1. Introduction
Kiwifruit (
Actinidia Lindl.), classified in the Actinidiaceae family and the genus
Actinidia, is commonly referred to as the "King of Fruits" due to its exceptionally high content of vitamin C, minerals, dietary fiber, and other beneficial metabolites [
1,
2,
3]. Originating in China, kiwifruit has become a significant cash crop, gaining popularity worldwide for its flavor and nutritional attributes [
1]. Given its high nutritional, medicinal and economic value, kiwifruit holds a promising future [
2,
3,
4,
5]. However, throughout the growth and development of kiwifruit, it is inevitably influenced by various biotic or abiotic stresses, such as infection by pathogenic bacteria, heat stress, waterlogging stress, and metal stress. Notably, the emergence of the
Pseudomonas syringae pv.
actinidiae (Psa) disease has proven to be a serious global threat to kiwifruit, leading to substantial economic losses in the industry on a worldwide scale [
6,
7]. Heat stress poses another challenge as it can adversely affect the growth and development of plants [
8]. Kiwifruit roots, being shallow and sensitive to water, can experience reduced crop yields and even plant fatalities [
9]. Therefore, it is essential to prioritize research on the resistance response mechanisms in kiwifruit. Investigating these mechanisms is crucial for developing strategies to ensure the economic viability and sustainability of kiwifruit cultivation in the face of diverse stress challenges.
Plants exist in a dynamically changing environment, where they must strike a balance between harnessing benefits from microorganisms and fending off pathogenic challenges. While beneficial microorganisms contribute to plant growth and agricultural production, pathogenic ones can result in diseases and economic losses. Unlike animals, plants lack a circulating immune system and depend on each cell's ability to deploy innate immune responses against potential pathogens [
10]. Plant immune receptors recognize the presence and activities of specific microorganisms, triggering coordinated downstream signaling pathways and initiating immune responses [
11,
12]. Pattern recognition receptors (PRRs) include receptor-like kinases (RLKs) and receptor-like proteins (RLPs) [
10,
13]. RLKs consist of an extracellular domain for ligand recognition, a single-pass transmembrane domain for intracellular and extracellular signaling and membrane localization. Additionally, they feature an intracellular kinase domain responsible for transmitting immune signals. In contrast, RLPs carry a short cytoplasmic tail and lack the intracellular kinase domain [
14,
15]. Due to the intracellular kinase domain, RLKs can independently trigger signals, whereas RLPs need to form a functional complex with RLKs to activate downstream signals [
16]. According to the different extracellular domains, PRRs can be categorized into LRR domain receptors, LysM domain receptors, Lectin domain receptors and EGF-like domain receptors [
17,
18]. Within the RLPs family, LRR-RLPs constitute the largest subfamily [
19].
LRR-RLP plays a crucial role in plant growth and development, signal transduction and stress responses. For instance, CLAVAT2 (CLV2) /AtRLP10 regulates shoot apical meristem maintenance and differentiation, impacting organ development [
20]. In
Arabidopsis thaliana, CLV2 is essential for meristem development, and its maize homolog FASCINATED EAR2 (FEA2) also governs meristem development [
21]. Another example is TOO MANY MOUTHS (TMM)/AtRLP17 in
Arabidopsis thaliana, which contributes to overall development and plays a key role in regulating stomatal distribution on the epidermis [
22,
23]. AtRLP41 modulates leaf sensitivity to abscisic acid (ABA) and potentially participates in ABA-induced senescence [
23]. AtRLP44 activates BR signaling by directly interacting with the BR coreceptor BAK1[
24]. In tomato, the Cf-9 gene is vital for pathogen defense and confers resistance to Avr9, an effector of Clavidium flavus [
25]. The expression of OsRLP1 significantly increases after infection with rice black-streaked dwarf virus (RBSDV), and its interaction with the receptor-like kinase OsSOBIR1 induces PTI response and enhances antiviral defense [
26]. Additionally, LRR-RLPs play a crucial role in abiotic stress tolerance. For instance, AtRLP28 overexpressed plants exhibit enhanced salt stress tolerance [
27].
The LRR-RLP gene family has undergone extensive study across various plant species. In the model plant
A.thaliana, the LRR-RLP gene family was identified, revealing 57 LRR-RLP members [
23]. A genome-wide analysis of wild bananas identified 78 members in the LRR-RLP family [
28]. Poplar has 82 identified LRR-RLPs [
29]. In tomato, a total of 176 LRR-RLP members were identified, nearly three times the number in
Arabidopsis [
21]. Rice has 90 LRR-RLPs [
30].
Brassica napus,
Brassica juncea,
Brassica rapa, and
Brassica nigra possess 276, 226, 63, and 175 LRR-RLPs members, respectively [
19,
31].
Kiwifruit is vulnerable to both biotic and abiotic stresses. LRR-RLP is crucial in plant growth and development, signal transduction, and stress responses. However, its study in kiwifruit has received limited attention. This study delves into the analysis of the LRR-RLP genes in kiwifruit, encompassing the identification of gene family members, phylogenetic analysis, and chromosomal localization. We explored the differential response of LRR-RLPs to biotic stress in various resistant kiwifruit varieties and analyzed the response of kiwifruit to multiple abiotic stresses. The results revealed a significant role of LRR-RLP in both biotic and abiotic stress responses in kiwifruit. Our findings contribute to a deeper understanding of the defense mechanisms employed by kiwifruit, holding crucial reference value for the genetic enhancement of kiwifruit varieties. Moreover, this study provides valuable insights for the development of resistant kiwifruit varieties.
2. Results
2.1. Identification of LRR-RLP Gene Family in Kiwifruit
To determine the number of LRR-RLPs family members in 'Hongyang', 'Huate' and 'Red5' kiwifruit, we retrieved 57 LRR-RLP sequences from Arabidopsis databases. BLASTP searches were conducted using these sequences as queries in three kiwifruit databases, with an e-value threshold set at 1e-5. The obtained candidate sequences were then submitted to the Pfam database to confirm the presence of the LRR domain in each candidate sequence. The results revealed the following counts: 101 LRR-RLPs in 'Hongyang' kiwifruit (HyLRR-RLPs), 164 LRR-RLPs in 'Huate' kiwifruit (HtLRR-RLPs), and 105 LRR-RLPs in 'Red5' kiwifruit (DhLRR-RLPs).
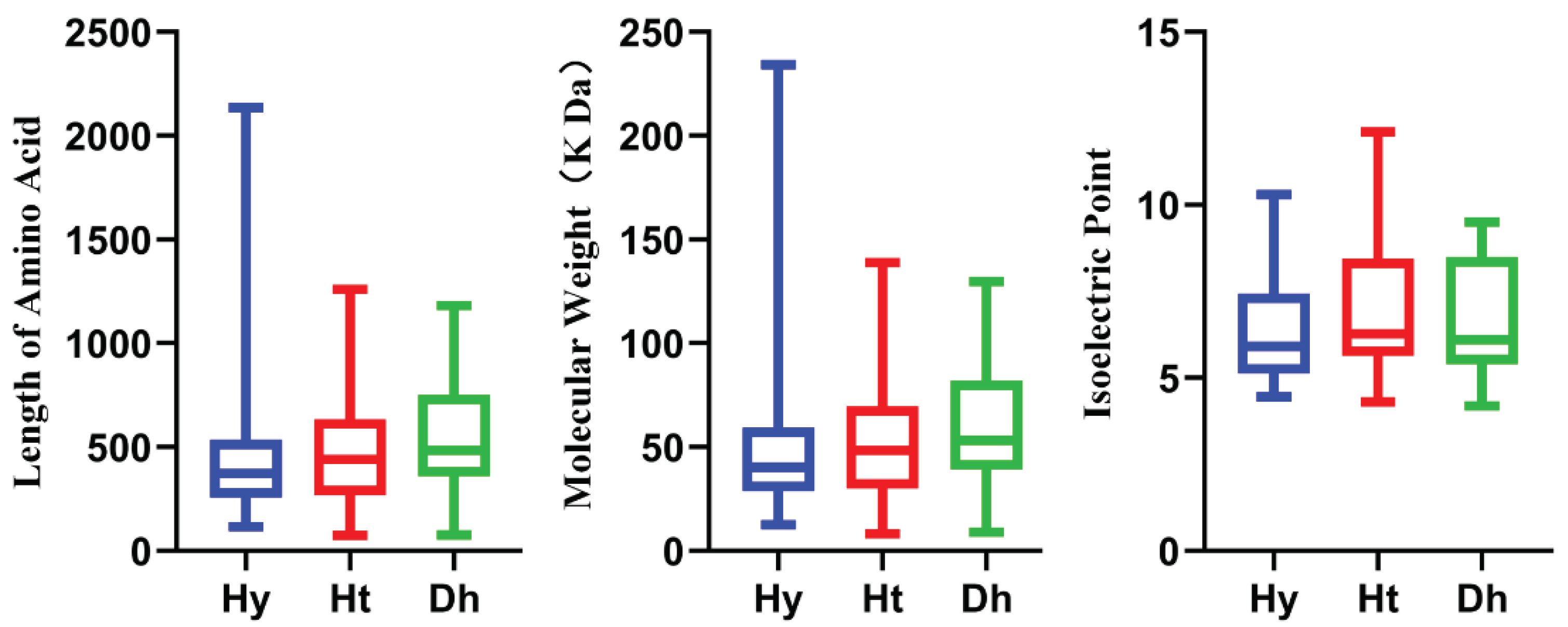
We also conducted an analysis of the physicochemical properties of LRR-RLP members in these three kiwifruit species (
Figure 1). Detailed physicochemical properties of HyLRR-RLP, HtLRR-RLPs and DhLRR-RLPs are provided in
Table S1, S2, and S3, respectively. The amino acid sequence lengths of HyLRR-RLPs ranged from 117(
Actinidia00252.t1) to 2136(
Actinidia29086.t1), with the protein molecular weights (MW) ranging from 12.60(
Actinidia00252.t1) to 234.25 kDa (
Actinidia29086.t1), and isoelectric points (PI) ranging between 4.45(
Actinidia38758.t1) and 10.3(
Actinidia08839.t1). For HtLRR-RLPs, amino acid sequence lengths ranged from 74(
DTZ79_29g13550) to 1260(
DTZ79_27g00620), protein molecular weights (MW) were from 8.29(
DTZ79_29g13550) to 138.89(
DTZ79_24g11220) kDa, and isoelectric points (PI) ranged between 4.3(
DTZ79_14g08180) and 12.11(
DTZ79_26g12390). The amino acid sequences of DhLRR-RLPs ranged from 77(
Acc26673) to 1180(
Acc26673), and the molecular weights of the proteins varied from 8.90(
Acc26673) to 129.82 kDa(
Acc03001), with isoelectric points between 4.19(
Acc10067) and 9.51(
Acc18523).
