1. Introduction
Kamchatka berry (
Lonicera caerulea L.) is one of the most ecologically important species. It is found mainly in the northern hemisphere, in countries such as Russia, Canada, Japan and China [
1].
The fruits of the Kamchatka berry (
Lonicera caerulea var. kamtschatica) are characterised by a blue-violet colouring of the skin, and have an ellipsoid cylindrical or slightly oval shape. They contain sugars such as glucose and fructose and minerals such as potassium, iron and calcium. They also contain organic acids such as citric acid, malic acid and phytic acid. Kamchatka berries owe their fame to their high content of biologically active compounds, including phenolic compounds or vitamins, which translates into their antibacterial, detoxifying and anti-inflammatory properties. In the food industry, they are mainly used in the production of jams, juices, tinctures, wines and as natural colourings [
2]. In contrast, no data were found in the literature on the use of Kamchatka berry pomace in cosmetic technology.
Bioferments are preparations produced from natural raw materials by a fermentation process using appropriate strains of bacteria and/or yeast [
3,
4]. They are mainly obtained by fermentation using lactic fermentation bacteria of the genus
Levilactobacillus, with the strains used showing potential probiotic activity [
5]. Fermentation increases the bioactivity of raw materials by breaking down high-molecular compounds into low-molecular compounds, so that fermented raw materials have a better effect on skin and hair than non-fermented raw materials [
6]. The use of fermentation also refers to the enrichment of organoleptic properties such as colour or aroma [
7,
8]. The range of applications of bioferments includes skin whitening, antioxidant, anti-aging, moisturising and anti-allergic properties [
9,
10].
The use of biomass waste from the agri-food industry has attracted the attention of researchers in many fields. Pomace, once used exclusively for the production of alcoholic beverages, vinegar or animal feed, now represents a potential for reuse as a valuable raw material [
11]. In addition to their biological and chemical properties, plant biomass wastes are also a rich source of wild microorganisms with interesting fermentation capabilities and faster adaptation to less favourable environmental conditions [
12].
A bioferment using
Saccharomyces cerevisiae,
Lacticaseibacillus paracasei strains was prepared from pomace of Kamchatka berry varieties Aurora and Indigo Gem. The formulation was used to formulate a cosmetic paste on a commercial COSMOS certified shampoo base [
13,
14].
2. Materials and Methods
2.1. Plant Material
The plant material consists of blue honeysuckle pomace, which is considered waste. Extrudates of the Kamchatka berry of the Aurora variety were obtained from cultivation in Łagiewniki, Poland.
The Aurora and Indigo Gem varieties were harvested in July 2022. The pressing of the fruit took place in the company "Dar Ogrodu" Błaszki, Poland.
2.2. Microorganisms
Microorganisms isolated in August 2022 and identified as Saccharomyces cerevisiae yeast and Lacticaseibacillus paracasei lactic acid fermentation bacteria were used for the study.
2.3. Physico-Chemical Tests
To check the basic physico-chemical parameters, pH (METTLER TOLEDO Five Essay Plus FP20), water activity (AQUALAB) and sugar content (TESSATM) were measured.
2.4. Organoleptic Tests
In order to observe the changes in the plant material during fermentation, an organoleptic evaluation was carried out, describing colour, aroma and texture.
2.5. Microbiological Tests
2.5.1. Agar Culture - Bacteria
Lactic acid fermentation bacterial cultures were performed on MRS Agar GranuCult® medium (Merck, Darmstadt, Germany). This medium is used for enrichment, cultivation and isolation of all Lactobacillus species from all types of material. Agar 12 g/L, dipotassium hydrogen phosphate 2 g/L, D(+)-glucose 20 g/L, magnesium sulphate (equivalent to 0.2 g/L heptahydrate) 0.1 g/L, manganous sulphate monohydrate 0.05 g/L, meat extract 5 g/L, sodium acetate 5 g/L, triammonium citrate 2 g/L, universal peptone 10 g/L, yeast extract 5 g/L.
Preparation: Suspend 61.15 g in 1000 ml distilled water containing 1 ml polysorbate 80 (TWEEN® 80; Sigma P8074). Boil to complete dissolution and mix thoroughly. Sterilise by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Adjust pH with glacial acetic acid after sterilisation if necessary.
2.5.2. Agar Yeast Cultures
Yeast cultures were performed on Sabouraud 4% dextrose agar GranuCult® (Merck, Darmstadt, Germany). This medium is used for the cultivation of fungi. Composition of the medium: casein peptone 5.0 g/L, meat peptone 5.0 g/L, D(+)glucose 40.0 g/L, agar 15.0 g/L.
Preparation: Suspend 65 g in 1000 ml. Boil to complete dissolution and mix thoroughly. Sterilise by autoclaving at 15 lbs pressure (121°C) for 15 minutes.
2.5.3. Inoculum Preparation
The inoculum for lactic acid fermentation bacteria was cultivated in MRS broth medium (Merck, Darmstadt, Germany). This medium is used for the cultivation of lactic acid bacteria of the species Lactobacillus. Medium composition: Dipotassium hydrogen phosphate 2 g/L, D(+)-glucose 20 g/L, magnesium sulfate (equivalent to 0.2 g/L heptahydrate) 0.1 g/L, manganous sulfate monohydrate 0.05 g/L, meat extract 5 g/L, sodium acetate 5 g/L, triammonium citrate 2 g/L, universal peptone 10 g/L, yeast extract 5 g/L.
Preparation: Suspend 50 g in 1000 ml distilled water containing 1 ml polysorbate 80 (TWEEN® 80; Sigma P8074). Boil to complete dissolution and mix thoroughly. Sterilise by autoclaving at 15 lbs pressure (121°C) for 15 minutes.
The yeast inoculum was cultivated on YPG medium (BTL, Lodz, Poland). The medium is used for yeast cultivation. Composition: yeast extract 10 g/L, peptone 20 g/L, glucose 20 g/L.
Preparation: Suspend 50 g in 1000 ml distilled water. Boil to dissolve the medium completely and mix thoroughly. Sterilise by autoclaving at 15 lbs pressure (121°C) for 15 minutes.
Single colonies were picked from the slanting surfaces of the MRS or Sabouraud agar medium using a sterile swab into liquid MRS or YPG medium respectively, the volume of the medium being 10 ml. The inoculum thus prepared was incubated at 30°C ± 1°C for 24 hours. After this time, the number of cfu/ml was checked using a Thom chamber. The target inoculum should be 106 cfu/ml.
2.6. Production of Bioferment
The method of Bioferment production is described in the patent application no. P.444531 at the Polish Patent Office. For this purpose, 75 g ± 0.1 g of fresh grape marc (Aurora and Indigo Gem varieties were mixed in a ratio of 1:1) was weighed, to which 75 g ± 0.1 g of water was added at near room temperature in the range of 22oC ± 1oC, the sample was mixed by shaking several times. Then 0,75 ml of inoculum in MRS medium of the bacterial strain Lacticaseibacillus paracasei with a cell count of 106 cfu/ml and 0,75 ml of inoculum in YPG medium of the yeast strain Saccharomyces cerevisiae were added. The sample was mixed by inversion several times. The inoculated sample was placed in an incubator under controlled conditions at a temperature of 22oC ± 1oC for a period of 5 days. The resulting pre-bioferment was then decanted into sterile 50 ml Falcon-type tubes and placed in an EppendorfTM Centrifuge 5804 R bench-top centrifuge, rotation 4200 rpm, time 10 minutes. The supernatant and the solid fraction were collected. The solid fraction was separated from the supernatant by pouring it into sterile falcons. The isolated solid fraction was then determined for its polyphenolic content, including anthocyanins, vitamins, water activity and subjected to physico-chemical and organoleptic tests.
