Submitted:
11 March 2024
Posted:
17 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Accelerated Aging and Characterization Techniques
3. Results
3.1. Materials Characterization
3.2. Accelerated and Natural Aging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saunders, K.J. Poly(Vinyl Acetate) and Related Polymers. In Organic Polymer Chemistry: An Introduction to the Organic Chemistry of Adhesives, Fibres, Paints, Plastics and Rubbers; Springer: Dordrecht, The Netherlands, 1988; pp. 113–124. [Google Scholar]
- Conner, A. Wood: Adhesives. Encycl. Mater. Sci. Technol. 2001, 9583–9599. [Google Scholar]
- Novak, M.; Ormsby, B. Poly(Vinyl Acetate) Paints: A Literature Review of Material Properties, Ageing Characteristics, and Conservation Challenges. Polymers 2023, 15, 4348. [Google Scholar] [CrossRef]
- Grassie, N. The Thermal Degradation of Polyvinyl Acetate: Part 2. - Determination of the Rate Constants of the Primary Processes Involved in the Elimination of Acetic Acid. Trans. Faraday Soc. 1953, 49, 835–842. [Google Scholar] [CrossRef]
- Buchanan, K.J.; McGill, W.J. Photodegradation of Poly(Vinyl Esters)-I. Formation and Quantitative Measurement of Volatile Products. Eur. Polym. J. 1980, 16, 309–312. [Google Scholar] [CrossRef]
- Toja, F.; Saviello, D.; Nevin, A.; Comelli, D.; Lazzari, M.; Levi, M.; Toniolo, L. The Degradation of Poly(Vinyl Acetate) as a Material for Design Objects: A Multi-Analytical Study of the Effect of Dibutyl Phthalate Plasticizer. Part 1. Polym. Degrad. Stab. 2012, 97. [Google Scholar] [CrossRef]
- Wei, S.; Pintus, V.; Schreiner, M. Photochemical Degradation Study of Polyvinyl Acetate Paints Used in Artworks by Py-GC/MS. J. Anal. Appl. Pyrolysis 2012, 97, 158–163. [Google Scholar] [CrossRef]
- Pintus, V.; Wei, S.; Schreiner, M. Accelerated UV Ageing Studies of Acrylic, Alkyd, and Polyvinyl Acetate Paints: Influence of Inorganic Pigments. Microchem. J. 2016, 124, 949–961. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Silva, M.F.; Aura-Castro, E.; Fuster-López, L.; Kröner, S.; Martínez-Bazán, M.L.; Más-Barberá, X.; Mecklenburg, M.F.; Osete-Cortina, L.; Doménech, A.; et al. Study of Behaviour on Simulated Daylight Ageing of Artists’ Acrylic and Poly(Vinyl Acetate) Paint Films. In Proceedings of the Analytical and Bioanalytical Chemistry; Springer, March 28 2011; Vol. 399, pp. 2921–2937.
