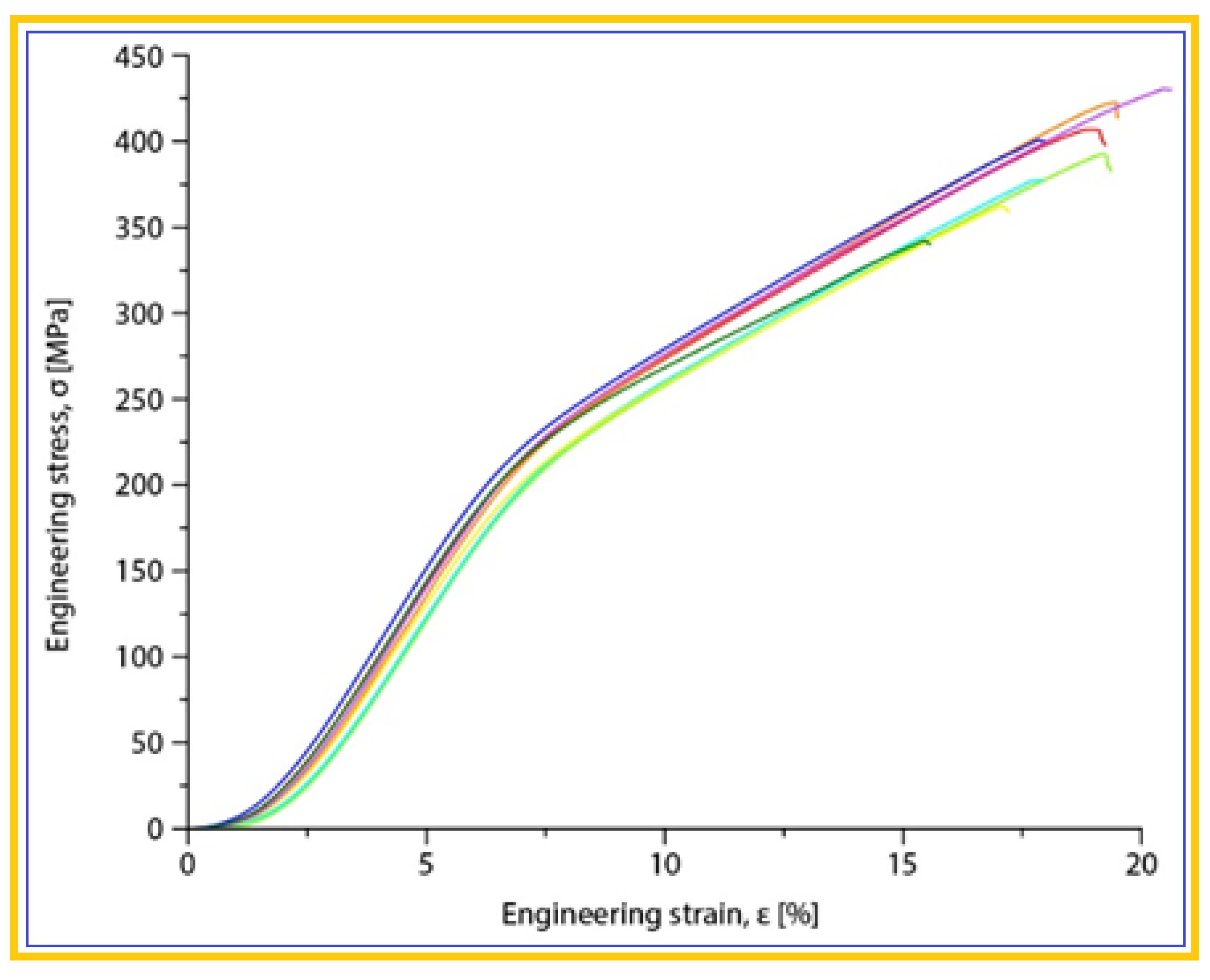
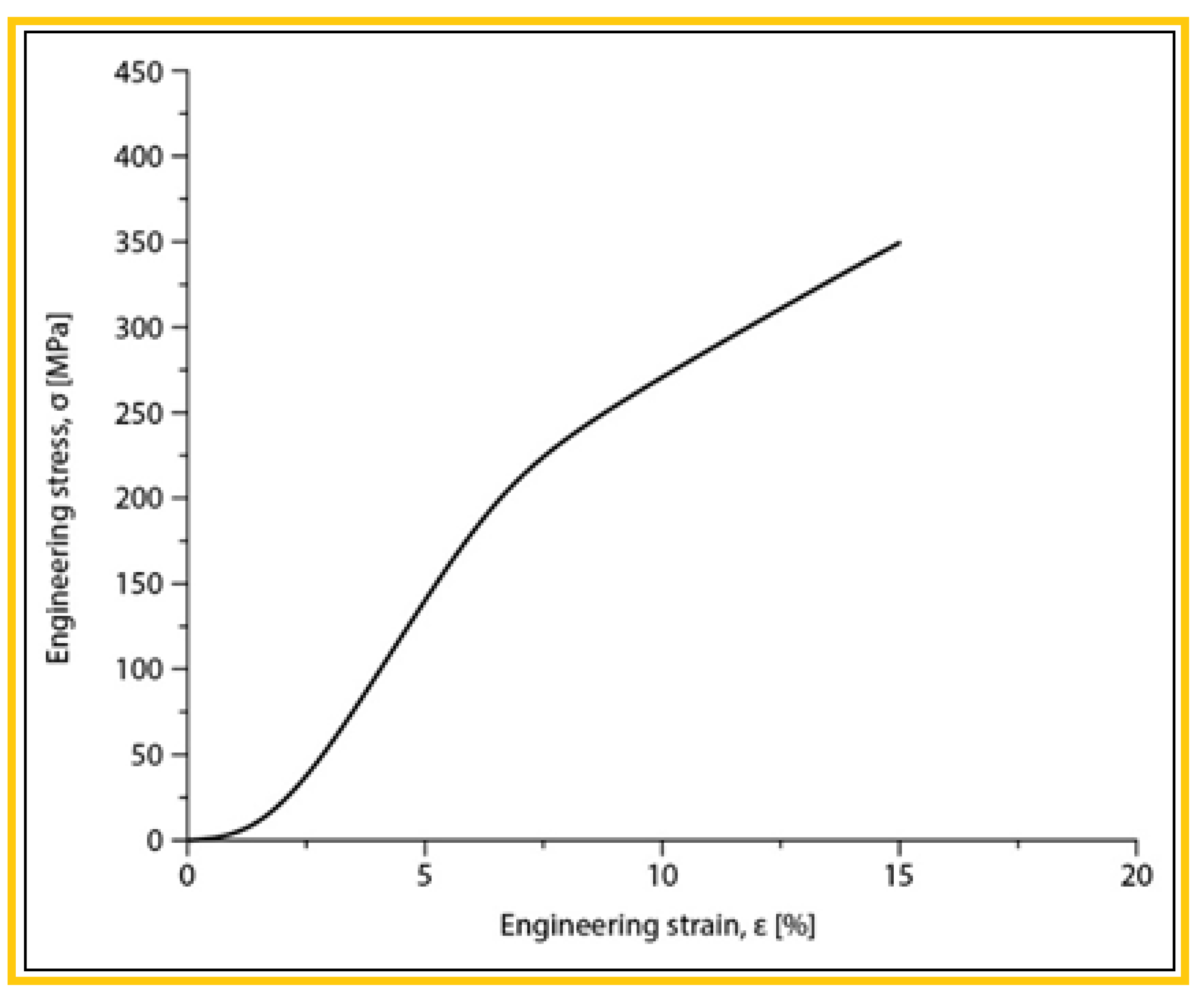
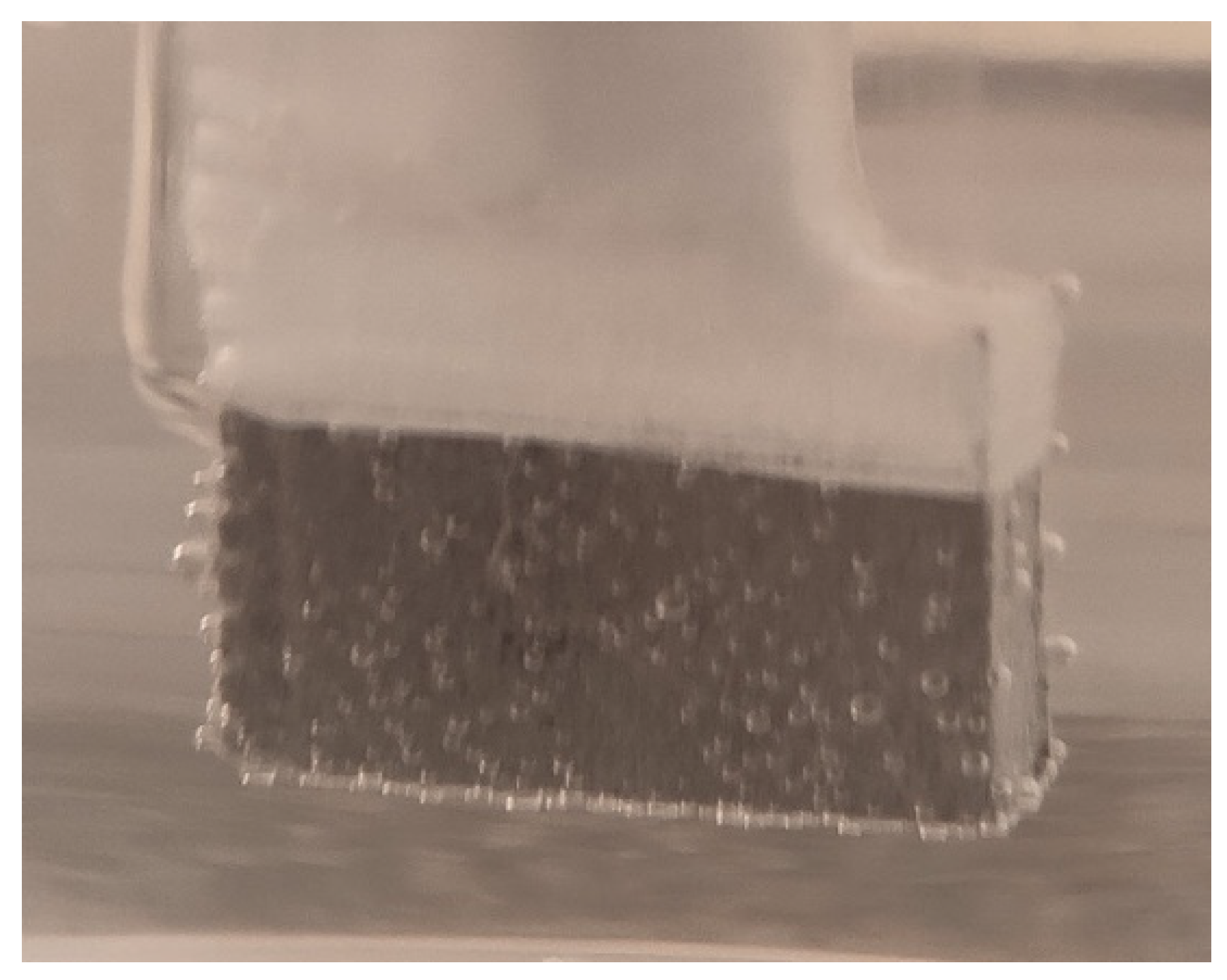
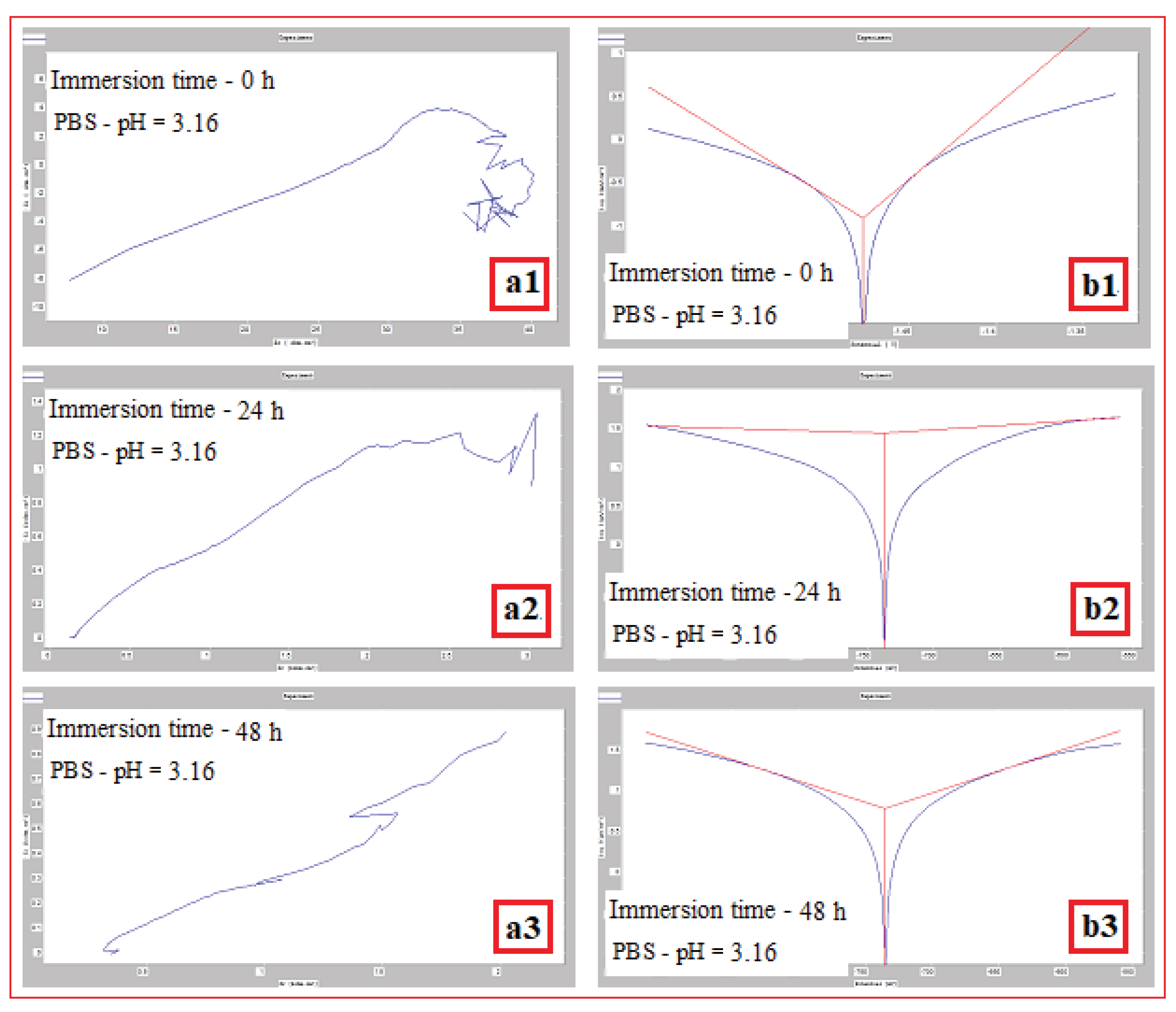
