Submitted:
14 March 2024
Posted:
15 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Main Pathogenetic Mechanisms of MNPs-Induced Cell Toxicity
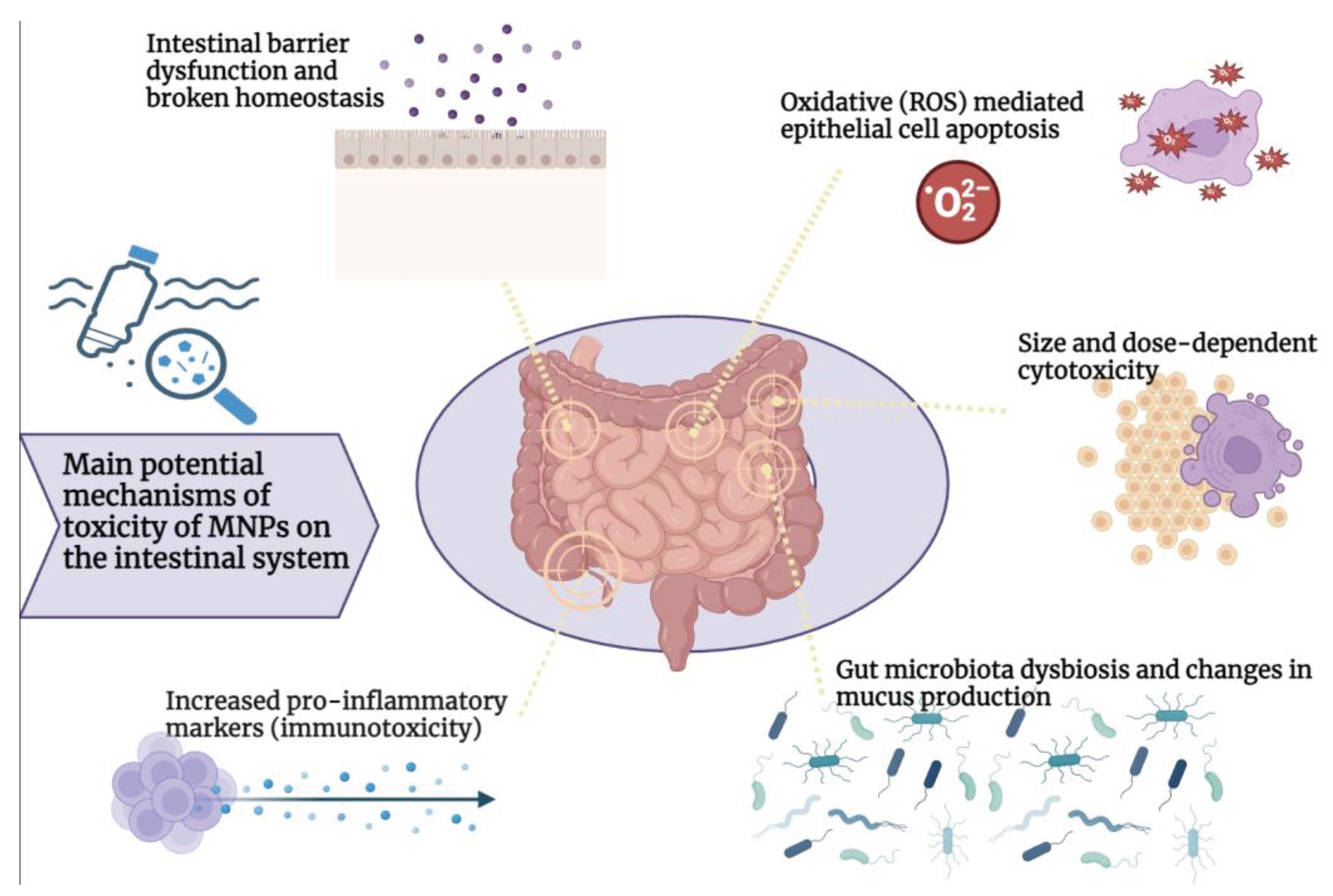
3. MNPs and the Intestinal System
3.1. Toxicity of Micro/Nanoplastics in the Intestine
3.2. MNPs Gut Immunological Impact
3.3. MNPs Effects on Gut Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodge, P.; Hellwich, K.-H.; Hiorns, R.C.; Jones, R.G.; Kahovec, J.; Luscombe, C.K.; Purbrick, M.D.; Wilks, E.S. A Concise Guide to Polymer Nomenclature for Authors of Papers and Reports in Polymer Science and Technology (IUPAC Technical Report). Pure and Applied Chemistry 2020, 92, 797–813. [CrossRef]
- Singh Jadaun, J.; Bansal, S.; Sonthalia, A.; Rai, A.K.; Singh, S.P. Biodegradation of Plastics for Sustainable Environment. Bioresour Technol 2022, 347, 126697. [CrossRef]
- Banaee, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Haghi, B.N.; Akhlaghi, M.; Derikvandy, A. Evaluation of Single and Combined Effects of Cadmium and Micro-Plastic Particles on Biochemical and Immunological Parameters of Common Carp (Cyprinus Carpio). Chemosphere 2019, 236, 124335. [CrossRef]
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci Prog 2018, 101, 207–260. [CrossRef]
- Shahul Hamid, F.; Bhatti, M.S.; Anuar, N.; Anuar, N.; Mohan, P.; Periathamby, A. Worldwide Distribution and Abundance of Microplastic: How Dire Is the Situation? Waste Management & Research: The Journal for a Sustainable Circular Economy 2018, 36, 873–897. [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric Microplastic Deposition in an Urban Environment and an Evaluation of Transport. Environ Int 2020, 136, 105411. [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the Marine Environment: Current Trends in Environmental Pollution and Mechanisms of Toxicological Profile. Environ Toxicol Pharmacol 2019, 68, 61–74. [CrossRef]
- Naegelen, I. Mikroplastik Als Gesundheitsgefahr Für Den Menschen. Biologie in unserer Zeit 2019, 49, 241–242. [CrossRef]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of Micro- and Nanoplastic Contamination in the Food Chain. Food Additives & Contaminants: Part A 2019, 36, 639–673. [CrossRef]
- Lambert, S.; Wagner, M. Microplastics Are Contaminants of Emerging Concern in Freshwater Environments: An Overview. In; 2018; pp. 1–23.