2.7. Freeze-Drying
The solid fraction of the bioferment and the pomace (Indigo Gem and Aurora 1:1) were freeze-dried according to the method described in the patent application no. P.443438 (Polish Patent Office). 440 g of the sample, after testing, was freeze-dried in a Labconco™ Triad freeze dryer, model: 794001030, nXDS Edwards dry spiral vacuum pump. The pomace tray was placed in the freeze dryer in manual mode, followed by a temperature setting of -15°C. After approximately 2.5 h, the sample was placed in the freeze dryer and the pressure in the freeze dryer was set to 0.15 Pa and the temperature to -10°C. The sample was freeze-dried for a period of 24 h, after which the storage temperature was set at 30°C and the raw material was heated under these conditions for approximately 24 h to prevent it from absorbing moisture from the environment.
The freeze-dried material obtained was subjected to grinding in a mortar to obtain the raw material in powder form, which was analysed for phenolic compounds, including anthocyanins, sugars and vitamins, and subjected to physico-chemical and organoleptic tests.
2.8. Chromatographic Analysis of B Vitamins (UHPLC-ESI-MS/MS)
Ultra-high performance reversed-phase liquid chromatography with tandem mass spectrometry (UHPLC-ESI-MS/MS) was used to determine the content of B vitamins (B1, B2, B3 and B6) in the samples [
15,
16].
2.8.1. Sample Preparation for B-Vitamin Analysis
Samples were weighed (0.1 ± 0.01 g) into 100 ml glass bottles and 25 ml of distilled water was added. The bottles were closed with screw caps and placed in a shaking water bath at 60°C. The extraction process was carried out at 60°C for 20 minutes. After this time, the samples were immediately cooled to 20°C and centrifuged at this temperature for 10 minutes at 4800 rpm. The supernatants were filtered through a 0.2 μm nylon syringe filter into glass autosampler vials. Samples were stored at -20°C until UHPLC-ESI-MS/MS analysis.
2.8.2. Conditions for UHPLC-ESI-MS/MS Analysis
The analysis of B vitamins in the samples was performed using a Q Exactive™ Hybrid Quadrupole-Orbitrap™ mass spectrometry system (Thermo Scientific™) coupled to a Thermo Scientific™ Transcend™ TLX-1 high-resolution UHPLC liquid chromatograph. An Acclaim™ Polar Advantage II HPLC column (2.1 × 150 mm, 3 µm) was used for the separation of vitamin C compounds. The following separation parameters were used for the compounds analysed: column temperature - 40°C, mobile phase flow - 0.25 ml/min, gradient elution, mobile phase A - 0.015% aqueous formic acid solution, mobile phase B - mixture of methanol and acetonitrile (v/v, 20:80), sample injection volume - 10 μl. The following gradient elution programme was used: initial conditions 100% A and 0% B; 0-4 min, 100% A and 0% B; 4-10 min, 55% A, 45% B; 10-11 min, 0% A, 100% B; 11-12 min, 0% A, 100% B; 12-15 min, 100% A, 0% B; 15-18 min, 100% A, 0% B. ESI-MS/MS analysis conditions: capillary voltage - 4000 V in positive ion scanning mode, capillary temperature - 250°C, drying gas temperature - 350°C, drying and collision gas - nitrogen, drying and collision gas flow rates - 10 and 8 L/min, collision energy - 25eV, mass spectrum sweep range - 50-50 m/z.
Full MS and MS2 fragmentation spectra were monitored in the m/z range 50 to 750. MS2 fragmentation spectra were obtained in Parallel Reaction Monitoring (PRM) mode using High Resolution Collisional Dissociation (HCD). Qexactive Tune 2.1, Aria 1.3.6 and Thermo Xcalibur software were used to control, record and analyse the results obtained.
Identification of B vitamins was based on comparison of retention times, UV-VIS spectra, full mass spectra (ESI-MS) and fragmentation spectra (MS/MS) of analytes with available standards. An external standard method was used to determine the concentration of vitamins B1, B2, B3 and B6.
2.9. Chromatographic Analysis of Vitamin C Content (UHPLC-ESI-MS/MS)
Ultra-high performance reversed-phase liquid chromatography with tandem mass spectrometry (UHPLC-ESI-MS/MS) was used to determine the vitamin C content of the samples [
15,
17].
2.9.1. Sample Preparation for Vitamin C Analysis
Samples were weighed (0.1 ± 0.01 g) into 100 ml glass bottles and 25 ml of distilled water was added. The bottles were closed with screw caps and placed in a shaking water bath at 60°C. The extraction process was carried out at 60°C for 20 minutes. After this time, the samples were immediately cooled to 20°C and centrifuged at this temperature for 10 minutes at 4800 rpm. The supernatants were filtered through a 0.2 μm nylon syringe filter into glass autosampler vials. Samples were stored at -20°C until UHPLC-ESI-MS/MS analysis.
2.9.2. UHPLC-ESI-MS/MS Analytical Conditions
Analysis of the vitamin C content of the samples was performed using a Q Exactive™ Hybrid Quadrupole-Orbitrap™ mass spectrometry system (Thermo Scientific™) coupled to a Thermo Scientific™ Transcend™ TLX-1 high-resolution UHPLC liquid chromatograph. An Acclaim™ Polar Advantage II HPLC column (2.1 × 150 mm, 3 µm) was used for the separation of vitamin C. The following separation parameters were used for the compounds analysed: column temperature - 40°C, mobile phase flow - 0.25 ml/min, gradient elution, mobile phase A - 0.015% aqueous formic acid solution, mobile phase B - mixture of methanol and acetonitrile (v/v, 20:80), sample injection volume - 10 μl. The following gradient elution programme was used: initial conditions 100% A and 0% B; 0-4 min, 100% A and 0% B; 4-10 min, 55% A, 45% B; 10-11 min, 0% A, 100% B; 11-12 min, 0% A, 100% B; 12-15 min, 100% A, 0% B; 15-18 min, 100% A, 0% B. ESI-MS/MS analysis conditions: capillary voltage - 4000 V in negative ion scan mode, capillary temperature - 250°C, drying gas temperature - 320°C, drying and collision gas - nitrogen, drying and collision gas flow rates - 10 and 8 L/min, collision energy - 25eV, mass spectrum sweep range - 50 - 750 m/z.
Full MS and MS2 fragmentation spectra were monitored in the m/z range 50 to 750. MS2 fragmentation spectra were obtained in Parallel Reaction Monitoring (PRM) mode using High Resolution Collisional Dissociation (HCD). Qexactive Tune 2.1, Aria 1.3.6 and Thermo Xcalibur software were used to control, record and analyse the results obtained.
Identification of vitamin C was based on comparison of retention times, UV-VIS spectra, full mass spectra (ESI-MS) and fragmentation spectra (MS/MS) of the analytes with available standards. An external standard method was used to determine the concentration of vitamin C.