2.2. Phylogenetic Relationships of the Kiwifruit LRR-RLP Gene Family Members
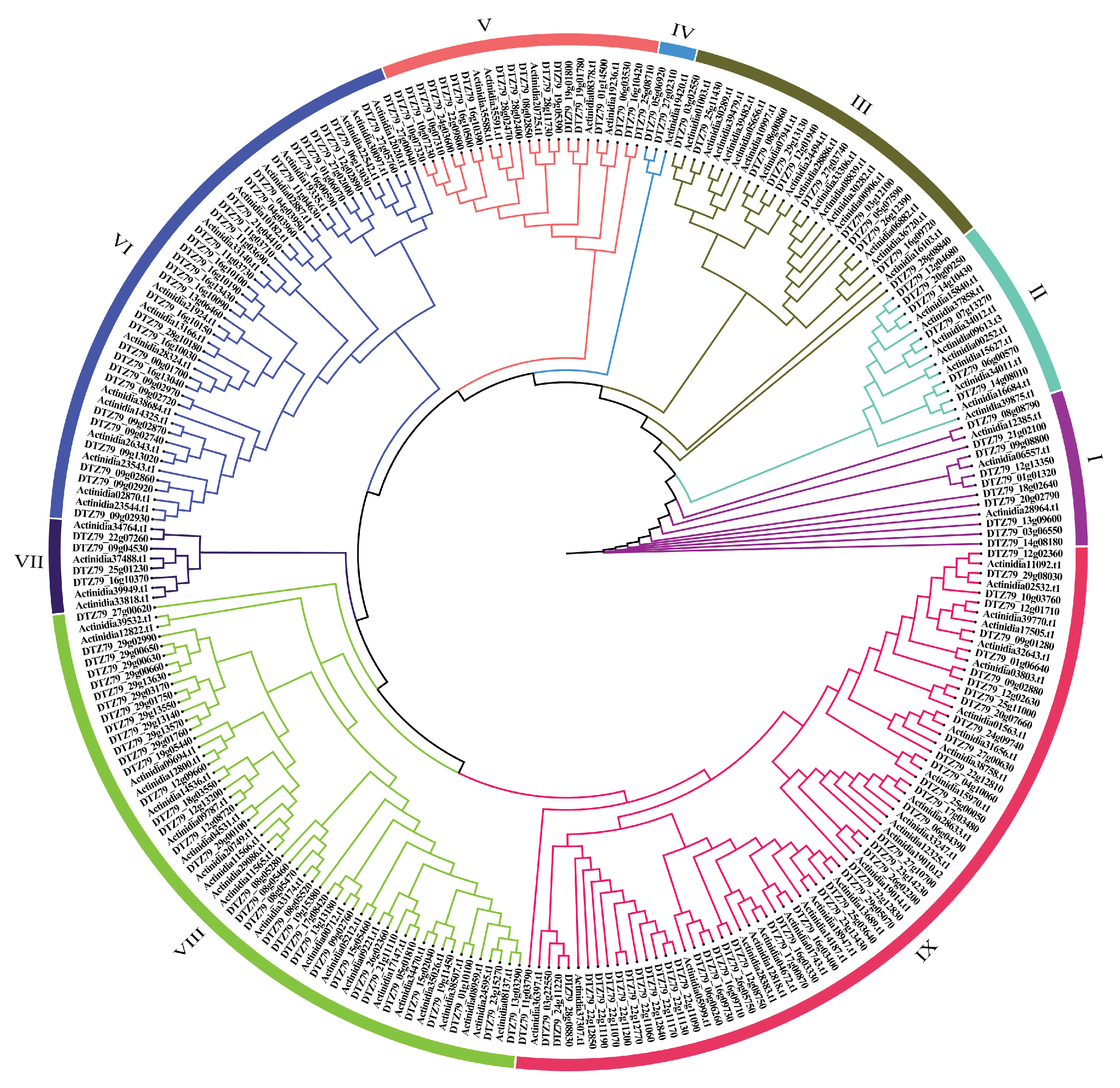
To understand the evolutionary relationship between 'Hongyang' and 'Huate' kiwifruit, we performed multiple sequence alignment using MAFFT7 for 101 HyLRR-RLPs and 164 HtLRR-RLPs identified in this study. Subsequently, a phylogenetic tree was consturcted using the maximum likelihood method with IQtree (
Figure 2). The clustering results were categorized into 9 groups. Based on the clustering results of the phylogenetic tree, we divided it into 9 groups. The distribution of 'Hongyang' kiwifruit and 'Huate' kiwifruit within each group was listed in
Table 1. Notably, group IX exhibited the highest count with 71 members, including 25 HyLRR-RLPs and 46 HtLRR-RLPs. Group VIII followed closely with 57 members, consisting of 22 HyLRR-RLPs and 35 HtLRR-RLPs. Conversely, group IV housed the fewest LRR-RLPs members, with 1 HyLRR-RLPs and 2 HyLRR-RLPs. The phylogenetic tree in
Figure 2 and
Table 1 illustrates a discernible clustering pattern, indicating a trend of convergent evolution of the kiwifruit LRR-RLP gene
.
2.3. Chromosomal Localization Analysis of Kiwifruit LRR-RLP Gene Family Members
We analyzed the chromosomal distribution of HyLRR-RLPs, HtLRR-RLPs, and DhLRR-RLPs identified in this study (
Figure S1). The results revealed that among the 101 HyLRR-RLP family members, 88 were situated in 28 linked groups, while the remaining 13 were found in 13 unlinked groups. Notably, LG07 of 'Hongyang' kiwifruit did not contain any LRR-RLP genes. As for HtLRR-RLP, all 164 genes were dispersed in 29 chromosomes. For DhLRR-RLPs, 100 genes were dispersed in 29 linked groups. The distribution pattern of kiwifruit LRR-RLPs appears extensive, with genes dispersed across linked groups and chromosomes.
2.4. Synteny Analysis of LRR-RLPs in Kiwifruit
We performed synteny analysis to unravel duplication events and possible collinear blocks within or between kiwifruit genomes. Collinearity analysis of LRR-RLPs between the genomes of Hy and
Arabidopsis revealed that 6 HyLRR-RLPs exhibited syntenic relationships with 4 AtLRR-RLPs, while 3 HtLRR-RLPs exhibited syntenic relationships with 4 AtLRR-RLPs. Additionally, 9 DhLRR-RLPs exhibited syntenic relationships with 8 AtLRR-RLPs (
Table S4). Synteny analysis between kiwifruit genomes showed that 37 HyLRR-RLPs and 36 HtLRR-RLP formed 47 pairs, 1 HyLRR-RLPs and 1 DhLRR-RLP formed 1 pair, and 43 HtLRR-RLPs and 52 DhLRR-RLPs formed 65 pairs (
Table S5). What’s more, we carried out synteny analysis within the kiwifruit. The results revealed that HyLRR-RLPs exhibited 47 pairs of segmental duplication and 3 pairs of tandem duplication, HtLRR-RLPs exhibited 56 pairs segmental duplication and 9 pairs tandem duplication, and DhLRR-RLPs exhibited 51 pairs segmental duplication and 7 pairs tandem duplication (
Table S6).
The Ka/Ks values were calculated to evaluate the selection pressure during evolution [
3]. We found that the Ka/Ks values of the duplicate pairs identified above were all less than 1, indicating that these genes evolved under purifying selection.
