1. Introduction
See the end of the document for further details on references. The tea plant originated from China's Guizhou Plateau [
1] and holds immense cultural significance and health benefits as a traditional beverage [
2]. China is the world's leading tea producer and exporter with a cultivation area of 3.165 million hectares in 2020 constituting 62.1% of global tea production [
3]. However, the pursuit of higher tea yields has led to a notable increase in the use of ammonia nitrogen fertilizers in tea gardens [
4,
5]. This heightened usage has accelerated Al biogeochemical cycling through soils and tea plants. The result was increased soil acidification especially in regions marked by abundant rainfall and acid rain [
6,
7]. As a consequence, the degree of soil acidification in tea cultivation systems surpasses that observed in other economically significant crop systems. Soil pH levels < 4.0 can significantly hinder tea plant growth and this lowers both the quantity and quality of tea leaf production [
8]. Additionally, soil acidification results in the leaching of crucial nutrients such as K, Na, Ca, Mg, and P that also reduce productivity and tea leaf quality [
9,
10,
11].
Among the nutrients prone to leaching in acidified soils, Mg is one of the necessary elements for plant growth and development and is an essential component of chlorophyll [
12]. Most soil Mg is bound within crystal mineral lattices and 90-98% is unavailable for direct plant uptake [
13]. Plants can only absorb the magnesium ion (Mg
2+) form of this element although its large size (ionic radius) results in weak associations with root cell walls and negatively charged soil particles and thus is susceptible to leaching in acidic soils [
13]. Plant Mg deficiency has become a significant factor inhibiting agricultural production [
14].
Previous studies have also demonstrated that Mg deficiency suppresses photosynthesis, leading to carbohydrate accumulation in source leaves, inhibiting root growth and altering both leaf and root morphologies [
15]. The activity of key enzymes in photosynthesis including ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) also decrease under Mg deficiency resulting in reduced photosynthetic rates [
16]. Mg deficiency also leads to a reduction in chlorophyll content, the occurrence of leaf chlorosis, changes in leaf ultrastructure including chloroplast swelling, deformation and disintegration as well as disruption of thylakoid continuity resulting in photooxidative damage [
17,
18,
19]. These structural changes adversely affect leaf functionality and photosynthetic efficiency. Moreover, Mg deficiency interferes with carbohydrate transport, preventing the effective transport of photosynthetic products to the roots and other non-photosynthetic tissues, thus limiting plant growth and development. Replenishing Mg rapidly enhances the export of sucrose from source leaves to the phloem [
20].
Both soil acidification and Mg deficiency present stress factors for plants and increase the production of reactive oxygen species (ROS) [
21,
22]. ROS are toxic by-products of aerobic plant processes including photosynthesis, photorespiration and respiration [
23] and ROS can react with lipids, proteins and nucleic acids resulting in lipid peroxidation, protein denaturation and genomic mutations, respectively [
24]. However, ROS also serve as signaling molecules in plant cells and play vital roles in programmed cell death regulation and other physiological activities. Under normal conditions, the production and removal of ROS are in dynamic equilibrium [
25]. The detrimental effects of excess ROS on cellular metabolism under stress conditions are countered in plants by an antioxidant defense system that minimizes lipid peroxidation of cellular and organelle membranes [
21,
26,
27]. Enzymatic antioxidant systems are integral to this defense system and under stress conditions, cells initially enhance their antioxidant defense system by increasing the activity of superoxide dismutase (SOD) that acts in the first line of defense from ROS. The activities of catalase (CAT) and peroxidase (POD) also increase under these conditions [
15]. SOD catalyzes the breakdown of superoxide anions (O
2•-) into hydrogen peroxide (H
2O
2) that is then converted to water and oxygen via CAT and POD among others [
28]. When ROS production exceeds the capacity of the clearance system, the latter cannot provide sufficient protection for the membrane to prevent photooxidation, This leads to oxidative stress and a significant increase in malondialdehyde (MDA) levels that are also used as an indicator of the degree of exposure of the plant to these types of processes.
Soil acidification has become an established reality. Without a focus on plant nutrition, including Mg nutrition, the combination of acid and nutrient stress could result in significant losses in productivity. Given that tea plants are predominantly grown in the rainy, acidic soils of southern China characterized by low cation exchange capacity and low Mg content, we investigated the importance of Mg nutrition on the antioxidant response and quality of one-year-old tea seedlings under acid stress as a model for remediation of these soils via Mg supplementation.
2. Materials and Methods
2.1. Plant Culture and Mg Treatments
We obtained one-year-old tea seedlings (Longjing 43) from the Tea Seedling Breeding Base located in Fuyang District, Hangzhou, Zhejiang Province, China. Longjing 43 is a commonly cultivated tea cultivar in China and is frequently used for biological research. These seedlings were propagated through asexual reproduction and were cultivated hydroponically at the Pingshan Experimental Base of Zhejiang Agriculture and Forestry University. Prior to cultivation, soil was thoroughly washed from the seedling roots using deionized water as part of a pre-cultivation process. The plants were then placed in black plastic containers (9 L) filled with deionized water, for 6 days and the water was then replaced with a 25% aerated nutrient solution. The nutrient solution concentration was gradually increased every 6 days until it reached 100%. The experiments were commences following a three-week acclimatization period. During the pre-cultivation phase, we adjusted the pH of the nutrient solution daily to 5.0 using 0.1 M NaOH and 0.1 M H
2SO
4. The nutrient solution was replaced every three days. Magnesium nutrition was provided using MgSO
4. The experiment consisted of nine different treatment levels (refer to
Table 1) and for treatments lacking SO
42-, we supplemented the missing ion with a Na
2SO
4 solution.
