Submitted:
15 March 2024
Posted:
15 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
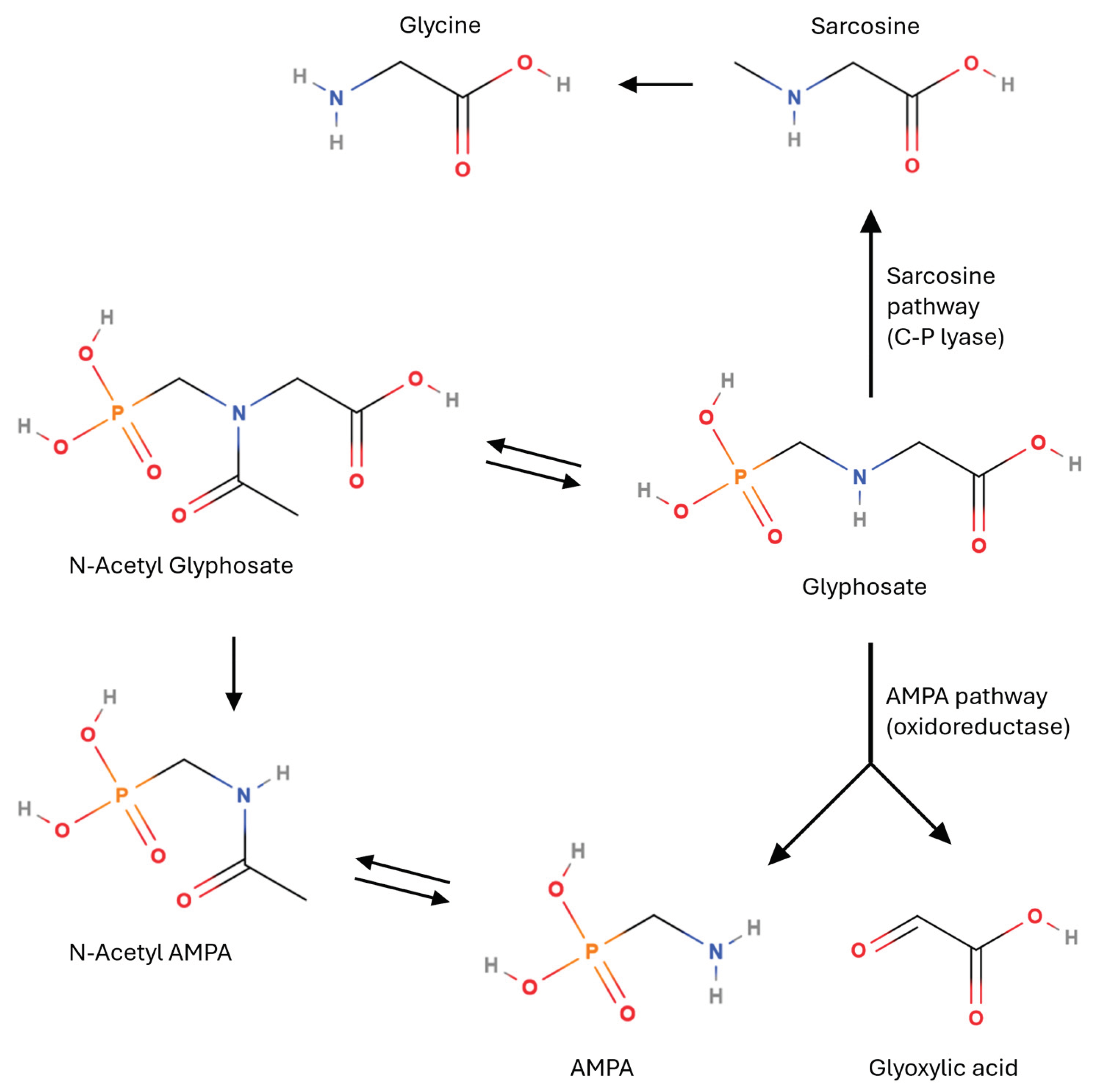
2. Glyphosate Degradation Pathways
3. Occurrence Data, Accumulation, Fate
3.1. Glyphosate Concentration in Straight Grade Flour vs. Concentration in Wholemeal Flour
3.2. Glyphosate Accumulation in Crops
3.3. Fate and Degradation of Glyphosate
4. Regulatory Status
5. Chemical Analytical Methods
5.1. Liquid Chromatography
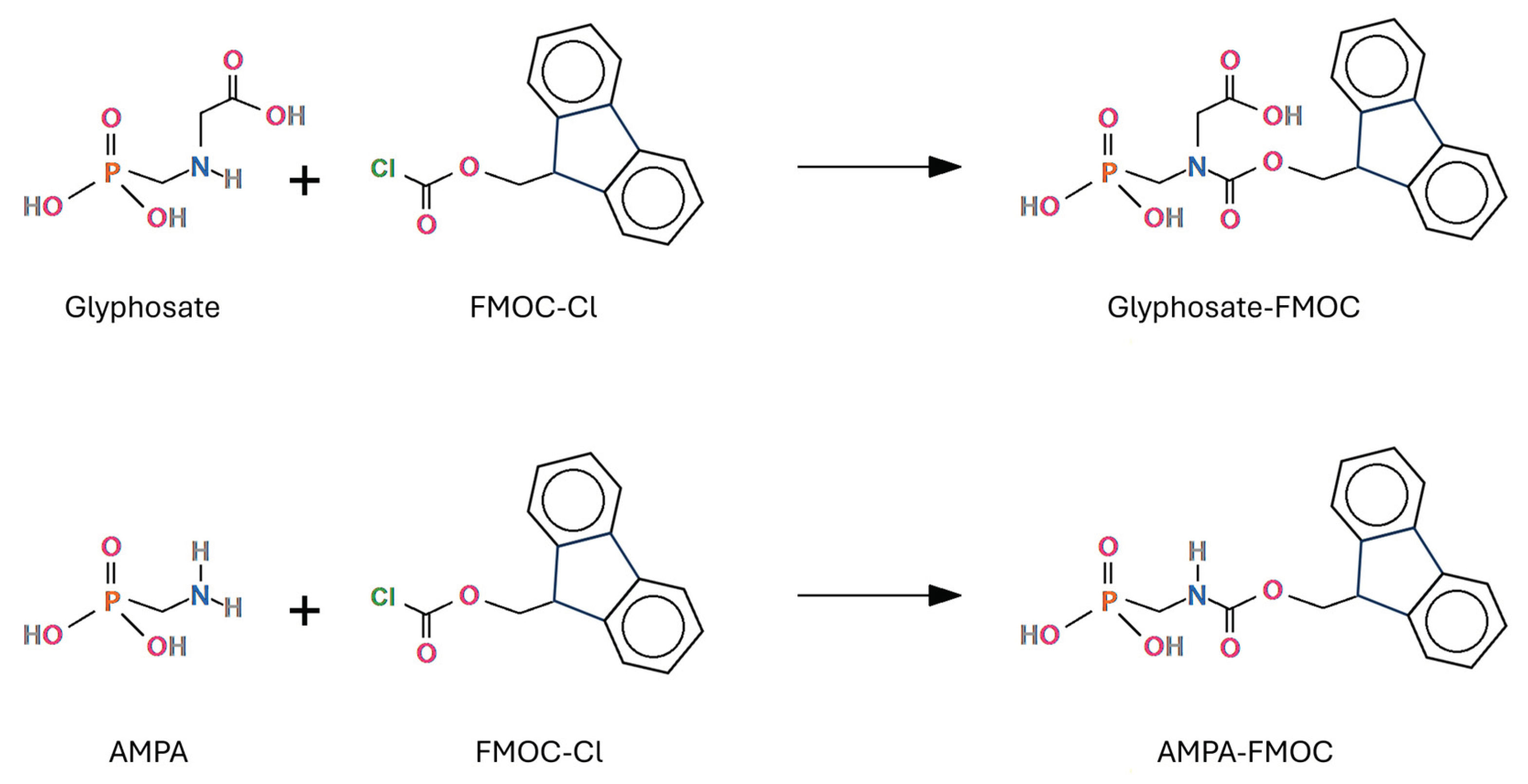
5.1.1. FMOC Derivatization
5.1.2. Sample Preparation Used with FMOC Derivatization
5.1.3. Direct vs. Indirect Determination
5.1.4. Glyphosate and Glufosinate
5.2. Gas Chromatography
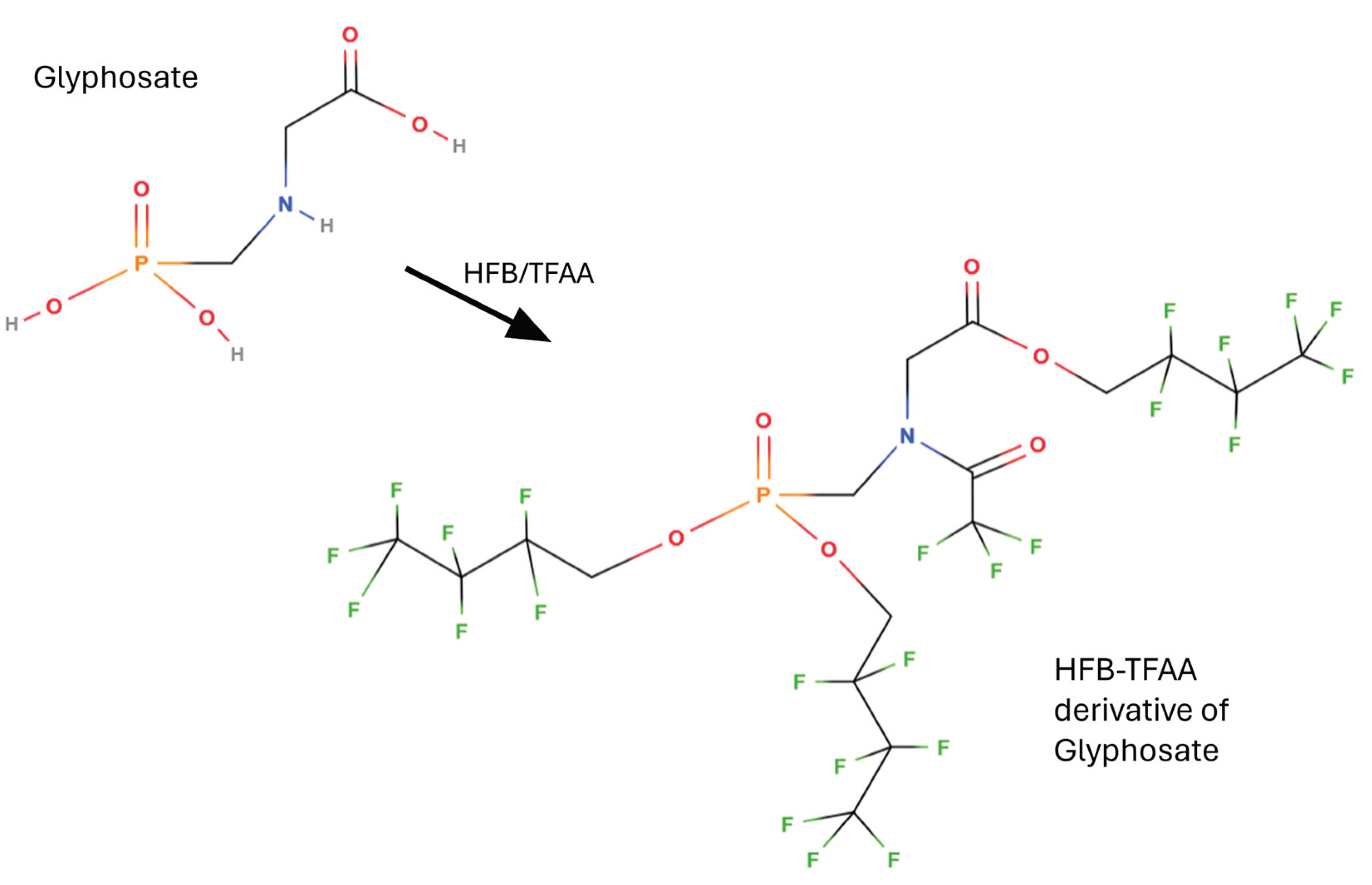
5.2.1. Sample Preparation and Perfluoroalcohol/TFAA Derivatization
5.2.2. Instrumental
5.2.3. Alkylsilyl Derivatization
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonetti, E.; Cartaud, G.; Quinn, R.M.; Marotti, I.; Dinelli, G. An Interlaboratory Comparative Study on the Quantitative Determination of Glyphosate at Low Levels in Wheat Flour. J. AOAC Int., 2015, 98, 1760–1768. [Google Scholar] [CrossRef]
- Benbrook, M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur., 2016, 28, 1–15. [Google Scholar] [CrossRef]
- Rigobello-Masini, M.; Oliveira Pereira, E.A.; Abate, G.; Masini, J.C. Solid-Phase Extraction of Glyphosate in the Analyses of Environmental, Plant, and Food Samples. Chromatographia, 2019, 82, 1121–1138. [Google Scholar] [CrossRef]
- Gélinas, P.; Gagnon, F.; McKinnon, C. Wheat preharvest herbicide application, whole-grain flour properties, yeast activity and the degradation of glyphosate in bread. Int. J. Food Sci. Tech., 2018, 53, 1597–1602. [Google Scholar] [CrossRef]
- Kadžienė, G.; Pranaitienė, S.; Auškalnienė, O.; Veršulienė, A.; Supronienė, S.; Žvirdauskienė, R.; Gecaitė, V.; Cesevičienė, J.; Semaškienė, R. Oilseed Rape, Wheat, and Barley Grain Contamination as Affected by Different Glyphosate Usage. Plants, 2023, 12, 1335. [Google Scholar] [CrossRef]
- Winters, J.F.M.; Foldager, L.; Krogh, U.; Nørskov, N.P.; Sørensen, M.T. Impact of glyphosate residues in sow diets on neonatal piglets: tail kinks, stillborn and diarrhoea. Livest. Sci., 2023, 269, 105172. [Google Scholar] [CrossRef]
- Andert, S.; de Mol, F.; Koning, L.; Gerowitt, B. Weed response in winter wheat fields on a gradient of glyphosate use in the recent past. Agric. Ecosyst. Environ., 2022, 333, 107977. [Google Scholar] [CrossRef]
- Malalgoda, M.; Ohm, J.-B.; Howatt, K.A.; Green, A.; Simsek, S. Effects of pre-harvest glyphosate use on protein composition and shikimic acid accumulation in spring wheat. Food Chem. 2020. [Google Scholar] [CrossRef]
- Alahmed, A.; Simsek, S. Pre-harvest glyphosate application effects on properties of β-glucan from oat groats. J. Cereal Sci., 2020, 96, 103119. [Google Scholar] [CrossRef]
- Malalgoda, M.; Ohm, J.-B.; Howatt, K.A.; Simsek, S. Pre-harvest glyphosate application and effects on wheat starch chemistry: Analysis from application to harvest. J. Food Biochem., 2020, 44, e13330. [Google Scholar] [CrossRef]
- Malalgoda, M.; Ohm, J.-B.; Ransom, J.K.; Howatt, K.; Simsek, S. Effects of Pre-Harvest Glyphosate Application on Spring Wheat Quality Characteristics. Agriculture, 2020, 10, 111. [Google Scholar] [CrossRef]
- Steinmann, H.-H. Glyphosate: A Herbicide in Discussion and the Quest for the “Necessary Extent”. Gesunde Pflanz., 2013, 65, 47–56. [Google Scholar] [CrossRef]
- Bøhn, T.; Cuhra, M.; Traavik, T.; Sanden, M. , Fagan, J.; Primicerio, R. Compositional differences in soybeans on the market: Glyphosate accumulates in Roundup Ready GM soybeans. Food Chem., 2014, 153, 207–215. [Google Scholar] [CrossRef]
- Masci, M.; Nevigato, T.; Caproni, R. Glifosato residuo nei cereali: generalità e aspetti chimico-analitici. Tecnica Molitoria, 2020, 71, 41–55. [Google Scholar]
- Steinrücken, H.C.; Amrhein, N. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvylshikimic acid-3-phosphate synthase. Biochem. Bioph. Res. Co., 1980, 94, 1207–1212. [Google Scholar] [CrossRef]
- Amrhein, N.; Schab, J.; Steinrücken, H.C. The mode of action of the herbicide glyphosate. Naturwissenschaften, 1980, 67, 356–357. [Google Scholar] [CrossRef]
- Schönbrunn, E.; Eschenburg, S.; Shuttleworth, W.A.; Schloss, J.V.; Amrhein, N.; Evans, J.N.S; Kabsch, W. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. P. Natl. Acad. Sci. Usa, 2001, 98, 1376–1380. [Google Scholar] [CrossRef]
- Pesticide Action Network, UK. Glyphosate fact sheet. Pesticides News, 1996, 33, 28–29. [Google Scholar]
- Silva, V.; Montanarella, L. , Jones, A., Fernández-Ugalde, O.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union. Sci. Total Environ., 2018, 621, 1352–1359. [Google Scholar] [CrossRef]
- Wang, M.; Rivenbark, K.J.; Phillips, T.D. Kinetics of glyphosate and aminomethylphosphonic acid sorption onto montmorillonite clays in soil and their translocation to genetically modified corn. J Environ Sci., 2024, 135, 669–680. [Google Scholar] [CrossRef]
- IARC, International Agency for Research on Cancer, World Health Organization. Some Organophosphate Insecticides and Herbicides. IARC monographs on the evaluation of carcinogenic risks to humans, 2017, 112, 398. https://publications.iarc.fr/Book–And.
