Submitted:
13 March 2024
Posted:
14 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Diets, and Cheese Manufacture and Sampling
2.2. Chemical Analyses of Milk and Cheeses
2.3. Data Treatment and Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Compounds
3.2. Fatty Acid Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado-Pertíñez, M.; Horcada, A. Better Animal Feeding for Improving the Quality of Ruminant Meat and Dairy. Foods 2021, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pertíñez, M.; Martín-García, I.; Mena, Y.; Zarazaga, L.Á.; Guzmán, J.L. Supplementing the Diet of Dairy Goats with Dried Orange Pulp throughout Lactation: II Effect on Milk Fatty Acids Profile, Phenolic Compounds, Fat-Soluble Vitamins and Antioxidant Capacity. Animals 2021, 11, 2421. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: http://www.fao.org/faostat/es/#home (accessed on 6 March 2024).
- Bampidis, V.; Robinson, P. Citrus by-products as ruminant feeds: A review. Anim. Feed. Sci. Technol. 2006, 128, 175–217. [Google Scholar] [CrossRef]
- Guzmán, J.; Perez-Ecija, A.; Zarazaga, L.; Martín-García, A.; Horcada, A.; Delgado-Pertíñez, M. Using dried orange pulp in the diet of dairy goats: Effects on milk yield and composition and blood parameters of dams and growth performance and carcass quality of kids. Animal 2020, 14, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, J.L.; Martín-García, I.; Pérez-Écija, A.; García-Brenes, M.D.; Zarazaga, L.Á.; Delgado-Pertíñez, M. Supplementing the Diet of Dairy Goats with Dried Orange Pulp throughout Lactation: I. Effect on Milk Performance, Nutrient Utilisation, Blood Parameters and Production Economics. Animals 2021, 11, 2601. [Google Scholar] [CrossRef]
- Guzmán, J.L.; Delgado-Pertíñez, M.; Beriain, M.J.; Pino, R.; Zarazaga, L.Á.; Horcada, A. The use of concentrates rich in orange by-products in goat feed and its effects on physico-chemical, textural, fatty acids, volatile compounds and sensory characteristics of the meat of suckling kids. Animals 2020, 10, 766. [Google Scholar] [CrossRef]
- McGrath, J.; Duval, S.M.; Tamassia, L.F.; Kindermann, M.; Stemmler, R.; Gouvêa, V.; Acedo, T.S.; Immig, I.; Williams, S.N.; Celi, P. Nutritional strategies in ruminants: A lifetime approach. Res. Veter. Sci. 2018, 116, 28–39. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Morales, F.A.; Castel-Genís, J.M.; Mena-Guerrero, Y. Current status, challenges and the way forward for dairy goat production in Europe. Asian-Australas. J. Anim. Sci. 2019, 32, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, J.L.; Delgado-Pertíñez, M.D.; Soldevilla, H.G.; Pérez-Cacho, P.R.; Polo, O.P.; Zarazaga, L.Á.; Ramírez, C.A. Effect of Citrus By-product on Physicochemical Parameters, Sensory Analysis and Volatile Composition of Different Kinds of Cheese from Raw Goat Milk. Foods 2020, 9, 1420. [Google Scholar] [CrossRef]
