Background
Improved survival rates for patients with hematological malignancies due to diagnostic and therapeutic advances have led to an increase in the number of survivors at risk for long-term side effects of chemotherapy [
1]. With an overall 5-year survival rate of around 70%, non-Hodgkin lymphoma (NHL) represents the seventh most frequent tumor worldwide, accounting for more than 4% of all cancers in the USA alone, with approximately 75,000 new cases per year, and a median age at diagnosis of 67 years [
2,
3] . In contrast, Hodgkin lymphoma (HL) presents overall 5-year survival rates of around 90%, and is less common, with around 80% of cases diagnosed in patients younger than 65 years. [
4]
Anthracyclines have been a mainstay of treatment for both NHL and HL since 1976, when they were first used as part of different immunochemotherapy regimens, including cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), before being combined with the anti-CD20 monoclonal antibody rituximab in 1995. Long-term cardiotoxicity such as heart failure (HF) is a well-described side effect of treatment with anthracyclines used in regimens with curative intent [
5], although other molecules used in lymphoma treatment (such as cyclophosphamide) are also capable of causing cardiotoxicity within the first days of treatment, leading to early HF [
6].
The classic model of anthracycline-induced toxicity involves hyperproduction of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS), producing DNA damage, protein carbonylation and lipid peroxidation and leading to cellular dysfunction and cardiomyocyte death. On the other hand, anthracyclines can also bind and block the functions of both topoisomerases 2A (TOP2A) and 2B (TOP2B). Tumor cells express high levels of TOP2A, whereas TOP2B is expressed ubiquitously, including by cardiomyocytes; this difference in topoisomerase expression leads CRTCD [
7]
Both the antitumor effect of anthracyclines and the probability of drug-related toxicity are dose-dependent. A lifetime cumulative dose of 400 mg/m2 has been associated with a 10% risk of developing congestive HF, while higher doses lead to an exponential increase in risk (up to 50% at 700 mg/m2) [
8,
9]. However, patient vulnerability to CTRCD is highly variable, with some patients developing HF at lower doses or long after ending chemotherapy.
The clinical spectrum of anthracycline-induced CTRCD related ranges from asymptomatic left ventricular systolic disfunction to advanced stage HF with massive pump failure, potentially ending in heart transplantation or even death[
10]. Other cardiotoxic events include myocardial Infarction, thromboembolism, conduction disorders, valvopathies and cardiomyopathies [
11]. Although the mechanisms behind these events are not fully understood, patients with previous cardiovascular comorbidities (hypertension, left ventricular hypertrophy, diabetes, alcohol intake, smoking, etc.) have a higher risk of developing CRTCD [
12].
Despite anthracycline-induced CRCTD being a well-described phenomenon, the use of anthracyclines as first-line treatment for patients with NHL is expected to increase [
13]. Careful assessment of the risk-benefit tradeoff between inducing HF and curing cancer is critical for patients and health systems[
10]. Our study aims to characterize patients with a high risk for anthracycline-associated CRCTD, in order to improve clinical management for these patients.
Material and Methods
Participants and Study Metods
We performed retrospective study in patients diagnosed with NHL or HL treated with anthracyclines as first line therapy between 1st January 2017 and 31st December 2019. Patients were identified using the hospital pharmacy department’s database, which prospectively collects data on patients receiving anthracycline-containing regimens. Findings were compared with data from the electronic medical record. The follow-up period ended on 15th February 2023.
Inclusion, Exclusion Criteria and Study Period
All patients from the hospital pharmacy department’s database aged 18 years or above with a diagnosis of HL or NHL, and who had received anthracycline-containing regimens as first-line therapy (R-CHOP, CHOP, R-mini-CHOP, mini-CHOP, R-COMP and R-ABVD, ABVD) were included. Patients who did not meet the main inclusion criteria were excluded. The anthracycline doses used were standard according to each chemotherapy protocol, and patients who had received concurrent therapy such as radiotherapy for lymphoma treatment were not included. Accumulated doses of anthracyclines were not recorded.
The baseline for the study period was immediately prior to starting a chemotherapy regimen with anthracyclines. Patients were followed until their last recorded appointment or death (until 15 February 2023).
Exposure and Covariates
A retrospective chart review, using data from the electronic medical record and the hospital pharmacy database, was carried out for all patients. We extracted treatment-related data, including chemotherapy regimen (R-CHOP, R-mini-CHOP, CHOP, mini-CHOP, R-COMP, ABVD, R-ABVD) and start date as well as lymphoma subtype (HL, diffuse large B cell lymphoma, follicular lymphoma, mantle cell lymphoma, mediastinal lymphoma, MALT lymphoma and marginal zone splenic lymphoma).
Data on potential confounding variables were also collected, including patient demographics (age, sex, BMI), cardiovascular risk factors (hypertension, dyslipidemia, alcohol intake and smoking status), and previous medical history of cardiomyopathy, myopericarditis, or arrhythmias, (defined as previous cardiovascular disease). Information on cardiovascular medication was also retrieved.
