Submitted:
14 March 2024
Posted:
14 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
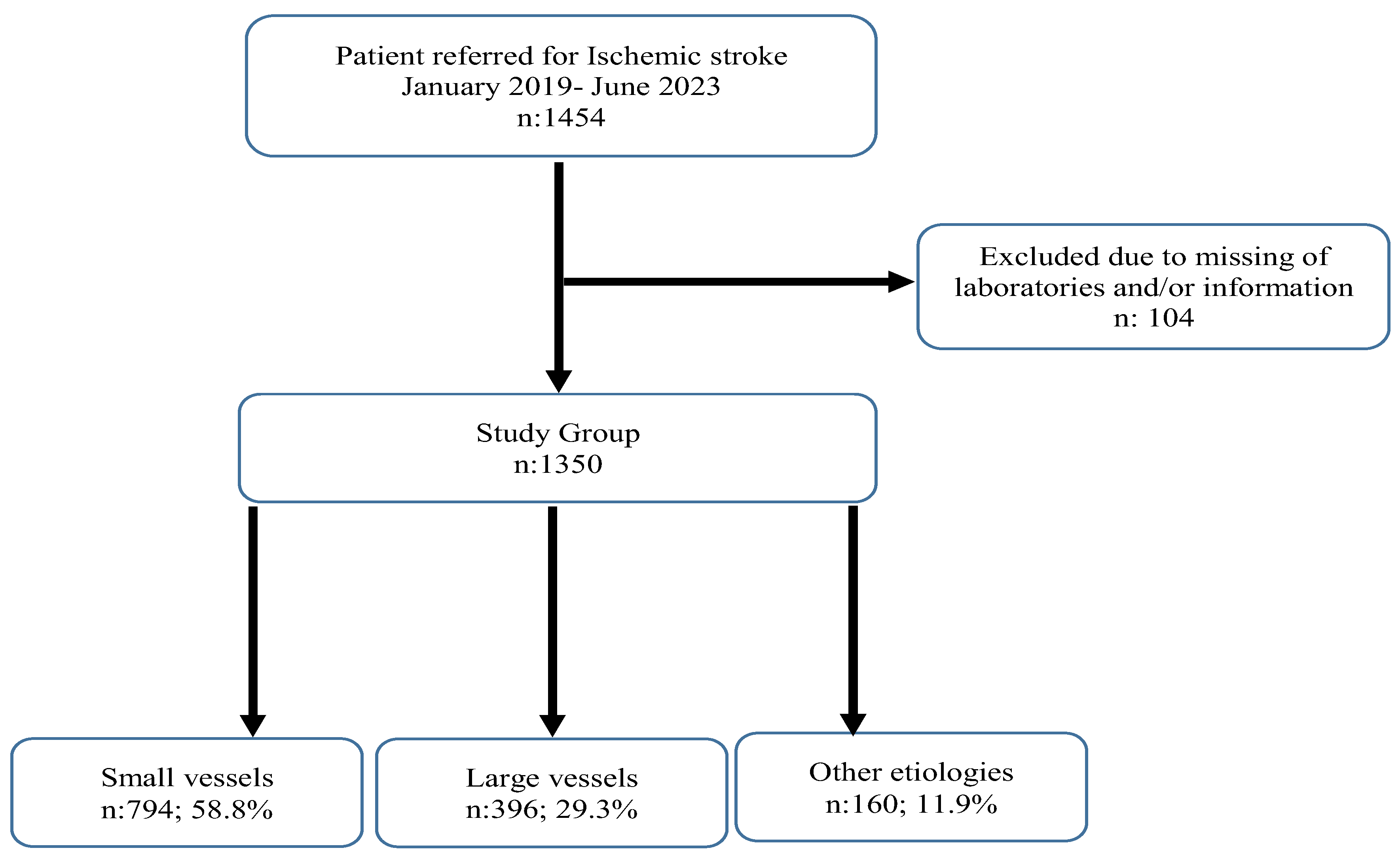
2.1. Study Design and Population
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Laboratory Parameters
2.5. Nutritional Indeces
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cardiovascular diseases (CVDs) fact sheets. 2021.
- O’Donnell, M.J.; Chin, S.L.; Rangarajan, S.; Xavier, D.; Liu, L.; Zhang, H.; Rao-Melacini, P.; Zhang, X.; Pais, P.; Agapay, S.; et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case-control study. Lancet 2016, 388, 761–775. [Google Scholar] [CrossRef]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44.
- Global regional. and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820.
- Veerbeek, J.M.; Kwakkel, G.; van Wegen, E.E.; Ket, J.C.; Heymans, M.W. Early Prediction of Outcome of Activities of Daily Living After Stroke. Stroke 2011, 42, 1482–1488. [Google Scholar] [CrossRef]
- Moretti Anfossi C, Ahumada Muñoz M, Tobar Fredes C, et al. Work Exposures and Development of Cardiovascular Diseases: A Systematic Review. Ann Work Expo Health. 2022;66(6):698-713.
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association [published correction appears in Circulation. 2023 Feb 21;147(8):e622] [published correction appears in Circulation. 2023 Jul 25;148(4):e4]. Circulation. 2023;147(8):e93-e621.