- Rincón, A.A.; Pino, V.; Fresno, M.; Jiménez, A.; Álvarez, S.; Ayala, J.; Afonso, A. Influence of vegetable coagulant and ripening time on the lipolytic and sensory profile of cheeses made with raw goat milk from Canary breeds. Food Sci. Technol. Int. 2017, 23, 254–264. [Google Scholar] [CrossRef]
- Llorente, B.E.; Obregón, W.D.; Avilés, F.X.; Caffini, N.O.; Vairo-Cavalli, S. Use of artichoke (Cynara scolymus) flower extract as a substitute for bovine rennet in the manufacture of Gouda-type cheese: Characterization of aspartic proteases. Food Chem. 2014, 159, 55–63. [Google Scholar] [CrossRef]
- Delgado-Pertíñez, M.; Gutiérrez-Peña, R.; Mena, Y.; Fernández-Cabanás, V.M.; Laberye, D. Milk production, fatty acid composition and vitamin E content of Payoya goats according to grazing level in summer on Mediterranean shrublands. Small Rumin. Res. 2013, 114, 167–175. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agri. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Juárez, M.; Polvillo, O.; Contò, M.; Ficco, A.; Ballico, S.; Failla, S. Comparison of four extraction/methylation analytical methods to measure fatty acid composition by gas chromatography in meat. J. Chromatogr. A 2008, 1190, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Barbudo, M.C.; Granado-Lorencio, F.; Blanco-Navarro, I.; Olmedilla-Alonso, B. Retinol, α-and γ -tocopherol and carotenoids in natural and vitamin A- and E-fortified dairy products commercialized in Spain. Int. Dairy J. 2005, 15, 521–526. [Google Scholar] [CrossRef]
- Chauveau-Duriot, B.; Doreau, M.; Nozière, P.; Graulet, B. Simultaneous quantification of carotenoids, retinol, and tocopherols in forages, bovine plasma, and milk: Validation of a novel UPLC method. Anal. Bioanal. Chem. 2010, 397, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Peña, R.; Fernández-Cabanás, V.M.; Mena, Y.; Delgado-Pertíñez, M. Fatty acid profile and vitamins A and E contents of milk in goat farms under Mediterranean wood pastures as affected by grazing conditions and seasons. J. Food Compost. Anal. 2018, 72, 122–131. [Google Scholar] [CrossRef]
- Fellegrini, N.; Ke, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2´-azinobis (3-ethylenebenzothiazoline-6-sulfonic acid) radical cation decolorization assay. Meth. Enzymol. 1999, 299, 379–389. [Google Scholar] [CrossRef]