- European Commission. Renewing the approval of the active substance glyphosate in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation [EU) No 540/2011. Official Journal of the European Union, 2017, L 333/10-L 333/16. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R2324 (accessed March 6, 2024).
- Gotti, R.; Fiori, J.; Bosi, S.; Dinelli, G. Field-amplified sample injection and sweeping micellar electrokinetic chromatography in analysis of glyphosate and aminomethylphosphonic acid in wheat. J. Chromatogr. A, 2019, 1601, 357–364. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2023/2660 of 28 November 2023 renewing the approval of the active substance glyphosate in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) No 540/2011. Official Journal of the European Union, 29.11.2023. https://eur-lex.europa.eu/eli/reg_impl/2023/2660 (accessed March 6, 2024).
- Álvarez, F.; Arena, M.; Auteri, D.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Crivellente, F.; Egsmose, M.; Fait, G.; Ferilli, F.; Gouliarmou, V.; Herrero Nogareda, L.; Ippolito, A.; Istace, F.; Jarrah, S.; Kardassi, D.; Kienzler, A.; Lanzoni, A.; Lava, R.; Linguadoca, A.; Lythgo, C.; Mangas, I.; Padovani, L.; Panzarea, M.; Parra Morte, J.M.; Rizzuto, S.; Romac, A.; Rortais, A.; Serafimova, R.; Sharp, R.; Szentes, C.; Terron, A.; Theobald, A.; Tiramani, M. , Vianello, G.; Villamar-Bouza, L. Peer review of the pesticide risk assessment of the active substance glyphosate. EFSA journal, 2023, 21, article. [Google Scholar] [CrossRef]
- Istituto Ramazzini, Italy. Global Glyphosate Study Reveals Glyphosate-Based Herbicides Cause Leukemia in Early Life. , 2023. https://glyphosatestudy.org/press-release/global-glyphosate-study-reveals-glyphosate-based-herbicides-cause-leukemia-in-early-life/ (accessed March 6, 2024).
- Mao, Q.; Manservisi, F.; Panzacchi, S.; Mandrioli, D.; Menghetti, I.; Vornoli, A.; Bua, L.; Falcioni, L.; Lesseur, C.; Chen, J.; Belpoggi, F.; Hu, J. The Ramazzini Institute 13-week pilot study on glyphosate and Roundup administered at human-equivalent dose to Sprague Dawley rats: effects on the microbiome. Environ. Health-Glob., 2018, 17, article. [Google Scholar] [CrossRef]
- Lesseur, C.; Pirrotte, P.; Pathak, K.V.; Manservisi, F.; Mandrioli, D.; Belpoggi, F.; Panzacchi, S.; Li, Q.; Barrett, E.S.; Nguyen, R.H.N.; Sathyanarayana, S.; Swan, S.H.; Chen, J. Maternal urinary levels of glyphosate during pregnancy and anogenital distance in newborns in a US multicenter pregnancy cohort. Environ. Pollut., 2021, 280, article. [Google Scholar] [CrossRef]
- Lacroix, R.; Kurrasch, D.M. Glyphosate toxicity: in vivo, in vitro, and epidemiological evidence. Toxicol. Sci., 2023, 192, 131–140. [Google Scholar] [CrossRef]
- Hao, C.; Morse, D.; Morra, F.; Zhao, X.; Yang, P.; Nunn, B. Direct aqueous determination of glyphosate and related compounds by liquid chromatography/tandem mass spectrometry using reversed-phase and weak anion-exchange mixed-mode column. J. Chromatogr. A, 2011, 1218, 5638–5643. [Google Scholar] [CrossRef]
- Tongur, T.; Ayranci, E. Investigation of the performance of activated carbon cloth to remove glyphosate, glufosinate, aminomethylphosphonic acid and bialaphos from aqueous solutions by adsorption/electrosorption. Environ. Monit. Assess., 2023, 195, article. [Google Scholar] [CrossRef]
- Leyva-Morales, J.B.; Cabrera, R.; Bastidas-Bastidas, P.d.J.; Valenzuela-Quintanar, A.I.; Pérez-Camarillo, J.P.; González-Mendoza, V.M.; Perea-Domínguez, X.P.; Márquez-Pacheco, H.; Amillano-Cisneros, J.M.; Badilla-Medina, C.N.; Ontíveros-García, L.A.; Cruz-Acevedo; E. Validation and application of liquid chromatography coupled with tandem mass spectrometry method for the analysis of glyphosate, aminomethylphosphonic acid (AMPA), and glufosinate in soil. Agriculture, 2023, 13, article. [Google Scholar] [CrossRef]
- Martin, P.J.; He, K.; Blaney, L.; Hobbs, S.R. Advanced liquid chromatography with tandem mass spectrometry method for quantifying glyphosate, glufosinate, and aminomethylphosphonic acid using pre-column derivatization. ACS ES and T Water, 2023, 3, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.V.; Kish, L.K. Determination of glyphosate, aminomethylphosphonic acid and glufosinate in tea samples of Russia. Asian J. Chem., 2023, 35, 1069–1073. [Google Scholar] [CrossRef]
- Zhang, Y.; Dang, Y.; Lin, X.; An, K.; Li, J.; Zhang, M. Determination of glyphosate and glufosinate in corn using multi-walled carbon nanotubes followed by ultra high performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A, 2020, 169, article. [Google Scholar] [CrossRef]
- Ehling, S.; Reddy, T.M. Analysis of glyphosate and aminomethylphosphonic acid in nutritional ingredients and milk by derivatization with fluorenylmethyloxycarbonyl chloride and liquid chromatography-mass spectrometry. J. Agr. Food Chem., 2015, 63, 10562–10568. [Google Scholar] [CrossRef] [PubMed]
- Rämö, S.; Välimäki, J.; Siimes, K.; Uusi-Kämppä, J. Determination of glyphosate and aminomethylphosphonic acid residues in Finnish soils by ultra-high performance liquid chromatography–tandem mass spectrometry. MethodsX, 2023, 11, article. [Google Scholar] [CrossRef]
- Małysiak, M.; Kiljanek, T. Method of Glyphosate, AMPA, and Glufosinate Ammonium Determination in Beebread by Liquid Chromatography—Tandem Mass Spectrometry after Molecularly Imprinted Solid-Phase Extraction. Molecules, 2022, 27, article. [Google Scholar] [CrossRef]