- Nishioka, S.; Okamoto, T.; Takayama, M.; Urushihara, M.; Watanabe, M.; Kiriya, Y.; Shintani, K.; Nakagomi, H.; Kageyama, N. Malnutrition risk predicts recovery of full oral intake among older adult stroke patients undergoing enteral nutrition: Secondary analysis of a multicentre survey (the APPLE study). Clin. Nutr. 2016, 36, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Buzby, G.P.; Mullen, J.L.; Matthews, D.C.; Hobbs, C.L.; Rosato, E.F. Prognostic nutritional index in gastrointestinal surgery. Am. J. Surg. 1980, 139, 160–167. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.P.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325:525-532.
- Naito, H.; Hosomi, N.; Nezu, T.; Kuzume, D.; Aoki, S.; Morimoto, Y.; Yoshida, T.; Shiga, Y.; Kinoshita, N.; Ueno, H.; et al. Prognostic role of the controlling nutritional status score in acute ischemic stroke among stroke subtypes. J. Neurol. Sci. 2020, 416, 116984. [Google Scholar] [CrossRef] [PubMed]
- López Espuela F, Roncero-Martín R, Zamorano JDP, et al. Controlling Nutritional Status (CONUT) Score as a Predictor of All-Cause Mortality at 3 Months in Stroke Patients. Biol Res Nurs. 2019;21(5):564-570.
- Lee, M.; Lim, J.-S.; Kim, Y.; Lee, J.H.; Kim, C.-H.; Lee, S.-H.; Jang, M.U.; Oh, M.S.; Lee, B.-C.; Yu, K.-H. Association between Geriatric Nutritional Risk Index and Post-Stroke Cognitive Outcomes. Nutrients 2021, 13, 1776. [Google Scholar] [CrossRef]
- Xiang, W.; Chen, X.; Ye, W.; Li, J.; Zhang, X.; Xie, D. Prognostic Nutritional Index for Predicting 3-Month Outcomes in Ischemic Stroke Patients Undergoing Thrombolysis. Front. Neurol. 2020, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Yan, D.; Xu, M.; Huang, Q.; Ren, W. Geriatric Nutritional Risk Index is related to the risk of stroke-associated pneumonia. Brain Behav. 2022, 12, e2718. [Google Scholar] [CrossRef]
- Gibson, P.H.; Cuthbertson, B.H.; Croal, B.L.; Rae, D.; El-Shafei, H.; Gibson, G.; Jeffrey, R.R.; Buchan, K.G.; Hillis, G.S. Usefulness of Neutrophil/Lymphocyte Ratio As Predictor of New-Onset Atrial Fibrillation After Coronary Artery Bypass Grafting. Am. J. Cardiol. 2010, 105, 186–191. [Google Scholar] [CrossRef]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske Lekarske Listy 2001, 102, 5–14. [Google Scholar]
- Celikbilek, A.; Ismailogullari, S.; Zararsiz, G. Neutrophil to Lymphocyte Ratio Predicts Poor Prognosis in Ischemic Cerebrovascular Disease. J. Clin. Lab. Anal. 2014, 28, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Gökhan, S.; Ozhasenekler, A.; Durgun, H.M.; Akil, E.; Ustündag, M.; Orak, M. Neutrophil lymphocyte ratios in stroke subtypes and transient ischemic attack. . 2013, 17, 653–7. [Google Scholar]
- Tokgoz S, Keskin S, Kayrak M, Seyithanoglu A, Ogmegul A. Is neutrophil/lymphocyte ratio predict to short-term mortality in acute cerebral infarct independently from infarct volume? J Stroke Cerebrovasc. 2014;23(8), 2163-8.
- Kwon, H.-M.; Lee, Y.-S.; Bae, H.-J.; Kang, D.-W. Homocysteine as a Predictor of Early Neurological Deterioration in Acute Ischemic Stroke. Stroke 2014, 45, 871–873. [Google Scholar] [CrossRef]
- Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014; 20: 6212–22.
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Ishii, N.; Iwata, Y.; Takata, R.; Nishimura, T.; Aizawa, N.; Sakai, Y.; Ikeda, N.; et al. The Relationship between Controlling Nutritional (CONUT) Score and Clinical Markers among Adults with Hepatitis C Virus Related Liver Cirrhosis. Nutrients 2018, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Iseki, Y.; Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Sugano, K.; Ikeya, T.; Muguruma, K.; Tanaka, H.; Toyokawa, T.; et al. Impact of the Preoperative Controlling Nutritional Status (CONUT) Score on the Survival after Curative Surgery for Colorectal Cancer. PLOS ONE 2015, 10, e0132488. [Google Scholar] [CrossRef]
- Bo, Y.; Wang, K.; Liu, Y.; You, J.; Cui, H.; Zhu, Y.; Lu, Q.; Yuan, L. The Geriatric Nutritional Risk Index Predicts Survival in Elderly Esophageal Squamous Cell Carcinoma Patients with Radiotherapy. PLOS ONE 2016, 11, e0155903–e0155903. [Google Scholar] [CrossRef] [PubMed]
- Al-Najjar, Y.; Clark, A.L. Predicting Outcome in Patients With Left Ventricular Systolic Chronic Heart Failure Using a Nutritional Risk Index. Am. J. Cardiol. 2012, 109, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai zasshi 1984, 85, 1001–1005. [Google Scholar]
- Corrigan ML, Escuro AA, Celestin J, et al. Nutrition in the stroke patient. Nutr Clin Pract 2011; 26(3): 242-252.