- Gutiérrez-Peña, R.; Avilés, C.; Galán-Soldevilla, H.; Polvillo, O.; Ruiz Pérez-Cacho, P.; Guzmán, J.L.; Horcada, A.; Delgado-Pertíñez, M. Physicochemical Composition, Antioxi-dant Status, Fatty Acid Profile, and Volatile Compounds of Milk and Fresh and Ripened Ewes’ Cheese from a Sustainable Part-Time Grazing System. Foods 2021, 10, 80. [Google Scholar] [CrossRef]
- Vázquez, C.V.; Rojas, M.G.; Ramirez, C.A.; Chávez-Servin, J.L.; García-Gasca, T.; Ferriz-Martínez, R.A.; Garcia, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I.; et al. Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin-Ciocalteu method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef]
- Di Trana, A.; Bonanno, A.; Cecchini, S.; Giorgio, D.; Di Grigoli, A.; Claps, S. Effects of Sulla forage (Sulla coronarium L.) on the oxidative status and milk polyphenol content in goats. J. Dairy Sci. 2015, 98, 37–46. [Google Scholar] [CrossRef]
- Chávez-Servín, J.L.; Andrade-Montemayor, H.M.; Vázquez, C.V.; Barreyro, A.A.; García-Gasca, T.; Martínez, R.A.F.; Ramírez, A.M.O.; de la Torre-Carbot, K. Effects of feeding system, heat treatment and season on phenolic compounds and antioxidant capacity in goat milk, whey and cheese. Small Rumin. Res. 2018, 160, 54–58. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fox, P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited Review: Bioactive Compounds Produced during Cheese Ripening and Health Effects Associated with Aged Cheese Consumption. J. Dairy Sci. 2018, 101, 3742–3757. [Google Scholar] [CrossRef]
- Santos, G.T.; Lima, L.S.; Schogor, A.L.B.; Romero, J.V.; De Marchi, F.E.; Grande, P.A.; Santos, N.W.; Santos, F.S.; Kazama, R. Citrus Pulp as a Dietary Source of Antioxidants for Lactating Holstein Cows Fed Highly Polyunsaturated Fatty Acid Diets. Australas. J. Anim. Sci. 2014, 27, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- López-Expósito, I.; Amigo, L.; Recio, I. A Mini-Review on Health and Nutritional Aspects of Cheese with a Focus on Bioactive Peptides. Dairy Sci. Technol. 2012, 92, 419–438. [Google Scholar] [CrossRef]
- Felix da Silva, D.; Matumoto-Pintro, P.T.; Bazinet, L.; Couillard, C.; Britten, M. Effect of commercial grape extracts on the cheese-making properties of milk. J. Dairy Sci. 2015, 98, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, A.; Picon, A.; Nuñez, M. Cheese supplementation with five species of edible seaweeds: Effect on microbiota, antioxidant activity, colour, texture and sensory characteristics. Int. Dairy J. 2018, 84, 36–45. [Google Scholar] [CrossRef]
- Lucas, A.; Rock, E.; Chamba, J.F.; Verdier-Metz, I.; Brachet, P.; Coulon, J.-B. Respective Effects of Milk Composition and the Cheese-Making Process on Cheese Compositional Variability in Components of Nutritional Interest. Le Lait 2006, 86, 21–41. [Google Scholar] [CrossRef]
