1. Introduction
Dengue has persistently afflicted Mexico, with recurring epidemic waves marked by the circulation of all four serotypes at varying intervals. As a result, Mexico is classified as hyperendemic for dengue. In June 2023, the Pan American Health Organization (PAHO) issued an alert, citing a surge in dengue, Zika, and chikungunya cases across several countries in the Americas in 2022 compared to the previous year. This trend persisted into the early months of 2023, with dengue emerging as the predominant arbovirus, characterized by cyclical outbreaks recurring every 3 to 5 years. Notably, Mexico, along with Honduras, Nicaragua, Guatemala, and Panama, [
1] reported a significant surge in severe dengue cases in 2023. All four dengue virus serotypes (DENV-1, DENV-2, DENV-3, and DENV-4) are endemic to this subregion. Subsequently, PAHO issued another alert due to sustained dengue circulation, declaring 2023 as the year with the highest historical record of dengue cases, surpassing 4.1 million new infections in the Americas. This surge was primarily attributed to DENV-3, which had not circulated in certain areas for several years. [
2]
The detection of these viruses’ introduction into the country relies on laboratory-based epidemiological surveillance, targeting individuals exhibiting clinical symptoms and a history of travel to regions with known disease transmission (during periods of autochthonous transmission). This surveillance hinges on precise case definitions and viral sampling within vector populations. [
3]
Mexico is endemic to dengue and other arboviruses, which have triggered epidemics across various regions and over time. According to data from the General Directorate of Epidemiology (DGE), in 2023, Mexico faced a dengue epidemic, with 64% of confirmed cases concentrated in Yucatan, Veracruz, Quintana Roo, Morelos, and Puebla. This marked a significant increase compared to the previous year, with a predominance of DENV-2.[
4] The incidence rate per 100,000 inhabitants until epidemiological week No. 49 of 2023 was 38.81 confirmed cases, with Yucatan reporting the highest incidence at 444.31 cases, followed by Quintana Roo (261.79), Morelos (194.84), Campeche (176.22), and Veracruz (117.85). [
4]
The circulation of all four serotypes has been observed in Mexico from 2016 to 2023, exhibiting cyclic behavior, primarily driven by DENV-1 and DENV-2. DENV-3 has not circulated for several years; DENV-1 predominated between 2011 and 2018, followed by DENV-2 from 2019 onwards. From 2016 to 2018, DENV-1 dominated, replaced by DENV-2 from 2019 to 2022, except in 2018 when both serotypes co-circulated. However, in 2023, DENV-3 co-circulated with DENV-2, detected in low proportions between 2016 and 2021. In 2023, the number of positive samples for DENV-1 is similar to the total reported the previous year. DENV-4 has consistently been the least predominant serotype; however, in 2023, it accounted for 2.4%, a higher value than reported from 2016 to 2022. The circulation of all four serotypes has been concentrated in the southern, Gulf, and central regions. [
4]
In Mexico, laboratory-based epidemiological surveillance is used for arbovirus diagnosis, employing RT-qPCR during the acute phase and serology during convalescence (for those not tested during the acute phase) and employing sampling schemes. [
5] Additionally, Mexico is experiencing a dengue epidemic in the southeastern and central regions of the country, with DENV-3 predominance and co-circulation with DENV-2. [
4] The presence of DENV-3 is associated with increased severity and has not been present in the country for decades, leading to higher incidence rates among young individuals. Consequently, the objective was to describe dengue serotypes by age group and their association with disease severity concerning sex, time, and the origin of clinical samples collected from units within the epidemiological surveillance system.
2. Materials and Methods
A retrospective, multicenter, national-based cross-sectional study was conducted to determine cumulative incidence rates, based on laboratory-based epidemiological surveillance. The source of dengue case records was the Laboratory Epidemiological Control System (SISCEP, for its acronym in Spanish), operational in all medical units of the Mexican Social Security Institute (IMSS). Cases registered in 2023 with molecular results for dengue virus serotyping were identified. Variables including age, sex, federal entity, date of symptom onset, type of care (outpatient or hospitalization), and clinical characteristics of dengue (non-severe, with warning signs, or severe) were collected.