- Marcelino, R.C.; Cardoso, R.M.; Domingues, E.L.B.C.; Gonçalves, R. V.; Lima, G.D.A.; Novaes, R.D. The Emerging Risk of Microplastics and Nanoplastics on the Microstructure and Function of Reproductive Organs in Mammals: A Systematic Review of Preclinical Evidence. Life Sci 2022, 295, 120404. [CrossRef]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic Sources, Formation, Toxicity and Remediation: A Review. Environ Chem Lett 2023, 21, 2129–2169. [CrossRef]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as Contaminants in the Soil Environment: A Mini-Review. Science of The Total Environment 2019, 691, 848–857. [CrossRef]
- Xu, B.; Liu, F.; Cryder, Z.; Huang, D.; Lu, Z.; He, Y.; Wang, H.; Lu, Z.; Brookes, P.C.; Tang, C.; et al. Microplastics in the Soil Environment: Occurrence, Risks, Interactions and Fate – A Review. Crit Rev Environ Sci Technol 2020, 50, 2175–2222. [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS One 2014, 9, e111913. [CrossRef]
- Schwarz, A.E.; Ligthart, T.N.; Boukris, E.; van Harmelen, T. Sources, Transport, and Accumulation of Different Types of Plastic Litter in Aquatic Environments: A Review Study. Mar Pollut Bull 2019, 143, 92–100. [CrossRef]
- Bouwmeester, H.; Hollman, P.C.H.; Peters, R.J.B. Potential Health Impact of Environmentally Released Micro- and Nanoplastics in the Human Food Production Chain: Experiences from Nanotoxicology. Environ Sci Technol 2015, 49, 8932–8947. [CrossRef]
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environmental Pollution 2018, 234, 115–126. [CrossRef]
- Chen, G.; Feng, Q.; Wang, J. Mini-Review of Microplastics in the Atmosphere and Their Risks to Humans. Science of The Total Environment 2020, 703, 135504. [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric Microplastic Deposition in an Urban Environment and an Evaluation of Transport. Environ Int 2020, 136, 105411. [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and Potential Risk Assessment of Suspended Atmospheric Microplastics in Shanghai. Science of The Total Environment 2019, 675, 462–471. [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric Transport and Deposition of Microplastics in a Remote Mountain Catchment. Nat Geosci 2019, 12, 339–344. [CrossRef]
- Zhang, Q.; Zhao, Y.; Du, F.; Cai, H.; Wang, G.; Shi, H. Microplastic Fallout in Different Indoor Environments. Environ Sci Technol 2020, 54, 6530–6539. [CrossRef]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating Human Exposure to Indoor Airborne Microplastics Using a Breathing Thermal Manikin. Sci Rep 2019, 9, 8670. [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E. V. Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS One 2018, 13, e0194970. [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It In? Curr Opin Environ Sci Health 2018, 1, 1–5. [CrossRef]
- Enyoh, C.E.; Shafea, L.; Verla, A.W.; Verla, E.N.; Qingyue, W.; Chowdhury, T.; Paredes, M. Microplastics Exposure Routes and Toxicity Studies to Ecosystems: An Overview. Environ Anal Health Toxicol 2020, 35, e2020004. [CrossRef]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [CrossRef]
- Amereh, F.; Babaei, M.; Eslami, A.; Fazelipour, S.; Rafiee, M. The Emerging Risk of Exposure to Nano(Micro)Plastics on Endocrine Disturbance and Reproductive Toxicity: From a Hypothetical Scenario to a Global Public Health Challenge. Environmental Pollution 2020, 261, 114158. [CrossRef]
- Lim, X. Microplastics Are Everywhere — but Are They Harmful? Nature 2021, 593, 22–25. [CrossRef]
- Wagner, S.; Reemtsma, T. Things We Know and Don’t Know about Nanoplastic in the Environment. Nat Nanotechnol 2019, 14, 300–301. [CrossRef]
- Guerrera, M.C.; Aragona, M.; Porcino, C.; Fazio, F.; Laurà, R.; Levanti, M.; Montalbano, G.; Germanà, G.; Abbate, F.; Germanà, A. Micro and Nano Plastics Distribution in Fish as Model Organisms: Histopathology, Blood Response and Bioaccumulation in Different Organs. Applied Sciences 2021, 11, 5768. [CrossRef]
- Sökmen, T.Ö.; Sulukan, E.; Türkoğlu, M.; Baran, A.; Özkaraca, M.; Ceyhun, S.B. Polystyrene Nanoplastics (20 Nm) Are Able to Bioaccumulate and Cause Oxidative DNA Damages in the Brain Tissue of Zebrafish Embryo (Danio Rerio). Neurotoxicology 2020, 77, 51–59. [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J Hazard Mater 2018, 344, 179–199. [CrossRef]
- Barrows, A.P.W.; Cathey, S.E.; Petersen, C.W. Marine Environment Microfiber Contamination: Global Patterns and the Diversity of Microparticle Origins. Environmental Pollution 2018, 237, 275–284. [CrossRef]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic Microfibers in the Marine Environment: A Review on Their Occurrence in Seawater and Sediments. Mar Pollut Bull 2018, 127, 365–376. [CrossRef]
- Woods, M.N.; Stack, M.E.; Fields, D.M.; Shaw, S.D.; Matrai, P.A. Microplastic Fiber Uptake, Ingestion, and Egestion Rates in the Blue Mussel (Mytilus Edulis). Mar Pollut Bull 2018, 137, 638–645. [CrossRef]
- Rebelein, A.; Int-Veen, I.; Kammann, U.; Scharsack, J.P. Microplastic Fibers — Underestimated Threat to Aquatic Organisms? Science of The Total Environment 2021, 777, 146045. [CrossRef]
- Banerjee, A.; Shelver, W.L. Micro- and Nanoplastic Induced Cellular Toxicity in Mammals: A Review. Science of The Total Environment 2021, 755, 142518. [CrossRef]
