1. Introduction
Aortic valve (AV) stenosis is a rare congenital cardiac malformation with a prevalence of 2.2 to 6 % [
1,
2]. “Critical” aortic valve stenosis (CAVS) implies a severe impairment of the left ventricle (LV) due to a high-grade stenosis and pressure overload of the LV. Of note, there is no standardised definition for CAVS [
3]. AV dysplasia and hypoplasia of the aortic annulus are the main causes. In most cases, the AV has a unicuspid or bicuspid morphology [
2,
4] due to congenital commissural fusions. Additional cardiac malformations may exist as for example aortic hypoplasia or coarctation of the aorta.
CAVS results in LV pressure overload with severely reduced LV systolic function, LV enlargement and eccentric dilatation, mitral regurgitation, and consecutive low output heart failure. Endocardial fibroelastosis of the LV may occur as well [
5]. Cardiomegaly and fetal hydrops may develop antenatally [
6].
After birth, clinical manifestations vary from mild symptoms to cardiogenic shock. However, floppiness, pallor, nutritional disturbance, and failure to thrive are common initial symptoms. A typical systolic murmur may be missing especially in case of high-grade stenosis with low cardiac output.
Frequently, the definitive diagnosis is only made after clinical manifestation in the neonate or infant as the rate of prenatally diagnosed CAVS by fetal ultrasound is still low [
7]. In case of (imminent) cardiogenic shock, intravenous prostaglandin application should be started immediately after birth respectively after diagnosis in order to maintain a sufficient systemic perfusion via a patent ductus arteriosus. Inotropic medication, mechanical ventilation, and balloon atrioseptostomy may also be indicated [
8,
9].
The further therapeutic approach can be challenging. It should always be aimed at LV pressure relief by enlargement of the aortic valvular orifice. It is still controversially discussed, which technique – transcatheter balloon aortic valvulotomy (BAV) or cardiac surgery – is more beneficial [
3,
10,
11,
12,
13,
14,
15]. Transcatheter BAV – first described by Labadibi et al.[
16] – has become standard of care in most centres. The intention is to quickly reduce the stenosis and thus to achieve improvement of LV function, yet avoiding open-heart surgery and cardio-pulmonary bypass in a critically ill neonate. Both retrograde and antegrade trans-valvular access are performed depending on the feasibility of probing the stenotic valve.
The aim of this retrospective study was to analyse patients’ characteristics, procedural parameters, effectiveness, complications, and outcome of transcatheter BAV in CAVS in our institution. Stable support for guidewires and valvuloplasty catheters is particularly challenging in CAVS due to the valvular dysplasia and the small anatomical features in the relevant age group. In this regard, we also present rarely described techniques for peri-interventional wire and catheter support.