2.5. Expression Profiles of LRR-RLPs in Response to Psa Infection
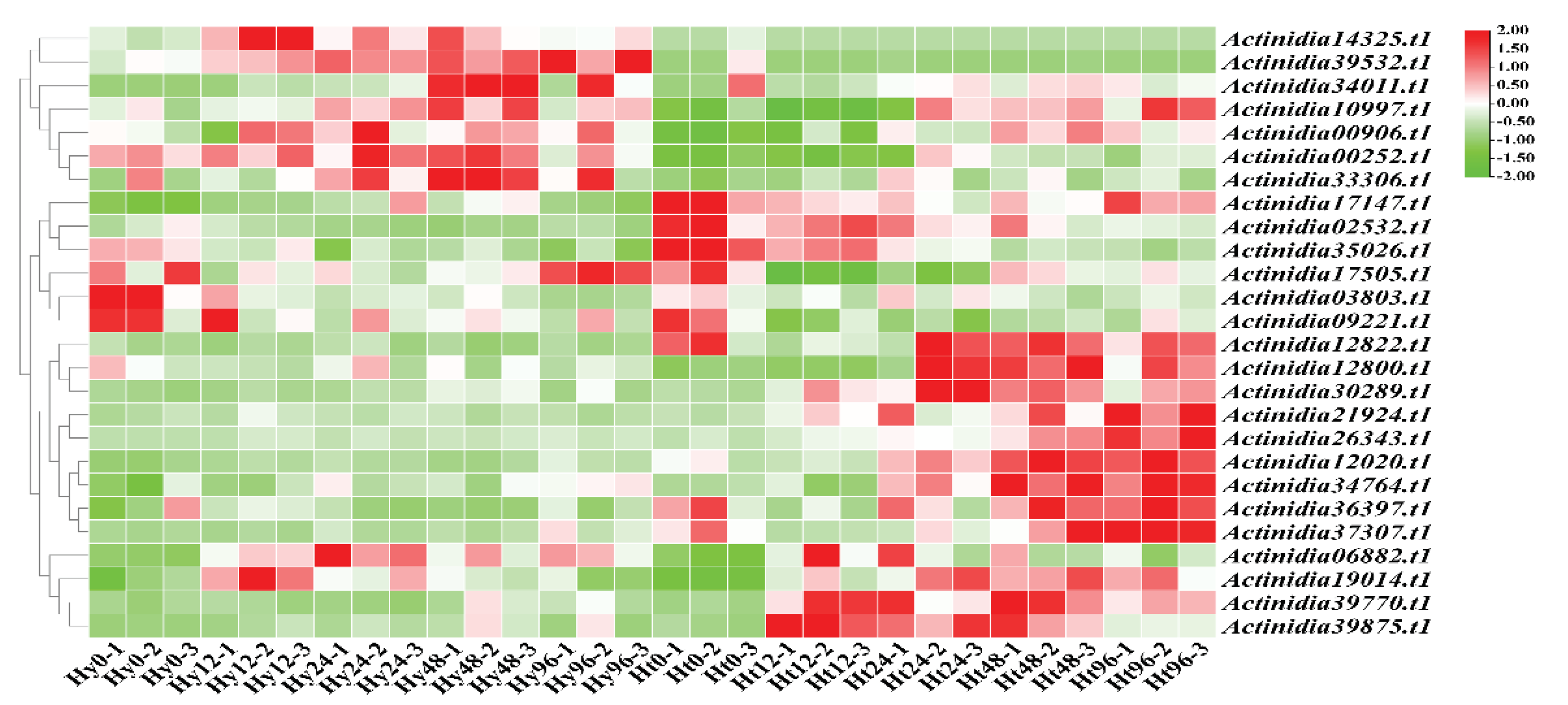
Based on the transcriptome data of kiwifruit infected with Psa, we analyzed the expression of LRR-RLP genes (
Figure 3). Our analysis revealed differentially expressed
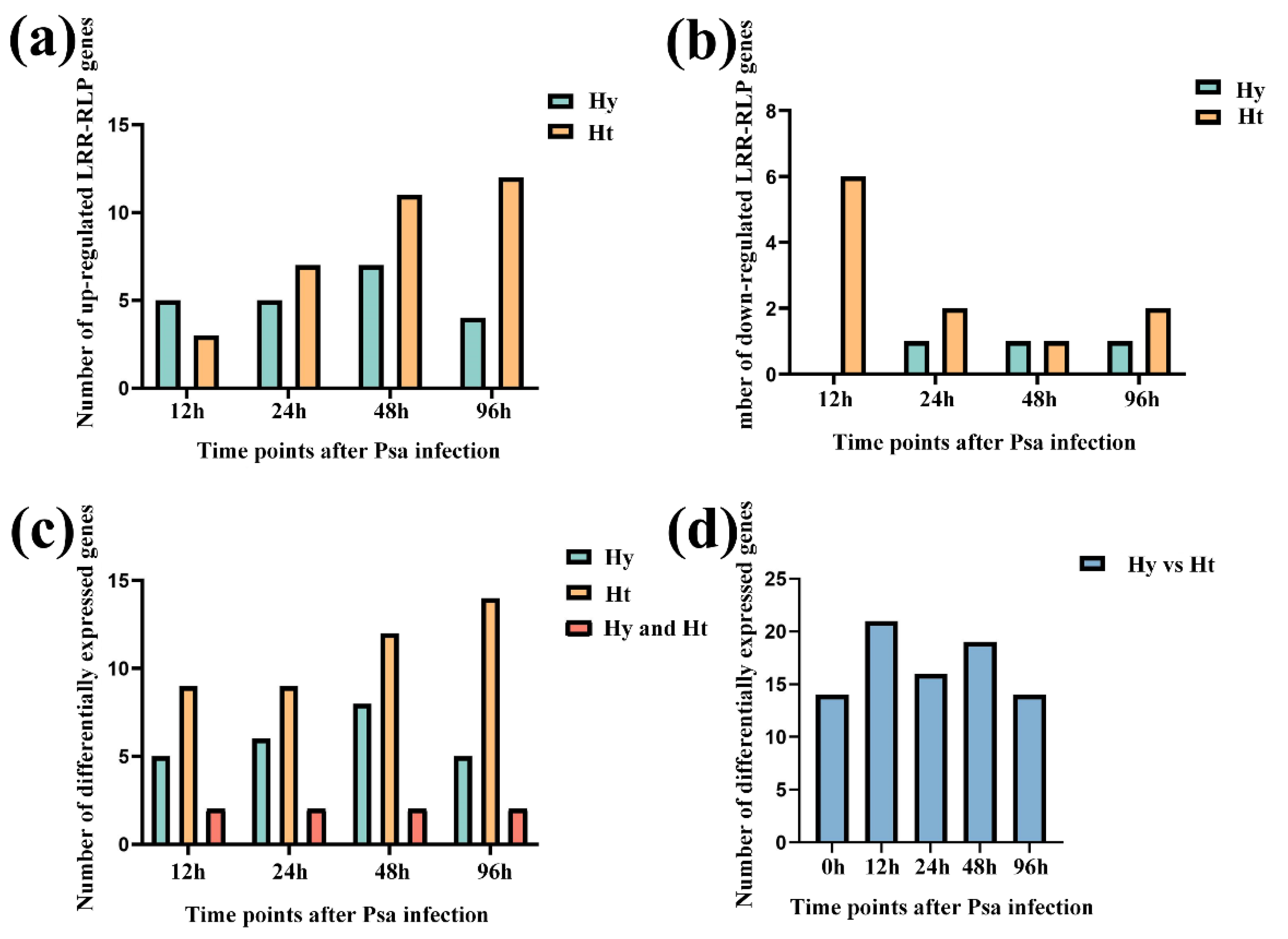
LRR-RLP genes in both resistant 'Huate' kiwifruit (Ht) and susceptible 'Hongyang' kiwifruit (Hy) during Psa infection. Specifically, 10 LRR-RLPs genes were differentially expressed in Hy, and 22 LRR-RLP genes were differentially expressed in Ht. We found that the number of LRR-RLP in Ht was more than that in Hy, regardless of the differentially up-regulated expression or differentially down-regulated expression (
Figure 4a and 4b). In addition, with the increase of infection time, the differentially up regulated LRR-RLP genes and the differentially down regulated LRR-RLP genes in Ht showed a gradual increase trend, but there was no such change in Hy.
Compared to the 0h time point, we analyzed the differentially expressed LRR-RLP genes in Hy and Ht at 12h, 24h, 48h and 96h, respectively (
Figure 4c). Simultaneously, we detected the differentially expressed LRR-RLP gene between two kiwifruit types at the same time point (
Figure 4d). In Hy, we identified 10 differentially expressed LRR-RLP genes, among which 5, 6, 8 and 5 genes were differentially expressed at 12h, 24h, 48h and 96h, respectively. In Ht, we found 22 differentially expressed LRR-RLP genes, with 9, 9, 12 and 14 differentially expressed genes at 12h, 24h, 48h and 96h, respectively. Of these genes, 4 are unique to Hy and 16 are unique to Ht. Compared to 0h time point, the number of LRR-RLP genes that were simultaneously differentially expressed in both Hy and Ht at the 12h, 24h, 48h and 96h time nodes was 2, 2, 2 and 2, and these two genes were respectively
Actinidia39875.t1 and
Actinidia06882.t1. Using Hy as a control, the comparison of differentially expressed LRR-RLP genes between Hy and Ht at the same time points revealed 14, 21, 16, 19, and 14 genes at 0h, 12h, 24h, 48h, and 96h, respectively (
Figure 4d).
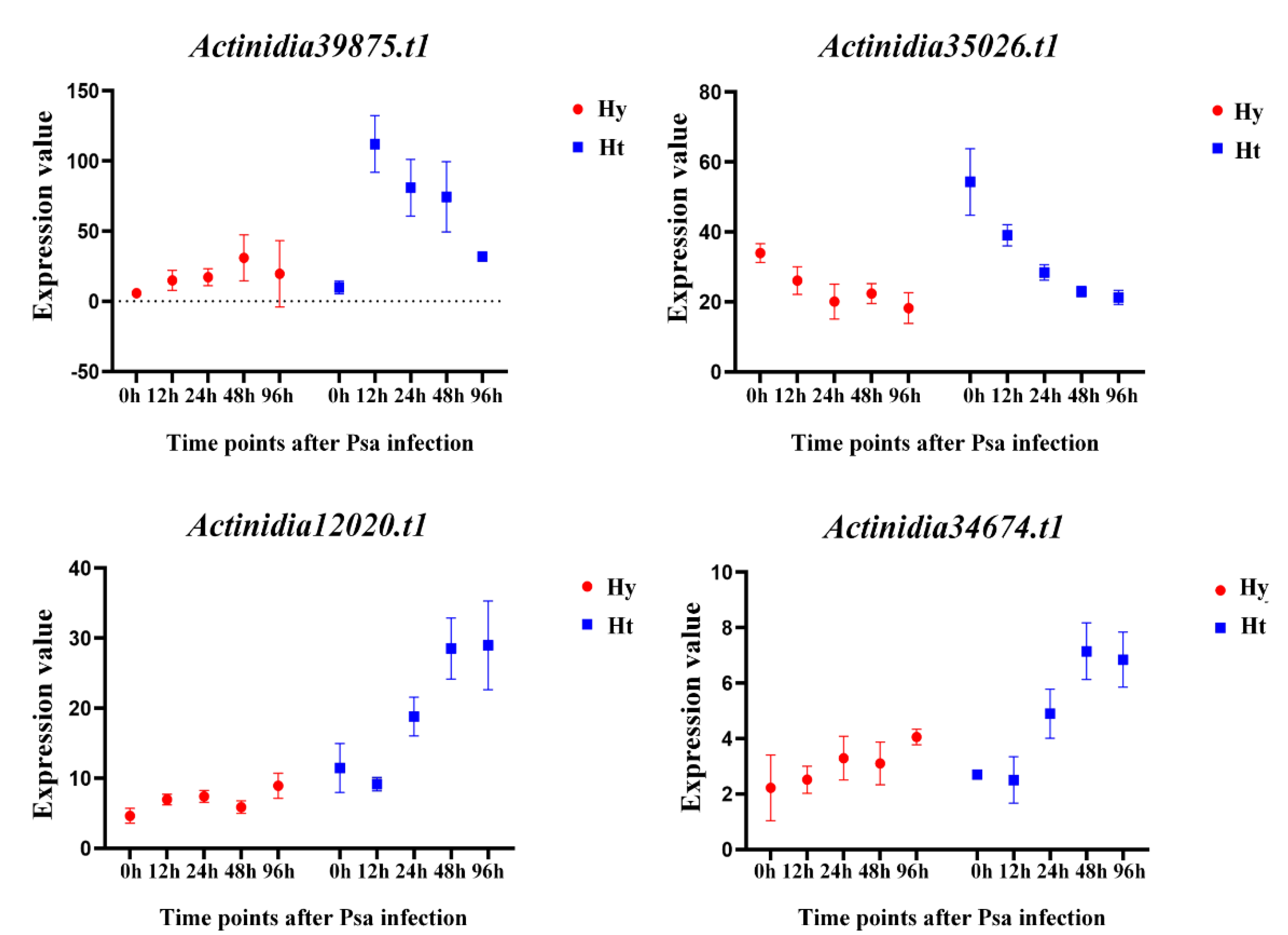
In
Figure 5, several genes exhibit interesting expression changes.
