Submitted:
13 March 2024
Posted:
15 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Participant Selection Criteria
2.4. Sample Size and Sampling Technique
2.5. Survey Instrument and Data Collection
2.6. Variables and Measurement
2.7. Data Analysis
2.8. Ethical Considerations
3. Results
3.1. Response Rate
3.2. Participant Characteristics
3.3. Knowledge about Influenza and Vaccination
3.4. Influenza Vaccine Hesitancy among the Participants
3.5. Vaccine Hesitancy Determinants
3.6. Factors Associated with Influenza Vaccine Hesitancy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Determinants | Items |
|---|---|
| a) Contextual factors: | |
|
|
|
|
|
|
|
|
| b) Individual/group influences: | |
|
|
|
|
|
|
|
|
| c) Influenza vaccination-specific issues | |
|
|
|
|
|
|
|
|
| Location | Expected respondents (N) | Respondents (n) |
Response rate (%) |
|---|---|---|---|
| Nakhon Phanom Hospital | 100 | 100 | 100.0 |
| That Phanom Hospital | 50 | 50 | 100.0 |
| Phonsawan Hospital | 50 | 50 | 100.0 |
| Plapak Hospital | 50 | 49 | 98.0 |
| Na Kae Hospital | 50 | 49 | 98.0 |
| Wang Yang Hospital | 50 | 40 | 80.0 |
| Overall | 350 | 338 | 96.6 |
| Item No | Recommendation | Page No |
|
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 1 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 1-2 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 2 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 2 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 2-3 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants | 3 |
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 4 |
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 4 |
| Bias | 9 | Describe any efforts to address potential sources of bias | |
| Study size | 10 | Explain how the study size was arrived at | 3 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 3-4 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 4 |
| (b) Describe any methods used to examine subgroups and interactions | N/A | ||
| (c) Explain how missing data were addressed | N/A | ||
| (d) If applicable, describe analytical methods taking account of sampling strategy | 4 | ||
| (e) Describe any sensitivity analyses | N/A | ||
| Results | |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 5 |
| (b) Give reasons for non-participation at each stage | 5,9 | ||
| (c) Consider use of a flow diagram | 5 | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 5-7 |
| (b) Indicate number of participants with missing data for each variable of interest | N/A | ||
| Outcome data | 15* | Report numbers of outcome events or summary measures | 8 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 9-13 |
| (b) Report category boundaries when continuous variables were categorized | 9 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | N/A | ||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | N/A |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 13 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 14 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 13-14 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 13-14 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 15 |
References
- Wilson, K.E.; Wood, S.M.; Schaecher, K.E.; Cromwell, K.B.; Godich, J.; Knapp, M.H.; et al. Nosocomial outbreak of influenza A H3N2 in an inpatient oncology unit related to health care workers presenting to work while ill. American Journal of Infection Control 2019, 47, 683–687. [Google Scholar] [CrossRef]
- Wilson, P.; Zumla, A. Transmission and prevention of acute viral respiratory tract infections in hospitals. Curr Opin Pulm Med. 2019, 25, 220–224. [Google Scholar] [CrossRef]
- Huzly, D.; Kurz, S.; Ebner, W.; Dettenkofer, M.; Panning, M. Characterisation of nosocomial and community-acquired influenza in a large university hospital during two consecutive influenza seasons. Journal of Clinical Virology 2015, 73, 47–51. [Google Scholar] [CrossRef]
- Fiore, A.E.; Shay, D.K.; Broder, K.; Iskander, J.K.; Uyeki, T.M.; Mootrey, G.; et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2009.