Analytical (hemoglobin, pro-BNP, troponin) and echocardiographic (Left Ventricle Ejection Fraction (LVEF) and presence of valve disease or cardiomyopathy) covariates were collected. Troponin levels were measured in relation to anthracycline administration as biochemical monitoring protocols before each anthracycline administration, as well as pro-BNP leves.
Outcomes
The primary objective of our study was to identify the incidence of CTRCD in patients with NHL or HL in first-line treatment with anthracyclines. Our secondary objective was to identify the prevalence of risk factors previously described in the literature in our cohort. Overall survival (OS) and time to treatment event (TTE) were also included as secondary endpoints.
We defined CTRCD as a composite of heart failure, myocardial infraction, thromboembolism, conduction disorders, and the development of valve disease or cardiomyopathy. This inclusive approach inclusive approach aims to capture the full spectrum of anthracycline-induced cardiac effects, acknowledging that while some events like valve disease and thromboembolism are less commonly attributed directly to anthracycline, their inclusion reflects the complex clinical presentations observed in practice.
Data Collection, Storage and Ethics
We designed a database in Excel format, pseudonymizing patient data by assignation of a numerical identification code following the order of recruitment. The database did not contain patients’ personal information.
The raw data obtained during the study is considered confidential and will be treated in accordance with the General Data Protection Regulation (May 25th, 2016) which has been transposed into our legislation as the Organic Law 3/2018 (December 5th, 2018), on the protection of personal data. The database will be kept for up to two years after the end of the study, after which the data will be deleted permanently.
This study was conducted in accordance with the ethics standards of the institutional research committee and the Declaration of Helsinki, Hospital Clinical Research Ethics Committee (EO047-19_FJD). Being a retrospective study, it was not necessary to request informed consent.
Stastical Analysis
A descriptive analysis of the population was performed. Qualitative variables were described using frequency tables. Continuous variables that followed a normal distribution were described by mean and standard deviation. Continuous variables that did not follow a normal distribution were described using the median and interquartile range.
Normality was verified using the Kolmogorov Smirnov test. To study the relationship between the different variables, Student's t tests or Analysis of Variance were used for variables with normal distribution, and the Mann-Whitney or Kruskal-Wallis tests for variables with non-normal distribution. Univariate analyzes were carried out and those variables with statistically significant results were entered into a multivariate regression model. Survival curves were traced by the Kaplan-Meier method and compared with the logarithm of the rank (LogRank) test. Statistical analyses were performed using SPSS v25 for Windows (SPSS Inc., Chicago, IL, USA).
Results
A total of 200 patients were included. Incidence of CTRCD and cardiovascular risk factors were identified by collecting data from participants’ electronic medical records. Patients’ characteristics are shown in
Table 1.
The incidence of CTRCD was 17.4% (35/200). Patients suffering from CTRCD were older, with a mean age of 65.17 ± 16.41 years vs 56.77 ± 17.18 years in the subgroup of patients who did not develop CT (p = 0.008). The distribution of cardiotoxic events is shown in
Table 2.
DL was more frequent in the group who developed an event (36.84 % vs 16.67%, p = <0.00001), as well as previous cardiovascular disease (61.05% vs 14.02%, p = <0.00001). We found no differences between other cardiovascular risk factors described in the literature such as female sex (21.54% vs 18.75%), hypertension (30.3% vs 16.67%), diabetes (20% vs 20.17%), obesity (21.15% vs 19.84%), smoking (21.43% vs 19.54%) or alcohol intake (13% vs 18.8%) (
Table 3).
We found no differences between patients receiving liposomal anthracyclines (all of whom presented previous cardiovascular disease) and those receiving conventional therapy (27.8% vs 17%, p= ns). Likewise, no differences in cardiotoxic events were found between patients with and without previous reduced LVEF (14.3% vs 18.1%, p= ns;
Table 4), or with and without valvulopathy (21.5% vs 15%, p=ns). However, we found significant differences between patients with and without previous history of cardiomyopathy at diagnosis (24.5% vs 11.8%, p= 0.026).
Prescriptions of cardiovascular drugs, such as angiotensin converting enzyme (ACE) inhibitors, beta blockers, or angiotensin receptor antagonists, were recorded as part of the data collection process. These prescriptions formed part of patients’ habitual treatment for underlying cardiovascular disease, with no differences in CTRCD incidence between groups.
Regarding cardiac biomarkers of myocardial infarction, no differences in troponin values were observed at baseline, although differences were found at intermediate (0.031-0.035 µg/L, p= 0.0009) and final (post-treatment) assessment (0.007-0-033 µg/L, p = 0.0082). Although these troponin values do not exceed our laboratory’s upper reference limit (N < 0,08µg/L), differences between values reach statistical significance, suggesting that, in our cohort, an increase in troponin levels during treatment could be considered a risk factor for developing CTRCD. (
Table 5)
Mean baseline NT-proBNP levels in the group of patients with cardiovascular events were 456.1 µg/L ±101.02, and 120.1 µg/L ±26.22 in the “no event” group (p = 0.052). Mean NT-proBNP at mid-treatment was 536.5 µg/L ±111.03 vs. 125.9 µg/L ±31.78 (p=0.03), while mean NT-proBNP after completion of treatment was 785.7 µg/L ±678.76 vs. 165.4 µg/L ±37.88 (p = 0.033). The upper reference limit in our laboratory is 250 µg/L (
Table 6).