Actinidia39875.t1 was differentially expressed in both Hy and Ht, with higher expression in Ht kiwifruit at each time point. In Hy, the expression of
Actinidia39875.t1 gradually increases, while in Ht, it initially increases, peaks at 12h, and then gradually decreased.
Actinidia35026.t1 is not differently expressed in Hy, but is differently expressed at 48h and 96h in Ht. The expression of
Actinidia35026.t1 remains stable in 'Hongyang' kiwifruit and gradually decreases in 'Huate' kiwifruit.
Actinidia12020.t1 and
Actinidia34674.t1 in Hy have relatively constant expression levels with no differential expression. However, their expression levels show a gradual increasing trend in Ht, and both are differentially up-regulated at 48h and 96h time points. This suggests that these two genes in Ht consistently respond to Psa infection. According to these results, we speculate that the four genes
Actinidia39875.t1,
Actinidia35026.t1,
Actinidia12020.t1, and
Actinidia34674.t1 play a role in the process of Psa infection.
Actinidia39875.t1, and
Actinidia35026.t1 play a role in the early stage of Psa infection, while
Actinidia12020.t1 and
Actinidia34674.t1 play a role in the late stage of Psa infection.
2.6. Analysis of Upstream Transcription Factors of LRR-RLP in Kiwifruit
To unravel the potential pathways governing the expression of LRR-RLP genes in kiwifruit, we intersected the genes differentially expressed in both Hy and Ht, and subsequently identified the upstream transcription factors for these genes using the PlantRegMap database. After filtering out the transcription factors with low expression and no significant changes, as well as those with no differential expression in both Hy and Ht, we obtained 40 transcription factors (
Figure 6). Compared with 0h time point, 9, 15, 15, and 8 transcription factors displayed differential expression in Hy at 12h, 24h, 48h, and 96h, respectively. In Ht, 24, 20, 16, and 15 transcription factors were differentially expressed at 12h, 24h, 48h, and 96h, respectively. Among these, 7 were unique to Hy, while 15 were unique to Ht (
Table 2). Notably, the number of unique differentially expressed transcription factors in Ht was approximately twice that in Hy.
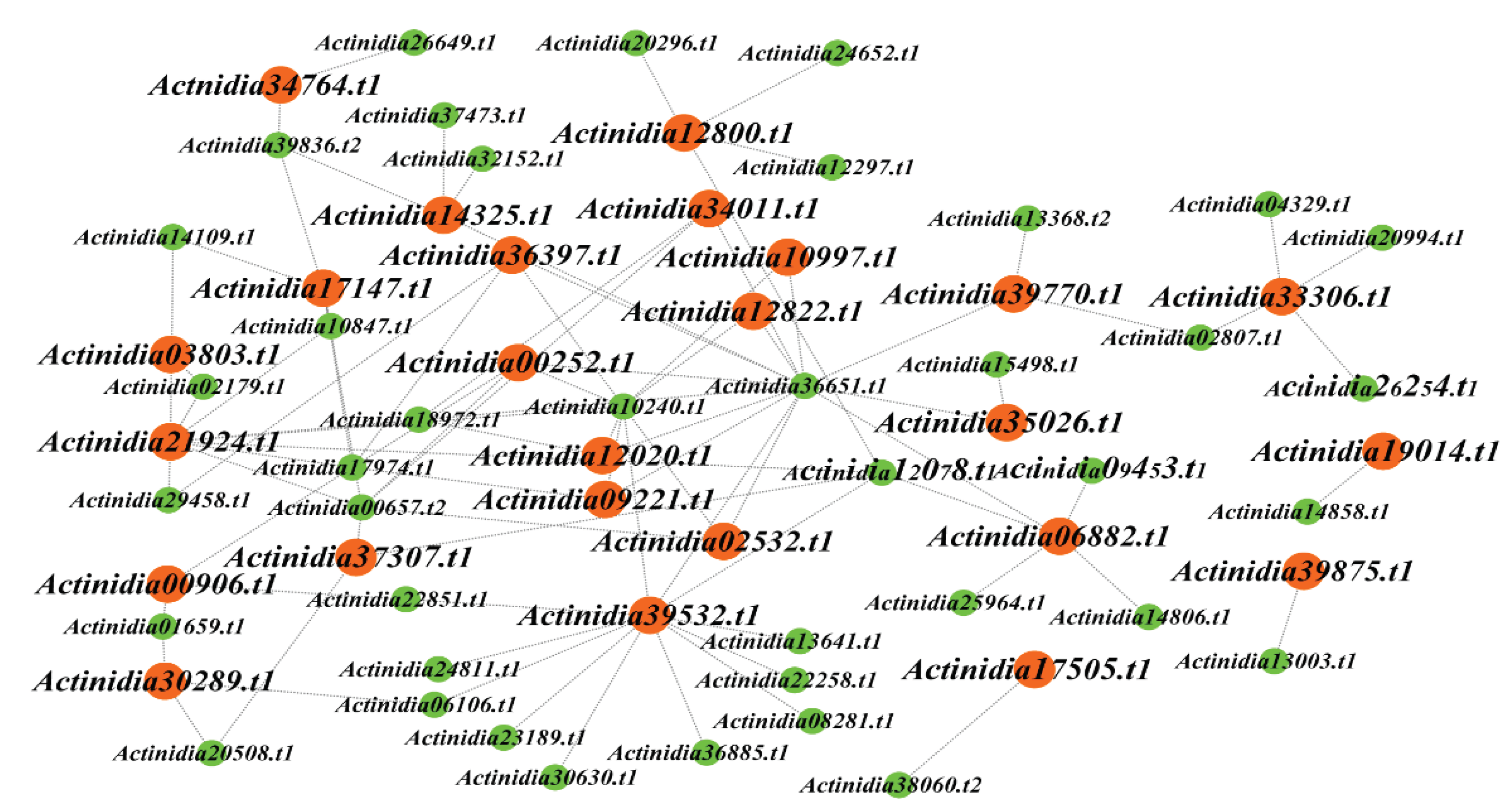
In order to intuitively observe the intricate relationship between the 40 transcription factors and 25 genes, we plotted a network diagram, as illustrated in
Figure 7. Utilizing the PlantRegMap database, we further obtained the families of these 40 transcription factors (
Table S7). Among them, 15 transcription factors exclusively differentially expressed in Ht belong to the MYB, TCP, WRKY, TALE, HD-ZIP, ERF, bZIP, and C2H2 families. Specially,
Actinidia29458.t1 serves as an upstream transcription factor of
Actinidia36397.t1 and
Actinidia21924.t1.
Actinidia14109.t1 is an upstream transcription factor for
Actinidia17147.t1 and
Actinidia21924.t1. Both
Actinidia29458.t1 and
Actinidia14109.t1 belong to the ERF family, and this family was involved in regulating plant development and tolerance to biotic/abiotic stresses [
32].
Actinidia10240.t1, a member of the TALE family, functions as the upstream transcription factor for
Actinidia02532.t1,
Actinidia00252.t1,
Actinidia09221.t1,
Actinidia10997.t1,
Actinidia12822.t1,
Actinidia36397.t1,
Actinidia39532.t1,
Actinidia21924.t1, and
Actinidia12020.t1. The TALE family is associated with meristem formation and the regulation of leaf morphology [
33]. Furthermore,
Actinidia37473.t1 is an upstream transcription factor of
Actinidia14325.t1.
Actinidia39836.t2 is an upstream transcription factor for
Actinidia14325.t1,
Actinidia17147.t1, and
Actinidia34764.t1.
Actinidia37473.t1 and
Actinidia39836.t2 belong to C2H2 gene family, which is involved in plant development, and can control chloroplast development and starch granule formation [
34]. These differentially expressed transcription factors have the potential to regulate the expression of kiwifruit LRR-RLP genes, thereby influencing plant resistance.