All patients included in the study met the epidemiological operational case definition used in Mexico, [
5] based on the clinical classification established by the Pan American Health Organization (PAHO). [
1,
2] This classification includes:
a) Probable non-severe dengue case: Individuals of any age with fever and at least two of the following groups of signs and symptoms (nausea/vomiting, rash, myalgia/arthralgia, headache/retro-orbital pain, petechiae/positive tourniquet test, leukopenia), residing in or coming from areas with disease transmission in the previous 14 days.
b) Probable dengue case with warning signs: In addition to the above criteria, presents one or more warning signs (intense and continuous abdominal pain, persistent vomiting, fluid accumulation, mucosal bleeding, lethargy, postural hypotension, hepatomegaly, progressive increase in hematocrit, platelet count <100,000/mm³ or progressive decrease in platelets, progressive decrease in hemoglobin).
c) Probable severe dengue case: Presents signs of shock, severe bleeding, or severe organ involvement (severe hepatitis, renal failure, central nervous system involvement, myocarditis, or others).
d) Confirmed dengue case: Confirmation of recent dengue virus infection by laboratory techniques recognized by the Institute of Epidemiological Diagnosis and Reference (InDRE).
The studied variables included socio-demographic factors (age, sex, federal entity). Age was summarized in years and grouped into the following age categories: under one year, 1 to 5 years, 6 to 18 years, 19 to 40 years, 41 to 60 years, and 61 years and older. Regarding temporal aspects, the date of onset of dengue symptoms was used to create infection quarters during the year: January to March, April to June, July to September, and October to December. Concerning geographical location, patient residence in a specific federal entity was used to confirm geographical zones based on the climate of each region, categorized into three climate regions: humid and sub-humid temperate, humid and sub-humid warm, and very dry and dry, as established by the National Institute of Statistics and Geography of Mexico. [
6]