- Lucas, A.; Andueza, D.; Rock, E.; Martin, B. Prediction of dry matter, fat, pH, vitamins, minerals, carotenoids, total antioxidant capacity, and color in fresh and freeze-dried cheeses by visible-near-infrared reflectance spectroscopy. J. Agric. Food Chem. 2008, 56, 6801–6808. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulou, I.; Zoidis, E.; Massouras, T.; Hadjigeorgiou, I. Terpenes transfer to milk and cheese after oral administration to sheep fed indoors. J. Anim. Physiol. Anim. Nutr. 2012, 96, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, I.; Bustamante, M.Á.; Buccioni, A.; Franci, O.; Ruíz de Gordoa, J.C.; de Renobales, M.; Barron, L.J.R. Commercial sheep flocks–fatty acid and fat-soluble antioxidant composition of milk and cheese related to changes in feeding management throughout lactation. J. Dairy Res. 2015, 82, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, M.A.M.; Ahmed, N.S. Cheese yield as affected by some parameters. Review. Acta Sci. Pol. Technol. Aliment. 2011, 10, 131–153. [Google Scholar]
- Revilla, I.; González-Martín, M.I.; Vivar-Quintana, A.M.; Blanco-López, M.A.; Lobos-Ortega, I.A.; Hernández-Hierro, J.M. Antioxidant capacity of different cheeses: Affecting factors and prediction by near infrared spectroscopy. J. Dairy Sci. 2016, 99, 5074–5082. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pertíñez, M.; Mancilla-Leytón, J.M.; Morales-Jerrett, E.; Muñoz-Vallés, S.; Cruz, V.; Mena, Y. A survey of the commercial traits and antioxidant status of goat milk in Mediterranean pastoral farm. Span. J. Agric. Res. 2024. under review. [Google Scholar]
- Timón, M.L.; Andrés, A.I.; Otte, J.; Petrón, M.J. Antioxidant Peptides (<3 kDa) Identified on Hard Cow Milk Cheese with Rennet from Different Origin. Food Res. Int. 2019, 120, 643–649. [Google Scholar] [CrossRef]
- Colombo, M.L.; Cimino, C.V.; Bruno, M.A.; Hugo, A.; Liggieri, C.; Fernández, A.; Vairo-Cavalli, S. Artichoke cv. Francés flower extract as a rennet substitute: Effect on textural, microstructural, microbiological, antioxidant properties, and sensory acceptance of miniature cheeses. J. Sci. Food Agric. 2021, 101, 1382–1388. [Google Scholar] [CrossRef]
- Khan, U.M.; Aadil, R.M.; Shabbir, M.A.; Shahid, M.; Decker, E.A. Interpreting the production, characterization and antioxidant potential of plant proteases. Food Sci. Technol, 2023, 43, e84922. [Google Scholar] [CrossRef]