- Bhattacharjee, S.; Ershov, D.; Islam, M.A.; Kämpfer, A.M.; Maslowska, K.A.; van der Gucht, J.; Alink, G.M.; Marcelis, A.T.M.; Zuilhof, H.; Rietjens, I.M.C.M. Role of Membrane Disturbance and Oxidative Stress in the Mode of Action Underlying the Toxicity of Differently Charged Polystyrene Nanoparticles. RSC Adv. 2014, 4, 19321–19330. [CrossRef]
- Feng, L.-J.; Li, J.-W.; Xu, E.G.; Sun, X.-D.; Zhu, F.-P.; Ding, Z.; Tian, H.; Dong, S.-S.; Xia, P.-F.; Yuan, X.-Z. Short-Term Exposure to Positively Charged Polystyrene Nanoparticles Causes Oxidative Stress and Membrane Destruction in Cyanobacteria. Environ Sci Nano 2019, 6, 3072–3079. [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int J Nanomedicine 2012, 5577. [CrossRef]
- Hollóczki, O.; Gehrke, S. Can Nanoplastics Alter Cell Membranes? ChemPhysChem 2020, 21, 9–12. [CrossRef]
- Wang, F.; Salvati, A.; Boya, P. Lysosome-Dependent Cell Death and Deregulated Autophagy Induced by Amine-Modified Polystyrene Nanoparticles. Open Biol 2018, 8, 170271. [CrossRef]
- Yong, C.; Valiyaveettil, S.; Tang, B. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int J Environ Res Public Health 2020, 17, 1509. [CrossRef]
- Rubio, L.; Marcos, R.; Hernández, A. Potential Adverse Health Effects of Ingested Micro- and Nanoplastics on Humans. Lessons Learned from in Vivo and in Vitro Mammalian Models. Journal of Toxicology and Environmental Health, Part B 2020, 23, 51–68. [CrossRef]
- Zheng, T.; Yuan, D.; Liu, C. Molecular Toxicity of Nanoplastics Involving in Oxidative Stress and Desoxyribonucleic Acid Damage. Journal of Molecular Recognition 2019, 32. [CrossRef]
- Liu, X.; Tian, X.; Xu, X.; Lu, J. Design of a Phosphinate-Based Bioluminescent Probe for Superoxide Radical Anion Imaging in Living Cells. Luminescence 2018, 33, 1101–1106. [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential Toxicity of Polystyrene Microplastic Particles. Sci Rep 2020, 10, 7391. [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and Importance of Microplastics in the Marine Environment: A Review of the Sources, Fate, Effects, and Potential Solutions. Environ Int 2017, 102, 165–176. [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in Freshwater and Terrestrial Environments: Evaluating the Current Understanding to Identify the Knowledge Gaps and Future Research Priorities. Science of The Total Environment 2017, 586, 127–141. [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ Sci Technol 2019, 53, 7068–7074. [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded Milks – Are They Immune from Microplastics Contamination? Science of The Total Environment 2020, 714, 136823. [CrossRef]
- Paul, M.B.; Stock, V.; Cara-Carmona, J.; Lisicki, E.; Shopova, S.; Fessard, V.; Braeuning, A.; Sieg, H.; Böhmert, L. Micro- and Nanoplastics – Current State of Knowledge with the Focus on Oral Uptake and Toxicity. Nanoscale Adv 2020, 2, 4350–4367. [CrossRef]
- Liebezeit, G.; Liebezeit, E. Non-Pollen Particulates in Honey and Sugar. Food Additives & Contaminants: Part A 2013, 30, 2136–2140. [CrossRef]
- Liebezeit, G.; Liebezeit, E. Synthetic Particles as Contaminants in German Beers. Food Additives & Contaminants: Part A 2014, 31, 1574–1578. [CrossRef]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ Sci Technol 2015, 49, 13622–13627. [CrossRef]
- Bai, Z.; Wang, N.; Wang, M. Effects of Microplastics on Marine Copepods. Ecotoxicol Environ Saf 2021, 217, 112243. [CrossRef]
- Hasan, J.; Islam, S.M.M.; Alam, M.S.; Johnson, D.; Belton, B.; Hossain, M.A.R.; Shahjahan, M. Presence of Microplastics in Two Common Dried Marine Fish Species from Bangladesh. Mar Pollut Bull 2022, 176, 113430. [CrossRef]
- Ahmadi, A.; Moore, F.; Keshavarzi, B.; Soltani, N.; Sorooshian, A. Potentially Toxic Elements and Microplastics in Muscle Tissues of Different Marine Species from the Persian Gulf: Levels, Associated Risks, and Trophic Transfer. Mar Pollut Bull 2022, 175, 113283. [CrossRef]
- Novotna, K.; Cermakova, L.; Pivokonska, L.; Cajthaml, T.; Pivokonsky, M. Microplastics in Drinking Water Treatment – Current Knowledge and Research Needs. Science of The Total Environment 2019, 667, 730–740. [CrossRef]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic Contamination of Drinking Water: A Systematic Review. PLoS One 2020, 15, e0236838. [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann Intern Med 2019, 171, 453–457. [CrossRef]
- van Raamsdonk, L.W.D.; van der Zande, M.; Koelmans, A.A.; Hoogenboom, R.L.A.P.; Peters, R.J.B.; Groot, M.J.; Peijnenburg, A.A.C.M.; Weesepoel, Y.J.A. Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain. Foods 2020, 9, 72. [CrossRef]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and Fate of Dietary Nanoparticles and Microparticles in the Gastrointestinal Tract. J Autoimmun 2010, 34, J226–J233. [CrossRef]
- Coméra, C.; Cartier, C.; Gaultier, E.; Catrice, O.; Panouille, Q.; El Hamdi, S.; Tirez, K.; Nelissen, I.; Théodorou, V.; Houdeau, E. Jejunal Villus Absorption and Paracellular Tight Junction Permeability Are Major Routes for Early Intestinal Uptake of Food-Grade TiO2 Particles: An in Vivo and Ex Vivo Study in Mice. Part Fibre Toxicol 2020, 17, 26. [CrossRef]
- Smith, M.; Thomas, N.; Jenkins, P.; Miller, N.; Cremaschi, D.; Porta, C. Selective Transport of Microparticles across Peyer’s Patch Follicle-associated M Cells from Mice and Rats. Exp Physiol 1995, 80, 735–743. [CrossRef]
- Galloway, T.S. Micro- and Nano-Plastics and Human Health. In Marine Anthropogenic Litter; Springer International Publishing: Cham, 2015; pp. 343–366.
