Submitted:
13 March 2024
Posted:
14 March 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Heart Failure
Insulin Resistance/Hyperinsulinemia
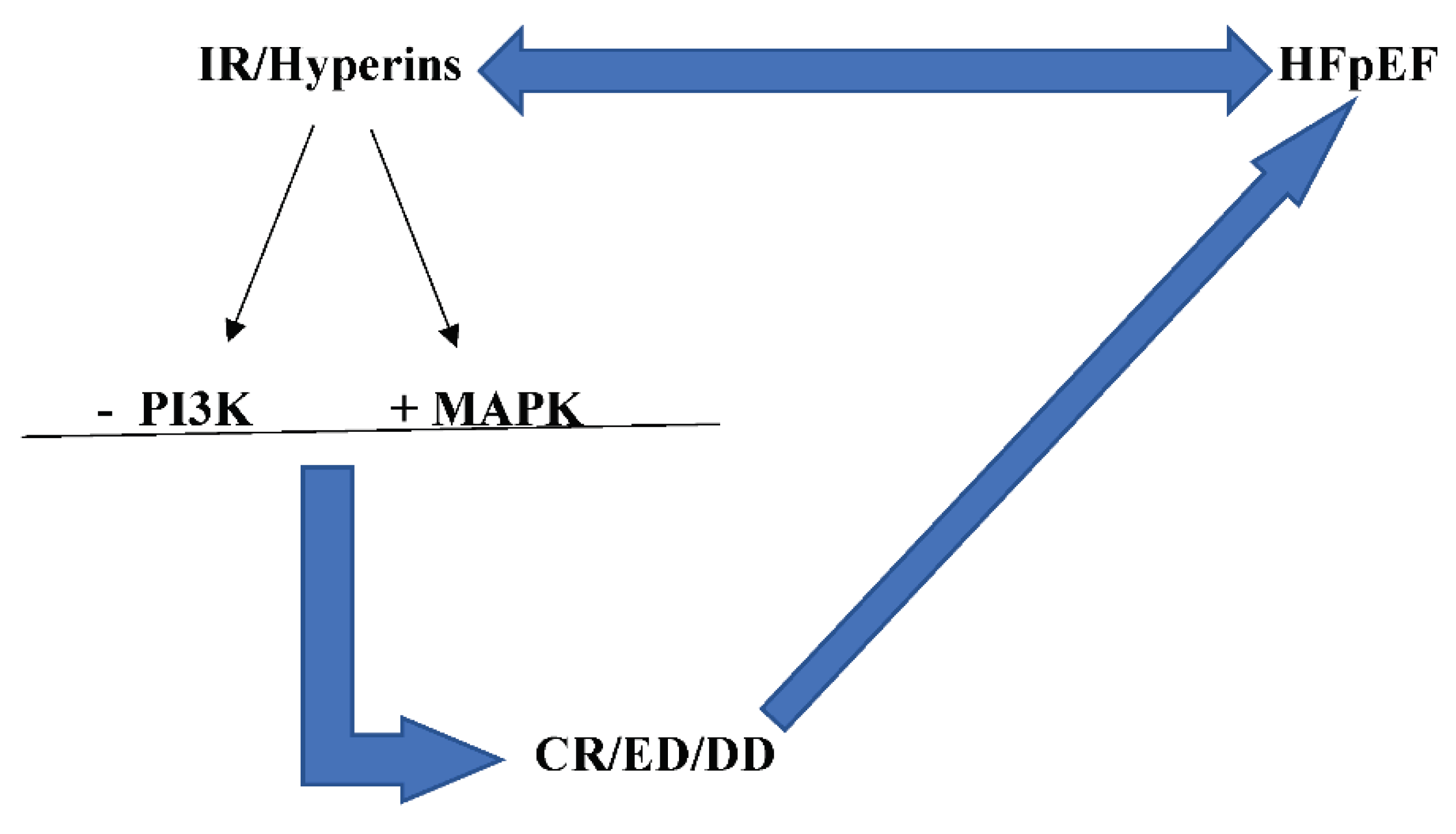
IR/Hyperins and HFpEF
Potential Treatments of IR/Hyperins
Conclusions
References
- Istituto Superiore di Sanità. Le Statistiche Delle Malattie Cardiovascolari in Europa per il 2008; Istituto Superiore di Sanità-EpiCentro: Roma, Italy, 2008. [GoogleScholar].
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1789–1858. Crossref PubMed WorldCat.
