1. Introduction
Cutaneous Lupus Erythematosus (CLE) is a chronic autoimmune skin disease characterized by various kinds of cutaneous manifestations that can range from mild skin rashes to more severe lesions and can significantly impact the quality of life of affected individuals [
1]. Classically, CLE is divided into different forms:
1) Acute Cutaneous Lupus Erythematosus (ACLE), that typically presents with a malar rash, which is a butterfly-shaped rash across the cheeks and bridge of the nose but that may also involve the scalp, neck, and upper chest [
2]. From a histopathological point of view, ACLE presents interface dermatitis, with vacuolar degeneration of basal keratinocytes, often accompanied by lymphocytic infiltration and a perivascular and periadnexal inflammation, with some degrees of dermal edema and, in some cases, signs of leukocytoclastic vasculitis. Furthermore, epidermal changes such as hyperkeratosis, focal parakeratosis and dyskeratosis can be appreciated. Finally, mucin deposition in the dermis is another potential feature of this form of CLE [
3].
2) Subacute Cutaneous Lupus Erythematosus (SCLE) that is characterized by nonscarring, psoriasiform, or annular lesions predominantly found on sun-exposed areas such as the upper back, shoulders, extensor surfaces of the arms, and neck and that can also present as widespread erythematous plaques [
4]. Histologically, SCLE presents interface dermatitis with basal cell vacuolization and hyperkeratosis with some degree of follicular plugging that is a common finding in SCLE and that contributes to the characteristic scaling and follicular papules seen in SCLE lesions. At the level of dermis, it’s possible to appreciate a dense inflammatory infiltrate around blood vessels (perivascular) and hair follicles (perifollicular) with lymphocytes, histiocytes, and occasionally eosinophils infiltrate these areas, contributing to the inflammatory response. Finally, in some cases of SCLE there are mucin deposition and dermal changes such as edema [
5].
3) Chronic Cutaneous Lupus Erythematosus (CCLE) encompasses several subtypes: Discoid Lupus Erythematosus (DLE) that presents with well-defined, scaly, erythematous plaques often with follicular plugging and atrophy and in which the lesions typically occur on the face, scalp, and ears, but can also affect other areas of the body.
The Hypertrophic variant of CCLE manifests as thickened, hyperkeratotic plaques, particularly on the scalp and other areas subject to trauma or friction while the mucosal Lupus Erythematosus can cause oral or nasal ulcerations and can occur in isolation or concurrently with other CLE subtypes.
Histologically, DLE presents epidermis with hyperkeratosis, variable epidermal atrophy alternating with acanthosis and follicular plugging [
6]. The basement membrane is often thickened and the inflammatory infiltrate has a superficial and deep pattern and frequently involves adnexal structures [
7].
Hypertrophic CLE presents hyperkeratosis, acanthosis, hypergranulosis with inflammatory infiltrate that, although less pronounced than in other variants of cutaneous lupus erythematosus, can be observed in the papillary and reticular dermis. Furthermore, there is almost an absence of interface dermatitis and there is follicular hypertrophy with absence of atrophy [
8].
Tumid lupus erythematosus (TLE) is a rare, chronic relapsing, photosensitive dermatosis of indolent clinical behaviour [
9,
10]. TLE has been traditionally classified under the umbrella definition of chronic cutaneous lupus erythematosus (CCLE) [
11]. CCLE includes other cutaneous manifestations that maybe seen infrequently in association with systemic lupus erythematosus (SLE) including discoid lupus erythematosus (DLE), lupus panniculitis and chilblains lupus erythematosus [
10,
11]. However, since the first use of the term ‘lupus erythematodes tumidus’ in the Berlin dermatological society in 1909 [
12], the classification of TLE and its genuine relation to lupus erythematosus (LE) has remained disputed in the literature [
9,
10,
13,
14,
15]. Indeed, TLE was not present in the classification by Gilliam in the 1970s that contemplated 3 main clinical types: chronic CLE (CCLE), which included discoid lupus erythematosus (DLE) as the most important subtype; subacute CLE (SCLE) and acute CLE (ACLE) [
16]. To complicate matters, a spectrum including TLE with lymphocytic infiltrate of Jessner and reticular erythematous mucinosis (REM) has been postulated [
17]. Anyway, it’s important to remind that TLE, particularly in European literature, has been neglected mainly because it has not always been considered as a separate entity.
In this review article, we discuss the clinicopathological characteristics of TLE with a focus on classification history, related debates, prognosis and therapeutic approaches.
2. Epidemiology
The exact incidence of TLE is unknown but it is less common than DLE [
17]. Informations regarding the prevalence and incidence of TLEare still lacking also for the absence of a specific categorization (code) in International Classification of Diseases (ICD), in which it is included in ICD L93.2 (other local lupus erythematosus, such as Chilblain LE and Lupus Erythematosus Profundus) [
18]. In contrast to CCLE which is more common in females, TLE appears to have equal sex incidence or a slight male predilection [
9,
13]. Age of presentation can be wide but mostly seen in the fourth and fifth decades [
9,
13,
14]. Presentation in children is rare [
19]. In 2013, the European Society of Cutaneous Lupus Erythematosus (EUSCLE) provided clinical data from 1002 CLE patients from 13 European countries and Brazil, and only 65 of these were diagnosed with TLE and a further 41 were diagnosed with TLE together with one or more different CLE subtypes, most commonly ACLE or DLE [
20].
3. Etiopathogenesis
Etiopathogenesis of TLE has not been yet fully elucidated, but is believed to involve complex interplay between genetic, environmental, and immunological factors [
19,
21,
22]. Ultraviolet (UV) radiation is a major triggering factor in most, but not all TLE patients [
15,
23]. In fact, TLE was found to be the most photosensitive type of cutaneous lupus erythematosus [
23]. UV radiation can lead to induction of keratinocytes apoptosis and exposure of autoantigens to circulating antibodies [
24]. Cigarette smoking has been associated with both TLE and DLE [
25]. Multiple drugs have been described to induce TLE such as tumor necrosis factor-alpha (TNF-a) inhibitors, angiotensin-converting-2 enzyme inhibitors, and bortezomib [
26,
27,
28,
29]. TLE has rarely been reported in association with DLE and SLE [
30,
31]. Indeed, TLE appears to be the least form of CCLE to be seen concomitantly with SLE. ANA titers are typically low (≤1:160), and higher titers should raise the concerns of systemic involvement with LE [
31]. Immune dysregulation, however, appears to play an important role in the pathogenesis of TLE. FOXP3+ and CD39+ T-regulatory cell and epidermal Langerhans cells were shown to be decreased in TLE [
24,
32]. Similar to other types of cutaneous lupus erythematosus, plasmacytoid dendritic cells (pDCs) recruitment is believed to be a major factor in the pathogenesis, with the resultant production of Interferon type I (IFN-I). The latter subsequently leads to activation of T-lymphocytes and induction of chemokines and cytokines [
22,
33].