2.2. Sample Preparation
Tea seedlings were separated into roots, stems and leaves at random intervals of 1, 7, 15 and 30 days and the plants were thoroughly rinsed with deionized water to remove soil. Chlorophyll concentrations were measured in fresh leaf samples. Prior to evaluation of quality parameters and nutrient element content of the tea leaves, fresh samples were blanched at 105°C for 15 minutes, then dried in an oven at 60°C to constant weight. The samples were ground to pass through a 40-mesh sieve and stored for later use. In addition, fresh tea leaf samples were promptly frozen in liquid nitrogen and preserved at -80°C.
2.3. Determination of Chlorophyll Concentration and Root Viability
The concentration of chlorophyll in fresh mature leaves was determined using a modified version previously described (Li et al., 2021). In brief, the leaves were washed, dried and primary veins were removed followed by chopping into small pieces and placement (0.20 g) in a 25 mL volumetric flask. The flask was filled with 95% ethanol and the samples were allowed to steep in the dark until the leaves turned completely white. Absorbance values were measured at 470, 649 and 665 nm and the content of chlorophyll a, chlorophyll b and carotenoids were calculated. Each treatment was replicated three times.
To determine root vitality, the triphenyl tetrazolium chloride (TTC) method was used. In brief, a 10 mL beaker was filled with 0.2 g of root tip sample and 5 mL of 4 g·L-1 TTC solution and 5 mL of phosphate buffer solution were added. The samples were kept in the dark at 37°C for 2 h and the reaction was terminated by adding 2 mL of 1 M sulfuric acid. Simultaneously, a control experiment was conducted, wherein sulfuric acid was initially added to the root sample, followed by the addition of other chemicals after 10 minutes. The procedural steps were consistent with those outlined in the previous experiment. Extract the roots, remove excess moisture, and grind them together with 3-4 ml of ethyl acetate and a small amount of quartz sand in a mortar. Transfer the red extract to a test tube, and wash the residue with a small amount of ethyl acetate two or three times, combining all washings in the test tube. Finally, adjust the total volume to 10 ml with ethyl acetate. After thorough mixing, the absorbance of the extraction solution was measured at 485 nm using the blank sample as a reference. TTC reduction was determined based on the absorbance values using a standard curve constructed using red formazan (1,3,5-triphenyltetrazolium formazan).
2.4. Antioxidant Enzyme and Malondialdehyde Determination
Antioxidant enzyme levels (SOD, POD and CAT) and MDA content were measured as previously described with slight modifications [
29]. In brief, 0.1 g of leaf tissue (excluding the main vein) and root tissue and 3 steel balls were ground using a mill into a paste in 2 mL centrifuge tubes containing 50 mM phosphate buffer pH 7.8 containing 1.0% polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 12,500 × g for 20 min at 4°C and antioxidant enzymes and MDA levels were measured in supernatants as follows (all assays were conducted at temperatures between 2-4°C): SOD activity was measured using the nitro-blue tetrazolium (NBT) photochemical reduction method; POD activity utilized the guaiacol method and CAT activity was determined via UV absorbance; MDA content was measured using the thiobarbituric acid (TBA) colorimetric method [
29].
2.5. Nutrient Content Measurements
Tea samples (0.2 g) were digested in 5 mL concentrated HNO3 and 2 mL 30% H2O2 at 180°C as previously described (Batista dos Santos Espinelli Junior et al., 2020) with slight adjustments. In brief, following digestion, the mineralized samples were transferred to volumetric flasks, diluted to a final volume of 30 mL with ultrapure water and filtered through 0.22 μm filters. The filtrate was used for flame atomic absorption analysis to quantify the Mg content. Dried samples of roots, stems and leaves (0.2 g each) were boiled in concentrated H2SO4-H2O2 until the solution was clear and then diluted with ultrapure water and adjusted to 50 mL. The N content was determined using indophenol blue colorimetry, K content using flame atomic absorption and the molybdenum antimony method was used to determine P content.
2.6. Tea Quality Determination
Following the standards of GB/T 8305-2013 "Determination of Water Extracts in Tea," the differential weighing method was employed to measure water extracts. Referring to GB/T 8312-2013 "Determination of Caffeine in Tea," the caffeine content was determined using ultraviolet spectrophotometry. According to GB/T 8314-2013 "Determination of Total Free Amino Acids in Tea," the total free amino acids were quantified using the ninhydrin colorimetric method. The content of tea polyphenols was determined through the iron tartrate colorimetric method.
2.7. Statistical Analysis
Data analyses were performed using IBM SPSS statistical software for Windows, Version 26.0 (IBM , Armonk, NY, USA). Significant differences between the data were processed on the basis of one-way analysis of variance and Duncan’s multiple range test (P < 0.05). Data are presented as mean ± SD.
3. Results
3.1. Effects of Mg Nutrition and pH on Overall Plant Health
We initially examined the effects of Mg supplementation and acidic conditions measured on the growth of our experimental tea seedlings. We tested pH 3.5, 5 and 6.5 with Mg additions of 0.01, 0.4 and 0.8 mM. We found that by 30 days, seedlings exposed to pH 5.0 exhibited the highest fresh weights. These mass increases were also positively correlated with Mg dose under similar pH conditions. We did not observe any significant distinctions between treatment groups (
Table 2). Similarly, seedling dry weights did not significantly differ between the treatment groups indicating a linkage between pH and Mg (
Table 3).
