Submitted:
14 March 2024
Posted:
15 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
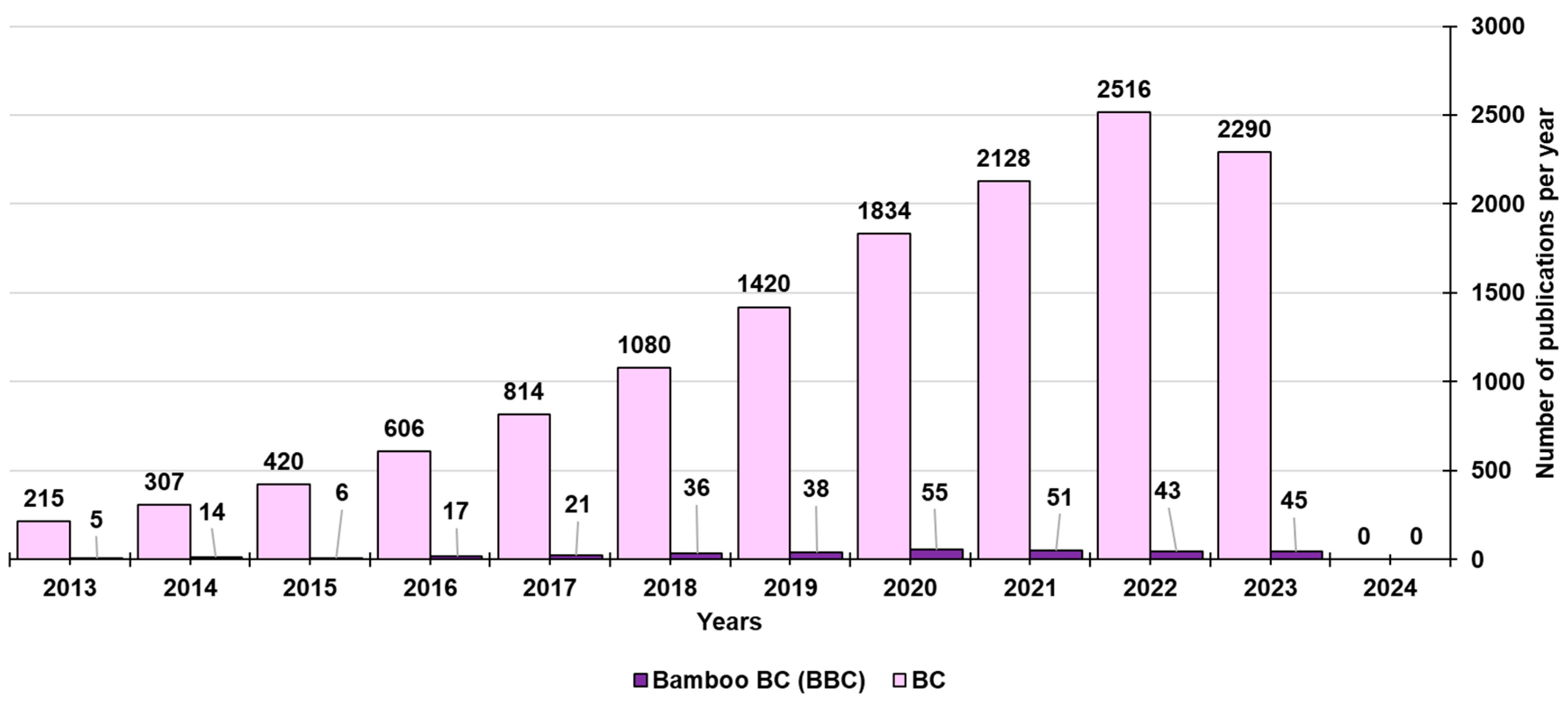
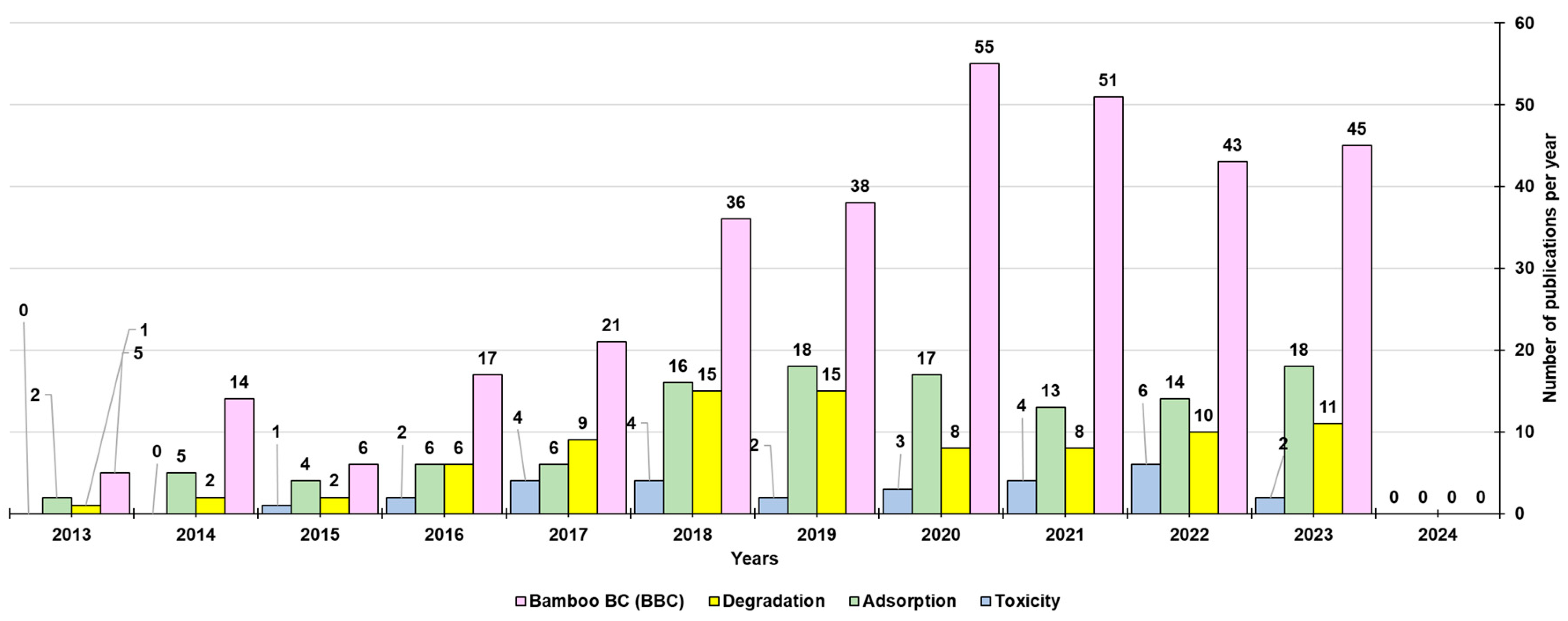
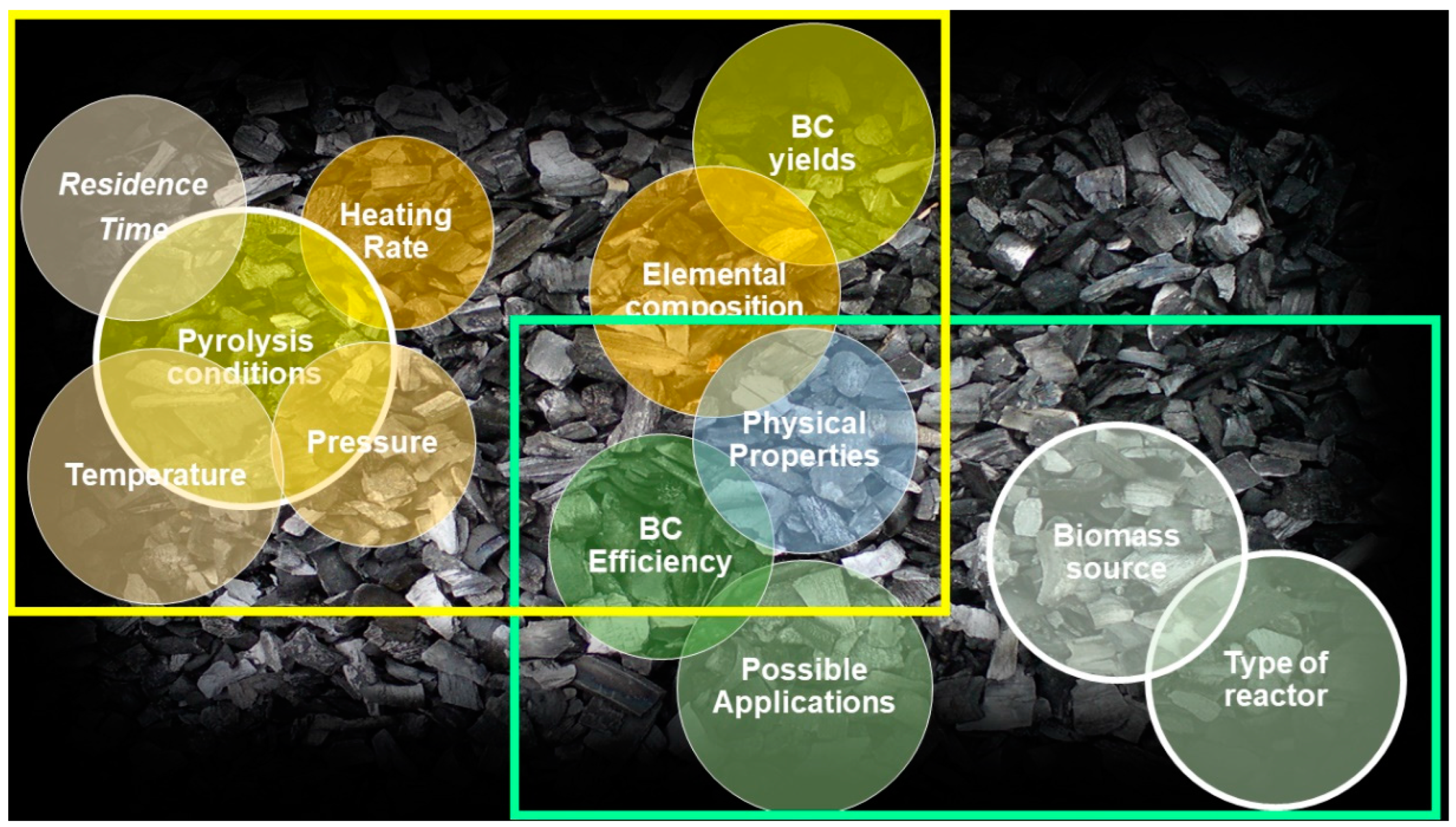
2. Biochar (BC)
2.1. Main Methods to Produce Biochar
2.1.1. Pyrolysis
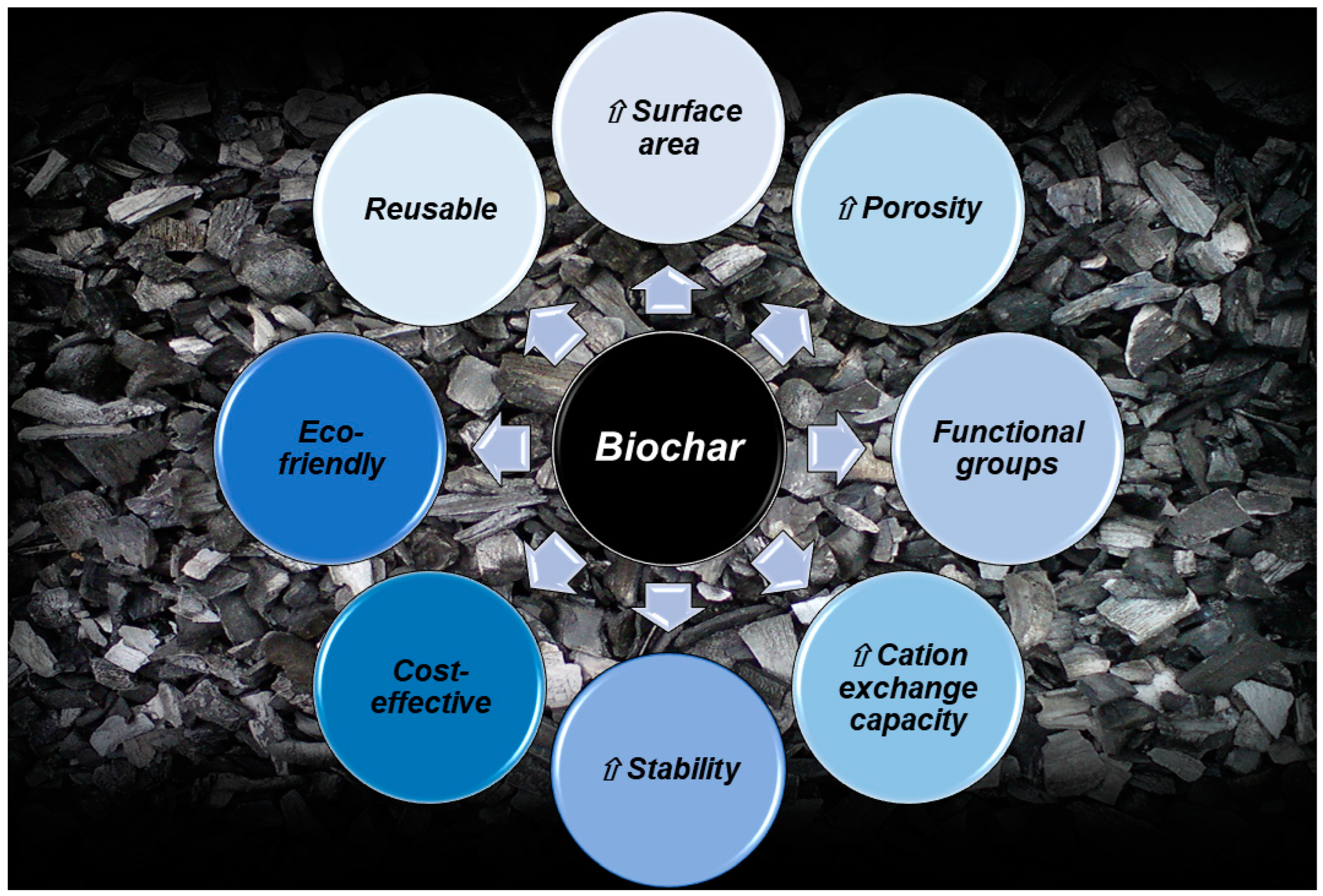
2.2. Biochar Characterization and Main Properties
2.2.1. The Question of Temperature
2.3. Possible Biochar Applications
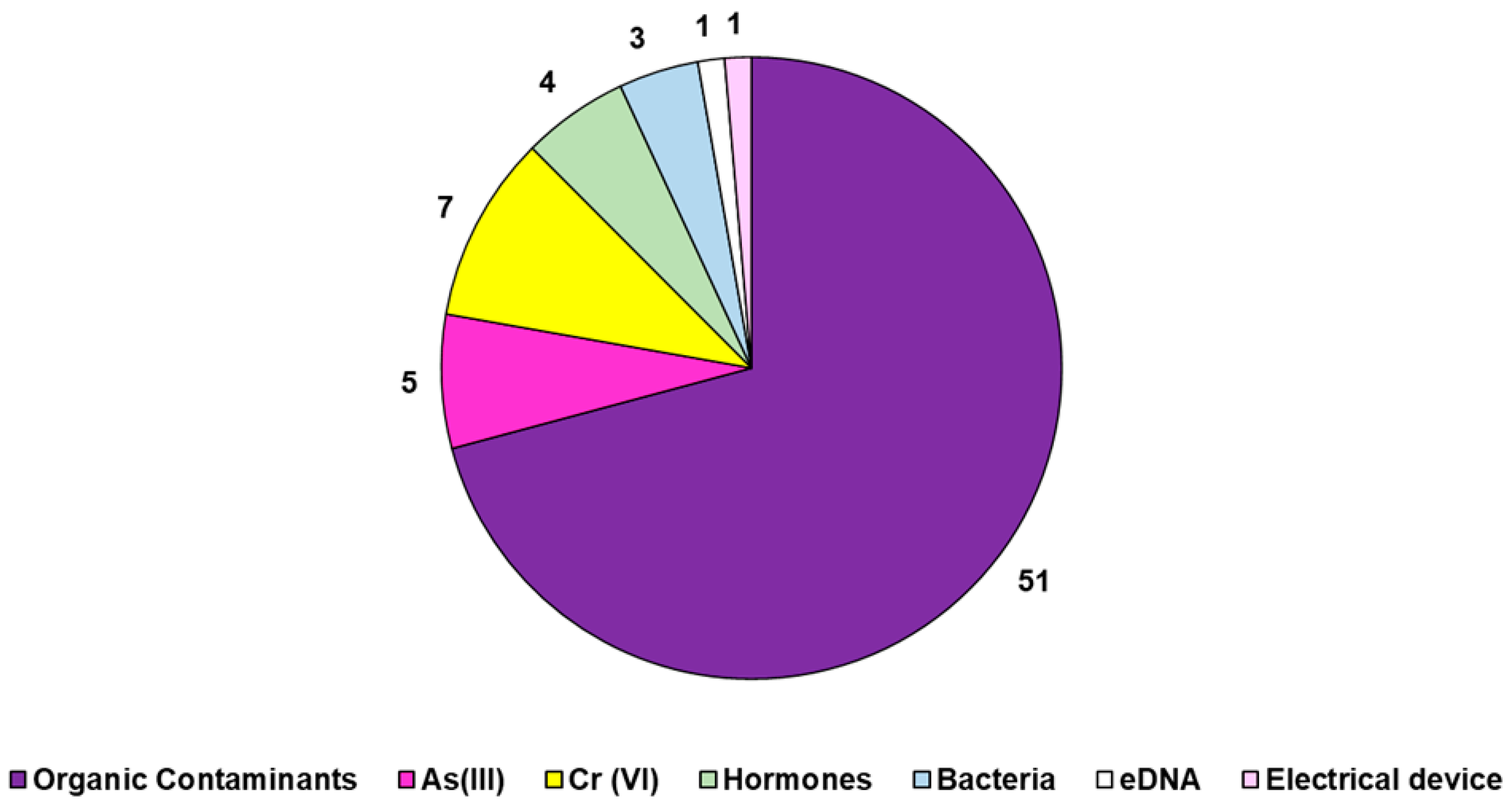
2.3.1. Xenobiotics Removal by Biochar (BC)
2.3.2. Not Only Adsorption
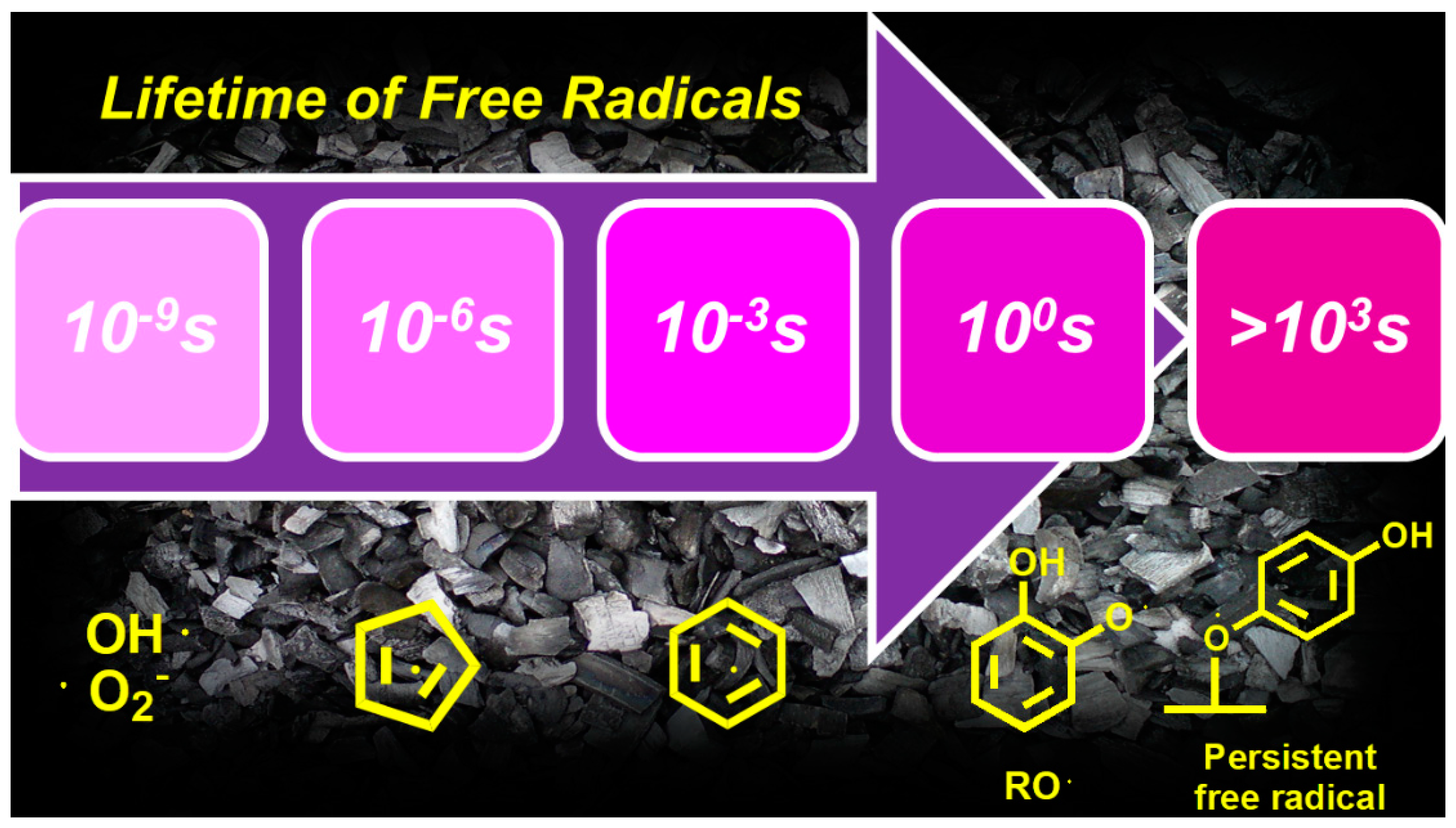
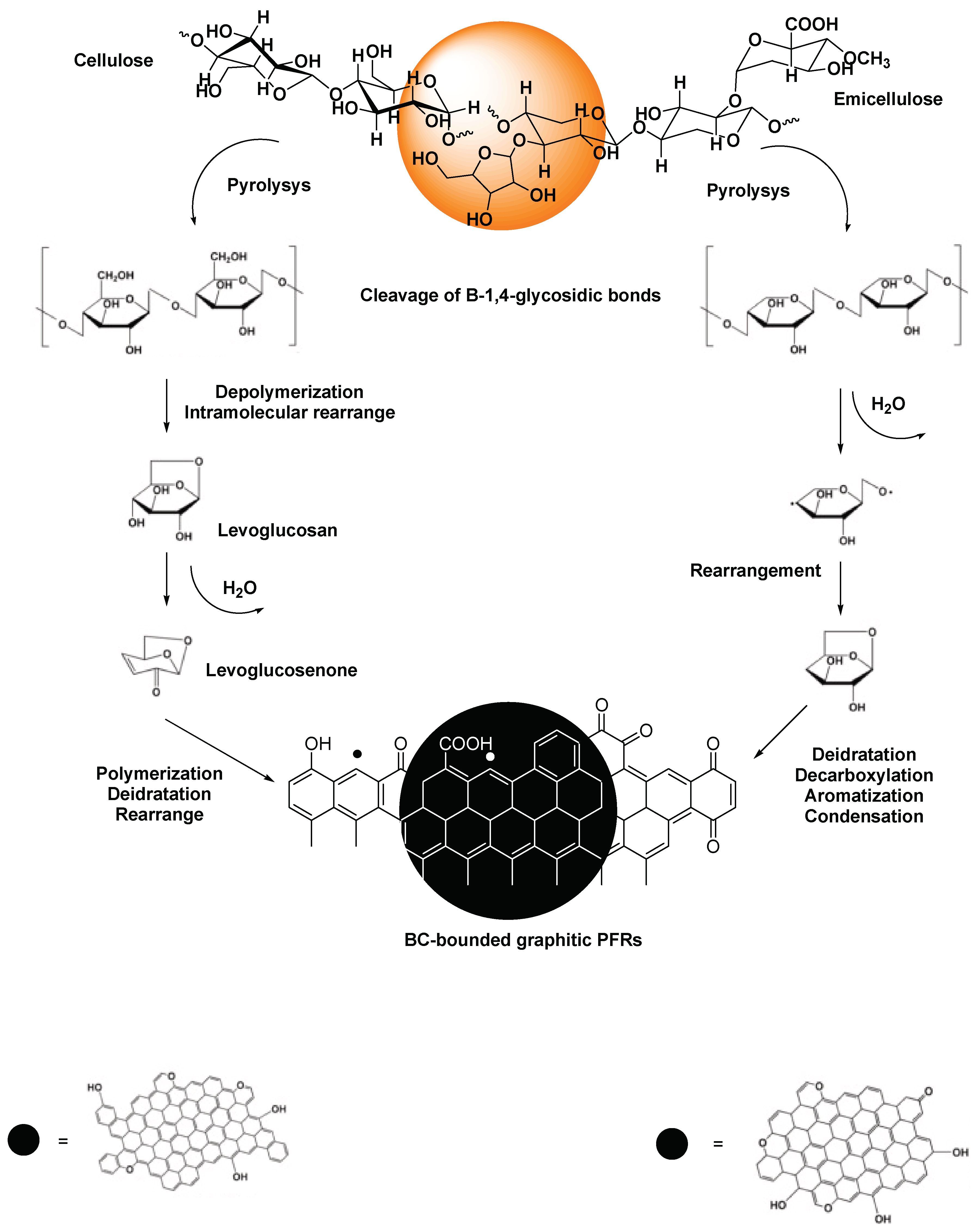
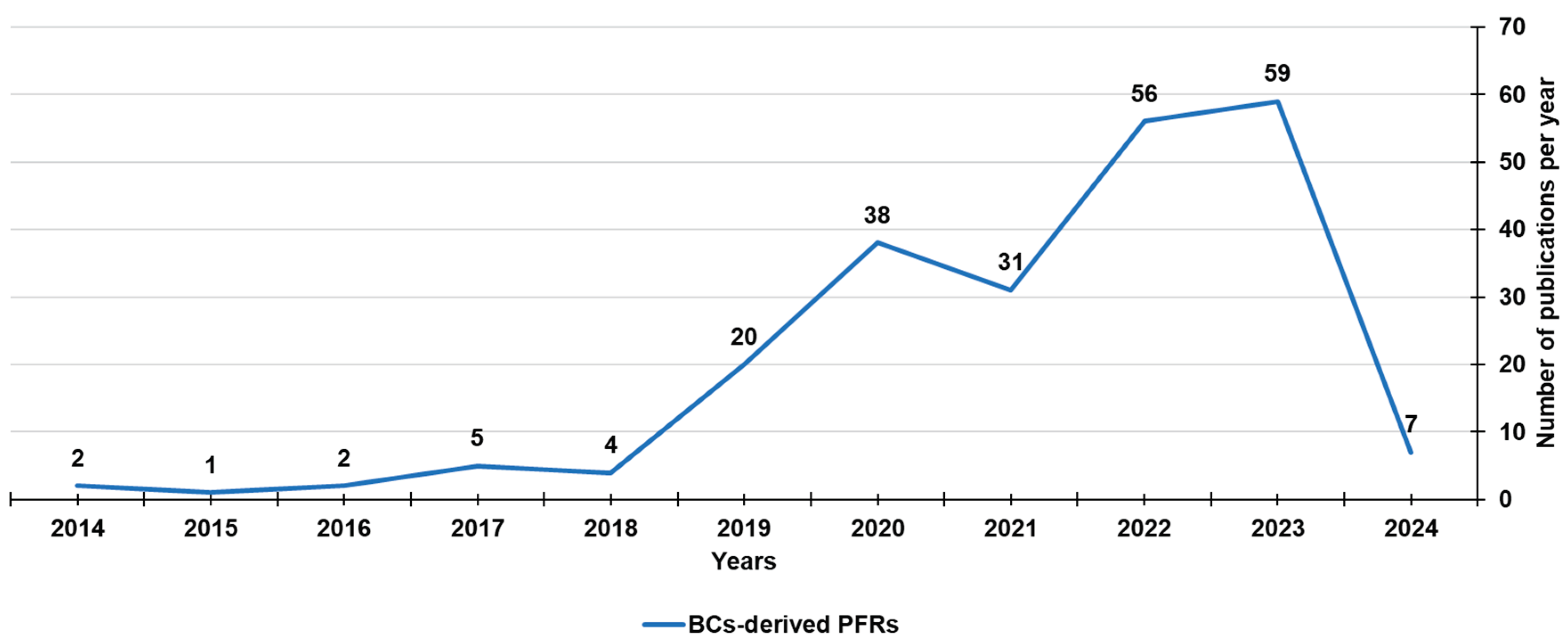
3. Biochar-Derived Free Radicals
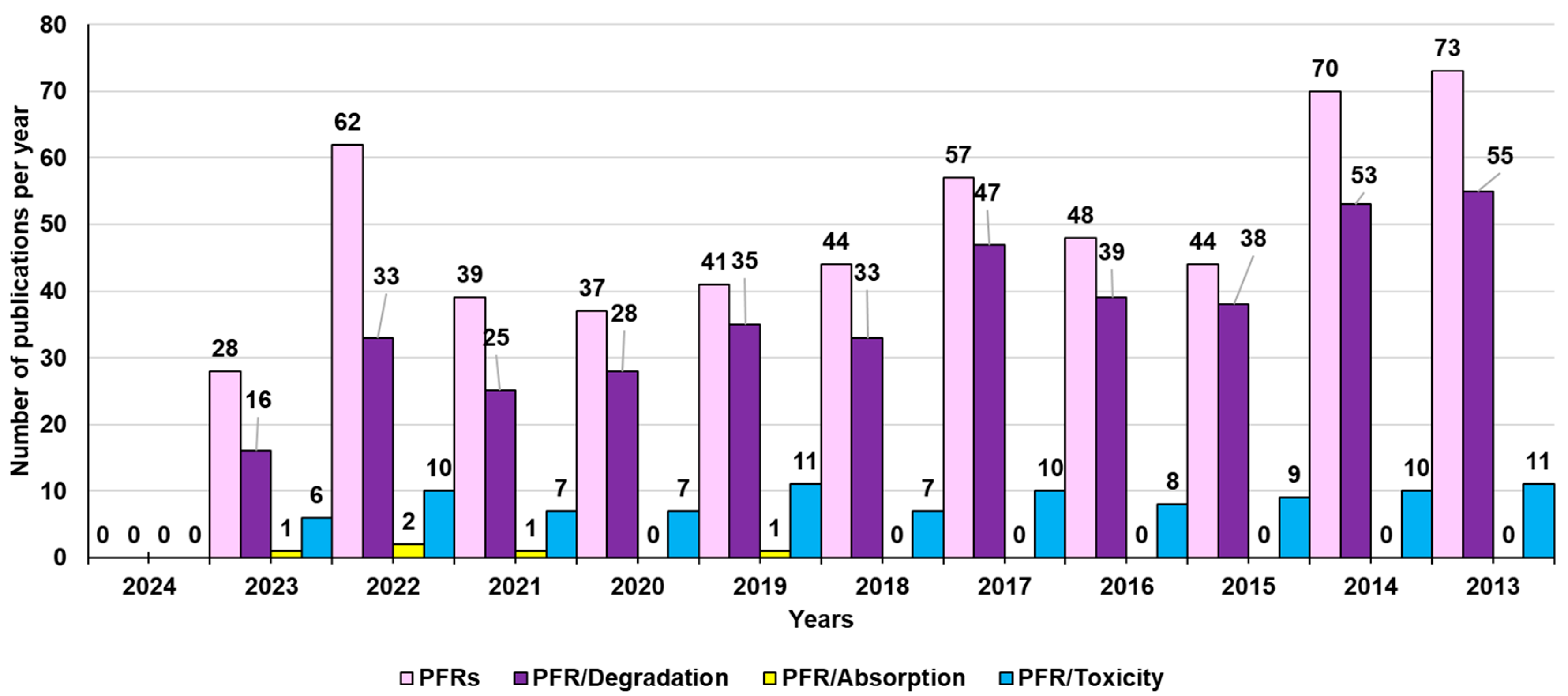
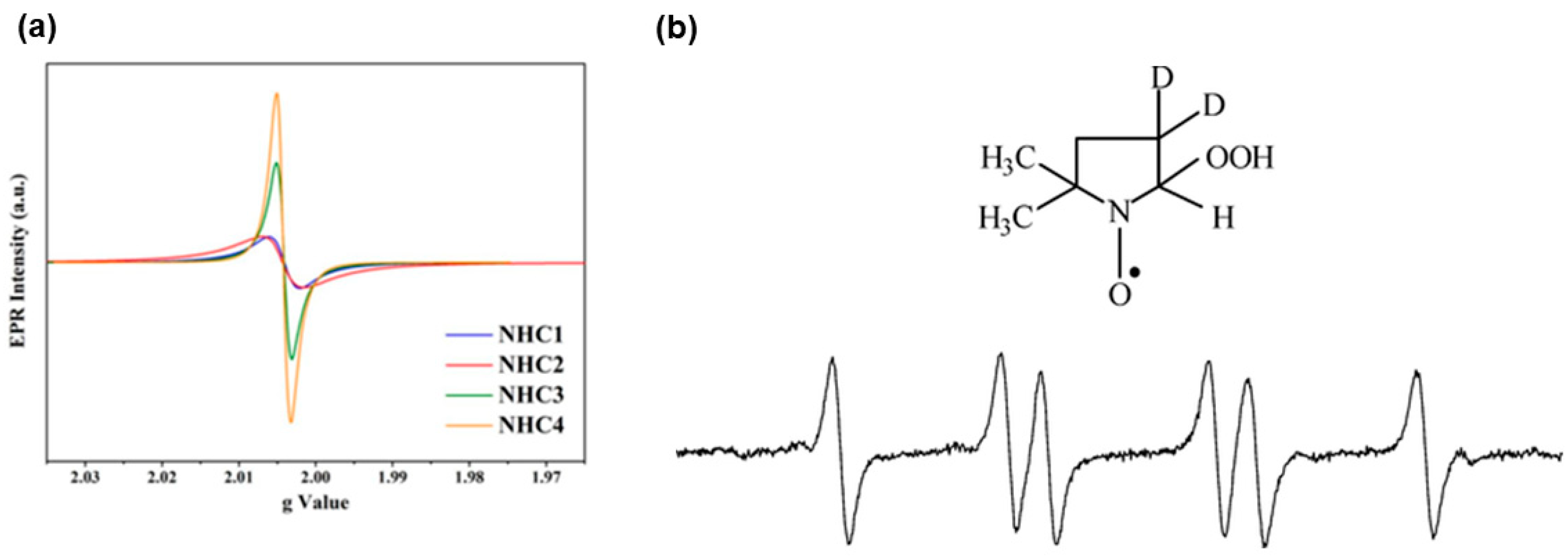
3.1. Persistent Free Radicals (PFRs)
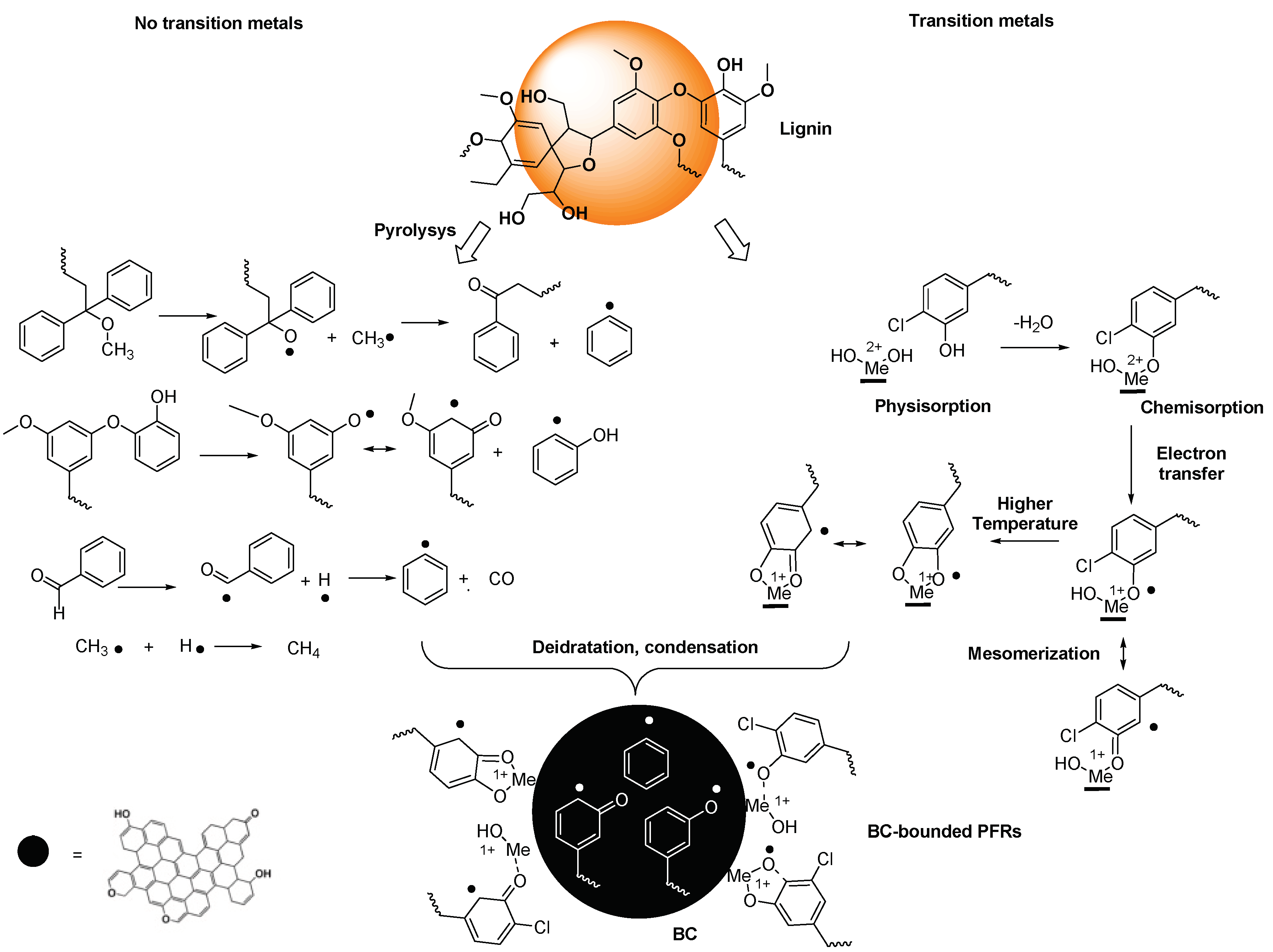
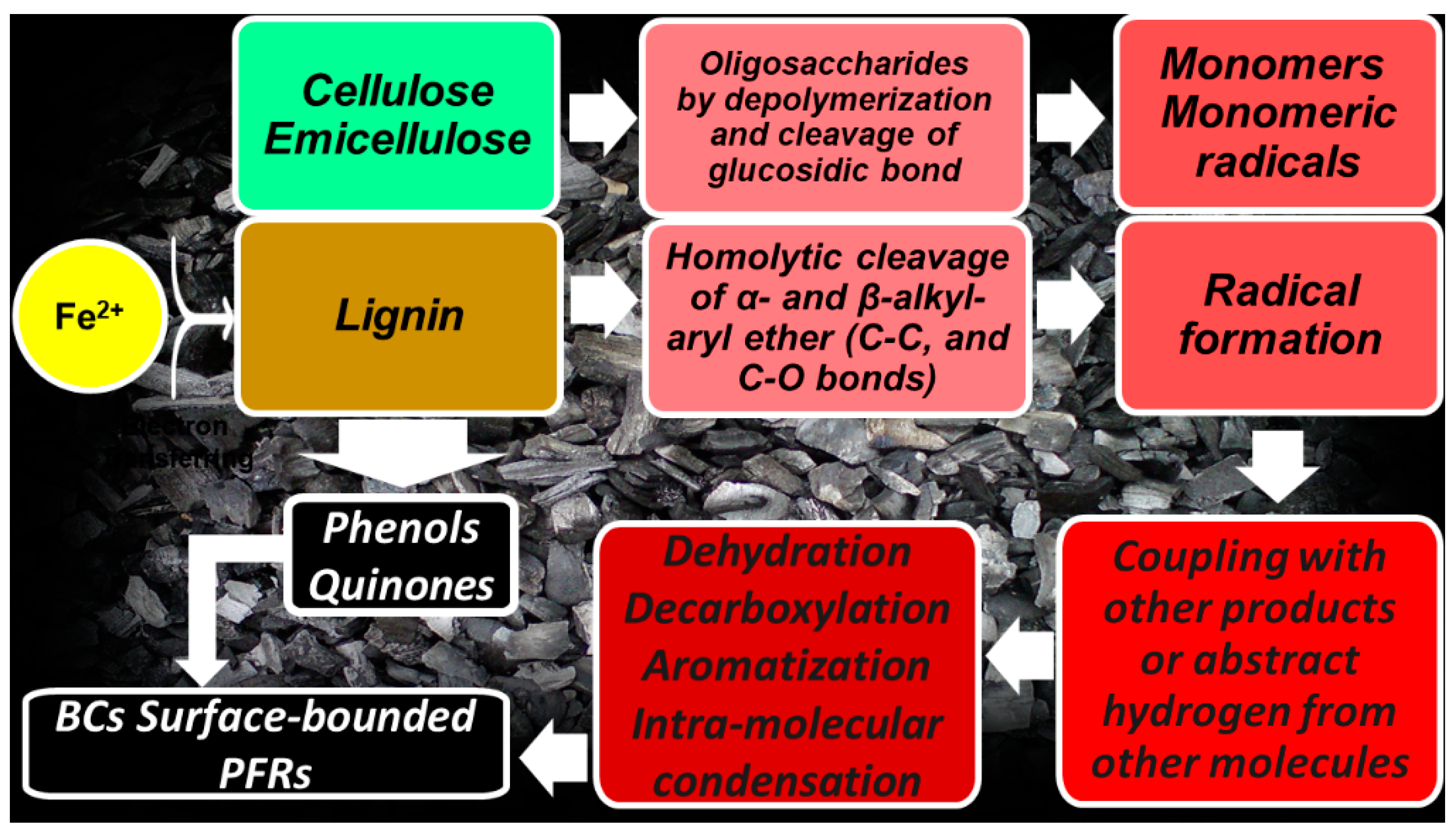
3.1.1. Mechanism Proposed for PFRs Formation during Biomass Pyrolysis
3.2. PFRs: Lights and Shadows
3.2.1. PFRs Lights
3.2.2. BC-Associated PFRs Shadows: Cytotoxicity and Biotoxicity
4. Risk Prevention Strategies and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhang, R.; Zhang, R.; Zimmerman, A.R.; Wang, H.; Gao, B. Applications, Impacts, and Management of Biochar Persistent Free Radicals: A Review. Environmental Pollution 2023, 327, 121543. [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification, and Environmental Application of Biochar: A Review. Journal of Cleaner Production 2019, 227, 1002–1022. [CrossRef]
- Premarathna, K.S.D.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-Based Engineered Composites for Sorptive Decontamination of Water: A Review. Chemical Engineering Journal 2019, 372, 536–550. [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A Review of Biochars’ Potential Role in the Remediation, Revegetation and Restoration of Contaminated Soils. Environmental Pollution 2011, 159, 3269–3282. [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agriculture, Ecosystems & Environment 2011, 144, 175–187. [CrossRef]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrologic Properties of Biochars Produced at Different Temperatures. Biomass and Bioenergy 2012, 41, 34–43. [CrossRef]
- Qin, Y.; Li, G.; Gao, Y.; Zhang, L.; Ok, Y.S.; An, T. Persistent Free Radicals in Carbon-Based Materials on Transformation of Refractory Organic Contaminants (ROCs) in Water: A Critical Review. Water Research 2018, 137, 130–143. [CrossRef]
- Vejerano, E.P.; Rao, G.; Khachatryan, L.; Cormier, S.A.; Lomnicki, S. Environmentally Persistent Free Radicals: Insights on a New Class of Pollutants. Environ. Sci. Technol. 2018, 52, 2468–2481. [CrossRef]
- Volpe, R.; Bermudez Menendez, J.M.; Ramirez Reina, T.; Volpe, M.; Messineo, A.; Millan, M.; Titirici, M.-M. Free Radicals Formation on Thermally Decomposed Biomass. Fuel 2019, 255, 115802. [CrossRef]
- Huang, Y.; Guo, X.; Ding, Z.; Chen, Y.; Hu, X. Environmentally Persistent Free Radicals in Biochar Derived from Laminaria Japonica Grown in Different Habitats. Journal of Analytical and Applied Pyrolysis 2020, 151, 104941. [CrossRef]
- Ruan, X.; Sun, Y.; Du, W.; Tang, Y.; Liu, Q.; Zhang, Z.; Doherty, W.; Frost, R.L.; Qian, G.; Tsang, D.C.W. Formation, Characteristics, and Applications of Environmentally Persistent Free Radicals in Biochars: A Review. Bioresource Technology 2019, 281, 457–468. [CrossRef]
- Wang, R.-Z.; Huang, D.-L.; Liu, Y.-G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.-M.; Gong, X.-M.; Duan, A.; Zhang, Q.; et al. Recent Advances in Biochar-Based Catalysts: Properties, Applications and Mechanisms for Pollution Remediation. Chemical Engineering Journal 2019, 371, 380–403. [CrossRef]