4. Clinical Picture
The characteristic clinical presentation of TLE is that of erythematous edematous urticarial plaques lesions on sun-exposed sites. The lesions may have an annular or arciform configuration. Epidermal changes such as ulceration, scaling, crusting are typically absent and the lesions tend to heal without secondary sequalae but can recur [
21,
31,
34]. As already mentioned, TLE usually interest sun-exposed areas such as the neckline, shoulders, face, and arms but a type of TLE in ‘blaschkoid’ distribution has been reported [
35] and also with periorbital edema, or scalp involvement that appears similar to alopecia areata (AA). Lesions of TLE persist for days or weeks and chronically recur but they have the potential to regress spontaneously; however, patients may report recurrence during the summer months [
34,
35].
Figure 1 presents an example of TLE lesion on the right cheek of a 25-years old female.
5. Histopathology
From a histopathological point of view, usually there are abundant dermal mucin deposition and a superficial and deep perivascular and peri-adnexal lymphocytic infiltrate, with sometimes edema in the papillary dermis. Typically, there is no involvement of the epidermis or dermo-epidermal junction with consequent lack of atrophy, scarring, follicular plugging, and dyspigmentation that represent clues for the diagnosis of TLE [
36].
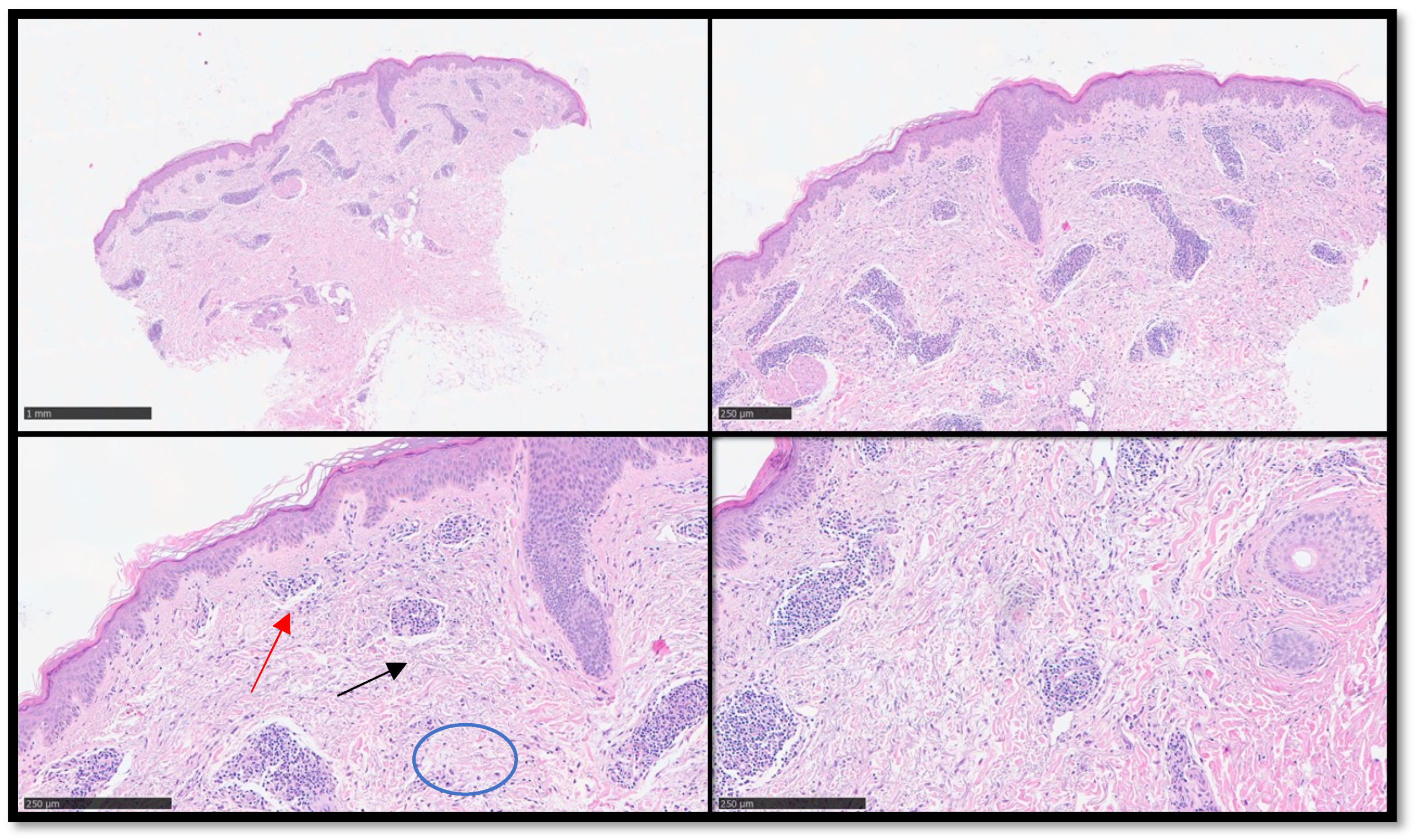
Figure 2.
(A) Panoramic histological view of an incisional biopsy of the previous clinical case: note the diffuse perivascular infiltration of inflammatory cells appreciable also to this magnification (Hematoxylin-Eosin, Original Magnification 2x). (B) In this photomicrograph it’s easier to appreciate a perivascular lymphoid inflammatory infiltrate with apparent uninvolved dermo-epidermal junction (Hematoxylin-Eosin, Original Magnification 4x). (C) Scanning magnification of the previous pictures showing the perivascular lympho-monocytic infiltrate (an example indicated by black arrow) without involving of junction (an example indicated by red arrow): note the presence of dermal mucin (an example indicated by blue circle) (Hematoxylin-Eosin, Original Magnification 10x). (D) Higher magnification of the previous (D) pictures (Hematoxylin-Eosin, Original Magnification 20x).