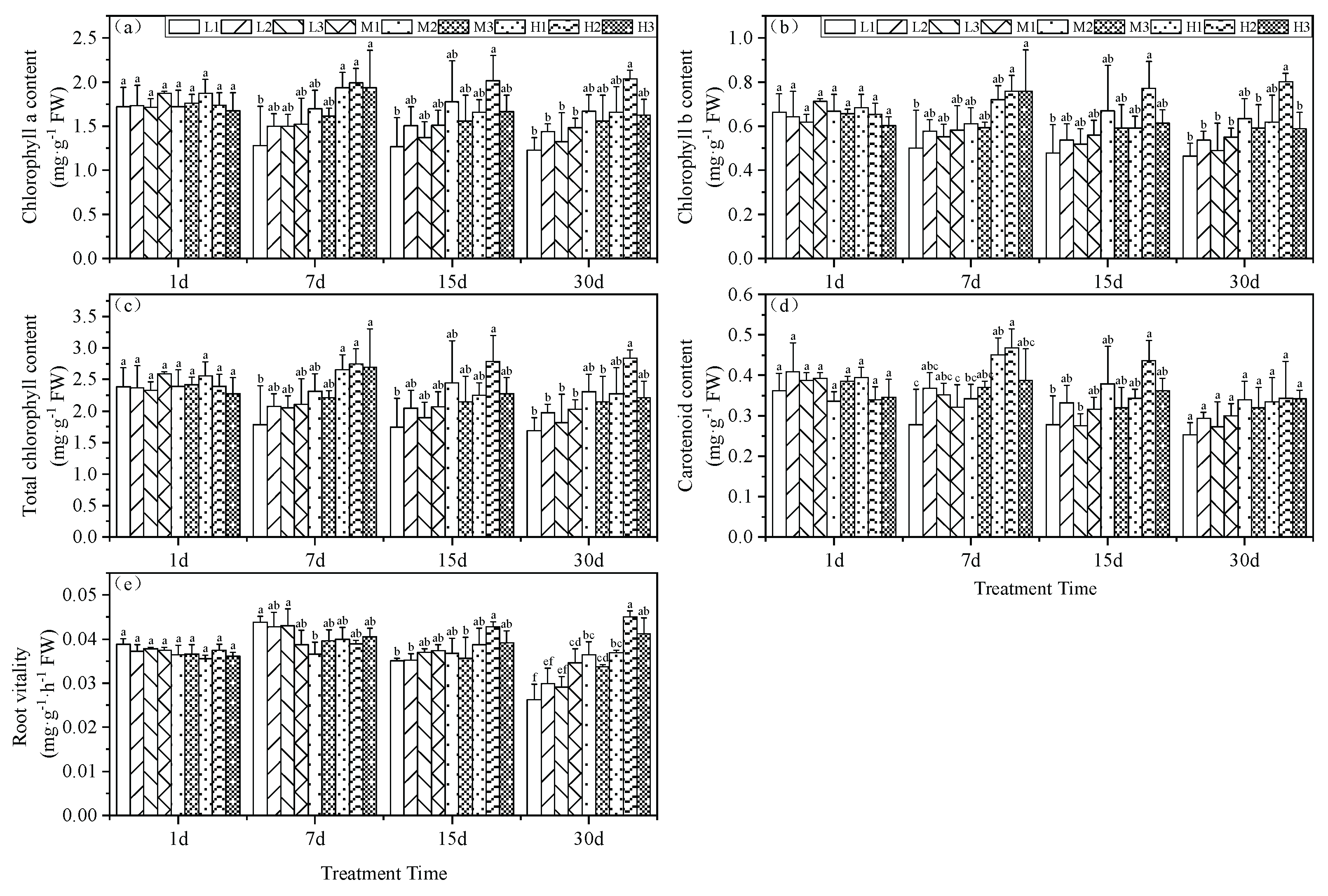
These growth effects of pH and Mg were further examined by measuring the chlorophyll content of the plant leaves. Beginning on day 7, we found a significant difference in chlorophyll a content between the pH groupings that increased with increasing Mg level. High (H) Mg treatments were similar and did not significantly differ and increased 13.9, 17.2 and 10.3% compared to the M2 treatment. Mg deficiency (L) conditions at pH 3.5 yielded the lowest chlorophyll a content; a decrease of 24.6%. By day 15, Mg treatments H1 and H3 generated chlorophyll a levels significantly lower than for the H2 treatment but did not significantly differ from the M2 treatment. On day 30, all low Mg treatments (L1, L2, and L3) and the M1 treatment yielded significantly lower chlorophyll a levels than the M2 treatment with reductions of 26.4, 13.8, 20.6 and 11.2%, respectively. The H2 treatment generated the highest chlorophyll a concentration that was significantly greater than the M2 treatment by 22.2% (
Figure 1a).
The chlorophyll b content of the experimental plants displayed a pattern similar to that of chlorophyll a. On day 7, the tea seedling leaves subjected to Mg deficiency at a low pH had the lowest chlorophyll b levels that were significantly lower than for the M2 treatment with a reduction of 18%. The high Mg treatments H2 and H3 displayed significantly higher chlorophyll b levels versus M2 with increases of 24.3 and 24.2%, respectively. On day 15, H3 and M2 did not significantly differ. At the end of the experiment at day 30, low Mg and pH (except for H1) had significantly lower chlorophyll b levels than the M2 treatment (
Figure 1b).