- Gains, K.K.K.; Roland, N’G. K.; Urbain, K.Y.; Ardjouma, D. Determination of the glyphosate content in liquid and dry formulations by HPLC-UV: pre-column derivation with 9-fluorenylmethyl chloroformate (FMOC). Chromatographia, 2022, 85, 655–664. [Google Scholar] [CrossRef]
- Alonso, B.; Griffero, L.; Bentos Pereira, H.; Pareja, L.; Pérez Parada, A. Determination of glyphosate and AMPA in freshwater and soil from agroecosystems by 9-fluorenylmethoxycarbonyl chloride derivatization and liquid chromatography - fluorescence detection and tandem mass spectrometry. MethodsX, 2022, 9, article. [Google Scholar] [CrossRef]
- Delhomme, O.; Rodrigues, A.; Hernandez, A.; Chimjarn, S.; Bertrand, C.; Bourdat-Deschamps, M.; Fritsch, C.; Pelosi, C.; Nélieu, S.; Millet, M. A method to assess glyphosate, glufosinate and aminomethylphosphonic acid in soil and earthworms. J. Chromatogr. A, 2021, 1651, article. [Google Scholar] [CrossRef] [PubMed]
- Garba, J.; Samsuri, A.W.; Othman, R.; Hamdani, M.S.A. Simplified method for derivatization of extractable glyphosate and aminomethylphosphonic acid and their determination by high performance liquid chromatography. Environmental Research & Technology, 2018; 1, 19–30. [Google Scholar]
- Junqué, E.; Fernández, P.; Filippi, I.; Grimalt, J.O. Determination of glyphosate and its derivative, aminomethylphosphonic acid, in human urine by gas chromatography coupled to tandem mass spectrometry and isotope pattern deconvolution. J. Chromatogr. Open, 2023, 4, article. [Google Scholar] [CrossRef]
- Steinborn, A.; Alder, L.; Michalski, B.; Zomer, P.; Bendig, P.; Martinez, S.A.; Mol, H.G.J.; Class, T.J.; Costa Pinheiro, N. Determination of glyphosate levels in breast milk samples from germany by LC-MS/MS and GC-MS/MS. J. Agr. Food Chem., 2016, 64, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Yang; Y. ; Feng, S.; Hu, Y.; Li, Y.; Wang, H.; Liu, J.; Zhong, H. Determination of glyphosate and aminomethyl phosphonic acid residue in green tea by gas chromatography-mass spectrometry. Journal of Tea Science, 2020, 40, 125–132. [Google Scholar]
- De Abreu, A.B.G.; Da Matta, M.H. De R.; Montagner, É. Development and validation of a method of analysis of glyphosate in soy grains. Quim. Nova, 2008, 31, 5–9. [Google Scholar] [CrossRef]
- Royer, A.; Beguin, S.; Tabet, J.C.; Hulot, S.; Reding, M.A.; Communal, P.Y. Determination of glyphosate and aminomethylphosphonic acid residues in water by gas chromatography with tandem mass spectrometry after exchange ion resin purification and derivatization. Application on vegetable matrixes. Anal. Chem., 2000, 72, 3826–3832. [Google Scholar] [CrossRef]
- Stajnko, A.; Tratnik, J.S. , Kosjek, T.; Mazej, D.; Jagodic, M.; Eržen, I.; Horvat, M. Seasonal glyphosate and AMPA levels in urine of children and adolescents living in rural regions of Northeastern Slovenia. Environ. Int., 2020, 143, article. [Google Scholar] [CrossRef]
- Conrad, A.; Schröter-Kermani, C.; Hoppe, H.W.; Rüther, M.; Pieper, S.; Kolossa-Gehring, M. Glyphosate in German adults – Time trend (2001 to 2015) of human exposure to a widely used herbicide. Int. J. Hyg. Envir. Heal., 2017, 220, 8–16. [Google Scholar] [CrossRef]
- Kittlaus, S.; Lipinski, J.; Speer, K. New approaches for determination of glyphosate and aminomethylphosphonic acid from different tea samples-prospects and limits of cleanup with molecularly imprinted polymer and titanium dioxide. J. Aoac Int., 2009, 92, 703–714. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Chen, C.-L.; Li, J.-Z. A simple method for the determination of glyphosate residues in soil by capillary gas chromatography with nitrogen phosphorus. J. Anal. Chem., 2008, 63, 371–375. [Google Scholar] [CrossRef]
- Li, S.; Li, P.; Li, X.; Wen, N.; Wang, Y.; Lu, W.; Lin, M.; Lang, Z. In maize, co-expression of GAT and GR79-EPSPS provides high glyphosate resistance, along with low glyphosate residues. aBIOTECH, 2023. [Google Scholar] [CrossRef]
- Hove-Jensen, B.; Zechel, D.L.; Jochimsen, B. Utilization of Glyphosate as Phosphate Source: Biochemistry and Genetics of Bacterial Carbon-Phosphorus Lyase. Microbiol. Mol. Biol. R., 2014, 78, 176–197. [Google Scholar] [CrossRef]
- Aslam, S.; Jing, Y.; Nowak, K.M. Fate of glyphosate and its degradation products AMPA, glycine and sarcosine in an agricultural soil: Implications for environmental risk assessment. J. Hazard. Mater., 2023, 447, article. [Google Scholar] [CrossRef] [PubMed]
- Kaczynski, P.; Lozowicka, B.; Wolejko, E.; Iwaniuk, P.; Konecki, R.; Dragowski, W.; Lozowicki, J.; Amanbek, N.; Rusilowska, J.; Pietraszko, A. Complex study of glyphosate and metabolites influence on enzymatic activity and microorganisms association in soil enriched with Pseudomonas fluorescens and sewage sludge. J. Hazard. Mater., 2020, 393, article. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; Harikumar, S.L.; Singh, J. Herbicide Glyphosate: Toxicity and Microbial Degradation. Int. J. Env. Res. Pub. He., 2020, 17, article. [Google Scholar] [CrossRef] [PubMed]
- Graf, H.G.; Biebl, S.M.; Müller, L.; Breitenstein, C.; Huhn, C. Capillary electrophoresis applied for the determination of acidity constants and limiting electrophoretic mobilities of ionizable herbicides including glyphosate and its metabolites and for their simultaneous separation. J. Sep. Sci., 2022, 45, 1128–1139. [Google Scholar] [CrossRef]