- World Health Organization. Vaccines against influenza WHO position paper—November 2012. Wkly Epidemiol Rec. 2012, 87, 461–476. [Google Scholar]
- Black, C.L.; Yue, X.; Ball, S.W.; Fink, R.V.; de Perio, M.A.; Laney, A.S.; et al. Influenza Vaccination Coverage Among Health Care Personnel - United States, 2017–2018 Influenza Season. MMWR Morb Mortal Wkly Rep. 2018, 67, 1050–1054. [Google Scholar] [CrossRef]
- Health Canada. Vaccine uptake in Canadian adults: Results from the 2014 adult National Immunization Coverage (aNIC) survey; Government of Canada, 2016. [Google Scholar]
- To, K.W.; Lai, A.; Lee, K.C.K.; Koh, D.; Lee, S.S. Increasing the coverage of influenza vaccination in healthcare workers: review of challenges and solutions. Journal of Hospital Infection 2016, 94, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Karafillakis, E.; Dinca, I.; Apfel, F.; Cecconi, S.; Wűrz, A.; Takacs, J.; et al. Vaccine hesitancy among healthcare workers in Europe: A qualitative study. Vaccine 2016, 34, 5013–5020. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, O.; Castle-Clarke, S.; Sevdalis, N.; Chataway, J. Attitudes to vaccination: a critical review. Social Science & Medicine 2014, 112, 1–11. [Google Scholar]
- Verger, P.; Fressard, L.; Collange, F.; Gautier, A.; Jestin, C.; Launay, O.; et al. Vaccine hesitancy among general practitioners and its determinants during controversies: a national cross-sectional survey in France. EBioMedicine 2015, 2, 891–897. [Google Scholar] [CrossRef]
- Dubé, E.; Vivion, M.; MacDonald, N.E. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Expert Review of Vaccines. 2015, 14, 99–117. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ten threats to global health in 2019; WHO, 2019. [Google Scholar]
- Kwon, D.S.; Kim, K.; Park, S.M. Factors associated with influenza vaccination coverage among the elderly in South Korea: the Fourth Korean National Health and Nutrition Examination Survey (KNHANES IV). BMJ Open 2016, 6, e012618. [Google Scholar] [CrossRef]
- Offeddu, V.; Tam, C.C.; Yong, T.T.; Tan, L.K.; Thoon, K.C.; Lee, N.; et al. Coverage and determinants of influenza vaccine among pregnant women: a cross-sectional study. BMC Public Health. 2019, 19, 890. [Google Scholar] [CrossRef]
- MacDonald, N.E.; Dubé, E. Unpacking Vaccine Hesitancy Among Healthcare Providers. EBioMedicine 2015, 2, 792–793. [Google Scholar] [CrossRef]
- MacDonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Paterson, P.; Meurice, F.; Stanberry, L.R.; Glismann, S.; Rosenthal, S.L.; Larson, H.J. Vaccine hesitancy and healthcare providers. Vaccine 2016, 34, 6700–6706. [Google Scholar] [CrossRef]
- Owusu, J.T.; Prapasiri, P.; Ditsungnoen, D.; Leetongin, G.; Yoocharoen, P.; Rattanayot, J.; et al. Seasonal influenza vaccine coverage among high-risk populations in Thailand, 2010–2012. Vaccine 2015, 33, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Chotpitayasunondh, T.; Sawanpanyalert, N.; Bumrungsak, R.; Chunthitiwong, P.; Chainatraporn, P. Influenza vaccination among health care workers in Thailand. BMC proceedings: Springer; 2011. p. 84.
- Chotpitayasunondh, C.; Patrasuwan, S.; Prontri, M.; Poiynok, S. Acceptance of pandemic influenza A (H1N1) 2009 vaccine among health care workers, Thailand. BMC proceedings: Springer; 2011. p. P83.
- Praphasiri, P.; Ditsungneon, D.; Greenbaum, A.; Dawood, F.S.; Yoocharoen, P.; Stone, D.M.; et al. Do Thai Physicians Recommend Seasonal Influenza Vaccines to Pregnant Women? A Cross-Sectional Survey of Physicians’ Perspectives and Practices in Thailand. PLOS ONE 2017, 12, e0169221. [Google Scholar]
- Tanavikrankoon, M.; Suwannapong, N.; Thipayamongkolkul, M.; Howteerakul, N. Acceptance of seasonal influenza vaccination among medical personnel in a super tertiary care hospital, Bangkok. Vajira Nursing Journal 2015, 17, 15–29. [Google Scholar]
- Goyal, S.; Prasert, K.; Praphasiri, P.; Chittaganpitch, M.; Waicharoen, S.; Ditsungnoen, D.; et al. The acceptability and validity of self-collected nasal swabs for detection of influenza virus infection among older adults in Thailand. Influenza and Other Respiratory Viruses 2017, 11, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Chittaganpitch, M.; Puthavathana, P.; Praphasiri, P.; Waicharoen, S.; Shrestha, M.; Mott, J.A.; et al. Antibody responses against influenza B lineages among community-dwelling individuals 65 years of age or older having received trivalent inactivated influenza vaccine during two consecutive seasons in Thailand. SouthEast Asian Journal of Tropical Medicine and Public Health 2019, 50, 500–513. [Google Scholar]
- Prasert, K.; Patumanond, J.; Praphasiri, P.; Siriluk, S.; Ditsungnoen, D.; Chittaganpich, M.; et al. Effectiveness of trivalent inactivated influenza vaccine among community-dwelling older adults in Thailand: A two-year prospective cohort study. Vaccine 2019, 37, 783–791. [Google Scholar] [CrossRef]
- Prapasiri, P.; Jareinpituk, S.; Keawpan, A.; Chuxnum, T.; Baggett, H.C.; Thamathitiwat, S.; et al. Epidemiology of radiographically-confirmed and bacteremic pneumonia in rural Thailand. The Southeast Asian journal of tropical medicine and public health 2008, 39, 706–718. [Google Scholar]
- Baggett, H.C.; Peruski, L.F.; Olsen, S.J.; Thamthitiwat, S.; Rhodes, J.; Dejsirilert, S.; et al. Incidence of Pneumococcal Bacteremia Requiring Hospitalization in Rural Thailand. Clinical Infectious Diseases 2009, 48, S65–S74. [Google Scholar] [CrossRef] [PubMed]
- Jordan, H.T.; Prapasiri, P.; Areerat, P.; Anand, S.; Clague, B.; Sutthirattana, S.; et al. A comparison of population-based pneumonia surveillance and health-seeking behavior in two provinces in rural Thailand. International Journal of Infectious Diseases 2009, 13, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Dal Poz, M.R.; Kinfu, Y.; Dräger, S.; Kunjumen, T.; Diallo, K. Counting health workers: definitions, data, methods and global results; World Health Organization: Geneva, 2006. [Google Scholar]
- World Health Organization. Health workers: a global profile. Working together for health: the World health report 2006. 2006.