Regarding clinical characteristics, patient type was classified as outpatient or hospitalized. Based on the operational case definitions of dengue, patients were classified as severe dengue based on clinical data from severe dengue cases and dengue with warning signs at the time of clinical care, whether hospitalized or outpatient. Additionally, the presence or absence of pregnancy and gestational weeks were considered. Finally, for serotyping, real-time RT-qPCR amplification kits were used.
Statistical Analysis
All samples with molecular results of dengue serotypes were included in the analysis. A descriptive analysis was conducted to determine the incidence of dengue virus serotypes by age group. Simple frequencies and measures of central tendency and dispersion were calculated. Bivariate analysis was performed using adjusted Chi-square tests, and multivariate analysis was conducted using logistic regression. Statistical significance was set at an alpha level of <0.05.
Ethical approval for this study was obtained from the Local Committee for Scientific Research of the Mexican Institute of Social Security (IMSS) and was granted with the registration number R-2023-3605-145.
3. Results
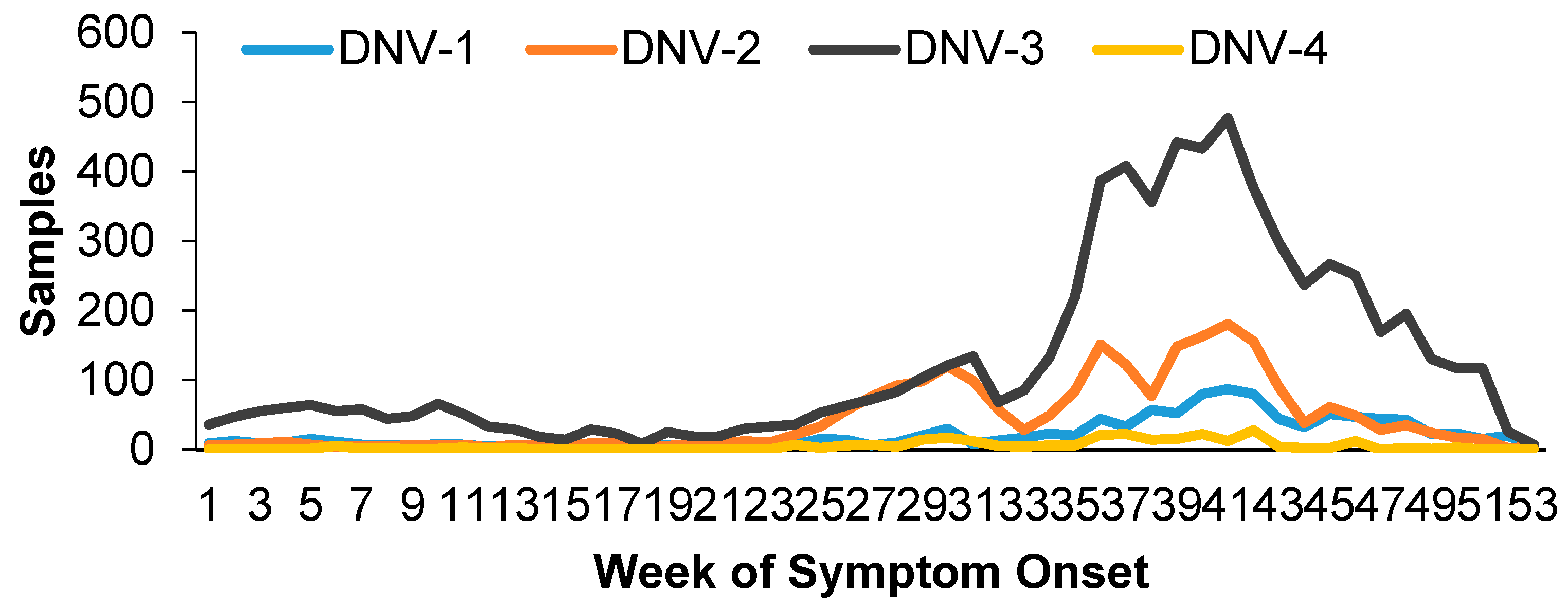
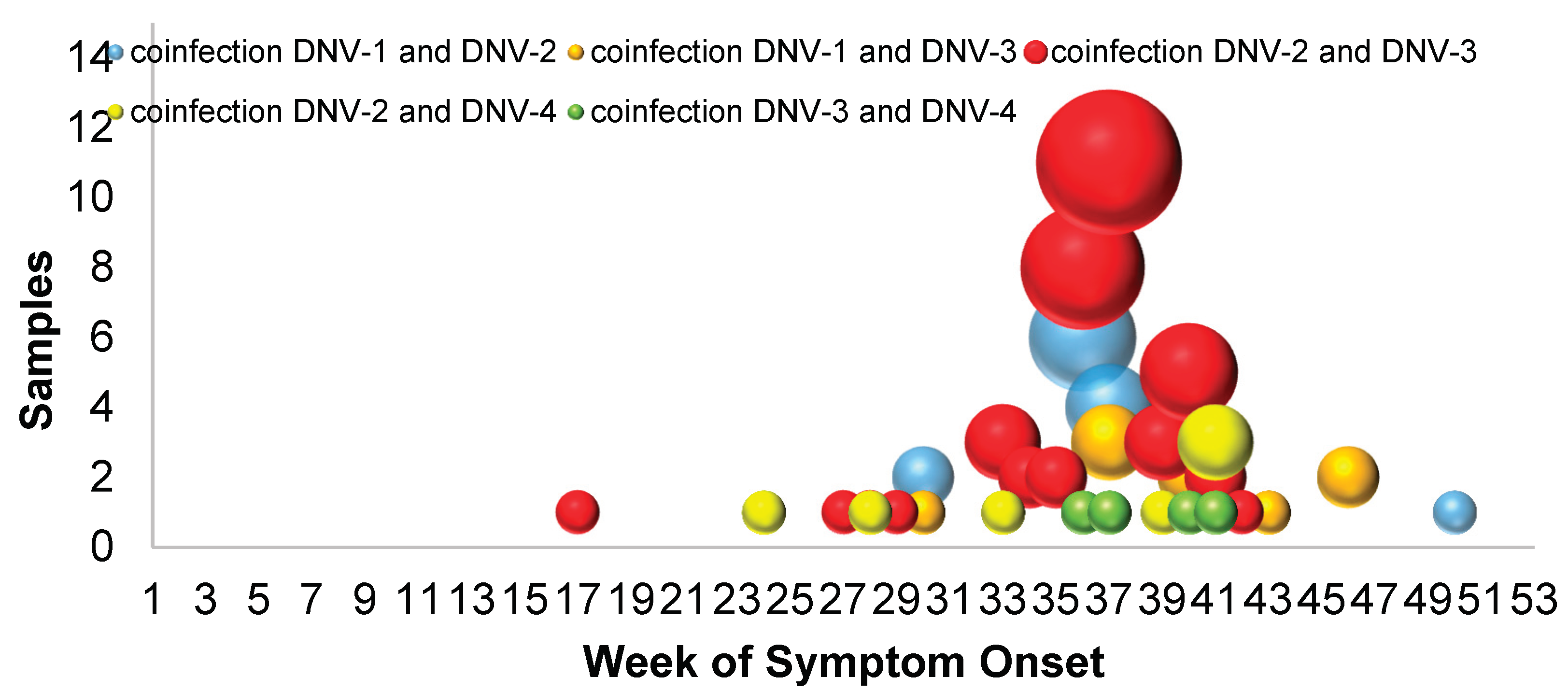
During 2023, a total of 10,530 samples were processed for dengue serotyping using RT-qPCR at IMSS. The identified serotypes, from most to least prevalent, were as follows: DNV-3 with 63.9% of samples (n=6,727), DNV-2 with 22.2% (n=2,336), DNV-1 with 10.6% (n=1,117), and DNV-4 with 2.6% (n=271). Additionally, 79 samples showed coinfection with two serotypes (0.75%), with the predominant combination being DNV-3/DNV-2. (See
Supplementary Material 1). There was a slightly higher number of samples from females, with no differences observed in circulating serotypes by sex (
Table 1).
Six out of ten laboratory samples were from individuals receiving outpatient care; however, DNV-3 predominated in outpatient management, while DNV-2 predominated in hospital management (
Table 2).
According to clinical classification, 56.7% were reported as non-severe dengue, 41.2% as dengue with warning signs, and only 2.1% as severe dengue. Regarding clinical classification, DNV-3 was found in 65.8% of non-severe dengue cases, 62.4% of cases with warning signs, and 63.0% of severe dengue cases (See
Supplementary Material 2). Men were hospitalized less frequently than women.
The behavior of the samples over time was constant throughout the year, with increases at the beginning of the year and from summer onwards, which continued until November (