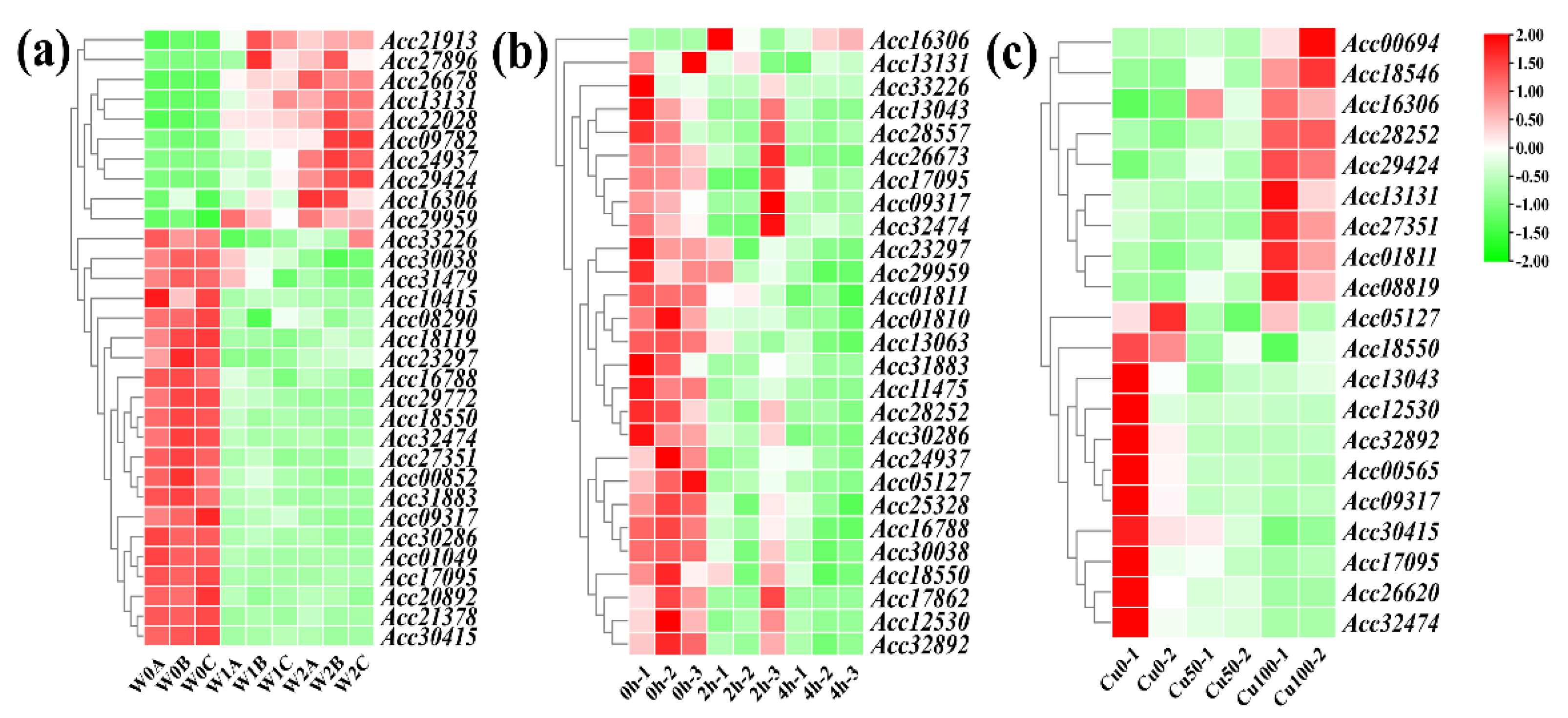
2.7. Expression Profiles of LRR-RLPs in Response to Abiotic Stresses
To explore the impact of kiwifruit LRR-RLPs under abiotic stress conditions, we conducted an analysis using transcriptome data from kiwifruit subjected to three different abiotic stresses: high temperature, waterlogging, and copper. The results revealed distinct responses of LRR-RLP genes in kiwifruit under these different stresses (
Figure 8). Specially, 31 LRR-RLP genes exhibited differentially expressed under waterlogging stress, 27 under heat stress, and 20 under copper stress. Notably, the genes
Acc09317,
Acc13131,
Acc16306,
Acc17095,
Acc18550, and
Acc32474 were all responsive to waterlogging stress, heat stress, and copper stress. This suggests that LRR-RLPs may be involved in the response to multiple abiotic stresses, highlighting their potential key regulatory role in enhancing kiwifruit resistance to adverse environmental conditions.
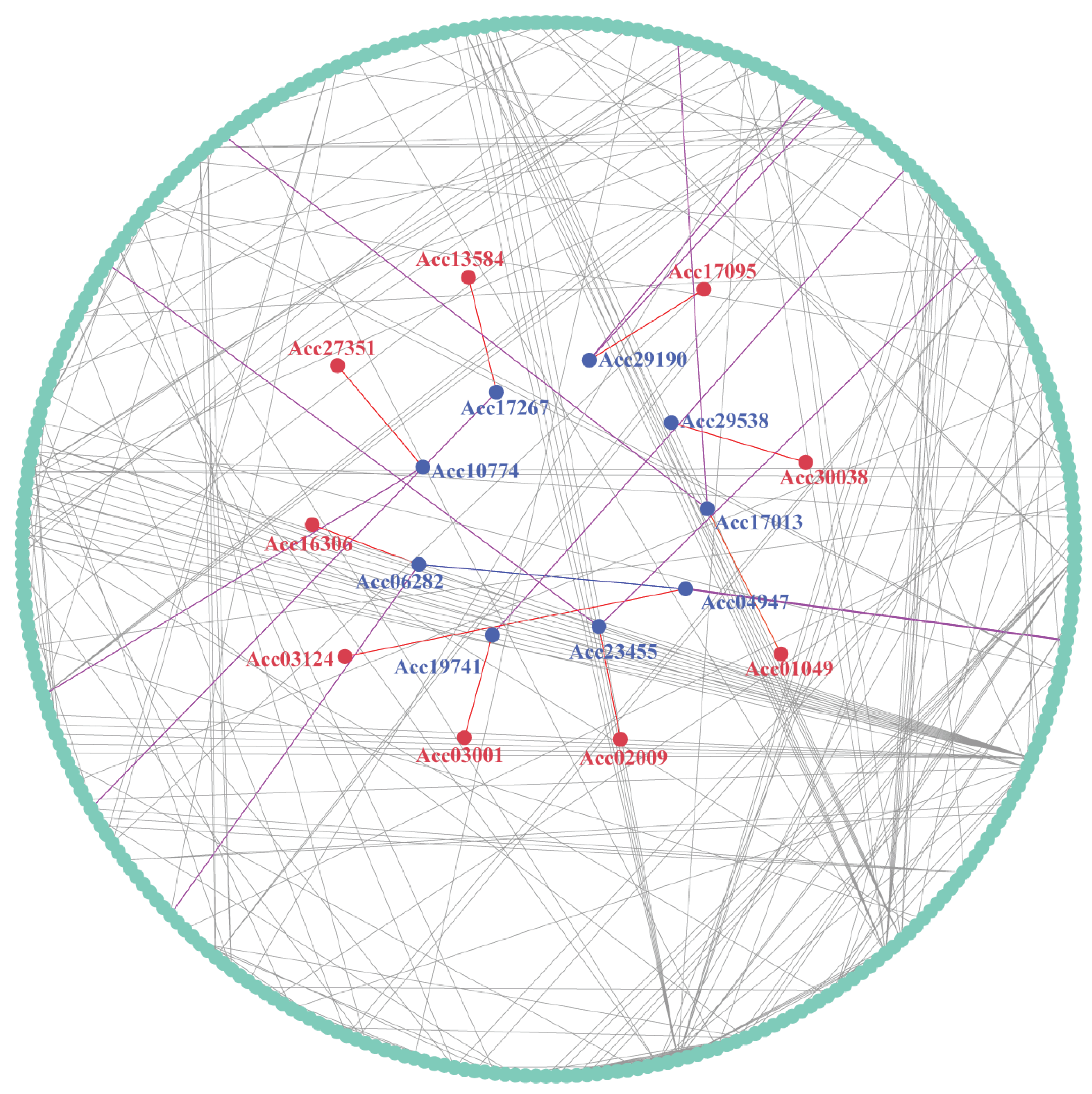
2.8. Protein Interaction Network
Taking the control group as reference, we compared the expression data under three abiotic stresses, i.e. heat, copper, and waterlogging, and screened the differentially expressed genes in each case. The identified differentially expressed genes were then overlapped. Subsequently, we constructed an interaction network between LRR-RLP and the proteins encoded by these differentially expressed genes in kiwifruit (
Figure 9). This protein interaction network contains 341 proteins, among which 9 are LRR-RLP proteins, i.e.
Acc03124,
Acc02009,
Acc13584,
Acc16306,
Acc27351,
Acc30038,
Acc01049,
Acc03001, and
Acc17095. Notably, interactions were observed between LRR-RLP and 9 other proteins in kiwifruit, i.e.
Acc17013,
Acc23455,
Acc19741,
Acc04947,
Acc17267,
Acc29190,
Acc06282,
Acc10774, and
Acc29538. Through interactions with these proteins, kiwifruit LRR-RLP regulates downstream proteins within the network. These LRR-RLP members may play a crucial role in regulating the abiotic stress tolerance of kiwifruit.
3. Discussion
Kiwifruit is known as the 'king of Vitamin C' due to its high nutritional, economic, and medicinal value. However, it faces challenges in yield and quality caused by adverse environmental conditions during growth and development. These conditions encompass biotic and abiotic stresses, including pathogen stress, cold stress, heat stress, and waterlogging stress, etc. LRR-RLP encounters various external and internal cellular signals during the growth and development stages, triggering signal transduction pathways and biological responses. As a pattern recognition receptor, LRR-RLP plays an important role in plant growth and development, signal transduction and stress response. Both
Arabidopsis CLV2 and its functional homologous protein FEA2 in maize regulate the maintenance and differentiation of meristem and the development of related organs [
20,
21]. AtRLP41 and AtRLP44 are involved in the signal transduction of plant hormones [
23,
24]. RBPG1\AtRLP42 serves as a novel microbial-associated molecular pattern receptor, recognizing fungal polygalacturonase (PG) [
35]. Several LRR-RLP proteins have been implicated in plant disease resistance, including the apple anti-blackstar protein HcrVf2, the anti-cf protein of tomato leaf mold, and the anti-ve receptor protein of tomato fungus [
21,
23]. However, the functions of LRR-RLPs and their response to stress in kiwifruit remain to be elucidated.