2. Material and Methods
After approval from the local ethics committee of the Saarland, Saarbruecken, Germany (file number 37/24), this single-centre retrospective observational cohort study was performed at the Saarland University Medical Center, Homburg/Saar, Germany.
We retrospectively analysed all patients with CAVS who underwent emergency transcatheter BAV as first-line therapy in our institution from January 2006 to December 2023. Criteria for the diagnosis of CAVS were depressed systolic LV-function and / or clinical symptoms of heart failure or cardiogenic shock. We reviewed the relevant patients’ files as well as the echocardiographic and angiographic records in order to obtain the following data:
- -
patients’ characteristics (sex, body weight, age at intervention) ,
- -
AV cuspidity and diameter of the AV annulus,
- -
balloon diameter and balloon / annulus ratio,
- -
direction of the AV access (i.e. antegrade or retrograde),
- -
left ventricular end-diastolic diameter (LVEDD, [mm]) before BAV,
- -
left ventricular shortening fraction (LVSF, [%]) before and after BAV,
- -
trans-valvular systolic pressure gradient [mmHg] before and after BAV,
- -
post-interventional aortic regurgitation and other BAV-related complications (cardio-vascular injuries, thrombosis, embolism, haemorrhage, mortality),
- -
time to AV surgery.
Z-scores were calculated according to Cantenotti et al. 2017 [
17] for the AV annulus and according to Cantenotti et al. 2014 [
18] for the LVEDD.
2.1. Statistics
The reduction of the trans-valvular pressure gradient as well as the improvement of the LVSF by BAV was evaluated using paired t-tests; the normality of data was assessed by Shapiro-Wilk test. A Kaplan-Meier analysis (time-to-event analysis) was performed to calculate the time to valvular surgery after BAV. IBM© SPSS 28.0 statistics software was used for statistical analyses.
2.2. Percutaneous Catheterization
Femoral venous and arterial access was chosen in all patients. 4 French introducer sheaths and 4 French angiographic catheters (Multipurpose A SPECIAL, Cordis
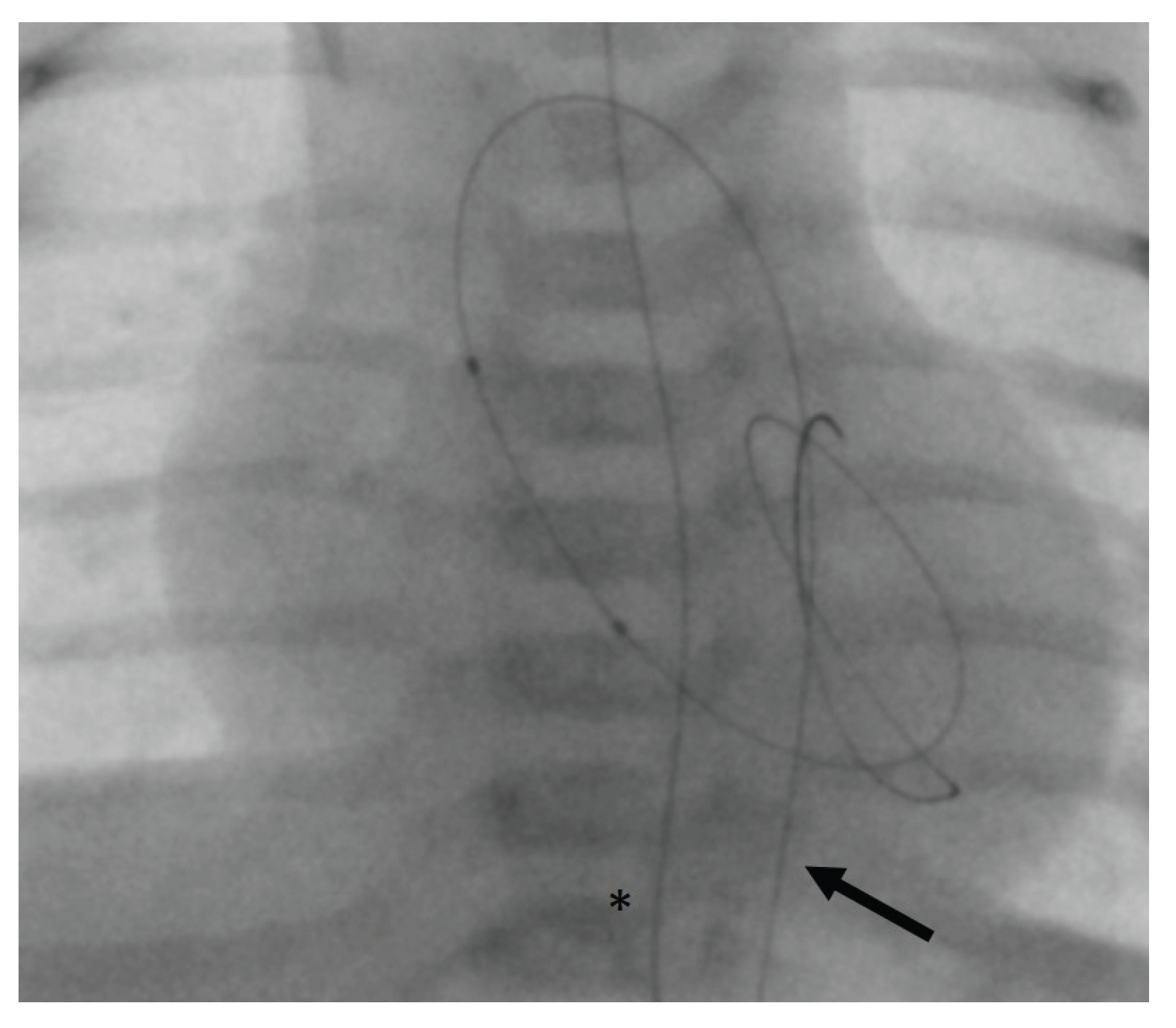
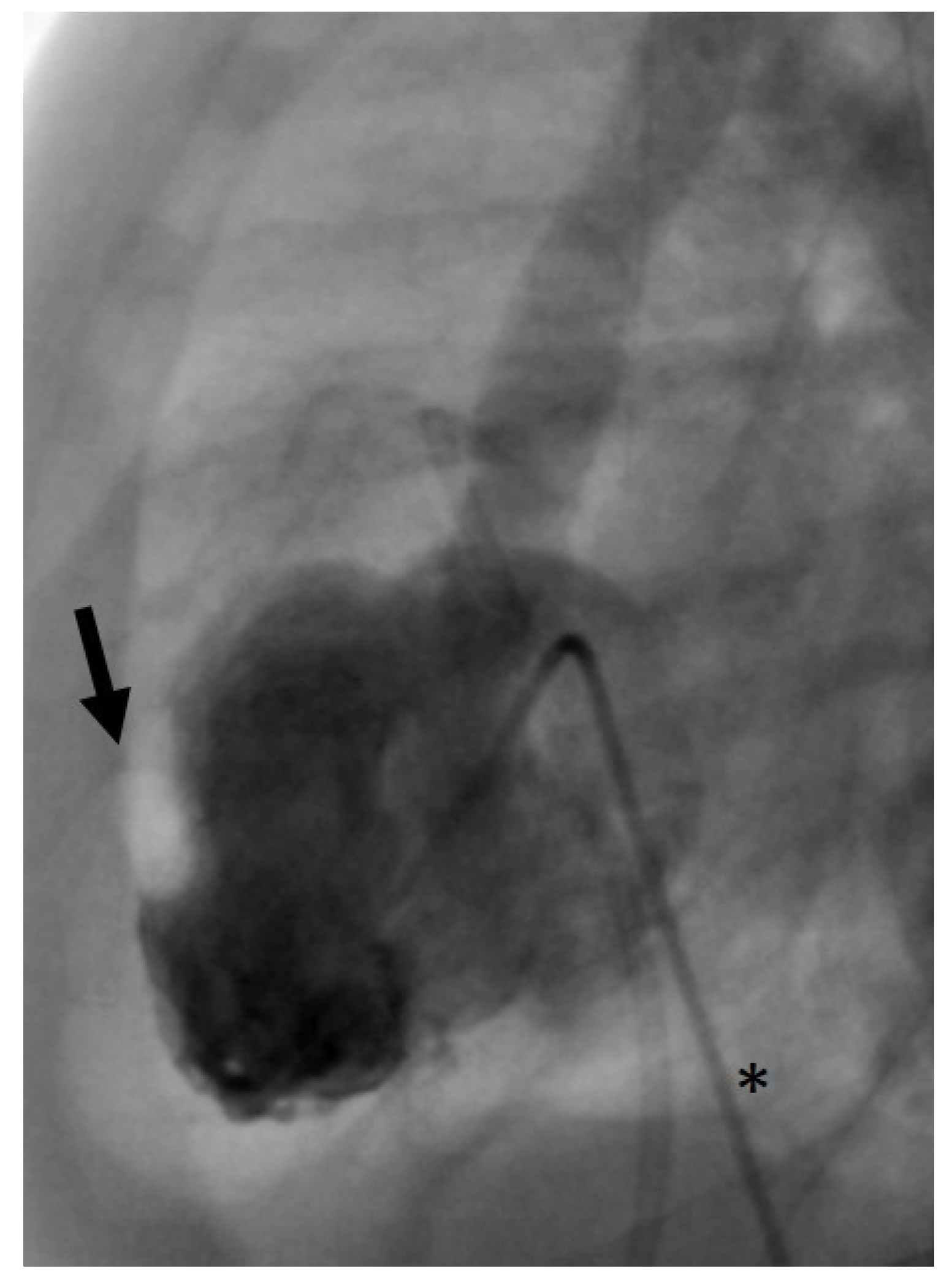
©, Miami Lakes, FL, USA) were used. First, we aimed at trans-arterial retrograde probing of the AV using a coronary guidewire. If this was successful, it may still have been difficult to pass the severely stenotic AV orifice with the angiographic catheter. In these cases, a 2.7 French microcatheter (Rebar™ 18, ev3 Neurovascular, Irvine, CA, USA) was introduced beforehand in order to provide more support for the angiographic catheter and thus to allow its trans-valvular passage. For retrograde BAV, the guidewire usually can be positioned stably within the LV (
Figure 1).