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning Microplastics into Nanoplastics through Digestive Fragmentation by Antarctic Krill. Nat Commun 2018, 9, 1001. [CrossRef]
- Ma, C.; Li, L.; Chen, Q.; Lee, J.-S.; Gong, J.; Shi, H. Application of Internal Persistent Fluorescent Fibers in Tracking Microplastics in Vivo Processes in Aquatic Organisms. J Hazard Mater 2021, 401, 123336. [CrossRef]
- Volkheimer, G. Hematogenous Dissemination of Ingested Polyvinyl Chloride Particles. Ann N Y Acad Sci 1975, 246, 164–171. [CrossRef]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.M.; Tromp, P.; Helsper, J.P.F.G.; van der Zande, M.; Rietjens, I.M.C.M.; Bouwmeester, H. Translocation of Differently Sized and Charged Polystyrene Nanoparticles in in Vitro Intestinal Cell Models of Increasing Complexity. Nanotoxicology 2015, 9, 453–461. [CrossRef]
- Hu, L.; Zhou, Y.; Wang, Y.; Zhang, D.; Pan, X. Transfer of Micro(Nano)Plastics in Animals: A Mini-Review and Future Research Recommendation. Journal of Hazardous Materials Advances 2022, 7, 100101. [CrossRef]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.M.; Helsdingen, R.; van der Zande, M.; Rietjens, I.M.C.M.; Bouwmeester, H. In Vitro Gastrointestinal Digestion Increases the Translocation of Polystyrene Nanoparticles in an in Vitro Intestinal Co-Culture Model. Nanotoxicology 2015, 9, 886–894. [CrossRef]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and Health Hazard Ranking and Assessment of Plastic Polymers Based on Chemical Composition. Science of The Total Environment 2011, 409, 3309–3324. [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic Particles Cause Intestinal Damage and Other Adverse Effects in Zebrafish Danio Rerio and Nematode Caenorhabditis Elegans. Science of The Total Environment 2018, 619–620, 1–8. [CrossRef]
- Wang, Y.; Mao, Z.; Zhang, M.; Ding, G.; Sun, J.; Du, M.; Liu, Q.; Cong, Y.; Jin, F.; Zhang, W.; et al. The Uptake and Elimination of Polystyrene Microplastics by the Brine Shrimp, Artemia Parthenogenetica, and Its Impact on Its Feeding Behavior and Intestinal Histology. Chemosphere 2019, 234, 123–131. [CrossRef]
- Jiang, X.; Chang, Y.; Zhang, T.; Qiao, Y.; Klobučar, G.; Li, M. Toxicological Effects of Polystyrene Microplastics on Earthworm (Eisenia Fetida). Environ Pollut 2020, 259, 113896. [CrossRef]
- Huang, J.-N.; Wen, B.; Zhu, J.-G.; Zhang, Y.-S.; Gao, J.-Z.; Chen, Z.-Z. Exposure to Microplastics Impairs Digestive Performance, Stimulates Immune Response and Induces Microbiota Dysbiosis in the Gut of Juvenile Guppy (Poecilia Reticulata). Science of The Total Environment 2020, 733, 138929. [CrossRef]
- Gu, H.; Wang, S.; Wang, X.; Yu, X.; Hu, M.; Huang, W.; Wang, Y. Nanoplastics Impair the Intestinal Health of the Juvenile Large Yellow Croaker Larimichthys Crocea. J Hazard Mater 2020, 397, 122773. [CrossRef]
- Kang, H.-M.; Byeon, E.; Jeong, H.; Kim, M.-S.; Chen, Q.; Lee, J.-S. Different Effects of Nano- and Microplastics on Oxidative Status and Gut Microbiota in the Marine Medaka Oryzias Melastigma. J Hazard Mater 2021, 405, 124207. [CrossRef]
- Ahrendt, C.; Perez-Venegas, D.J.; Urbina, M.; Gonzalez, C.; Echeveste, P.; Aldana, M.; Pulgar, J.; Galbán-Malagón, C. Microplastic Ingestion Cause Intestinal Lesions in the Intertidal Fish Girella Laevifrons. Mar Pollut Bull 2020, 151, 110795. [CrossRef]
- de Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the Effects of Microplastics on Aquatic Organisms: What Do We Know and Where Should We Focus Our Efforts in the Future? Science of The Total Environment 2018, 645, 1029–1039. [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics Induce Intestinal Inflammation, Oxidative Stress, and Disorders of Metabolome and Microbiome in Zebrafish. Science of The Total Environment 2019, 662, 246–253. [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of Different Shapes of Microplastics Initiates Intestinal Injury and Gut Microbiota Dysbiosis in the Gut of Zebrafish. Chemosphere 2019, 236, 124334. [CrossRef]
- Pedà, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal Alterations in European Sea Bass Dicentrarchus Labrax (Linnaeus, 1758) Exposed to Microplastics: Preliminary Results. Environmental Pollution 2016, 212, 251–256. [CrossRef]
- Limonta, G.; Mancia, A.; Benkhalqui, A.; Bertolucci, C.; Abelli, L.; Fossi, M.C.; Panti, C. Microplastics Induce Transcriptional Changes, Immune Response and Behavioral Alterations in Adult Zebrafish. Sci Rep 2019, 9, 15775. [CrossRef]
- Abdelkhaliq, A.; van der Zande, M.; Punt, A.; Helsdingen, R.; Boeren, S.; Vervoort, J.J.M.; Rietjens, I.M.C.M.; Bouwmeester, H. Impact of Nanoparticle Surface Functionalization on the Protein Corona and Cellular Adhesion, Uptake and Transport. J Nanobiotechnology 2018, 16, 70. [CrossRef]
- Magrì, D.; Sánchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P.; et al. Laser Ablation as a Versatile Tool To Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment. ACS Nano 2018, 12, 7690–7700. [CrossRef]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Büchel, C.; et al. Multi-Endpoint Toxicological Assessment of Polystyrene Nano- and Microparticles in Different Biological Models in Vitro. Toxicology in Vitro 2019, 61, 104610. [CrossRef]
- Huang, W.; Yin, H.; Yang, Y.; Jin, L.; Lu, G.; Dang, Z. Influence of the Co-Exposure of Microplastics and Tetrabromobisphenol A on Human Gut: Simulation in Vitro with Human Cell Caco-2 and Gut Microbiota. Science of The Total Environment 2021, 778, 146264. [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and Effects of Orally Ingested Polystyrene Microplastic Particles in Vitro and in Vivo. Arch Toxicol 2019, 93, 1817–1833. [CrossRef]