- Laskaridis, K.; Serafeimidou, A.; Zlatanos, S.; Gylou, E.; Kontorepanidou, E.; Sagredos, A. Changes in fatty acid profile of feta cheese including conjugated linoleic acid. J. Sci. Food Agric. 2013, 93, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Bocquel, D.; Marquis, R.; Dromard, M.P.; Salamin, A.; Rey-Siggen, J.; Héritier, J.; Kosinska-Cagnazzo, A.; Andlauer, W. Effect of flaxseed supplementation of dairy cows’ forage on physicochemical characteristic of milk and Raclette cheese. Int. J. Dairy Technol. 2016, 69, 129–136. [Google Scholar] [CrossRef]
- Schiavon, S.; Cesaro, G.; Cecchinato, A.; Cipolat-Gotet, C.; Tagliapietra, F.; Bittante, G. The influence of dietary nitrogen reduction and conjugated linoleic acid supply to dairy cows on fatty acids in milk and their transfer to ripened cheese. J. Dairy Sci. 2016, 99, 8759–8778. [Google Scholar] [CrossRef]
- Valdivielso, I.; Bustamante, M.Á.; Buccioni, A.; Franci, O.; Ruíz de Gordoa, J.C.; de Renobales, M.; Barron, L.J.R. Commercial sheep flocks–fatty acid and fat-soluble antioxidant composition of milk and cheese related to changes in feeding management throughout lactation. J. Dairy Res. 2015, 82, 334–343. [Google Scholar] [CrossRef]
- Bodkowski, R.; Czyz˙, K.; Kupczyn´ ski, R.; Patkowska-Sokoła, B.; Nowakowski, P.; Wiliczkiewicz, A. Lipid complex effect on fatty acid profile and chemical composition of cow milk and cheese. J. Dairy Sci. 2016, 99, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Tejada, L.; Abellan, A. , Prados, F.; Cayuela, J.M. Compositional characteristics of Murcia al Vino goat’s cheese made with calf rennet and plant coagulant. Int. J. Dairy Technol. 2008, 61, 119–125. [Google Scholar] [CrossRef]
- Ordiales, E.; Benito, M.J.; Martín, A.; Fernández, M.; Hernández, A.; de Guia Córdoba, M. Proteolytic effect of Cynara cardunculus rennet for use in the elaboration of ‘Torta del Casar’ cheese. J. Dairy Res. 2013, 80, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Collins, Y.F.; McSweeney, P.L.H.; Wilkinson, M.G. Lipolysis and free fatty acid catabolism in cheese: A review of current knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Addis, M.; Piredda, G.; Pes, M.; Di Salvo, R.; Scintu, M.F.; Pirisi, A. Effect of the use of three different lamb paste rennets on lipolysis of the PDO Pecorino Romano Cheese. Int. Dairy J. 2005, 15, 563–569. [Google Scholar] [CrossRef]
- Addis, M.; Pirisi, A.; Di Salvo, R.; Podda, F.; Piredda, G. The influence of the enzymatic composition of lamb rennet paste on some properties of experimentally produced PDO Fiore Sardo cheese. Int. Dairy J. 2005, 15, 1271–1278. [Google Scholar] [CrossRef]
- Oštarić, F.; Antunac, N.; Cubric-Curik, V.; Curik, I.; Jurić, S.; Kazazić, S.; Kiš, M.; Vinceković, M.; Zdolec, N.; Špoljarić, J.; et al. Challenging Sustainable and Innovative Technologies in Cheese Production: A Review. Processes 2022, 10, 529. [Google Scholar] [CrossRef]