- Shahim B., Kapelios C.J., Savarese G., Lund L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card Fail Rev. 2023; 9: e11. [CrossRef]
- Bozkurt B., Coats A.J.S., Tsutsui H., Abdelhamid C.M., Adamopoulos S., Albert N., Anker S.D., Atherton J., Bohm M., Butler J., Drazner M.H., Michael Felker G., Filippatos G., Fiuzat M., Fonarow G.C., Gomez-Mesa J.E., Heidenreich P., Imamura T., Jankowska E.A., Januzzi J., Khazanie P., Kinugawa K., Lam C.S.P., Matsue Y., Metra M., Ohtani T., Francesco Piepoli M., Ponikowski P., Rosano G.M.C., Sakata Y., Seferovic P., Starling RC., Teerlin J.R., Vardeny O., Yamamoto K., Yancy C., Zhang J., Zieroth S. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352–380.Google Scholar Crossref PubMed WorldCat.
- McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., Burri H., Butler J., Čelutkienė J., Chioncel O., et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023;44:3627–3639. [CrossRef]
- Riehle C., Abel E.D. Insulin Signaling and Heart Failure. Circ. Res. 2016;118:1151–1169. [CrossRef]
- Freeman A.M., Pennings N. StatPearls. StatPearls; Treasure Island, FL, USA: 2023. Insulin Resistance. [PubMed] [GoogleScholar].
- Fahed M., Jaoudeh M.G.A., Merhi S., Mosleh J.M.B., Ghadieh R., Al Hayek S., El Hayek Fares J.E. Evaluation of risk factor for insulin resistance: a cross sectional study among employees at a private university in Lebanon. BMC Endocr Disord. 2020;20:85. [CrossRef]
- Writing Committee; Maddox T.M., Januzzi J.L. Jr, Allen L.A., Breathett K., Butler J., Davis L.L., Fonarow G.C., Ibrahim N.E., Lindenfeld J., Masoudi F.A., Motiwala S.R., Oliveros E., Patterson J.H., Walsh M.N., Wasserman A., Yancy C.W., Youmans Q.R. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021 Feb 16;77(6):772-810. [CrossRef]
- Pfeffer M.A., Shah A.M., Borlaug B.A.. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res. 2019 May 24;124(11):1598-1617. PMID: 31120821; PMCID: PMC6534165. [CrossRef]
- Ma C., Luo H., Fan L., Liu X., Gao C. Heart failure with preserved ejection fraction: an update on pathophysiology, diagnosis, treatment, and prognosis. Braz J Med Biol Res. 2020 Jun 5;53(7):e9646. Erratum in: Braz J Med Biol Res. 2021 Feb 26;54(4):e9646erratum. [CrossRef]
- Larson K.F., Malik A., Brozovich F.V. Aging and Heart Failure with Preserved Ejection Fraction. Compr Physiol. 2022 Aug 11;12(4):3813-3822. [CrossRef]
- Kristensen S.L., Jhund P.S., Lee M.M.Y., Køber L., Solomon S.D., Granger C.B., Yusuf S., Pfeffer M.A., Swedberg K., McMurray J.J.V.; CHARM Investigators and Committees. Prevalence of Prediabetes and Undiagnosed Diabetes in Patients with HFpEF and HFrEF and Associated Clinical Outcomes. Cardiovasc Drugs Ther. 2017 Dec;31(5-6):545-549. [CrossRef]
- Alzadjali M.A., Godfrey V., Khan F., Choy A., Doney A.S., Wong A.K., Petrie J.R., Struthers A.D., Lang C.C. Insulin Resistance Is Highly Prevalent and Is Associated with Reduced Exercise Tolerance in Nondiabetic Patients with Heart Failure. J. Am. Coll. Cardiol. 2009;53:747–753. [CrossRef]
- Chun S., Tu J.V., Wijeysundera H.C., Austin P.C., Wang X., Levy D., Lee D.S.. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail. 2012 Jul 1;5(4):414-21. [CrossRef]
- Packer M. Differential Pathophysiological Mechanisms in Heart Failure with a Reduced or Preserved Ejection Fraction in Diabetes. JACC Heart Fail. 2021;9:535–549. [CrossRef]
- Hasegawa H., Komuro I. CHARM study-new strategy for the treatment of heart failure. Nihon Rinsho. Jpn. J. Clin. Med. 2004;62:995–1002. [PubMed] [GoogleScholar].