No significant differences in hemoglobin levels were observed between groups at baseline and during follow-up, although a tendency towards lower hemoglobin counts was found in the cardiotoxic event group before, during and after treatment. Mean baseline hemoglobin was 11.8 gr/dL ± 0.34 for patients with a cardiotoxic event and 12.6 gr/dL ± 0.16 in those without (p = 0.503) (
Table 7).
After performing the univariate analysis and identifying statistically significant variables, we conducted a multivariate analysis (
Table 8), identifying dyslipidemia, age 72 years or older, previous cardiovascular disease and basal NT-proBNP as risk factors for cardiotoxicity in patients receiving treatment with anthracyclines as first-line treatment for lymphoma.
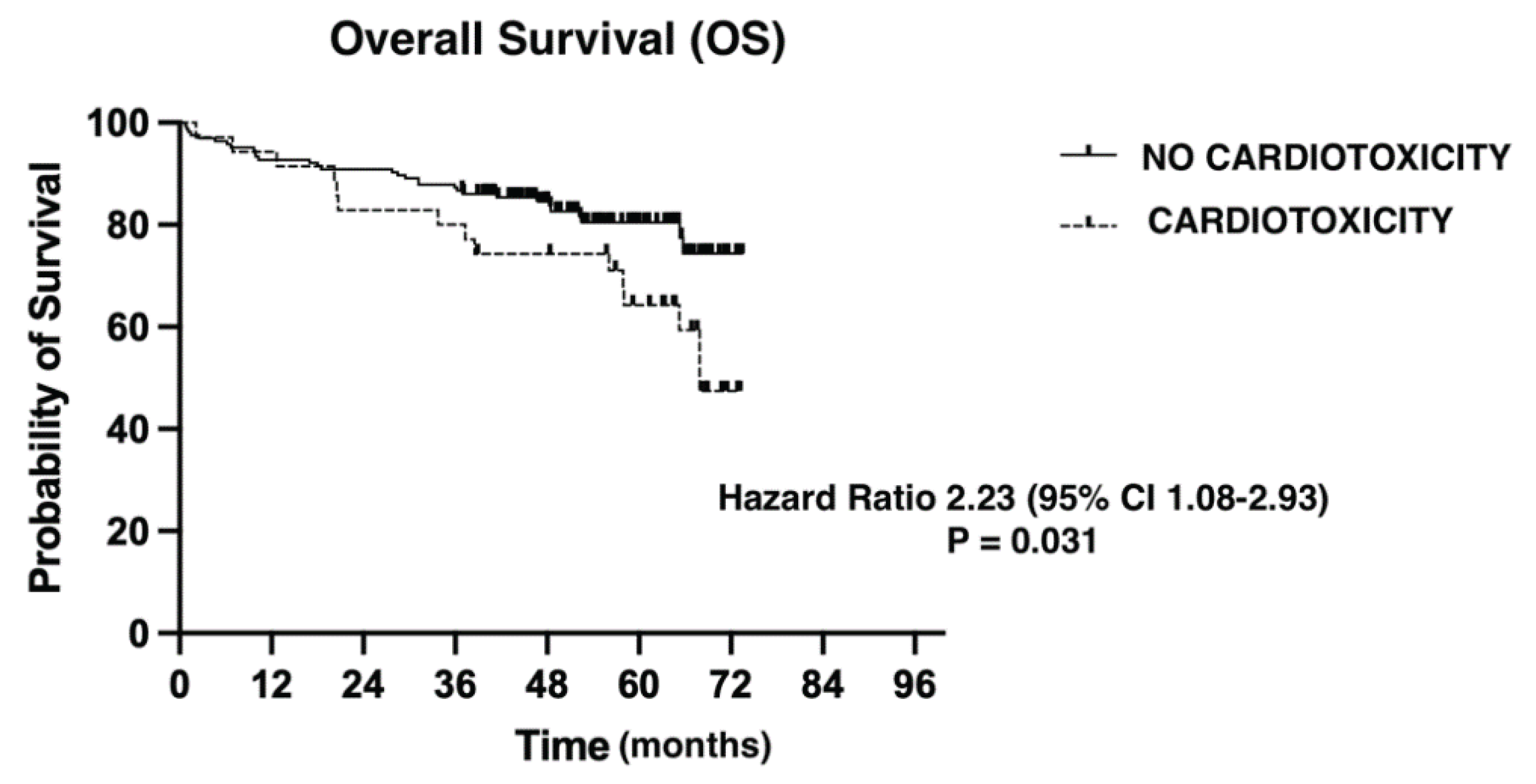
We evaluated overall survival using the Kaplan-Maier method (
Figure 1). Median follow-up was 51.83 months (0.76-73.49). The presence of a CT event had a negative impact on OS from any cause (Hazard Ratio= 2.23 (95% CI 1.08-2.93), p = 0.031).