- Simonsen, L.; Fomsgaard, I.S.; Svensmark, B.; Spliid, N.H. Fate and availability of glyphosate and AMPA in agricultural soil. J. Environ. Sci. Heal. B, 2008, 43, 365–375. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Silva, T.L.; Andreani, T.; Silva, A.M. Glyphosate vs. Glyphosate-Based Herbicides Exposure: A Review on Their Toxicity. J. Xenobiot., 2022, 12, 21–40. [Google Scholar] [CrossRef]
- Santos Barreto, L.; Lima de Souza, T.; Pereira de Morais, T.; De Oliveira Ribeiro, C.A. Toxicity of glyphosate and aminomethylphosphonic acid (AMPA) to the early stages of development of Steindachneridion melanodermatum, an endangered endemic species of Southern Brazil. Environ. Toxicol. Phar., 2023, 102, article. [Google Scholar] [CrossRef]
- Granby, K.; Johannesen, S.; Vahl, M. Analysis of glyphosate residues in cereals using liquid chromatography-mass spectrometry (LC-MS/MS). Food Addit. Contam., 2003, 20, 692–698. [Google Scholar] [CrossRef]
- Zoller, O.; Rhyn, P.; Rupp, H.; Zarn, J.A.; Geiser, C. Glyphosate residues in Swiss market foods: monitoring and risk evaluation. Food Addit. Contam. B, 2018, 11, 83–91. [Google Scholar] [CrossRef]
- Rodrigues, N.R.; Ferreira de Souza, A.P.; Morais, P.P.P.; Braga, D.P.V.; Crivellari, A.C.; Favoretto, L.R.G.; Berger, G.U. Residues of glyphosate and aminomethylphosphonic acid (AMPA) in genetically modified glyphosate tolerant soybean, corn and cotton crops. Cienc. Rural, 2021, 51, 1–13. [Google Scholar] [CrossRef]
- Bou-Mitri, C.; Mekanna, A.N.; Dagher, S.; Moukarzel, S.; Farhat, A. Occurrence and exposure to glyphosate present in bread and flour products in Lebanon. Food Control, 2022, 136, article. [Google Scholar] [CrossRef]
- Rodrigues, N.R.; Ferreira de Souza, A.P. Occurrence of glyphosate and AMPA residues in soy-based infant formula sold in Brazil. Food Addit. Contam. A, 2018, 35, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Arregui, M.C.; Leonardón, A.; Sanchez, D.; Maitre, M.I.; Scotta, R.; Enrique, S. Monitoring glyphosate residues in transgenic glyphosate-resistant soybean. Pest Manag. Sci., 2004, 60, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Kolakowski, B.M.; Miller, L.; Murray, A.; Leclair, A.; Bietlot, H.; Van de Riet, J.M. Analysis of glyphosate residues in foods from the Canadian retail markets between 2015 and 2017. J. Agr. Food Chem., 2020, 68, 5201–5211. [Google Scholar] [CrossRef]
- Liao, Y.; Berthion, J.-M.; Colet, I.; Merlo, M.; Nougadère, A.; Hu, R. Validation and application of analytical method for glyphosate and glufosinate in foods by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A, 2018, 1549, 31–38. [Google Scholar] [CrossRef]
- Soares, D.; Silva, L.; Duarte, S.; Pena, A.; Pereira, A. Glyphosate use, toxicity and occurrence in food. Food, 2021, 10, article. [Google Scholar] [CrossRef] [PubMed]
- Granby, K.; Vahl, M. Investigation of the herbicide glyphosate and the plant growth regulators chlormequat and mepiquat in cereals produced in Denmark. Food Addit. Contam., 2001, 18, 898–905. [Google Scholar] [CrossRef]
- Xu, J.; Smith, S.; Smith, G.; Wang, W.; Li, Y. Glyphosate contamination in grains and foods: An overview. Food Control, 2019, 106, article. [Google Scholar] [CrossRef]
- Tittlemier, S.A; Bestvater, L.; Carlson, J.; Kletke, J.; Izydorczyk, M.; Fu, B.X. Fate of glyphosate in wheat during milling and bread production. Cereal Chem., 2021, 98, 100–108. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Bestvater, L.; Chan, J.; Timofeiev, V.; Richter, A.; Wang, K.; Ruan, Y.; Izydorczyk, M.; Fu, B.X. Diverging fates of cadmium and glyphosate during pasta cooking. Food Addit. Contam. A, 2023, 40, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Amvrazi, E.G. Fate of Pesticide Residues on Raw Agricultural Crops after Postharvest Storage and Food Processing to Edible Portions. Pesticides - Formulations, Effects, Fate, Prof. Margarita Stoytcheva (Ed. 9: ISBN; -7. [CrossRef]
- Ashley-Martin, J.; Huang, R.; MacPherson, S.; Brion, O.; Owen, J.; Gaudreau, E.; Bienvenu, J.-F.; Fisher, M.; Borghese, M.M; Bouchard, M.F.; Lanphear, B.; Foster, W.G.; Arbuckle, T.E. Urinary concentrations and determinants of glyphosate and glufosinate in pregnant Canadian participants in the MIREC study. Environ. Res., 2023, 217, article. [Google Scholar] [CrossRef] [PubMed]
- Mellen, P.B.; Walsh, T.F.; Herrington, D.M. Whole grain intake and cardiovascular disease: A meta-analysis. Nutr. Metab. Cardiovasc. Dis., 2008, 18, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of cohort studies. Eur. J. Epidemiol., 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Slavin, G. Why whole grains are protective: biological mechanisms. P. Nutr. Soc., 2003, 62, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Björck, I.; Östman, E.; Kristensen, M.; Mateo Anson, N.; Price, R.K.; Haenen, G.R.M.M.; Havenaar, R.; Bach Knudsen, K.E.; Frid, A.; Mykkänen, H.; Welch, R.B.; Riccardi, G. Cereal grains for nutrition and health benefits: Overview of results from in vitro, animal and human studies in the HEALTHGRAIN project. Trends Food Sci. Tech., 2012, 25, 87–100. [Google Scholar] [CrossRef]
- Slavin, J. ; Whole grains and human health. Nutr. Res. Rev., 2004, 17, 99–110. [Google Scholar] [CrossRef]