- Odinga, E.S.; Waigi, M.G.; Gudda, F.O.; Wang, J.; Yang, B.; Hu, X.; Li, S.; Gao, Y. Occurrence, Formation, Environmental Fate and Risks of Environmentally Persistent Free Radicals in Biochars. Environment International 2020, 134, 105172. [CrossRef]
- Fang, G.; Liu, C.; Gao, J.; Dionysiou, D.D.; Zhou, D. Manipulation of Persistent Free Radicals in Biochar To Activate Persulfate for Contaminant Degradation. Environ. Sci. Technol. 2015, 49, 5645–5653. [CrossRef]
- He, J.; Xiao, Y.; Tang, J.; Chen, H.; Sun, H. Persulfate Activation with Sawdust Biochar in Aqueous Solution by Enhanced Electron Donor-Transfer Effect. Science of The Total Environment 2019, 690, 768–777. [CrossRef]
- Wang, H.Z.; Guo, W.Q.; Yin, R.L.; Du, J.S.; Wu, Q.L.; Luo, H.C.; Liu, B.H.; Sseguya, F.; Ren, N.Q. Biochar-induced Fe(III) reduction for persulfate activation in sulfamethoxazole degradation: insight into the electron transfer, radical oxidation and degradation pathways. Chem. Eng. J. 2019, 362, 561-569.
- Liang, J.; Xu, X.; Qamar Zaman, W.; Hu, X.; Zhao, L.; Qiu, H.; Cao, X. Different Mechanisms between Biochar and Activated Carbon for the Persulfate Catalytic Degradation of Sulfamethoxazole: Roles of Radicals in Solution or Solid Phase. Chemical Engineering Journal 2019, 375, 121908. [CrossRef]
- Fang, G.; Gao, J.; Liu, C.; Dionysiou, D.D.; Wang, Y.; Zhou, D. Key Role of Persistent Free Radicals in Hydrogen Peroxide Activation by Biochar: Implications to Organic Contaminant Degradation. Environ. Sci. Technol. 2014, 48, 1902–1910. [CrossRef]
- Fang, G.; Zhu, C.; Dionysiou, D.D.; Gao, J.; Zhou, D. Mechanism of Hydroxyl Radical Generation from Biochar Suspensions: Implications to Diethyl Phthalate Degradation. Bioresource Technology 2015, 176, 210–217. [CrossRef]
- Fang, G.; Liu, C.; Wang, Y.; Dionysiou, D.D.; Zhou, D. Photogeneration of Reactive Oxygen Species from Biochar Suspension for Diethyl Phthalate Degradation. Applied Catalysis B: Environmental 2017, 214, 34–45. [CrossRef]
- Luo, K.; Yang, Q.; Pang, Y.; Wang, D.; Li, X.; Lei, M.; Huang, Q. Unveiling the Mechanism of Biochar-Activated Hydrogen Peroxide on the Degradation of Ciprofloxacin. Chemical Engineering Journal 2019, 374, 520–530. [CrossRef]
- Zhang, Y.; Xu, M.; He, R.; Zhao, J.; Kang, W.; Lv, J. Effect of Pyrolysis Temperature on the Activated Permonosulfate Degradation of Antibiotics in Nitrogen and Sulfur-Doping Biochar: Key Role of Environmentally Persistent Free Radicals. Chemosphere 2022, 294, 133737. [CrossRef]
- Liao, S.; Pan, B.; Li, H.; Zhang, D.; Xing, B. Detecting Free Radicals in Biochars and Determining Their Ability to Inhibit the Germination and Growth of Corn, Wheat and Rice Seedlings. Environ. Sci. Technol. 2014, 48, 8581–8587. [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [CrossRef]
- Qin, J.; Chen, Q.; Sun, M.; Sun, P.; Shen, G. Pyrolysis Temperature-Induced Changes in the Catalytic Characteristics of Rice Husk-Derived Biochar during 1,3-Dichloropropene Degradation. Chemical Engineering Journal 2017, 330, 804–812. [CrossRef]
- Yang, J.; Pignatello, J.J.; Pan, B.; Xing, B. Degradation of P-Nitrophenol by Lignin and Cellulose Chars: H2O2-Mediated Reaction and Direct Reaction with the Char. Environ. Sci. Technol. 2017, 51, 8972–8980. [CrossRef]
- Ginoble Pandoli, O.; Santos de Sá, D.; Nogueira Barbosa Junior, M.; Paciornik, S. Bamboo-Based Lignocellulose Biomass as Catalytic Support for Organic Synthesis and Water Treatments. In: Palombini, F.L.; Nogueira, F.M. (eds) Bamboo Science and Technology. Environmental Footprints and Eco-design of Products and Processes. 2023, Springer, Singapore, pp. 297-327. [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis —A Technological Review. Energies 2012, 5, 4952–5001. [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S.J.; Saravanan, A. A Review on Photochemical, Biochemical and Electrochemical Transformation of CO2 into Value-Added Products. Journal of CO2 Utilization 2019, 33, 131–147. [CrossRef]