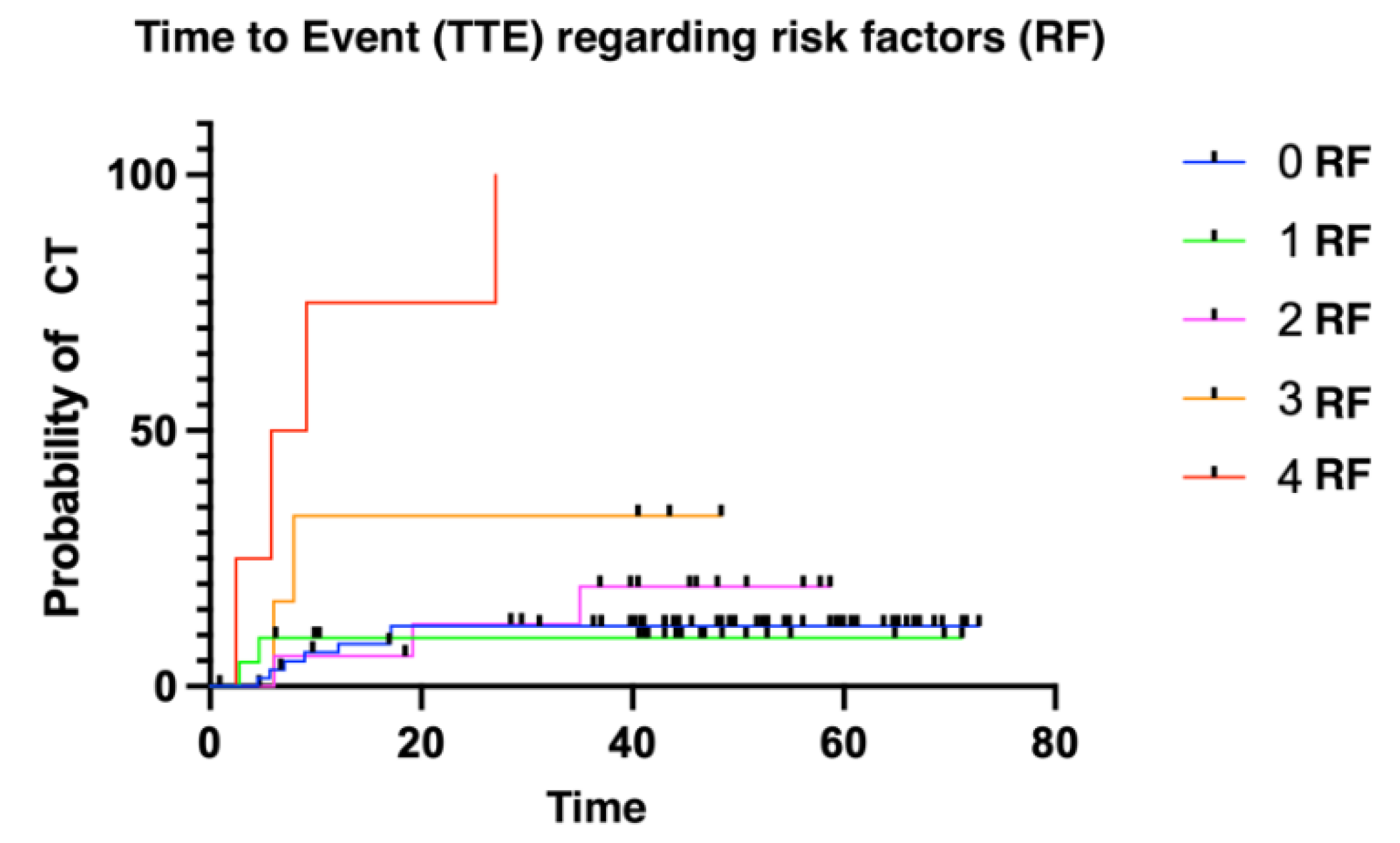
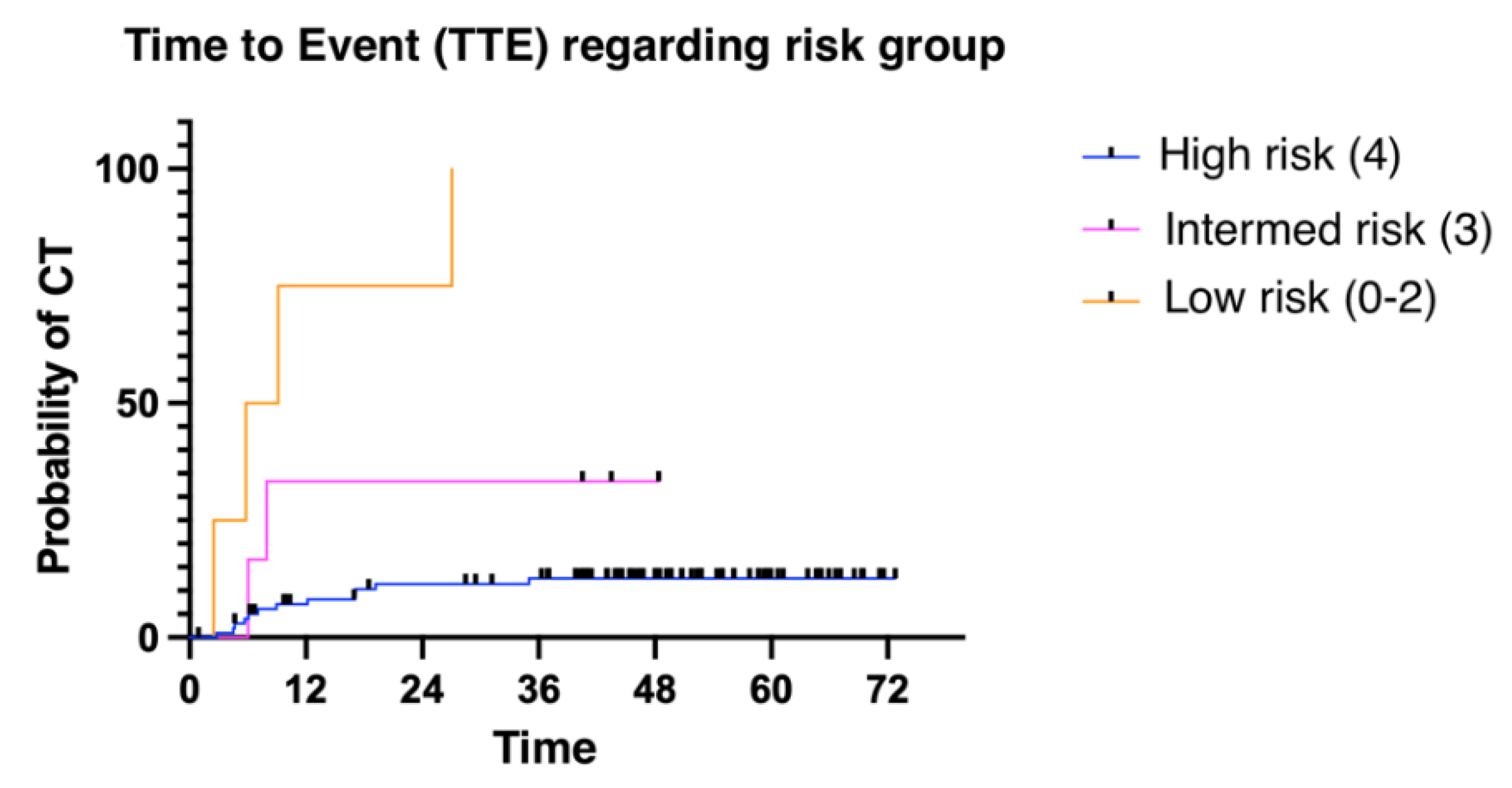
The median time to presenting a cardiotoxic event was 6.11 months (1.02 - 52.86). We divided patients into three groups: low risk (≤ 2 risk factors for CT); intermediate risk (3 risk factors); and high risk (4 risk factors). Patients presenting more risk factors for cardiotoxicity presented with cardiotoxic events earlier than patients with fewer risk factors (
Figure 2 and
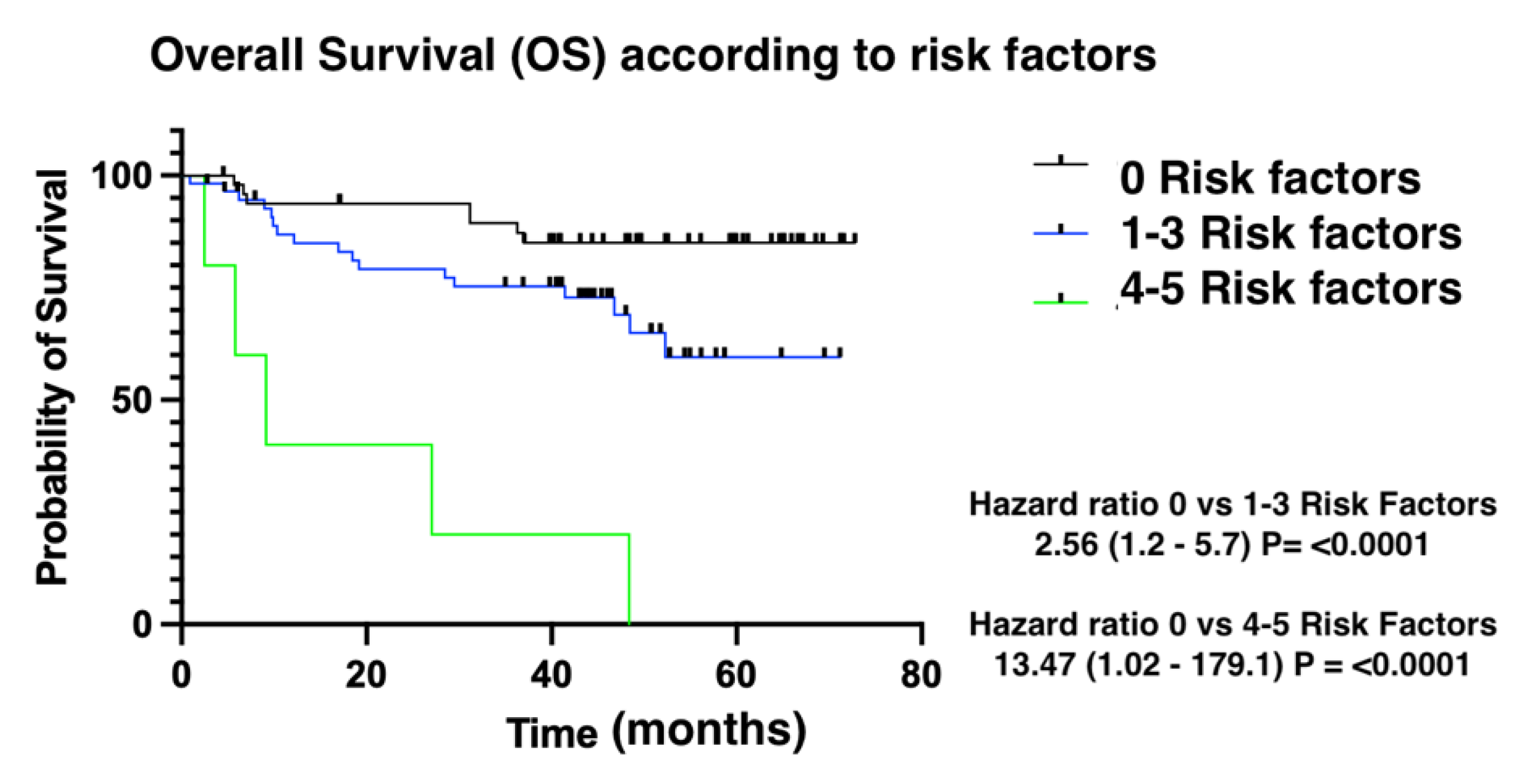
Figure 3). Finally, overall survival was also influenced by the number of risk factors for CTRCD. In this case, low-risk patients were identified as those with no risk factors, intermediate-risk patients as those with between 1 and 3 risk factors, and finally, high-risk patients as those with 4 risk factors (
Figure 4).
Discussion
Anthracycline-induced cardiotoxicity is a growing challenge for public health, due to the ageing population with an increasing prevalence of cardiovascular risk factors and the widespread use of anthracyclines in lymphoma therapy, with high response rates and improved overall survival [
14]. Our study reports incidence and risk factors for cardiotoxicity in a cohort of 200 lymphoma patients undergoing first-line treatment with anthracyclines.