- Low, F.L. , Shaw, I.C.; Gerrard, J.A. The effect of Saccharomyces cerevisiae on the stability of the herbicide glyphosate during bread leavening. Lett. Appl. Microbiol., 2005, 40, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Rendón-von Osten, J.; Dzul-Caamal, R. Glyphosate residues in groundwater, drinking water and urine of subsistence farmers from intensive agriculture localities: A survey in Hopelchén, Campeche, Mexico. Int. J. Env. Res. Pub. He., 2017, 14, article. [Google Scholar] [CrossRef]
- Carles, L.; Gardon, H.; Joseph, L.; Sanchís, J.; Farré, M.; Artigas, J. Meta-analysis of glyphosate contamination in surface waters and dissipation by biofilms. Environ. Int., 2019, 124, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Lutri, V.F.; Matteoda, E.; Blarasin, M. , Aparicio, V.; Giacobone, D., Maldonado, L., Becher Quinodoz, F.; Cabrera, A.; Giuliano Albo, J. Hydrogeological features affecting spatial distribution of glyphosate and AMPA in groundwater and surface water in an agroecosystem. Córdoba, Argentina. Sci. Total Environ., 2020, 711, article. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.B.; Boëchat, I.G.; Fernandes, M.D.; Monteiro, J.A.F.; Rivaroli, L.; Gücker, B. Glyphosate pollution of surface runoff, stream water, and drinking water resources in Southeast Brazil. Environ. Sci. Pollut. R., 2023, 30, 27030–27040. [Google Scholar] [CrossRef]
- El Baroudi, Y. , Ouazzani, C., Er-Ramly, A., Moustaghfir, A.; Essebbahi, I., El Baroudi, H., Dami, A., Balouch, L. Glyphosate Contamination of Well Water from Various Agricultural Areas – A Case Study in Morocco. Ecological Engineering and Environmental Technology E.E.E.T., 2023, 24, 247–257. [Google Scholar] [CrossRef]
- Peruzzo, P.J.; Porta, A.A.; Ronco, A.E. Levels of glyphosate in surface waters, sediments and soils associated with direct sowing soybean cultivation in north pampasic region of Argentina. Environ. Pollut., 2008, 156, 61–66. [Google Scholar] [CrossRef]
- Ruiz-Toledo, J.; Castro, R.; Rivero-Pérez, N.; Bello-Mendoza, R.; Sánchez, D. Occurrence of glyphosate in water bodies derived from intensive agriculture in a tropical region of southern Mexico. B. Environ. Contam. Tox., 2014, 93, 289–293. [Google Scholar] [CrossRef]
- Poiger, T.; Buerge, I.J.; Bächli, A.; Müller, M.D.; Balmer, M.E. Occurrence of the herbicide glyphosate and its metabolite AMPA in surface waters in Switzerland determined with on-line solid phase extraction LC-MS/MS. Environ. Sci. Pollut. R., 2017, 24, 1588–1596. [Google Scholar] [CrossRef]
- Ulrich, J.C.; Hoffman, K. , Gunasekara, T.D.K.S.C.; Sandamini, P.M.M.A.; Jackson, B.P.; De Silva, P. Mangala C.S.; Jayasundara, N.; Ferguson, P.L. Glyphosate and Fluoride in High-Hardness Drinking Water Are Positively Associated with Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Environ. Sci. Technol. Lett., 2023, 10, 916–923. [Google Scholar] [CrossRef]
- Bento, C.P.M.; Yang, X.; Gort, G.; Xue, S.; Van Dam, R.; Zomer, P.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Persistence of glyphosate and aminomethylphosphonic acid in loess soil under different combinations of temperature, soil moisture and light/darkness. Sci. Total Environ., 2016, 572, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Lydon, J.; Koskinen, W.C.; Moorman, T.B. , Chaney, R.L.; Hammerschmidt, R. Glyphosate effects on plant mineral nutrition, crop rhizosphere microbiota, and plant disease in glyphosate-resistant crops. J. Agr. Food Chem., 2012, 60, 10375–10397. [Google Scholar] [CrossRef]
- Regulation (EC) No 396/2005 of the European Parliament and of the Council on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Official Journal of the European Union. Current consolidated version: 21/10/2023. https://eur-lex.europa.eu/eli/reg/2005/396/oj (accessed March 11, 2024).
- Review of the existing maximum residue levels for glyphosate according to Article 12 of Regulation (EC) No 396/2005 – revised version to take into account omitted data. EFSA Journal, 2019, 17, article. [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO). Codex Alimentarius, International Food Standards, Glyphosate. https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticide-detail/en/?p_id=158 (accessed March 11, 2024).
- United States Environmental Protection Agency (US EPA). Regulation of Pesticide Residues on Food, 40 CFR § 180.364 - Glyphosate; tolerances for residues. July 1, 2022. https://www.govinfo.gov/app/details/CFR-2022-title40-vol26/CFR-2022-title40-vol26-sec180-364 (accessed March 11, 2024).
- Health Canada, Government of Canada, Pesticide Product Information Database. Glyphosate. https://pest-control.canada.ca/pesticide-registry/en/mrl-search.html (accessed March 11, 2024).
- Hogendoorn, E.A.; Ossendrijver, F.M.; Dijkman, E.; Baumann, R.A. Rapid determination of glyphosate in cereal samples by means of pre-column derivatisation with 9-fluorenylmethyl chloroformate and coupled-column liquid chromatography with fluorescence detection. J. Chromatogr. A, 1999, 833, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.H.; Bille, R.L.L.; Granby, K. An intercomparison study of the determination of glyphosate, chlormequat and mepiquat residues in wheat. Food Addit. Contam., 2007, 24, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Ayoola, R.T.; Olujimi, O.O.; Bada, B.S.; Dedeke, G.A. Seasonal variations in the levels of glyphosate in soil, water and crops from three farm settlements in Oyo state, Nigeria. Heliyon, 2023, 9, article. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Kong, D.; Gu, W.; Guo, X.; Tao, W.; Shan, Z.; Wang, Y.; Wang, N. Determination of glyphosate in soil/sludge by high performance liquid chromatography. J. Chromatogr. A, 2017, 1502, 8–13. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, Y.; Ma, L.; An, J.; Zhang, H.; Cao, M.; Zhu, H.; Kang, W.; Lian, K. A method for determining glyphosate and its metabolite aminomethyl phosphonic acid by gas chromatography-flame photometric detection. J. Chromatogr. A, 2019, 1589, 116–121. [Google Scholar] [CrossRef]