In this study, we investigated the identification, classification, chromosomal localization, synteny analysis of LRR-RLPs in three kiwifruit species. A total of 101, 164, and 105 LRR-RLPs were identified in 'Hongyang', 'Huate' and 'Red5' kiwifruit, respectively. HyLRR-RLPs and HtLRR-RLPs were classified into nine groups, with the highest and lowest numbers observed in group IX and group IV, respectively. The LRR-RLP of the three species of kiwifruit were widely distributed and dispersed in their chromosomes, linked groups or unlinked groups. Specifically, HyLRR-RLPs, HtLRR-RLPs, and DhLRR-RLPs exhibited the highest abundance on LG07, Chr16, and LG01, respectively, with counts of 7, 17, and 9. Collinearity analysis revealed 6 pairs of collinear relationships between AtLRR-RLP and HyLRR-RLPs, involving 10 genes (4 AtLRR-RLPs and 6 HyLRR-RLPs). HtLRR-RLPs exhibited 4 pairs of collinear relationships with AtLRR-RLPs, involving 7 genes (4 AtLRR-RLPs and 3 HtLRR-RLPs). AtLRR-RLPs and DhLRR-RLPs displayed 11 collinearity relationships, involving 17 genes (8 AtLRR-RLPs and 9 DhLRR-RLPs). There were 47 covariate pairs between HyLRR-RLPs and HtLRR-RLPs involving 73 genes. Only 1 collinear relationship involving two genes was observed between HyLRR-RLP and DhLRR-RLP. Meanwhile, HtLRR-RLPs and DhLRR-RLPs exhibited 65 pairs of collinearity, involving 95 genes. There were 47, 56, 51 pairs of segmental duplications in the Hy, Ht and Dh genomes, respectively. Additionally, there were 3, 9, and 7 pairs of tandem duplications in the genomes of Hy, Ht, and Dh, respectively. The results suggested that the amplification of the LRR-RLP gene family in kiwifruit was mainly due to segmental duplication. Furthermore, the Ka/Ks values for the identified duplicate pairs were less than 1, indicating that these genes evolved under purifying selection.
Using RNA-seq data obtained from both resistant and susceptible kiwifruits infected Psa, we analyzed the differentially expressed LRR-RLP genes at various time points. Furthermore, we analyzed the differentially expressed LRR-RLP genes in both kiwifruit species at different time points compared to 0h time point. Notably, we observed a higher number of differentially expressed LRR-RLP genes in Ht compared to Hy, with the genes unique to Ht being four times more abundant than those in Hy. Furthermore, we analyzed the differentially expressed LRR-RLP genes between two kiwifruit species at the same time point. Interestingly, some genes exhibited insignificant changes in expression in Hy, but showed a significant trend in Ht, specifically
Actinidia39875.t1,
Actinidia35026.t1,
Actinidia12020.t1, and
Actinidia34674.t1. These findings suggested a pivotal role for LRR-RLP in the process of Psa infection in kiwifruit. Moreover, our analysis extended to abiotic stresses, revealing that 31, 27, and 20 LRR-RLP genes were differentially expressed under waterlogging, heat, and copper stress, respectively. This indicated a crucial role for LRR-RLPs in enhancing the resistance of kiwifruit to abiotic stresses. The comprehensive examination of both biotic and abiotic stress responses highlighted the multifaceted involvement of LRR-RLPs in shaping the defense mechanisms of kiwifruit. Most of the upstream transcription factors of the differentially expressed LRR-RLP genes in Hy and Ht belonged to families associated with plant growth, development, and stress response. In the interaction network involved in kiwifruit LRR-RLP, there were 341 proteins, including 9 LRR-RLP proteins. Homologous genes coding for these proteins were searched and annotated in Arabidopsis TAIR and Uniprot databases. For instance, the homologous gene
SERK2 of
Acc04947 played an important role in zygotic embryo development [
36]. The
Acc17267 homolog
ATLOX1 highly expressed in
Arabidopsis roots and seedlings under the induction of pathogens and plant hormones, and was involved in lateral root development and defense response [
37]. These findings suggest potential functional implications for the identified homologous genes in kiwifruit LRR-RLP-associated processes.
4. Materials and Methods
4.1. Data Resource
The genome files and gff3 files of 'Hongyang', 'Red5', and 'Huate' kiwifruit were sourced from the kiwifruit genome database (
http://kiwifruitgenome.org/) [
38]. Fifty-seven Arabidopsis LRR-RLPs (AtLRR-RLP) sequences were retrieved from the
Arabidopsis database (
https://www.arabidopsis.org/). Transcriptome data were obtained from NCBI database (
http://www.ncbi.nlm.nih.gov/). The transcriptome data registration numbers are as follows:
Pseudomonas syringae infected kiwifruit: PRJNA514180; kiwifruit under heat stress: PRJNA796069; kiwifruit under waterlogging stress: PRJNA765913.
4.2. Genome-Wide Identification of LRR-RLP Genes in Kiwifruit
The 57 AtLRR-RLPs obtained were subjected to a BLASTP search against the protein databases of 'Hongyang', 'Huat', and 'Red5' kiwifruit, respectively. The e-value threshold was set to 1e-5 to reduce false positives. The putative 'Hongyang' LRR-RLPs (HyLRR-RLPs), 'Huate' LRR-RLPs (HtLRR-RLPs) and 'Red5' LRR-RLPs (DhLRR-RLPs) were then submitted to Pfam database to identify protein sequences containing LRR domains [
39]. Key features, such as amino acid length, isoelectric point, and molecular weight of HyLRR-RLPs, HtLRR-RLPs and DhLRR-RLPs proteins, were analyzed using ProtParam online tool (
http://au.expasy.org/tools/protparam.html) [
40].
4.3. Multiple Sequence Alignment and Phylogenetic Tree Analysis
We used MAFFT7 to perform multiple sequence alignment of HyLRR-RLPs and HtLRR-RLPs, with the parameters set to default [
41]. Subsequently, we employed IQtree software to construct phylogenetic trees for both HyLRR-RLP and HtLRR-RLP using the maximum likelihood method [
42]. The resulting tree was further visualized and modified by Figtree v1.4.4 (
http://tree.bio.ed.ac.uk/software/figtree/).
4.4. Chromosomal Localization and Collinearity Analysis
The chromosome coordinates of HyLRR-RLPs, HtLRR-RLPs and DhLRR-RLPs were extracted from the genome files and GFF3 annotation files of 'Hongyang', 'Huate' and 'Red5' kiwifruit. To comprehend their distribution on the chromosomes, we generated distribution map using the MG2C v2.0 online tool (
http://mg2c.iask.in/mg2c_v2.1/) [
43].
For collinearity analysis, we employed the one-step MCScanX-super Fast plugin in TBtools to investigate collinearity between
Arabidopsis and the three kiwifruit species, as well as between the three kiwifruit species, with an E-value threshold of 0.001[
44]. Additionally, block analysis of the three kiwifruit species was carried out using the same method.
4.5. Transcriptome Data Analysis
To investigate the expression patterns of kiwifruit LRR-RLPs in response to biotic and abiotic stresses, we conducted a comprehensive analysis of multiple transcriptome datasets. For the examination of LRR-RLP expression in kiwifruit responding to Psa infection, we performed a detailed analysis. The Psa infection time points were divided into 0h, 12h, 24h, 48h, and 96h, with three biological replicates for each time point. Heat stress treatment included 0h, 2h, and 4h at 50 degrees Celsius, with three biological replicates for each time node. Waterlogging stress was treated over 0, 1, and 2 days, each with three biological replicates.
We performed the subsequent processing steps on the provided transcriptome data. Firstly, we assessed the quality of the transcriptome data with the FastQC tool [
45], and then applied fastp for read clipped to obtain clean reads [
46]. Secondly, we aligned the clean reads from each sample data with the kiwifruit reference genome using HISAT2, resulting in SAM files [
47]. Thirdly, we employed SAMtools to convert the SAM files obtained earlier into BAM files, followed by sorting [
48,
49]. Lastly, we used Stringtie for further transcriptome assembly and reads sequence quantification [
50]. The gene expression levels were measured by FPKM (transcribed fragments per kilobase per million of labeled reads). Differential gene expression analysis was performed using EdgeR[
51]. The threshold to identify differentially expressed genes was p-value<0.05 and logFC>= 1. Subsequently, based on the LRR-RLPs gene expression matrix, cluster analysis was performed by HeatMap plug-in in TBtools[
44].
4.6. Analysis of Upstream Transcription Factors of LRR-RLP in Kiwifruit
We compiled a concatenated set of genes that exhibited differential expression in ‘Hongyang’ and ‘Huate’. Subsequently, we identified the upstream transcription factors associated with these genes using the PlantRegMap database (
http://plantregmap.gao-lab.org/) [
33]. This tool facilitates the inference of potential interactions between input genes and transcription factors. Finally, we visualized potential regulatory relationships by Cytoscape [
52].
4.7. Construction of a Protein Interaction Network
Taking the control group as a reference, we performed a comparative analysis of the expression data under three abiotic stresses, i.e. heat, copper, and waterlogging. Differentially expressed genes were identified through screening, and the overlapping set of these genes was determined. In the STRING database (
https://cn.string-db.org/), the protein sequences of kiwifruit LRR-RLPs and the previously identified differentially expressed genes were used as input files.
A.thaliana was chosen as the reference species for the comparison of protein sequences. The protein sequences of homologous genes were subsequently mapped to their interactions in the database, leading to the formation of the comprehensive interaction network. The parameters were set to a 70% reliability threshold and a 5% error detection rate.