Figure 1).
Also at that time, the highest number of coinfections occurred (69%), that is, the greater the circulation of DNV-2 and DNV-3, the number of samples with coinfections increased (
Figure 2). DNV-2 was associated with warm humid weather and the quarter that was associated with the presence of more serious cases and hospitalizations was from April to June.
The southeast of Mexico was where the largest number of samples were presented, specifically in the states of Quintana Roo (16.9%), Yucatán (23.0%) and Veracruz (18.6%); The first two had the predominant presence of DNV-3, with 25.3% and 34.7% respectively, while in Veracruz the DNV-2 serotype was more frequent, with 51.7% of the samples; which has circulation of all serotypes of the virus. (
S3). A higher median age was observed in DNV-2 and in the coinfections of DNV-1/DNV-2, DNV-2/DNV-4, with 35, while in the coinfection of DNV-3/DNV-4 it was younger than the median with 11.4 years. Hospitalized patients had a median age of 21 years. By serotype, DNV-1 samples had the youngest average age; however, when determined by coinfection, the youngest was DNV-3/DNV-4 coinfection. (
S4) No statistically significant difference was found between serotypes in relation to sex; except for DNV-1 and DNV-2, which was minimal, with a predominance of DNV-2 in women (23.34% vs 20.39%, p= 0.003).
Significant associations were found between hospitalization and being male, as well as serotypes 1, 2, and 3. DNV-2 exhibited a stronger association with hospitalization. Similarly, statistically significant associations were observed between severe dengue and being male, as well as serotypes 1, 2, and 3. DNV-2 showed a stronger association with the presentation of severe dengue (See
Table 3).
The mean Ct value of positive results was obtained, with no differences observed by age group and sex. However, lower values were observed in pregnant individuals, outpatients, and non-severe dengue cases (See
Supplementary Material 5). Regarding the risk of coinfection, it was more likely in the last trimester of pregnancy (OR = 4.65, 95%CI: 2.74; 7.88), as well as in individuals residing in the state of Veracruz (OR = 4.11, 95%CI: 2.64; 6.42), and with the presence of DNV-2 (OR = 13.67, 95%CI: 7.88; 23.72) (See
Table 4).