- Larson, H.J.; Jarrett, C.; Schulz, W.S.; Chaudhuri, M.; Zhou, Y.; Dube, E.; et al. Measuring vaccine hesitancy: the development of a survey tool. Vaccine 2015, 33, 4165–4175. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Y.H.; Ibrahim, M.H.; Badran, S.G.; Eldeeb, S.M. Hesitancy for influenza vaccine among healthcare workers and mothers of preschool children: A cross-sectional study in Zagazig, Egypt. Journal of Family and Community Medicine 2022, 29, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.O.; Li, K.K.; Wei, W.I.; Tang, A.; Wong, S.Y.; Lee, S.S. Influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: A survey. International journal of nursing studies 2021, 114, 103854. [Google Scholar] [CrossRef] [PubMed]
- Alobwede, S.M.; Kidzeru, E.B.; Katoto, P.D.M.C.; et al. Influenza Vaccination Uptake and Hesitancy among Healthcare Workers in Early 2021 at the Start of the COVID-19 Vaccine Rollout in Cape Town, South Africa. Vaccines (Basel) 2022, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Sibanda, M.; Meyer, J.C.; Godman, B.; Burnett, R.J. Low influenza vaccine uptake by healthcare workers caring for the elderly in South African old age homes and primary healthcare facilities. BMC Public Health 2023, 23, 91. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, W.; Little, M.; Markham, P.; Gordon, J.; Kerridge, I. Doctors on Status and Respect: A Qualitative Study. Journal of Bioethical Inquiry 2013, 10, 205–217. [Google Scholar] [CrossRef]
- Strömgren, M.; Eriksson, A.; Ahlstrom, L.; Bergman, D.K.; Dellve, L. Leadership quality: a factor important for social capital in healthcare organizations. Journal of Health Organization and Management 2017, 31, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Corace, K.; Prematunge, C.; McCarthy, A.; Nair, R.C.; Roth, V.; Hayes, T.; et al. Predicting influenza vaccination uptake among health care workers: what are the key motivators? Am J Infect Control 2013, 41, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Llupià, A.; García-Basteiro, A.L.; Olivé, V.; Costas, L.; Ríos, J.; Quesada, S.; et al. New interventions to increase influenza vaccination rates in health care workers. American Journal of Infection Control 2010, 38, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Slaunwhite, J.M.; Smith, S.M.; Fleming, M.T.; Strang, R.; Lockhart, C. Increasing vaccination rates among health care workers using unit "champions" as a motivator. Can J Infect Control 2009, 24, 159–164. [Google Scholar]
- Abramson, Z.H.; Avni, O.; Levi, O.; Miskin, I.N. Randomized Trial of a Program to Increase Staff Influenza Vaccination in Primary Care Clinics. The Annals of Family Medicine 2010, 8, 293–298. [Google Scholar] [CrossRef]
- Bourdieu, P. Distinction: A Social Critique of the Judgement of Taste 1984.