2.10. Chromatographic Analysis of Polyphenolic Content (UHPLC-DAD, UHPLC-ESI-MS/MS).
Ultra-high performance reversed-phase liquid chromatography with spectrophotometric detection (UHPLC-DAD) and tandem mass spectrometry (UHPLC-ESI-MS/MS) were used to identify and analyse the polyphenolic content of the samples [
18,
19].
2.10.1. Sample Preparation for UHPLC-DAD and UHPLC-ESI-MS/MS Analysis
Samples were weighed (0.1 ± 0.01 g) into 100 ml glass bottles and 25 ml of 70% methanol solution was added. The bottles were sealed with screw caps and placed in an ultrasonic bath. Extraction was carried out at 45°C for 30 minutes. The resulting extracts were centrifuged at 20°C for 10 min at 4800 rpm. The supernatants were filtered through a 0.2 μm nylon syringe filter into glass autosampler vials. Samples were stored at -20°C until analysis by UHPLC-DAD and UHPLC-ESI-MS/MS.
2.10.2. UHPLC-DAD Analytical Conditions
UHPLC-DAD analyses of the samples were performed on a Dionex UltiMate 3000 high-resolution UHPLC liquid chromatograph equipped with a diode array detector (DAD). An Accucore™ C18 column (2.6 μm, 150 × 2.1 mm) was used for reversed-phase separation of the compounds identified. Phase A was a 0.1% aqueous solution of formic acid and phase B was 0.1% formic acid in acetonitrile. The column was thermostatted at 30°C. Gradient elution was used with a mobile phase flow rate of 0.35 ml/min. The separation programme was as follows: 0-8 min, 1-5% B; 8-15 min, 5-8% B; 15-20 min, 8-10% B; 20-25 min, 10-15% B; 25-35 min, 15-20% B; 35-40 min, 20-25% B; 40-50 min, 25-90% B; 50-53 min, 90% B; 53-58 min, 90-1% B; and finally the initial conditions were maintained for 7 min until the column was rebalanced. The volume of sample injected was 10 μL. Spectrophotometric detection was performed at different wavelengths: flavan-3-ols, hydroxybenzoic acids - 280 nm, hydroxycinnamic acids - 320 nm, flavonols - 365 nm and anthocyanins - 520 nm. To check, record and analyse the results obtained
2.10.3. Conditions for the UHPLC-ESI-MS/MS Analysis
UHPLC-ESI-MS/MS analyses of the samples were performed using a Q Exactive™ Hybrid Quadrupole-Orbitrap™ mass spectrometry system (Thermo Scientific™) coupled to a Thermo Scientific™ Transcend™ TLX-1 high-resolution UHPLC liquid chromatograph. An Accucore™ C18 column (2.6 μm, 150 × 2.1 mm) was used to separate the compounds identified in a reversed phase system. Phase A was a 0.1% aqueous solution of formic acid and phase B was 0.1% formic acid in acetonitrile. The column was thermostatted at 30°C. Gradient elution was used with a mobile phase flow rate of 0.35 ml/min. The separation programme was identical to the UHPLC-DAD analysis. The volume of the sample injected was 10 μL. The following ESI-MS/MS analytical conditions were used: capillary voltage - 4500 V in negative ion scanning mode and 4500 V in positive ion scanning mode, capillary temperature - 325°C, gas drying temperature - 400°C, drying and collision gas - nitrogen, drying gas flow - 10 L/min, Parallel Reaction Monitoring (PRM) mode, collision energy - 25eV, resolution - 35,000. Full MS and MS2 fragmentation spectra were monitored in the m/z range 50 to 750. MS2 fragmentation spectra were obtained in Parallel Reaction Monitoring (PRM) mode using High Resolution Collisional Dissociation (HCD). Qexactive Tune 2.1, Aria 1.3.6 and Thermo Xcalibur software were used to control, record and analyse the results obtained.
Polyphenolic compounds present in the samples were identified based on retention times, UV-VIS spectra, full mass spectra (ESI-MS) and fragmentation spectra (MS/MS) compared with available standards and literature data.
Quantitative analysis was performed using calibration curves of standard solutions of polyphenolic compounds. Based on the obtained chromatograms of standard solutions, the concentration dependence was plotted as a function of the peak area of the standard compound.
2.11. Preparation of Cosmetic Mass
After analysis of the freeze-dried solid fraction of the bioferment, it was used as an ingredient in the cosmetic formulation of a natural shampoo. For this purpose, 950 g of unperfumed COSMOS-certified, commercially available shampoo base was measured, a dipole was placed, and a magnetic stirrer was switched on. Then, 50 g of the freeze-dried solid fraction of the bioferment was added to the base (the content of the additional ingredient in the compound recommended by the manufacturer is up to 5%) and stirred for 10 minutes. The cosmetic mass thus prepared was poured into sterile 15 ml vials and further tested.
Composition: Aqua, Aloe vera leaf juice, decyl glucoside, lauryl glucoside, xanthan gum, potassium sorbate, sodium benzoate, citric acid.
2.12. Stability Testing
One method of assessing stability is to expose samples to different temperature conditions to accelerate the changes that occur in a normal environment. Such a test makes it possible to predict the stability and the effect of temperature on the cosmetic mass. The relationship is described by the Arrhenius equation [
20], which shows that a 10°C increase in temperature doubles the reaction rate, resulting in faster transformations. To this end, stability tests were carried out on a natural shampoo manufactured with the addition of bioferment. The tests lasted 12 weeks, during which the cosmetic was exposed to increased temperature (40°C ± 1°C), decreased temperature (5°C ± 1°C), UV light (UV lamp) and room temperature (21°C ± 1°C).
Temperature variations during transport or storage can significantly affect the stability of a cosmetic product. In order to accurately determine the stability to temperature changes, a so-called shock test is performed in which the cosmetic product is exposed to elevated temperature conditions (40°C ± 1°C) for 24 hours, followed by reduced temperature conditions (5°C ± 1°C) for 24 hours and elevated temperature conditions (40°C ± 1°C) for the last 24 hours. A further shock test is carried out in a similar manner, but the cosmetic product is first exposed to a reduced temperature and then to an increased temperature.
2.13. Statistical Analysis
All data are presented as mean ± standard deviation (SD), with three replicates for each sample (n = 3).
3. Results
3.1. Bioferment
3.1.1. Physico-Chemical Analysis
The pH, total sugars and water activity were measured in triplicate for Kamchatka berry pomace of the Aurora and Indigo Gem varieties (mixed in a ratio of 1:1), the solid fraction of the bioferment, and for the freeze-dried pomace and the solid fraction of the bioferment. The average results of the measurements together with the standard deviation are given in the table below.
The data in
Table 1 show that the pH changed after the fermentation process, there was a decrease of 0.08 ± 0.01. The content of monosaccharides also decreased, which means that the microorganisms inoculated in the bioferment used sugars as a carbon source in the fermentation process, and their low content in the bioferment (0.12 g/100 g ± 0.02) shows that 5 days is the optimal fermentation time.
The activity of the water in the bioferment increased due to the dissolution of the pomace in water before the fermentation process, which translates into better conditions for lactic bacteria and yeast to carry out the fermentation process. High water activity also increases the likelihood of negative effects of external factors on the solid fraction of the bioferment, such as microbial contamination, negative effects of environmental factors such as heat or UV radiation, so this fraction was freeze-dried. The water activity in the freeze-dried fraction was reduced by more than 87% (0.770 ± 0.02), resulting in a much higher stability of the formulation.