- Sánchez-Moreno, C, Jiménez-Escrig A, Martín A. Stroke: Roles of B vitamins, homocysteine and antioxidants. Nutr Res Rev 2009; 22(1): 49-67.
- Mosselman MJ, Kruitwagen CL, Schuurmans MJ, et al, Malnutrition and risk of malnutrition in patients with stroke: Prevalence during hospital stay. J Neurosci Nurs 2013; 45(4): 194-204.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Velez, L.; Toffel, S.; Trejo-Lopez, J.; Kresak, J.L.; Beal, S.G. Educational Case: Etiologies, Mechanisms, and Treatment of Stroke. Acad. Pathol. 2020, 7, 2374289520901817. [Google Scholar] [CrossRef]
- Blank-Stein, N.; Mass, E. Macrophage and monocyte subsets in response to ischemic stroke. Eur. J. Immunol. 2023, 53, e2250233. [Google Scholar] [CrossRef]
- Chamorro A, Hallenbeck J. The harms and benefits of inflamatory and immune responses in vascular disease. Stroke. 2006;37:291-3.
- Worthmann, H.; Tryc, A.; Deb, M.; Goldbecker, A.; Ma, Y.; Tountopoulou, A.; Lichtinghagen, R.; Weissenborn, K. Linking infection and inflammation in acute ischemic stroke. Ann. New York Acad. Sci. 2010, 1207, 116–122. [Google Scholar] [CrossRef]
- Buck BH, Liebeskind DS, Saver JL, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. 2008;39(2):355-360.
- Xue, J.; Huang, W.; Chen, X.; Li, Q.; Cai, Z.; Yu, T.; Shao, B. Neutrophil-to-Lymphocyte Ratio Is a Prognostic Marker in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 650–657. [Google Scholar] [CrossRef]
- Maestrini I, Tagzirt M, Gautier S, et al. Analysis of the association of MPO and MMP-9 with stroke severity and outcome: Cohort study. Neurology. 2020;95(1):e97-e108.
- Mureșan, E.-M.; Golea, A.; Vesa. C.; Lenghel, M.; Csutak, C.; Perju-Dumbravă, L. Emergency department point-of-care biomarkers and day 90 functional outcome in spontaneous intracerebral hemorrhage: A single-center pilot study. Exp. Ther. Med. 2022, 23, 200. [Google Scholar] [CrossRef]
- Xie, M.; Yuan, K.; Zhu, X.; Chen, J.; Zhang, X.; Xie, Y.; Wu, M.; Wang, Z.; Liu, R.; Liu, X. Systemic Immune-Inflammation Index and Long-Term Mortality in Patients with Stroke-Associated Pneumonia. J. Inflamm. Res. 2023, 16, 1581–1593. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Yin, X.-S.; Li, Z.-P. Association of the systemic immune-inflammation index (SII) and clinical outcomes in patients with stroke: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 1090305. [Google Scholar] [CrossRef]
- Yang, R.; Chang, Q.; Meng, X.; Gao, N.; Wang, W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J. Cancer 2018, 9, 3295–3302. [Google Scholar] [CrossRef]
- Agard, T.A.; Hass, R.; Cavrak, M.E.; Foual, N.S.; Byrum, C.; Adcock, A.K.; Gehan, D.; Petrone, A.B. Neutrophil lymphocyte ratio (NLR) and systemic immune inflammatory index (SII) for the differential diagnosis of CT-negative mild acute ischemic stroke and transient ischemic attack. Int. J. Neurosci. 2023, 1–8. [Google Scholar] [CrossRef]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.L.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163–164, 144–171. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Zhu, S.; Wang, H.; Chen, J.; Zhang, X.; Xu, P.; Xie, Y.; Zhu, X.; Zhu, W.; Sun, W.; et al. Association between malnutrition and long-term mortality in older adults with ischemic stroke. Clin. Nutr. 2021, 40, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Raposeiras Roubín S, Abu Assi E, Cespón Fernandez M, et al. Prevalence and Prognostic Significance of Malnutrition in Patients With Acute Coronary Syndrome. J Am Coll Cardiol. 2020;76(7):828-840.
- akmak EÖ, Öcal L, Erdoğan E, et al. Prognostic Value of 3 Nutritional Screening Tools to Predict 30-Day Outcome in Patients Undergoing Carotid Artery Stenting. Angiology. 2022;73(3):225-233.
- Pikula A, Howard BV, Seshadri S. Stroke and Diabetes. In: Cowie CC, Casagrande SS, Menke A, et al., eds. Diabetes in America. 3rd ed. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); August 2018.