| Items | Experimental Diets1 | |||
|---|---|---|---|---|
| CD | DOP40 | DOP80 | ||
| Ration ingredients, % dry matter (DM) basis | ||||
| Alfalfa hay | 20.2 | 20.3 | 20.4 | |
| Concentrate | ||||
| Dehydrated orange pulp (pellets) | 0.00 | 19.4 | 38.6 | |
| Oats | 21.4 | 12.8 | 4.24 | |
| Barley | 8.28 | 4.96 | 1.65 | |
| Corn | 18.8 | 11.3 | 3.77 | |
| Soy flour, 44% | 7.09 | 9.92 | 12.6 | |
| Sunflower pellets, 28% | 12.5 | 12.1 | 13.5 | |
| Peas | 10.0 | 7.87 | 3.93 | |
| Salt | 0.39 | 0.39 | 0.39 | |
| Stabilised lard | 0.39 | 0.00 | 0.00 | |
| Vitamins and minerals2 | 1.01 | 1.01 | 1.02 | |
| Proximate composition and nutritive value, % DM | ||||
| DM, % | 87.1 | 87.1 | 88.1 | |
| Crude protein | 20.9 | 18.7 | 18.3 | |
| Neutral detergent fibre | 29.8 | 26.6 | 28.3 | |
| Acid detergent fibre | 14.7 | 15.2 | 16.8 | |
| Acid detergent lignin | 3.09 | 3.13 | 3.43 | |
| Ether extract | 2.63 | 1.85 | 1.43 | |
| Ash | 6.50 | 7.47 | 8.64 | |
| Gross energy, kcal/g DM | 4.37 | 4.31 | 4.25 | |
| Forage unit for lactation, UFL/kg | 0.98 | 0.98 | 0.96 | |
| Protein digestible in the intestine (PDI) | 10.4 | 10.4 | 11.4 | |
| Item2 | Diet1 | SEM | ||
|---|---|---|---|---|
| Control | DOP40 | DOP80 | ||
| Dry matter (DM), % | 11.7 | 12.3 | 12.6 | 0.16 |
| Crude protein, % | 3.17 | 3.18 | 3.34 | 0.06 |
| Fat, % | 3.50 | 4.09 | 4.15 | 0.13 |
| Retinol, μg/100 g | 2.82 | 5.98 | 6.15 | 0.64 |
| α-Tocopherol, μg/100 g | 17.9 b | 28.6 ab | 58.0 a | 5.88 |
| TPC, mg GA equivalents/L | 55.6 c | 72.8 b | 98.8 a | 4.48 |
| TAC, μmol Trolox® equivalents/mL | 6.19 c | 8.95 b | 11.86 a | 0.61 |
| Fatty acids (FAs), mg/g DM | ||||
| C4:0 | 7.99 | 9.33 | 8.30 | 0.51 |
| C6:0 | 10.82 | 12.41 | 10.29 | 0.75 |
| C8:0 | 9.37 | 10.65 | 8.77 | 0.65 |
| C10:0 | 24.4 | 27.1 | 23.2 | 1.84 |
| C12:0 | 12.6 | 14.3 | 11.8 | 0.88 |
| C14:0 | 15.5 | 17.6 | 14.6 | 1.08 |
| C14:1 | 0.42 | 0.48 | 0.40 | 0.03 |
| C15:0 | 1.19 | 1.30 | 1.13 | 0.09 |
| C16:0 | 54.8 | 62.6 | 50.7 | 3.90 |
| C16:1 | 2.31 | 2.51 | 2.18 | 0.17 |
| C17:0 | 0.73 | 0.84 | 0.69 | 0.05 |
| C17:1 | 0.20 | 0.23 | 0.19 | 0.01 |
| C18:0 | 24.8 | 27.0 | 23.4 | 1.79 |
| C18:1 n-9 trans | 2.17 | 2.46 | 2.03 | 0.15 |
| C18:1 n-11 trans (VA) | 1.54 | 1.75 | 1.44 | 0.11 |
| C18:1 n-9 cis | 45.2 | 49.2 | 42.6 | 3.27 |
| C18:2 n-6 trans | 0.33 | 0.38 | 0.31 | 0.02 |
| C18:2 n-6 cis | 7.57 | 8.60 | 7.08 | 0.53 |
| α -C18:3 n-3 | 0.48 | 0.55 | 0.45 | 0.03 |
| γ -C18:3 n-6 | 0.20 | 0.26 | 0.19 | 0.02 |
| CLA cis-9, trans-11 (RA) | 1.58 | 1.72 | 1.49 | 0.11 |
| C20:0 | 0.48 | 0.55 | 0.45 | 0.03 |