3.1.2. Organoleptic Analysis
The organoleptic analysis was carried out in a special room designed for this type of analysis by an independent assessor experienced in this type of testing.
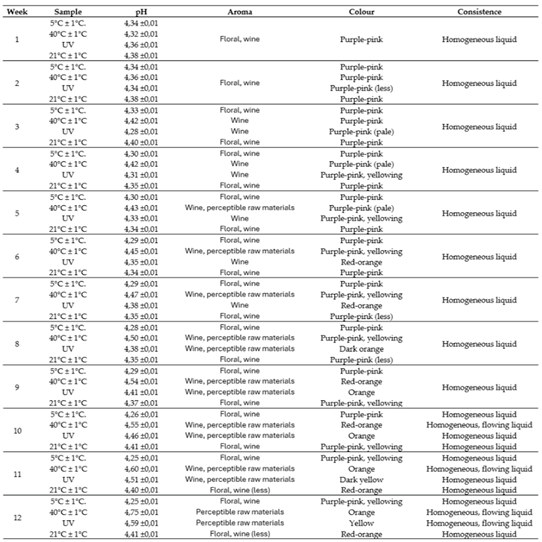
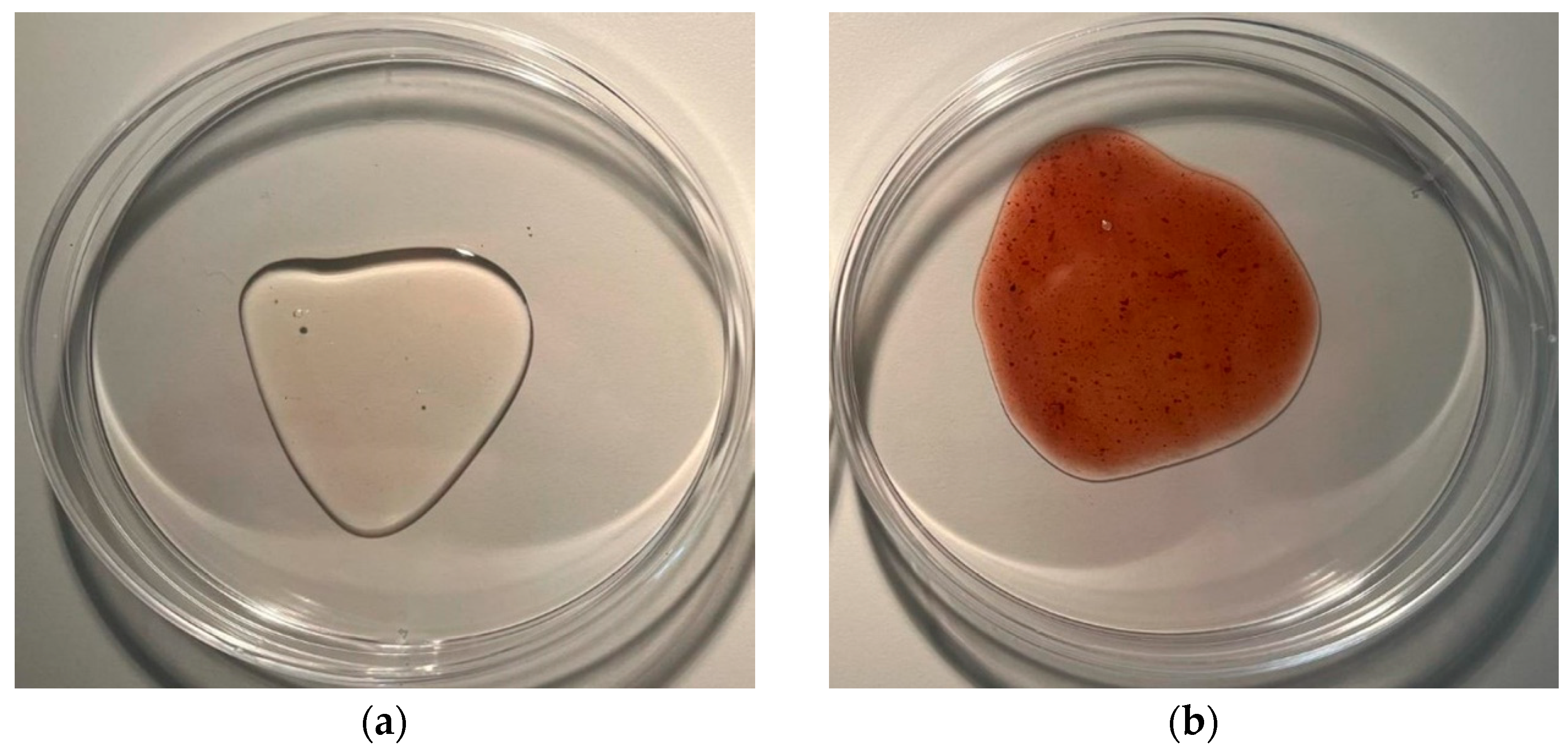
Aroma, colour and texture were assessed. The first differences between the pomace (
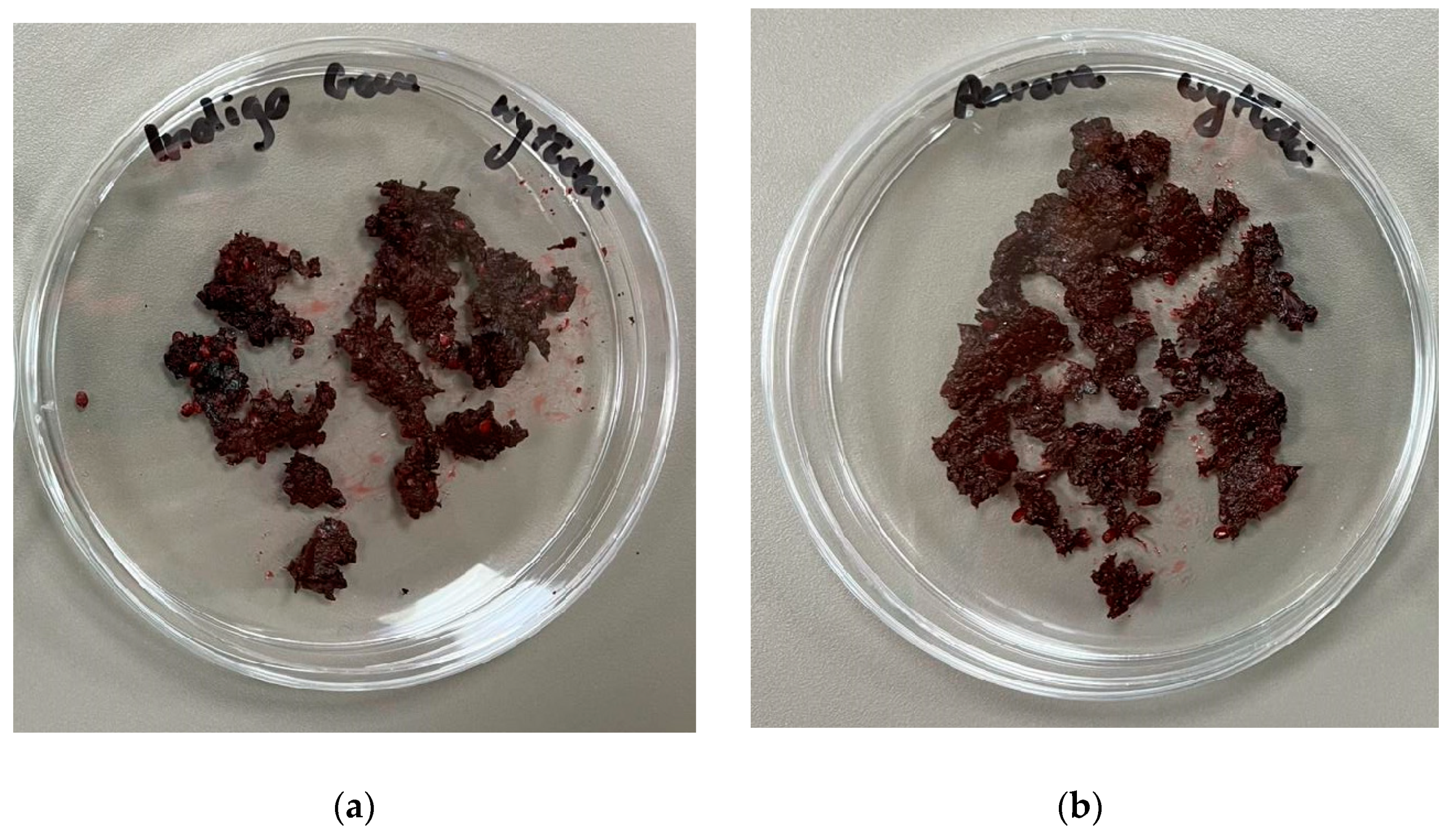
Figure 1.) and the solid fraction of the bioferment (
Figure 2) are already evident in the changes in aroma, as the laboratory technician noted that the lactic acid in the bioferment is palpable, which can be translated into the next parameter, colour, since the high content of anthocyanins gives colour to the raw material and the change in pH due to lactic fermentation directly affects the change in colour.