The total chlorophyll levels under Mg deficiency and acid stress (L1) were significantly less than for M2 at days 7, 15 and 30 with reductions of 22.9, 28.8 and 26.6 %, respectively. On day 7, all three high Mg treatments (H1, H2, and H3) were significantly higher than M2 with increases of 14.9, 19.1 and 16.5%, respectively. At the end of the experiment, the provision of high Mg nutrition under the same pH conditions mitigated the reduction of total chlorophyll under acid stress. H1 levels were greater that for L1 and M1 by 34.4 and 10.7%; H2 exceeded L2 and M2 by 43.8 and 23.3 % and H3 surpassed L3 and M3 by 22 and 3%, respectively. These results indicated that the combination treatment of pH 5.0 and high Mg significantly increased total chlorophyll (
Figure 1c). These findings collectively suggest that acid stress is detrimental to the accumulation of total chlorophyll in tea leaf tissues.
Carotenoid content was the lowest under acid stress and Mg-deficient conditions and at days 7 and 15, the levels were significantly enhanced under high Mg and in particular, at pH 5. In contrast, by day 30 there were no significant differences in carotenoid content among the various treatment groups (
Figure 1d).
Root vitality is another measure of plant health and this parameter peaked on day 7 under Mg deficiency but steadily declined thereafter in our experimental groups. By day 30, the pH 5.0 group (series 2) levels were significantly greater than for the other treatments at the same Mg level. This indicated that a continuous decrease in root environment pH reduces tea seedling root vitality. Furthermore, under the same pH conditions, increasing Mg levels enhanced tea seedling root vitality. In particular, these effects could be ranked as H1 > M1 and L1 with increases of 6.5 and 40.2%; H2 > M2 and L2 with increases of 23.6 and 50.7% and H3 > M3 and L3 with increases of 22.2 and 41.4, respectively (
Figure 1e).
3.2. Mg and pH effects on Plant Oxidative Stress Indicators
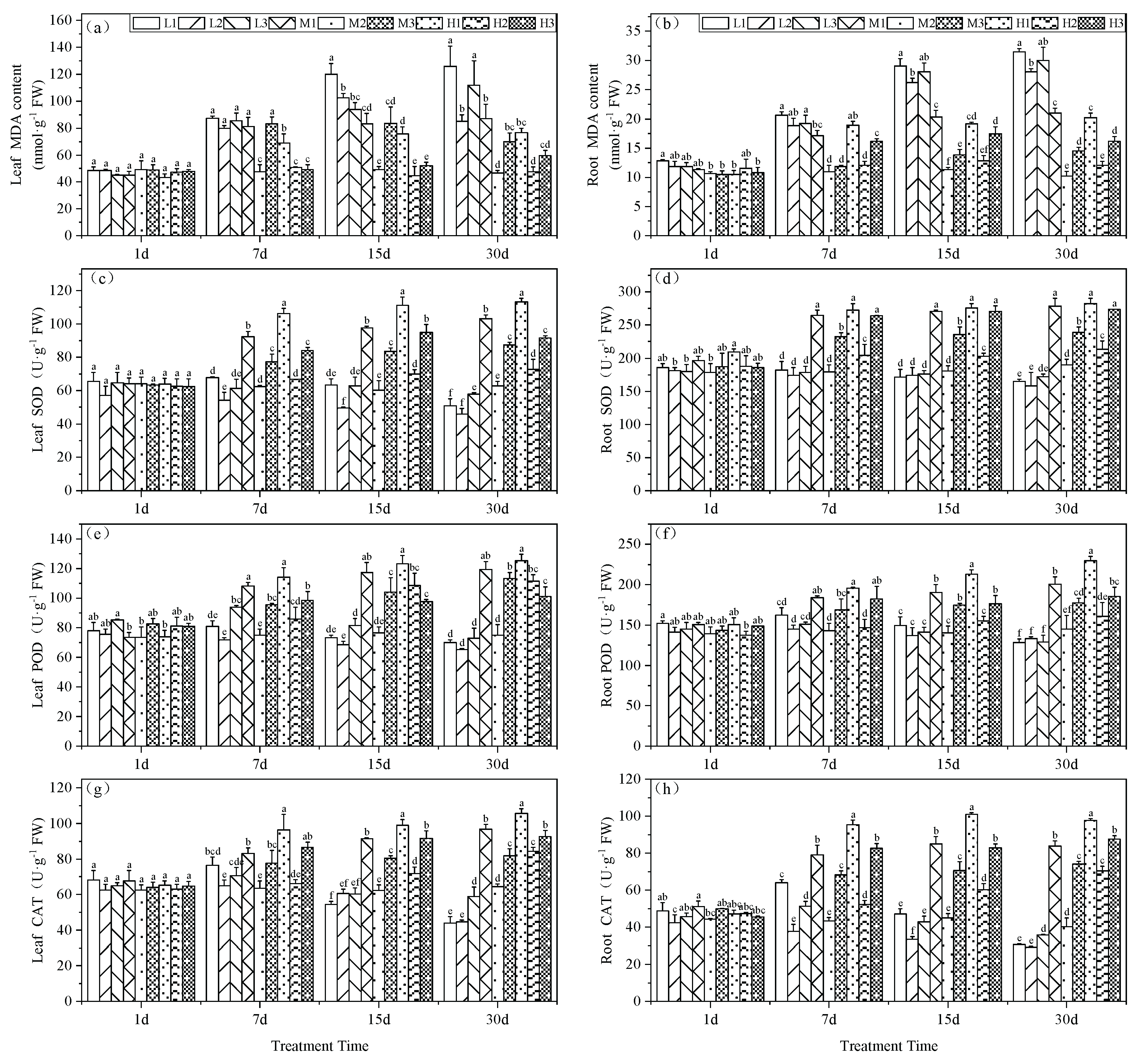
MDA levels are an indicator of prior exposure to oxidative stress conditions. The MDA content in the leaves of our experimental plants significantly increased under Mg deficiency and acid stress (L1). For all treatment groups at days 7, 15 and 30, MDA levels were maximal at 87.33, 119.90 and 125.86 nmol·g
-1, respectively. Particularly on days 15 and 30, L1 plants increased by 144.6 and 169.6%, respectively, compared to the M2 treatment. At low pH, Mg supplementation effectively reduced MDA accumulation in the tea seedling leaves. On day 30, H1 MDA levels were reduced by 64% compared to L1 and by 13.6% compared to M1 while H3 decreased by 88% compared to L3 and by 17.2% compared to M3. Therefore, Mg deficiency led to greater levels of MDA i.e., L2 > M2 by 82.6% indicating the past exposure of greater levels of oxidative stress (
Figure 2a).