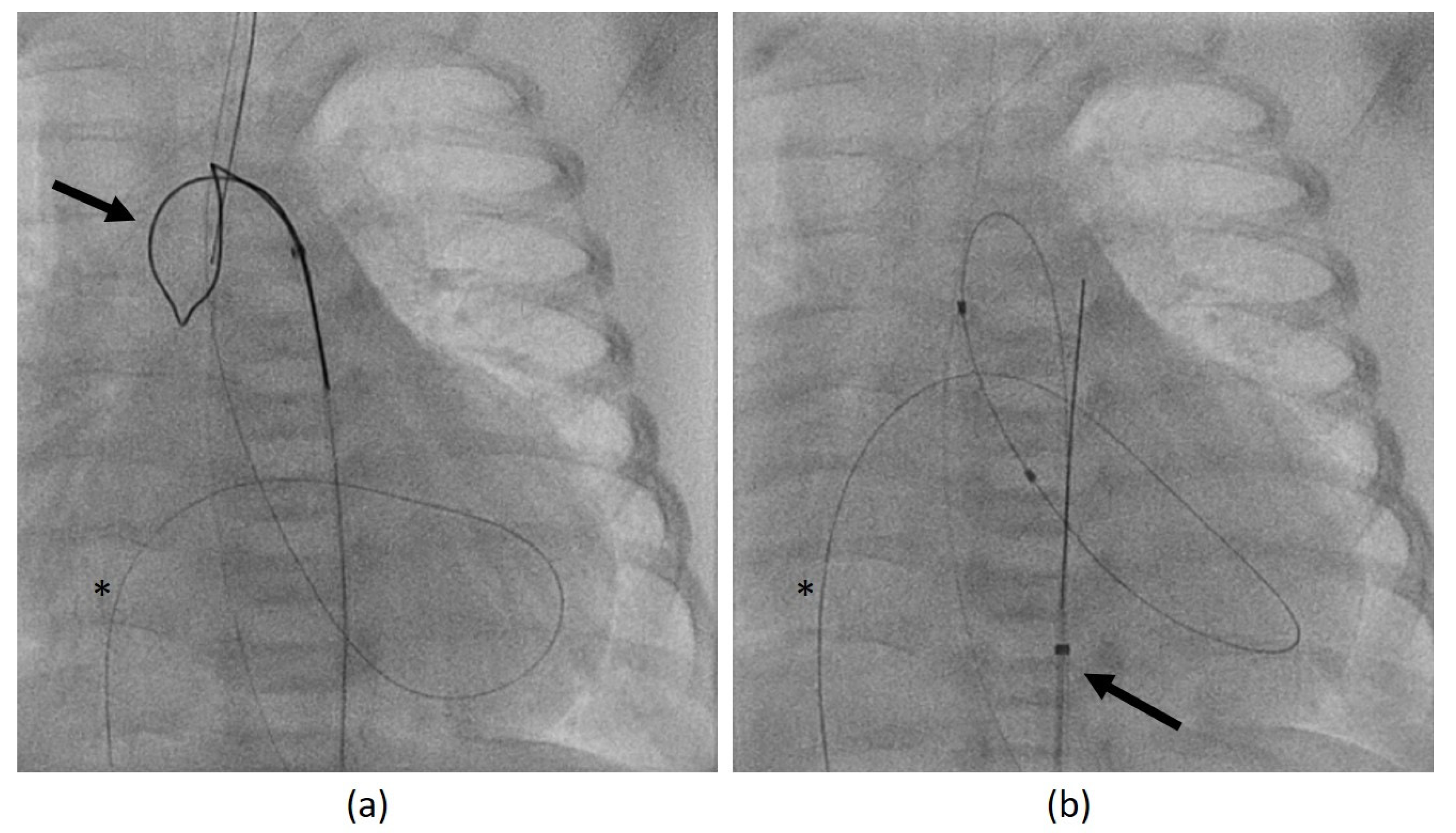
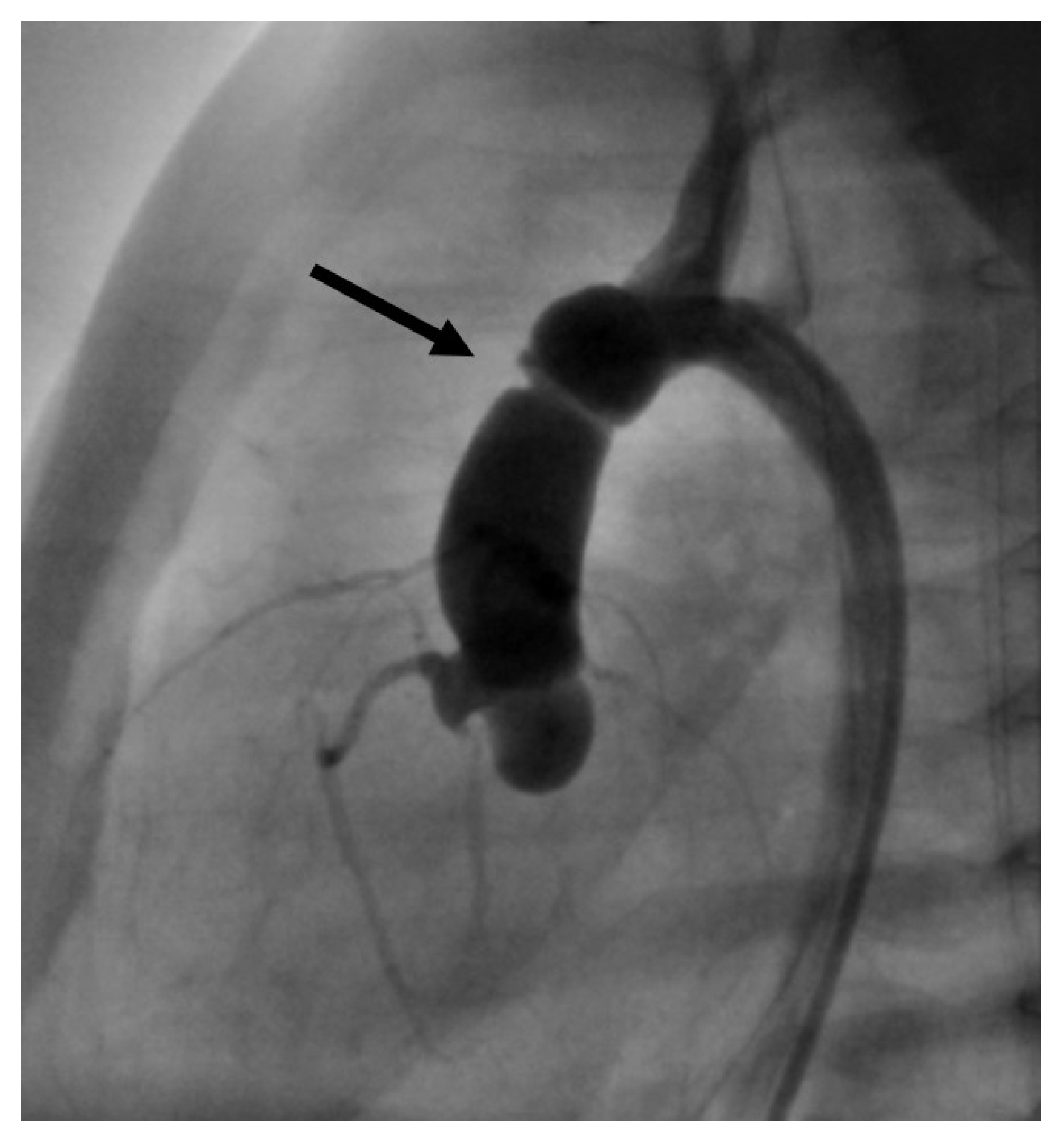
If retrograde catheterization was not feasible, a trans-venous antegrade approach was accomplished via lower vena cava, right atrium, foramen ovale, left atrium, and LV. After passing the AV into the ascending aorta, the guidewire was caught using a retrieval loop (Multi-Snare
©, pfm medical mepro gmbh, Nonnweiler-Otzenhausen, Germany, 5 or 10 mm diameter) that was inserted retrogradely into the aorta (
Figure 2a). Then, it is possible to pull the wire into the descending aorta and fixate it stably during the BAV (
Figure 2b).