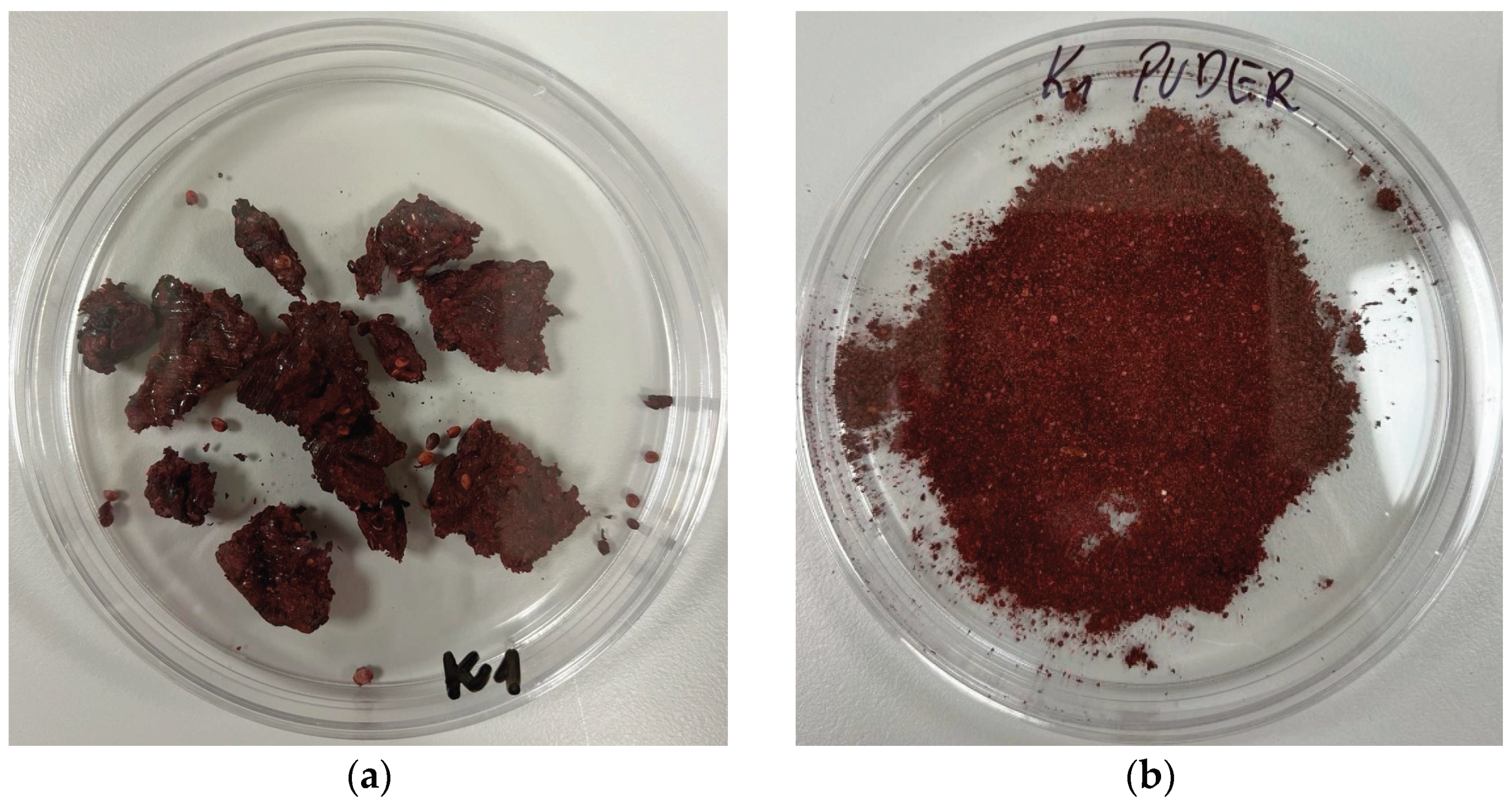
The results collected in the table also show that the freeze-drying process does not lead to a change in the aroma palette, neither in the pomace nor in the solid fraction of the bioferment (
Figure 3), which is very beneficial. The colour change in the freeze-dried product is also related to the pH, as it was lower than in the samples not subjected to freeze-drying. The process also affected the consistency, which is technologically more suitable for the mass production of cosmetics, as it is homogeneous. This is due to the fact that freeze-dried samples do not contain plant parts such as seeds, peels and stem fragments.
Table 2.
Results of organoleptic analysis of Kamchatka berry pomace and solid fraction of bioferment before and after freeze-drying.
Table 2.
Results of organoleptic analysis of Kamchatka berry pomace and solid fraction of bioferment before and after freeze-drying.
| Sample |
Aroma |
Colour |
Consistency |
| Pomace |
Floral, wine, sweet |
Purple |
Mashed spread |
| Bioferment |
Floral, sweet, lactic acid |
Purple-pink |
Mashed spread |
| Freeze-dried pomace |
Floral, wine, sweet |
Pink-purple |
Powder |
| Freeze-dried bioferment |
Floral, sweet, lactic acid |
Pink-purple |
Powder |
3.1.3. Analysis of Bioactive Compounds
Table 3,
Table 4,
Table 5,
Table 6,
Table 7 and
Table 8 show that Kamchatka berry pomace and the solid fraction of the bioferment are a source of many bioactive compounds, including phenolic compounds, including anthocyanins, and B vitamins and vitamin C.
The fermentation process influences the profile of bioactive compounds in Kamchatka berry pomace.