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A Review on Biomass as a Fuel for Boilers. Renewable and Sustainable Energy Reviews 2011, 15, 2262–2289. [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnology Reports 2020, 28, e00570. [CrossRef]
- Lehmann, J. Biochar for environmental management: an introduction. Biochar for Environmental Management. Sci. Technol. 2009, 25, 15801-15811.
- Mohanty, S.K.; Valenca, R.; Berger, A.W.; Yu, I.K.M.; Xiong, X.; Saunders, T.M.; Tsang, D.C.W. Plenty of Room for Carbon on the Ground: Potential Applications of Biochar for Stormwater Treatment. Science of The Total Environment 2018, 625, 1644–1658. [CrossRef]
- Ayla Norris Smidth. A New Boost for Biochar as a Natural Climate Solution. 2023. Available online at https://blog.nature.org/science-brief/a-new-boost-for-biochar-as-a-natural-climate-solution/#:~:text=Biochar%20is%20a%20type%20of%20charcoal%20made%20from,other%20biomass%20would%2C%20leading%20to%20more%20carbon%20sequestration (accessed on December 27, 2023).
- Yu, D.; Yu, Y.; Tang, J.; Li, X.; Ke, C.; Yao, Z. Application Fields of Kitchen Waste Biochar and Its Prospects as Catalytic Material: A Review. Science of The Total Environment 2022, 810, 152171. [CrossRef]
- Yu, S.; Zhang, W.; Dong, X.; Wang, F.; Yang, W.; Liu, C.; Chen, D. A Review on Recent Advances of Biochar from Agricultural and Forestry Wastes: Preparation, Modification and Applications in Wastewater Treatment. Journal of Environmental Chemical Engineering 2024, 12, 111638. [CrossRef]
- Wijitkosum, S. Biochar Derived from Agricultural Wastes and Wood Residues for Sustainable Agricultural and Environmental Applications. International Soil and Water Conservation Research 2022, 10, 335–341. [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Biochar Derived from Anaerobically Digested Sugar Beet Tailings: Characterization and Phosphate Removal Potential. Bioresource Technology 2011, 102, 6273–6278. [CrossRef]
- Puettmann, M.; Sahoo, K.; Wilson, K.; Oneil, E. Life Cycle Assessment of Biochar Produced from Forest Residues Using Portable Systems. Journal of Cleaner Production 2020, 250, 119564. [CrossRef]
- Papageorgiou, A.; Azzi, E.S.; Enell, A.; Sundberg, C. Biochar Produced from Wood Waste for Soil Remediation in Sweden: Carbon Sequestration and Other Environmental Impacts. Science of The Total Environment 2021, 776, 145953. [CrossRef]
- Laird, D. Biochar Amendments Make the Harvesting of Crop Residue for Bioenergy Production Sustainable. Nutrient Cycling in Agroecosystems 2023. [CrossRef]
- Li, N.; He, M.; Lu, X.; Yan, B.; Duan, X.; Chen, G.; Wang, S.; Hou, L. Municipal Solid Waste Derived Biochars for Wastewater Treatment: Production, Properties and Applications. Resources, Conservation and Recycling 2022, 177, 106003. [CrossRef]
- Shi, W.; Wang, H.; Yan, J.; Shan, L.; Quan, G.; Pan, X.; Cui, L. Wheat Straw Derived Biochar with Hierarchically Porous Structure for Bisphenol A Removal: Preparation, Characterization, and Adsorption Properties. Separation and Purification Technology 2022, 289, 120796. [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Chin, B.L.F.; Lock, S.S.M.; Yee, C.Y.; Yiin, C.L.; Peng, W.; Lam, S.S. Production of Biochar from Rice Straw and Its Application for Wastewater Remediation − An Overview. Bioresource Technology 2022, 360, 127588. [CrossRef]
- Gunamantha, M.; Widana, G.A.B. IOP Conf. Ser.: Earth Environ. Sci. 2018, 131 012055.