5. Conclusions
In this study, a total of 101,164, and 105 LRR-RLP genes were identified in the genomes of three kiwifruit species, and their physicochemical properties, evolutionary relationships and collinearity relationships were elucidated. Segmental duplication emerged as the primary driver for the expansion of the LRR-RLP gene family in kiwifruit. The differentially expressed LRR-RLP genes in resistant and susceptible kiwifruits infected with Psa highlighted their involvement in biotic stress responses. Additionally, the expression patterns of LRR-RLP genes under three abiotic stresses underscore their significance in abiotic stress tolerance. The protein interaction network elucidated the regulatory relationships between LRR-RLP members and associated proteins, shedding light on the complex network governing abiotic stress responses in kiwifruit. These findings provided valuable reference information for understanding protein interactions within the LRR-RLP gene family and contributed to the study of resistant kiwifruit varieties.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Chromosomal localization of three kiwifruit species LRR-RLP genes; Table S1: Detailed information for LRR-RLP genes in Actinidia chinensis 'Hongyang' genome; Table S2: Detailed information for LRR-RLP genes in the Actinidia eriantha genome;Table S3: Detailed information for LRR-RLP genes in the Actinidia chinensis 'Red5' genome;Table S4: The Ka/Ks results for LRR-RLP collinear pairs between Arabidopsis and kiwifruit;Table S5: The Ka/Ks results for LRR-RLP collinear pairs between different species kiwifruit;Table S6: The Ka/Ks results for LRR-RLP duplicate pairs within same kiwifruit genome;Table S7: Transcription factor corresponding family.
Author Contributions
Conceptualization, Y.C., X.Z., L.L and A.Z.; methodology, Y.C. and C.Z.; software, C.Z.; validation, Y.C.; writing—original draft preparation, Y.C.; writing—review and editing, Y.C., C.Z., D.L. and F.L.; visualization, X.Z., L.L and A.Z.; supervision, X.Z., L.L and A.Z.; project administration, X.Z.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32070682, 32200504), the International Science and Technology Cooperation Program of Hubei Province (2023EHA048), the Key Research and Development Program of Hubei Province (2022BBA0076), the National Science & Technology Innovation Zone Project (1816315XJ00100216), and CAS Pioneer Hundred Talents Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw reads used in this work were deposited in NCBI Bio-Project under the accession numbers PRJNA796069, PRJNA514180, and PRJNA765913.
Acknowledgments
We would like to thank the members of the Bioinformatics Group of Wuhan Botanical Garden, Chinese Academy of Sciences for the discussion and suggestions to improve the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pereira, C.; Costa, P.; Pinheiro, L.; Balcão, V.M.; Almeida, A. Kiwifruit bacterial canker: an integrative view focused on biocontrol strategies. Planta 2021, 253, 49. [Google Scholar] [CrossRef]
- Huang, S.; Ding, J.; Deng, D.; Tang, W.; Sun, H.; Liu, D.; Zhang, L.; Niu, X.; Zhang, X.; Meng, M.; et al. Draft genome of the kiwifruit Actinidia chinensis. Nat. Commun. 2013, 4, 2640. [Google Scholar] [CrossRef]
- Zhang, A.; Xiong, Y.; Liu, F.; Zhang, X. A Genome-Wide Analysis of the Pentatricopeptide Repeat Protein Gene Family in Two Kiwifruit Species with an Emphasis on the Role of RNA Editing in Pathogen Stress. Int. J. Mol. Sci. 2023, 24, 13700. [Google Scholar] [CrossRef]
- Satpal, D.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia deliciosa (Kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J. Food Process. Preserv. 2021, 45, e15588. [Google Scholar] [CrossRef]
- Gao, Z.; Deng, G.; Li, Y.; Huang, H.; Sun, X.; Shi, H.; Yao, X.; Gao, L.; Ju, Y.; Luo, M. Actinidia chinensis Planch prevents proliferation and migration of gastric cancer associated with apoptosis, ferroptosis activation and mesenchymal phenotype suppression. Biomed. Pharmacother. 2020, 126, 110092. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, J.; Kay, C.; Onorato, R.; Yu, J.; Cornish, D.; Spinelli, F.; Max, S. Recent advances in the characterisation and control of Pseudomonas syringae pv. actinidiae, the causal agent of bacterial canker on kiwifruit. Acta Hortic. 2011, 913, 443–455. [Google Scholar] [CrossRef]
- Cameron, A.; Sarojini, V. Pseudomonas syringae pv. actinidiae: chemical control, resistance mechanisms and possible alternative. Plant Pathol. 2013, 63, 1–11. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2020, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Wang, W.; Liu, J.; Zhu, C.; Zhong, Y.; Zhang, H.; Liu, X.; Yin, X. Transcription factors AcERF74/75 respond to waterlogging stress and trigger alcoholic fermentation-related genes in kiwifruit. Plant Sci. 2022, 314, 111115. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-Triggered Immunity: From Pathogen Perception to Robust Defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tsuda, K. Intimate Association of PRR- and NLR-Mediated Signaling in Plant Immunity. Mol. Plant Microbe Interact. 2021, 34, 3–14. [Google Scholar] [CrossRef]
- Jamieson, P.A.; Shan, L.; He, P. Plant cell surface molecular cypher: Receptor-like proteins and their roles in immunity and development. Plant Sci. 2018, 274, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Boutrot, F.; Zipfel, C. Function, Discovery, and Exploitation of Plant Pattern Recognition Receptors for Broad-Spectrum Disease Resistance. Annu. Rev. Phytopathol. 2017, 55, 257–286. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant–microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- Liebrand, T.W.; van den Berg, G.C.; Zhang, Z.; Smit, P.; Cordewener, J.H.; America, A.H.; Sklenar, J.; Jones, A.M.; Tameling, W.I.; Robatzek, S.; et al. Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proc. Natl. Acad. Sci. USA 2013, 110, 10010–10015. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J. Receptor-Like Kinases in Plant Innate Immunity. J. Integr. Plant Biol. 2013, 55, 1271–1286. [Google Scholar] [CrossRef]
- Macho, A.P.; Zipfel, C. Plant PRRs and the Activation of Innate Immune Signaling. Mol. Cell 2014, 54, 263–272. [Google Scholar] [CrossRef]
- Yang, H.; Bayer, P.E.; Tirnaz, S.; Edwards, D.; Batley, J. Genome-Wide Identification and Evolution of Receptor-Like Kinases (RLKs) and Receptor like Proteins (RLPs) in Brassica juncea. Biology 2020, 10, 17. [Google Scholar] [CrossRef]
- Pan, L.; Lv, S.; Yang, N.; Lv, Y.; Liu, Z.; Wu, J.; Wang, G. The Multifunction of CLAVATA2 in Plant Development and Immunity. Front. Plant Sci. 2016, 7, 1573. [Google Scholar] [CrossRef]
- Kang, W.-H.; Yeom, S.-I. Genome-wide Identification, Classification, and Expression Analysis of the Receptor-Like Protein Family in Tomato. Plant Pathol. J. 2018, 34, 435–444. [Google Scholar] [CrossRef]
- Nadeau, J.A.; Sack, F.D. Control of Stomatal Distribution on the Arabidopsis Leaf Surface. Science 2002, 296, 1697–1700. [Google Scholar] [CrossRef]
- Wang, G.; Ellendorff, U.; Kemp, B.; Mansfield, J.W.; Forsyth, A.; Mitchell, K.; Bastas, K.; Liu, C.-M.; Woods-Tör, A.; Zipfel, C.; et al. A Genome-Wide Functional Investigation into the Roles of Receptor-Like Proteins in Arabidopsis. Plant Physiol. 2008, 147, 503–517. [Google Scholar] [CrossRef]
- Wolf, S.; van der Does, D.; Ladwig, F.; Sticht, C.; Kolbeck, A.; Schürholz, A.-K.; Augustin, S.; Keinath, N.; Rausch, T.; Greiner, S.; et al. A receptor-like protein mediates the response to pectin modification by activating brassinosteroid signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 15261–15266. [Google Scholar] [CrossRef]
- Jones, D.A.; Thomas, C.M.; Hammond-Kosack, K.E.; Balint-Kurti, P.J.; Jones, J.D.G. Isolation of the Tomato Cf-9 Gene for Resistance to Cladosporium fulvum by Transposon Tagging. Science 1994, 266, 789–793. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Li, L.; Tan, X.; Wei, Z.; Li, Y.; Li, J.; Yan, F.; Chen, J.; Sun, Z. A rice LRR receptor-like protein associates with its adaptor kinase OsSOBIR1 to mediate plant immunity against viral infection. Plant Biotechnol. J. 2021, 19, 2319–2332. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Zhang, Z.; Lv, Y.; Yang, N.; Zhang, G.; Wu, M.; Lv, S.; Pan, L.; Joosten, M.H.A.J.; et al. Transcriptional regulation of receptor-like protein genes by environmental stresses and hormones and their overexpression activities inArabidopsis thaliana. J. Exp. Bot. 2016, 67, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- lvarez-López, D.; Herrera-Valencia, V.A.; Góngora-Castillo, E.; Garcia-Laynes, S.; Puch-Hau, C.; López-Ochoa, L.A.; Lizama-Uc, G.; Peraza-Echeverria, S. Genome-wide analysis of the LRR-RLP gene family in a wild banana (Musa acuminata ssp. malaccensis) uncovers multiple fusarium wilt resistance gene candidates. Genes 2022, 13, 638. [Google Scholar] [CrossRef] [PubMed]
- Petre, B.; Hacquard, S.; Duplessis, S.; Rouhier, N. Genome analysis of poplar LRR-RLP gene clusters reveals RISP, a defense-related gene coding a candidate endogenous peptide elicitor. Front. Plant Sci. 2014, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Fritz-Laylin, L.K.; Krishnamurthy, N.; Tör, M.; Sjölander, K.V.; Jones, J.D. Phylogenomic Analysis of the Receptor-Like Proteins of Rice and Arabidopsis. Plant Physiol. 2005, 138, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, J.; Yang, C.; Xia, S. Identification of receptor-like proteins induced by Sclerotinia sclerotiorum in Brassica napus. Front. Plant Sci. 2022, 13, 944763. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, S.; Verma, S.; Rahman, M.H.; Kav, N.N.V. Functional characterization of four APETALA2-family genes (RAP2.6, RAP2.6L, DREB19 and DREB26) in Arabidopsis. Plant Mol. Biol. 2011, 75, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Jin, J.; Gao, G. PlantRegMap: charting functional regulatory maps in plants. Nucleic Acids Res. 2020, 48, D1104–D1113. [Google Scholar] [CrossRef]
- Ingkasuwan, P.; Netrphan, S.; Prasitwattanaseree, S.; Tanticharoen, M.; Bhumiratana, S.; Meechai, A.; Chaijaruwanich, J.; Takahashi, H.; Cheevadhanarak, S. Inferring transcriptional gene regulation network of starch metabolism in Arabidopsis thaliana leaves using graphical Gaussian model. BMC Syst. Biol. 2012, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kars, I.; Essenstam, B.; Liebrand, T.W.H.; Wagemakers, L.; Elberse, J.; Tagkalaki, P.; Tjoitang, D.; van den Ackerveken, G.; van Kan, J.A.L. Fungal Endopolygalacturonases Are Recognized as Microbe-Associated Molecular Patterns by the Arabidopsis Receptor-Like Protein RESPONSIVENESS TO BOTRYTIS POLYGALACTURONASES1. Plant Physiol. 2014, 164, 352–364. [Google Scholar] [CrossRef]
- Li, H.; Cai, Z.; Wang, X.; Li, M.; Cui, Y.; Cui, N.; Yang, F.; Zhu, M.; Zhao, J.; Du, W.; et al. SERK Receptor-like Kinases Control Division Patterns of Vascular Precursors and Ground Tissue Stem Cells during Embryo Development in Arabidopsis. Mol. Plant 2019, 12, 984–1002. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, W.; Guan, C.; Guan, M.; He, X. Evolution and functional diversity of lipoxygenase (LOX) genes in allotetraploid rapeseed (Brassica napus L.). Int. J. Biol. Macromol. 2021, 188, 844–854. [Google Scholar] [CrossRef]
- Yue, J.; Liu, J.; Tang, W.; Wu, Y.Q.; Tang, X.; Li, W.; Yang, Y.; Wang, L.; Huang, S.; Fang, C.; et al. Kiwifruit Genome Database (KGD): a comprehensive resource for kiwifruit genomics. Hortic. Res. 2020, 7, 117. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: a user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.; Pirrung, M.; McCue, L.A. FQC Dashboard: integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 2017, 33, 3137–3139. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. ; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Physicochemical properties of LRR-RLPs in kiwifruit. Different colors represent distinct kiwifruit species. Blue, red, and green represent ‘Hongyang’, ‘Huate’, and ‘Red5’ kiwifruit, respectively.