Morelos showed a particularity of being the state with the highest risk of developing severe dengue, requiring hospitalization, and mortality. A similar situation was observed with pregnancy, where the risk of mortality in the last trimester was double. Coinfection was not a significant determinant for hospitalization or death (See
Supplementary Material 6). Most of the results remained consistent in the multivariate analysis with minimal variation (See Supplementary Material 7). In the multinomial regression analysis with serotype as the dependent variable, no association was found with the individual
’s sex for any dengue virus serotype. However, serotype 1 was associated with the age groups of 1 to 5 years and 6 to 18 years, with ORs of 4.3 (95%CI: 1.5-11.9) and 2.6 (95%CI: 1.3-4.8), respectively, with p-values less than 0.05. DNV-2 did not associate with any age group, while for DNV-3, the associated age groups were 6 to 18 years and 19 to 40 years, with ORs of 2.0 (1.2-3.5) and 1.8 (1.04-2.97), respectively, with p-values less than 0.05 (
Table 5).
4. Discussion
In this study, we examined the behavior of samples processed for dengue by RT-qPCR in the population with social security in Mexico, with a focus on the year 2023.During this period, we observed an introduction and continuous rise of serotype DNV-3, particularly prevalent in the southern and southeastern regions of the country. Notably, an increase in cases was also noted in the state of Veracruz, situated in the Gulf of Mexico region, which, despite being hyperendemic and having identified all four serotypes previously, has also been documented in other areas of the world such as Brazil [
7] and Southeast Asia. [
8]
Our findings indicate a rise in cases during the summer months, consistent with trends observed in other countries like Italy, [
9] reflecting a second peak of activity in Mexico. However, an additional increase in activity was observed during the winter and at the beginning of the following year, as well as in the fall, This pattern echoes the dengue epidemic in Mexico from 2016 to 2022. . [
4] where similar fluctuations were noted in seasonal increases, albeit with variations among states. In our study, half of the samples were from individuals under 24 years old, and it was observed that serotypes DNV-3 and DNV-1 affected younger individuals compared to DNV-2. [
4] This trend aligns with previous reports in Mexico,, where most cases of non-severe dengue and with warning signs occurred in individuals under 24 years old. However, the median age in our study was similar to that published in Italy.[
9] Additionally, the median age for DNV-2 was 30 years, which is consistent with previous findings. [
8] In summary, dengue appears to mainly affect economically active age groups and adolescents, underscoring the importance of prevention and control strategies specifically targeting these demographic groups.
In a 19-year cohort study in Nicaragua, it was demonstrated that prior immunity to flaviviruses and pre-existing antibody titers differentially affected the risk of disease for incoming serotypes, increasing the risk for DNV-2 and DNV-4, while providing protection against DNV-1. However, for DNV-3, low titers of pre-existing antibodies were observed due to its absence in circulation from 2014 to 2022 (peaks in 2009 to 2012 for DNV-3). [
10] In Mexico, Chikungunya was introduced in 2014, followed by Zika in 2015, [
5] along with epidemics of these viruses and Guillain-Barré syndrome from 2014 to 2017. [
11,
12] In 2019, during a dengue epidemic in Mexico caused by DNV-2, the most affected age groups were 10-14 and 15-19 years old. [
13] This serotype co-circulated predominantly with DNV-1 between 2011 and 2013 and had low circulation in 2016 and 2017. These observations reflect the dynamic nature of dengue in Mexico over the years, despite constant efforts to control the vector and mitigate virus transmission. Environmental factors such as temperature and humidity, with modifications in ecological conditions, have contributed to the expansion of vectors. [
14,
15] On the other hand, demographic factors such as population density, human migration, and tourism also favor dengue transmission. The metropolitan area of Mexico City is of concern, as it mainly detects imported cases from other states but is surrounded by federative entities with active vector circulation and high dengue transmission, such as Morelos, Puebla, and parts of the State of Mexico. [
16,
17] Although it was considered an area with a low probability of Aedes aegypti establishment due to its altitude, temperature, and humidity characteristics, recent evidence shows intermittent presence of the mosquito in the city. [
14,
15] Causal factors for the introduction and colonization of Aedes aegypti include continuous urbanization (both legal and illegal), poor housing conditions and overcrowding, inadequate access to clean water and sanitation, insufficient waste management, and increasing water and air temperatures in urban areas. [
18,
19] Additionally, population mobility from areas with permanent mosquito presence can induce vector and virus dispersion. [
20,
21] In Mexico, it has been documented that there are higher probabilities of secondary infection in the Southeast [2,19 (95%CI: 1.76, 2.73)] and Northwest [3,10 (95%CI: 2.30, 4.51)] regions compared to the Central region. [
22] However, the probability of severe dengue is higher for the Central region compared to all others, suggesting that an epidemic in the central part of the country would lead to high demand for healthcare services and a high fatality rate. This was reflected in this study, particularly in Morelos, a state located in the central part of the country with a higher clinical severity risk.