Table 3 summarises the results of the content analysis of caffeic acid (CA) and its derivatives: 3-O-caffeoylquinic acid (3-CQA), 5-O-caffeoylquinic acid (5-CQA), p-coumaric acid (pCuA), 3,4-Di-O-caffeoylquinic acid (3,4-DiCQA) caffeic acid derivative (CA derivative). After fermentation, (4.47 ± 0.07 mg/100g) caffeic acid (CA) was determined, which was not found in the pomace, and a 484% increase in caffeic acid derivative (CA derivative) was observed. A decrease of 5-O-caffeoylquinic acid (5-CQA) by more than 91%, 3-O-caffeoylquinic acid (3-CQA) by 31%, p-coumaric acid (p-CuA) by 94%, 3,4-Di-O-caffeoylquinic acid (3,4-DiCQA) by 91% was also observed in the solid fraction of the bioferment compared to the pomace.
The fermentation process also decreased vanillic acid (VA), syringic acid (SA) and ellagic acid (EA) by about 17%, 58% and 63% respectively, while protocatechuic acid (PCA) increased by about 50%. Gallic acid (GA) and 4-hydroxybenzoic acid (4-HBA) were not affected by fermentation.
3.2. Cosmetic Mass
3.2.1. Physico-Chemical and Organoleptic Analysis
The pH measurement and organoleptic analysis showed that the addition of the bioferment to the cosmetic mass lowered its pH by 1.15 ± 0.1, which means that the bioferment acidifies the environment of the cosmetic formulation, so in addition to a number of bioactive properties, it can also act as a pH regulator.
Organoleptic evaluation showed that the bioferment had a positive effect on the sensory profile of the cosmetic compound, enriching its aroma (floral, wine) and giving it a more attractive colour, without altering its consistency.
Table 9.
Analysis of a cosmetic product with a freeze-dried bioferment solid fraction compared to a shampoo base.
Table 9.
Analysis of a cosmetic product with a freeze-dried bioferment solid fraction compared to a shampoo base.
| Sample |
pH |
Aroma |
Colour |
Consistency |
| Shampoo base |
5,53 ±0,01 |
Herbal |
Yellow |
Homogeneous liquid |
| Cosmetic product |
4,38 ±0,01 |
Floral, wine |
Purple/pink |
Homogeneous liquid |
3.2.2. Stability Tests
Stability tests were carried out on the cosmetic preparation with the addition of the freeze-dried bioferment solid fraction and the cosmetic base from which the formulation was made, in order to check whether the bioferment was responsible for any changes in mass or whether the formulation of the cosmetic base was also affected.
After 12 weeks of analysis, it was found that the cosmetic formulation with the freeze-dried solid fraction of the bioferment showed the least changes for samples placed under reduced temperature conditions (5°C ± 1°C), the pH value decreased by 0.13 ± 0.01, while the other parameters showed no change.
Samples incubated at elevated temperature (40°C ± 1°C) and exposed to UV light showed the greatest changes, with pH increasing by 0.37 ± 0.01 (elevated temperature) and 0.21 ± 0.01 (UV). In both cases, the aroma changed adversely, cosmetic ingredients were detectable and the colour changed from violet-pink to yellow (UV) and orange (elevated temperature).
At room temperature (21°C ± 1°C), the pH did not change significantly, increasing by only 0.03 ± 0.01. The aroma changed from a floral, wine, aroma to a less floral, wine aroma. The most significant change was in colour from violet-pink to red-orange (
Figure 4 and
Figure 5).
Stability tests carried out on the cosmetic base without the freeze-dried bioferment solid fraction showed that the cosmetic mass itself also changes at a given time and under given conditions. The first changes in the mass were observed much earlier than in the mass with the bioferment, as early as week 3, when the aroma changed to odourless under elevated temperature (40°C ± 1°C) and UV radiation, and the colour changed from yellow to straw in the test at UV radiation. The aroma of raw materials appeared in the base after 4 weeks in a test at elevated temperature (40°C ± 1°C).
After 12 weeks, the pH of the sample decreased by 0.12 ± 0.01 at reduced temperature (5°C ± 1°C), increased by 0.24 ± 0.01 at elevated temperature (40°C ± 1°C), decreased by 0.26 ± 0.01 at UV radiation and decreased by 0.07 ± 0.01 at room temperature (21°C ± 1°C).
Table 10.
Stability test results for a cosmetic product containing the freeze-dried solid fraction of the bioferment.
Table 10.
Stability test results for a cosmetic product containing the freeze-dried solid fraction of the bioferment.
Table 11.
Stability test results for a commercial shampoo base.
Table 11.
Stability test results for a commercial shampoo base.
Shock tests have shown that both the cosmetic base and the shampoos produced are stable under varying temperature conditions, which translates into stability during storage and transport.
Table 12.
Results of the shock test for the base without bio-ferments and the shampoos with bio-ferments.
Table 12.
Results of the shock test for the base without bio-ferments and the shampoos with bio-ferments.
| Sample |
Conditions |
pH |
Colour |
Aroma |
Consistency |
| Shampoo base |
L/H/L1
|
5,46 ± 0,01 |
Yellow |
Herbal |
Homogeneous liquid |
| H/L/H2
|
5,38 ± 0,01 |
Yellow |
Herbal |
Homogeneous liquid |
| Cosmetic product |
L/H/L |
4,38 ± 0,01 |
Purple/pink |
Floral, wine |
Homogeneous liquid |
| H/L/H |
4,39 ± 0,01 |
Purple/pink |
Floral, wine |
Homogeneous liquid |
4. Discussion
Comparing the data obtained, the highest content of the anthocyanin group was shown by cyanidin-3-O-glucoside in Kamchatka berry pomace. It has been confirmed in the literature that Kamchatka berry is the main source of this phenolic compound. Depending on the variety, it ranges from 68 to 649 mg/100 g fresh fruit weight. From the data obtained, it was 1653.80 ± 0.15 mg/100g in the pomace before freeze-drying. This shows that the pomace, which is the waste product in this case, is more valuable than the fruit itself. [
21].
During the fermentation process, the content of polyphenols, especially those in complex form, can change. It has been reported in the literature that after the fermentation process, the composition showed a higher amount of lower molecular weight flavonoid derivatives (di- and triglycosides) as well as flavonoids and hydroxycinnamone aglycones than in fresh Pak Choi and Chinese mustard leaves [
22].
After fermentation, the content of flavonoid derivatives and hydroxycinnamic acid derivatives decreases, but the total polyphenol content increases due to the release of bound sugar groups and the formation of free hydroxyl groups. Simpler hydroxycinnamic acid derivatives (e.g. caffeoylquinic acid) were more stable under fermentation conditions, whereas the more complex derivatives were more easily degraded [
22].
Referring to the above scientific reports, it can be concluded that the formation of caffeic acid after fermentation may have been due to the breakdown of the caffeic acid derivative 5-O-cavoylquinic acid, while the increase in 3-O-cavoylquinic acid may indicate that it is more stable and does not degrade during the fermentation process.
In addition, the presence of 4.47 ± 0.07 mg/100g of caffeic acid, which had not been demonstrated before fermentation, was shown after fermentation of pomace of the Kamchatka berry cultivars Indigo Gem and Aurora. In cosmetic products, coffee acid and its derivatives are potent active ingredients in the treatment of skin diseases, as they exhibit a range of anti-inflammatory and antioxidant properties. In addition, they have been shown to have anti-wrinkle effects in vivo [
23].