The MDA content in the roots indicated that Mg deficiency generated significantly higher levels at pH 5.0 on days 15 and 30 that were increases of 131.5 and 174.4 %, respectively. Acid stress exacerbated the impact of Mg deficiency resulting in a 10.8% increase at pH 3.5 and a 6.9% increase at pH 6.5 compared to the pH 5.0 treatment on day 15 and by day 30, these levels were 12 and 7%, respectively. An increase in Mg in the roots significantly alleviated the increase in MDA content under acid stress. In the final phase of the experiment, 0.4 and 0.8 mM Mg under acid stress reduced MDA levels by 33.2 and 35.8%, respectively compared to the 0.01 mM Mg. Elevated pH conditions further reduced MDA levels with decreases of 51.6 and 46.2%, respectively (
Figure 2b).
Mg levels also correlated with SOD activities in the tea seedling leaves. Under acid stress, high Mg generated significant increases in SOD activity versus all other treatments starting from day 7 and this high activity was maintained for the remainder of the experiments. On day 30, SOD levels were significantly increased by 60 % versus M2 treatment that surpassed L2 and H2 by 122 and 9.9%, respectively. Conversely, low Mg did not lead to elevations in SOD activity. By day 30, the low Mg group reduced SOD activity by 27.2% (
Figure 2c).
The SOD activity in the roots of tea seedlings versus controls displayed significantly higher levels for M1, M3, H1, H2, and H3. These findings indicated that supplying Mg can offset the effects of acid stress. Furthermore, by day 30, Mg deficiency resulted in a significant reduction in SOD activity by 13.1, 16.4 and 9.43% compared to the M2 treatment, respectively (
Figure 2d).
The POD activity in tea seedling leaves increased to a maximum at day 7 that was followed by a gradual decline. At pH 5.0, the POD activity in tea leaves consistently decreased although Mg supplementation could enhance POD activity in the leaves (
Figure 2e). In contrast, POD root activity under Mg deficiency significantly decreased and by day 30, groups L1, L2 and L3 exhibited reductions of 11.6, 8.1 and 11.1%, respectively versus M2. Furthermore, POD activity for low Mg treatments continually decreased over the experimental period and conversely, Mg addition under acid stress significantly enhanced POD activity that peaked at 0.8 mM;. H1 levels increased by 58.2% increase compared to M2 and by 44.1% and 12.7% compared to L1 and M1, respectively (
Figure 2f).
In tea seedling leaves, CAT activity reached its peak on day 7 day under Mg deficiency (0.01 mM) and gradually declined thereafter. On day 30, leaf CAT reached its minimum and decreased by 31.7, 30.6 and 8.6%, respectively, compared with M2. Therefore, Mg addition could increase CAT activity in tea seedling leaves and by day 30, the high-Mg, low-pH (H1) treatment possessed CAT levels significantly higher than for the other treatments and 64.1% greater versus M2 (
Figure 2g). In the seedling roots, Mg deficiency initially led to an increase and subsequent decrease in CAT activity under acid stress with a decline in CAT activity over time when at pH 5.0. By day 30, CAT activity in the Mg -deficient tea seedling roots was consistently lower than for M2 . Specifically, the L1 and L2 treatments exhibited reductions of 23.9 and 27.5%, respectively. Furthermore, Mg addition had a significant enhancing effect on CAT activity in tea seedling roots and activity markedly increased as Mg was increased under similar pH conditions (
Figure 2h).