The incidence of cardiotoxicity in our cohort is 17.4%, higher than that found in previous studies. Boddicker et al. report cardiotoxicity rates of 10.7% [
15], while Curigliano et al. describe an incidence of 3-5% [
16]. This difference may be due to several factors. First, the aforementioned studies do not include venous thrombosis in their definition of cardiotoxic event [
17]. Secondly, discrepancy exists between different echocardiographic criteria for cardiotoxicity[
10]. Our institution uses the classification published by the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI), which defines cardiotoxicity as a decrease in left ventricular ejection fraction (LVEF) of more than 10 percentage points below the normal reference value (53%), independently of the presence or absence of symptoms[
18].
Incorporating microangiopathy could significantly enhance understanding of its mechanisms, especially in light of findings from the AIM PILOT Study which suggests a critical role of microcirculation disorders in anthracycline-induced cardiotoxicity. Furthermore, observations around liposomal anthracyclines offer intriguing insights into their potential protective effects against CTRCD, warranting further investigation. Highlighting these aspects not only broadens the discourse on cardiotoxicity but also underscores the importance of continued research into protective strategies and the underlying pathophysiology of CTRCD[
19]
Age has been reported as a risk factor for CTRCD. Our study reports statistically significant differences in the mean age of patients with and without CT. Aging is associated with aortic stiffness, left ventricle remodeling due to myocardial hypertrophy, myocardial fibrosis, and cardiac amyloidosis. Increased susceptibility to toxicity is determined by complex interactions between the cardiovascular aging process and cardiovascular risk factors, comorbidities (such as anemia or kidney disease), and disease modifiers such as sex[
20].
Several cardioprotective interventions using classical HF drug therapies (beta-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers) have recently been tested in controlled trials, although none have shown robust beneficial effects to date. Although these studies have many limitations, including important heterogeneity, some indicate a potential clinical benefit for the prevention of HF [
20,
21]. In our cohort, however, patients taking classical HF drug therapies did not present a lower risk of CT, although it is important to highlight that the medication formed part of chronic treatment for underlying PCVD instead of primary prevention.