- Attwell, K.; Meyer, S.B.; Ward, P.R. The Social Basis of Vaccine Questioning and Refusal: A Qualitative Study Employing Bourdieu's Concepts of 'Capitals' and 'Habitus'. International journal of environmental research and public health 2018, 15, 1044. [Google Scholar] [CrossRef]
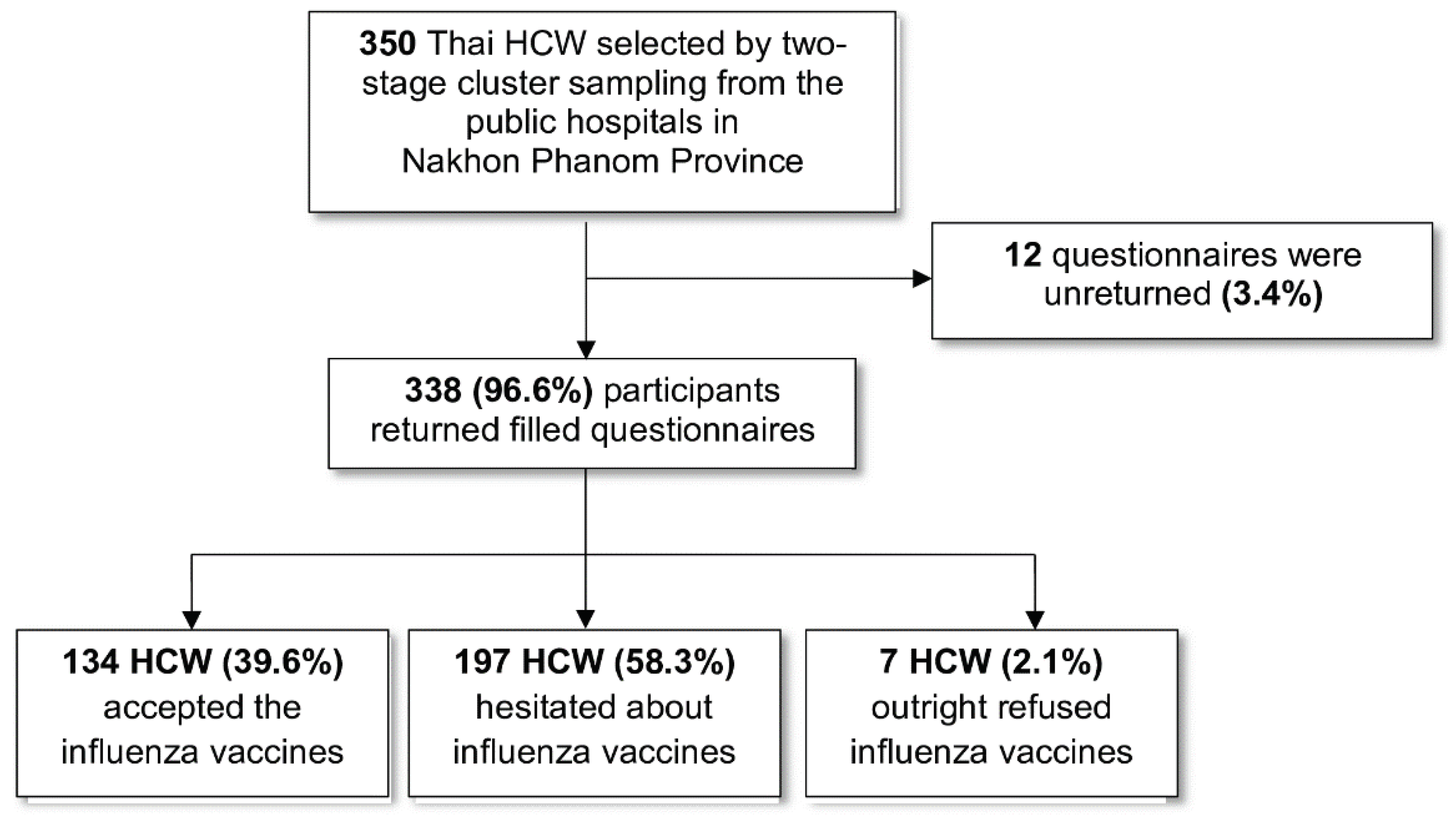
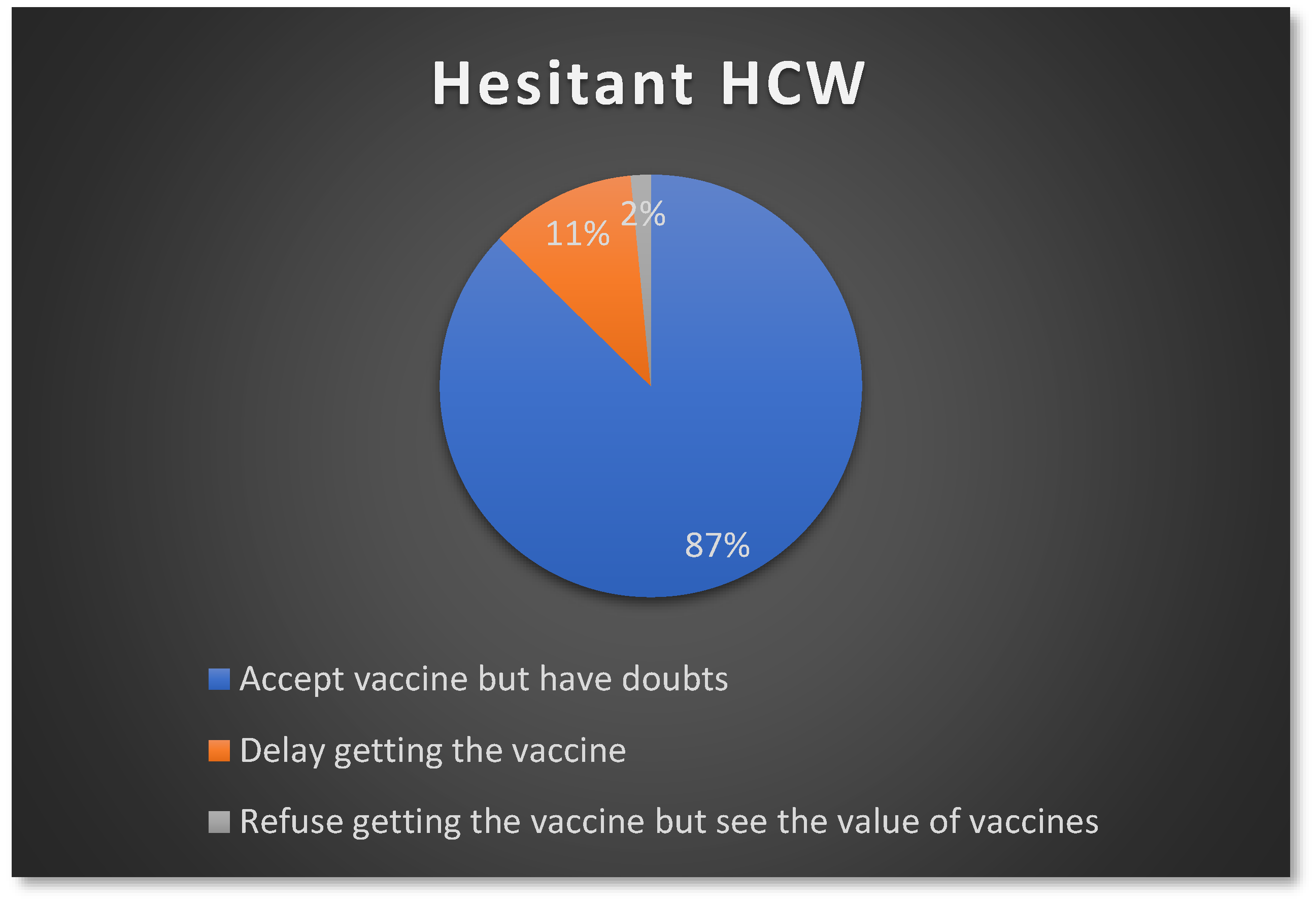
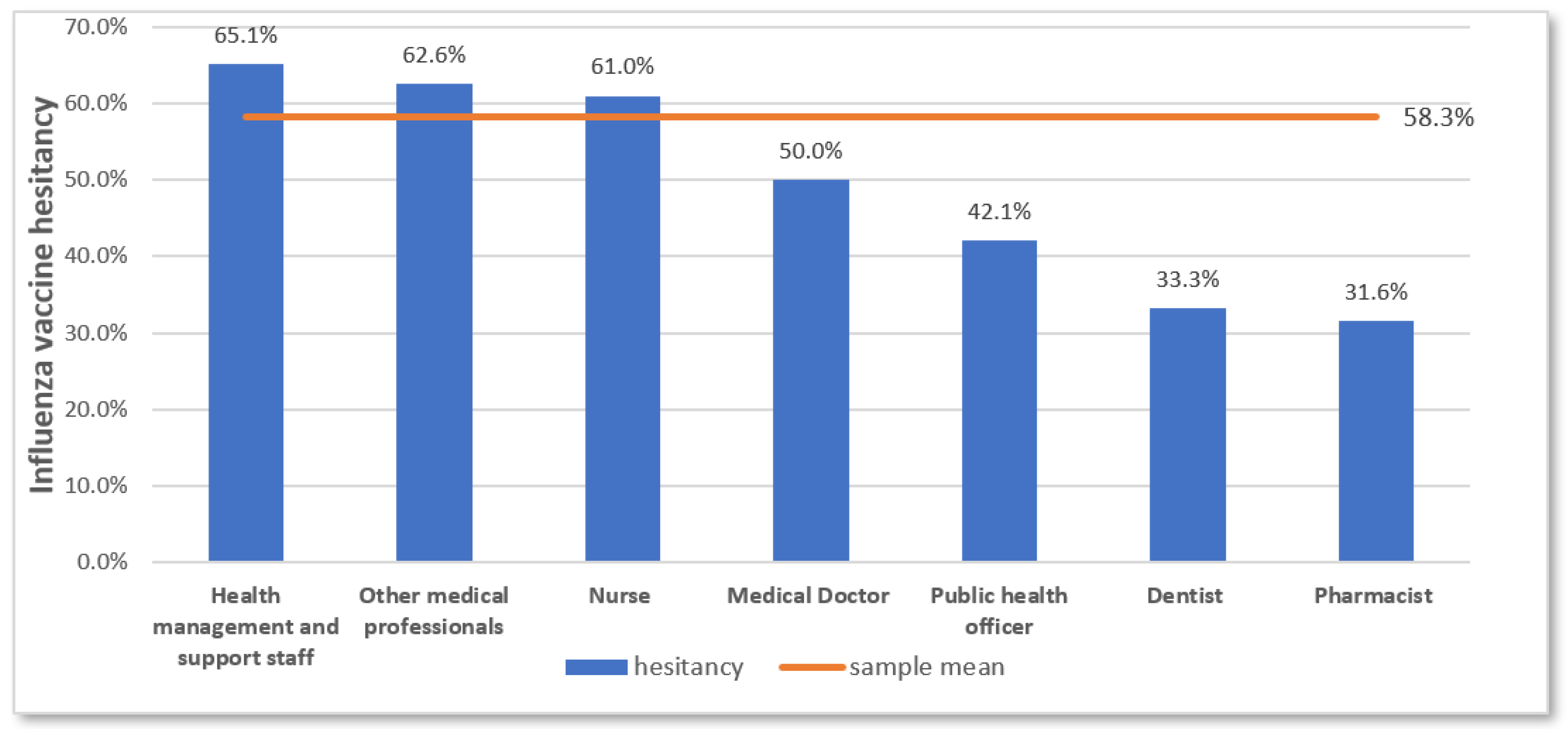
| Characteristic | Number | Percentage |
|---|---|---|
| Age group | ||
| ≤30 years | 102 | 30.2 |
| 31-40 years | 121 | 35.8 |
| 41-50 years | 72 | 21.3 |
| >50 years | 43 | 12.7 |
| Mean (SD): 37.2 years (9.7); Min: 20, Max: 60 | ||
| Sex | ||
| Female | 280 | 82.8 |
| Male | 58 | 17.2 |
| Religion | ||
| Buddhism | 333 | 98.5 |
| Christianity | 5 | 1.5 |
| Level of hospital | ||
| Provincial | 100 | 29.6 |
| District | 238 | 70.4 |
| HCW type | ||
| Health service providers | 295 | 87.3 |
| Nurse | 136 | 40.2 |
| Pharmacist | 19 | 5.6 |
| Public health officer | 19 | 5.6 |
| Medical doctor | 16 | 4.7 |
| Dentist | 6 | 1.8 |
| Other medical personnel | 99 | 29.3 |
| Hospital assistant staff | 55 | 16.3 |
| Patient assistants | 27 | 8.0 |
| Emergency medical technicians (EMT)/paramedic | 7 | 2.1 |
| Laboratory staff | 7 | 2.1 |
| Nurse aids | 3 | 0.9 |
| Health management and support staff | 43 | 12.7 |
| Average daily exposure with patients | ||
| High (<1 meter) | 172 | 50.9 |
| Moderate (1 meter or more) | 106 | 31.4 |
| Low (no direct patient contact) | 60 | 17.8 |
| Work experience as HCW | ||
| ≤5 years | 74 | 21.9 |
| >5 years to <20 years | 166 | 49.1 |
| ≥20 years | 98 | 29.0 |
| Mean (SD): 13.8 years (9.8); Min:1, Max: 37 | ||
| Highest educational attainment | ||
| High school or below | 36 | 10.7 |