- Huhn, C. More and enhanced glyphosate analysis is needed. Anal. Bioanal. Chem., 2018, 410, 3041–3045. [Google Scholar] [CrossRef]
- ISO 16308:2014, International Organization for Standardization. Water quality — Determination of glyphosate and AMPA — Method using high performance liquid chromatography (HPLC) with tandem mass spectrometric detection. https://www.iso.org/standard/56140.html (accessed March 12, 2024).
- Cruz, J.M.; Murray, J.A. Determination of glyphosate and AMPA in oat products for the selection of candidate reference materials. Food Chem., 2021, 342, article. [Google Scholar] [CrossRef]
- Sancho, J.V.; Hidalgo, C.; Hernández, F.; López, F.J.; Dijkman, E.; Hogendoorn, E.A. Rapid Determination of Glyphosate Residues and Its Main Metabolite Ampa in Soil Samples by Liquid Chromatography. Int. J. Environ. An. Ch., 1996, 62, 53–63. [Google Scholar] [CrossRef]
- Piestansky, J.; Olesova, D.; Matuskova, M.; Cizmarova, I.; Chalova, P.; Galba, J.; Majerova, P.; Mikus, P.; Kovac, A. Amino acids in inflammatory bowel diseases: Modern diagnostic tools and methodologies. Adv. Clin. Chem., 2022, 107, 139–213. [Google Scholar] [CrossRef]
- Franke, A.A.; Li, X.; Lai, J.F. Analysis of glyphosate, aminomethylphosphonic acid, and glufosinate from human urine by HRAM LC-MS. Anal. Bioanal. Chem., 2020, 412, 8313–8324. [Google Scholar] [CrossRef]
- Chen, M.X.; Cao, Z.Y.; Jiang, Y.; Zhu, Z.-W. Direct determination of glyphosate and its major metabolite, aminomethylphosphonic acid, in fruits and vegetables by mixed-mode hydrophilic interaction/weak anion-exchange liquid chromatography coupled with electrospray tandem mass spectrometry. J. Chromatogr. A, 2013, 1272, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; Huo, Z.; Sun, H.; Zhang, F.; Zhu, B. An ion chromatography tandem mass spectrometry (IC-MS/MS) method for glyphosate and amino methyl phosphoric acid in serum of occupational workers. Microchem. J., 2021, 170, article. [Google Scholar] [CrossRef]
- Amberger, M.A.; Schröder, M.; Kuballa, J.; Jantzen, E. Direct determination of glyphosate, aminomethylphosphonic acid and glufosinate in food samples with ion chromatography coupled to electrospray ionization tandem mass spectrometry. J. Chromatogr. A, 2023, 1687, article. [Google Scholar] [CrossRef] [PubMed]
- Feltracco, M.; Barbaro, E.; Morabito, E.; Zangrando, R.; Piazza, R.; Barbante, C.; Gambaro, A. Assessing glyphosate in water, marine particulate matter, and sediments in the Lagoon of Venice. Environ. Sci. Pollut. R., 2022, 29, 16383–16391. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.K.; Dayan, F.E. Glufosinate-ammonium: a review of the current state of knowledge. Pest Manag. Sci., 2020, 76, 3911–3925. [Google Scholar] [CrossRef] [PubMed]
- Hoerlein, G. Glufosinate (phosphinothricin), a natural amino acid with unexpected herbicidal properties. Rev. Environ. Contam. T., 1994, 138, 73–145. [Google Scholar] [CrossRef]
- Koskinen, W.C.; Marek, L.J.; Hall, K.E. Analysis of glyphosate and aminomethylphosphonic acid in water, plant materials and soil. Pest Manag. Sci. 2016; 423–432. [Google Scholar] [CrossRef]
- Gauglitz, G.; Wimmer, B.; Melzer, T.; Huhn, C. Glyphosate analysis using sensors and electromigration separation techniques as alternatives to gas or liquid chromatography. Anal. Bioanal. Chem., 2018, 410, 725–746. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Guo, H.; Liu, W.-w.; Zhang, W.-w.; Wang, J.-w. Current progress on the detection of glyphosate in environmental samples. J. Sci. Appl: BioMed., 2015, 3, 88–95. [Google Scholar]
- Deyrup, C.L.; Chang, S.M. , Weintraub, R.A.; Moye, H.A. Simultaneous Esterification and Acylation of Pesticides for Analysis by Gas Chromatography. 1. Derivatization of Glyphosate and (Aminomethyl)phosphonic Acid with Fluorinated Alcohols-Perfluorinated Anhydrides. J. Agr. Food Chem., 1985, 33, 944–947. [Google Scholar] [CrossRef]
- Konar, S.K.; Roy, D.N. Method for the determination of residues of the herbicide glyphosate and its principal metabolite, aminomethylphosphonic acid, in plant materials by nitrogen-selective gas chromatography. Anal. Chim. Acta, 1990, 229, 277–280. [Google Scholar] [CrossRef]
- Eberbach, P.L.; Douglas, L.A. Method for the Determination of Glyphosate and (Aminomethyl)phosphonic Acid in Soil Using Electron Capture Gas Chromatography. J. Agr. Food Chem., 1991, 39, 1776–1780. [Google Scholar] [CrossRef]
- AOAC INTERNATIONAL, Association of Official Analytical Collaboration International (formerly Association of Official Analytical Chemists). Determination of Glyphosate and Aminomethylphosphonic Acid (AMPA) in Crops. Gas Chromatography with Mass-Selective Detection. First Action 2000.