- Ferreira, J.L.; Melo, M.J.; Ramos, A.M. Poly(Vinyl Acetate) Paints in Works of Art: A Photochemical Approach. Part 1. Polym. Degrad. Stab. 2010, 95, 453–461. [Google Scholar] [CrossRef]
- Toja, F.; Saviello, D.; Nevin, A.; Comelli, D.; Lazzari, M.; Valentini, G.; Toniolo, L. The Degradation of Poly(Vinyl Acetate) as a Material for Design Objects: A Multi-Analytical Study of the Cocoon Lamps. Part 2. Polym. Degrad. Stab. 2013, 98. [Google Scholar] [CrossRef]
- Chelazzi, D.; Chevalier, A.; Pizzorusso, G.; Giorgi, R.; Menu, M.; Baglioni, P. Characterization and Degradation of Poly(Vinyl Acetate)-Based Adhesives for Canvas Paintings. Polym. Degrad. Stab. 2014, 107, 314–320. [Google Scholar] [CrossRef]
- Ploeger, R.; René De La Rie, E.; McGlinchey, C.W.; Palmer, M.; Maines, C.A.; Chiantore, O. The Long-Term Stability of a Popular Heat-Seal Adhesive for the Conservation of Painted Cultural Objects. Polym. Degrad. Stab. 2014, 107, 307–313. [Google Scholar] [CrossRef]
- Chiozza, F.; Santoni, I.; Pizzo, B. Discoloration of Poly(Vinyl Acetate) (PVAc) Gluelines in Wood Assemblies. Polym. Degrad. Stab. 2018, 157, 90–99. [Google Scholar] [CrossRef]
- Down, J.L.; MacDonald, M.A.; Tétreault, J.; Williams, S. Adhesive Testing at the Canadian Conservation Institute - An Evaluation of Selected Poly(Vinyl Acetate) and Acrylic Adhesives. Stud. Conserv. 1996, 41, 19–44. [Google Scholar] [CrossRef]
- Gómez, M.; Kadkhodazadeh, S.; Lazzari, M. Surface Enhanced Raman Scattering (SERS) in the Visible Range on Scalable Aluminum-Coated Platforms. Chem. Commun. 2018, 54, 10638–10641. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Reggio, D.; Lazzari, M. Detection of Degradation Markers from Polymer Surfaces by a Novel SERS-Based Strategy. Talanta 2019, 191, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, M.; Reggio, D. What Fate for Plastics in Artworks? An Overview of Their Identification and Degradative Behaviour. Polymers 2021, 13, 883. [Google Scholar] [CrossRef]
- De Sá, S.F.; Viana, C.; Ferreira, J.L. Tracing Poly(Vinyl Acetate) Emulsions by Infrared and Raman Spectroscopies: Identification of Spectral Markers. Polym. 2021, 13, 3609. [Google Scholar] [CrossRef]
- Silva, M.F.; Doménech-Carbó, M.T.; Fuster-Lopéz, L.; Martín-Rey, S.; Mecklenburg, M.F. Determination of the Plasticizer Content in Poly(Vinyl Acetate) Paint Medium by Pyrolysis-Silylation-Gas Chromatography-Mass Spectrometry. J. Anal. Appl. Pyrolysis 2009, 85, 487–491. [Google Scholar] [CrossRef]
- Silva, M.F.; Doménech-Carbó, M.T.; Osete-Cortina, L. Characterization of Additives of PVAc and Acrylic Waterborne Dispersions and Paints by Analytical Pyrolysis-GC-MS and Pyrolysis-Silylation-GC-MS. J. Anal. Appl. Pyrolysis 2015, 113, 606–620. [Google Scholar] [CrossRef]
- Clough, R.; Billingham, N.; Gillen, K. Polymer Durability: Degradation, Stabilization, and Lifetime Prediction; ACS Advances in Chemistry Series 249; American Chemical Society: Washington, DC, USA, 1996. [Google Scholar]
- Rodríguez-Vázquez, M.; Liauw, C.M.; Allen, N.S.; Edge, M.; Fontan, E. Degradation and Stabilisation of Poly(Ethylene-Stat-Vinyl Acetate): 1 - Spectroscopic and Rheological Examination of Thermal and Thermo-Oxidative Degradation Mechanisms. Polym. Degrad. Stab. 2006, 91, 154–164. [Google Scholar] [CrossRef]
- Pereira, A.I.M.L. The Perfect Paint in Modern Art Conservation: A Comparative Study of 21st Century Vinyl Emulsions, NOVA School of Science and Technology, Lisbon, Portugal, 2015.
- J. Brandrup, E.H.I. and E.A.G. Polymer Handbook; 4th ed.; John Wiley & Sons, Inc., 1999; ISBN 978-0-471-47936-9.