Figure 1.
Physicochemical properties of LRR-RLPs in kiwifruit. Different colors represent distinct kiwifruit species. Blue, red, and green represent ‘Hongyang’, ‘Huate’, and ‘Red5’ kiwifruit, respectively.
Figure 2.
Phylogenetic tree of LRR-RLP members in ‘Hongyang’ and ‘Huate’ kiwifruit. The phylogenetic tree, derived from multiple sequence alignment with the maximum likelihood (ML) method, illustrates the relationships among LRR-RLP members in 'Hongyang' and 'Huate' kiwifruit. The tree is categorized into 9 groups, with distinct colors representing different phylogenetic groups.
Figure 2.
Phylogenetic tree of LRR-RLP members in ‘Hongyang’ and ‘Huate’ kiwifruit. The phylogenetic tree, derived from multiple sequence alignment with the maximum likelihood (ML) method, illustrates the relationships among LRR-RLP members in 'Hongyang' and 'Huate' kiwifruit. The tree is categorized into 9 groups, with distinct colors representing different phylogenetic groups.
Figure 3.
Expression of LRR-RLPs after Psa infection in susceptible (Hy) and resistant (Ht) kiwifruit. Heat maps of differentially expressed LRR-RLPs in susceptible and resistant kiwifruit after Psa infection. The X-axis represents the hours after Psa infection (0h, 12h, 24h, 48h, 96h), and the Y-axis represents the LRR-RLP genes.
Figure 3.
Expression of LRR-RLPs after Psa infection in susceptible (Hy) and resistant (Ht) kiwifruit. Heat maps of differentially expressed LRR-RLPs in susceptible and resistant kiwifruit after Psa infection. The X-axis represents the hours after Psa infection (0h, 12h, 24h, 48h, 96h), and the Y-axis represents the LRR-RLP genes.
Figure 4.
Differentially expressed LRR-RLPs after Psa infection. (a) Number of up-regulated LRR-RLP genes after infection by the Psa in two kiwifruit species. (b) Number of down-regulated LRR-RLP genes after infection by the Psa in two kiwifruit species. (c) Number of differentially expressed LRR-RLPs after Psa infection in two kiwifruit species. (d) Number of differentially expressed LRR-RLPs between the two kiwifruit types at the same time point.
Figure 4.
Differentially expressed LRR-RLPs after Psa infection. (a) Number of up-regulated LRR-RLP genes after infection by the Psa in two kiwifruit species. (b) Number of down-regulated LRR-RLP genes after infection by the Psa in two kiwifruit species. (c) Number of differentially expressed LRR-RLPs after Psa infection in two kiwifruit species. (d) Number of differentially expressed LRR-RLPs between the two kiwifruit types at the same time point.
Figure 5.
Expression levels of representative differentially expressed LRR-RLPs between susceptible (Hy) and resistant (Ht) kiwifruit after Psa infection. The X-axis represents the time point after Psa infection, and the Y-axis represents the gene expression value.
Figure 5.
Expression levels of representative differentially expressed LRR-RLPs between susceptible (Hy) and resistant (Ht) kiwifruit after Psa infection. The X-axis represents the time point after Psa infection, and the Y-axis represents the gene expression value.
Figure 6.
Heatmap of differentially expressed upstream transcription factors associated with differentially expressed LRR-RLP genes in two kiwifruit species.
Figure 6.
Heatmap of differentially expressed upstream transcription factors associated with differentially expressed LRR-RLP genes in two kiwifruit species.
Figure 7.
Regulatory network between LRR-RLPs and their corresponding transcription factors. The highlighted genes in this network are those that exhibit differential expression in at least one kiwifruit species. LRR-RLP genes are represented in orange, while transcription factors are denoted in green.
Figure 7.
Regulatory network between LRR-RLPs and their corresponding transcription factors. The highlighted genes in this network are those that exhibit differential expression in at least one kiwifruit species. LRR-RLP genes are represented in orange, while transcription factors are denoted in green.
Figure 8.
The expression profiles of differentially expressed LRR-RLP genes in kiwifruit waterlogging, heat, and copper stress. (a) Heatmap depicting differentially expressed kiwifruit LRR-RLPs under waterlogging stress. (b) Heatmap illustrating differentially expressed kiwifruit LRR-RLPs under heat stress. (c) Heatmap presenting differentially expressed kiwifruit LRR-RLPs under copper stress.
Figure 8.
The expression profiles of differentially expressed LRR-RLP genes in kiwifruit waterlogging, heat, and copper stress. (a) Heatmap depicting differentially expressed kiwifruit LRR-RLPs under waterlogging stress. (b) Heatmap illustrating differentially expressed kiwifruit LRR-RLPs under heat stress. (c) Heatmap presenting differentially expressed kiwifruit LRR-RLPs under copper stress.
Figure 9.
Protein interaction network involving kiwifruit LRR-RLP. Red circles represent the LRR-RLP proteins, while blue circles represent other proteins associated with LRR-RLP. The red lines connect the LRR-RLP protein to other associated proteins, purple lines connect other proteins associated with LRR-RLP and other proteins associated with it, and blue lines link to other proteins associated with LRR-RLP.
Figure 9.
Protein interaction network involving kiwifruit LRR-RLP. Red circles represent the LRR-RLP proteins, while blue circles represent other proteins associated with LRR-RLP. The red lines connect the LRR-RLP protein to other associated proteins, purple lines connect other proteins associated with LRR-RLP and other proteins associated with it, and blue lines link to other proteins associated with LRR-RLP.
Table 1.
Number of LRR-RLPs for kiwifruit in groups.
Table 1.
Number of LRR-RLPs for kiwifruit in groups.
| Groups |
Number of HyLRR-RLPs |
Number of HtLRR-RLPs |
Total |
| I |
3 |
10 |
13 |
| II |
9 |
6 |
15 |
| III |
16 |
11 |
27 |
| IV |
1 |
2 |
3 |
| V |
5 |
18 |
23 |
| VI |
16 |
32 |
48 |
| VII |
4 |
4 |
8 |
| VIII |
22 |
35 |
57 |
| IX |
25 |
46 |
71 |
| Total |
101 |
164 |
265 |
Table 2.
Transcription factors exclusively differentially expressed in one kiwifruit species.
Table 2.
Transcription factors exclusively differentially expressed in one kiwifruit species.
| Species |
Exclusively differentially expressed transcription factors (Gene ID) |
| ‘Hongyang’(Hy) |
Actinidia17974.t1, Actinidia13641.t1, Actinidia32152.t1,
Actinidia20296.t1, Actinidia14858.t1, Actinidia22851.t1,
Actinidia15498.t1
|
| ‘Huate’(Ht) |
Actinidia01659.t1, Actinidia04329.t1, Actinidia08281.t1,
Actinidia10240.t1, Actinidia13003.t1, Actinidia14109.t1,
Actinidia20508.t1, Actinidia20994.t1, Actinidia24811.t1,
Actinidia26254.t1, Actinidia26649.t1, Actinidia29458.t1,
Actinidia36885.t1, Actinidia37473.t1, Actinidia39836.t2
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).