Figure 2.
(A) Panoramic histological view of an incisional biopsy of the previous clinical case: note the diffuse perivascular infiltration of inflammatory cells appreciable also to this magnification (Hematoxylin-Eosin, Original Magnification 2x). (B) In this photomicrograph it’s easier to appreciate a perivascular lymphoid inflammatory infiltrate with apparent uninvolved dermo-epidermal junction (Hematoxylin-Eosin, Original Magnification 4x). (C) Scanning magnification of the previous pictures showing the perivascular lympho-monocytic infiltrate (an example indicated by black arrow) without involving of junction (an example indicated by red arrow): note the presence of dermal mucin (an example indicated by blue circle) (Hematoxylin-Eosin, Original Magnification 10x). (D) Higher magnification of the previous (D) pictures (Hematoxylin-Eosin, Original Magnification 20x).
Direct immunofluorescence studies are usually negative (differential diagnosis with “lupus band test” of ACLE/SLE patients), and it’s important to underline that histopathological findings of this disease may show certain variations. The body area biopsied, the specific location on the plaque selected (central or peripheral) and the time of taking a biopsy (recent or older lesion) are factors that may play a role in the histologic interpretation of skin specimens in TLE, as in many other inflammatory conditions [
37].
Furthermore, the staining with anti-CD123 (IL-3 receptor alpha-chain) antibody is important to confirm the histological diagnosis of TLE, since usually there are clusters of PDCs that are quite characteristic for lupus dermatitis. More in details, recently, the role of PDCs in the pathogenesis of TLE has been underlined and there are robust evidences that the PDCs are important actors in the inflammatory mechanisms of pathogenesis of TLE [
1,
66].
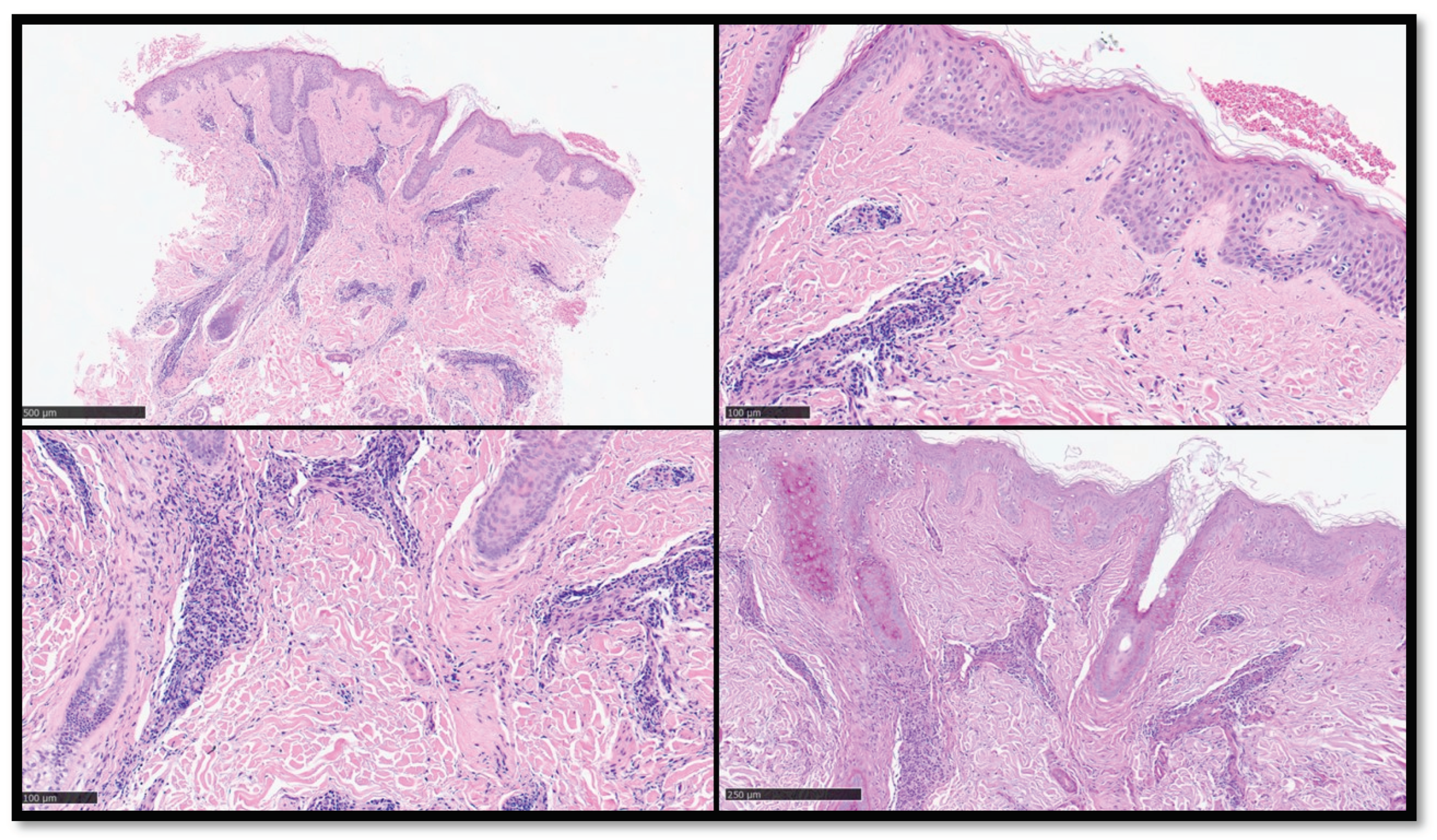
Figure 3.
(A) Panoramic histological view of an incisional biopsy of another clinical case of TLE: note the diffuse perivascular infiltration of inflammatory cells appreciable also to this magnification (Hematoxy-lin-Eosin, Original Magnification 2x). (B) Histopathological microphotograph showing complete absence of inflammation along the dermal-epidermal junction (Hematoxylin-Eosin, Original Magnification 10x). (C) Details of the previous images (Hematoxylin-Eosin, Original Magnification 20x). (D) PAS staining showing mild thickening of the basal membrane zone (PAS histochemical staining, Original Magnification 10x).