- Lebovitz H.E. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. 2001;109 Suppl 2:S135-48. [CrossRef]
- Janssen J.A.M.J.L. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int J Mol Sci. 2021 Jul 21;22(15):7797. [CrossRef]
- Jia G., DeMarco V.G., Sowers J.R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016 Mar;12(3):144-53. Epub 2015 Dec 18. PMID: 26678809; PMCID: PMC4753054. [CrossRef]
- Reaven G.M. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J Clin Endocrinol Metab. 2003 Jun;88(6):2399-403. PMID: 12788834. [CrossRef]
- Ginsberg H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000;106:453–458. [CrossRef]
- Abel E.D. Insulin signaling in the heart. Am. J. Physiol. Endocrinol. Metab. 2021;321:E130–E145. [CrossRef]
- Ziaee A., Esmailzadehha N., Oveisi S., Ghorbani A., Ghanei L. The threshold value of homeostasis model assessment for insulin resistance in Qazvin Metabolic Diseases Study (QMDS): Assessment of metabolic syndrome. J. Res. Health Sci. 2015;15:94–100. [PubMed] [GoogleScholar].
- Guerrero-Romero F., Simental-Mendía L.E., González-Ortiz M., Martínez-Abundis E., Ramos-Zavala M.G., Hernández-González S.O., Jacques-Camarena O., Rodríguez-Morán M. The Product of Triglycerides and Glucose, a Simple Measure of Insulin Sensitivity. Comparison with the Euglycemic-Hyperinsulinemic Clamp. J. Clin. Endocrinol. Metab. 2010;95:3347–3351. [CrossRef]
- Cai W., Sakaguchi M., Kleinridders A., Pino G.G.-D., Dreyfuss J.M., O’neill B.T., Ramirez A.K., Pan H., Winnay J.N., Boucher J., et al. Domain-dependent effects of insulin and IGF-1 receptors on signalling and gene expression. Nat. Commun. 2017;8:14892. [CrossRef]
- Chopra I., Li H.F., Wang H., Webster K.A. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia. 2012;55:783–794. [CrossRef]
- Petersen M.C., Shulman G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018;98:2133–2223. [CrossRef]
- Mercurio V., Carlomagno G., Fazio V., Fazio S. Insulin resistance: Is it time for primary prevention? World J. Cardiol. 2012;4:1–7. [CrossRef]
- Bachmann K.N., Deger S.M., Alsouqi A., Huang S., Xu M., Ferguson J.F., Su Y.R., Niswender K.D., Ikizler T.A., Wang T.J. Acute effects of insulin on circulating natriuretic peptide levels in humans. PLoS ONE. 2018;13:e0196869. [CrossRef]
- Stout R.W. Insulin as a mitogenic factor: Role in the pathogenesis of cardiovascular disease. Am. J. Med. 1991;90:S62–S65. [CrossRef]
- Mohan M., Dihoum A., Mordi I.R., Choy A.-M., Rena G., Lang C.C. Left Ventricular Hypertrophy in Diabetic Cardiomyopathy: A Target for Intervention. Front. Cardiovasc. Med. 2021;8:746382. [CrossRef]
- Okwuosa T.M., Soliman E.Z., Lopez F., Williams K.A., Alonso A., Ferdinand K.C. Left ventricular hypertrophy and cardiovascular disease risk prediction and reclassification in blacks and whites: the Atherosclerosis Risk in Communities Study. Am Heart J. 2015 Jan;169(1):155-61.e5. Epub 2014 Oct 16. PMID: 25497261; PMCID: PMC4269255. [CrossRef]
- Shah R.V., Abbasi S.A., Heydari B., Rickers C., Jacobs D.R., Jr., Wang L., Kwong R.W., Bluemke D.A., Lima J.A.C., Jerosch-Herold M. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis) J. Am. Coll. Cardiol. 2013;61:1698–1706. [CrossRef]
- Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl. J. Med. 1997;336:525–533. [CrossRef]
- Massie B.M., Carson P.E., McMurray J.J., Komajda M., McKelvie R., Zile M.R., Andresen S., Donovan M., Iverson E., Staiger C., et al. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008;359:2456–2467. [CrossRef]
- . Lindman B.R., Davila-Roman V.G., Mann D.L., McNulty S., Semigran M.J., Lewis G.D., de la Fuentes L., Vader J., Hernanderz A.H., Redfield M.M., et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: A RELAX trial ancillary study. J. Am. Coll. Cardiol. 2014;64:541–549. [CrossRef]