The effect of the fermentation process on gallic acid content has not been demonstrated. This compound has excellent properties in cosmetic terms, such as strong antioxidant, anti-inflammatory, antimicrobial and anticancer effects. Gallic acid and its derivatives are widely used in many therapeutic preparations, e.g. as a substitute for hydrocortisone in children with atopic dermatitis. This compound has USFDA GRAS status showing low systemic toxicity and associated mortality at high concentrations in many experimental models [
24].
Polyphenols are a large group of chemicals found in plants. Their main sources are fruits, vegetables, berries and red wine. The most common polyphenolic substances are phenolic acids, flavonoids, stilbenes or lignans. Many studies have confirmed that various polyphenolic substances have anti-glycemic functions in vivo and in vitro. This is related to the structure of flavonoids, which contains three benzene rings and an -OH group, giving them antioxidant properties, which may be related to the anti-glycation properties of flavonoids [
25].
Antioxidants in the form of plant extracts not only have the function of protecting the cosmetic preparation from oxidative processes, but also have an excellent effect on skin physiology. Studies by many authors show that plant extracts, e.g. oils and ethanolic extracts of lavender, have a free radical scavenging capacity (DPPH) of 90-93%, while synthetic BHA (hydroxybenzoic acid) has a free radical scavenging capacity (DPPH) of 95-98% [
26,
27,
28,
29].
However, it is important to note that antioxidant activity varies from one natural resource to another. Furthermore, this factor is influenced by many other parameters such as climate and soil conditions or harvest time, which makes standardisation of natural products very difficult. Due to their ability to inhibit oxidation processes and the growth of microorganisms, including many pathogenic ones such as Salmonella spp. and Escherichia coli, natural antioxidants are increasingly used as natural preservatives, e.g. in foods. In recent years, much evidence has been published that they increase the stability of edible oils, carotenoid colours and fruit juice flavourings, successfully replacing artificial preservatives and stabilisers. This is not only for environmental or economic reasons, but also because they have a positive impact on human health [
30].
Investigating the content of active ingredients in natural preparations can help to assess their potential dermatological activity. One group of compounds that play a key role in the action of preparations applied to the skin are phenolic acids. Phenolic acids are valuable compounds due to their antioxidant and anti-inflammatory properties [
31].
Vitamins are used as active ingredients in cosmetic products. According to the Overview of Ingredients Used in Cosmetic Products, B vitamins are a group of water-soluble vitamins that are used in cosmetics for their beneficial properties in dermatological care (i.e. skin and hair conditioners, antiseborrhoea, antistatic and emollient properties). As a result, these groups of compounds are included in cosmetic formulations as high value-added cosmetic ingredients. In addition to its dermatological properties, riboflavin (vitamin B2) can also be used as a cosmetic colouring agent and its maximum allowable concentration is limited by the European Cosmetics Regulation (EU Regulation 1223/2009) [
32].
The vitamin C content of the biofermentable solid fraction of Kamchatka berry pomace increased dramatically (141%) compared to the pomace before the fermentation process. As one of the most potent antioxidants, vitamin C has been shown to protect the skin against photo-aging, UV-induced immunosuppression and photocarcinogenesis. It also has anti-ageing effects by increasing collagen synthesis, stabilising collagen fibres and reducing collagen degradation. It reduces the formation of melanin, thus reducing pigmentation, i.e. it acts as a bleaching agent and evens out skin tone. Vitamin C is the main complement to vitamin E and acts synergistically with vitamin E to protect against oxidative damage [
33,
34].
The addition of the freeze-dried solid fraction of the Kamchatka berry pomace bioferment to cosmetic mass lowers the pH by 0.15 ± 0.01. The pH of cosmetic products has an effect on the condition of the skin. It has been shown that a lower pH of cosmetics reduces water loss through the skin, supports the skin barrier and reduces peeling of the epidermis [
32]. The chosen cosmetic base has a simple composition and does not contain aggressive cleansing agents such as SLS or SLES, as skin cleansing products containing mainly anionic surfactants can cause increased dryness and skin irritation [
33].
The use of pomace may not only bring environmental benefits through its reduction but has also found its potential application in the cosmetics industry as a raw material for the production of valuable preparations. Further research and development in this field may open new perspectives for the use of Kamchatka berry marc as a valuable biologically active raw material.
5. Conclusions
A bioferment was prepared from the pulp of the Kamchatka berry cv. Aurora and Indigo Gem and strains of Saccharomyces cerevisiae and Lacticaseibacillus paracasei, which was used to formulate a cosmetic preparation at a concentration of 5%, in accordance with the guidelines of the COSMOS certification body.
Searching the SciFinder database, no reports of this type of microbiological valorisation of Kamchatka berry marc were found in the literature, so the solution was registered as an invention at the Patent Office of the Republic of Poland.
The conducted researches showed that Kamchatka berry pomace is a valuable source of biologically active compounds. Experiments conducted confirmed the presence of substances with potential health benefits, such as phenolic compounds, vitamins, antioxidants.
In a cosmetic product they not only play the role of a biologically active raw material, but also help to enrich the fragrance palette, the colour of the cosmetic product and even regulate its pH by acidifying the formulation environment or consistency due to the low water activity of the freeze-dried product.
The results of stability tests showed that in the process of physico-chemical analysis of a cosmetic preparation with freeze-dried solid fraction of bioferment from Kamchatka berry pomace, the pH should oscillate in the range of 4.38-4.76 ± 0.01. The tests showed that the cosmetic should be protected from high temperature and UV radiation and proved that the product is stable under different conditions, which translates into lower transport and storage costs.
6. Patents
Methodology of producing bioferment was described in patent application no. P.444531 to the Patent Office of the Republic of Poland.
Methodology for the production of a bioactive preparation was described in patent application no. P.443438 to the Patent Office of the Republic of Poland.
Author Contributions
Conceptualization, W.M., K.Ś. and I.M.; methodology, W.M., J.O.; formal analysis, W.M.; data curation, W.M., J.O.; writing—original draft preparation, W.M, K.S, S.M..; writing—review and editing, W.M., K.Ś.; All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Raudonė, L.; Liaudanskas, M.; Vilkickytė, G.; Kviklys, D.; Žvikas, V.; Viškelis, J.; Viškelis, P. Phenolic Profiles, Antioxidant Activity and Phenotypic Characterization of Lonicera caerulea L. Berries, Cultivated in Lithuania. Antioxidants 2021, 10, 115. [Google Scholar] [CrossRef]
- Belcar, J.; Kapusta, I.; Sekutowski, T.R.; Gorzelany, J. Impact of the Addition of Fruits of Kamchatka Berries (L. caerulea var. kamtschatica) and Haskap (L. caerulea var. emphyllocalyx) on the Physicochemical Properties, Polyphenolic Content, Antioxidant Activity and Sensory Evaluation Craft Wheat Beers. Molecules 2023, 28, 4011. [Google Scholar] [CrossRef]
- Choi, N.M.; Choi, J.I. Fermented Cosmetics. Korean Patent KR1430591, 18 August 2014. [Google Scholar]
- Yoseph, C.; Avanti, C.; Hadiwidjaja, M.; Briliani, K. The importance of fermented plant extract for anti aging in cosmetics product. World J. Pharm. Res. 2021, 10, 1330–1343. [Google Scholar]
- Joon, S.S.; Song, H.X.; So, H.P.; Keon, S.L.; Young, M.P.; Soo, N.P. Antioxidative and antiaging activities and component analysis of Lespedeza cuneata G. don extracts fermented with Lactobacillus pentosus. J. Microbiol. Biotechnol. 2017, 27, 1961–1970. [Google Scholar]
- Sivamaruthi, B.S.; Chaiyasut, C.; Kesika, P. Cosmeceutical importance of fermented plant extracts: A short review. Int. J. Appl. Pharm. 2018, 10, 31–34. [Google Scholar] [CrossRef]
- Wending Chen, Caiyun Xie, Qianqian He, Jianxia Sun, Weibin Bai,Improvement in color expression and antioxidant activity of strawberry juice fermented with lactic acid bacteria: A phenolic-based research. Food Chemistry: X 2023, 17, 100535.