- Jensen, M.; Thomalla, G. Causes and Secondary Prevention of Acute Ischemic Stroke in Adults. 2019, 40, 022–030. [CrossRef]
- Bae, D.J.; Willey, J.Z.; Ibeh, C.; Yuzefpolskaya, M.; Colombo, P.C. Stroke and Mechanical Circulatory Support in Adults. Curr. Cardiol. Rep. 2023, 25, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
| All Groups | Small vessels(n:794; 58.8%) | Large vessels(n:396; 29.3%) | Other etiologies(n:160; 11.9%) | ||
|---|---|---|---|---|---|
| Gender | n(%) | n(%) | n(%) | n(%) | p value |
| Male | 710 (52.60%) | 413 (52.00%)a | 155 (39.10%)b | 142 (88.80%)c | <0.001 |
| Female | 640 (47.40%) | 381 (48.00%) | 241 (60.90%) | 18 (11.30%) | |
| Hypertension | 1046 (77.50%) | 630 (79.30%) | 293 (74.00%) | 123 (76.90%) | 0.112 |
| Diabetes | 649 (48.10%) | 399 (50.30%)a | 168 (42.40%)b | 82 (51.30%)a,b | 0.027 |
| Dyslipidemia | 1101 (81.60%) | 667 (84.00%) a | 309 (78.00%) b | 125 (78.10%) a,b | 0.021 |
| Smoking | 784 (58.10%) | 457 (57.60%) a | 201 (50.80%)a | 126 (78.80%)b | <0.001 |
| Alcoholism | 353 (26.10%) | 202 (25.40%) a | 82 (20.70%) a | 69 (43.10%) b | <0.001 |
| Survival | 919 (68.10%) | 637 (80.20%) a | 154 (38.90%)b | 128 (80.00%) a | <0.001 |
| All Groups | Small vessels | Large vessels | Other etiologies | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean±Std | Median(25p-75p) | Mean±Std | Median(25p-75p) | Mean±Std | Median(25p-75p) | Mean±Std | Median(25p-75p) | p value | |
| Age (years) | 64.38±16.43 | 66(54-77) | 64.8±16.59 | 67(54-77) | 64.3±16.65 | 66(54-78) | 62.54±14.96 | 64(54.5-72.5) | 0,139 |