Figure 3.
(A) Panoramic histological view of an incisional biopsy of another clinical case of TLE: note the diffuse perivascular infiltration of inflammatory cells appreciable also to this magnification (Hematoxy-lin-Eosin, Original Magnification 2x). (B) Histopathological microphotograph showing complete absence of inflammation along the dermal-epidermal junction (Hematoxylin-Eosin, Original Magnification 10x). (C) Details of the previous images (Hematoxylin-Eosin, Original Magnification 20x). (D) PAS staining showing mild thickening of the basal membrane zone (PAS histochemical staining, Original Magnification 10x).
6. Classification of TLE
As part of the spectrum of SLE, the nosographic evolution of TLE is truly long lasting. Whereas rheumatological classifications for LE are based on symptomatic views to assess systemic involvement, from a dermatological perspective, classifications have been established in order to categorize the disease according to the morphology of skin manifestations [
22,
38,
39].
In classification systems from a dermatological purpose, chilblain lupus, lupus tumidus and lupus profundus are predominantly classified as cutaneous-limited LE [
22,
38]. Since the first case described in the early 1900 only several years later, Gougerot and Burnier [
40] described five patients with similar clinical features, such as erythematous, indurated, non-scarring lesions on the face with minimal surface changes. During the following years the real incidence of TLE was obviously underestimated. The next case reports of TLE were published in the 1950s in the European literature [
41,
42]. This might have been due to the fact that authors did not consider TLE as a separate entity differing from other variants of cutaneous lupus erythematosus. In 1981 the classification proposed by Gilliam and Sontheimer, histologically, differentiates LE cutaneous lesions in specific and nonspecific ones [
11]. The specific ones, defined by the presence of dermo-epidermal interface dermatitis, are exclusive to LE, with or without systemic disease. They are subdivided into three categories based on clinical characteristics: acute cutaneous Lupus Erythematous (ACLE), Subacute cutaneous Lupus Erythematous (SCLE) and chronic cutaneous Lupus Erythematous (CCLE) [
9,
15,
43]. The nonspecific lesions include other cutaneous manifestations associated with SLE. In 2004, the Düsseldorf classification added another subtype, the intermittent CLE (ICLE), which corresponds to tumid LE, previously considered as a variant of CCLE [
44]. However, with increasing evidence, interface dermatitis, used as a criterion to define specific CLE lesions, were found to lack specificity, as it may be present in other conditions, such as dermatomyositis, graft-versus-host disease (GVHD), and drug reactions [
14,
43]. Significantly, in the last two decades, the extensive works of Kuhn and co-workers on TLE demonstrated several significant differences between TLE and other subtypes of CLE [
13,
45]. These differences were based on clinical, histological and laboratory parameters and indicate that TLE should be defined as a separate entity in the classification of CLE. They defined diagnostic criteria for the classification of the disease ruling out that a correct diagnosis requires attention to subtle details and identification of the characteristic signs as well as the course of the disease [
46].
7. Prognosis
Although TLE is currently considered to be a subtype of cutaneous lupus erythematosus, TLE differs from the other subtypes of cutaneous lupus erythematosus in that an association with SLE is rare [
13,
47]. Because of this weak association with SLE and a relative lack of serologic abnormalities in patients with TLE, it has been defined as a disease with a benign course [
13,
34,
47]. Long-term remission is observed in some patients. TLE lesions have a favorable prognosis than lesions of discoid lupus erythematosus or subacute cutaneous lupus erythematosus [
13,
14,
34,
47]. Spontaneous resolution of the lesions without residual dyspigmentation or scarring may be noted within days or weeks, despite the relapses [
34]. Solitary lesions are mostly self-limiting, often without any need for topical or systemic therapy. However, recurrences are frequent with a relatively long disease-free period in between.
8. Therapy
Although singular TLE lesions are frequently self-limiting, there is a high relapse rate [
34]. Singular lesions responding quickly to topical therapies may not need any further treatment [
48]. Sunscreens, topical corticosteroids and systemic antimalarials are the most common and most frequently highly effective therapeutic measures. Sun protection is recommended in all patients with TLE. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic anti-malarials [
49,
50]. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for TLE. Its response rate varies among studies and may be influenced by dosage [
34,
49,
50]. Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically. Data regarding the efficacy of all systemic agents used as second-line treatment of CLE are lacking in terms of therapy for TLE. Such treatments include methotrexate, retinoids such as acitretin, dapsone, mycophenolate mofetil, thalidomide, all of which are preferably used in combination with antimalarials [
51]. The treatment of TLE with pulsed dye lasers has been evaluated in a monocentric prospective study. All of the ten patients showed clinical improvement. However, relapses were not prevented, and new lesions developed in 50% of the patients so that PDL is not considered as a treatment option for patients with TLE [
34]. Recently, anti-CD20 monoclonal antibody rituximab, was successful used to treat relapsing TLE lesions [
52], but the effectivenesss of this approach has to be fully demonstrated since B-lymphocytes targeted through Rituximab are not recognized as important players in the immunologic response in TLE [
53]. The elimination of photosensitizing drugs should be also considered, especially in refractory disease, as well as vitamin D supplementation, since vitamin D deficiency can follow lack of sun exposure, as suggested by the S2 guidelines for the treatment of CLE [
20]. Last but not least TLE patients are advised to join smoking cessation programs because of the evidence-based association of their disease with smoking [
54]. Moreover patients might be prone to vitamin D deficiency by avoiding sun exposure. Hence evaluation of the vitamin D deficiency with 25-hydroxyvitamin D level and adequate supplementation with at least 400 IU of cholecalciferol is suggested [
51].