Laevocardiography was conducted in order to localize and to measure the diameter of the aortic annulus in all cases. For aortic valvuloplasty, we used either coronary balloon catheters or valvuloplasty catheters (Tyshak mini©, NuMED, Inc., Hopkinton, NY, USA) that were introduced over the guidewire. BAV was conducted stepwise with gradually increasing balloon diameters. We administered Heparin (100 IU per kg body weight) and antibiotic prophylaxis during the procedure.
3. Results
3.1. Patients’ Characteristics, Cardiac Parameters, and Procedural Parameters
For underlying CAVS, BAV had been performed in 12 patients (83.3 % male) of whom 9 patients underwent a second procedure, and 2 patients underwent a third procedure. In total, 23 BAV had been performed. Renewed increase of the pressure gradient and / or worsening of the left ventricular systolic function were indications for re-intervention.
A unicuspid AV was present in 9/12 patients (75 %), a bicuspid AV in 3/12 (25 %). At the time of the first BAV, median z-score for AV annulus was -1.25 (range: -4.1 to +2.8), and median z-score for LVEDD was +1.8 (range: -2.0 to +6.2). Retrograde BAV could be achieved in 6/12 (50 %) patients in the first intervention, in 6/9 (66.7 %) patients in the second, and 2/2 (100 %) patients in the third BAV. Antegrade BAV was performed for all other procedures. The balloon / AV annulus ratio for the first BAV was in median 0.90 (range: 0.7 – 1.1), for the second BAV in median 1.0 (range: 0.84 – 1.16), and for the third BAV 1.23 in both interventions.
The patients’ characteristics, morphological and functional cardiac parameters, as well as procedural parameters are summarised in
Table 1 and described case-based in
Table 2.
3.2. Effectiveness of the BAV
3.2.1. First BAV
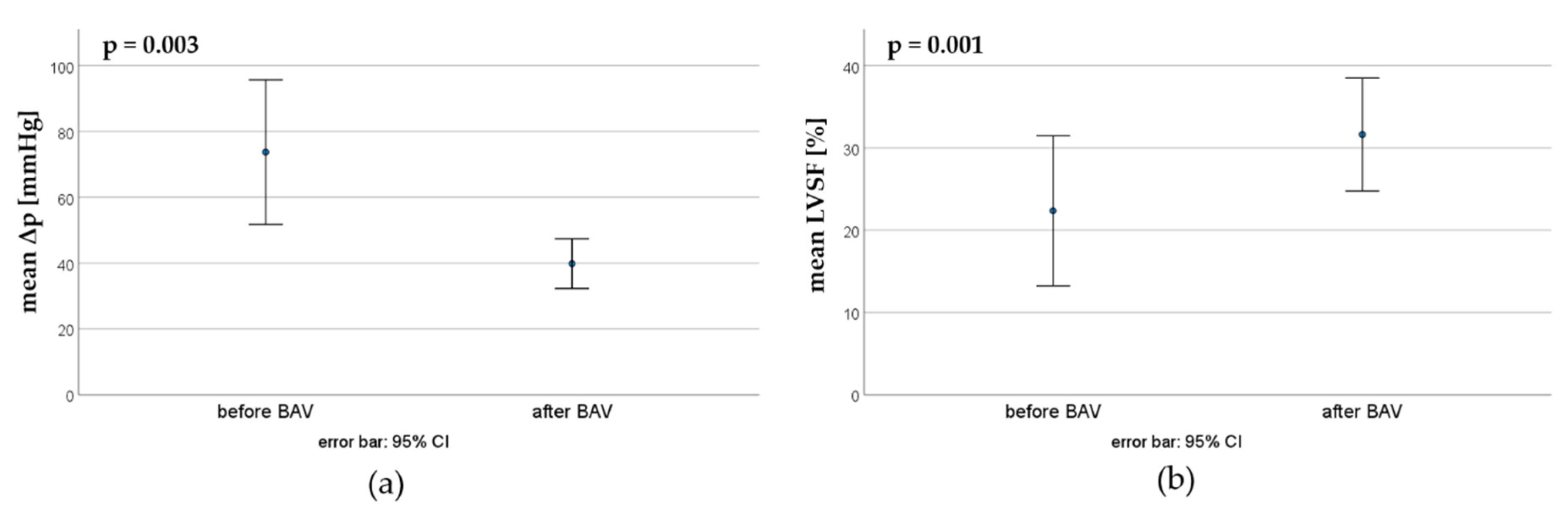
In the first BAV, the maximal pressure gradient could be reduced from 73.7 ±34.5 mmHg to 39.8 ±11.9 mmHg, whereas the mean LVSF increased from 22.3 ±13.5 % to 31.6 ±10.2 % (
Figure 3).