| C20:4 n-6 (ARA) | 0.44 | 0.50 | 0.41 | 0.03 |
| C20:5 n-3 (EPA) | 0.06 | 0.07 | 0.06 | 0.00 |
| C22:0 | 0.26 | 0.30 | 0.25 | 0.02 |
| C22:5 n-3 (DPA) | 0.13 | 0.14 | 0.12 | 0.01 |
| C22:6 n-3 (DHA) | 0.05 | 0.06 | 0.05 | 0.00 |
| Others | 1.00 | 1.14 | 0.95 | 0.01 |
| SFA | 163.4 | 184.4 | 154.0 | 11.5 |
| MUFA | 52.1 | 56.9 | 49.1 | 3.75 |
| PUFA | 11.2 | 12.6 | 10.5 | 0.78 |
| Total CLA | 1.61 | 1.75 | 1.52 | 0.12 |
| n-6 | 8.61 | 9.82 | 8.08 | 0.60 |
| n-3 | 0.78 | 0.88 | 0.74 | 0.06 |
| n-6:n-3 | 11.0 | 11.2 | 11.0 | 0.06 |
| Item2 | Diet1 (D) | Rennet (R) | SEM | p-Values | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | DOP40 | DOP80 | Animal | Vegetable | D | R | D × R | ||
| Retinol, μg/100 g | 26.9 | 26.9 | 23.2 | 22.8 | 28.6 | 2.62 | 0.803 | 0.291 | 0.209 |
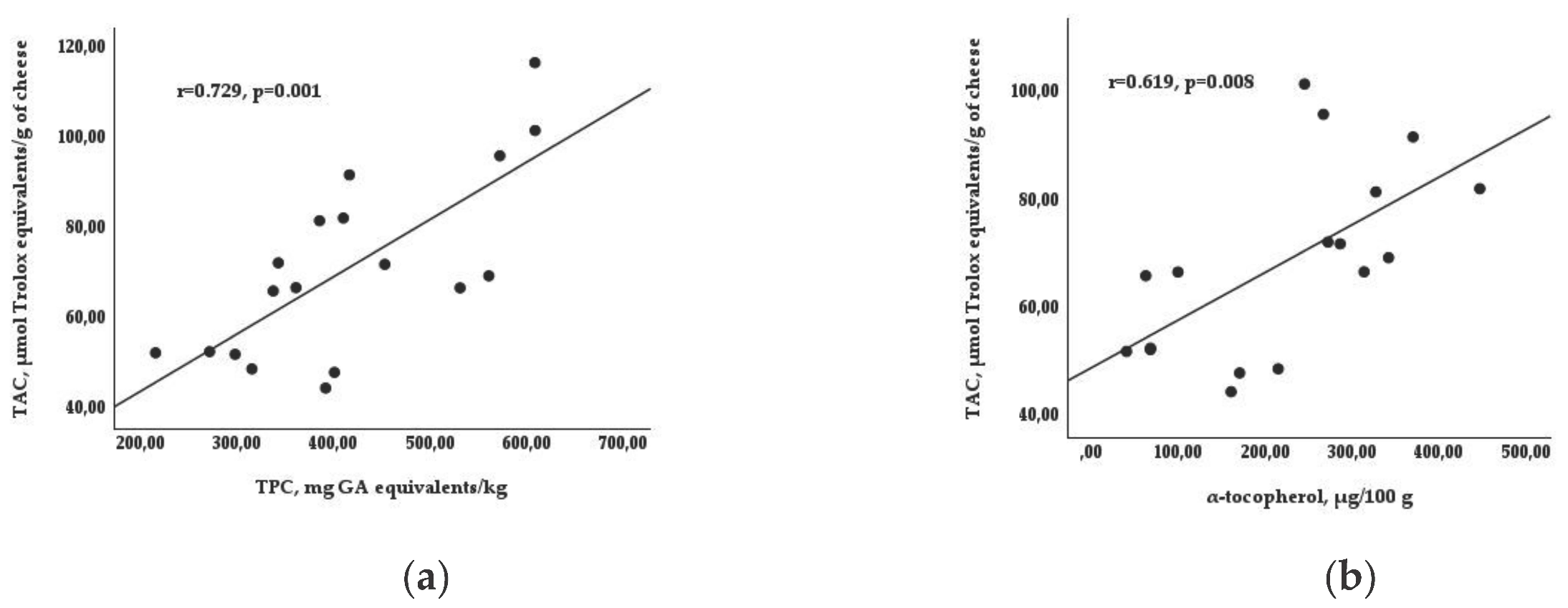
| α-Tocopherol, μg/100 g | 120.3 b | 229.2 ab | 331.1 a | 223.1 | 218.6 | 29.9 | 0.009 | 0.848 | 0.097 |
| TPC, mg GA equivalents/kg | 315.2 c | 430.7 b | 499.9 a | 493.2 a | 337.3 b | 27.7 | 0.000 | 0.000 | 0.158 |
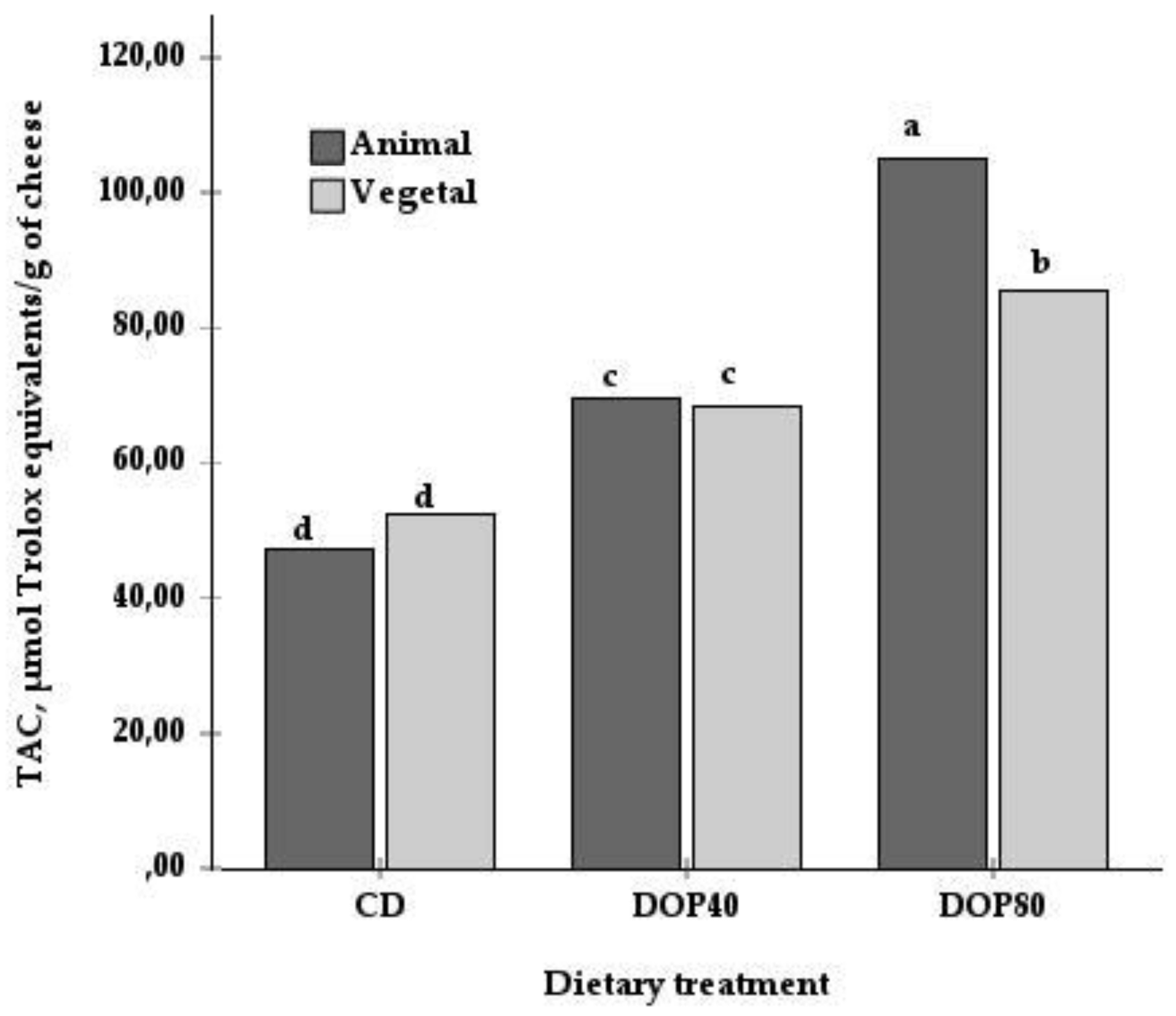
| TAC, μmol Trolox® equivalents/g | 49.9 c | 69.0 b | 95.1 a | 73.9 | 68.8 | 4.83 | 0.000 | 0.065 | 0.004 |
| Fatty Acids2, mg/g DM | Diet1 (D) | Rennet (R) | SEM | p-Values | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | DOP40 | DOP80 | Animal | Vegetable | D | R | D × R | ||
| C4:0 | 17.0 | 17.1 | 16.9 | 17.8 | 16.2 | 0.75 | 0.996 | 0.366 | 0.496 |
| C6:0 | 25.3 | 21.9 | 21.4 | 23.6 | 22.1 | 1.05 | 0.327 | 0.527 | 0.777 |
| C8:0 | 22.1 | 18.9 | 19.6 | 20.8 | 19.6 | 0.96 | 0.418 | 0.568 | 0.676 |
| C10:0 | 89.8 | 79.5 | 86.4 | 93.2 | 77.3 | 5.01 | 0.700 | 0.141 | 0.450 |
| C11:0 | 1.08 | 0.98 | 1.23 | 1.06 | 1.14 | 0.06 | 0.259 | 0.507 | 0.120 |
| C12:0 | 48.4 | 43.6 | 53.3 | 51.1 | 45.7 | 2.83 | 0.409 | 0.363 | 0.446 |
| C13:0 | 0.87 | 0.94 | 0.97 | 1.05 a | 0.80 b | 0.06 | 0.769 | 0.048 | 0.923 |
| C14:0 | 88.1 | 78.2 | 82.7 | 89.8 | 76.2 | 4.92 | 0.724 | 0.194 | 0.360 |
| C14:1 | 2.49 | 1.96 | 2.29 | 2.73 a | 1.76 b | 0.19 | 0.364 | 0.006 | 0.231 |
| C15:0 | 5.53 | 4.70 | 5.89 | 5.63 | 5.12 | 0.31 | 0.328 | 0.438 | 0.619 |
| C15:1 | 1.43 | 1.42 | 1.78 | 1.30 b | 1.79 a | 0.10 | 0.123 | 0.006 | 0.199 |
| C16:0 | 250.2 | 227.2 | 236.6 | 262.5 | 213.5 | 13.9 | 0.793 | 0.100 | 0.439 |
| C16:1 | 11.4 | 10.8 | 11.9 | 10.0 | 12.8 | 0.68 | 0.789 | 0.058 | 0.583 |
| C17:0 | 3.69 | 3.64 | 3.32 | 3.21 | 3.89 | 0.22 | 0.773 | 0.158 | 0.577 |
| C17:1 | 1.93 b | 2.33 ab | 2.86 a | 2.65 | 2.10 | 0.17 | 0.048 | 0.067 | 0.299 |
| C18:0 | 62.1 | 53.7 | 57.8 | 65.4 a | 50.4 b | 3.67 | 0.625 | 0.050 | 0.462 |