9. Differential Diagnosis
9.1. Jessner-Kanof Infiltrate (Pseudolymphoma)
In terms of differential diagnosis, it is important to emphasise the main clinico-pathological entities with which TLE must be differentiated in order not to risk confusing diagnoses. In the first instance, it should be mentioned that the relationship between TLE and the Jessner-Kanof lymphocytic infiltrate has not been linear. In particular, although from the initial description by Jessner [
55], the lymphocytic infiltrate (pseudolymphoma) has always been considered as a different entity to TLE, Weber F. et al. [
56] performed a photobiology study including 10 patients who had Jessner lymphocytic infiltration. The investigation revealed that all the patients experienced a latency period exceeding 48 hours prior to the onset of lesions, which is common in all types of CLE. The patients also acquired lesions upon photoprovocation. Examining the biopsies from the lesions showed that the epidermis was unaffected and that the perivascular and periadnexal infiltration was identical to that of TLE. They concluded that there were no appreciable variations between Jessner lymphocytic infiltration and TLE in terms of clinical, pathological, or photobiological aspects. Furthermore, Rémy-Leroux et al. [
57] compared 14 cases of TLE with 32 cases of Jessner lymphocytic infiltration. They came to the conclusion that Jessner lymphocytic infiltration and TLE are interchangeable after examining the clinical, microscopic, and response characteristics of the two patient groups in the photobiology investigation. The results of those investigations led to the current consensus that Jessner lymphocytic infiltration is not a distinct illness, but rather a subtype of TLE.
9.2. Polymorphous Light Eruption (PLE)
Another differential diagnosis is with the Polymorphous light eruption (PLE), a photodermatosis that can result in a wide range of skin lesions, although these are often monomorphic in a given patient. Skin lesions can be vesicular or pseudovesicular, or they can resemble papules or plaques that are hard to distinguish from TLE. In the latter instance, determining the differential diagnosis requires an understanding of specific variations in the lesions' clinical course. Unlike TLE, polymorphous light eruption lesions appear shortly after being exposed to sunlight and heal on their own in a few days if no additional exposure occurs. Additionally, if exposure is prolonged, a highly distinctive tolerance phenomena develops, and the attacks become less severe [
9,
58]. Another important differentiating features is represented by CD123 immunostaining, because there was a lot of clustering PDC highlighted by the antibody in cases of TLE but few or no stained cells in cases of PLE.
9.3. Reticular Erythematous Mucinosis (REM)
Steigleder G.K. et al [
59] presented cases in 1974 that were indistinguishable from those presented of TLE until then, but who called “Reticular erythematous mucinosis” and many authors published their observations under the name of REM [
60,
61,
62,
63]. Young women are the majority of patients with reticulated erythematous mucinosis, which usually manifests as papular erythema or reticulated macular. A periadnexal and perivascular lymphocytic infiltration linked to interstitial mucin deposits is found after lesion biopsy [
58]. Additionally, most patients experience significant photosensitivity. This has led some authors to believe that reticular erythematous mucinosis is a form of CCLE or TLE [
9]. However, it's crucial to remember that immunoglobulin M deposits have been seen on the basement membrane in certain reticular erythematous mucinosis examples [
64]. Nevertheless, no published comparison studies between the 2 circumstances have been conducted to provide confirmation of this notion.
9.4. Granuloma Faciale (GF)
Another important differential diagnosis is with Granuloma Faciale (GF), in which there are violaceous nodule or plaque on the face and the lesions are, usually, asymptomatic. Histologically, a
grenz zone is the characteristic histologic finding that distinguishes it from TLE, a thin zone of the uninvolved papillary dermis that divides the dermal inflammatory infiltrate constituted by lymphocytes, histiocytes, plasma cells, neutrophils, and eosinophils [
65].
9.5. Cutaneous Manifestations of B-Chronic Lymphocytic Leukemia (B-CLL)
Importantly, differential diagnosis with B-CLL is of paramount importance and the co-expression of CD20, CD5 and CD23 by neoplastic cells of B-CLL as well as the detection of a monoclonal rearrangement of the Ig genes in contrast to the polyclonality of T lymphocytes admixed with CD123+ PDC in TLE, allows to reach the correct diagnosis [
66].
10. Associations Rarely Reported
TLE has rarely been reported in association with uncommon histopathological features that deserve specific mention in this review. Firstly, in 2022, Georgiadou N. et al. [
67] reported a case of an 85-year-old patient who presented with four indurated erythematous plaques on her face and upper chest. A 4-mm punch biopsy had been taken of one of these lesions, which showed, along with histological features compatible with lupus dermatitis, also aspects of haemophagocytosis (mainly erythrophagocytosis, lymphophagocytosis and cellular debris). Another paper [
68] described the occurrence of neutrophilic granulocytes in the context of the typical dermal lymphocytic infiltrate of CLE. Furthermore, Boggio F et al. [
69], in 2018, reported the largest published series in the literature of 21 cases of cutaneous haemophagocytosis in patients who had underlying pathological conditions not ascribable to cutaneous T-cell lymphoma and cutaneous Rosai-Dorfman diseases (cRDD) and presented, among other cases, 2 patients with CLE who had histological evidence of inflammatory lymphocytic infiltrate with consensual haemophagocytosis, most represented by aspects of neutrophil phagocytosis. As detailed previously [
34,
67], although there is still no unifying theory explaining the pathogenesis of TLE, it is important to remember that LE is a complex pathology, with a broad spectrum of clinico-pathological manifestations reflecting the two main components at play in its aetiology: 1) dysregulation of cell-mediated immunity and 2) immunocomplex deposition pathology. It is therefore plausible to hypothesise that the impairment of this labile balance between the innate and adaptive immune system is also responsible for the pathogenesis of TLE, with the possibility of the development of cutaneous haemophagocytosis, which has also been observed in the course of autoimmune diseases, infections and malignant neoplasms, among others [
70].
11. The Larger Series of TLE Reported in Literature
The largest series of patients with TLE reported in the literature so far was published by Magana M. et al. in 2022 [
71]. These authors reported data on 20 patients (11 men and 9 women, with an average age of 43.5 years) who had been diagnosed in the previous 16 years of histological activity. In detail, all reported patients presented erythematous, non-scarring urticarial-like plaques, of which 8 only in the head region, 8 at the trunk/limbs level; head and trunk/limbs in 2 cases and topographic data for 2 patients were not recoverable. Only 1 patient out of 20 reported (1/20) had developed SLE. Histologically the biopsies presented the classic features of TLE and, interestingly, the differential count of CD123+ PDCs carried out on 10/20 patients with TLE was much higher compared to 5 cases of DLE and 5 cases of normal skin, suggesting more robustly that this marker plays a role of some importance in the differential diagnosis of TLE compared to its histological mimics.