- Alferness, P.L.; Wiebe, L.A. Determination of glyphosate and aminomethylphosphonic acid in crops by capillary gas chromatography with mass-selective detection: Collaborative study. J. AOAC Int., 2001, 84, 823–846. [Google Scholar] [CrossRef]
- Alferness, P.L.; Iwata, Y. Determination of Glyphosate and (Aminomethyl)phosphonic Acid in Soil, Plant and Animal Matrixes, and Water by Capillary Gas Chromatography with Mass-Selective Detection. J. Agr. Food Chem., 1994, 42, 2751–2759. [Google Scholar] [CrossRef]
- Börjesson, E.; Torstensson, L. New methods for determination of glyphosate and (aminomethyl)phosphonic acid in water and soil. J. Chromatogr. A, 2000, 886, 207–216. [Google Scholar] [CrossRef]
- Hori, Y.; Fujisawa, M.; Shimada, K.; Hirose, Y. Determination of the herbicide glyphosate and its metabolite in biological specimens by gas chromatography-mass spectrometry. A case of poisoning by Roundup® herbicide. J. Anal. Toxicol., 2003, 27, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Motojyuku, M.; Saito, T.; Akieda, K.; Otsuka, H.; Yamamoto, I.; Inokuchi, S. Determination of glyphosate, glyphosate metabolites, and glufosinate in human serum by gas chromatography–mass spectrometry. J. Chromatogr. B, 2008, 875, 509–514. [Google Scholar] [CrossRef]
- Aris, A.; Leblanc, S. Maternal and fetal exposure to pesticides associated to genetically modified foods in Eastern Townships of Quebec, Canada. Reprod. Toxicol. 2011; 31, 528–533. [Google Scholar] [CrossRef]
- Saito, T.; Miura, N.; Namera, A.; Oikawa, H.; Miyazaki, S.; Nakamoto, A.; Inokuchi, S. Mixed-mode C–C18 monolithic spin-column extraction and GC–MS for simultaneous assay of organophosphorus compounds, glyphosate, and glufosinate in human serum and urine. Forensic Toxicol., 2012, 30, 1–10. [Google Scholar] [CrossRef]
- Tsunoda, N. Simultaneous determination of the herbicides glyphosate, glufosinate and bialaphos and their metabolites by capillary gas chromatography-ion-trap mass spectrometry. J. Chromatogr., 1993, 637, 167–173. [Google Scholar] [CrossRef]
- Ngim, K.K.; Green, J.; Cuzzi, J.; Ocampo, M.; Gu, Z. Optimized derivatization procedure for characterizing (Aminomethyl) phosphonic acid impurities by GC-MS. J. Chromatogr. Sci., 2011, 49, 8–14. [Google Scholar] [CrossRef]