- Mattioda, V.; Benedetti, V.; Tessarolo, C.; Oberto, F.; Favole, A.; Gallo, M.; Martelli, W.; Crescio, M.I.; Berio, E.; Masoero, L.; et al. Pro-Inflammatory and Cytotoxic Effects of Polystyrene Microplastics on Human and Murine Intestinal Cell Lines. Biomolecules 2023, 13, 140. [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Science of The Total Environment 2019, 649, 308–317. [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene Microplastics Induce Gut Microbiota Dysbiosis and Hepatic Lipid Metabolism Disorder in Mice. Science of The Total Environment 2018, 631–632, 449–458. [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene Microplastics Affect the Distribution of Gut Microbiota and Inflammation Development in Mice. Chemosphere 2020, 244, 125492. [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics Release Phthalate Esters and Cause Aggravated Adverse Effects in the Mouse Gut. Environ Int 2020, 143, 105916. [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated Health Risks: Polystyrene Micro- and Nanoplastics Jointly Induce Intestinal Barrier Dysfunction by ROS-Mediated Epithelial Cell Apoptosis. Part Fibre Toxicol 2021, 18, 20. [CrossRef]
- Park, S.B.; Jung, W.H.; Choi, K.J.; Koh, B.; Kim, K.Y. A Comparative Systematic Analysis of The Influence of Microplastics on Colon Cells, Mouse and Colon Organoids. Tissue Eng Regen Med 2023, 20, 49–58. [CrossRef]
- Lehner, R.; Wohlleben, W.; Septiadi, D.; Landsiedel, R.; Petri-Fink, A.; Rothen-Rutishauser, B. A Novel 3D Intestine Barrier Model to Study the Immune Response upon Exposure to Microplastics. Arch Toxicol 2020, 94, 2463–2479. [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An Assessment of the Toxicity of Polypropylene Microplastics in Human Derived Cells. Sci Total Environ 2019, 684, 657–669. [CrossRef]
- Park, E.-J.; Han, J.-S.; Park, E.-J.; Seong, E.; Lee, G.-H.; Kim, D.-W.; Son, H.-Y.; Han, H.-Y.; Lee, B.-S. Repeated-Oral Dose Toxicity of Polyethylene Microplastics and the Possible Implications on Reproduction and Development of the next Generation. Toxicol Lett 2020, 324, 75–85. [CrossRef]
- Ma, J.; Wan, Y.; Song, L.; Wang, L.; Wang, H.; Li, Y.; Huang, D. Polystyrene Nanobeads Exacerbate Chronic Colitis in Mice Involving in Oxidative Stress and Hepatic Lipid Metabolism. Part Fibre Toxicol 2023, 20, 49. [CrossRef]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ Sci Technol 2019, 53, 10978–10992. [CrossRef]
- Jiang, P.; Yuan, G.; Jiang, B.; Zhang, J.; Wang, Y.; Lv, H.; Zhang, Z.; Wu, J.; Wu, Q.; Li, L. Effects of Microplastics (MPs) and Tributyltin (TBT) Alone and in Combination on Bile Acids and Gut Microbiota Crosstalk in Mice. Ecotoxicol Environ Saf 2021, 220, 112345. [CrossRef]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of Gut Microbiota Plays an Important Role in Micro/Nanoplastics-Induced Gut Barrier Dysfunction. Nanoscale 2021, 13, 8806–8816. [CrossRef]
- Liu, J.; Lv, M.; Sun, A.; Ding, J.; Wang, Y.; Chang, X.; Chen, L. Exposure to Microplastics Reduces the Bioaccumulation of Sulfamethoxazole but Enhances Its Effects on Gut Microbiota and the Antibiotic Resistome of Mice. Chemosphere 2022, 294, 133810. [CrossRef]
- Montano, D. Chemical and Biological Work-Related Risks across Occupations in Europe: A Review. Journal of Occupational Medicine and Toxicology 2014, 9, 28. [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J Hazard Mater 2018, 344, 179–199. [CrossRef]
- Lyche, J.L., 2011. C. 48-Phthalates.I.G.R.C. (Ed. ), R. and D.Toxicology.A.P.S.Diego. .
- Eales, J.; Bethel, A.; Galloway, T.; Hopkinson, P.; Morrissey, K.; Short, R.E.; Garside, R. Human Health Impacts of Exposure to Phthalate Plasticizers: An Overview of Reviews. Environ Int 2022, 158, 106903. [CrossRef]
- Cirillo, T.; Latini, G.; Castaldi, M.A.; Dipaola, L.; Fasano, E.; Esposito, F.; Scognamiglio, G.; Francesco, F. Di; Cobellis, L. Exposure to Di-2-Ethylhexyl Phthalate, Di- N -Butyl Phthalate and Bisphenol A through Infant Formulas. J Agric Food Chem 2015, 63, 3303–3310. [CrossRef]
- Kıralan, S.S.; Toptancı, İ.; Öncül Abacıgil, T.; Ramadan, M.F. Phthalates Levels in Olive Oils and Olive Pomace Oils Marketed in Turkey. Food Additives & Contaminants: Part A 2020, 37, 1332–1338. [CrossRef]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and Diet: A Review of the Food Monitoring and Epidemiology Data. Environmental Health 2014, 13, 43. [CrossRef]
- Zhang, Y.-J.; Guo, J.-L.; Xue, J.; Bai, C.-L.; Guo, Y. Phthalate Metabolites: Characterization, Toxicities, Global Distribution, and Exposure Assessment. Environmental Pollution 2021, 291, 118106. [CrossRef]
- Polinski, K.J.; Dabelea, D.; Hamman, R.F.; Adgate, J.L.; Calafat, A.M.; Ye, X.; Starling, A.P. Distribution and Predictors of Urinary Concentrations of Phthalate Metabolites and Phenols among Pregnant Women in the Healthy Start Study. Environ Res 2018, 162, 308–317. [CrossRef]
- Wang, G.; Chen, Q.; Tian, P.; Wang, L.; Li, X.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W. Gut Microbiota Dysbiosis Might Be Responsible to Different Toxicity Caused by Di-(2-Ethylhexyl) Phthalate Exposure in Murine Rodents. Environmental Pollution 2020, 261, 114164. [CrossRef]