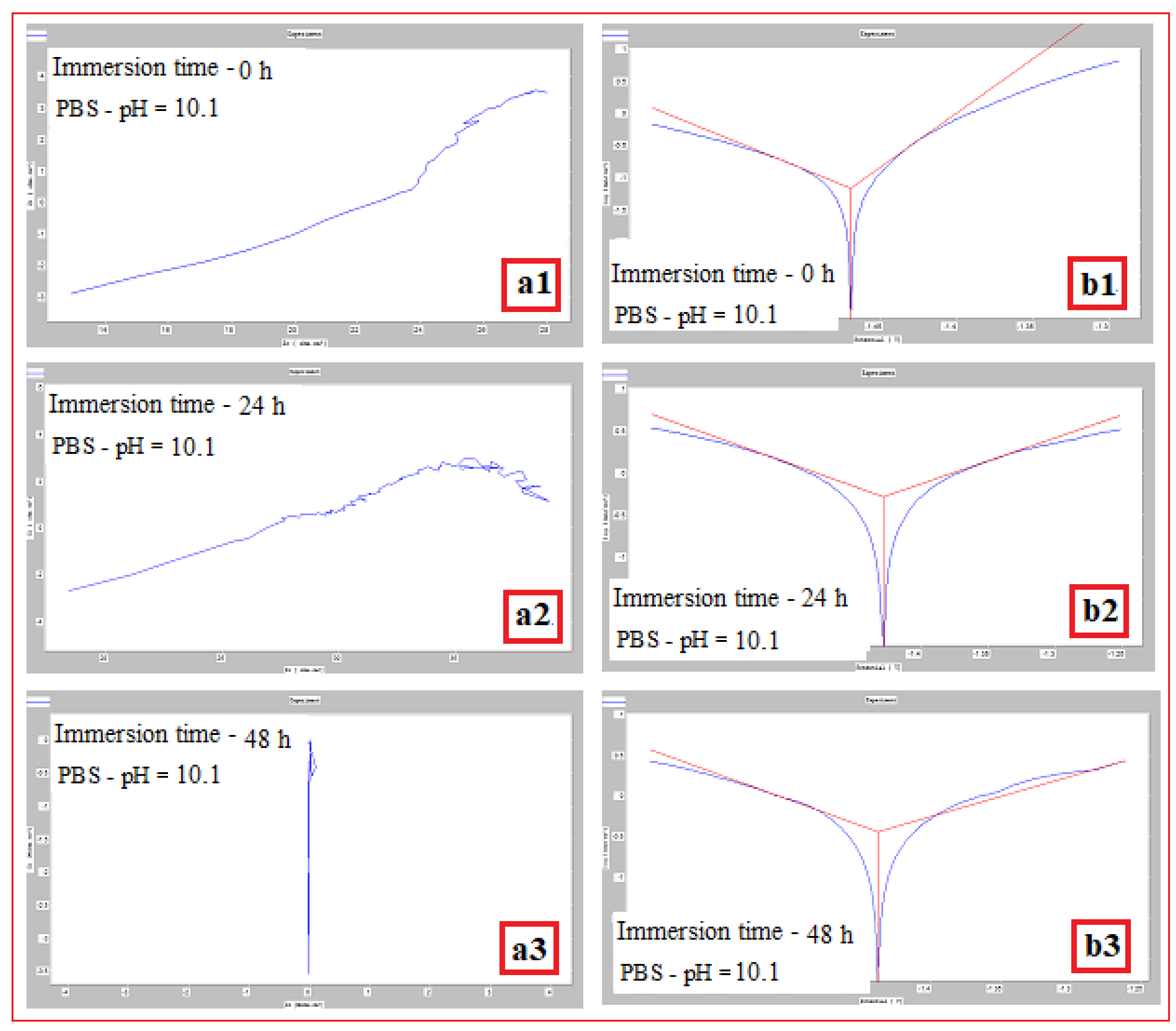
| The alloy in SLM condition | σmax [MPa] | εmax [%] | E [GPa] |
| Mg-10Zn-0.8Ca-0.5Zr (%wt.) | 