- Rathnayake, D.; Schmidt, H.-P.; Leifeld, J.; Mayer, J.; Epper, C.A.; Bucheli, T.D.; Hagemann, N. Biochar from Animal Manure: A Critical Assessment on Technical Feasibility, Economic Viability, and Ecological Impact. GCB Bioenergy 2023, 15, 1078–1104. [CrossRef]
- Drané, M.; Zbair, M.; Hajjar-Garreau, S.; Josien, L.; Michelin, L.; Bennici, S.; Limousy, L. Unveiling the Potential of Corn Cob Biochar: Analysis of Microstructure and Composition with Emphasis on Interaction with NO2. Materials 2024, 17. [CrossRef]
- Luo, L.; Wang, J.; Lv, J.; Liu, Z.; Sun, T.; Yang, Y.; Zhu, Y.-G. Carbon Sequestration Strategies in Soil Using Biochar: Advances, Challenges, and Opportunities. Environ. Sci. Technol. 2023, 57, 11357–11372. [CrossRef]
- Brassard, P.; Godbout, S.; Lévesque, V.; Palacios, J.H.; Raghavan, V.; Ahmed, A.; Hogue, R.; Jeanne, T.; Verma, M. 4 - Biochar for Soil Amendment. In Char and Carbon Materials Derived from Biomass; Jeguirim, M., Limousy, L., Eds.; Elsevier, 2019; pp. 109–146 ISBN 978-0-12-814893-8.
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for Composting Improvement and Contaminants Reduction. A Review. Bioresource Technology 2017, 246, 193–202. [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar Technology in Wastewater Treatment: A Critical Review. Chemosphere 2020, 252, 126539. [CrossRef]
- Rawat, S.; Wang, C.-T.; Lay, C.-H.; Hotha, S.; Bhaskar, T. Sustainable Biochar for Advanced Electrochemical/Energy Storage Applications. Journal of Energy Storage 2023, 63, 107115. [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of Biochar for the Adsorption of Organic Pollutants from Wastewater: Modification Strategies, Mechanisms and Challenges. Separation and Purification Technology 2022, 300, 121925. [CrossRef]
- Kalu, S.; Kulmala, L.; Zrim, J.; Peltokangas, K.; Tammeorg, P.; Rasa, K.; Kitzler, B.; Pihlatie, M.; Karhu, K. Potential of Biochar to Reduce Greenhouse Gas Emissions and Increase Nitrogen Use Efficiency in Boreal Arable Soils in the Long-Term. Frontiers in Environmental Science 2022, 10. [CrossRef]
- Jiang, T.; Wang, B.; Gao, B.; Cheng, N.; Feng, Q.; Chen, M.; Wang, S. Degradation of Organic Pollutants from Water by Biochar-Assisted Advanced Oxidation Processes: Mechanisms and Applications. Journal of Hazardous Materials 2023, 442, 130075. [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of Biochar and Its Composites in Catalysis. Chemosphere 2020, 240, 124842. [CrossRef]
- Gayathri, R.; Gopinath, K.P.; Kumar, P.S. Adsorptive Separation of Toxic Metals from Aquatic Environment Using Agro Waste Biochar: Application in Electroplating Industrial Wastewater. Chemosphere 2021, 262, 128031. [CrossRef]
- Hemavathy, R.V.; Kumar, P.S.; Kanmani, K.; Jahnavi, N. Adsorptive Separation of Cu(II) Ions from Aqueous Medium Using Thermally/Chemically Treated Cassia Fistula Based Biochar. Journal of Cleaner Production 2020, 249, 119390. [CrossRef]
- El-Naggar, A.; El-Naggar, A.H.; Shaheen, S.M.; Sarkar, B.; Chang, S.X.; Tsang, D.C.W.; Rinklebe, J.; Ok, Y.S. Biochar Composition-Dependent Impacts on Soil Nutrient Release, Carbon Mineralization, and Potential Environmental Risk: A Review. Journal of Environmental Management 2019, 241, 458–467. [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar Application to Low Fertility Soils: A Review of Current Status, and Future Prospects. Geoderma 2019, 337, 536–554. [CrossRef]
- Hu, X.; Xu, J.; Wu, M.; Xing, J.; Bi, W.; Wang, K.; Ma, J.; Liu, X. Effects of Biomass Pre-Pyrolysis and Pyrolysis Temperature on Magnetic Biochar Properties. Journal of Analytical and Applied Pyrolysis 2017, 127, 196–202. [CrossRef]
- Senthil Kumar, P.; Abhinaya, R.V.; Gayathri Lashmi, K.; Arthi, V.; Pavithra, R.; Sathyaselvabala, V.; Dinesh Kirupha, S.; Sivanesan, S. Adsorption of Methylene Blue Dye from Aqueous Solution by Agricultural Waste: Equilibrium, Thermodynamics, Kinetics, Mechanism and Process Design. Colloid Journal 2011, 73, 651–661. [CrossRef]
- Kumar, P.S.; Senthamarai, C.; Deepthi, A.S.L.S.; Bharani, R. Adsorption Isotherms, Kinetics and Mechanism of Pb(II) Ions Removal from Aqueous Solution Using Chemically Modified Agricultural Waste. Canadian Journal of Chemical Engineering 2013, 91, 1950–1956.
- Varjani, S.; Kumar, G.; Rene, E.R. Developments in Biochar Application for Pesticide Remediation: Current Knowledge and Future Research Directions. Journal of Environmental Management 2019, 232, 505–513. [CrossRef]
- Bridgwater, A.V. Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass and Bioenergy 2012, 38, 68–94. [CrossRef]
- Ng, W.C.; You, S.; Ling, R.; Gin, K.Y.-H.; Dai, Y.; Wang, C.-H. Co-Gasification of Woody Biomass and Chicken Manure: Syngas Production, Biochar Reutilization, and Cost-Benefit Analysis. Energy 2017, 139, 732–742. [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of Pyrolysis Temperature and Manure Source on Physicochemical Characteristics of Biochar. Bioresource Technology 2012, 107, 419–428. [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels, Bioproducts and Biorefining 2010, 4, 160–177. [CrossRef]
- Klinghoffer, N.B.; Castaldi, M.J.; Nzihou, A. Influence of Char Composition and Inorganics on Catalytic Activity of Char from Biomass Gasification. Fuel 2015, 157, 37–47. [CrossRef]
- P.C.A. Bergman, A.R. Boersma, R.W.R. Zwart, J.H.A. Kiel, Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations; Report No. ECNC05013, Energy Research Centre of The Netherlands (ECN), Petten, The Netherlands, 2005, pp. 71.
- Nunoura, T.; Wade, S.R.; Bourke, J.P.; Antal, M.J. Studies of the Flash Carbonization Process. 1. Propagation of the Flaming Pyrolysis Reaction and Performance of a Catalytic Afterburner. Ind. Eng. Chem. Res. 2006, 45, 585–599. [CrossRef]
- Basu, P. Chapter 5 - Pyrolysis. In Biomass Gasification, Pyrolysis and Torrefaction (Third Edition); Basu, P., Ed.; Academic Press, 2018; pp. 155–187 ISBN 978-0-12-812992-0.
- Devi, M.; Rawat, S.; Sharma, S. A Comprehensive Review of the Pyrolysis Process: From Carbon Nanomaterial Synthesis to Waste Treatment. Oxford Open Materials Science 2021, 1, itab014. [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and Utilization of Biochar: A Review. Journal of Industrial and Engineering Chemistry 2016, 40, 1–15. [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S.J.; Saravanan, A. Advances in Production and Application of Biochar from Lignocellulosic Feedstocks for Remediation of Environmental Pollutants. Bioresource Technology 2019, 292, 122030. [CrossRef]
- Gontijo, L.O.L.; Junior, M.N.B.; Santos de Sá, D.; Letichevsky, S.; Pedrozo-Peñafiel, M.J.; Aucélio, R.Q.; Bott, I.S.; Diniz Lopes Alves, H.; Fragneaud, B.; Oliveira Maciel, I.; et al. 3D Conductive Monolithic Carbons from Pyrolyzed Bamboo for Microfluidic Self-Heating System. Carbon 2023, 213, 118214. [CrossRef]
- Swagathnath, G.; Rangabhashiyam, S.; Parthsarathi, K.; Murugan, S.; Balasubramanian, P. Modeling Biochar Yield and Syngas Production During the Pyrolysis of Agro-Residues. In Proceedings of the Green Buildings and Sustainable Engineering; Drück, H., Pillai, R.G., Tharian, M.G., Majeed, A.Z., Eds.; Springer Singapore: Singapore, 2019; pp. 325–336.
- Ibitoye, S.E.; Mahamood, R.M.; Jen, T.-C.; Loha, C.; Akinlabi, E.T. An Overview of Biomass Solid Fuels: Biomass Sources, Processing Methods, and Morphological and Microstructural Properties. Journal of Bioresources and Bioproducts 2023, 8, 333–360. [CrossRef]
- Tan, H.; et al. A Review On The Comparison Between Slow Pyrolysis And Fast Pyrolysis On The Quality Of Lignocellulosic And Lignin-Based Biochar IOP Conf. Ser.: Mater. Sci. Eng. 2021, 1051 012075. [CrossRef]
- Kumar, A.; Bhattacharya, T. Biochar and Its Application 2018.
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Reviews in Environmental Science and Bio/Technology 2020, 19, 191–215. [CrossRef]
- Ding, W.; Dong, X.; Ime, I.M.; Gao, B.; Ma, L.Q. Pyrolytic Temperatures Impact Lead Sorption Mechanisms by Bagasse Biochars. Chemosphere 2014, 105, 68–74. [CrossRef]
- Banik, C.; Lawrinenko, M.; Bakshi, S.; Laird, D.A. Impact of Pyrolysis Temperature and Feedstock on Surface Charge and Functional Group Chemistry of Biochars. Journal of Environmental Quality 2018, 47, 452–461. [CrossRef]
- Zhao, S.-X.; Ta, N.; Wang, X.-D. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10. [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Pérez, M. The Role of Biochar Porosity and Surface Functionality in Augmenting Hydrologic Properties of a Sandy Soil. Science of The Total Environment 2017, 574, 139–147. [CrossRef]
- Gontijo, L.O.; Junior, M.N.B.; de Sá, D.S.; Letichevsky, S.; Pedrozo-Peñafiel, M.J.; Aucélio, R.Q.; ... Pandoli, O.G. 3D conductive monolithic carbons from pyrolyzed bamboo for microfluidic self-heating system. Carbon, 2023, 118214.
- Sudarsan, J.S.; Prasanna, K.; Shiam Babu, R.; Sai Krishna, V.M.V. Chapter 11 - Biochar: A Sustainable Solution for Organic Waste Management a Way Forward towards Circular Economy. In Recent Trends in Solid Waste Management; Ravindran, B., Gupta, S.K., Bhat, S.A., Chauhan, P.S., Tyagi, N., Eds.; Elsevier, 2023; pp. 215–230 ISBN 978-0-443-15206-1.
- Zhou, B.; Liu, Q.; Shi, L.; Liu, Z. Electron Spin Resonance Studies of Coals and Coal Conversion Processes: A Review. Fuel Processing Technology 2019, 188, 212–227. [CrossRef]
- Zhang, S.; Cui, J.; Wu, H.; Zheng, Q.; Song, D.; Wang, X.; Zhang, S. Organic Carbon, Total Nitrogen, and Microbial Community Distributions within Aggregates of Calcareous Soil Treated with Biochar. Agriculture, Ecosystems & Environment 2021, 314, 107408. [CrossRef]
- Ma, L.; Song, D.; Liu, M.; Li, Y.; Li, Y. Effects of Earthworm Activities on Soil Nutrients and Microbial Diversity under Different Tillage Measures. Soil and Tillage Research 2022, 222, 105441. [CrossRef]
- Hu, W.; Gao, W.; Tang, Y.; Zhang, Q.; Tu, C.; Cheng, J. Remediation via Biochar and Potential Health Risk of Heavy Metal Contaminated Soils. Environmental Earth Sciences 2022, 81, 482.
- Kant Bhatia, S.; Palai, A.K.; Kumar, A.; Kant Bhatia, R.; Kumar Patel, A.; Kumar Thakur, V.; Yang, Y.-H. Trends in Renewable Energy Production Employing Biomass-Based Biochar. Bioresource Technology 2021, 340, 125644. [CrossRef]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, Activation, and Applications of Biochar in Recent Times. Biochar 2020, 1–33.
- Nguyen, H.N.; Pignatello, J.J. Laboratory Tests of Biochars as Absorbents for Use in Recovery or Containment of Marine Crude Oil Spills. Environmental Engineering Science 2013, 30, 374–380. [CrossRef]
- Jothirani, R.; Kumar, P.S.; Saravanan, A.; Narayan, A.S.; Dutta, A. Ultrasonic Modified Corn Pith for the Sequestration of Dye from Aqueous Solution. Journal of Industrial and Engineering Chemistry 2016, 39, 162–175. [CrossRef]
- Suganya, S.; Senthil Kumar, P.; Saravanan, A.; Sundar Rajan, P.; Ravikumar, C. Computation of Adsorption Parameters for the Removal of Dye from Wastewater by Microwave Assisted Sawdust: Theoretical and Experimental Analysis. Environmental Toxicology and Pharmacology 2017, 50, 45–57. [CrossRef]
- Saravanan, A.; Kumar, P.S.; Renita, A.A. Hybrid Synthesis of Novel Material through Acid Modification Followed Ultrasonication to Improve Adsorption Capacity for Zinc Removal. Journal of Cleaner Production 2018, 172, 92–105. [CrossRef]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel Insights into the Adsorption of Organic Contaminants by Biochar: A Review. Chemosphere 2022, 287, 132113. [CrossRef]
- Lou, L.; Wu, B.; Wang, L.; Luo, L.; Xu, X.; Hou, J.; Xun, B.; Hu, B.; Chen, Y. Sorption and Ecotoxicity of Pentachlorophenol Polluted Sediment Amended with Rice-Straw Derived Biochar. Bioresource Technology 2011, 102, 4036–4041. [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of Biochar for the Removal of Pollutants from Aqueous Solutions. Chemosphere 2015, 125, 70–85. [CrossRef]
- Yu, X.-Y.; Ying, G.-G.; Kookana, R.S. Reduced Plant Uptake of Pesticides with Biochar Additions to Soil. Chemosphere 2009, 76, 665–671. [CrossRef]
- Liang, C.; Niu, H.-Y.; Guo, H.; Niu, C.-G.; Yang, Y.-Y.; Liu, H.-Y.; Tang, W.-W.; Feng, H.-P. Efficient Photocatalytic Nitrogen Fixation to Ammonia over Bismuth Monoxide Quantum Dots-Modified Defective Ultrathin Graphitic Carbon Nitride. Chemical Engineering Journal 2021, 406, 126868. [CrossRef]
- Kumbhar, D.; Palliyarayil, A.; Reghu, D.; Shrungar, D.; Umapathy, S.; Sil, S. Rapid Discrimination of Porous Bio-Carbon Derived from Nitrogen Rich Biomass Using Raman Spectroscopy and Artificial Intelligence Methods. Carbon 2021, 178, 792–802. [CrossRef]
- Zhang, K.; Sun, P.; Faye, M.C.A.S.; Zhang, Y. Characterization of Biochar Derived from Rice Husks and Its Potential in Chlorobenzene Degradation. Carbon 2018, 130, 730–740. [CrossRef]
- Chen, D.; Xu, J.; Ling, P.; Fang, Z.; Ren, Q.; Xu, K.; Jiang, L.; Wang, Y.; Su, S.; Hu, S.; et al. Formation and Evolution Mechanism of Persistent Free Radicals in Biochar during Biomass Pyrolysis: Insights from Biochar’s Element Composition and Chemical Structure. Fuel 2024, 357, 129910. [CrossRef]
- Wu, C.; Fu, L.; Li, H.; Liu, X.; Wan, C. Using Biochar to Strengthen the Removal of Antibiotic Resistance Genes: Performance and Mechanism. Science of The Total Environment 2022, 816, 151554. [CrossRef]
- Huang, C.; Qin, F.; Zhang, C.; Huang, D.; Tang, L.; Yan, M.; Wang, W.; Song, B.; Qin, D.; Zhou, Y.; et al. Effects of Heterogeneous Metals on the Generation of Persistent Free Radicals as Critical Redox Sites in Iron-Containing Biochar for Persulfate Activation. ACS EST Water 2023, 3, 298–310. [CrossRef]
- Yu, J.; Zhu, Z.; Zhang, H.; Shen, X.; Qiu, Y.; Yin, D.; Wang, S. Persistent Free Radicals on N-Doped Hydrochar for Degradation of Endocrine Disrupting Compounds. Chemical Engineering Journal 2020, 398, 125538. [CrossRef]
- Ma, L.; Syed-Hassan, S.S.A.; Tong, Y.; Xiong, Z.; Chen, Y.; Xu, J.; Jiang, L.; Su, S.; Hu, S.; Wang, Y.; et al. Interactions of Cellulose- and Lignin-Derived Radicals during Pyrolysis: An in-Situ Electron Paramagnetic Resonance (EPR) Study. Fuel Processing Technology 2023, 239, 107536. [CrossRef]
- Retcofsky, H.L.; Hough, M.R.; Clarkson, R.B. Nature of the Free Radicals in Coals, Pyrolyzed Coals, and Liquefaction Products, 1979. Journal Volume: 24:1; Conference: 177. national meeting of the American Chemical Society, Honolulu, HI, USA, 1 Apr 1979.