3.2.2. Second BAV
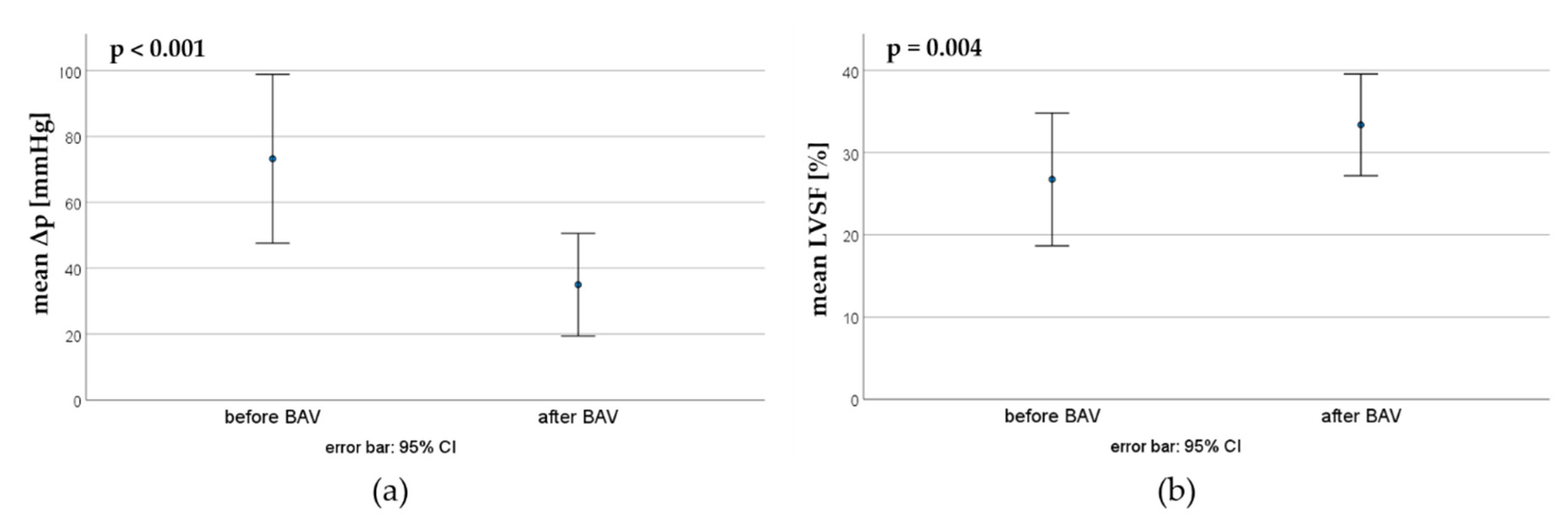
Nine patients underwent a second BAV. Here, the mean pressure gradient could be reduced from 73.2 ±33.3 mmHg to 35.0 ±20.2 mmHg, whereas the mean LVSF increased from 26.7 ±9.6 % to 33.3 ±7.4 % (
Figure 4).
3.2.3. Third BAV
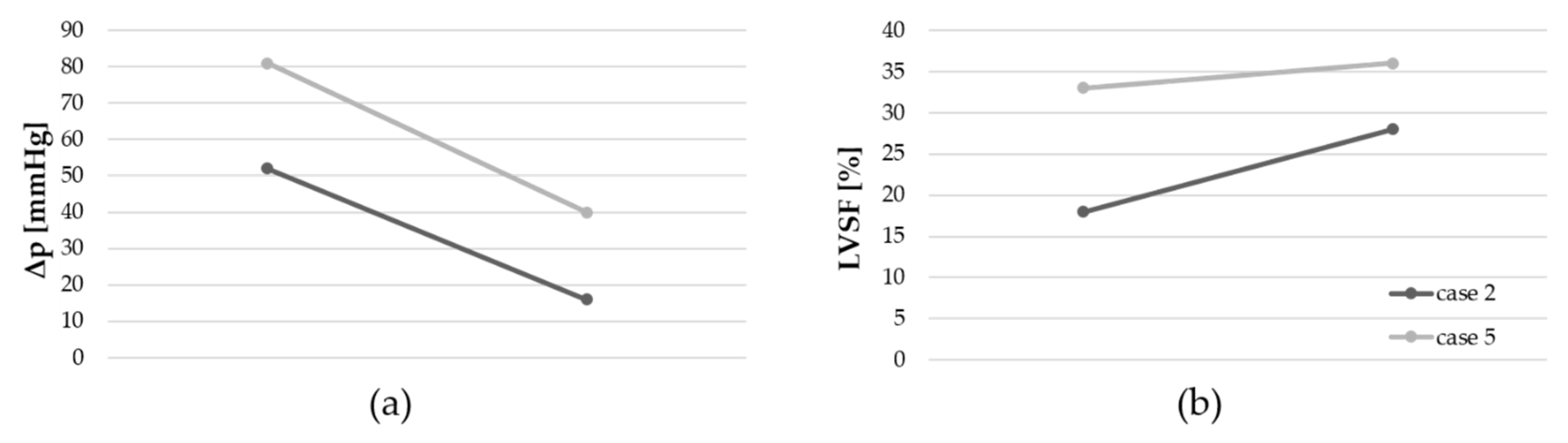
A third BAV was performed in only two patients (cases 2 and 5,
Table 2). A reduction of the pressure gradient and an increase of the LVSF were achieved in both interventions (
Figure 5).
3.3. Complications
3.3.1. Aortic Regurgitation
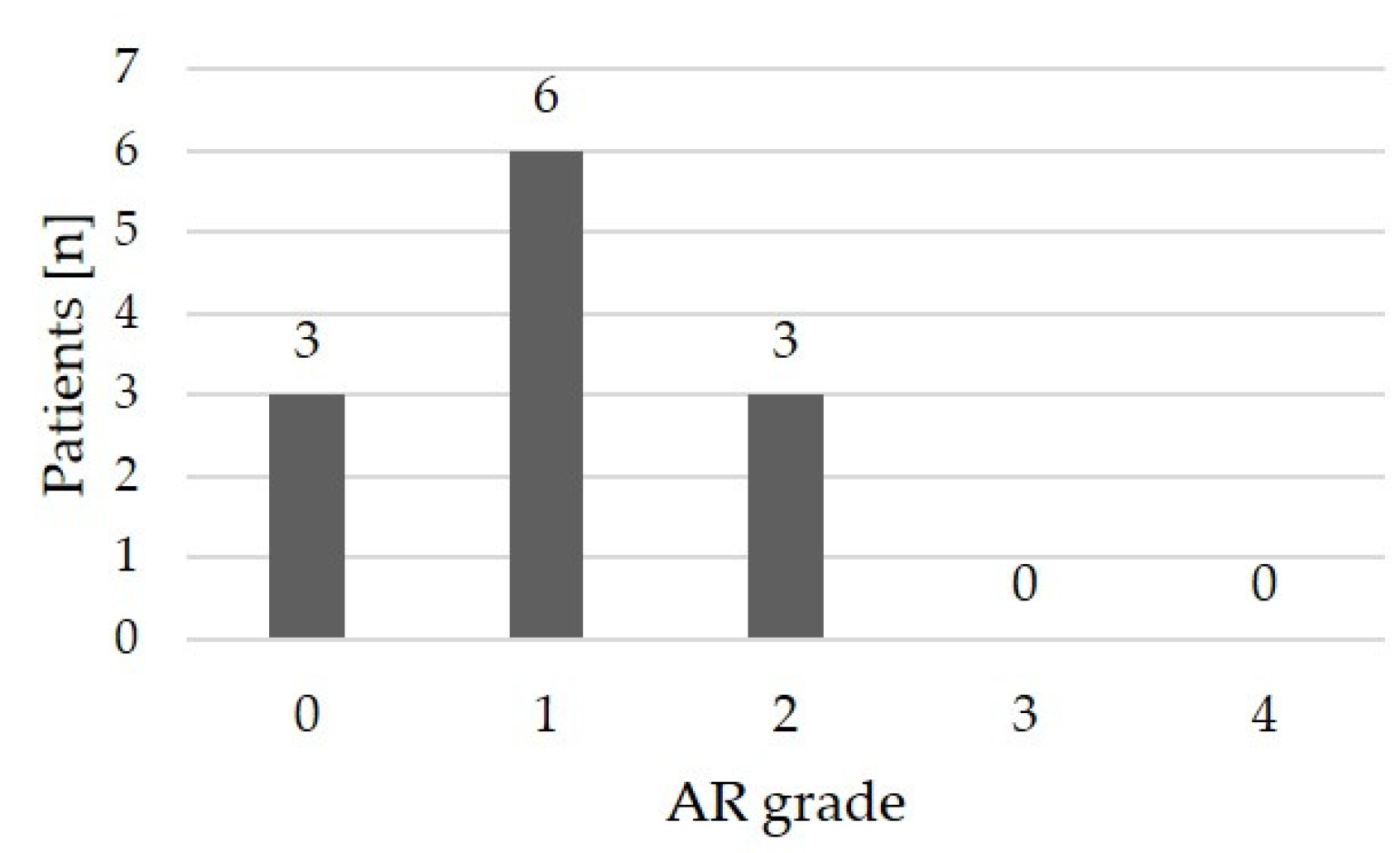
Echocardiographic assessment following the most recent BAV revealed no or only mild aortic regurgitation in 9/12 (75 %) patients; 3/12 (25 %) patients had moderate aortic regurgitation (
Table 2,
Figure 6). High-grade regurgitation did not occur.