| Diploma or vocational college | 43 | 12.7 |
| Bachelor’s degree | 223 | 65.9 |
| Master’s degree or higher | 36 | 10.7 |
| Pre-existing chronic medical condition | ||
| Yes | 74 | 21.9 |
| No | 264 | 78.1 |
| Knowledge items | Correct response | Number | % |
|---|---|---|---|
|
True | 302 | 89.3 |
|
True | 273 | 80.8 |
|
True | 240 | 71.0 |
|
False | 219 | 64.8 |
|
True | 176 | 52.1 |
|
True | 140 | 41.4 |
|
False | 102 | 30.2 |
| Overall knowledge categories | |||
| Need improvement (Overall score <60%) | 0-3 | 104 | 30.8 |
| Fair (Overall score 60-79%) | 4-5 | 149 | 44.1 |
| Good (Overall score ≥80%) | 6-7 | 85 | 25.1 |
| Determinant | Total Mean (SD) |
Influenza vaccine hesitancy | p-value* | |||
|---|---|---|---|---|---|---|
| Yes (n=197) | No (n=134) | |||||
| Mean | SD | Mean | SD | |||
| Contextual factors | ||||||
| Negative influence of media/ social media | 2.7 (0.8) | 2.6 | 0.8 | 2.8 | 0.9 | 0.154 |
| Negative perception of politics/policies | 1.9 (0.9) | 1.9 | 0.8 | 1.8 | 0.9 | 0.057 |
| Negative perception of pharmaceutical industry | 2.8 (1.0) | 2.8 | 0.9 | 2.7 | 1.1 | 0.435 |
| Lack of trust in health system | 2.5 (1.0) | 2.7 | 0.7 | 2.3 | 1.1 | 0.008 |
| Individual/Group influences | ||||||
| Negative past experience of vaccination | 2.3 (1.1) | 2.4 | 1.1 | 2.1 | 1.0 | 0.017 |
| Negative beliefs, attitudes about influenza and vaccination | 2.2 (0.9) | 2.2 | 0.8 | 2.1 | 1.0 | 0.614 |
| Negative perception of vaccination as a social norm | 2.2 (0.9) | 2.3 | 0.8 | 2.0 | 0.9 | 0.011 |
| Negative influence of other HCW | 2.0 (1.0) | 2.2 | 0.9 | 1.9 | 1.0 | 0.006 |
| Influenza vaccine specific factors | ||||||
| Perceived risk of influenza infection and lack of benefit of vaccination | 2.2 (0.7) | 2.4 | 0.6 | 2.1 | 0.7 | 0.001 |