- Huan Zhang, Jiajia Wang, Dandan Zhang, Li Zeng, Yanan Liu, Wen Zhu, Gaixiang Lei, Youyi Huang,Aged fragrance formed during the post-fermentation process of dark tea at an industrial scale. Food Chemistry 2021, 342, 128175. [CrossRef] [PubMed]
- Otsuka, A.; Moriguchi, C.; Shigematsu, Y.; Tanabe, K.; Haraguchi, N.; Iwashita, S.; Tokudome, Y.; Kitagaki, H. Fermented Cosmetics and Metabolites of Skin Microbiota—A New Approach to Skin Health. Fermentation 2022, 8, 703. [Google Scholar] [CrossRef]
- Majchrzak, W.; Motyl, I.; Śmigielski, K. Biological and Cosmetical Importance of Fermented Raw Materials: An Overview. Molecules 2022, 27, 4845. [Google Scholar] [CrossRef] [PubMed]
- European Parliament Circular economy: definition, importance and benefits. Available online: https://www.europarl.europa.eu/topics/en/article/20151201STO05603/circular-economy-definition-importance-and-benefits (accessed on 20 February 2024).
- Hungría, J.; Siles, J. A.; Chica, A. F.; Gil, A.; Martín, M. A. Anaerobic co-digestion of winery waste: comparative assessment of grape marc waste and lees derived from organic crops. Environmental Technology 2021, 42, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- ECOSPA Baza szamponowa EKOLOGICZNA. Available online https://ecospa.pl/baza-szamponowa-ekologiczna (accessed on 20 February 2024).
- COSMOS Natural and Organic Certification for Cosmetics. Available online: https://www.cosmos-standard.org/ (accessed on 20 February 2024).
- Thermo Fisher Scientific. Determination of water- and fat-soluble vitamins by HPLC. TECHNICAL NOTE 72488, 2017. https://assets.thermofisher.com/TFS-Assets/CMD/Technical-Notes/tn-72488-hplc-water-fat-soluble-vitamins-tn72488-en.pdf.
- Kahoun, D.; Fojtíková, P.; Vácha, F.; Čížková, M.; Vodička, R.; Nováková, E.; Hypša, V. Development and validation of an LC-MS/MS method for determination of B vitamins and some its derivatives in whole blood. PLoS One 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Szultka, M.; Buszewska-Forajta, M.; Kaliszan, R.; Buszewski, B. ; Determination of ascorbic acid and its degradation products by high-performance liquid chromatography-triple quadrupole mass spectrometry. Electrophoresis. 2014, 35(4), 585–92. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Żyżelewicz, D.; Pacholczyk-Sienicka, B. ; UHPLC-DAD-ESI-HRMS/MS profile of phenolic compounds in northern red oak (Quercus rubra L. , syn. Q. borealis F. Michx) seeds and its transformation during thermal processing, Industrial Crops and Products, 2022, 189, 115860. [Google Scholar]
- Khattab, R.; Brooks, M.S.L.; Ghanem, A. Phenolic Analyses of Haskap Berries (Lonicera caerulea L.): Spectrophotometry versus High Performance Liquid Chromatography. International Journal of Food Properties 2016, 19, 1708–1725. [Google Scholar] [CrossRef]
- Thais Guaratini, Mirela D. Gianeti, Patrícia M.B.G.M. Campos, Stability of cosmetic formulations containing esters of Vitamins E and A: Chemical and physical aspects, International Journal of Pharmaceutics, 2006, 327, 12–16.
- Bylinowska, J. Jagoda kamczacka- superowoc, który czeka na odkrycie. Available online: https://dietetycy.org.pl/jagoda-kamczacka/ (accessed on 21 February 2024).
- Šalić, A.; Šamec, D. Changes in the content of glucosinolates, polyphenols and carotenoids during lactic-acid fermentation of cruciferous vegetables: A mini review. Food Chemistry 2022, 16, 100457. [Google Scholar] [CrossRef] [PubMed]
- Bastianini, M.; Faffa, C.; Sisani, M.; Petracci, A. Caffeic Acid-layered Double Hydroxide Hybrid: A New Raw Material for Cosmetic Applications. Cosmetics 2018, 5, 51. [Google Scholar] [CrossRef]
- Khan, B.A.; Mahmood, T.; Menaa, F.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; Ray, S.D. New Perspectives on the Efficacy of Gallic Acid in Cosmetics & Nanocosmeceuticals. Curr Pharm Des 2018, 24, 5181–5187. [Google Scholar]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J Food Drug Anal 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Allemann, B.; Baumann, L. ; Antioxidants used in skin care formulations. Skin Therapy Lett., 2008, 13, 5. [Google Scholar]
- Glina, R.; Góra, J. Związki naturalne w kosmetyce. Wydawnictwo Warsaw Voice 2000. [Google Scholar]
- Martini, M.C. Kosmetologia i farmakologia skóry. PZWL, Warszawa 2007.
- Seun-Ah, Y.; Sang-Kyung, J.; Eun-Jung, L.; Chang-Hyun, S.; In-Seon, L. ; Comparitive study of the chemical composition and antioxidant activity of six essential oils and their components. Natural Product Research, 2010, 24, 140–151. [Google Scholar]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials (Basel) 2021, 14, 4135. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Bujak, T. et al. Effect of fermentation time on the content of bioactive compounds with cosmetic and dermatological properties in Kombucha Yerba Mate extracts. Sci Rep 2021, 11, 18792. [Google Scholar] [CrossRef]
- Vállez-Gomis, V.; Peris-Pastor, G.; Benedé, J.l.; Chisvert, A.; Salvador, A. Green determination of eight water-soluble B vitamins in cosmetic products by liquid chromatography with ultraviolet detection. Journal of Pharmaceutical and Biomedical Analysis 2021, 205, 114308. [Google Scholar] [CrossRef]
- Al-Niaimi, F.; Chiang, N.Y.Z. Topical Vitamin C and the Skin: Mechanisms of Action and Clinical Applications. J Clin Aesthet Dermatol. 2017, 10, 14–17. [Google Scholar]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants (Basel) 2022, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Lichterfeld-Kottner, A.; El Genedy, M.; Lahmann, N.; Blume-Peytavi, U.; Büscher, A.; Kottner, J. Maintaining Skin Integrity in the Aged: A Systematic Review. Int. J. Nurs. Stud. 2020, 103, 103509. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.; Dasgupta, B.R.; Ananthapadmanabhan, K.P. Role of pH in Skin Cleansing. Int. J. Cosmet. Sci. 2021, 43, 474–483. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).