| Body Mass Index (kg/m2) | 28.3±5.26 | 27.78(24.28-32.27) | 27.23±4.97b | 26.57(23.41-30.74)b | 31.01±4.33a | 31.31(27.77-33.93)a | 26.94±6.24b | 25.87(22.46-31.74)b | <0.001 |
| Temperature (0C) | 36.99±0.46 | 37.1(36.7-37.5) | 37±0.46 | 37.1(36.7-37.5) | 36.99±0.44 | 36.9(36.7-37.5) | 36.94±0.45 | 36.9(36.6-37.3) | 0,337 |
| Systolik Blood Preasure (mmHg) | 157±20.2 | 153(143-163) | 155.22±19.28 a | 153(141-157) a | 159.61±20.88b | 154(144-164) b | 159.39±22.03a,b | 154(144-163) a,b | 0,005 |
| Diastolik Blood Preasure(mmHg) | 85.35±8.88 | 85(79-88) | 84.46±8.58 a | 84(79-88) a | 86.77±9.18 b | 85(80-89) b | 86.21±9.09 a,b | 85(79-88) a,b | 0,003 |
| NIHSS | 6.46±1.6 | 7(6-8) | 6.39±1.62 | 7(5-8) | 6.52±1.55 | 7(6-8) | 6.65±1.6 | 7(6-8) | 0,160 |
| Lymphocytes count | 2297.93±1539.62 | 1743.5(1459-2074) | 2041.91±1368.65 a | 1693.5(1459-1973) a | 2024.12±1327.16 a | 1667(1348.5-1998) a | 4246.06±1435.3 | 4200(2985-5370)b | <0.001 |
| Neutrophils count | 7260.04±1943.96 | 7786(6343-8212) | 7498.5±1369.85 a | 7832.5(7535-8194) a | 7536.37±2102.73 a | 7871(6330-8457) a | 5392.8±2781.42 b | 4670(3510-6900) b | <0.001 |
| Neutrophil lymphocyte ratio (NLR) | 4.06±1.81 | 4.23(2.96-5.16) | 4.35±1.4 a | 4.46(3.81-5.18) a | 4.54±1.93 a | 4.63(3.15-5.69) a | 1.44±0.95 b | 1.27(0.75-1.83) b | <0.001 |
| Systemic immune-inflammation index (SII) | 816.54±421.87 | 807.68(506.32-1092.19) | 872.76±346.11 a | 871.04(650.62-1102.69) a | 917.22±470.13 a | 898.17(565.27-1165.79) a | 288.36±204.25 b | 243.34(142.97-367.66) b | <0.001 |