- Catrinck, T.C.P.G.; Aguiar, M.C.S.; Dias, A.; Silvério, F.O.; Fidêncio, P.H.; Paulino de Pinho, G. Study of the Reaction Derivatization Glyphosate and Aminomethylphosphonic Acid (AMPA) with N,O-Bis(trimethylsilyl)trifluoroacetamide. American Journal of Analytical Chemistry, 2013, 4, 647–652. [Google Scholar] [CrossRef]
- Arkan, T.; Csámpai, A.; Molnár-Perl, I. Alkylsilyl derivatization of glyphosate and aminomethylphosphonic acid followed by gas chromatography mass spectrometry. Microchem. J., 2016, 125, 219–223. [Google Scholar] [CrossRef]
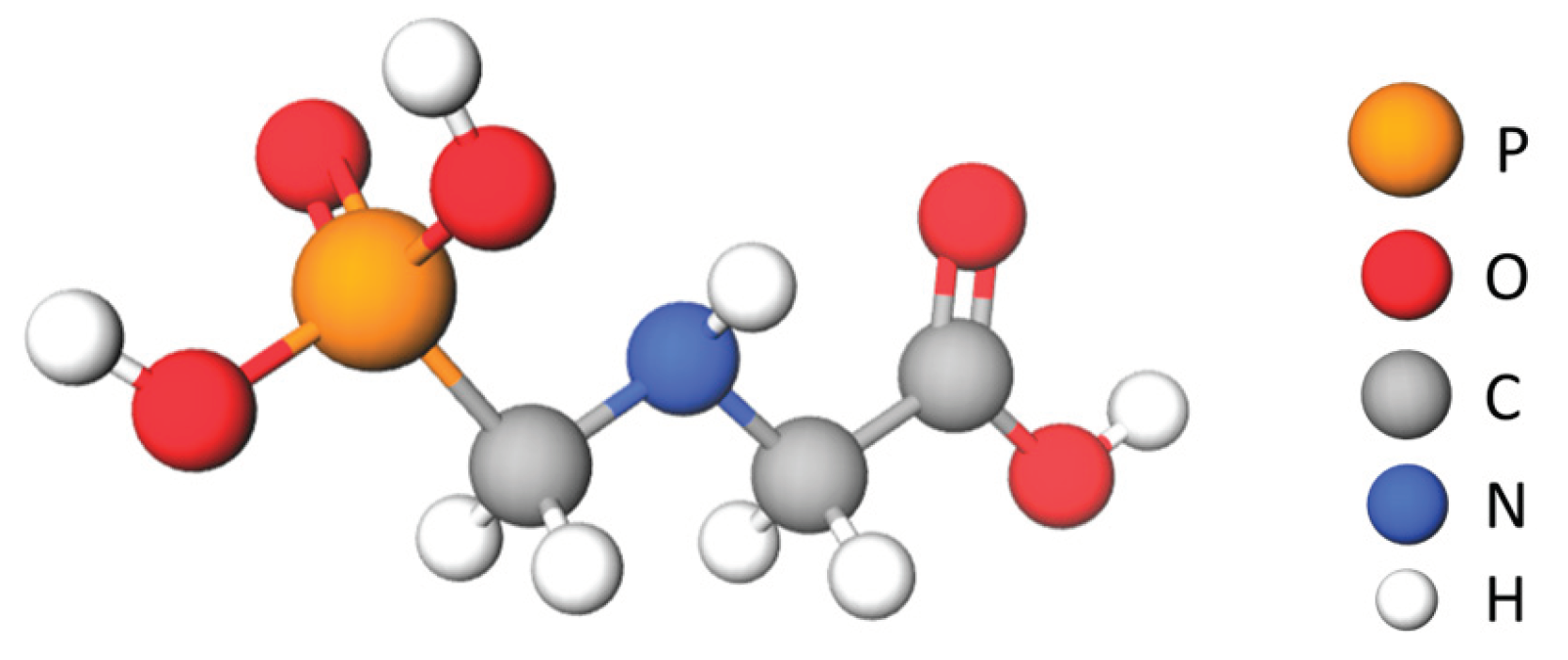


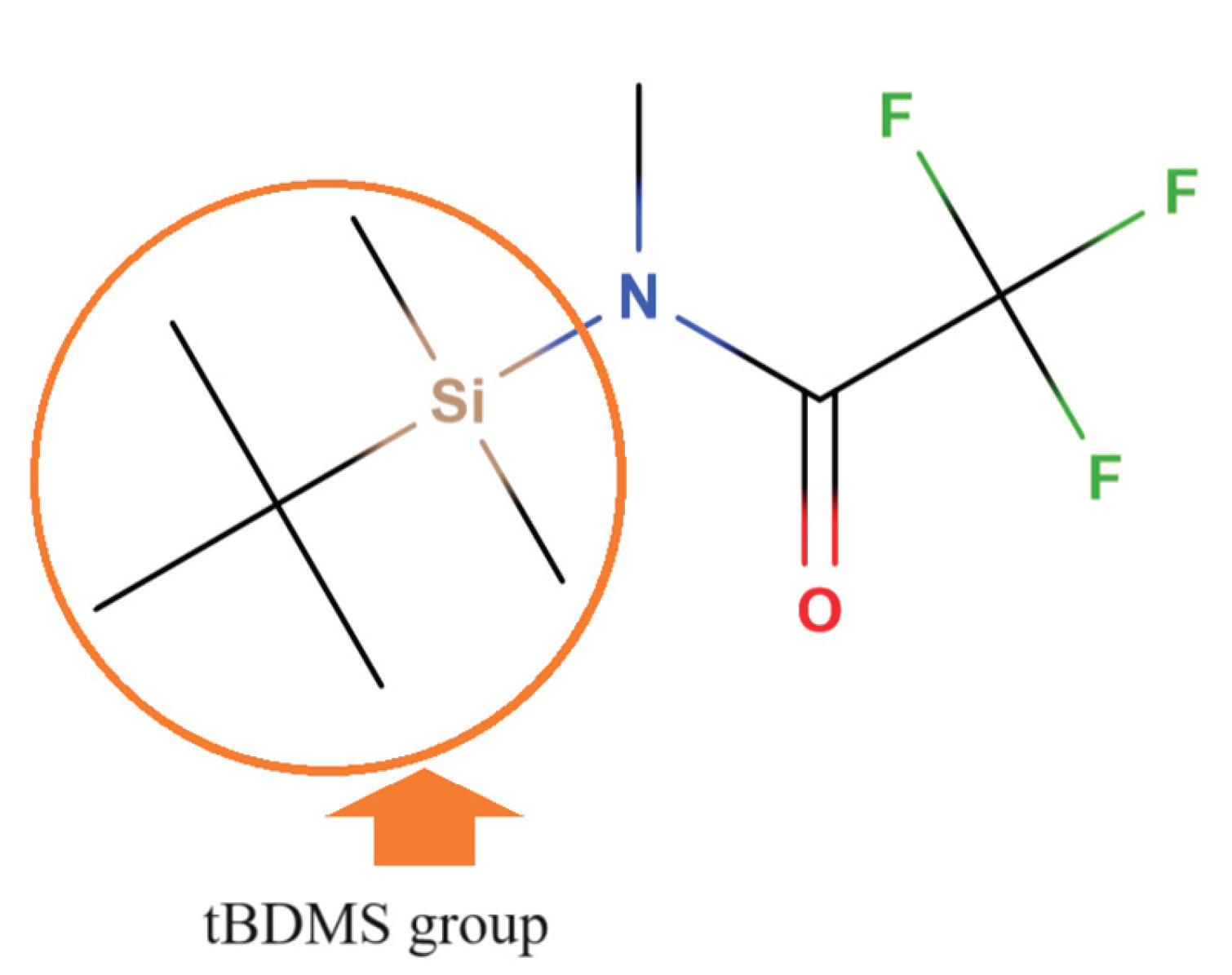
| Cereals and cereal-based foods | Glyphosate concentration (mg kg-1) |
AMPA concentration (mg kg-1) |
Reference |
|---|---|---|---|
| Barley | < 0.45 | n.a. 1 | [61] |
| Oats | < 0.08 | n.a. 1 | [61] |
| Rye | < 0.04 | n.a. 1 | [61] |
| Durum wheat | 0.421 (max.) | 0.0247 (max.) | [62] |
| Wheat | < 0.13 | n.a. 1 | [61] |
| Wheat | 6.1 – 11.1 | n.a. 1 | [4] |
| Wheat bran | < 0.7 | n.a. 1 | [61] |
| Wheat flour | 0.02 | n.a. 1 | [61] |
| Bread | 0.0458 (max.) | traces | [62] |
| Breakfast cereals | 0.291 (max.) | 0.01 (max.) | [62] |
| Flour and baking mixtures | 0.133 (max.) | traces | [62] |
| GM soybean | 0.4 – 8.8 | 0.7 – 10 | [13] |
| GM corn | 0.15 | 0.49 | [63] |
| Wheat | 0.373 | 0.034 | [5] |
| Barley | 2.15 | 0.041 | [5] |
| Whole grain | 0.0257 | n.a. 1 | [64] |
| White bread | 0.0149 | n.a. 1 | [64] |
| Soy-based infant formulas | 0.03 – 1.08 | 0.02 – 0.17 | [65] |
| GM soybean | 0.1 – 1.8 | 0.9 (max.) | [66] |
| Corn flour | 0.0052 – 0.3 2 | [67] | |
| Breakfast cereals | 0.006 – 0.034 | n.a. 1 | [68] |
| Wheat flour | < 0.03 | n.a. 1 | [69] |
| Wheat bran | 1.62 (max.) | n.a. 1 | [70] |
| Cereals and related crops | European Union [93,94] |
FAO / WHO Codex [94,95] |
U.S. EPA 1 [96] |
Health Canada [97] |
|---|---|---|---|---|
| Barley | 20 | 30 | 30 | 10 |
| Buckwheat | 0.1 | 30 | 30 | |
| Maize/corn grains | 1 | 5 | 5 | 3 |
| Millet | 0.1 | 30 | 30 | |
| Oats | 20 | 30 | 30 | 15 |
| Rice | 0.1 | 0.1 | ||
| Rye | 10 | 30 | 30 | |
| Sorghum | 20 | 30 | 30 | |
| Soya beans | 20 | 20 | 20 | 20 |
| Wheat | 10 | 30 | 30 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