- Nørbygaard, T.; Berg, R.W. Analysis of Phthalate Ester Content in Poly(Vinyl Chloride) Plastics by Means of Fourier Transform Raman Spectroscopy. Appl. Spectrosc. 2004, 58, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Hilles, H.; Monroy, F. Dilational Creep Compliance in Langmuir Polymer Films. Soft Matter 2011, 7, 7790–7796. [Google Scholar] [CrossRef]
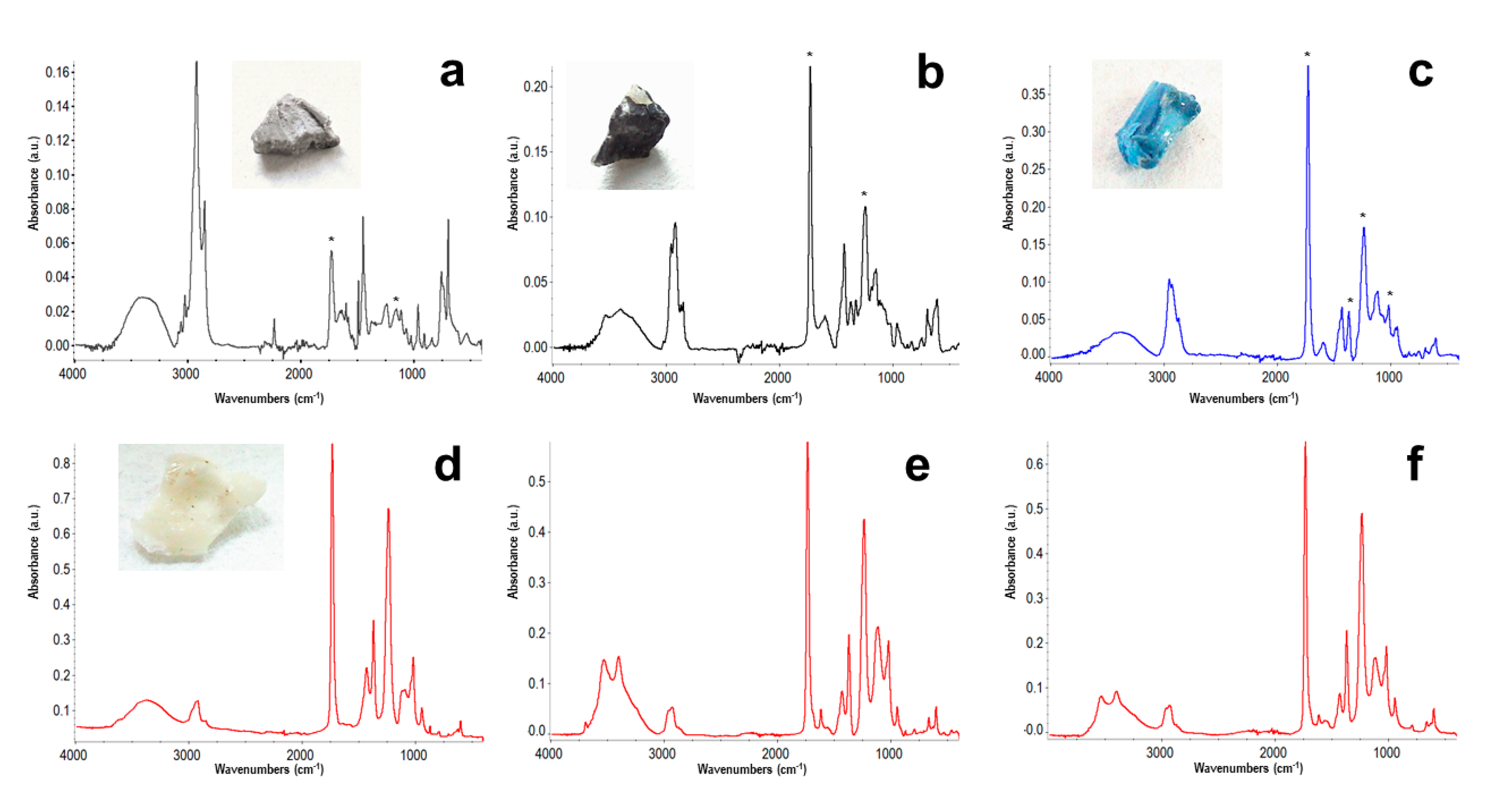
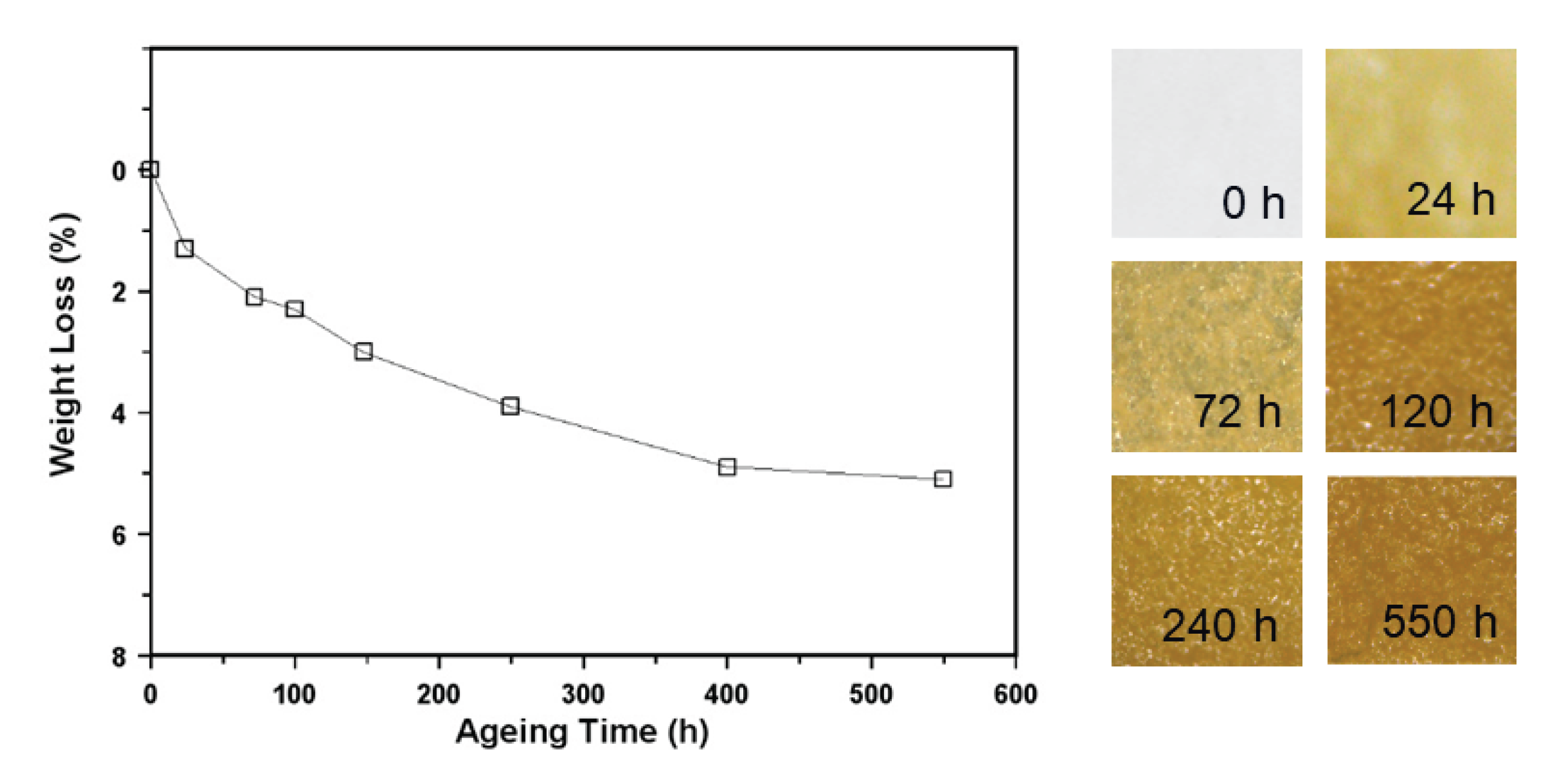
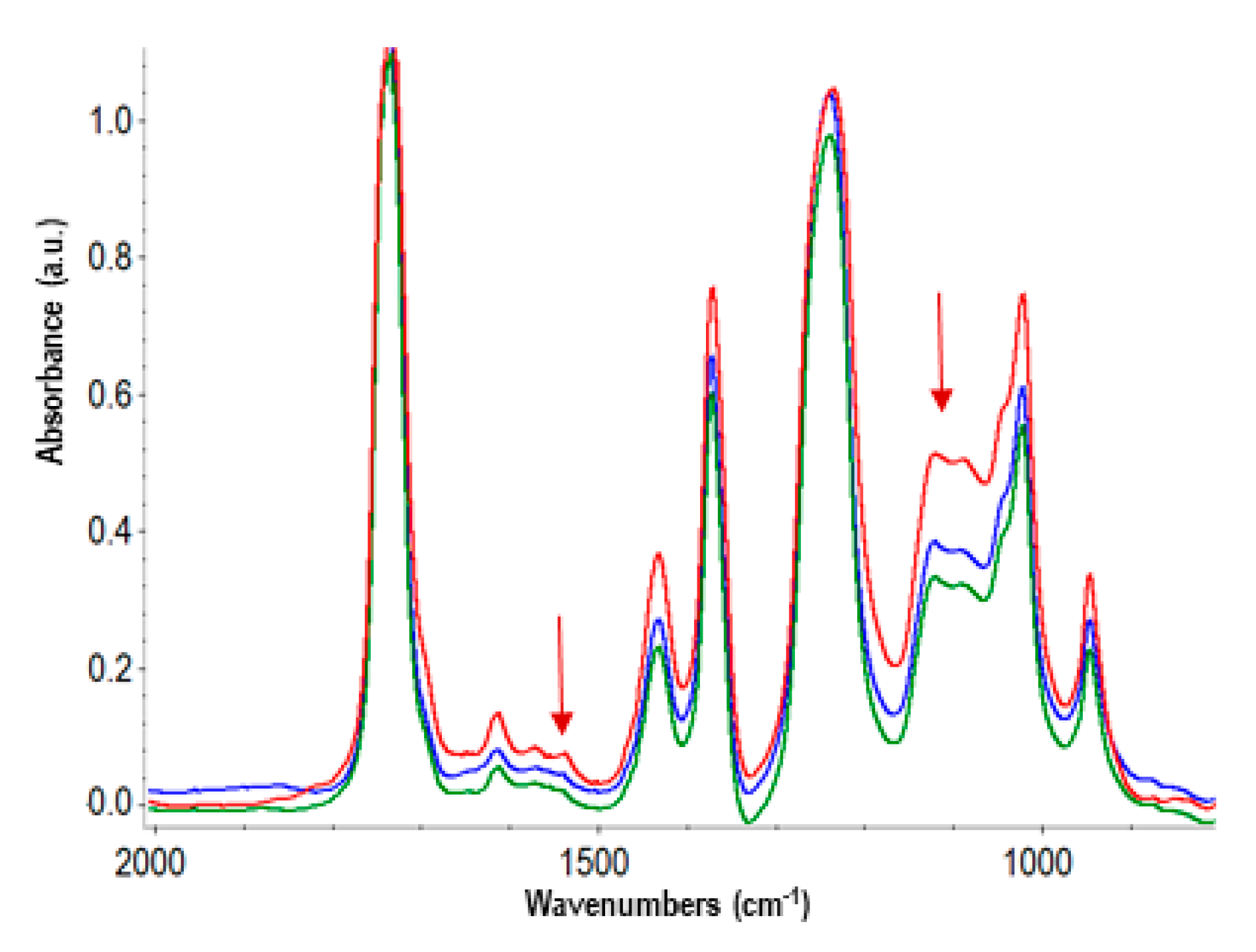
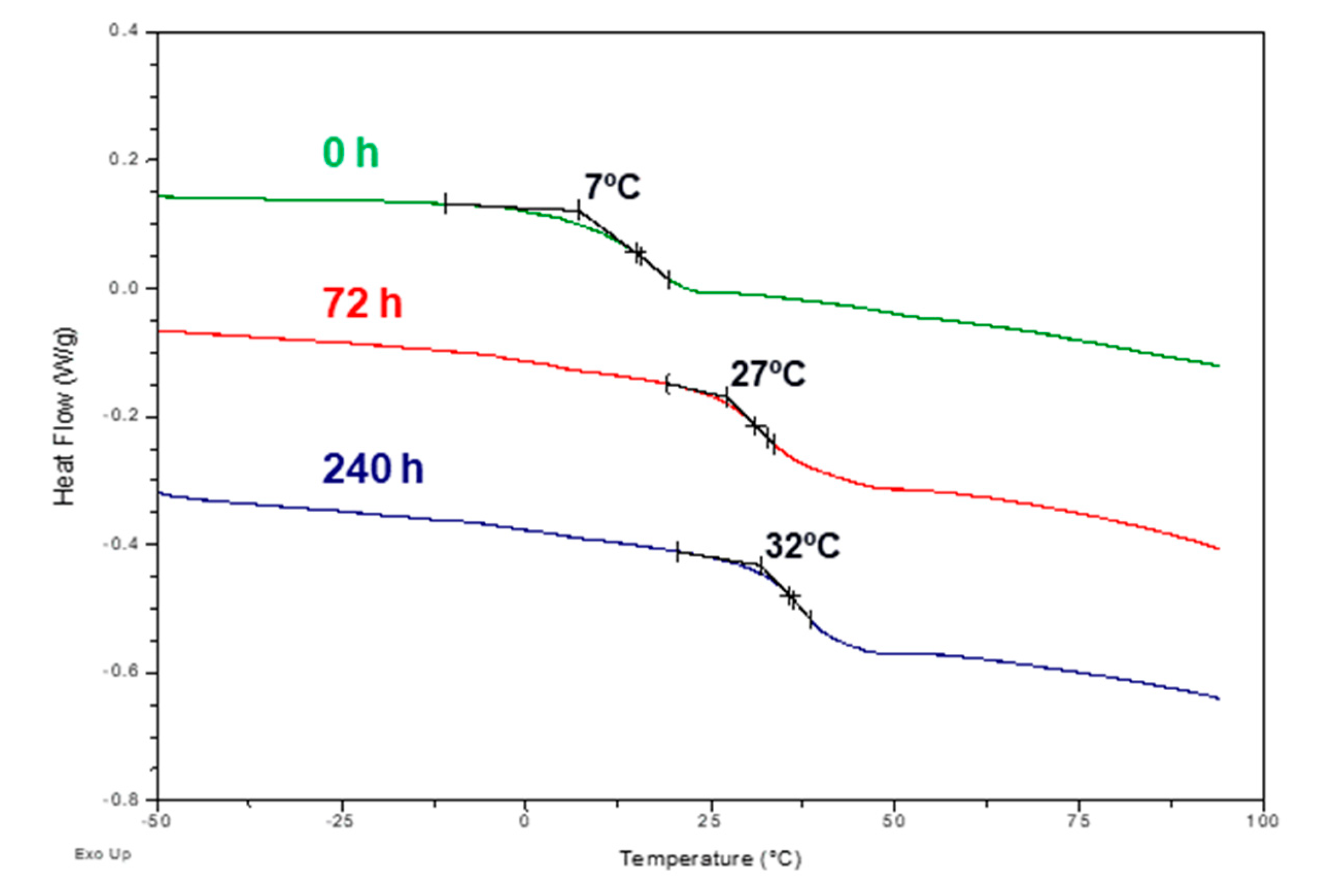
| Time (h) | Tg (ºC) |
|---|---|
| 0 | 7 |
| 24 | 19 |
| 72 | 27 |
| 120 | 30 |
| 240 | 32 |
| 550 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