| White blood cells count | 9.27±2.03 | 9(7.6-11) | 9.29±2.04 | 9(7.6-11) | 9.15±1.99 | 8.9(7.55-10.75) | 9.53±2.07 | 9.2(7.85-11.45) | 0,157 |
| Platelet count | 201.55±47.81 | 205.65(164.9-242.1) | 201.38±47.31 | 203.95(165.1-241.3) | 202.1±49.05 | 208.75(163.95-243.25) | 201.05±47.44 | 201.25(166.55-241.85) | 0,926 |
| Fasting glucose (mg/dL) | 134.35±56.77 | 120(102-145) | 134.07±56.61 | 120(102-145) | 134.57±56.08 | 120(100-145) | 135.18±59.52 | 120(102-145) | 0,971 |
| Albumin (g/dL) | 2.62±0.34 | 2.61(2.55-2.7) | 2.7±0.25 b | 2.62(2.59-2.72) b | 2.47±0.44 a | 2.6(2.29-2.69) a | 2.61±0.34 b | 2.61(2.54-2.71) b | <0.001 |
| Prognostic nutritional index (PNI) | 37.71±8.45 | 35.34(33.32-39.96) | 37.25±7.23 a | 35.29(33.69-37.25) a | 34.78±8.16 b | 34.11(30.23-36.87) b | 47.3±8.06 c | 47.03(41.33-52.85) c | <0.001 |
| Nutritional risk index (NRI) | 57.23±9.53 | 56.46(49.95-64.40) | 55.33±9.06 b | 54.07(48.53-61.57) b | 62.02±7.70 a | 62.33(56.23-67.06) a | 54.78±11.49 b | 52.96(46.26-63.54)b | <0.001 |
| Total cholesterol (mg/dL) | 232.72±145.96 | 159(146-267) | 149.02±11.41 a | 149(140-156) a | 346.79±174.67 b | 276(194-527) b | 365.78±138.26 a,b | 317.5(278-395) a,b | <0.001 |
| C-reactive protein (CRP) mg/L | 89.57±87.25 | 66.42(33.32-100.84) | 56.36±57.96 a | 46.5(18.97-82.84) a | 155.69±102.42 b | 108.83(66.42-247.63) b | 90.78±73.38 | 69.2(42.09-99.83) c | <0.001 |