381.25±17.19 | 17.92±0.44 | 42.10±1.08 |
|
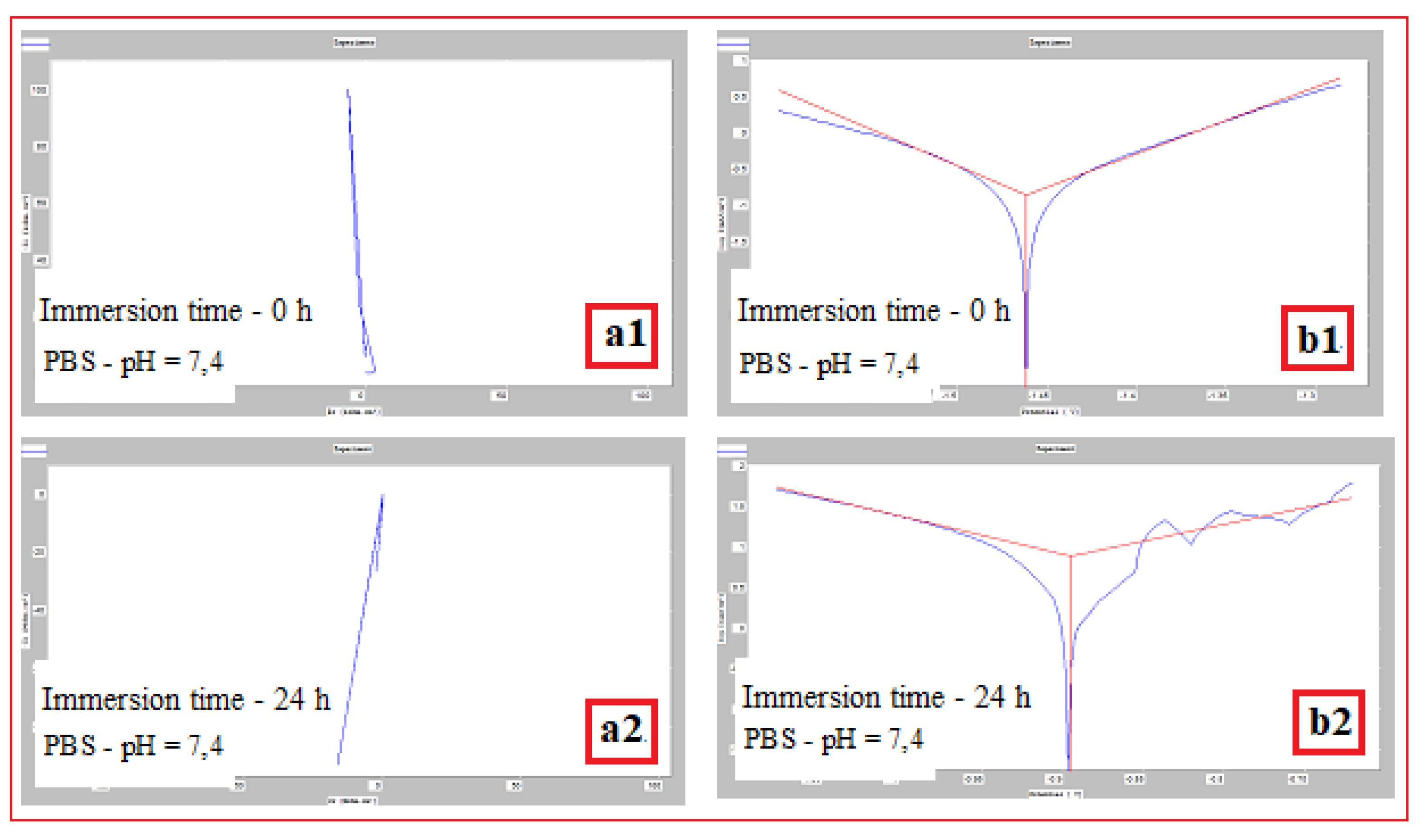
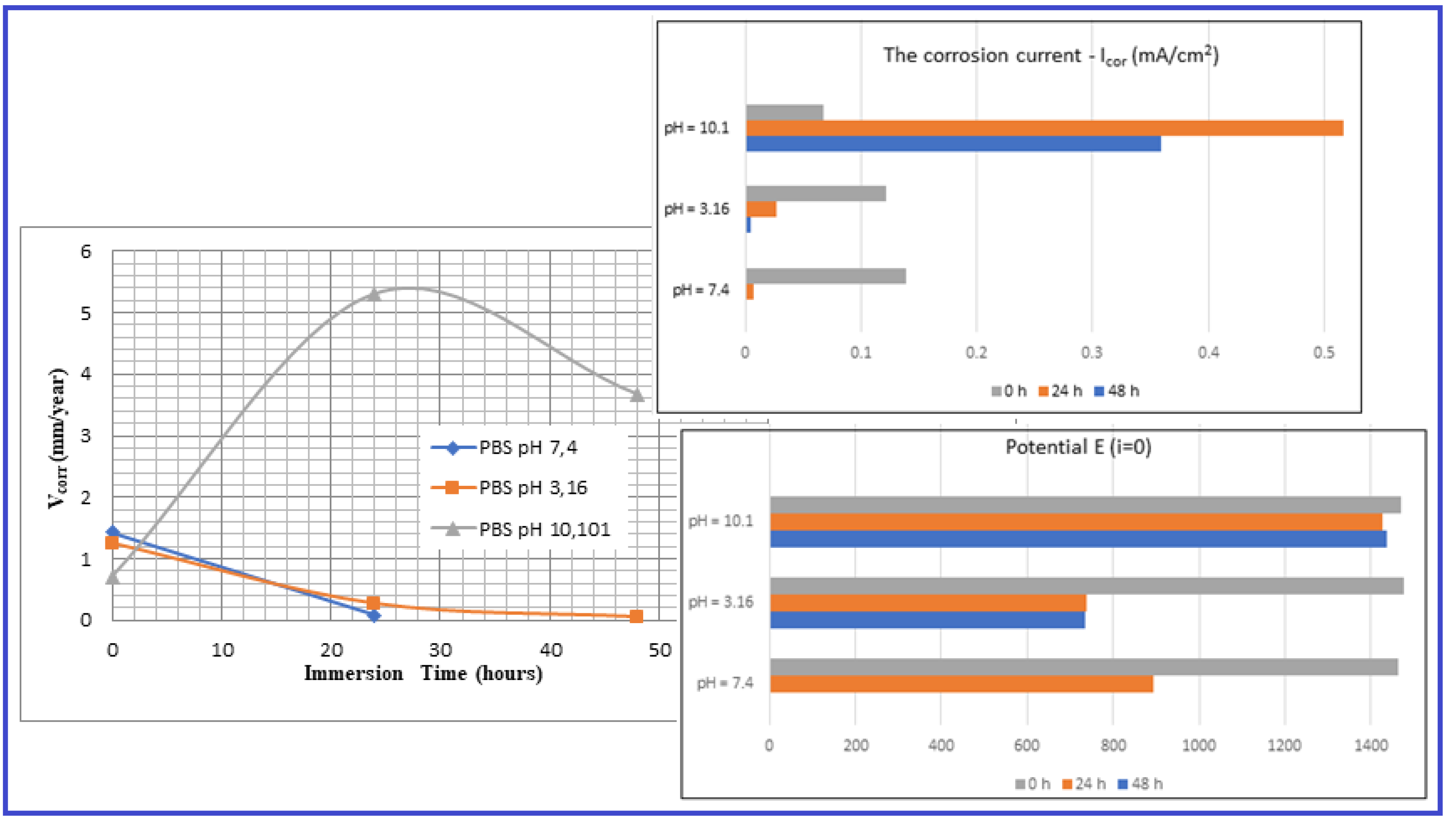
Mg-alloy, SLM processed, Immersed in PBS with pH: |
Immersion time (h) | Vcor (mm/year) | Icor (mA/cm2) |
Potential E(i=o) (mV) |
| pH = 7,4 | 0 | 1,426±0.04 | 0,139±0.026 | -1462,1±2.1 |
| 24 | 0,079±0.01 | 0,007±0.001 | -895±1.3 | |
| 48 | - | - | - | |
| pH = 3,16 | 0 | 1,252±0.04 | 0,122±0.016 | -1476,4±2.1 |
| 24 | 0,278±0.02 | 0,027±0.004 | -737,9±1.1 | |
| 48 | 0,060±0.01 | 0,005±0.001 | -735±1.1 | |
| pH = 10,1 | 0 | 0,703±0.06 | 0,068±0.002 | -1469,6±2.8 |
| 24 | 5,305±0.12 | 0,517±0.022 | -1428±2.2 | |
| 48 | 3,679±0.10 | 0,359±0.013 | -1437,6±2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