- Liu, J.; Jiang, X.; Shen, J.; Zhang, H. Chemical Properties of Superfine Pulverized Coal Particles. Part 1. Electron Paramagnetic Resonance Analysis of Free Radical Characteristics. Advanced Powder Technology 2014, 25, 916–925. [CrossRef]
- Luo, K.; Pang, Y.; Wang, D.; Li, X.; Wang, L.; Lei, M.; Huang, Q.; Yang, Q. A Critical Review on the Application of Biochar in Environmental Pollution Remediation: Role of Persistent Free Radicals (PFRs). Journal of Environmental Sciences 2021, 108, 201–216. [CrossRef]
- Zhang, Y.; Sun, X.; Bian, W.; Peng, J.; Wan, H.; Zhao, J. The Key Role of Persistent Free Radicals on the Surface of Hydrochar and Pyrocarbon in the Removal of Heavy Metal-Organic Combined Pollutants. Bioresource Technology 2020, 318, 124046. [CrossRef]
- Qin, J.; Cheng, Y.; Sun, M.; Yan, L.; Shen, G. Catalytic Degradation of the Soil Fumigant 1,3-Dichloropropene in Aqueous Biochar Slurry. Science of The Total Environment 2016, 569–570, 1–8. [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible Role of Biomass Types and Its Compositions on the Formation of Persistent Free Radicals in Biochar: Insight into the Influences on Fenton-like Process. Chemical Engineering Journal 2019, 361, 353–363. [CrossRef]
- Wang, Y.; Gu, X.; Huang, Y.; Ding, Z.; Chen, Y.; Hu, X. Insight into Biomass Feedstock on Formation of Biochar-Bound Environmentally Persistent Free Radicals under Different Pyrolysis Temperatures. RSC Adv. 2022, 12, 19318–19326. [CrossRef]
- Tao, W.; Duan, W.; Liu, C.; Zhu, D.; Si, X.; Zhu, R.; Oleszczuk, P.; Pan, B. Formation of Persistent Free Radicals in Biochar Derived from Rice Straw Based on a Detailed Analysis of Pyrolysis Kinetics. Science of The Total Environment 2020, 715, 136575. [CrossRef]
- Xiang, C.; Liu, Q.; Shi, L.; Liu, Z. A Study on the New Type of Radicals in Corncob Derived Biochars. Fuel 2020, 277, 118163. [CrossRef]
- Chintala, R.; Subramanian, S.; Fortuna, A.-M.; Schumacher, T.E. Examining Biochar Impacts on Soil Abiotic and Biotic Processes and Exploring the Potential for Pyrosequencing Analysis. Chapter 6, In Biochar Application Essential Soil Microbial Ecology, Komang Ralebitso-Senior, T.; Orr, C.H. Eds; Elsevier, 2016, pp. 133-162, ISBN 9780128034330. [CrossRef]
- Takeshita, A.; Uemura, Y.; Onoe, K. Quantification of Persistent Free Radicals (PFRs) Formed in Thermally Decomposed Cellulose and Its Correlation with Residual Carbon Amount. Journal of Analytical and Applied Pyrolysis 2020, 150, 104883. [CrossRef]
- Pan, B.; Li, H.; Lang, D.; Xing, B. Environmentally Persistent Free Radicals: Occurrence, Formation Mechanisms and Implications. Environmental Pollution 2019, 248, 320–331. [CrossRef]
- Lomnicki, S.M.; Truong, H.; Vejerano, E.P.; Dellinger, B. Copper Oxide-Based Model of Persistent Free Radical Formation on Combustion-Derived Particulate Matter. Environmental science & technology 2008, 42 13, 4982–4988.
- Gasim, M.F.; Choong, Z.-Y.; Koo, P.-L.; Low, S.-C.; Abdurahman, M.-H.; Ho, Y.-C.; Mohamad, M.; Suryawan, I.W.; Lim, J.-W.; Oh, W.-D. Application of Biochar as Functional Material for Remediation of Organic Pollutants in Water: An Overview. Catalysts 2022, 12. [CrossRef]
- Zhong, D.; Jiang, Y.; Zhao, Z.; Wang, L.; Chen, J.; Ren, S.; Liu, Z.; Zhang, Y.; Tsang, D.C.W.; Crittenden, J.C. pH Dependence of Arsenic Oxidation by Rice-Husk-Derived Biochar: Roles of Redox-Active Moieties. Environ. Sci. Technol. 2019, 53, 9034–9044. [CrossRef]
- Zhang, K.; Sun, P.; Zhang, Y. Decontamination of Cr(VI) Facilitated Formation of Persistent Free Radicals on Rice Husk Derived Biochar. Frontiers of Environmental Science & Engineering 2019, 13, 22. [CrossRef]
- Zhu, S.; Huang, X.; Yang, X.; Peng, P.; Li, Z.; Jin, C. Enhanced Transformation of Cr(VI) by Heterocyclic-N within Nitrogen-Doped Biochar: Impact of Surface Modulatory Persistent Free Radicals (PFRs). Environ. Sci. Technol. 2020, 54, 8123–8132. [CrossRef]
- Wang, X.; Xu, J.; Liu, J.; Liu, J.; Xia, F.; Wang, C.; Dahlgren, R.A.; Liu, W. Mechanism of Cr(VI) Removal by Magnetic Greigite/Biochar Composites. Science of The Total Environment 2020, 700, 134414. [CrossRef]
- Tang, Z.; Kong, Y.; Zhao, S.; Jia, H.; Vione, D.; Kang, Y.; Gao, P. Enhancement of Cr(VI) Decontamination by Irradiated Sludge Biochar in Neutral Conditions: Evidence of a Possible Role of Persistent Free Radicals. Separation and Purification Technology 2021, 277, 119414. [CrossRef]
- Zhu, Y.; Wei, J.; Li, J. Decontamination of Cr(VI) from Water Using Sewage Sludge-Derived Biochar: Role of Environmentally Persistent Free Radicals. Chinese Journal of Chemical Engineering 2023, 56, 97–103. [CrossRef]
- Baltrėnaitė-Gedienė, E.; Lomnicki, S.; Guo, C. Impact of Biochar, Fertilizers and Cultivation Type on Environmentally Persistent Free Radicals in Agricultural Soil. Environmental Technology & Innovation 2022, 28, 102755. [CrossRef]
- Tai, Y.; Sun, J.; Tian, H.; Liu, F.; Han, B.; Fu, W.; Liu, Z.; Yang, X.; Liu, Q. Efficient Degradation of Organic Pollutants by S-NaTaO3/Biochar under Visible Light and the Photocatalytic Performance of a Permonosulfate-Based Dual-Effect Catalytic System. Journal of Environmental Sciences 2023, 125, 388–400. [CrossRef]
- Kelley, M.A.; Hebert, V.Y.; Thibeaux, T.M.; Orchard, M.A.; Hasan, F.; Cormier, S.A.; Thevenot, P.T.; Lomnicki, S.M.; Varner, K.J.; Dellinger, B.; et al. Model Combustion-Generated Particulate Matter Containing Persistent Free Radicals Redox Cycle to Produce Reactive Oxygen Species. Chem. Res. Toxicol. 2013, 26, 1862–1871. [CrossRef]
- Squadrito, G.L.; Cueto, R.; Dellinger, B.; Pryor, W.A. Quinoid Redox Cycling as a Mechanism for Sustained Free Radical Generation by Inhaled Airborne Particulate Matter. Free Radical Biology and Medicine 2001, 31, 1132–1138. [CrossRef]
- Alburquerque, J.A.; Salazar, P.; Barrón, V.; Torrent, J.; del Campillo, M. del C.; Gallardo, A.; Villar, R. Enhanced Wheat Yield by Biochar Addition under Different Mineral Fertilization Levels. Agronomy for Sustainable Development 2013, 33, 475–484. [CrossRef]
- Jiang, S.-F.; Ling, L.-L.; Chen, W.-J.; Liu, W.-J.; Li, D.-C.; Jiang, H. High Efficient Removal of Bisphenol A in a Peroxymonosulfate/Iron Functionalized Biochar System: Mechanistic Elucidation and Quantification of the Contributors. Chemical Engineering Journal 2019, 359, 572–583. [CrossRef]
- Luo, H.; Lin, Q.; Zhang, X.; Huang, Z.; Liu, S.; Jiang, J.; Xiao, R.; Liao, X. New Insights into the Formation and Transformation of Active Species in nZVI/BC Activated Persulfate in Alkaline Solutions. Chemical Engineering Journal 2019, 359, 1215–1223. [CrossRef]
- Pi, Z.; Li, X.; Wang, D.; Xu, Q.; Tao, Z.; Huang, X.; Yao, F.; Wu, Y.; He, L.; Yang, Q. Persulfate Activation by Oxidation Biochar Supported Magnetite Particles for Tetracycline Removal: Performance and Degradation Pathway. Journal of Cleaner Production 2019, 235, 1103–1115. [CrossRef]
- He, J.; Xiao, Y.; Tang, J.; Chen, H.; Sun, H. Persulfate Activation with Sawdust Biochar in Aqueous Solution by Enhanced Electron Donor-Transfer Effect. Science of The Total Environment 2019, 690, 768–777. [CrossRef]
- Danping, W.U.; Fangfang, L.I.; Jing, Z.; Peng, W.; Min, W.U. Photocatalysis degradation of rhodamine B by dissolved organic matter of biochars[J]. Chinese Journal of Environmental Engineering 2019, 13(11), 2562-2569. [CrossRef]
- Wang, H.; Guo, W.; Yin, R.; Du, J.; Wu, Q.; Luo, H.; Liu, B.; Sseguya, F.; Ren, N. Biochar-Induced Fe(III) Reduction for Persulfate Activation in Sulfamethoxazole Degradation: Insight into the Electron Transfer, Radical Oxidation and Degradation Pathways. Chemical Engineering Journal 2019, 362, 561–569. [CrossRef]
- Zhu, K.; Wang, X.; Geng, M.; Chen, D.; Lin, H.; Zhang, H. Catalytic Oxidation of Clofibric Acid by Peroxydisulfate Activated with Wood-Based Biochar: Effect of Biochar Pyrolysis Temperature, Performance and Mechanism. Chemical Engineering Journal 2019, 374, 1253–1263. [CrossRef]
- Li, L.; Lai, C.; Huang, F.; Cheng, M.; Zeng, G.; Huang, D.; Li, B.; Liu, S.; Zhang, M.; Qin, L.; et al. Degradation of Naphthalene with Magnetic Bio-Char Activate Hydrogen Peroxide: Synergism of Bio-Char and Fe–Mn Binary Oxides. Water Research 2019, 160, 238–248. [CrossRef]
- Luo, K.; Pang, Y.; Yang, Q.; Wang, D.; Li, X.; Wang, L.; Lei, M.; Liu, J. Enhanced Ciprofloxacin Removal by Sludge-Derived Biochar: Effect of Humic Acid. Chemosphere 2019, 231, 495–501. [CrossRef]
- Deng, R.; Luo, H.; Huang, D.; Zhang, C. Biochar-Mediated Fenton-like Reaction for the Degradation of Sulfamethazine: Role of Environmentally Persistent Free Radicals. Chemosphere 2020, 255, 126975. [CrossRef]
- Liu, G.; Zhang, Y.; Yu, H.; Jin, R.; Zhou, J. Acceleration of Goethite-Catalyzed Fenton-like Oxidation of Ofloxacin by Biochar. Journal of Hazardous Materials 2020, 397, 122783. [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. Activation of Peroxymonosulfate by Cobalt-Impregnated Biochar for Atrazine Degradation: The Pivotal Roles of Persistent Free Radicals and Ecotoxicity Assessment. Journal of Hazardous Materials 2020, 398, 122768. [CrossRef]
- Grilla, E.; Vakros, J.; Konstantinou, I.; Manariotis, I.D.; Mantzavinos, D. Activation of Persulfate by Biochar from Spent Malt Rootlets for the Degradation of Trimethoprim in the Presence of Inorganic Ions. Journal of Chemical Technology & Biotechnology 2020, 95, 2348–2358. [CrossRef]
- Feng, Z.; Zhou, B.; Yuan, R.; Li, H.; He, P.; Wang, F.; Chen, Z.; Chen, H. Biochar Derived from Different Crop Straws as Persulfate Activator for the Degradation of Sulfadiazine: Influence of Biomass Types and Systemic Cause Analysis. Chemical Engineering Journal 2022, 440. [CrossRef]
- Kim, D.-G.; Ko, S.-O. Effects of Thermal Modification of a Biochar on Persulfate Activation and Mechanisms of Catalytic Degradation of a Pharmaceutical. Chemical Engineering Journal 2020, 399, 125377. [CrossRef]
- He, W.; Zhu, Y.; Zeng, G.; Zhang, Y.; Wang, Y.; Zhang, M.; Long, H.; Tang, W. Efficient Removal of Perfluorooctanoic Acid by Persulfate Advanced Oxidative Degradation: Inherent Roles of Iron-Porphyrin and Persistent Free Radicals. Chemical Engineering Journal 2020, 392, 123640. [CrossRef]
- Cao, W.; Zeng, C.; Guo, X.; Liu, Q.; Zhang, X.; Mameda, N. Enhanced Electrochemical Degradation of 2,4-Dichlorophenol with the Assist of Hydrochar. Chemosphere 2020, 260, 127643. [CrossRef]
- Xu, H.; Zhang, Y.; Li, J.; Hao, Q.; Li, X.; Liu, F. Heterogeneous Activation of Peroxymonosulfate by a Biochar-Supported Co3O4 Composite for Efficient Degradation of Chloramphenicols. Environmental Pollution 2020, 257, 113610. [CrossRef]
- Li, X.; Jia, Y.; Zhou, M.; Su, X.; Sun, J. High-Efficiency Degradation of Organic Pollutants with Fe, N Co-Doped Biochar Catalysts via Persulfate Activation. Journal of Hazardous Materials 2020, 397, 122764. [CrossRef]
- Xie, Y.; Hu, W.; Wang, X.; Tong, W.; Li, P.; Zhou, H.; Wang, Y.; Zhang, Y. Molten Salt Induced Nitrogen-Doped Biochar Nanosheets as Highly Efficient Peroxymonosulfate Catalyst for Organic Pollutant Degradation. Environmental Pollution 2020, 260, 114053. [CrossRef]
- Yan, J.; Yang, L.; Qian, L.; Han, L.; Chen, M. Nano-Magnetite Supported by Biochar Pyrolyzed at Different Temperatures as Hydrogen Peroxide Activator: Synthesis Mechanism and the Effects on Ethylbenzene Removal. Environmental Pollution 2020, 261, 114020. [CrossRef]
- Mian, M.M.; Liu, G.; Zhou, H. Preparation of N-Doped Biochar from Sewage Sludge and Melamine for Peroxymonosulfate Activation: N-Functionality and Catalytic Mechanisms. Science of The Total Environment 2020, 744, 140862. [CrossRef]
- Veiga, P.A. da S.; Schultz, J.; Matos, T.T. da S.; Fornari, M.R.; Costa, T.G.; Meurer, L.; Mangrich, A.S. Production of High-Performance Biochar Using a Simple and Low-Cost Method: Optimization of Pyrolysis Parameters and Evaluation for Water Treatment. Journal of Analytical and Applied Pyrolysis 2020, 148, 104823. [CrossRef]
- Sun, P.; Zhang, K.-K.; Zhang, Y.; Zhang, Y. Sunflower-Straw-Derived Biochar-Enhanced Fe(Ⅲ/S2O82- System for Degradation of Benzoic Acid. Environmental Science 2020, (05), 2301-2309.