| CONUT | 5.23±1.33 | 5(4-6) | 5.49±1.2 a | 5(5-6) a | 5.12±1.46 b | 5(4-6) b | 4.22±1.11 | 4(4-4) c | <0.001 |
| Univariate | Enter | Backward | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Etiology (ref: Other etiology) | <0.001 | <0.001 | <0.001 | |||
| Small Vessels | 0.986(0.645-1.508) | 0.948 | 5.379(2.652-10.910) | <0.001 | 5.207(2.67-10.15) | <0.001 |
| Large vessels | 6.286(4.061-9.73) | <0.001 | 154.3(67.7-351.6) | <0.001 | 145.9(65.1-327.0) | <0.001 |
| Diabetes | 1.159(0.922-1.457) | 0.207 | 1.407(1.050-1.886) | 0.022 | 1.404(1.048-1.880) | 0.023 |
| Dyslipidemia | 1.643(1.196-2.258) | 0.002 | 2.067(1.308-3.268) | 0.002 | 1.910(1.286-2.837) | 0.001 |
| Age (years) | 1.008(1.001-1.015) | 0.024 | 1.014(1.005-1.023) | 0.003 | 1.014(1.005-1.023) | 0.003 |
| Body Mass Index (kg/m2) | 1.015(0.993-1.037) | 0.180 | 0.908(0.481-1.712) | 0.765 | 0.941(0.911-0.971) | <0.001 |
| Diastolik Blood Preasure (mmHg) | 0.989(0.976-1.002) | 0.088 | 0.982(0.965-0.998) | 0.030 | 0.981(0.965-0.997) | 0.023 |
| NIHSS | 1.059(0.985-1.138) | 0.121 | 0.949(0.857-1.051) | 0.317 | - | - |
| NLR | 0.846(0.793-0.903) | <0.001 | 0.984(0.820-1.179) | 0.858 | - | - |
| SII | 0.999(0.999-1.000) | <0.001 | 1.000(0.999-1.000) | 0.575 | - | - |
| PNI | 1.057(1.042-1.072) | <0.001 | 1.214(1.164-1.265) | <0.001 | 1.224(1.180-1.269) | <0.001 |
| NRI | 1.008(0.996-1.020) | 0.187 | 1.019(0.718-1.447) | 0.915 | - | - |
| CONUT | 0.950(0.872-1.035) | 0.244 | 1.743(1.483-2.049) | <0.001 | 1.742(1.513-2.007) | <0.001 |
| All Group | |||||