| Risk of adverse events due to vaccination | 2.5 (0.9) | 2.7 | 0.9 | 2.3 | 1.0 | 0.001 |
| Fear of painful injections | 2.4 (0.7) | 2.5 | 0.8 | 2.2 | 0.8 | 0.007 |
| Access to vaccines in the hospital | 2.4 (0.7) | 2.5 | 0.7 | 2.2 | 0.7 | <0.001 |
| Factor | Influenza vaccine hesitancy | Crude OR | 95% CI | Adjusted ORa | 95% CI | |
|---|---|---|---|---|---|---|
| Yes (n=197) | No (n=134) | |||||
| n (%) | n (%) | |||||
| Age-groups (in years) | ||||||
| ≤30 | 55 (53.9) | 47 (46.1) | Ref | Ref | ||
| 31-40 | 65 (55.1) | 53 (44.9) | 1.0 | 0.6-1.8 | 1.1 | 0.6-2.1 |
| 41–50 | 44 (64.7) | 24 (35.3) | 1.6 | 0.8-2.9 | 1.9 | 0.9-4.1 |
| >50 | 33 (76.7) | 10 (23.3) | 2.8 | 1.2-6.3* | 3.2 | 1.3-8.5* |
| Sex | ||||||
| Male | 34 (59.6) | 23 (40.4) | Ref | |||
| Female | 163 (59.5) | 111 (40.5) | 0.9 | 0.6-1.8 | ||
| Level of hospital | ||||||
| Provincial | 54 (55.7) | 43 (44.3) | Ref | |||
| District | 143 (61.1) | 91 (38.9) | 1.2 | 0.8-2.0 | ||
| HCW type | ||||||
| Health service providers | 169 (58.3) | 121 (41.7) | 0.6 | 0.3-1.3 | ||
| Health management and support staff | 28 (68.3) | 13 (31.7) | Ref | |||
| Average daily patient exposure | ||||||
| High | 96 (56.8) | 73 (43.2) | 0.8 | 0.4-1.5 | ||
| Moderate | 65 (63.1) | 38 (36.9) | 1.1 | 0.6-2.1 | ||
| Low | 36 (61.0) | 23 (39.0) | Ref | |||
| Work experience | ||||||
| ≤5 years | 37 (50.0) | 37 (50.0) | Ref | |||
| >5 years to <20 years | 95 (58.3) | 68 (41.7) | 1.4 | 0.8-2.4 | ||
| ≥20 years | 65 (69.1) | 29 (30.9) | 2.2 | 1.2-4.2* | ||
| Highest educational attainment | ||||||
| High school or below | 25 (69.4) | 11 (30.6) | Ref | |||
| Diploma/ vocational college | 31 (72.1) | 12 (27.9) | 1.1 | 0.4-3.0 | ||