- Zeng, L.; Chen, Q.; Tan, Y.; Lan, P.; Zhou, D.; Wu, M.; Liang, N.; Pan, B.; Xing, B. Dual Roles of Biochar Redox Property in Mediating 2,4-Dichlorophenol Degradation in the Presence of Fe3+ and Persulfate. Chemosphere 2021, 279, 130456. [CrossRef]
- Yang, F.; Zhu, Q.; Gao, Y.; Jian, H.; Wang, C.; Sun, H. Effects of Biochar-Dissolved Organic Matter on the Photodegradation of Sulfamethoxazole and Chloramphenicol in Biochar Solutions as Revealed by Oxygen Reduction Performances and Free Radicals. Science of The Total Environment 2021, 781, 146807. [CrossRef]
- Min, L.; Zhang, P.; Fan, M.; Xu, X.; Wang, C.; Tang, J.; Sun, H. Efficient Degradation of P-Nitrophenol by Fe@pomelo Peel-Derived Biochar Composites and Its Mechanism of Simultaneous Reduction and Oxidation Process. Chemosphere 2021, 267, 129213. [CrossRef]
- Mer, K.; Sajjadi, B.; Egiebor, Nosa.O.; Chen, W.-Y.; Mattern, Daniell.L.; Tao, W. Enhanced Degradation of Organic Contaminants Using Catalytic Activity of Carbonaceous Structures: A Strategy for the Reuse of Exhausted Sorbents. Journal of Environmental Sciences 2021, 99, 267–273. [CrossRef]
- Cao, Y.; Jiang, S.; Kang, X.; Zhang, H.; Zhang, Q.; Wang, L. Enhancing Degradation of Atrazine by Fe-Phenol Modified Biochar/Ferrate(VI) under Alkaline Conditions: Analysis of the Mechanism and Intermediate Products. Chemosphere 2021, 285, 131399. [CrossRef]
- Zhang, R.; Zheng, X.; Zhang, D.; Niu, X.; Ma, J.; Lin, Z.; Fu, M.; Zhou, S. Insight into the Roles of Endogenous Minerals in the Activation of Persulfate by Graphitized Biochar for Tetracycline Removal. Science of The Total Environment 2021, 768, 144281. [CrossRef]
- Yi, Y.; Luo, J.; Fang, Z. Magnetic Biochar Derived from Eichhornia Crassipes for Highly Efficient Fenton-like Degradation of Antibiotics: Mechanism and Contributions. Journal of Environmental Chemical Engineering 2021, 9, 106258. [CrossRef]
- Zhang, Y.; Xu, M.; Liang, S.; Feng, Z.; Zhao, J. Mechanism of Persulfate Activation by Biochar for the Catalytic Degradation of Antibiotics: Synergistic Effects of Environmentally Persistent Free Radicals and the Defective Structure of Biochar. Science of The Total Environment 2021, 794, 148707. [CrossRef]
- Wang, X.; Zhang, P.; Wang, C.; Jia, H.; Shang, X.; Tang, J.; Sun, H. Metal-Rich Hyperaccumulator-Derived Biochar as an Efficient Persulfate Activator: Role of Intrinsic Metals (Fe, Mn and Zn) in Regulating Characteristics, Performance and Reaction Mechanisms. Journal of Hazardous Materials 2022, 424, 127225. [CrossRef]
- Luo Kun; Yang Chen; Li Xue; Pang Ya; Yang Qi Mn-Doped Biochar Derived from Sewage Sludge for Ciprofloxacin Degradation. Journal of Environmental Engineering 2022, 148, 04022048. [CrossRef]
- Li, H.; Liu, Y.; Jiang, F.; Bai, X.; Li, H.; Lang, D.; Wang, L.; Pan, B. Persulfate Adsorption and Activation by Carbon Structure Defects Provided New Insights into Ofloxacin Degradation by Biochar. Science of The Total Environment 2022, 806, 150968. [CrossRef]
- Dai, Z.; Zhao, L.; Peng, S.; Yue, Z.; Zhan, X.; Wang, J. Removal of Oxytetracycline Promoted by Manganese-Doped Biochar Based on Density Functional Theory Calculations: Comprehensive Evaluation of the Effect of Transition Metal Doping. Science of The Total Environment 2022, 806, 150268. [CrossRef]
- Rangarajan, G.; Farnood, R. Role of Persistent Free Radicals and Lewis Acid Sites in Visible-Light-Driven Wet Peroxide Activation by Solid Acid Biochar Catalysts – A Mechanistic Study. Journal of Hazardous Materials 2022, 438, 129514. [CrossRef]
- An, X.; Chen, Y.; Ao, M.; Jin, Y.; Zhan, L.; Yu, B.; Wu, Z.; Jiang, P. Sequential Photocatalytic Degradation of Organophosphorus Pesticides and Recovery of Orthophosphate by Biochar/α-Fe2O3/MgO Composite: A New Enhanced Strategy for Reducing the Impacts of Organophosphorus from Wastewater. Chemical Engineering Journal 2022, 435, 135087. [CrossRef]
- Jiang, Xinyi and Xiao, Ye and Xiao, Jiana and Zhang, Weihua and Rongliang, Qiu, The Effect of Persistent Free Radicals in Sludge Derived Biochar on the Removal of P-Chlorophenol. Available at SSRN: https://ssrn.com/abstract=3992617 (accessed on 04 January 2024).
- Pan, Y.; Peng, Z.; Liu, Z.; Shao, B.; Liang, Q.; He, Q.; Wu, T.; Zhang, X.; Zhao, C.; Liu, Y.; et al. Activation of Peroxydisulfate by Bimetal Modified Peanut Hull-Derived Porous Biochar for the Degradation of Tetracycline in Aqueous Solution. Journal of Environmental Chemical Engineering 2022, 10, 107366. [CrossRef]
- Yin, Q.; Yan, H.; Liang, Y.; Jiang, Z.; Wang, H.; Nian, Y. Activation of Persulfate by Blue Algae Biochar Supported FeOX Particles for Tetracycline Degradation: Performance and Mechanism. Separation and Purification Technology 2023, 319, 124005. [CrossRef]
- Badiger, S.M.; Nidheesh, P.V. Applications of Biochar in Sulfate Radical-Based Advanced Oxidation Processes for the Removal of Pharmaceuticals and Personal Care Products. Water Science and Technology 2023, 87, 1329–1348. [CrossRef]
- Yu, Y.; Zhong, Z.; Guo, H.; Yu, Y.; Zheng, T.; Li, H.; Chang, Z. Biochar–Goethite Composites Inhibited/Enhanced Degradation of Triphenyl Phosphate by Activating Persulfate: Insights on the Mechanism. Science of The Total Environment 2023, 858, 159940. [CrossRef]
- Pei, S.; Zhao, Y.; Li, W.; Qu, C.; Ren, Y.; Yang, Y.; Liu, J.; Wu, C. Critical Impact of Pyrolysis Temperatures on Biochars for Peroxymonosulfate Activation: Structural Characteristics, Degradation Performance and Mechanism. Chemical Engineering Journal 2023, 477, 147274. [CrossRef]
- Zhang, Y.; He, R.; Zhao, J.; Zhang, X.; Bildyukevich, A.V. Effect of Aged Biochar after Microbial Fermentation on Antibiotics Removal: Key Roles of Microplastics and Environmentally Persistent Free Radicals. Bioresource Technology 2023, 374, 128779. [CrossRef]
- Yang, Z.; An, Q.; Deng, S.; Xu, B.; Li, Z.; Deng, S.; Zhao, B.; Ye, Z. Efficient Activation of Peroxydisulfate by Modified Red Mud Biochar Derived from Waste Corn Straw for Levofloxacin Degradation: Efficiencies and Mechanisms. Journal of Environmental Chemical Engineering 2023, 11, 111609. [CrossRef]
- Zhang, Y.; He, R.; Zhao, J. Removal Mechanism of Tetracycline-Cr(Ⅵ) Combined Pollutants by Different S-Doped Sludge Biochars: Role of Environmentally Persistent Free Radicals. Chemosphere 2023, 317. [CrossRef]
- Luo, K.; Lin, N.; Li, X.; Pang, Y.; Wang, L.; Lei, M.; Yang, Q. Efficient Hexavalent Chromium Removal by Nano-Cerium Oxide Functionalized Biochar: Insight into the Role of Reduction. Journal of Environmental Chemical Engineering 2023, 11, 110004. [CrossRef]
- Zhong, D.; Zhao, Z.; Jiang, Y.; Yang, X.; Wang, L.; Chen, J.; Guan, C.-Y.; Zhang, Y.; Tsang, D.C.W.; Crittenden, J.C. Contrasting Abiotic As(III) Immobilization by Undissolved and Dissolved Fractions of Biochar in Ca2+-Rich Groundwater under Anoxic Conditions. Water Research 2020, 183, 116106. [CrossRef]
- Huang, Y.; Gao, M.; Deng, Y.; Khan, Z.H.; Liu, X.; Song, Z.; Qiu, W. Efficient Oxidation and Adsorption of As(III) and As(V) in Water Using a Fenton-like Reagent, (Ferrihydrite)-Loaded Biochar. Science of The Total Environment 2020, 715, 136957. [CrossRef]
- Zhu, Y.; Wei, J.; Li, J. Biochar-Activated Persulfate Oxidation of Arsenic(III): Nonnegligible Roles of Environmentally Persistent Free Radicals. Journal of Environmental Chemical Engineering 2023, 11, 111033. [CrossRef]
- Yu, J.; Zhu, Z.; Zhang, H.; Chen, T.; Qiu, Y.; Xu, Z.; Yin, D. Efficient Removal of Several Estrogens in Water by Fe-Hydrochar Composite and Related Interactive Effect Mechanism of H2O2 and Iron with Persistent Free Radicals from Hydrochar of Pinewood. Science of The Total Environment 2019, 658, 1013–1022. [CrossRef]
- Zhu, N.; Li, C.; Bu, L.; Tang, C.; Wang, S.; Duan, P.; Yao, L.; Tang, J.; Dionysiou, D.D.; Wu, Y. Bismuth Impregnated Biochar for Efficient Estrone Degradation: The Synergistic Effect between Biochar and Bi/Bi2O3 for a High Photocatalytic Performance. Journal of Hazardous Materials 2020, 384, 121258. [CrossRef]
- Chen, Y.; Duan, X.; Zhang, C.; Wang, S.; Ren, N.; Ho, S.-H. Graphitic Biochar Catalysts from Anaerobic Digestion Sludge for Nonradical Degradation of Micropollutants and Disinfection. Chemical Engineering Journal 2020, 384, 123244. [CrossRef]
- Xu, H.; Han, Y.; Wang, G.; Deng, P.; Feng, L. Walnut Shell Biochar Based Sorptive Remediation of Estrogens Polluted Simulated Wastewater: Characterization, Adsorption Mechanism and Degradation by Persistent Free Radicals. Environmental Technology & Innovation 2022, 28, 102870. [CrossRef]
- Wang, T.; Zheng, J.; Cai, J.; Liu, Q.; Zhang, X. Visible-Light-Driven Photocatalytic Degradation of Dye and Antibiotics by Activated Biochar Composited with K+ Doped g-C3N4: Effects, Mechanisms, Actual Wastewater Treatment and Disinfection. Science of the Total Environment 2022, 839. [CrossRef]
- Shi, J.; Wang, J.; Liang, L.; Xu, Z.; Chen, Y.; Chen, S.; Xu, M.; Wang, X.; Wang, S. Carbothermal Synthesis of Biochar-Supported Metallic Silver for Enhanced Photocatalytic Removal of Methylene Blue and Antimicrobial Efficacy. Journal of Hazardous Materials 2021, 401, 123382. [CrossRef]
- Lian, F.; Yu, W.; Zhou, Q.; Gu, S.; Wang, Z.; Xing, B. Size Matters: Nano-Biochar Triggers Decomposition and Transformation Inhibition of Antibiotic Resistance Genes in Aqueous Environments. Environ. Sci. Technol. 2020, 54, 8821–8829. [CrossRef]
- Liang, Z.; Min, W.U.; Guo-juan, W.U. Electron exchange capacity of rice biochar at different preparation temperatures[J]. CHINA ENVIRONMENTAL SCIENCECE, 2019, 39(10), 4329-4336.
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential Hazards of Biochar: The Negative Environmental Impacts of Biochar Applications. Journal of Hazardous Materials 2021, 420, 126611. [CrossRef]
- Kharisov, B.I.; Kharissova, O.V. Carbon Allotropes in the Environment and Their Toxicity. In Carbon Allotropes: Metal-Complex Chemistry, Properties and Applications. Springer, Cham, 2019. [CrossRef]
- El-Naggar, A.; Lee, M.-H.; Hur, J.; Lee, Y.H.; Igalavithana, A.D.; Shaheen, S.M.; Ryu, C.; Rinklebe, J.; Tsang, D.C.W.; Ok, Y.S. Biochar-Induced Metal Immobilization and Soil Biogeochemical Process: An Integrated Mechanistic Approach. Science of The Total Environment 2020, 698, 134112. [CrossRef]
- Cui, H.; Li, D.; Liu, X.; Fan, Y.; Zhang, X.; Zhang, S.; Zhou, J.; Fang, G.; Zhou, J. Dry-Wet and Freeze-Thaw Aging Activate Endogenous Copper and Cadmium in Biochar. Journal of Cleaner Production 2021, 288, 125605. [CrossRef]
- Rombolà, A.G.; Fabbri, D.; Baronti, S.; Vaccari, F.P.; Genesio, L.; Miglietta, F. Changes in the Pattern of Polycyclic Aromatic Hydrocarbons in Soil Treated with Biochar from a Multiyear Field Experiment. Chemosphere 2019, 219, 662–670. [CrossRef]
- Joseph, S.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.M.; Zwieten, L.V.; Kimber, S.; Cowie, A.L.; Singh, B.P.; et al. An Investigation into the Reactions of Biochar in Soil. Soil Research 2010, 48, 501–515.
- Kim, H.-B.; Kim, S.-H.; Jeon, E.-K.; Kim, D.-H.; Tsang, D.C.W.; Alessi, D.S.; Kwon, E.E.; Baek, K. Effect of Dissolved Organic Carbon from Sludge, Rice Straw and Spent Coffee Ground Biochar on the Mobility of Arsenic in Soil. Science of The Total Environment 2018, 636, 1241–1248. [CrossRef]
- Jia, C.; Luo, J.; Fan, J.; Clark, J.H.; Zhang, S.; Zhu, X. Urgently Reveal Longly Hidden Toxicant in a Familiar Fabrication Process of Biomass-Derived Environment Carbon Material. Journal of Environmental Sciences 2021, 100, 250–256. [CrossRef]
- Liu, J.; Gao, N.; Wen, X.; Jia, H.; Lichtfouse, E. Plant and Algal Toxicity of Persistent Free Radicals and Reactive Oxygen Species Generated by Heating Anthracene-Contaminated Soils from 100 to 600 °C. Environmental Chemistry Letters 2021, 19, 2695–2703. [CrossRef]
- Xu, Y.; Lu, X.; Su, G.; Chen, X.; Meng, J.; Li, Q.; Wang, C.; Shi, B. Scientific and Regulatory Challenges of Environmentally Persistent Free Radicals: From Formation Theory to Risk Prevention Strategies. Journal of Hazardous Materials 2023, 456, 131674. [CrossRef]
- Yi, J.-F.; Lin, Z.-Z.; Li, X.; Zhou, Y.-Q.; Guo, Y. A Short Review on Environmental Distribution and Toxicity of the Environmentally Persistent Free Radicals. Chemosphere 2023, 340, 139922. [CrossRef]
- Jaligama, S.; Patel, V.S.; Wang, P.; Sallam, A.; Harding, J.; Kelley, M.; Mancuso, S.R.; Dugas, T.R.; Cormier, S.A. Radical Containing Combustion Derived Particulate Matter Enhance Pulmonary Th17 Inflammation via the Aryl Hydrocarbon Receptor. Particle and Fibre Toxicology 2018, 15, 20. [CrossRef]
- Harmon, A.C.; Hebert, V.Y.; Cormier, S.A.; Subramanian, B.; Reed, J.R.; Backes, W.L.; Dugas, T.R. Particulate Matter Containing Environmentally Persistent Free Radicals Induces AhR-Dependent Cytokine and Reactive Oxygen Species Production in Human Bronchial Epithelial Cells. PLOS ONE 2018, 13, e0205412. [CrossRef]
- Reed, J.R.; dela Cruz, A.L.N.; Lomnicki, S.M.; Backes, W.L. Environmentally Persistent Free Radical-Containing Particulate Matter Competitively Inhibits Metabolism by Cytochrome P450 1A2. Toxicology and Applied Pharmacology 2015, 289, 223–230. [CrossRef]
- Chuang, G.C.; Xia, H.; Mahne, S.E.; Varner, K.J. Environmentally Persistent Free Radicals Cause Apoptosis in HL-1 Cardiomyocytes. Cardiovascular Toxicology 2017, 17, 140–149. [CrossRef]
- Zhao, S.; Miao, D.; Zhu, K.; Kelin, T.; Wang, C.; Sharma, V.; Jia, H. Interaction of Benzo[a]Pyrene with Cu(II)-Montmorillonite: Generation and Toxicity of Environmentally Persistent Free Radicals and Reactive Oxygen Species. Environment International 2019, 129, 154–163. [CrossRef]
- Thevenot, P.; Saravia, J.; Jin, N.; Giaimo, J.; Chustz, R.; Mahne, S.; Kelley, M.; Hebert, V.; Dellinger, B.; Dugas, T.; et al. Radical-Containing PM0.2 Initiates Epithelial-to-Mesenchymal Transitions in Airway Epithelial Cells. American journal of respiratory cell and molecular biology 2012, 48. [CrossRef]
- Zhang, X.; Gu, W.; Ma, Z.; Liu, Y.; Ru, H.; Zhou, J.; Zang, Y.; Xu, Z.; Qian, G. Short-Term Exposure to ZnO/MCB Persistent Free Radical Particles Causes Mouse Lung Lesions via Inflammatory Reactions and Apoptosis Pathways. Environmental Pollution 2020, 261, 114039. [CrossRef]
- Balakrishna, S.; Saravia, J.; Thevenot, P.; Ahlert, T.; Lominiki, S.; Dellinger, B.; Cormier, S.A. Environmentally Persistent Free Radicals Induce Airway Hyperresponsiveness in Neonatal Rat Lungs. Particle and Fibre Toxicology 2011, 8, 11. [CrossRef]
- Huang, H.-S.; Ma, M.-C.; Chen, J.; Chen, C.-F. Changes in Renal Hemodynamics and Urodynamics in Rats with Chronic Hyperoxaluria and after Acute Oxalate Infusion: Role of Free Radicals. Neurourology and Urodynamics 2003, 22, 176–182. [CrossRef]
- Reinke, L.A.; Moore, D.R.; Nanji, A.A. Pronounced Hepatic Free Radical Formation Precedes Pathological Liver Injury in Ethanol-Fed Rats. Alcoholism: Clinical and Experimental Research 2000, 24, 332–335. [CrossRef]
- Burn, B.R.; Varner, K.J. Environmentally Persistent Free Radicals Compromise Left Ventricular Function during Ischemia/Reperfusion Injury. American Journal of Physiology-Heart and Circulatory Physiology 2015, 308, H998–H1006. [CrossRef]
- Wang, P.; You, D.; Saravia, J.; Shen, H.; Cormier, S.A. Maternal Exposure to Combustion Generated PM Inhibits Pulmonary Th1 Maturation and Concomitantly Enhances Postnatal Asthma Development in Offspring. Particle and Fibre Toxicology 2013, 10, 29. [CrossRef]
- Guan, X.; Truong, L.; Lomnicki, S.; Tanguay, R.; Cormier, S. Developmental Hazard of Environmentally Persistent Free Radicals and Protective Effect of TEMPOL in Zebrafish Model. Toxics 2021, 9, p12.
- Zhang, Y.; Guo, X.; Si, X.; Yang, R.; Zhou, J.; Quan, X. Environmentally Persistent Free Radical Generation on Contaminated Soil and Their Potential Biotoxicity to Luminous Bacteria. Science of The Total Environment 2019, 687, 348–354. [CrossRef]
- Zhang, X.; Chen, Y.; Hu, D.; Zhao, L.; Wang, L.; Wu, M. Neurotoxic effect of environmental persistent free radicals in rice biochar to Caenorhabditis elegans. China Environ. Sci. 2019, 39, 2644-2651.