|---|---|---|---|---|---|
| Variables | SII | PNI | NRI | CONUT | |
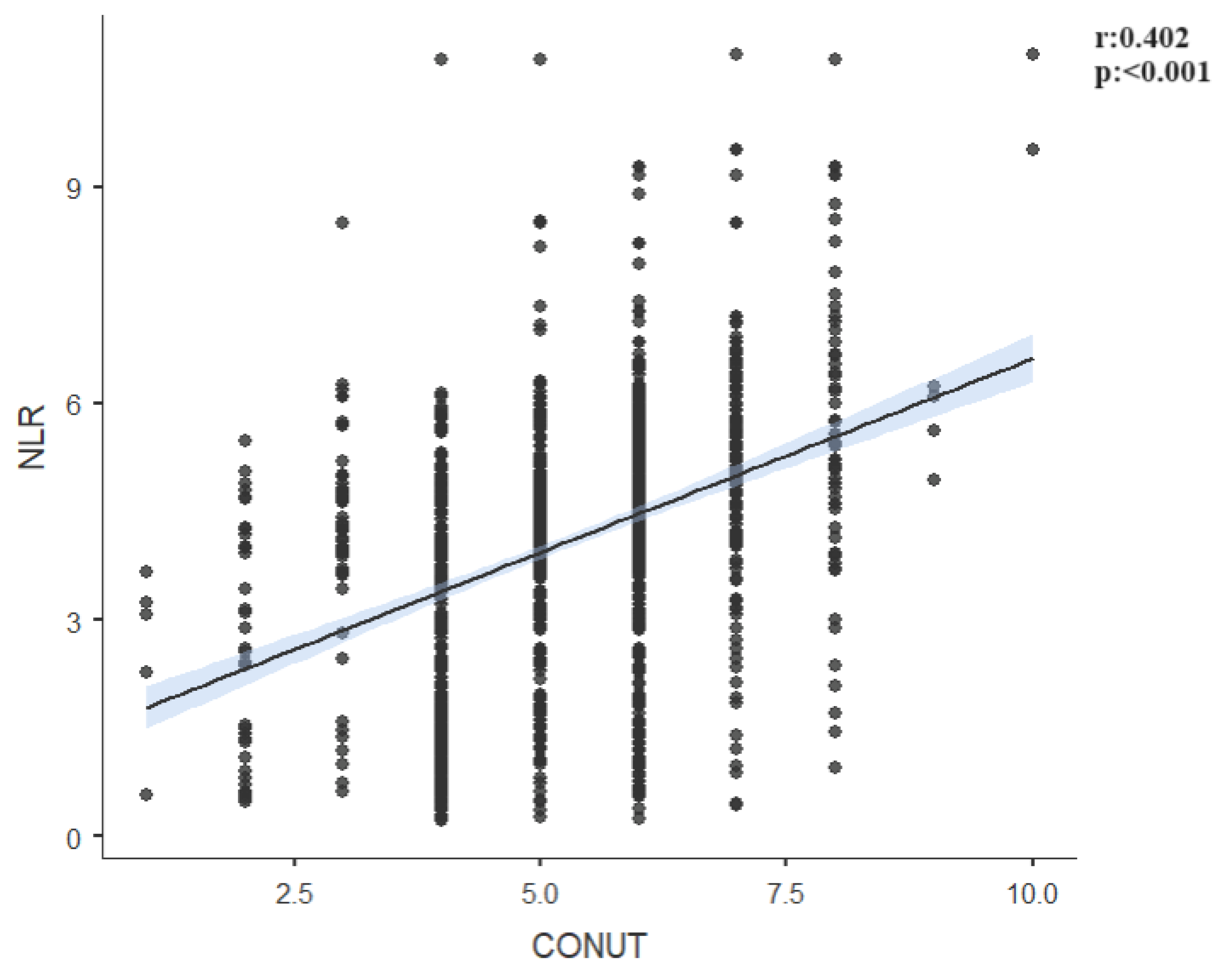
| NLR | r | 0.826 | -0.692 | 0.034 | 0.402 |
| p | <0.001 | <0.001 | 0.208 | <0.001 | |
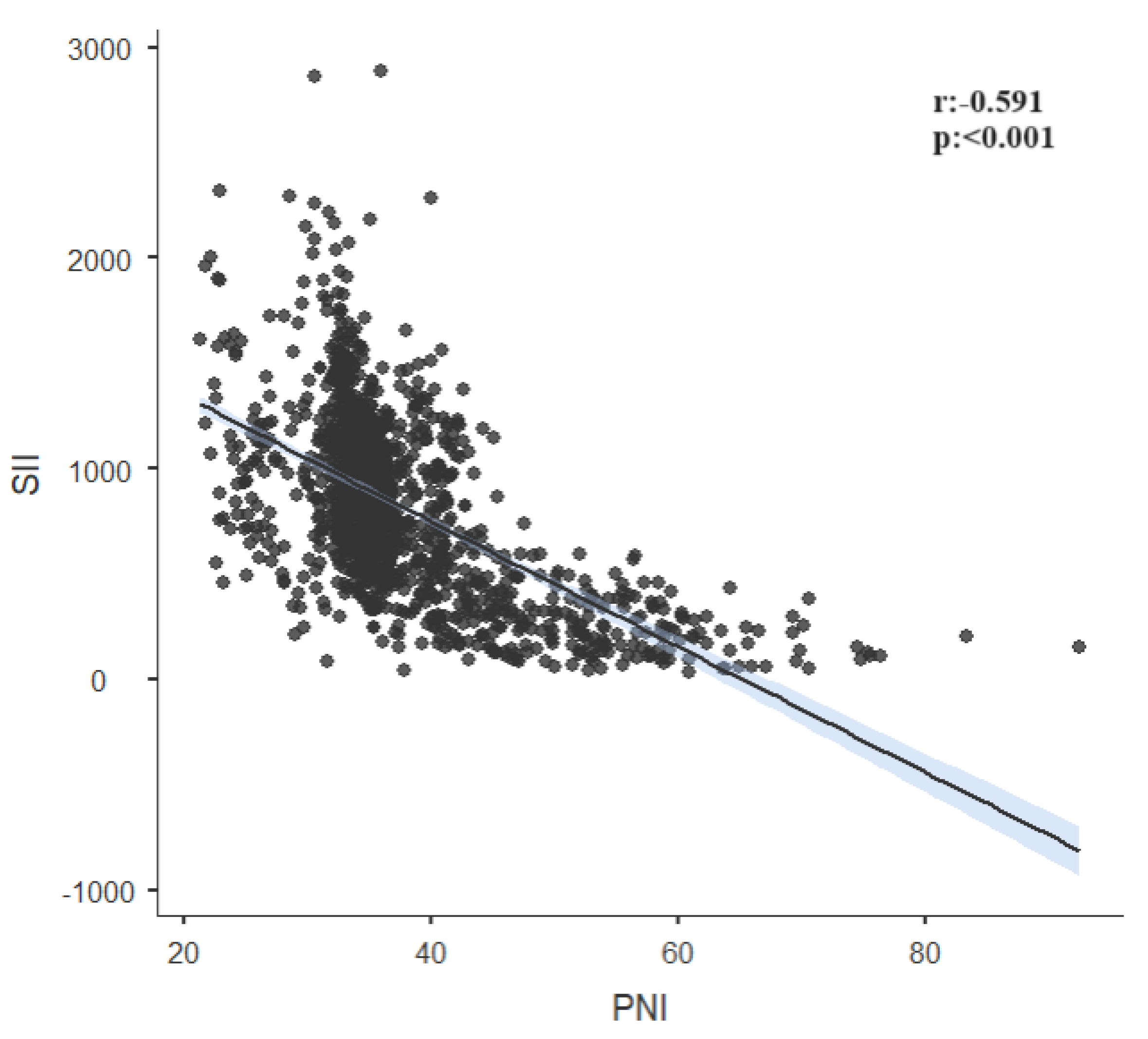
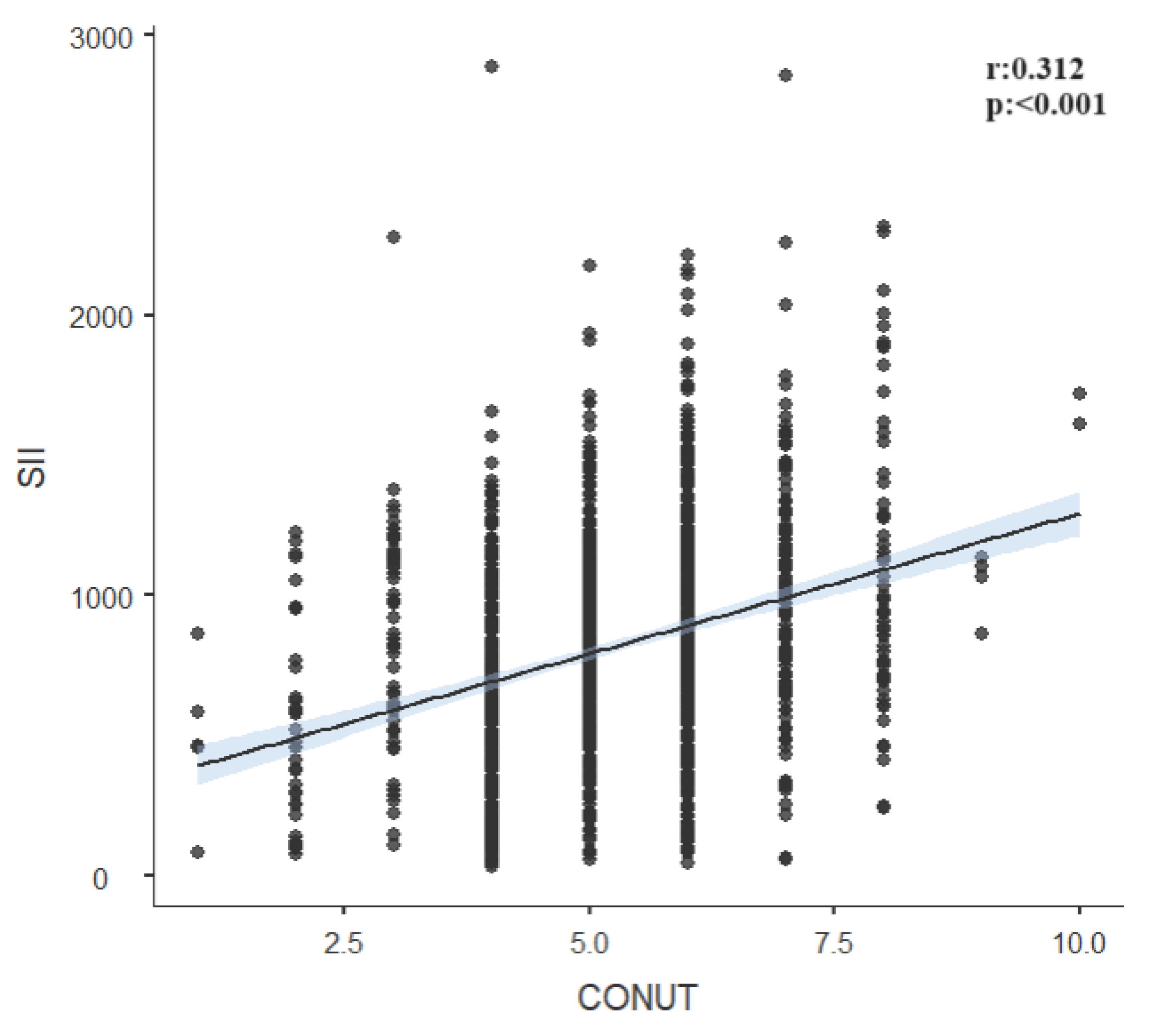
| SII | r | -0.591 | 0.039 | 0.312 | |
| p | <0.001 | 0.148 | <0.001 | ||
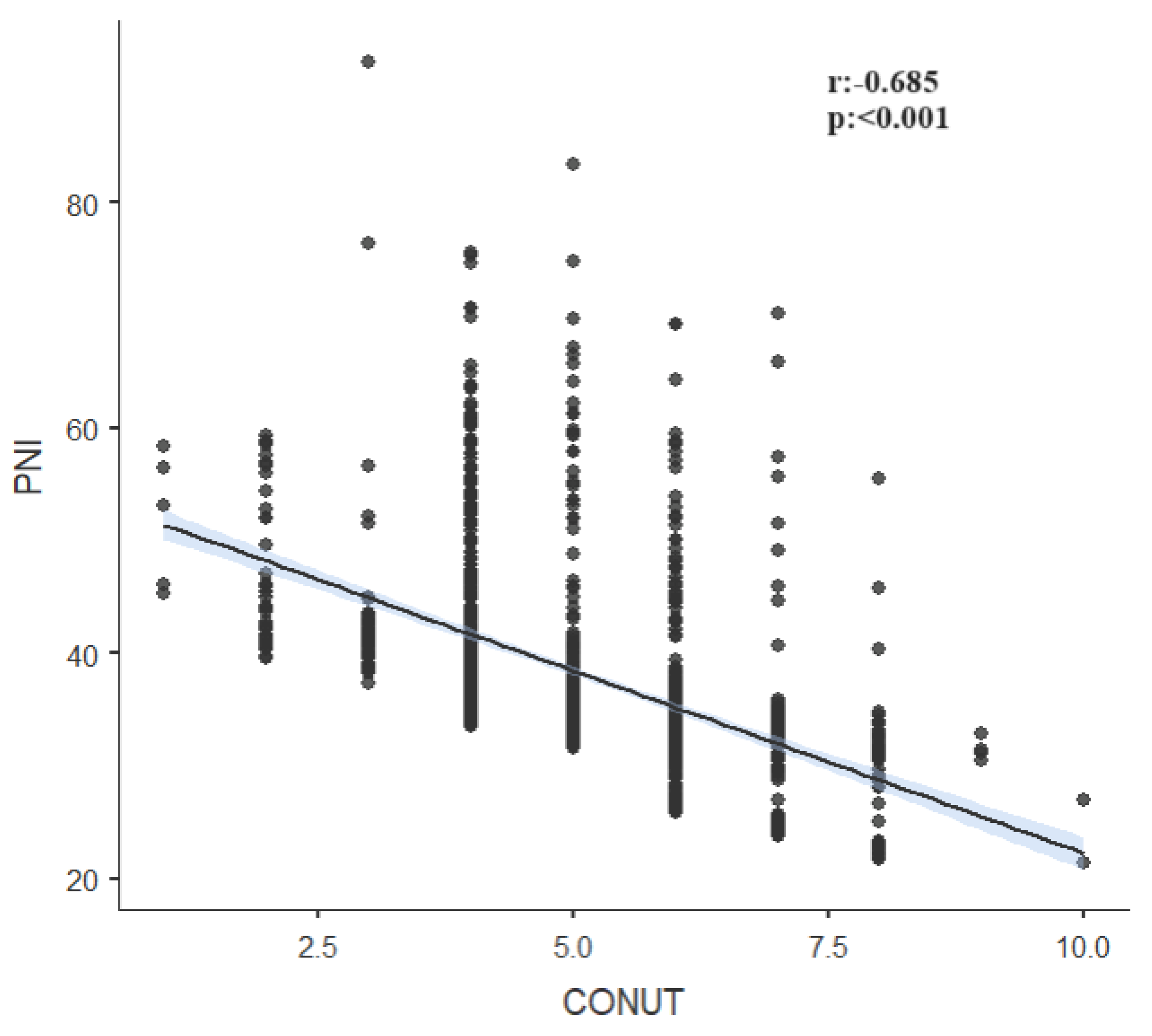
| PNI | r | -0.095 | -0.685 | ||
| p | <0.001 | <0.001 | |||
| NRI | r | -0.029 | |||
| p | 0.289 | ||||
| Small Vessels | |||||
| SII | PNI | NRI | CONUT | ||
| NLR | r | 0.694 | -0.653 | -0.036 | 0.335 |
| p | <0.001 | <0.001 | 0.311 | <0.001 | |
| SII | r | -0.455 | -0.015 | 0.164 | |
| p | <0.001 | 0.674 | <0.001 | ||
| PNI | r | 0.022 | -0.676 | ||
| p | 0.544 | <0.001 | |||
| NRI | r | -0.024 | |||
| p | 0.497 | ||||
| Large Vessels | |||||
| SII | PNI | NRI | CONUT | ||
| NLR | r | 0.837 | -0.577 | 0.004 | 0.389 |
| p | <0.001 | <0.001 | 0.937 | <0.001 | |
| SII | R | -0.504 | 0.023 | 0.321 | |
| p | <0.001 | 0.650 | <0.001 | ||
| PNI | r | -0.029 | -0.825 | ||
| p | 0.570 | <0.001 | |||
| NRI | r | -0.018 | |||
| p | 0.720 | ||||
| Other Etiologies | |||||
| SII | PNI | NRI | CONUT | ||
| NLR | R | 0.919 | -0.531 | 0.107 | 0.079 |
| p | <0.001 | <0.001 | 0.178 | 0.319 | |
| SII | r | -0.509 | 0.090 | 0.064 | |
| p | <0.001 | 0.258 | 0.424 | ||
| PNI | r | -0.188 | -0.434 | ||
| p | 0.017 | <0.001 | |||
| NRI | r | 0.035 | |||
| p | 0.659 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