Regarding echocardiography, general speaking only patients with normal LVEF are prescribed anthracyclines, pointing to a selection bias in our cohort, as only patients with normal LVEF were selected to receive anthracyclines. This same bias occurred in patients treated with liposomal anthracyclines, as only patients with previous cardiac disorders were prescribed this variation, aimed at reducing the risk of CTRCD, probably underestimating its protective factor for the development of this toxicity.[
22] Of the biomarkers of myocardial damage featured in our cohort, only NT-proBNP proved a risk factor for cardiotoxicity in the multivariate analysis. Troponin I levels were significantly different between groups during and after treatment, although not at baseline, with mean values within the laboratory’s normal reference limits.
it is vital to align with the 2022 European Society of Cardiology (ESC) guidelines, which introduce comprehensive criteria for diagnosing cardiotoxicity. These guidelines emphasize the importance of left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) as critical parameters. LVEF, a traditional measure of cardiac function, and GLS, a sensitive indicator of subtle myocardial deformation, together provide a robust framework for early detection and diagnosis of cancer therapy-related cardiac dysfunction (CTRCD), ensuring timely intervention and management
An important finding of our study is that the number of risk factors for cardiotoxicity impacts both time to treatment event as well as overall survival related to death from any cause. This emphasizes the importance of closely monitoring lymphoma patients receiving anthracycline therapy and implementing strategies for optimal management of risk factors such as dyslipidemia and previous cardiovascular disease.
Our study is limited by its retrospective design, leading to missing information on the control of cardiovascular risk factors during treatment. Also, our analysis did not consider variables that may directly influence plasma levels of biomarkers for myocardial damage. The influence of factors such as renal failure or obesity on troponin I plasma levels has been described in the literature and may be a confounding factor in our study [
23,
24].
Cardio-oncology is a complex field which requires individual patient assessment as well as the creation of multidisciplinary teams. Where future developments are concerned, the recent publication of the European Society of Cardiology’s guideline on cardio-oncology [
25] and the application of techniques such as cardiac magnetic resonance imaging in oncology patients [
26]will help to identify patients at risk of developing anthracycline-associated cardiotoxicity in a more homogeneous and precise manner, allowing clinicians to develop effective preventive strategies.
Conclusions
Our study demonstrates that age is a risk factor for anthracycline-induced cardiotoxicity. Dyslipidemia, previous cardiovascular disease and increased NT-proBNP levels at baseline and follow-up are also predictors of cardiotoxicity. On the other hand, reduced LVEF was not associated with an increased risk of CTRCD, probably due to positive selection of patients with normal ventricular function at the start of treatment. Patients with a previous history of cardiomyopathy presented a higher incidence of cardiotoxicity, while no differences were found in patients with valvulopathy.
In our cohort, the use of cardioprotective drugs was not associated with reduced rates of cardiotoxicity. A possible explanation is the existence of a certain selection bias, as the drugs had been prescribed to treat underlying conditions, instead of as primary prevention. Selection bias may also explain why patients with normal baseline LVEF and those receiving liposomal anthracyclines showed similar rates of cardiotoxicity to those with reduced LVEF and those receiving standard anthracycline treatment.
Patients who developed CTRCD during treatment with anthracyclines presented higher all-cause mortality rates than patients without cardiotoxic events. The number of risk factors is correlated with time to event, as well as with overall survival.
CTRCD is an important challenge for cardio-oncologists worldwide. Actions geared towards reducing modifiable risk factors such as dyslipidemia and optimizing management of cardiovascular comorbidities are key for preventing cardiotoxic events in patients with lymphoma.
Author Contributions
ALG literature search, conception and design, data collection, analysis and interpretation of data, manuscript preparation and writing, tables, approval of final document. EM conception and design, data interpretation of data, approval of final document. SGM conception and design, data interpretation of data, approval of final document. DM and JT approval of final document. BI study design, analysis and interpretation of data, approval of final document. RC study design, literature search, statistical analysis, analysis and interpretation of data, approval of final document. All authors read and approved the final manuscript.
Sources of Funding:
This study did not receive funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee in Fundación Jimenez Diaz Instituto de Investigacion Santiaria (protocol code EO047-19_FJD 12th March 2019)
Acknowledgments
Thanks to the physicians of the Hematology and Cardiology Departments of the Fundación Jimenez Diaz University Hospital for facilitating the study and making teamwork easy. We also thank Cristina Fernández MD (Department of Epidemiology) for her support. Special thanks to Arantxa Garcia for her unconditional support during the drafting process. We acknowledge Bernadette Pfang MD for her help with the English language revision of this manuscript.
Disclosures: The authors have nothing to disclose.