3.3.2. Miscellaneous Complications
3.4. Follow Up
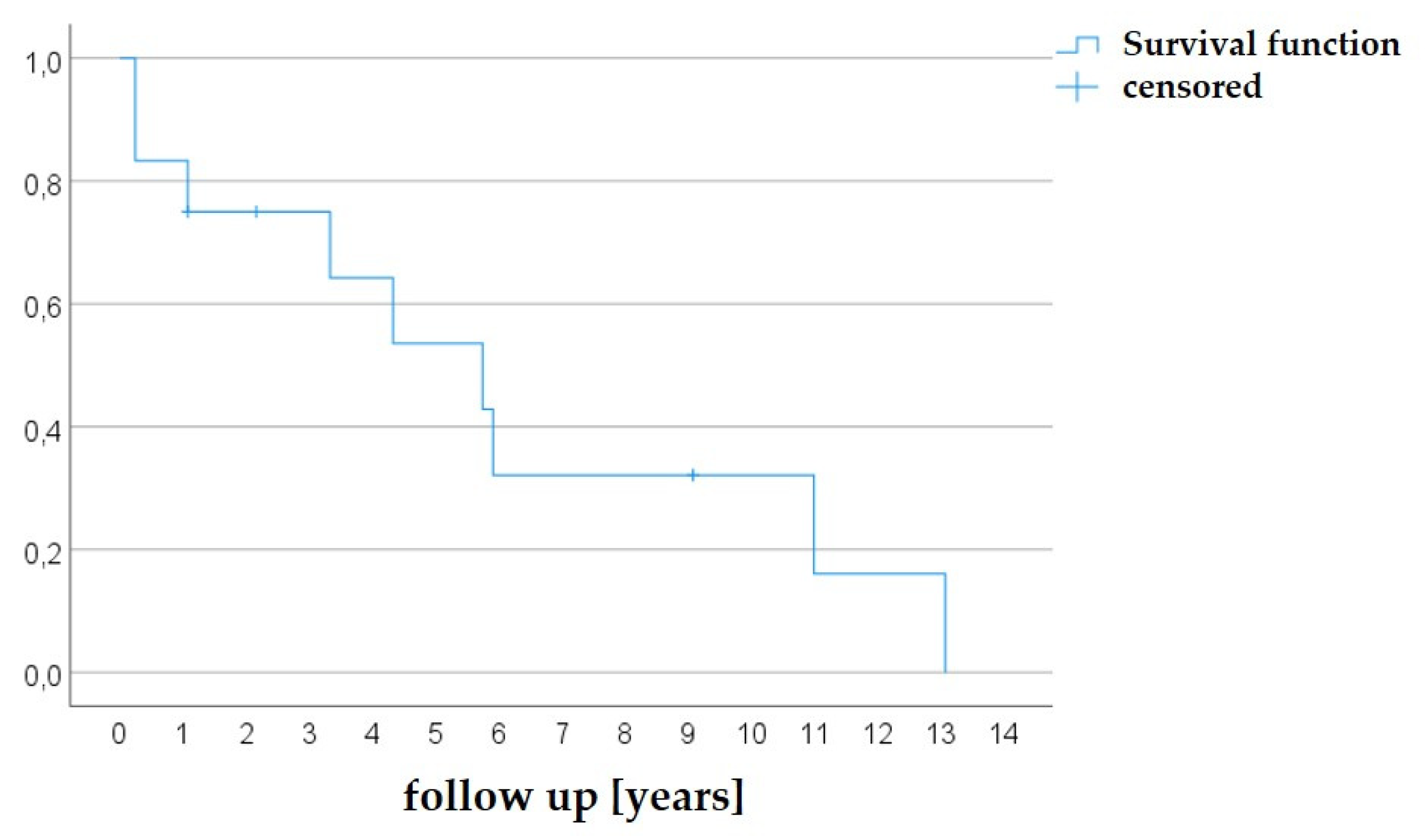
To date, 9/12 patients (75.0 %) had to undergo a surgical AV reconstruction due to progressive aortic regurgitation, aortic re-stenosis, or combined stenosis and regurgitation. The earliest time for an operation was after 3 months. Median time to surgery (Kaplan Meier estimator) was 5.75 years (
Figure 9).
4. Discussion
In case of CAVS, high-grade LV dysfunction with diminished contractility and consecutive low cardiac output usually lead to rapid clinical deterioration up to cardiogenic shock of the neonate [
9,
19]. Therefore, urgent intervention is indicated. Regarding the further prognosis, saving the depressed LV has the topmost priority. Both open heart surgery and transcatheter BAV are possible approaches for initial treatment [
10]. Nevertheless, the optimal first-line therapy is still controversial [
3].
In the present study, we analyzed a case series of 12 children with CAVS who underwent BAV. In all patients, aortic valve dysplasia in terms of either unicuspid or bicuspid AV was diagnosed. At the time of the first BAV, the median age was 3 days. In a recent study, Sylwestrzak et al. demonstrated that the best outcome is achieved if CAVS is diagnosed prenatally and postnatal BAV is performed within the first days of life [
20].