12. Conclusions
TLE is a type of lupus erythematosus that differs from the traditional types of CLE because to its unique clinical presentation. While the initial accounts of the histological characteristics of TLE emphasized the lack of the distinct epidermal modifications typical of lupus, certain cases do exhibit these changes, but they are always mild or moderate. In order to prevent cases of TLE in which damaged epidermis is found in the biopsy from being mistakenly identified as CCLE or SCLE, the sporadic occurrence of epidermal changes in TLE should be considered in clinical practice, although without crust and/or scarring occurrence. It’s almost important to say that today, after the pioneering papers by Kuhn and more recent by Magana, TLE can be totally considered as a distinct, highly photosensitive disease, with characteristic and peculiar clinical, histopathological and immunophenotypic features that confirm it as a distinct type of CLE.
Future papers with more cases are needed to improve knowledge of this entity that has been neglected for some time in the literature and has come to the attention of the dermatologist and dermatopathologist in recent years.
Author Contributions
Conceptualization, G.C., C.F. and M.D.; methodology, G.C.; software, F.A.; validation, G.C., F.A. and A.F.; formal analysis, G.C.; investigation, M.D.; resources, F.A.; data curation, G.C.; writing—original draft preparation, G.C.; writing—review and editing, G.C., F.A., M.D., C.F. and A.F.; visualization, G.C.; supervision, C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
In memory of Antonietta Cimmino (A.C.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Petty, A.J.; Floyd, L.; Henderson, C.; Nicholas, M.W. Cutaneous Lupus Erythematosus: Progress and Challenges. Curr Allergy Asthma Rep. 2020 Apr 4;20(5):12. 1. [CrossRef]
- Walling, H.W.; Sontheimer, R.D. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10(6):365-81. [CrossRef]
- Moura Filho, J.P.; Peixoto, R.L.; Martins, L.G.; Melo, S.D.; Carvalho, L.L.; Pereira, A.K.; Freire, E.A. Lupus erythematosus: considerations about clinical, cutaneous and therapeutic aspects. An Bras Dermatol. 2014 Jan-Feb;89(1):118-25. [CrossRef]
- Niebel, D.; de Vos, L.; Fetter, T.; Brägelmann, C.; Wenzel, J. Cutaneous Lupus Erythematosus: An Update on Pathogenesis and Future Therapeutic Directions. Am J Clin Dermatol. 2023 Jul;24(4):521-540. [CrossRef]
- Poh, Y.J.; Alrashid, A.; Sangle, S.R.; Higgins, E.; Benton, E.; McGibbon, D.; D'Cruz, D.P. Proton pump inhibitor induced subacute cutaneous lupus erythematosus: Clinical characteristics and outcomes. Lupus. 2022 Aug;31(9):1078-1083. [CrossRef]
- Zhou, W.; Wu, H.; Zhao, M.; Lu, Q. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020 Aug;16(8):829-837. [CrossRef]
- Joseph, A.K.; Windsor, B.; Hynan, L.S.; Chong, B.F. Discoid lupus erythematosus skin lesion distribution and characteristics in Black patients: a retrospective cohort study. Lupus Sci Med. 2021 Nov;8(1):e000514. [CrossRef]
- Green, P.J.; Pasternak, S. Hypertrophic and ulcerated discoid lupus erythematosus. J Cutan Med Surg. 2012 Nov-Dec;16(6):453-7. [CrossRef]
- Kuhn, A.; Richter-Hintz, D.; Oslislo, C.; Ruzicka, T.; Megahed, M.; Lehmann, P. Lupus erythematosus tumidus--a neglected subset of cutaneous Lupus erythematosus: report of 40 cases. Arch Dermatol. 2000, 136, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Alexiades-Armenakas, M.R.; Baldassano, M.; Bince, B.; Werth, V.; Bystryn, J.C.; Kamino, H, Soter, N.A.; Franks, A.G. Tumid lupus erythematosus: criteria for classification with immunohistochemical analysis. Arthritis Rheum. 2003, 49, 494–500. [CrossRef]
- Sontheimer, R.D. The lexicon of cutaneous lupus erythematosus--a review and personal perspective on the nomenclature and classification of the cutaneous manifestations of lupus erythematosus. Lupus. 1997, 6, 84–95. [Google Scholar] [CrossRef]
- Hoffmann, E. Demonstrationen: lupus erythematodes tumidus. Derm Zeitschr 1909, 16, 159–160. [Google Scholar]
- Vieira, V.; Del Pozo, J.; Yebra-Pimente,l M. T.; Martínez, W.; Fonseca, E. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006, 45, 512–517. [Google Scholar] [CrossRef]
- Schmitt, V.; Meuth, A.M.; Amler, S.; Kuehn, E.; Haust, M.; Messer, G.; Bekou, V.; Sauerland, C.; Metze, D.; Köpcke, W.; Bonsmann, G.; Kuhn, A. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010, 162, 64–73. [Google Scholar] [CrossRef]
- Kuhn, A.; Sonntag, M.; Ruzicka, T.; Lehmann, P.; Megahed, M. Histopathologic findings in lupus erythematosus tumidus: review of 80 patients. J Am Acad Dermatol. 2003, 48, 901–908. [Google Scholar] [CrossRef]
- Gilliam, J.N.; Sontheimer, R.D. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol 1981, 4, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.; Grubbs, H.; Koritala, T.; Crane, J.S. Tumid Lupus Erythematosus. StatPearls Publishing; 2023.
- Available online: https://icd.who.int/browse10/2019/en#/L93.2 (accessed on 17 February 2024).