Regarding gender, there is controversy regarding the development of the disease. On one hand, it has been documented that women have a protective factor against dengue (RR= 0.704, 95% CI: 0.622; 0.796), while in Brazil the opposite was observed, although after adjusting the model, the result remained imprecise (OR: 2.80, 95% CI 0.78; 10.0).[
7] In the studied population, no association was found between dengue serotype and gender, except for a slight predominance of DNV-2 in women. A study conducted in Mexico from 2012 to 2017 revealed that 60% of dengue cases were in women with an average age of 33 years; however, men have 0.91 (95% CI: 0.86, 0.97) times more chances than women of having a secondary infection, and they tend to have higher odds [1.40 (95% CI: 1.35, 1.46)] of severe infection. [
22]
Regarding clinical presentation, DNV-3 was mainly identified, being higher in non-severe dengue; this was probably due to the predominant circulation of this serotype, after years without circulation in Mexico, which clinically manifested as an increase in cases of dengue with warning signs compared to the previous four years. [
23]
The cycles (Ct) for RT-qPCR for dengue were 26, except for DNV-4, which was 25.97, corresponding to approximately 1x105 copies, according to published data. [
24] There is no reported low Ct during pregnancy, although one hypothesis could be related to immunological changes during this period. The high Ct in severe cases and deceased patients could be associated with pre-existing conditions of the patients that were not explored in this study. The Ct values detected in this study were also higher compared to another study in Nepal for DNV-1, DNV-2, and DNV-3; the average Ct value for all serotypes was 17.6, and for DNV-3, it had the lowest Ct value of 16.6, indicating high viremia and a probable re-emergence of this serotype after years of absence of circulation. However, the Ct values detected for the rest of the serotypes were not significantly different to confirm this hypothesis. [
25]
In our study, logistic regression models revealed that the greatest risk for coinfection was associated with the peak period of an outbreak, residing in a hyperendemic area with circulation of all serotypes, and the predominant serotype at that time, as previously described.[
26] Similarly, a study in Mexico uncovered asymptomatic arbovirus infections, which could explain why individuals living in an endemic region may become re-infected, leading to silent infections with positive laboratory diagnoses. In the case of dengue, asymptomatic infections could signify reinfections by homologous serotypes or indicate the potential risk of developing severe forms with infections by heterologous serotypes.[
27]
The risk of hospitalization, severe dengue, and death remained consistent for the state of Morelos, an area with circulation of all four dengue serotypes and endemicity. However, this risk was not higher than that observed for the state of Veracruz. Unfortunately, other variables were not available to conduct a more in-depth analysis of the causes, although it has been documented that the risk is increased by concomitant diseases, such as cardiac conditions.[
28]
One of the limitations of this study included: 1) absence of variables regarding clinical conditions, 2) pathological history to establish better associations, 3) additionally, there was a low incidence of coinfections and lethality, which hindered a better stratification of the data. Despite this, the strengths include: 1) a study conducted on a population with social security coverage across a significant portion of the national territory, 2) with samples from cases reported in the epidemiological surveillance system, 3) confirmed infection through RT-qPCR and determination of serotype. It is one of the first studies in a population with social security coverage in Mexico to reveal the circulation of DNV-3 during 2023.
Lastly, DNV-2 was associated with age for hospitalization and severe dengue, and the presence of coinfection increases proportionally with an increase in transmission, with age differences observed for serotype infection.