| Bachelor’s degree | 122 (56.2) | 95 (43.8) | 0.6 | 0.3-1.2 | ||
| Master’s degree or higher | 19 (54.3) | 16 (45.7) | 0.5 | 0.2-1.4 | ||
| Pre-existing chronic condition | ||||||
| Yes | 44 (60.3) | 29 (29.7) | 1.0 | 0.6-1.8 | ||
| No | 153 (59.3) | 105 (40.7) | Ref | |||
| Knowledge of influenza and vaccination | ||||||
| Need improvement | 71 (69.6) | 31 (30.4) | Ref | Ref | ||
| Fair | 74 (51.4) | 70 (48.6) | 0.5 | 0.3-0.8* | 0.4 | 0.2-0.8* |
| Good | 52 (61.2) | 33 (38.8) | 0.7 | 0.4-1.3 | 0.9 | 0.5-2.0 |
| Contextual factors | ||||||
| Negative influence of media/ social media | ||||||
| High | 41 (57.7) | 30 (42.3) | 0.7 | 0.4-1.4 | ||
| Moderate | 93 (57.1) | 70 (42.9) | 0.7 | 0.4-1.2 | ||
| Low | 63 (64.9) | 34 (35.1) | Ref | |||
| Negative perception of politics/policies | ||||||
| High | 76 (69.1) | 34 (30.9) | 2.6 | 1.5-4.7* | 1.8 | 0.8-4.0 |
| Moderate | 78 (61.4) | 49 (38.6) | 1.9 | 1.1-3.2* | 1.2 | 0.6-2.3 |
| Low | 43 (45.7) | 51 (54.3) | Ref | Ref | ||
| Negative perception of pharmaceutical industry | ||||||
| High | 58 (65.9) | 30 (34.1) | 1.6 | 0.9-2.9 | ||
| Moderate | 89 (58.6) | 63 (41.4) | 1.1 | 0.7-1.9 | ||
| Low | 50 (54.9) | 41 (45.1) | Ref | |||
| Lack of trust in health system | ||||||
| High | 52 (69.3) | 23 (30.7) | 2.6 | 1.3-4.9* | 1.3 | 0.5-3.5 |
| Moderate | 106 (61.3) | 67 (38.7) | 1.8 | 1.1-3.0* | 1.2 | 0.6-2.4 |
| Low | 39 (47.0) | 44 (53.0) | Ref | Ref | ||
| Individual/group factors | ||||||
| Negative past experience of vaccination | ||||||
| High | 65 (67.0) | 32 (33.0) | 2.1 | 1.2-3.7* | 1.4 | 0.6-3.2 |
| Moderate | 80 (62.5) | 48 (37.5) | 1.7 | 1.0-2.9* | 1.2 | 0.6-2.5 |
| Low | 52 (49.1) | 54 (50.9) | Ref | Ref | ||
| Negative beliefs, attitudes about influenza and vaccination | ||||||