- Yu, Z.; Shi, Z.; Zheng, Z.; Han, J.; Yang, W.; Lu, R.; Lin, W.; Zheng, Y.; Nie, D.; Chen, G. DEHP Induce Cholesterol Imbalance via Disturbing Bile Acid Metabolism by Altering the Composition of Gut Microbiota in Rats. Chemosphere 2021, 263, 127959. [CrossRef]
- Fu, X.; Han, H.; Li, Y.; Xu, B.; Dai, W.; Zhang, Y.; Zhou, F.; Ma, H.; Pei, X. Di-(2-ethylhexyl) Phthalate Exposure Induces Female Reproductive Toxicity and Alters the Intestinal Microbiota Community Structure and Fecal Metabolite Profile in Mice. Environ Toxicol 2021, 36, 1226–1242. [CrossRef]
- Lei, M.; Menon, R.; Manteiga, S.; Alden, N.; Hunt, C.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Environmental Chemical Diethylhexyl Phthalate Alters Intestinal Microbiota Community Structure and Metabolite Profile in Mice. mSystems 2019, 4. [CrossRef]
- Chiu, K.; Bashir, S.T.; Nowak, R.A.; Mei, W.; Flaws, J.A. Subacute Exposure to Di-Isononyl Phthalate Alters the Morphology, Endocrine Function, and Immune System in the Colon of Adult Female Mice. Sci Rep 2020, 10, 18788. [CrossRef]
- Xiong, Z. e; Zeng, Y.; Zhou, J.; Shu, R.; Xie, X.; Fu, Z. Exposure to Dibutyl Phthalate Impairs Lipid Metabolism and Causes Inflammation via Disturbing Microbiota-Related Gut&Ndash;Liver Axis. Acta Biochim Biophys Sin (Shanghai) 2020, 52, 1382–1393. [CrossRef]
- Fu, X.; Han, H.; Li, Y.; Xu, B.; Dai, W.; Zhang, Y.; Zhou, F.; Ma, H.; Pei, X. Di-(2-ethylhexyl) Phthalate Exposure Induces Female Reproductive Toxicity and Alters the Intestinal Microbiota Community Structure and Fecal Metabolite Profile in Mice. Environ Toxicol 2021, 36, 1226–1242. [CrossRef]
- Goyal, S.P.; Saravanan, C. An Insight into the Critical Role of Gut Microbiota in Triggering the Phthalate-Induced Toxicity and Its Mitigation Using Probiotics. Science of The Total Environment 2023, 904, 166889. [CrossRef]
- Dekant, W. Grouping of Phthalates for Risk Characterization of Human Exposures. Toxicol Lett 2020, 330, 1–6. [CrossRef]
- Zoeller, R.T.; Birnbaum, L.S.; Collins, T.J.; Heindel, J.; Hunt, P.A.; Iguchi, T.; Kortenkamp, A.; Myers, J.P.; Vom Saal, F.S.; Sonnenschein, C.; et al. European Medicines Agency Conflicts With the European Food Safety Authority (EFSA) on Bisphenol A Regulation. J Endocr Soc 2023, 7, bvad107. [CrossRef]
- Liao, Y.; Yang, J. Microplastic Serves as a Potential Vector for Cr in an In-Vitro Human Digestive Model. Science of The Total Environment 2020, 703, 134805. [CrossRef]
- Banerjee, A.; Shelver, W.L. Micro- and Nanoplastic Induced Cellular Toxicity in Mammals: A Review. Science of The Total Environment 2021, 755, 142518. [CrossRef]
- Nickens, K.P.; Patierno, S.R.; Ceryak, S. Chromium Genotoxicity: A Double-Edged Sword. Chem Biol Interact 2010, 188, 276–288. [CrossRef]
- Ahmad, R.; Sorrell, M.F.; Batra, S.K.; Dhawan, P.; Singh, A.B. Gut Permeability and Mucosal Inflammation: Bad, Good or Context Dependent. Mucosal Immunol 2017, 10, 307–317. [CrossRef]
- Sadler, D.E.; Brunner, F.S.; Plaistow, S.J. Temperature and Clone-Dependent Effects of Microplastics on Immunity and Life History in Daphnia Magna. Environmental Pollution 2019, 255, 113178. [CrossRef]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of Nanoplastics on Mytilus Galloprovincialis after Individual and Combined Exposure with Carbamazepine. Science of The Total Environment 2018, 643, 775–784. [CrossRef]
- Auguste, M.; Balbi, T.; Ciacci, C.; Canonico, B.; Papa, S.; Borello, A.; Vezzulli, L.; Canesi, L. Shift in Immune Parameters After Repeated Exposure to Nanoplastics in the Marine Bivalve Mytilus. Front Immunol 2020, 11. [CrossRef]
- Auguste, M.; Lasa, A.; Balbi, T.; Pallavicini, A.; Vezzulli, L.; Canesi, L. Impact of Nanoplastics on Hemolymph Immune Parameters and Microbiota Composition in Mytilus Galloprovincialis. Mar Environ Res 2020, 159, 105017. [CrossRef]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.-L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of Marine Mussels Mytilus Spp. to Polystyrene Microplastics: Toxicity and Influence on Fluoranthene Bioaccumulation. Environmental Pollution 2016, 216, 724–737. [CrossRef]
- Murano, C.; Agnisola, C.; Caramiello, D.; Castellano, I.; Casotti, R.; Corsi, I.; Palumbo, A. How Sea Urchins Face Microplastics: Uptake, Tissue Distribution and Immune System Response. Environmental Pollution 2020, 264, 114685. [CrossRef]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Chen, M.; Zhao, Y. Effects of Microplastics on the Innate Immunity and Intestinal Microflora of Juvenile Eriocheir Sinensis. Science of The Total Environment 2019, 685, 836–846. [CrossRef]
- Shi, W.; Han, Y.; Sun, S.; Tang, Y.; Zhou, W.; Du, X.; Liu, G. Immunotoxicities of Microplastics and Sertraline, Alone and in Combination, to a Bivalve Species: Size-Dependent Interaction and Potential Toxication Mechanism. J Hazard Mater 2020, 396, 122603. [CrossRef]
- Tang, Y.; Rong, J.; Guan, X.; Zha, S.; Shi, W.; Han, Y.; Du, X.; Wu, F.; Huang, W.; Liu, G. Immunotoxicity of Microplastics and Two Persistent Organic Pollutants Alone or in Combination to a Bivalve Species. Environmental Pollution 2020, 258, 113845. [CrossRef]
- Greven, A.; Merk, T.; Karagöz, F.; Mohr, K.; Klapper, M.; Jovanović, B.; Palić, D. Polycarbonate and Polystyrene Nanoplastic Particles Act as Stressors to the Innate Immune System of Fathead Minnow ( Pimephales Promelas ). Environ Toxicol Chem 2016, 35, 3093–3100. [CrossRef]