Nine patients (75.0 %) underwent a second BAV, two patients (16.7 %) needed a third one. Previous studies also described frequent need for re-intervention after successful first BAV, especially the younger the children are [
13,
21]. In our patients, both significant improvement of the LV function and significant reduction of the trans-aortic pressure gradient could be achieved. Petit et al. suggest that BAV is particularly successful in case of high-grade stenosis due to small valve orifice areas and unicuspid AVs [
22], which is typical for CAVS. Repeated BAV may be required because AV stenosis tends to recur following the initial BAV due to the recovery of the LV function and consecutively increasing LV output. The development of scar tissue may as well promote re-stenosis. Moreover, it is advisable to start with low balloon / annulus ratios using coronary balloon catheters to prevent AV injury and high-grade aortic regurgitation. The balloon diameters can then be increased gradually in order to achieve a stepwise enlargement of the AV orifice area. Usually recommended balloon / annulus ratios are 0.7 – 1.0. Nevertheless, higher ratios might be required for an effective dilatation due to the rigidity of severely dysplastic AVs [
8,
10,
14,
23]. In our analysis, maximum balloon / annulus ratio was 1.23.
By applying an interventional method as rescue procedure, cardiac surgery including cardio-pulmonary bypass could be avoided in neonates who are a particularly vulnerable cohort with high perioperative mortality [
24] that is even higher in case of cardiogenic shock. In our study, the estimated time to AV surgery was 5.75 years. Among others, severe left ventricular dysfunction is a risk factor for AV surgery following neonatal BAV according to Kido et al. [
25]. However, the gain of time until first surgery is worthwhile because patients with congenital AV stenosis are exposed to a high life-time risk for multiple AV operations including AV reconstruction, Ross procedure or AV prosthesis [
26,
27,
28,
29,
30]. Thus, future perioperative risks due to typical intrathoracic adhesions [
31,
32] might be reduced by postponing surgery to later childhood. On the other hand, there are data that valve replacement cannot be postponed by BAV [
33]. Moreover, some authors generally perform primary cardiac surgery [
34] because it offers a precise AV commissurotomy and reconstruction in contrast to arbitrary valve dilatation [
11] and a longer freedom of re-intervention [
35]. Hill et al. described no differences in long-term outcome for both, primary BAV and a surgical approach but a higher rate for re-interventions after BAV [
15]. However, both are palliative approaches and AV surgery will be necessary in most cases anyway. Our data support the hypothesis of rescue interventional procedure to improve the survival in critically ill neonates with primarily diminished LV contractility.
When performing BAV, probing of highly restrictive AVs by catheter often is challenging. We describe a technique to provide more support for the angiographic catheter by inserting it via guide wire and an additional microcatheter. If a retrograde approach is unfeasible, trans-venous antegrade catheterization should be considered which provides equally effective results [
36]. Here, a stable and safe positioning of the balloon during BAV can be technically delicate. For this purpose, it is useful to fixate the guiding wire in the descending aorta using a retrieval loop as first described by Rao et al. [
37] in a trans-umbilical approach. The antegrade approach is associated with mitral valve injury whereas there is risk for arterial thrombosis in retrograde catheterization [
36,
38]. Both did not occur in our population. In our study, few BAV-associated complications occurred. The greatest risk exists for the development of aortic regurgitation especially in repeated BAV [
21]. In 75.0 % of our patients, no or mild aortic regurgitation was detectable, and in 25.0 % moderate regurgitation. There was no case of severe aortic valve insufficiency which usually only increases over time according to Rao [
39]. Another adverse event was an air embolism into the LV. Due to the poor LV function with a significantly reduced contractility, the air remained in the LV without embolization into systemic arteries. Therefore, the bubble could be aspirated by catheter. The most remarkable complication was an intimal injury of the distal ascending aorta which was also described as typical in a study by Brown et al. [
40]. In our case, the lesion was electively corrected in combination with the surgical AV reconstruction. There was no mortality peri-interventionally or in the follow-up in our study.
Limitations of our study are the retrospective approach as well as the limited number of patients which is based on either the low incidence of CAVS and the single center analysis.
5. Conclusions
In critical aortic valve stenosis, balloon valvuloplasty is a successful primary treatment resulting in prompt left ventricular relief and stabilization of the critically ill neonate. Thereby, cardiac surgery can be avoided during the neonatal period and postponed until later childhood. No mortality was observed in our cohort. Thus, transcatheter approach represents an effective emergency, yet palliative rescue procedure for the LV with an acceptable low rate of complications. Nevertheless, it seems advisable to choose the first-line therapy according to the experience of the respective treatment team.
Author Contributions
Conceptualization, J.P. and H.A.-K.; methodology, J.P., A.R., M.P. and H.A.-K.; validation, J.P., A.R., M.P. and H.A.-K.; formal analysis, J.P., M.P.; investigation, J.P., A.R., M.P. and H.A.-K.; resources, H.A.-K.; data curation, J.P.; writing—original draft preparation, J.P.; writing—review and editing, J.P., A.R., M.P. and H.A.-K; visualization, J.P.; supervision, H.A-K.; project administration, J.P. and H.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved on 6 March 2024 by the local ethics committee of the Saarland, Saarbruecken, Germany (file number 37/24).
Informed Consent Statement
Informed consent for BAV was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying the present study are available on request (corresponding author).
Acknowledgments
We thank Gudrun Wagenpfeil (Institute of Medical Biometry, Epidemiology and Medical Informatics, Saarland University) for her help with the statistical analysis.
Conflicts of Interest
The authors declare no conflicts of interest
References
- Lindinger, A., G. Schwedler, and H.W. Hense, Prevalence of congenital heart defects in newborns in Germany: Results of the first registration year of the PAN Study (July 2006 to June 2007). Klin Padiatr, 2010, 222, 321-6. 20 July.
- Singh, G.K., Congenital Aortic Valve Stenosis. Children (Basel), 2019, 6(5).
- Olofsson, C.K., et al., Outcomes in neonatal critical and non-critical aortic stenosis: a retrospective cohort study. Arch Dis Child, 2023, 108, 398-404.