3.3. Effects of Mg Nutrition on Tea Quality under Acid Stress
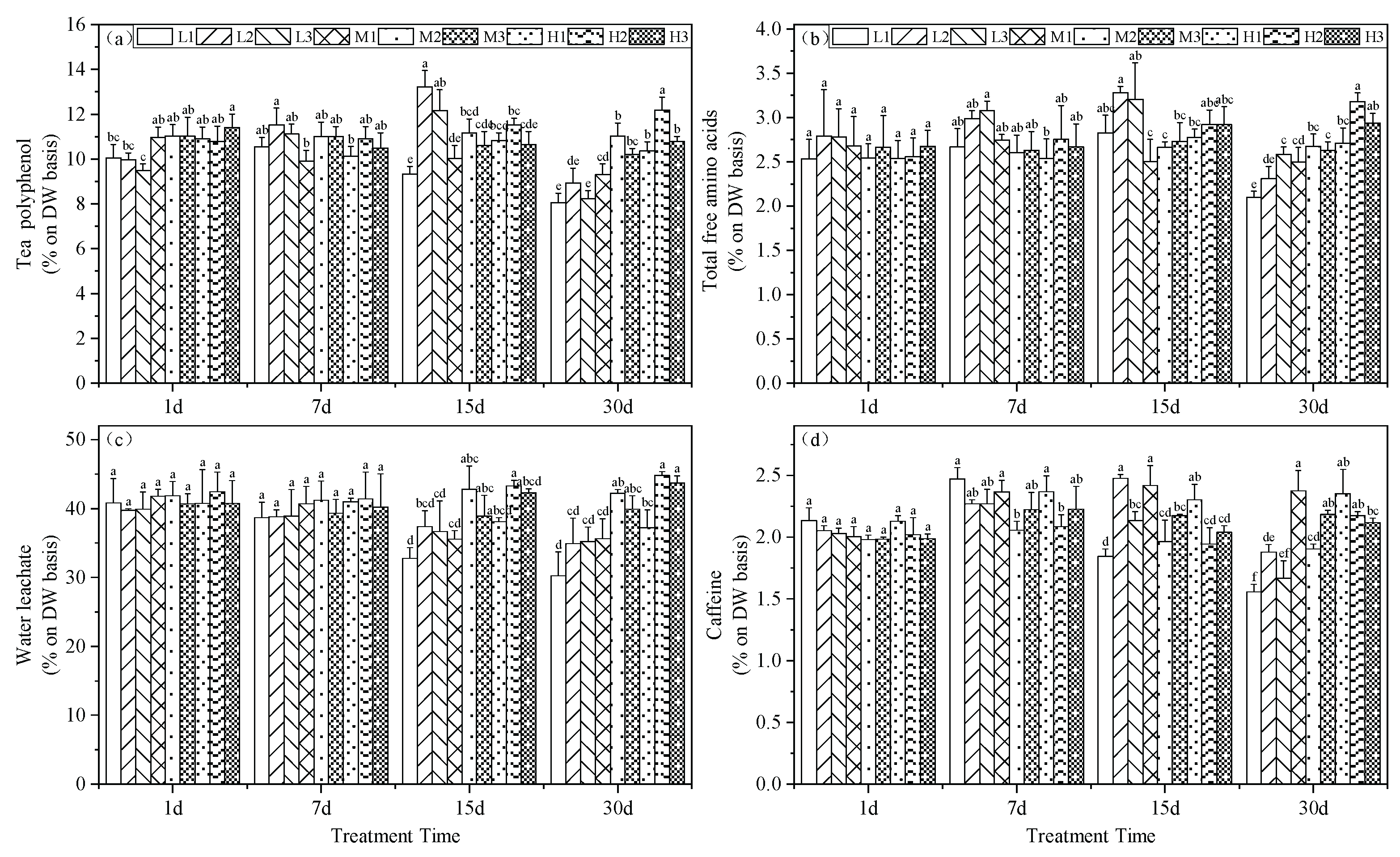
Tea polyphenols are complex compounds of polyhydroxy phenols found in tea leaves. Under similar Mg concentrations, the polyphenol content in tea leaves was significantly higher at pH 5.0 compared to other treatments. Interestingly, at day 15, Mg deficiency without acid stress led to maximal polyphenol values among all periods, exceeding the normal treatment by 2.07% and by day 30, the levels were stable at 2.09%, but were still higher than the other two treatment groups. By day 30, high Mg correlated with greater polyphenol content that increased by 8.1, 9.3 and 10.4 % under acid stress conditions (
Figure 3a).
The total free amino acid content in the tea leaves indicated that Mg deficiency led to significantly higher levels for treatments L1, L2 and L3 that increased by 0.17, 0.62 and 0.54%, respectively. However, by day 30, all Mg deficient treatments were significantly lower than for the 0.4 mM Mg with reductions of 0.58, 0.37 and 0.09%, respectively. Additionally, at high Mg levels, acid stress did not decrease the total free amino acid content in tea leaves. Furthermore, at this point, the increase in Mg content led to 0.51 and 0.26% increases at pH 5.0 and 6.5, respectively, compared to the M2 treatment. In contrast, moderate Mg levels resulted in a reduction of total free amino acid content under acid stress with reductions of 0.18 and 0.05% in acid stress and high pH treatments, respectively (
Figure 3b).
Acid stress led to a decrease in tea leaf extractable water and at days 7 and 15, L1, M1 and H1 treatments were significantly lower than M2 with reductions of 10, 7.2 and 4.7% as well as 12, 6.6 and 5%, respectively. By day 30, increased Mg could enhance the tea leaf extractable water. Specifically, H1 was 18.65% higher than L1 and M1, H2 were 22.1% higher than L2 and M2 and H3 was 19.4% higher than L3 and M3 (
Figure 3c).
Caffeine content also varied with our experimental treatments of the seedlings. Starting from day 7, all treatments except for H2 exhibited a significant increase compared to M2. However, at days 15 and 30 the caffeine content decreased continuously under Mg deficiency and acid stress conditions. In addition, on day 30, all three Mg -deficient treatments yielded caffeine levels significantly lower than M2 with reductions of 0.35, 0.03 and 0.24% while the remaining treatments were significantly higher than M2 with increases of 0.47, 0.28, 0.45, 0.27 and 0.22%, respectively. These data indicated that Mg deficiency and acid stress promoted accumulation of caffeine in the tea plant leaves (
Figure 3d).
3.4. Nutrient Accumulation in Tea Leaves
The influence of root zone pH and Mg on plant nitrogen content indicated that by day 30 under equivalent Mg concentrations, N levels decreased with increasing pH. Under Mg deficiency, acid stress increased the N content by 9.6 and 13.4% compared to the other two groups. At Mg level of 0.4 mM, the increase was 3.3 and 5.5% and at 0.8 mM, the increases were 4.7 and 5.1%, respectively. Additionally, under Mg -deficient conditions, the N content in tea leaves was significantly lower than in the other treatments. At pH 5, the N content for M1, M2 and M3 were 3.6, 4.3 and 4.4%, respectively (
Figure 4a).