- Banaee, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Haghi, B.N.; Akhlaghi, M.; Derikvandy, A. Evaluation of Single and Combined Effects of Cadmium and Micro-Plastic Particles on Biochemical and Immunological Parameters of Common Carp (Cyprinus Carpio). Chemosphere 2019, 236, 124335. [CrossRef]
- Mancia, A.; Chenet, T.; Bono, G.; Geraci, M.L.; Vaccaro, C.; Munari, C.; Mistri, M.; Cavazzini, A.; Pasti, L. Adverse Effects of Plastic Ingestion on the Mediterranean Small-Spotted Catshark (Scyliorhinus Canicula). Mar Environ Res 2020, 155, 104876. [CrossRef]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ Sci Technol 2022, 56, 414–421. [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut Biogeography of the Bacterial Microbiota. Nat Rev Microbiol 2016, 14, 20–32. [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European Consensus Conference on Faecal Microbiota Transplantation in Clinical Practice. Gut 2017, 66, 569–580. [CrossRef]
- Urbanek, A.K.; Rymowicz, W.; Mirończuk, A.M. Degradation of Plastics and Plastic-Degrading Bacteria in Cold Marine Habitats. Appl Microbiol Biotechnol 2018, 102, 7669–7678. [CrossRef]
- Yeom, S.-J.; Le, T.-K.; Yun, C.-H. P450-Driven Plastic-Degrading Synthetic Bacteria. Trends Biotechnol 2022, 40, 166–179. [CrossRef]
- Gambarini, V.; Pantos, O.; Kingsbury, J.M.; Weaver, L.; Handley, K.M.; Lear, G. Phylogenetic Distribution of Plastic-Degrading Microorganisms. mSystems 2021, 6. [CrossRef]
- García-Depraect, O.; Bordel, S.; Lebrero, R.; Santos-Beneit, F.; Börner, R.A.; Börner, T.; Muñoz, R. Inspired by Nature: Microbial Production, Degradation and Valorization of Biodegradable Bioplastics for Life-Cycle-Engineered Products. Biotechnol Adv 2021, 53, 107772. [CrossRef]
- Maity, W.; Maity, S.; Bera, S.; Roy, A. Emerging Roles of PETase and MHETase in the Biodegradation of Plastic Wastes. Appl Biochem Biotechnol 2021, 193, 2699–2716. [CrossRef]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic Biodegradation: Frontline Microbes and Their Enzymes. Science of The Total Environment 2021, 759, 143536. [CrossRef]
- Jacquin, J.; Cheng, J.; Odobel, C.; Pandin, C.; Conan, P.; Pujo-Pay, M.; Barbe, V.; Meistertzheim, A.-L.; Ghiglione, J.-F. Microbial Ecotoxicology of Marine Plastic Debris: A Review on Colonization and Biodegradation by the “Plastisphere.” Front Microbiol 2019, 10. [CrossRef]
- Lear, G.; Kingsbury, J.M.; Franchini, S.; Gambarini, V.; Maday, S.D.M.; Wallbank, J.A.; Weaver, L.; Pantos, O. Plastics and the Microbiome: Impacts and Solutions. Environ Microbiome 2021, 16, 2. [CrossRef]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of Polyethylene Microplastic Particles by the Fungus Aspergillus Flavus from the Guts of Wax Moth Galleria Mellonella. Science of The Total Environment 2020, 704, 135931. [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.-M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 2. Role of Gut Microorganisms. Environ Sci Technol 2015, 49, 12087–12093. [CrossRef]
- Plaza Oñate, F.; Le Chatelier, E.; Almeida, M.; Cervino, A.C.L.; Gauthier, F.; Magoulès, F.; Ehrlich, S.D.; Pichaud, M. MSPminer: Abundance-Based Reconstitution of Microbial Pan-Genomes from Shotgun Metagenomic Data. Bioinformatics 2019, 35, 1544–1552. [CrossRef]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig Dis Sci 2020, 65, 695–705. [CrossRef]
| MOLECULES | SPECIES | DESIGN | SIZE/EXPOSURE | EFFECTS | STUDY |
|---|---|---|---|---|---|
| Nanoplastics | Human | Human colon adenocarcinoma Caco-2 cell, in vitro design | PS particles between 50 and 200 nm at a concentration of 250 mg/mL for 10 to 120 minutes | Absence of cellular toxicity | Abdelkhaliq 2018 [88] |
| Nanoplastics | Human | Human colon adenocarcinoma Caco-2 cell, in vitro design | 100 nm PE terephthalate particles at a concentration between 1 and 30 mg/ml for an incubation time of 24 hours | No evidence of increased inflammatory factors | Magrì 2018 [89] |
| Nanoplastics, Microplastics | Human | Human Caco-2 and HT29-MTX-E12 cells, in vitro design | 50 nm and 0.5 μm COOH-modified PS particles, concentration (0,01 μg/mL – 100 μg/mL) for an incubation time of 24 hours | Absence of cellular toxicity | Hesler 2019 [90] |
| Microplastics | Human | Human Caco-2 cells and gut microbiota, in vitro design | PE microplastics between 30 and 140 um tested at various concentrations for 48 hours | Significant reduction in Caco-2 cell viability, only for high concentrations (1000 mg/L) | Huang 2021 [91] |
| Microplastics | Human, Mice | -Human colon adenocarcinoma Caco-2 cell, in vitro design -Male reporter gene mice, in vivo design |
-4 μm and 10 μm PS particles, variable concentration for an incubation time of 48 hours -Mixture of 1 µm to 10 µm PS microplastics at a volume of 10 ml/kg and a total of one dose for 3 weeks |
-Reduction in cell vitality for high concentrations (1x108 particles/mL), no effect on cell polarization -Absence of histologically detectable lesions and inflammatory responses |
Stock 2019 [92] |
| Microplastics | Human | HRT-18 and CMT-93 epithelial human cell lines, in vitro design |
PS microparticles of 4.8-5.8 µm for a concentration of 1mg/ml and a time between 6 and 48 hours | Significant cytotoxicity in both cell lines Oxidative stress activity was increased only in CMT-93 cells |
Mattioda 2023 [93] |
| Microplastics | Mice | IRC mice divided into control and exposed group, in vivo design | PS microparticles of 5 µm for a concentration of 100 and 1000 µg/L for six weeks | Reduced mucus production and damage to the intestinal barrier Decreased Actinobacteria content and altered microbial alpha diversity. At the genus level, a total of 15 types of bacteria changed significantly |