- Schulz, A. , et al., Aortic Valve Repair in Neonates With Aortic Stenosis and Reduced Left Ventricular Function. Semin Thorac Cardiovasc Surg, 2023, 35, 713–721. [Google Scholar] [CrossRef]
- Aldawsari, K.A. , et al., Endocardial fibroelastosis in infants and young children: a state-of-the-art review. Heart Fail Rev, 2023, 28, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Tulzer, A., W. Arzt, and G. Tulzer, Fetal aortic valvuloplasty may rescue fetuses with critical aortic stenosis and hydrops. Ultrasound Obstet Gynecol, 2021, 57, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Freud, L.R. , et al., Low rate of prenatal diagnosis among neonates with critical aortic stenosis: insight into the natural history in utero. Ultrasound Obstet Gynecol, 2015, 45, 326–32. [Google Scholar] [CrossRef]
- Hraska, V. and M. Schneider, Critical aortic stenosis with severe left ventricular dysfunction. Eur J Cardiothorac Surg, 2013, 43, 148–9. [Google Scholar] [CrossRef]
- Affolter, J.T. and N.S. Ghanayem, Preoperative management of the neonate with critical aortic valvar stenosis. Cardiol Young, 2014, 24, 1111–6. [Google Scholar] [CrossRef] [PubMed]
- Zain, Z. , et al., Neonatal isolated critical aortic valve stenosis: balloon valvuloplasty or surgical valvotomy. Heart Lung Circ, 2006, 15, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Hraška, V. , Neonatal Aortic Stenosis Is a Surgical Disease. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu, 2016, 19, 2–5. [Google Scholar] [CrossRef]
- Benson, L. , Neonatal Aortic Stenosis is a Surgical Disease: An Interventional Cardiologist View. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu, 2016, 19, 6–9. [Google Scholar] [CrossRef]
- McElhinney, D.B. , et al., Left heart growth, function, and reintervention after balloon aortic valvuloplasty for neonatal aortic stenosis. Circulation, 2005, 111, 451–8. [Google Scholar] [CrossRef]
- Stapleton, G.E. , Transcatheter management of neonatal aortic stenosis. Cardiol Young, 2014, 24, 1117–20. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.D., et al., Surgical Valvotomy Versus Balloon Valvuloplasty for Congenital Aortic Valve Stenosis: A Systematic Review and Meta-Analysis. J Am Heart Assoc, 2016, 5(8).
- Lababidi, Z., J. R. Wu, and J.T. Walls, Percutaneous balloon aortic valvuloplasty: results in 23 patients. Am J Cardiol, 1984, 53, 194–7. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M. , et al., Nomograms for two-dimensional echocardiography derived valvular and arterial dimensions in Caucasian children. J Cardiol, 2017, 69, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M. , et al., Echocardiographic nomograms for chamber diameters and areas in Caucasian children. J Am Soc Echocardiogr, 2014, 27, 1279–92. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M. , et al., Acute therapy of newborns with critical congenital heart disease. Transl Pediatr, 2019, 8, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Sylwestrzak, O. , et al., Fetal echocardiography and early neonatal balloon valvuloplasty improved overall survival in prenatally detected aortic stenosis over 25 years of tertiary center experience. J Clin Ultrasound, 2022, 50, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.J. , et al., Repeat balloon aortic valvuloplasty effectively delays surgical intervention in children with recurrent aortic stenosis. Catheter Cardiovasc Interv, 2013, 82, 549–55. [Google Scholar] [CrossRef]
- Petit, C.J. , et al., Relation of Aortic Valve Morphologic Characteristics to Aortic Valve Insufficiency and Residual Stenosis in Children With Congenital Aortic Stenosis Undergoing Balloon Valvuloplasty. Am J Cardiol, 2016, 117, 972–9. [Google Scholar] [CrossRef]
- Varan, B. , et al., Aortic balloon valvuloplasty and mid-term results in newborns: a single center experience. Turk J Pediatr, 2020, 62, 233–243. [Google Scholar] [CrossRef]
- Bobillo-Perez, S. , et al., Risk stratification models for congenital heart surgery in children: Comparative single-center study. Congenit Heart Dis, 2019, 14, 1066–1077. [Google Scholar] [CrossRef]
- Kido, T. , et al., Aortic Valve Surgery After Neonatal Balloon Aortic Valvuloplasty in Congenital Aortic Stenosis. Circ Cardiovasc Interv, 2021, 14, e009933. [Google Scholar] [CrossRef]
- Vida, V.L. , et al., Critical aortic stenosis in early infancy: surgical treatment for residual lesions after balloon dilation. Ann Thorac Surg, 2005, 79, 47–51. [Google Scholar] [CrossRef]
- Tyc, F. , et al., Balloon aortic valvuloplasty in neonates: short- and long-term effects and predictors of successful outcome. Postepy Kardiol Interwencyjnej, 2022, 18, 154–161. [Google Scholar] [PubMed]
- Wallace, F. , et al., Long-term outcomes of primary aortic valve repair for isolated congenital aortic stenosis in children. J Thorac Cardiovasc Surg, 2022, 164, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Kjellberg Olofsson, C. , et al., Treatment of valvular aortic stenosis in children: a 20-year experience in a single institution. Interact Cardiovasc Thorac Surg, 2018, 27, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Auld, B. , et al., Balloon Aortic Valvuloplasty for Congenital Aortic Stenosis: A 14-Year Single Centre Review. Heart Lung Circ, 2019, 28, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y. , et al., Adhesion barrier reduces postoperative adhesions after cardiac surgery. Asian Cardiovasc Thorac Ann, 2012, 20, 257–62. [Google Scholar] [CrossRef]
- Walther, T. , et al., A novel adhesion barrier facilitates reoperations in complex congenital cardiac surgery. J Thorac Cardiovasc Surg, 2005, 129, 359–63. [Google Scholar] [CrossRef]
- Vergnat, M. , et al., Aortic stenosis of the neonate: A single-center experience. J Thorac Cardiovasc Surg, 2019, 157, 318–326. [Google Scholar] [CrossRef]
- Kjellberg Olofsson, C. , et al., A national study of the outcome after treatment of critical aortic stenosis in the neonate. Cardiol Young, 2020, 30, 1321–1327. [Google Scholar] [CrossRef]
- Siddiqui, J. , et al., Surgical valvotomy and repair for neonatal and infant congenital aortic stenosis achieves better results than interventional catheterization. J Am Coll Cardiol, 2013, 62, 2134–40. [Google Scholar] [CrossRef] [PubMed]
- Mozumdar, N. , et al., A Comparison of Anterograde Versus Retrograde Approaches for Neonatal Balloon Aortic Valvuloplasty. Pediatr Cardiol, 2018, 39, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Rao, S. and S.B. Jureidini, Transumbilical venous, anterograde, snare-assisted balloon aortic valvuloplasty in a neonate with critical aortic stenosis. Cathet Cardiovasc Diagn, 1998, 45, 144–8. [Google Scholar] [CrossRef]
- Magee, A.G. , et al., Balloon dilation of severe aortic stenosis in the neonate: comparison of anterograde and retrograde catheter approaches. J Am Coll Cardiol, 1997, 30, 1061–6. [Google Scholar] [CrossRef] [PubMed]
- Rao, S. , Balloon aortic valvuloplasty. Indian Heart J, 2016, 68, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W. , et al., Aortic wall injury as a complication of neonatal aortic valvuloplasty: incidence and risk factors. Circ Cardiovasc Interv, 2008, 1, 53–9. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).