The P content in leaves were also significantly increased as the Mg levels were increased although at 0.8 mM Mg, P levels decreased with time. On day 30, Mg deficiency without acid stress (L2 and L3) and high pH treatments under normal Mg conditions (M3 and H3) facilitated the accumulation of P that increased by 14.1, 14, 6.5 and 10.9%, respectively, compared to M2. Furthermore, under conditions of equivalent Mg, acid stress had a detrimental impact on the accumulation of P in the leaves (
Figure 4b). In contrast, K levels did not vary for any of the experimental treatments (
Figure 4c).
Mg is a necessary element for photosynthesis and is an essential component of chlorophyll. For our experimental treatments, tea leaf Mg significantly increased as Mg and pH levels in the root environment increased. At days 7, 15 and 30, leaf Mg content increased with Mg and concurrently, pH also significantly affected leaf Mg levels. On day 15 under Mg deficiency, an increase in pH led to a significant enhancement in leaf Mg level with the M3 treatment exhibiting a 9.92% increase compared to M1 and a 6% increase compared to M2. The H3 treatment displayed an 8.7% increase compared to H1 and a 6.2% increase compared to H2. The trends in leaf Mg content at days 7 and 30 followed a similar pattern (
Figure 4d). In summary, the Mg content in tea leaves significantly increased with the rise of supplemented Mg and pH.
4. Discussion
4.1. Chlorophyll and Root Vitality in Tea Seedlings
Mg is an essential nutrient in plant life processes and is present in the octahedral chlorophyll structure and acts as a regulator of chlorophyll synthesis [
30]. In our study, we observed an increase in chlorophyll content that positively correlated with Mg concentration while acid stress led to a decrease. Notably, Mg deficiency generated a significant reductions in chlorophyll content within 30 days indicating that the two are tightly linked as previously reported [
31]. In agricultural practice, reduced chlorophyll content results in interveinal chlorosis in mature plant leaves [
32] although our experimental plants displayed no significant chlorosis at any time.
Root vitality is a critical physiological indicator reflecting a plant's ability to withstand adverse environmental stress and directly affects its growth status [
33,
34,
35,
36]. Previous studies have shown that under hydroponic conditions,
Mimosa pudica root vitality is closely linked to pH level in the root environment [
37]. Consistent with this, our experiments revealed that Mg deficiency significantly inhibited root viability [
38] and higher Mg (0.8 mM) enhanced the root vitality of our tea seedlings.
4.2. Oxidative Stress
Numerous environmental stressors can produce ROS in plants and this is a major contributor to significant crop productivity losses. It is estimated that approximately 1% of the O
2 consumed by plants is redirected to different subcellular sites (such as chloroplasts, mitochondria, peroxisomes) to generate ROS [
39]. ROS can impact numerous cellular functions through mechanisms including nucleic acid damage, protein oxidation and membrane lipid peroxidation [
40]. The effective removal of ROS generated under various environmental stress conditions relies on the action of several non-enzymatic and enzymatic antioxidants present in plant tissues [
21]. Among these, the antioxidant enzyme system plays a significant role in the removal of ROS in plants and includes antioxidant enzymes and cell protective enzymes. We found that POD, SOD and CAT activities were induced in the leaves and roots of tea seedlings under Mg deficiency and acid stress. Prolonged Mg deficiency also resulted in reduced antioxidant enzyme activities and this has been previously reported [
15,
41,
42,
43]. For example, Mg deficiency leads to a widespread suppression of the antioxidant defense system accompanied by an increase in ROS. Therefore, under conditions of moderate Mg deficiency, the antioxidant defense system is generally inhibited [
43]. In our research, we observed an initial increase and subsequent decrease in the activities of POD and CAT in both tea seedling leaves and roots under Mg deficiency and acid stress at 7 days. The decrease in CAT activity may be a reflection of the reduced Rubisco activity in response to Mg deficiency, leading to a reduced rate of photorespiration [
44]. Additionally, our study indicated that increases in Mg supplementation also led to higher antioxidant enzyme activities. Enhancing the Mg supply to maize plants has been shown to increase their antioxidant capacity and promote expression of SOD, CAT and POD [
45]. This suggests that Mg applications can be beneficial under acid stress to thereby improve the plants ability to eliminate ROS, stabilize leaf growth and enhance their adaptability to adverse rhizospheric acid environments.
When the production of ROS exceeds the clearance capacity of the antioxidant metabolic system, it fails to provide adequate protection for membrane lipids, resulting in a significant increase in the levels of MDA, a toxic metabolic byproduct of lipid peroxidation [
15]. This type of elevation is thus a marker of oxidative stress exposure [
46]. In our study, we found that long-term acid stress and Mg deficiency significantly increased the MDA content in tea seedling leaves and roots. This, coupled with the corresponding low antioxidant enzyme activities, indicates a notable decrease in the ability of tea seedlings to eliminate ROS under these conditions. This suggests that the rate of ROS production may exceed the cell's clearance capacity. Moreover, our findings revealed a significant reduction in MDA content with increasing Mg supplementation. This has been previously reported for Mg deficient corn [
47], rice [
48] and
Citrus grandis and
Citrus sinensis [
49] leaves. Adequate Mg application can mitigate damage to cell membranes to improve to the ability to withstand environmental stressors.
4.3. Tea Quality and Nutrient Uptake in Tea Seedlings
Tea polyphenols, free amino acids and caffeine are three essential chemical components of tea leaves. Tea polyphenol content plays a significant role in determining the richness and health benefits of tea leaves and so contributes to the taste profile, offering attributes such as acidity, sweetness, and freshness. However, excessively high levels of tea polyphenols can lead to a bitter taste in the tea infusion[
50,
51,
52]. Free amino acids, especially in green tea, are primary contributors to its taste profile. They provide a refreshing flavor, alleviate bitterness and enhance sweetness making them a key evaluation criterion for green tea quality [
53,
54,
55]. Caffeine is the most abundant alkaloid in tea leaves, extensively studied and known for its health benefits including reducing fatigue [
56].