- Sonntag, M.; Lehmann, P.; Megahed, M.; Ruzicka, T.; Kuhn, A. Lupus erythematosus tumidus in childhood. Report of 3 patients. Dermatology. 2003, 207, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Biazar, C.; Sigges, J.; Patsinakidis, N.; Ruland, V.; Amler, S.; Bonsmann, G.; Kuhn, A; EUSCLE co-authors. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Daze, R.P.; Moon, S. Tumid Lupus Erythematosus: A Rare and Distinctive Variant of Cutaneous Lupus Erythematosus Masquerading as Urticarial Vasculitis. Cureus. 2020, 12, e8305. [Google Scholar] [CrossRef]
- Vale, E.C. S, do.; Garcia, L.C. Cutaneous lupus erythematosus: a review of etiopathogenic, clinical, diagnostic and therapeutic aspects. An Bras Dermatol. 2023, 98, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Sonntag, M.; Richter-Hintz, D.; Oslislo, C.; Megahed, M.; Ruzicka, T.; Lehmann, P. Phototesting in lupus erythematosus: a 15-year experience. J Am Acad Dermatol. 2001, 45, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chang, C.; Zhang, J. Immunologic and genetic considerations of cutaneous lupus erythematosus: a comprehensive review. J Autoimmun. 2013, 41, 34–45. [Google Scholar] [CrossRef]
- Böckle, B.C.; Sepp, N.T. Smoking is highly associated with discoid lupus erythematosus and lupus erythematosus tumidus: analysis of 405 patients. Lupus. 2015, 24, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.W.; Staender, S.; Schlüter, B.; Luger, T.A.; Bonsmann, G. Infliximab-induced lupus erythematosus tumidus in a patient with rheumatoid arthritis. Arch Dermatol. 2006, 142, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Sohl, S.; Renner, R.; Winter, U.; Bodendorf, M.; Paasch, U.; Simon, J.C.; Treudler, R. [Drug-induced lupus erythematosus tumidus during treatment with adalimumab]. Hautarzt. 2009, 60, 826–829. [Google Scholar] [CrossRef]
- Schepis, C.; Lentini, M.; Siragusa, M.; Batolo, D. ACE-inhibitor-induced drug eruption resembling lymphocytic infiltration (of Jessner-Kanof) and Lupus erythematosus tumidus. Dermatology. 2004, 208, 354–355. [Google Scholar] [CrossRef] [PubMed]
- Böckle, B.C.; Baltaci, M.; Weyrer, W.; Sepp, N.T. Bortezomib-induced lupus erythematosus tumidus. Oncologist. 2009, 14, 637–639. [Google Scholar] [CrossRef]
- Abadías-Granado, I.; Sánchez-Bernal, J.; Felipo-Berlanga, F.; Ara-Martín, M. Coexistence of Tumid Lupus Erythematosus and Discoid Lupus Erythematosus. Actas Dermo-Sifiliográficas (English Edition). 2019, 110, 253–255. [Google Scholar] [CrossRef]
- Jatwani, K.; Chugh, K.; Osholowu, O.S.; Jatwani, S. Tumid Lupus Erythematosus and Systemic Lupus Erythematosus: A Report on Their Rare Coexistence. Cureus. 2020, 12, e7545. [Google Scholar] [CrossRef]
- Gambichler, T.; Pätzholz, J.; Schmitz, L.; Lahner, N.; Kreuter, A. FOXP3+ and CD39+ regulatory T cells in subtypes of cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 2015, 29, 1972–1977. [Google Scholar] [CrossRef]
- Rodríguez-Caruncho, C.; Bielsa, I. Lupus erythematosus tumidus: A clinical entity still being defined. Actas Dermosifiliogr. 2011, 102, 668–674. [Google Scholar] [CrossRef]
- Patsinakidis, N.; Kautz, O.; Gibbs, B.F.; Raap, U. Lupus erythematosus tumidus: clinical perspectives. CCID. 2019, 12, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Fisher, J.M.; Kuklinski, L.F.; Hogeling, M. A teen with blaschkolinear tumid lupus erythematosus. JAAD Case Rep. 2022;21:1-5. [CrossRef]
- Available online: https://www.pathologyoutlines.com/topic/skinnontumorlupussle.html (accessed on 17 February 2024).
- The Lives of Lesions: Chronology in Dermatopathology. Antoinette F. Hood, MD. The Lives of Lesions: Chronology in Dermatopathology. Antoinette F. Hood, MD. Arch Dermatol. 1985;121(6):809. [CrossRef]
- Weldemann, A.; Ziepert, M.; Kreuz, M.; Dumann, K.; Simon, J.C.; Kunz, M.; Ziemer, M. Lupus erythematosus: correlation of clinical and histological findings and proposal for a modified disease classification. JDDG 2021, 19, 11. [Google Scholar] [CrossRef]
- Tan, B.C.H.; Tang, I.; Bonin, J.; Koelmeyer, R.; Hoi, A. The performance of different classification criteria for systemic lupus erythematosus in a real-world rheumatology department. Rheumatology (Oxford). 2022 Nov 2;61(11):4509-4513. [CrossRef]
- Gougerot, H.; Burnier, R. Lupus érythémateux tumidus. Bull Soc Fr Dermatol Syphil. 1930, 37, 1219–1292. [Google Scholar]
- Rockl, H. Erythematodes tumidus; a case history with reference to the problem of erythematodic lymphocytoma. Hautarzt. 1954, 5, 422–423. [Google Scholar] [PubMed]
- Vilanova, X. [The profundus and tumidus forms of lupus erythe-matosus; report of a case and comments]. Rev Clin Sep 1950, 36, 388–393. [Google Scholar]
- Kuhn, A.; Sonntag, M.; Ruzicka, T.; Lehmann, P.; Megahed, M. Histopathologic findings in lupus erythematosus tumidus: review of 80 patients. J Am Acad Dermatol. 2003, 48, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Ruzicka, T. Classification of cutaneous lupus erythematosus. In: Kuhn A, Lehmann P, Ruzicka T, editors. Cutaneous Lupus Erythematosus. New York: Springer; 2005:53–57.