Abbreviations
| ABVD: doxorubicin, bleomycin, vinblastine and dacarbazine |
| ACE: angiotensin-converting enzyme |
| ARBs: angiotensin II receptor blockers |
| ASE: American Society of Echocardiography |
| BEACOPP: bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazina, prednisolone |
| CHOEP: cyclophosphamide, doxorubicin, vincristine, ethopoxide and prednisone, |
| CT: cardiotoxicityCTRCD: cancer therapy-related cardiac dysfunction" CI: Confidence Interval |
| DL: dyslipidemia |
| EACVI: European Association of Cardiovascular Imaging |
| HF: heart failure HT: Hypertension |
| HL: Hodgkin lymphoma |
| LV: left ventricular |
| LVEF: Left Ventricular Ejection Fraction |
| MALT: mucosa-associated lymphoid tissue |
| NHL: non-Hodgkin lymphoma |
| NT PROBNP: N-terminal B-type natriuretic peptideOR: Odds ratio OS: Overall survivalPCVD: Previous cardiovascular disease |
| R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. |
| R-DAEPOCH: etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituximab |
| RNS: Reactive Nitrogen species |
| ROS: Reactive Oxygen Species |
| TOP2A: topoisomerases 2A |
| TOP2B: topoisomerases 2B TTE: Time to event |
| VR-CAP: bortezomib, rituximab, cyclophosphamide, doxorrubicin, prednisone |
References
- Pulte D, Jansen L, Castro FA, Brenner H. Changes in the survival of older patients with hematologic malignancies in the early 21st century. Cancer. 2016, 122, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol 280, 280, 280–280. [Google Scholar] [CrossRef]
- Von Hoff, DD. Risk Factors for Doxorubicin-lnduced Congestive Heart Failure. Ann Intern Med. 1979, 91, 710. [Google Scholar] [CrossRef] [PubMed]
- Townsend W, Linch D. Hodgkin’s lymphoma in adults. Lancet Lond Engl. 2012, 380, 836–847. [Google Scholar] [CrossRef]
- Bansal N, Adams MJ, Ganatra S, et al. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardio-Oncol Lond Engl. 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet Lond Engl. 2013, 381, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Carrasco R, Castillo RL, Gormaz JG, Carrillo M, Thavendiranathan P. Role of Oxidative Stress in the Mechanisms of Anthracycline-Induced Cardiotoxicity: Effects of Preventive Strategies. Oxid Med Cell Longev. 2021, 2021, 8863789. [Google Scholar] [CrossRef]
- Mercurio V, Cuomo A, Della Pepa R, et al. What Is the Cardiac Impact of Chemotherapy and Subsequent Radiotherapy in Lymphoma Patients? Antioxid Redox Signal. 2019, 31, 1166–1174. [Google Scholar] [CrossRef]
- Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000, 342, 1077–1084. [Google Scholar] [CrossRef]
- Perez IE, Taveras Alam S, Hernandez GA, Sancassani R. Cancer Therapy-Related Cardiac Dysfunction: An Overview for the Clinician. Clin Med Insights Cardiol. 2019, 13, 117954681986644. [Google Scholar] [CrossRef]
- Cardinale D, Iacopo F, Cipolla CM. Cardiotoxicity of Anthracyclines. Front Cardiovasc Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010, 115, 155–162. [Google Scholar] [CrossRef]
- Wang, JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002, 3, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Our World in Data. Our World in Data. Accessed April 1, 2023. https://ourworldindata.
- Boddicker NJ, Larson MC, Castellino A, et al. Anthracycline treatment, cardiovascular risk factors and the cumulative incidence of cardiovascular disease in a cohort of newly diagnosed lymphoma patients from the modern treatment era. Am J Hematol. 2021, 96, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Curigliano G, Cardinale D, Dent S, et al. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. 2016, 66, 309–325. [Google Scholar] [CrossRef]
- Antoniak S, Phungphong S, Cheng Z, Jensen BC. Novel Mechanisms of Anthracycline-Induced Cardiovascular Toxicity: A Focus on Thrombosis, Cardiac Atrophy, and Programmed Cell Death. Front Cardiovasc Med. 2021, 8, 817977. [Google Scholar] [CrossRef]
- Plana JC, Galderisi M, Barac A, et al. Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy: A Report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef] [PubMed]
- Klotzka A, Iwańczyk S, Ropacka-Lesiak M, Misan N, Lesiak M. Anthracycline-induced microcirculation disorders: AIM PILOT Study. Kardiol Pol 2023, 81, 766–768. [Google Scholar] [CrossRef]
- Triposkiadis F, Xanthopoulos A, Butler J. Cardiovascular Aging and Heart Failure. J Am Coll Cardiol. 2019, 74, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006, 114, 2474–2481. [Google Scholar] [CrossRef]
- Henriksen, PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart Br Card Soc. 2018, 104, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Bracun V, Aboumsallem JP, van der Meer P, de Boer RA. Cardiac Biomarkers in Patients with Cancer: Considerations, Clinical Implications, and Future Avenues. Curr Oncol Rep. 2020, 22, 67. [Google Scholar] [CrossRef] [PubMed]
- Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Martin-Garcia A, Diaz-Pelaez E, Lopez-Corral L, et al. T2 Mapping Identifies Early Anthracycline-Induced Cardiotoxicity in Elderly Patients With Cancer. JACC Cardiovasc Imaging. 2020, 13, 1630–1632. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).