Jin 2019 [94] |
| Microplastics | Mice | Male mice exposed to two different MP sizes, in vivo design | Oral exposure to 1000 μg/L of 0.5 and 50 μm PS-MP, for five weeks | Decreased mucus secretion in the intestine in both sizes of treated groups Decreased relative abundance of Firmicutes and α-Proteobacteria in the feces. Significant changes in the richness and diversity of the caecal intestinal microbiota |
Lu 2018 [95] |
| Microplastics | Mice | SPF grade C57BL/6 male mice were divided into four groups, in vivo design | Exposure to different amounts of PE microplastics between 10 and 150 μm (6, 60, and 600 μg/day for 5 consecutive weeks) | Induction of histologic inflammation in the colon and duodenum (a higher expression of TLR4, AP-1 and IRF5) Changes of IL1α and granulocyte colony-stimulating factor (G-CSF) in the blood, decrease in the count of regulatory T-lymphocytes, and an increase in the proportion of Th17 cells in the spleen Increased number of intestinal microbial species, bacterial abundance and diversity of flora. Significant increase in Staphylococcus abundance along with a significant decrease in Parabacteroides abundance. |
Li 2020 [96] |
|
Microplastics, Phthalate esters |
Mice | Male mice (Mus musculus CD-1) divided into 12 groups and exposed to MPs and MPs contaminated with phthalate esters; in vivo design | Virgin PE spheres of size between 45 and 53 μm and concentration of 0.2 g/L (about 1.5 × 105 particles/L) for 30 days of exposure | Disruption of intestinal permeability Increased abundance of phylum Actinobacteria and genera Lactobacillus, Adlercreutzia, Butyricimonas and Parabacteroides |
Deng 2020 [97] |
| Microplastics, Nanoplastics | Mice | 6-weeks old C57BL/6 J mice, in vivo design | Combined exposure to PS-NP and PS-MP (50 nm, 500 and 5000 nm, respectively, at a concentration of 20 mL/kg body weight for 28 days) | Gut barrier dysfunction by apoptosis of epithelial cells through ROS production | Liang 2021 [98] |
| Microplastics, Nanoplastics | Human, Mice | -CCD18-Co cells from normal human colon fibroblasts, human colon organoids; in vitro design -Seven-week-old male C57BL/6 mice; in vivo design |
-Exposure to 50- and 100-nm MNPs particles at varying concentrations for 48 hours of incubation -50 nm MNPs at the concentration at which the highest toxicity was found in colonic organoids, for 7 days |
Concentrations of 5 mg/mL induced > 20% decrease in colonic organoid viability and increased expression of genes related to inflammation, apoptosis, and immunity 50 nm MNPs accumulate in various mouse organs, including colon, liver, pancreas, and testes after 7 days of exposure |
Park 2023 [99] |
| Microplastics | Human | 3D in vitro intestinal model comprising human intestinal epithelial cell lines Caco-2 and HT29-MTX-E12 | Exposure to 50–500 µm MP at the concentration of 823.5-1380.0 μg/cm2 for 24 hours | No induction of cytotoxicity nor pro-inflammatory response | Lehner 2020 [100] |
| Microplastics | Human, Mice/Sheep |
Murine and sheep blood and immune cells; human-derived cell lines, in vitro design | Polypropylene MPs (50-500 µm) at various concentrations | Induction of proinflammatory cytokines in a size- and concentration-dependent manner | Hwang 2019 [101] |
| Microplastics | Mice | Six-week-old male and female ICR mice, in vivo design | 40−48 μm PE-MPs (0.125, 0.5, 2 mg/day/mouse) by gavage to mice (10 mice/sex/dose) for 90 days | Increase in the number of blood neutrophils and immunoglobulin IgA levels, alteration of spleen lymphocytes | Park 2020 [102] |
| Nanoplastics | Mice | Mice with chronic colitis, in vivo design | 100 nm polystyrene nanospheres (PS-NPs, at concentrations of 1 mg/kg, 5 mg/kg and 25 mg/kg) for 28 consecutive days |
Increase in oxidative stress and intestinal inflammation by activating the MAPK signaling pathway | Ma 2023 [103] |
| Microplastics | Mice | Male and female ICR mice, in vivo design | PS-MPs (0.5 µmand 5 µm) at a concentration of 100 µg/L and1000 µg/L, from the day 1 of gestation to the day of birth |
Abundance of Actinobacteria increased while that of Proteobacteria and Firmicutes remained unchanged | Luo 2019 [104] |
| Microplastics | Mice | Seven-week-old male C57BL/6J mice, in vivo design | Oral exposure of 5um MPs (0.1mg/day), for 33 days | Increased relative abundance of Proteobacteria Decrease in Bacteroides and Marvinbryantia and increase in Bifidobacterium |
Jiang 2021 [105] |
|
Microplastics, Nanoplastics |
Mice | C57/B6 mice (male, 8 weeks old), in vivo design | PS M/NPLs, carboxyl-modified (PS-COOH) and aminomodified (PS-NH2) PS M/NPLs (70 nm, 5 μm in diameter), at a concentration between 2 mg and 0.2 mg/kg, for 28 days | Increased relative abundance of Proteobacteria Increase of Verrucomicrobia at a high concentration Reduced several short-chain fatty acid (SCFA) -producing genera |
Qiao 2021[106] |
| Microplastics | Mice | 4 weeks old female mice (KM mice), in vivo design | PET-MPs (2 μm to 631 μm) at a concentration of 500 mg/kg for 28 days | Decreased abundances of Bacteroidetes and increased that of Firmicutes Increased abundance of Lactobacillus and decreased abundance of Parabacteroides |
Liu 2022 [107] |
| MP: microplastic; NP: nanoplastic; MNPs: micro- and nanoplastics; PS: polystyrene; PE: polyethylene; PET: polyethylene terephthalate; | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