- Stephenson, E.J.; Ragauskas, A.; Jaligama, S.; Redd, J.R.; Parvathareddy, J.; Peloquin, M.J.; Saravia, J.; Han, J.C.; Cormier, S.A.; Bridges, D. Exposure to Environmentally Persistent Free Radicals during Gestation Lowers Energy Expenditure and Impairs Skeletal Muscle Mitochondrial Function in Adult Mice. American Journal of Physiology-Endocrinology and Metabolism 2016, 310, E1003–E1015. [CrossRef]
- Lee, G.I.; Saravia, J.; You, D.; Shrestha, B.; Jaligama, S.; Hebert, V.Y.; Dugas, T.R.; Cormier, S.A. Exposure to Combustion Generated Environmentally Persistent Free Radicals Enhances Severity of Influenza Virus Infection. Part Fibre Toxicol 2014, 11, 57. [CrossRef]
- Briedé, J.J.; Godschalk, R.W.L.; Emans, M.T.G.; de Kok, T.M.C.M.; van Agen, E.; van Maanen, J.M.S.; van Schooten, F.-J.; Kleinjans, J.C.S. In Vitro and In Vivo Studies on Oxygen Free Radical and DNA Adduct Formation in Rat Lung and Liver during Benzo[a]Pyrene Metabolism. Free Radical Research 2004, 38, 995–1002. [CrossRef]
- Balakrishna, S.; Lomnicki, S.; McAvey, K.M.; Cole, R.B.; Dellinger, B.; Cormier, S.A. Environmentally Persistent Free Radicals Amplify Ultrafine Particle Mediated Cellular Oxidative Stress and Cytotoxicity. Particle and Fibre Toxicology 2009, 6, 11. [CrossRef]

| Source biomass | Ref. | Applications | Refs. |
|---|---|---|---|
| Crop residue | [34] | Carbon sequestration | [48] |
| Kitchen waste | [35] | ||
| Forestry | [36] | Soil amendment | [49] |
| Agricultural waste | [37] | ||
| Sugar beet tailings | [38] | Composting | [50] |
| Forest residues | [39] | ||
| Waste wood | [40] | Wastewater treatment | [51] |
| Bioenergy crops | [41] | ||
| Municipal solid waste | [42] | Energy production/storage | [52] |
| Wheat straw | [43] | Adsorbing xenobiotics | [53] |
| Rice straw | [44] | Reducing greenhouse emission gas | [54] |
| Food manure | [45] | Xenobiotics degradation | [55] |
| Animal manure | [46] | Catalysis | [56] |
| Corn cob | [47] |
| Pyrolysis | Temperature (°C) | Residence Time | Biochar (%) | Bio-oil (%) | Syngas (%) | Refs |
| 200−700 | 0.5-2 sec | 35 | 30 | 35 | [67] | |
| 500−1000 | Hours/day | 12 | 75 | 13 | ||
| HC | 180−300 | 1−16 h | 50−80 | 5−20 | 2−5 | [68] |
| Gasification | 750−900 | 10−20 s | 10 | 5 | 85 | [69] |
| Torrefaction | 290 | 10−60 min | 80 | 0 | 20 | [70] |
| Flash carbonization | 300−600 | < 30 min | 37 | -- | -- | [71] |
| Fast Pyrolysis | Slow Pyrolysis | Ref. | |
|---|---|---|---|
| Target Product | Bio-oils | Biochar | [79] |
| Reactors | Bubbling fluidized bed Ablative reactor Rotary cone |
Fixed bed pyrolysis reactor Auger pyrolysis reactor |
|
| Warming rate | 10-10000°C/min | 0.1-10°C/min | |
| RT at⇧ temperature | 0.5-2 seconds | > 1 hour | |
| Aeration | Oxygen-free | Oxygen-free or limited | |
| Advantages | ⇧ Yield of bio-oil | ⇧ Yield of BC Accepted a wide range of particle size |
|
| Disadvantages | ⇓ BC yield Required fine particles of biomass feed (1-2 mm) Prefer biomass with low moisture content (<10%) |
Further treatment of gases is needed due to high CO concentrations |
|
| Applications | ⇧ Potential for energy applications | Improvement of soil quality |
| Biochar characterization techniques | |||||
|---|---|---|---|---|---|
| Physicochemical Characterization | Surface Characterization | Structural/Molecular Characterization | |||
| Chemical | Physical | Investigation | Technique | Investigation | Technique |
| pH | Surface area (BET) | Morphology | SEM | Thermal behavior | TGA |
| Cation exchange capacity (CEC) |
Size (nm) (TEM) | Functional groups | FTIR/Raman | Structural arrangements | XRD |
| Electrical conductivity |
Bulk density | Surface elements | SEM, EDX, XFR | Aromaticity | NMR/Raman |
| Pore size (BET) | Surface oxygen | Boehm titration | Free radicals | EPR/ESR | |
| Properties | Discussion | |
|---|---|---|
| Chemical properties | Atomic ratio | ⇓ O/C and H/C ratio for untreated biomass |
| Elemental composition | ⇑ Carbon content (>95%) *⇓ Hydrogen content (<5%) *⇓ Oxygen content (<2%) * | |
| Energy content | ⇑ Energy content with temperature (From 15-20 MJ/kg a to 30–35 MJ/kg b at 700 °C | |
| Fixed carbon (FC) **Volatile matter (VM) | ⇑ in FC (from 10% a to 90%b at 700 °C)⇓ in VM (from 90% a to 10% b at 700°C) | |
| Structural composition | Partially decomposed cellulose cNear totally decomposed hemicellulose cPartially decomposed lignin c | |
| Release of O2 and H2⇓ Oxygenated functional groups in BC (OH and C=O groups) *⇑ Highly stable aromatic structures in BC *(the maximum aromaticity at 500-800 °C)⇑ Alkalinity and ability to neutralize acids in soils *⇑ Unpaired negative charges that enable BC to accept protons | ||
| pH value | ⇑ pH-value (from 5-7.5 a to 10-12 b at > 500 °C)⇑ Ash | |
| Cation exchange capacity (CEC) | ⇑ CEC for BCs produced at relatively ⇓ low temperatures | |
| Ash content (SiO2, CaO, K2O, P2O5, Al2O3, MgO) | ⇑ With temperature | |
| Self-heating degradation during storage | ⇓ Highly volatile content in BC⇓ Risk of self-heating⇑ Thermal stability⇓ Risk of spontaneous combustion⇓ Water content and microbial | |
| Physical properties | Density and porosity | ⇑ Weight-based energy density * at ⇑ temperature⇓ Bulk density * (the volume-specific weight of a bulk material in a heap or pile)⇑ Porosities at ⇑ temperature |
| Surface area | ⇑ Total surface area * (< 800°C)⇓ Total surface area * (> 800-1000°C) | |
| Pore volume distributionPore size distribution | ⇑ Total pore volume * with ⇑ temperatureMacropores (1000–0.05 μm)Mesopores (0.05–0.002 μm)Micropores (0.05–0.0001 μm (more than 80% of the total pore volume) | |
| HydrophobicityWater holding capacity (WHC) | ⇑ Hydrophobicity⇓ Affinity to water⇑ Porosity and amount of water that can be absorbed | |
| Mechanical stability | ⇓ Mechanical stability during carbonatization⇓ Structural complexity during carbonization | |
| Grindability | ⇑ Grindability compared to the parent material | |
| Thermal conductivityHeat capacity | ⇓ Thermal conductivity in BC(from 1300 J/(kgK) a to 1000 J/(kgK) b at 500°C) | |
| Electromagnetic properties | ⇑ Conductivity⇑ Electromagnetic shielding efficiency | |
| Feedstock | Pyrolysis temp. | Yield | Ash | pH | C | H | O | N | Surface area |
|---|---|---|---|---|---|---|---|---|---|
| (∘C) | (%) | (%) | (%) | (%) | (%) | (%) | (m2 g 1) | ||
| Canola straw | 400 | 27.4 | – | – | 45.7 | – | – | 0.19 | – |
| Corn cobs | 500 | 18.9 | 13.3 | 7.8 | 77.6 | 3.05 | 5.11 | 0.85 | 0.0 |
| Corn stover | 450 | 15.0 | 58.0 | – | 33.2 | 1.40 | 8.60 | 0.81 | 12.0 |
| Corn stover | 500 | 17.0 | 32.8 | 7.2 | 57.3 | 2.86 | 5.45 | 1.47 | 3.1 |
| Cottonseed hull | 200 | 83.4 | 3.1 | – | 51.9 | 6.00 | 40.5 | 0.60 | – |
| Cottonseed hull | 800 | 24.2 | 9.2 | – | 90.0 | 0.60 | 7.00 | 1.90 | 322.0 |
| Fescue straw | 100 | 99.9 | 6.9 | – | 48.6 | 7.25 | 44.1 | 0.64 | 1.8 |
| Fescue straw | 700 | 28.8 | 19.3 | – | 94.2 | 1.53 | 3.60 | 0.70 | 139.0 |
| Oak bark | 450 | – | 11.1 | – | 71.2 | 2.63 | 12.9 | 0.46 | 1.9 |
| Oakwood | 400–450 | – | 2.9 | – | 82.8 | 2.70 | 8.05 | 0.31 | 2.7 |
| Orange peel | 150 | 82.4 | 0.5 | – | 50.6 | 6.20 | 41.0 | 1.75 | 22.8 |
| Orange peel | 700 | 22.2 | 2.8 | – | 71.6 | 1.76 | 22.2 | 1.72 | 201.0 |
| Peanut shell | 300 | 36.9 | 1.2 | 7.8 | 68.27 | 3.85 | 25.89 | 1.91 | 3.1 |
| Peanut shell | 700 | 21.9 | 8.9 | 10.6 | 83.76 | 1.75 | 13.34 | 1.14 | 448.2 |
| Peanut straw | 400 | 28.2 | – | – | 42.90 | – | – | 1.50 | – |
| Pine needles | 100 | 91.2 | 1.1 | – | 50.87 | 6.15 | 42.27 | 0.71 | 0.7 |
| Pine needles | 700 | 14.0 | 2.2 | – | 86.51 | 1.28 | 11.08 | 1.13 | 490.8 |
| Pine shaving | 100 | 99.8 | 1.2 | – | 50.60 | 6.68 | 42.70 | 0.05 | 1.6 |
| Pine shaving | 700 | 22.0 | 1.7 | – | 92.30 | 1.62 | 6.00 | 0.08 | 347.0 |
| Pinewood | 700 | – | 38.8 | 6.6 | 95.30 | 0.82 | 3.76 | 0.12 | 29.0 |
| Poplar wood | 400 | 32.0 | 3.5 | 9.0 | 67.30 | 4.42 | – | 0.78 | 3.0 |
| Rice husk | 500 | – | 42.2 | – | 42.10 | 2.20 | 12.10 | 0.50 | 34.4 |
| Saw dust | 450 | – | 1.1 | 5.9 | 72.00 | 3.50 | 24.41 | 0.08 | – |
| Saw dust | 550 | – | 2.8 | 12.1 | 85.00 | 1.00 | 13.68 | 0.30 | – |
| Soybean stover | 300 | 37.0 | 10.4 | 7.3 | 68.81 | 4.29 | 24.99 | 1.88 | 5.6 |
| Soybean stover | 700 | 21.6 | 17.2 | 11.3 | 81.98 | 1.27 | 15.45 | 1.30 | 420.3 |
| Soybean straw | 400 | 24.7 | – | – | 44.10 | – | – | 2.38 | – |
| Spruce wood | 400 | 36.0 | 1.9 | 6.9 | 63.50 | 5.48 | – | 1.02 | 1.8 |
| Spruce wood | 525 | – | 4.7 | 8.6 | 78.30 | 3.04 | – | 1.17 | 40.4 |
| Wheat straw | 400 | 34.0 | 9.7 | 9.1 | 65.70 | 4.05 | – | 1.05 | 4.8 |
| Wheat straw | 525 | – | 12.7 | 9.2 | 74.40 | 2.83 | – | 1.04 | 14.2 |
| Chicken litter | 620 | 43-49 | 53.2 | - | 41.50 | 1.20 | 0.70 | 2.77 | - |
| Poultry litter | 350 | 54.3 | 30.7 | 8.7 | 51.07 | 3.79 | 15.63 | 4.45 | 3.9 |
| Poultry litter | 700 | 36.7 | 46.2 | 10.3 | 45.91 | 1.98 | 10.53 | 2.07 | 50.9 |
| Tire rubber | 200 | 93.5 | 15.0 | - | 74.70 | 6.38 | 3.92 | - | - |
| Tire rubber | 800 | 43.0 | 10.5 | - | 86.0 | 0.87 | 2.16 | 0.47 | 50.0 |
| Application | Mechanisms | Refs. |
|---|---|---|
| Climate change mitigation | Sequestering carbon in soil ⇓ CO2 emissions into the atmosphere ⇓ NO2 emissions ⇓ CH4 emissions Tackling 12% of current anthropogenic carbon emissions |
[54] |
| Soil improvement | ⇑ Physicochemical and biological properties of soils ⇑ Water retention capacity of soil ⇓ Nutrient leaching ⇓ Acids in soils ⇑ Microbial population and microbial activity in soils Positive impacts on the earthworm population Preventing desiccation |
[49] |
| Waste management | By pyrolyzing waste biomass * | [87] |
| Energy production | By conversion of waste biomass to BC by fast pyrolysis, thus providing liquid fuel (bio-oil) |
[52] |
| Capturing contaminants | By adsorption of both organic pollutants and/or metal ions from soil and water |
[53,55] |
| Composting | ⇑ Physicochemical properties of composting ⇑ Composting microbial activities ⇑ Organic matter decomposition |
[50] |
| Advantages | Disadvantages |
|---|---|
| Obviate to significant modification on Earth | Gaseous aerosol emissions during improper pyrolysis |
| Enhanced soil productivity | Environmental pollution from dust, erosion and leaching of BC particles |
| Higher food security | Ash could be at risk for respiratory diseases. |
| Solution of xenobiotics danger | BC can sequester water and nutrients no further available for crops |
| Addressing waste management | Not desired sorption of residual herbicides and pesticides |
| Reduced utilization of fossil fuels | Long-term removal of crop residues for producing BCs can reduce overall soil health by diminishing the number of soil microorganisms and disrupting internal nutrient cycling |
| Less expensive than activated carbon (AC) | Possible negative impact on soil biota |
| Improvement of living microbiology in soil | Short-term adverse effects on earthworm population density |
| Greater WHC than AC | No universal reduction in nitrous oxide emissions |
| Enhanced food web in soil | |
| Improved aeration in the soil | |
| Reduced loss of nutrients through leaching |
| Capturing Mechanism | Influencing factors #, Details °, Examples § | Ref. |
|---|---|---|
| Sorption * | ⇑ Surface area # Microporosity of BC # pH # |
[98] |
| Hydrogen bond formation ** | For polar compounds °,** | |
| Electrostatic attraction/repulsion | For cationic compounds ° Interaction between positively charged cationic organic contaminants and negatively charged BC surfaces °,** |
|
| Electrostatic outer sphere complexation |
Due to metallic exchange with K+ and Na+ available in BC °,** | |
| Hydrophobic interactions *** | For non-polar compounds ° | |
| Diffusion | Non-ionic compounds can diffuse into the non-carbonized and carbonized fractions of BC ° |
|
| Formation of surface complexes ** | pH # Ionic radius # Between metal cations and -OH, -COOH on BCs ° |
|
| Precipitation | Lead precipitates as lead-phosphate-silicate in BC § Co-precipitates and inner-sphere complexes can form between metals and organic matter/mineral oxides of BC § |
| Parameter | Influencing Factors | Specifications | Observations | Ref. |
|---|---|---|---|---|
| PFRs concentration |
Biomass type | Cow manure, rice husk, others (< 500°C) | ≠ Concentrations | [114,115] |
| Non-lignocellulosic biomass with ⇓ H/C and O/C | ⇓ Concentration | [116] | ||
| Lignocellulosic biomass | ⇑ Concentration | |||
| Temperature | 300°C, 700°C | ≠ Concentrations | [114] | |
| Maximum concentration at 600°C | [117] | |||
| Maximum of concentration at 500-600°C | [10,118] | |||
| Transition metals | Adsorb onto biomass and transfers electrons from polymer to metal center during pyrolysis | ⇑ Concentration | [18] | |
| Type of PFRs | Temperature | 200-300°C | Oxygen centered radicals | [10] |
| 400°C | A mixture of oxygen and carbon-centered radicals |
|||
| 500-700°C | Exclusively carbon centered radicals |
| Radicals | g-value | Features |
|---|---|---|
| Carbon-centered radicals | < 2.003 | Susceptible to oxidation in air |
| Carbon-centered radicals adjacent to an oxygen atom (oxygenated carbon-centered radicals) |
2.003–2.004 | Susceptible to oxidation in air |
| Oxygen-centered radicals | > 2.004 | More stable in an atmospheric environment |
| Semiquinone radicals (oxygen-centered) | > 2.0045 | More resistant to react with molecular oxygen in ambient environment |
| Phenoxy radicals (oxygenated carbon-centered radicals) |
2.0030–2.0040 | Susceptible to oxidation in air |
| Cyclopentadienyls (carbon-centered radicals) |
< 2.003 | Susceptible to react with molecular oxygen in ambient environment |
| EPFRs actions | Degraded substances * | Mechanism | Refs. |
|---|---|---|---|
| Activation of H2O2 by single electron transferring |
SMX, CIP, SMT, TC, OG, MNZ, ERF benzene |