| High | 62 (59.0) | 43 (41.0) | 1.4 | 0.8-2.5 | 0.7 | 0.3-1.5 |
| Moderate | 86 (67.2) | 42 (32.8) | 2.0 | 1.2-3.5* | 1.4 | 0.7-2.7 |
| Low | 49 (50.0) | 49 (50.0) | Ref | Ref | ||
| Negative perception of vaccination as a social norm | ||||||
| High | 78 (68.4) | 36 (31.6) | 2.2 | 1.3-3.9* | 0.9 | 0.4-2.3 |
| Moderate | 70 (59.3) | 48 (40.7) | 1.5 | 0.9-2.5 | 1.1 | 0.5-2.2 |
| Low | 49 (49.5) | 50 (50.5) | Ref | Ref | ||
| Negative influence of other HCW | ||||||
| High | 92 (70.2) | 39 (29.8) | 3.3 | 1.9-5.7* | 2.3 | 1.1-4.8* |
| Moderate | 60 (65.9) | 31 (34.1) | 2.7 | 1.5-4.9* | 2.1 | 1.1-4.4* |
| Low | 45 (41.3) | 64 (58.7) | Ref | Ref | ||
| Influenza vaccination specific factors | ||||||
| Perceived risk of influenza infection and lack of benefit of vaccination | ||||||
| Low | 73 (68.2) | 34 (31.8) | 2.5 | 1.4-4.5* | 1.0 | 0.4-2.5 |
| Moderate | 82 (61.7) | 51 (38.3) | 1.9 | 1.1-3.2* | 0.9 | 0.4-1.8 |
| High | 42 (46.2) | 49 (53.8) | Ref | Ref | ||
| Risk of adverse events due to vaccination | ||||||
| High | 55 (73.3) | 20 (26.7) | 2.8 | 1.5-5.3* | 1.6 | 0.6-4.1 |
| Moderate | 84 (60.9) | 54 (39.1) | 1.6 | 0.9-2.6 | 0.9 | 0.5-1.9 |
| Low | 58 (49.2) | 60 (50.8) | Ref | Ref | ||
| Fear of painful injections | ||||||
| High | 104 (63.8) | 59 (36.2) | 1.7 | 1.1-2.7* | 0.9 | 0.5-1.6 |
| Moderate | 28 (70.0) | 12 (30.0) | 2.3 | 1.0-4.8* | 1.8 | 0.7-4.5 |
| Low | 65 (50.8) | 63 (49.2) | Ref | Ref | ||
| Access to vaccines in the hospital | ||||||
| Low | 64 (66.0) | 33 (34.0) | 2.0 | 1.2-3.5* | 1.3 | 0.6-2.8 |
| Moderate | 71 (66.4) | 36 (33.6) | 2.1 | 1.2-3.5* | 1.4 | 0.7-2.8 |
| High | 62 (48.8) | 65 (51.2) | Ref | Ref | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