- Rodriguez-Caruncho, C.; Bielsa, I.; Fernández-Figueras, M.T.; Roca, J.; Carrascosa, J.M.; Ferrándiz, C. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015 Jun;24(7):751-5. [CrossRef]
- Kuhn, A.; Bein, D.; Bonsmann, G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009 May;8(6):441-8. [CrossRef]
- Stead, J.; Headley, C.; Ioffreda, M.; Kovarik, C.; Werth, V. Coexistence of tumid lupus erythematosus with systemic lupus erythematosus and discoid lupus erythematosus: a report of two cases of tumid lupus. J Clin Rheumatol. 2008, 14, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Sigges, J.; Biazar, C.; Landmann, A.; Ruland, V.; Patsinakidis, N.; Amler, S.; Bonsmann, G. Therapeutic strategies evaluated by the European Society of Cutaneous Lupus Erythematosus (EUSCLE) Core Set Questionnaire in more than 1000 patients with cutaneous lupus erythematosus. Autoimmun Rev. 2013 May;12(7):694-702. [CrossRef]
- Pona, A.; Cardenas-de la Garza, J.A.; Broderick, A.; Sangueza, O.P.; Niehaus, A.G.; Bowers, N.; Pichardo, R.O. Lupus Erythematosus Tumidus Clinical Characteristics and Treatment: A Retrospective Review of 25 Patients. Cutis. 2022 Jun;109(6):330-332. [CrossRef]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Melles, R.B.; Mieler, W.F; American Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology. 2016 Jun;123(6):1386-94. [CrossRef]
- Kuhn, A.; Aberer, E.; Bata-Csörgő, Z.; Caproni, M.; Dreher, A.; Frances, C.; Gläser, R.; Klötgen, H.W.; Landmann, A.; Marinovic, B.; Nyberg, F.; Olteanu, R.; Ranki, A.; Szepietowski, J.C.; Volc-Platzer, B. S2k guideline for treatment of cutaneous lupus erythematosus - guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2017 Mar;31(3):389-404. [CrossRef]
- Kreuter, A.; Tigges, C.; Hunzelmann, N.; Oellig, F.; Lehmann, P.; Hofmann, S.C. Rituximab in the treatment of recalcitrant generalized lupus erythematosus tumidus. J Dtsch Dermatol Ges. 2017 Jul;15(7):729-731. [CrossRef]
- Verdelli, A.; Coi, A.; Marzano, A.V.; Antiga, E.; Cozzani, E.; Quaglino, P.; La Placa, M.; Benucci, M.; De Simone, C.; Papini, M.; Parodi, A.; Bianchi, F.; Caproni, M. Autoantibody profile and clinical patterns in 619 Italian patients with cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 2019 Apr;33(4):742-752. [CrossRef]
- Böckle, B.C.; Sepp, N.T. Smoking is highly associated with discoid lupus erythematosus and lupus erythematosus tumidus: analysis of 405 patients. Lupus. 2015, 24, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Jessner, M.; Kanof, N.B. Lymphocytic infiltration of the skin. Arch Dermatol 1953, 68, 447–449. [Google Scholar]
- Weber, F.; Schmuth, M.; Fritsch, P.; Sepp, N. Lymphocytic infiltration of the skin is a photosensitive variant of lupus erythematosus: evidence by phototesting. Br J Dermatol. 2001 Feb;144(2):292-6. [CrossRef]
- Rémy-Leroux, V.; Léonard, F.; Lambert, D.; Wechsler, J.; Cribier, B.; Thomas, P.; Adamski, H.; Marguery, M.C.; Aubin, F.; Leroy, D.; Bernard, P. Comparison of histopathologic-clinical characteristics of Jessner's lymphocytic infiltration of the skin and lupus erythematosus tumidus: Multicenter study of 46 cases. J Am Acad Dermatol. 2008 Feb;58(2):217-23. [CrossRef]
- Superficial and deep perivascular inflammatory dermatoses. En: McKee PH, Calonje E, Granter SR, editors. Pathology of the skin with clinical correlations. 3rd edition. Philadephia: Elsevier; 2005. p. 269–71.
- Steigleder, G.K.; Gartmann, H.; Linker, U. REM syndrome: reticular erythematous mucinosis (round-cell erythematosis), a new entity? Br J Dermatol. 1974 Aug;91(2):191-9. [CrossRef]
- PERRY, H.O.; KIERLAND, R.R.; MONTGOMERY, H. Plaque-like form of cutaneous mucinosis. Arch Dermatol. 1960 Dec;82:980-5. [CrossRef]
- Bleehen, S.S.; Slater, D.N.; Mahood, J.; Church, R.E. Reticular erythematous mucinosis: light and electron microscopy, immunofluorescence and histochemical findings. Br J Dermatol. 1982 Jan;106(1):9-18. [CrossRef]
- Vanuytrecht-Henderickx, D.; Dewolf-Peeters, C.; Degreef, H. Morphological study of the reticular erythematous mucinosis syndrome. Dermatologica. 1984;168(4):163-9. [CrossRef]
- Izumi, T.; Tajima, S.; Harada, R.; Nishikawa, T. Reticular erythematous mucinosis syndrome: glycosaminoglycan synthesis by fibroblasts and abnormal response to interleukin-1 beta. Dermatology. 1996;192(1):41-5. [CrossRef]
- Del Pozo, J.; Martínez, W.; Almagro, M.; Yebra, M.T.; García-Silva, J.; Fonseca, E. Reticular erythematous mucinosis syndrome. Report of a case with positive immunofluorescence. Clin Exp Dermatol. 1997 Sep;22(5):234-6. [CrossRef]
- Al Dhafiri, M.; Kaliyadan, F. Granuloma Faciale. 2023 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–.
- Skin Lymphoma, The Illustrated Guide. Lorenzo Cerroni. Wiley Blackwell. 5th Edition. 2020.
- Georgiadou, N.; Singh, S.; Singh, M. Lupus Erythematosus Tumidus Associated With Hemophagocytosis. Am J Dermatopathol. 2022 Jul 1;44(7):519-522. [CrossRef]
- Choonhakarn, C.; Poonsriaram, A.; Chaivoramukul, J. Lupus erythematosus tumidus. Int J Dermatol. 2004 Nov;43(11):815-8. [CrossRef]
- Boggio, F.; Lora, V.; Cota, C.; Pereira, A.; Müllegger, R.; Prieto-Torres, L.; Cerroni, L. Cutaneous hemophagocytosis: Clinicopathologic features of 21 cases. J Am Acad Dermatol. 2018 Feb;78(2):377-382. [CrossRef]
- Kerl, K.; Wolf, I.H.; Cerroni, L.; Wolf, P.; French, L.E.; Kerl, H. Hemophagocytosis in Cutaneous Autoimmune Disease. Am J Dermatopathol. 2015 Jul;37(7):539-43. [CrossRef]
- Magaña, M.; Castellanos, G.; Meurehg, C.C.; Magaña-Mainero, M.R. Lupus Erythematosus Tumidus: Clinical and Pathological Features in a Series of 20 Patients. Am J Dermatopathol. 2022 Jul 1;44(7):469-477. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).