| C18:1 n-9 trans | 3.35 | 2.90 | 3.86 | 3.04 | 3.71 | 0.20 | 0.127 | 0.083 | 0.710 |
| C18:1 n-11 trans (VA) | 3.38 | 2.82 | 3.63 | 4.02 a | 2.54 b | 0.30 | 0.337 | 0.006 | 0.073 |
| C18:1 n-9 cis | 185.8 | 152.5 | 149.5 | 184.5 | 140.7 | 11.2 | 0.318 | 0.056 | 0.663 |
| C18:2 n-6 trans | 3.99 | 3.57 | 3.89 | 4.60 a | 3.02 b | 0.29 | 0.709 | 0.003 | 0.087 |
| C18:2 n-6 cis | 19.5 | 20.7 | 21.3 | 21.1 | 19.9 | 1.03 | 0.813 | 0.620 | 0.559 |
| α -C18:3 n-3 | 2.61 | 2.72 | 3.41 | 3.30 a | 2.52 b | 0.20 | 0.148 | 0.038 | 0.262 |
| γ -C18:3 n-6 | 0.93 | 0.84 | 0.89 | 0.96 | 0.82 | 0.06 | 0.850 | 0.346 | 0.461 |
| CLA cis-9, trans-11 (RA) | 5.22 | 4.42 | 4.38 | 4.83 | 4.51 | 0.27 | 0.442 | 0.590 | 0.713 |
| CLA n-10 trans, n-12 cis | 0.46 | 0.33 | 0.35 | 0.45 a | 0.31 b | 0.03 | 0.093 | 0.009 | 0.156 |
| C20:0 | 0.41 | 0.30 | 0.31 | 0.39 | 0.29 | 0.03 | 0.095 | 0.055 | 0.349 |
| C20:1 n-9 | 0.27 | 0.23 | 0.24 | 0.28 | 0.22 | 0.02 | 0.538 | 0.098 | 0.519 |
| C20:2 | 0.32 | 0.32 | 0.30 | 0.34 | 0.29 | 0.02 | 0.923 | 0.219 | 0.833 |
| C20:3 n-3 | 0.99 | 0.83 | 0.84 | 0.99 | 0.78 | 0.06 | 0.447 | 0.092 | 0.282 |
| C20:3 n-6 | 0.41 | 0.36 | 0.37 | 0.41 | 0.35 | 0.02 | 0.716 | 0.274 | 0.306 |
| C20:4 n-6 (ARA) | 4.22 | 4.14 | 4.80 | 4.87 | 3.90 | 0.27 | 0.523 | 0.076 | 0.302 |
| C20:5 n-3 (EPA) | 1.06 | 0.99 | 1.04 | 1.16 | 0.91 | 0.06 | 0.903 | 0.056 | 0.259 |
| C21:0 | 0.33 | 0.27 | 0.27 | 0.34 a | 0.24 b | 0.02 | 0.247 | 0.011 | 0.251 |
| C22:0 | 0.92 | 0.72 | 0.81 | 0.92 | 0.72 | 0.06 | 0.328 | 0.074 | 0.256 |
| C22:1 n-9 | 0.18 | 0.16 | 0.19 | 0.20 | 0.15 | 0.01 | 0.469 | 0.055 | 0.475 |
| C22:2 | 0.09 | 0.10 | 0.10 | 0.11 | 0.09 | 0.01 | 0.499 | 0.126 | 0.478 |
| C22:5 n-3 (DPA) | 1.03 | 0.99 | 1.04 | 1.15 a | 0.89 b | 0.06 | 0.931 | 0.045 | 0.512 |
| C22:6 n-3 (DHA) | 0.91 | 0.87 | 0.93 | 0.97 | 0.83 | 0.05 | 0.864 | 0.202 | 0.466 |
| C23:0 | 0.19 | 0.17 | 0.17 | 0.20 a | 0.15 b | 0.01 | 0.796 | 0.042 | 0.946 |
| C24:0 | 0.26 | 0.25 | 0.26 | 0.27 | 0.24 | 0.01 | 0.925 | 0.273 | 0.498 |
| C24:1 | 0.18 | 0.15 | 0.19 | 0.19 | 0.16 | 0.01 | 0.260 | 0.091 | 0.523 |
| SFA | 616.4 | 552.1 | 588.0 | 637.3 | 533.7 | 33.2 | 0.740 | 0.148 | 0.465 |
| MUFA | 210.3 | 175.4 | 176.5 | 208.9 | 165.9 | 12.1 | 0.408 | 0.091 | 0.636 |
| PUFA | 41.7 | 41.1 | 43.7 | 45.2 | 39.1 | 2.25 | 0.903 | 0.227 | 0.527 |
| CLA total | 5.67 | 4.75 | 4.73 | 5.28 | 4.82 | 0.23 | 0.402 | 0.476 | 0.666 |
| n-6 | 29.1 | 29.6 | 31.3 | 31.9 | 28.0 | 1.57 | 0.855 | 0.265 | 0.527 |
| n-3 | 6.59 | 6.40 | 7.27 | 7.58 | 5.93 | 0.42 | 0.643 | 0.057 | 0.353 |
| n-6: n-3 | 4.46 ab | 4.62 a | 4.37 b | 4.24 b | 4.72 a | 0.07 | 0.037 | 0.000 | 0.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