- Szamosi A., Czinner A., Szamosi T., Sallai A., Hatunic M., Berla Z., Tomsits E., Almássy Z., Nolan J.J. Effect of diet and physical exercise treatment on insulin resistance syndrome of schoolchildren. J Am Coll Nutr. 2008 Feb;27(1):177-83. PMID: 18460496. [CrossRef]
- Ryan A.S. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000 Nov;30(5):327-46. [CrossRef]
- Butler J., Usman M.S., Khan M.S., Greene S.J., Friede T., Vaduganathan M., Filippatos G., Coats A.J.S., Anker S.D. Efficacy and safety of SGLT2 inhibitors in heart failure: Systematic review and meta-analysis. ESC Heart Fail. 2020;7:3298–3309. [CrossRef]
- Vaduganathan M., Docherty K.F., Claggett B.L., Jhund P.S., de Boer R.A., Hernandez A.F., Inzucchi S.E., Kosiborod M.N., Lam C.S.P., Martinez F., et al. SGLT2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757–767. [CrossRef]
- Hosokawa Y., Ogawa W. SGLT2 inhibitors for genetic and acquired insulin resistance: Considerations for clinical use. J. Diabetes Investig. 2020;11:1431–1433. [CrossRef]
- Salah H.M., Verma S., Santos-Gallego C.G., Bhatt A.S., Vaduganathan M., Khan M.S., Lopes R.D., Al’aref S.J., McGuire D.K., Fudim M. Sodium-Glucose Cotransporter 2 Inhibitors and Cardiac Remodeling. J. Cardiovasc. Transl. Res. 2022;15:944–956. [CrossRef]
- Dhingra N.K., Mistry N., Puar P., Verma R., Anker S., Mazer C.D., Verma S. SGLT2 inhibitors and cardiac remodelling: A systematic review and meta-analysis of randomized cardiac magnetic resonance imaging trials. ESC Heart Fail. 2021;8:4693–4700. [CrossRef]
- Herman R., Kravos N.A., Jensterle M., Janež A., Dolžan V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int J Mol Sci. 2022 Jan 23;23(3):1264. PMID: 35163187; PMCID: PMC8836112. [CrossRef]
- Halabi A., Sen J., Huynh Q., Marwick T.H. Metformin treatment in heart failure with preserved ejection fraction: A systematic review and meta-regression analysis. Cardiovasc. Diabetol. 2020;19:124. [CrossRef]
- Kamel A.M., Sabry N., Farid S. Effect of metformin on left ventricular mass and functional parameters in non-diabetic patients: A meta-analysis of randomized clinical trials. BMC Cardiovasc. Disord. 2022;22:405. [CrossRef]
- Shen Y., Zhang X., Ma W., Song H., Gong Z., Wang Q., Che L., Xu W., Jiang J., Xu J., et al. VE/VCO2 slope and its prognostic value in patients with chronic heart failure. Exp. Ther. Med. 2015;9:1407–1412. [CrossRef]
- Wong A.K., Symon R., AlZadjali M.A., Ang D.S., Ogston S., Choy A., Petrie J.R., Struthers A.D., Lang C.C. The effect of metformin on insulin resistance and exercise parameters in patients with heart failure. Eur. J. Heart Fail. 2012;14:1303–1310. [CrossRef]
- Cao C., Su M. Effects of berberine on glucose-lipid metabolism, inflammatory factors and insulin resistance in patients with metabolic syndrome. Exp Ther Med. 2019 Apr;17(4):3009-3014. Epub 2019 Feb 22. PMID: 30936971; PMCID: PMC6434235. [CrossRef]
- Imenshahidi M., Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): A clinical review. Phytother Res. 2019 Mar;33(3):504-523. Epub 2019 Jan 13. PMID: 30637820. [CrossRef]
- Zeng X.-H., Li Y.-Y. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2003;92:173–176. [CrossRef]
- Carlomagno G., Affuso F., Napoli R., Mercurio V., Fazio V., Micillo F., Pirozzi C., Ruvolo A., Saccá L., Fazio S. A nutraceutical combination improves insulin sensitivity in patients with metabolic syndrome. World J. Cardiol. 2012;4:77–83. [CrossRef]
- Mercurio V., Pucci G., Bosso G., Fazio V., Battista F., Iannuzzi A., Brambilla N., Vitalini C., D’Amato M., Giacovelli G., et al. A nutraceutical combination reduces left ventricular mass in subjects with metabolic syndrome and left ventricular hypertrophy: A multicenter, randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2020;39:1379–1384. [CrossRef]
- Fogacci F., Grassi D., Rizzo M., Cicero A.F.G. Metabolic effect of berberine-silymarin association: A meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Phytother Res. 2019 Apr;33(4):862-870. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