Oxidation by the production of ROS (OH•#, HO2•, O2•-) |
[18,123] |
| Activation of O2 by single electron transferring | Degradation of organic compounds Chloro-biphenyl Phenolic compounds Polychlorinated biphenyls Diethyl phthalate Thiacloprid Bisphenol A |
Oxidation by the production of radical superoxide (O2•-) |
[7,11] [19,104] |
| Activation of persulfate (S2O82−) | X-3B, SMT, CTC, SMX, TC, MB, SDZ, OG | Oxidation by the production of sulfate radicals (SO4 −•) |
[123] |
| Direct activity of macromolecular radicals on the BC surface |
Direct degradation of organic chemicals | Oxidation | [55] |
| Direct activity of semiquinone-type radicals |
As (III) removal | Oxidation | [124] |
| Direct activity of PFRs | Removal of Cr (VI) | Reduction to Cr (III) | [125,126,127,128,129] |
| Catalytic effects | Detoxification of environmental xenobiotics |
Generation of activated species Stimulation of the microbial biotransformation |
[55] |
| Ions’ exchange | Enhancement of agricultural soil performance |
Maintenance of CEC in soils | [130] |
| Electron-hole pair formation |
Photo-catalytically degradation of contaminants under Vis irradiation | Electrons in free radicals can be transformed from the valence band to the conduction band under irradiation | [131] |
| Biomass | Pyrolysis °C/Time | BC-name | Active radicals | Radical Mechanisms | Application 1 Degraded compound 2 |
Refs. |
|---|---|---|---|---|---|---|
| Sawdust | 700°C/1h | Fe0-BC-700 | SO4•- PFRs OH• | Activation of PMS by Fe0 Activation of PMS by PFRs |
BPA 2 | [135] |
| Waste wood | 500°C 700°C | Fe0-BC | SO4•- PFRs OH• | Production of PFRs by Fe0 Activation of PS by Fe0 Activation of PS by PFRs |
TDWW 2 | [136] |
| Camellia seed husks | 400°C/2h | OBC-Fe3O4 | SO4•- PFRs OH• | Activation of PS | TC 2 | [137] |
| Sawdust | 300°C 700°C | SBC | SO4•- PFRs OH• | Activation of PS | AO-7 2 | [138] |
| N.R. | 200°C 500°C | N.R. | PFRs • O2- | UV-induced interaction PFRs/DOM and • O2- production | RhB 2 | [139] |
| Sewage sludge | 500°C/4h | HNO3-BC | PFRs • O2- •OH •O2H | Activation of H2O2 | CIP 2 | [21] |
| Wheat straw | 500°C/2h | BC/Fe (III) | SO4•- PFRs OH• | Activation of PS by PFRs | SMX 2 | [140] |
| Sawdust | 700°C | BC700 | SO4•- PFRs OH• | Activation of PDS by PFRs | CA 2 | [141] |
| Pine needle | 500°C/2h | Fe/Mn/BC | •OH | Activation of H2O2 by Fe (II), Mn (II) and PFRs (FeMn/BC/H2O2 photo-Fenton system) |
Naphthalene 2 | [142] |
| Sewage sludge | 500°C/4h | SS-BC | PFRs • O2- •OH •O2H | Activation of O2 and H2O2 by PFRs Degradation of PNP by PFRs |
CIP 2 | [143] |
| Swine manure | 600°C | SBC | OCPFRs CCPFRs-O • OH •O2H |
Activation of oxygenated species by OCPFRs and CCPFRs-O (heterogeneous Fenton-like systems SBC/ H2O2) |
SMT 2 | [144] |
| Wheat straw | 300°C 600°C | BC300 BC600 | •OH •O2H | Goethite (Gt)-mediated activation of H2O2 (Fenton-like system) |
OFX2 | [145] |
| Wheat straw | 500°C/2h 800°C/2h | CoBCX | SO4•- PFRs OH• | Cobalt and PFRs mediated activation of PMS via O2 | ATZ2 | [146] |
| Various crop straws | 450, 550 650°C |
BC450,550 BC650 |
SO4•- • O2- OH• | BC mediated activation of PS by electron transferring | SDZ2 | [147] |
| Tobacco steam | 300℃ 500℃ 700℃ |
T-BC | ROS | OCPFRs mediated activation of O2 in the water | PNP2 | [148] |
| Pruning wastes of apple trees | 400°C550°C 700°C |
BC400, BC550 BC700 | SO4•− PFRs | BC and PFRs mediated activation of PS | ACT2 | [149] |
| Camphor leaves | 400°C/6h | Fe (TPFPP)/BC | SO4•- PFRs OH• | PFRs-mediated electrons transferring to iron porphyrin-loaded BC 3 | PFOA2 | [150] |
| Corn stalks | 240°C/4h | hydrochar | •OH | Electrode and PFRs mediated generation of ROS | 2,4-DCP2 | [151] |
| Wheat straw | 450°C/4h | Co3O4-BC | SO4•- PFRs OH• | Co3O4-BCmediated activation of PMS | CAP2 FF2 TAP2 | [152] |
| Wheat straw Urea Iron salts |
800°C/1h | Fe-N-BC | SO4•- PFRs •OH • O2- | Fe, N co-doped BC and PFRs mediated activation of O2 and PS | AO72 | [153] |
| Candida utilis | 700°C/2h | NCS-x | SO4•- PFRs OH• | Activation of PMS by nitrogen-doped biochar nanosheets (NCS-x) using molten salt (NaCl and KCl) in the pyrolysis process | BPA2 BPF2 BPS2 BPAF2 | [154] |
| Pine needles | 500°C | nFe3O4/BC | PFRs •O2H •OH • O2- | Activation of H2O2 by nano-magnetite supported biochar via Fe (III)/Fe (II) cycling and electron transfer with the PFRs | Ethylbenzene2 | [155] |
| Sewage sludge | 800°C/3h | SM-(0.5:1) | SO4•- PFRs OH• | Activation of PMS by nitrogen-doped sludge biochar with different ratios of melamine in acidic | Cationic/anionic dyes2 | [156] |
| Elefant grass | 350°C 600°C 900°C 30-120 min |
EG | OCPFRs | OCPFRs mediated oxidation | CV2 | [157] |
| Sunflower-straw | N.R. | SSBC | SO4•- PFRs OH• | Enhanced Fe (II) activation of PS via BC and PFRs | Benzoic acid2 | [158] |
| Pine chips | 500°C | OP5 RP5 |
SO4•- PFRs •OH • O2- •O2H |
EDC-involved structures, Fe3+ and BC (PFRs) mediated activation of PS in a Fenton-like reaction system using H2O2 and NaBH4 | 2,4-DCP2 | [159] |
| Rice straw | 350°C 500°C 700°C |
BCs MBCs BDOMs |
PFRs •OH | Direct photocatalytic degradation in BCs and MBCs solutions by Xenon-lamp Oxygen reduction by FPRs of BCs and MBCs BDOMs mediated generation of ROS |
SMX2 CAP2 | [160] |
| Pomelo peels | 600°C | Fe@PP-Hy-Py | PFRs •OH • O2- | Amorphous Fe (0) mediated formation of PFRs Fe (0) mediated reduction of PNP EPFRs mediated oxidation of PNP via ROS (O2 and H2O2) activation |
PNP2 | [161] |
| Softwood pine | 823-873 K | US-BC BC-P BC-P-DEA US-BC-P-DEA US-BC-P-DEA |
PFRs •OH • O2- •O2H | Reinforcement of PFRs concentration doping BCs with Ni and Pb Activation of H2O2 by PFRs |
Phenol2 | [162] |
| Camphor leaves | 500 °C/1h | Fe (VI)/BC-2 | Fe(Ⅴ)/Fe(Ⅳ) PFRs •OH | Fe (VI)-BC (PFRs) mediated electron transferring and generation of ROS | AZT2 | [163] |
| Bagasse powder | 800 °C | DBC800 PBC800-A | SO4•- PFRs •OH• O2- •O2H |
Enhanced BCs mediated activation of PS Improved PFRs generation by natural endogenous minerals |
TC2 | [164] |
|
Eichhornia crassipes Iron salts |
400 °C/2h | MBC | PFRs •OH• O2-•O2H | Fe (II)-BC mediated activation of H2O2 (Fenton-like system) |
MNZ2 | [165] |
| Poplar and pine sawdust | 300-500°C | PO xxx PI xxx |
SO4•- PFRs •OH• O2- | Activation of PMS by CCEPFRs-O and CCEPFRs in BC | TC2 CTC2 DOX2 | [166] |
|
S. alfredii |
Air-dried | Metal@P | •O2H | PFRs generation by the thermochemical behaviour of Mn and Zn Electron transfer Activation of PDS by PFRs in Fe/Zn@PB9/PDS system AOPs |
Imidacloprid2 | [167] |
| Sludge |
N.R. | N.R. | SO4•- PFRs •OH• O2- | Production of ROS via PFRs Mn-mediated electron transfer through Mn-doped sludge-based biochar (BC) mediated the | CIP2 | [168] |
| Cellulose Lignin |
200-1000°C | C200, C500 C1000 L200, L500 L1000 |
SO4•-PFRs • O2- | Activation of PS adsorbed onto BCs via PFRs, oxygen-containing functional groups, and defective structures of BCs | OFX2 | [169] |
| Chestnut shell KMnO4 |
700 °C/1h 400 °C/1h |
Mn-BC | PFRs | Mn-improved electron-transfer | OTC2 | [170] |
| Spent coffee TiO2 |
300 °C 500 °C 600 °C |
SBC500 | PFRs •OH• O2-•O2H | Activation of H2O2 by Ti-doped H2SO4-modified biochar (SBCs) (Photo-Fenton-like system) |
MO2 | [171] |
| RS | 550 °C/2h |
BC-α-Fe2O3/MgO | PFRs •OH• O2-•O2H | UV light activation of PFRs Production of O2 upon NPA degradation O2 activation by PFRs |
NPA2 | [172] |
| Sewage sludge | 400 °C/2h | SDBC | PFRs •OH• O2-•O2H | O2 activation by PFRs promoted by HNO3 or NaOH environmental |
p-Chlorophenol2 | [173] |
| Peanut hull | 700 °C/2h | BC-Fe-1-Zn | SO4•- PFRs •OH | Activation of PS by bimetal-modified peanut hull-derived biochar via Fe and Zn oxides and oxygen-containing functional groups active sites | TC2 | [174] |
| Blue algae | 700 °C | Z-700 FeOX@BC |
SO4•- PFRs •OH •O2-•O2H | • O2- production by FeOX (zero-valent iron and iron oxide) C=C, C=O, O-C=O, Fe-O functional groups and PFRs promoted the activation of PDS |
TC2 | [175] |
| Biomasses | 300-1000 °C | N.R. | SO4•- PFRs •OH • O2- | Activation of PS and PMS by physical and chemical modified BCs using acid/alkali treatment and metal doping via PFRs | PPCPs2 | [176] |
| Chicken feathers | 350°C/4h 800°C/4h |
MBC35@FH MBC80@FH | SO4•- PFRs •OH • O2- | Activation of PDS by the transformation of Fe species, oxygen-containing functional groups, pyrrolic nitrogen, and PFRs to produce ROS | TPhP2 | [177] |
| Pine needles | 300-900 °C | BC300-900 | SO4•- PFRs •OH • O2- | Activation of PMS by BC via ROS production or electron transfer capability | OFX2 ENR2 FLE2 | [178] |
| PolyS | 220°C/2h $ 500°C /2h # 900°C /2h # |
BC500 + PS BC900 + PS BC500 BC900 |
SO4•- PFRs •OH • O2- | Activation of PMS using CCEPFRs on BC-aged by microbial fermentation for ROS production | SDZ2 OFX2 DOX2 | [179] |
| Red mud Wheat crop |
700°C /2h | MRBC | SO4•-PFRs • OH | PDS activation by the active sites of MRBC, such as Fe (II) and PFRs | LFX2 | [180] |
| Various sludges | 300-900°C 2h |
S-HPBC S-PBC S-HBC |
SO4•- PFRs •OH • O2- | Activation of PS by PFRs mediated electrons transferring activity Electrons transferring to Cr (VI) by PFRs |
TC2 Cr (VI) ⇒ Cr (III) 1 |
[181] |
| Peanut shells | 500 °C/4 h | BC-Ce | OFGs, CCPFRs | Electrons transferring to Cr (VI) by OFGs, CCPFRs, oxygen vacancies and graphitic structure in BC-Ce promoted by CeO2 | Cr (VI) ⇒ Cr (III) 1 | [182] |
| Rice husk | 400°C/1h | BC400 | OH• H2O2 | (pH acid) Activation of O2 by phenol OH and semiquinone types PFRs |
As (III) ⇒ As (V) 1 | [124] |
| Semiquinone-type PFRs Quinoid C═O H2O2 |
(pH alkaline) Activation of O2 by phenol OH and semiquinone types PFRs |
|||||
| Rice husk | 550°C | RH-BC | PRFs | Promotion of OCPFRs by BC-inducted Cr (VI) degradation | Cr (VI) ⇒ Cr (III) 1 | [125] |
| Stalk | 450°C/90 min | NBC | PFRs | N-doped BC mediated evolution of PFRs for the transformation of Cr (VI) | Cr (VI) ⇒ Cr (III) 1 | [126] |
| Rice husk | 500°C/2h | MGBs | PFRs •OH • O2- | Efficient surface Fe (III)/Fe (II) cycling via electron transfer with the PFRs of magnetic greigite/BC composites (MGBs) | Cr (VI) ⇒ Cr (III) 1 | [127] |
| Sludge | 220 °C/2h | BC | OCPFRs | UV-Vis photo-irradiation enhanced the production of PFRs Action of OCPFRs as electron donors to transform Cr (VI) into Cr (III) |
Cr (VI) ⇒ Cr (III) 1 | [128] |
| Sludge | 120 °C | SBC120 | OCPFRs | OCPFRs mediated electrons transferring to Cr (VI) in neutral solutions | Cr (VI) ⇒ Cr (III) 1 | [129] |
| 270 °C | SBC270 | CCPFRs | CCPFRs mediated electrons transferring to Cr (VI) in neutral solutions | |||
| Rice husk | 400°C/1h | rUBC, rDBC | Quinoid C=O PFRs | Quinoid C=O and PFRs mediated oxidation of As (III) | As (III) ⇒ As (V) 1 | [183] |
| Maize straw powder |
500°C/2h | FhBC | PFRs • O2- •OH | Fe and PFRs mediated activation of O2 and H2O2 | As (III) ⇒ As (V)1 | [184] |
| Sewage sludge | 270 °C/2h | SBC | SO4•- PFRs •OH • O2- | Activation of PS by SBC via PFRs mediated electrons transferring | As (III) ⇒ As (V) 1 | [185] |
| Pinewood | 600°C/1h | Fe/HC | • O2- •OH | Activation of O2 and H2O2 by CCPFRs | Estrogens 2 | [186] |
| Rice straw | 500°C/1h | BiPB | •OH PFRs | Generation of •OH by Bi/Bi2O3 and PFRs | Estrone 2, * | [187] |
| Anaerobic digestion sludge | 400°C 600°C 800°C 1000°C |
ADSBC 400 ADSBC 600 ADSBC 800 ADSBC 1000 |
SO4•- PFRs OH• | BC-mediated activation of PDS | Dyes2 Estrogens2 Sulfonamides2 E. coli2 Others2 |
[188] |
| Walnut shell | 700 °C/1h | BC700 | PFRs | Oxidation by PFRs-mediated electron transfer | E12 E22 E32 | [189] |
|
Caragana korshinskii |
650 °C/3h | ACB-K-gC3N | PFRs h+•OH• O2- | Electron photogeneration and PFRs mediated H2O and O2 activation |
S. aureus2E. coli2 RhB2 TA2 NOR2 CAP2 |
[190] |
| Pinewood | 600°C | Ag0-PBC | PFRs •OH • O2- | UV-light promoted excitation of the electron-hole pairs and Subsequently, the production of ROS Enhanced ROS generation by PFRs |
MB2 E. coli2 | [191] |
| Rice straw | 400°C 700°C 120 min |
Nano-BC | PFRs •OH • O2- | Oxidation and damage by ROS | eDNA2 | [192] |
| Rice straw | 500℃ | RS-BC | Quinones Phenols PFRs | By electron acceptor capacity (EAC) By electron donor capacity (EDC) |
⇆ Redox property 1 Electronic storage 1 |
[193] |
| Target | Danger | Material source | Refs. |
|---|---|---|---|
| Cells | ⇑ Lungs’ T (Th1, Th2, Th17) cells | PM, DCB230, MCP230 | [205,206] |
| ⇓ P450 activity | PM, MCP230 | [207] | |
| Cardiomyocytes’ apoptosis | DCB230 | [208] | |
| ⇓ Survival of gastric epithelial cells | BaP–Na montmorillonite | [209] | |
| Loss of normal morphology of pulmonary epithelial cells | DCB230 | [210] | |
| Mitochondrial depolarization | DCB230 | [206] | |
| Changes in VEGF | ZnO/MCB | [211] | |
| Enzymes Proteins Genes |
Altered expression activity of Cyp1a, Cyp2b, Cyp2e1, Cyp2d2, Cyp3a and other genes | DCB230, MCP230 | [205] |
| ⇑ Expression levels of peroxiredoxin-6 Cofilin 1, annexin A8 |
MCP230, CGUFP, ZnO/MCB | [206] | |
| ⇓ of GSH, GPx, SOD | ZnO/MCB | [212] | |
| Organs and tissues | Altered normal renal hemodynamics and urodynamics | N.R. | [213] |
| Liver damage | N.R. | [214] | |
| Impair left ventricular function | DCB230 | [215] | |
| Airway hyperresponsiveness Lung inflammation |
MCP230 | [216] | |
| Individuals | Abnormalities in zebrafish | DCB230 | [217] |
| ⇓ Growth and reproduction of luminescent bacteria | PM | [218] | |
| Altered behavior of Caenorhabditis elegans | Biochar | [219] | |
| ⇓ Energy consumption | MCP230 | [220] | |
| Disease | ⇑ Severity of the flu | DCB230 | [221] |
| Asthma | MCP230 | [206] | |
| Cardiovascular disease and dysfunction | DCB230 | [208] | |
| Other damage | Oxidative stress | DCB230, ZnO/MCB | [212] |
| DNA damage | BaP | [222] | |
| Lipid peroxidation | MCP230 | [223] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