In our study, the content of tea polyphenols, free amino acids and caffeine exhibited a brief increase within the first 15 days under Mg deficiency but subsequently decreased significantly by day 30. Furthermore, this decrease could be counteracted by increasing Mg levels and prevented the decrease in tea polyphenol and free amino acid content under acid stress. This indicates that an appropriate Mg dosage supports the maintenance of tea leaf quality under acid stress. However, it is worth noting that some studies have suggested that tea plantations with soil pH levels > 6.0 may not require Mg fertilization. Interestingly, caffeine content significantly increased under prolonged acid stress with increased Mg concentration in the nutrient solution. Field experiments have also indicated that Mg fertilization substantially increased caffeine content in black, oolong and green teas [
57].
The content of tea leaf water extract is regarded as a crucial quality indicator. It reflects the abundance of soluble substances in tea leaves, influencing the tea's taste profile in terms of thickness, strength and richness and these have a decisive impact on tea quality [
58,
59]. Generally, higher water extract content results in a more robust tea flavor [
60]. Our research found that both Mg deficiency and acid stress significantly reduced the content of tea leaf water extract. Interestingly, we observed that an increase in Mg relatively enhanced the content of tea leaf water extract. This implied that Mg supplementation can stabilize and even improve the water extract content of tea leaves in the root zone environment under acid stress.
Nutrient deficiency in plants can lead to an imbalance in ion metabolism and inhibit plant growth [
61]. Our study indicated that N, P and Mg in tea leaves increased as Mg levels were increased. Some nutrient elements in tea leaves have been found to decrease under Mg due to reduced transport of photosynthetic products to the roots but also increases the lignification of transport tissues in roots and stems [
62]. This contributes to reduced nutrient absorption capacity that can further alter nutrient uptake. Our study demonstrated that Mg supplementation can increase the accumulation of N, P and Mg in tea leaves.
Interestingly, Mg deficiency without acid stress generated P levels in leaves that were significantly higher than that for the M2 treatment. This suggests that under these conditions, tea plants may promote P uptake and transport, possibly due to Mg deficiency to alter ion balance leading to changes in ion distribution [
19]. During adverse environmental stress, ion metabolism in plants is disrupted that adversely affect normal metabolism. Acid stress can markedly inhibit root growth and even lead to root tip cell death [
63]. Our study results indicated that low pH environments were not conducive to the accumulation of P, N and Mg in tea leaves and increased pH facilitated Mg absorption while N absorption tends to favor a low pH root zone environment.
5. Conclusions
Soil acidification is gaining increased attention in agricultural economic crop cultivation systems. However, Mg, as a crucial nutrient element in agricultural soils appears to be relatively overlooked, especially within the context of tea plantation systems. Given that tea plantation soils are often acidic with low cation exchange capacity and Mg content, special consideration must be given to Mg supplementation. In these experiments we found that Mg deficiency negatively affected antioxidant capacity, tea quality, chlorophyll content and root vitality when tea seedlings were exposed to acid stress. The provision of Mg nutrition significantly improved the physiological status and quality of tea seedlings.
Author Contributions
Conceptualization, X.L. and J.M.; methodology, X.L. and Y.L.; software, X.L. and Y.L.; validation, D.L., W.L. and H.K.; formal analysis, X.L.; investigation, D.W.; resources, D.L. and Z.Y.; data curation, D.L. and J.M.; writing—original draft preparation, X.L.; writing—review and editing, X.L., J.M. and D.D.; visualization, X.L.; supervision, D.L. and J.M.; project administration, J.M.; funding acquisition, D.L., J.M. and Z.Y. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This work was financed by the National Natural Science Foundation of China (31670617), the Key Research and Development Project of Science and Technology Department of Zhejiang Province (2022C02022), and Zhejiang High-level Talents Special Support Program (2020R52026).
Institutional Review Board Statement
No humans or animals were involved in this study.
Informed Consent Statement
No applicable.
Data Availability Statement
No applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, S.; Li, H.; Yang, C.; Wang, Y.; Xue, H.; Niu, Y. Rates of soil acidification in tea plantations and possible causes. Agriculture, Ecosystems & Environment 2016, 233, 60–66. [Google Scholar]
- Ye, J.-H.; Ye, Y.; Yin, J.-F.; Jin, J.; Liang, Y.-R.; Liu, R.-Y.; Tang, P.; Xu, Y.-Q. Bitterness and astringency of tea leaves and products: Formation mechanism and reducing strategies. Trends in Food Science & Technology 2022, 123, 130–143. [Google Scholar]
- Dai, W.; Lou, N.; Xie, D.; Hu, Z.; Song, H.; Lu, M.; Shang, D.; Wu, W.; Peng, J.; Yin, P.; et al. N-Ethyl-2-Pyrrolidinone-Substituted Flavan-3-Ols with Anti-inflammatory Activity in Lipopolysaccharide-Stimulated Macrophages Are Storage-Related Marker Compounds for Green Tea. Journal of agricultural and food chemistry 2020, 68(43), 12164–12172. [Google Scholar] [CrossRef]
- Peng, Y.; WenYan, H.; Xin, L.; LiPing, Z.; Lan, Z. Present Situation and Analysis of Soil Acidification in Chinese Tea Garden. Scientia Agricultura Sinica 2020, 795–801. (in Chinese). [Google Scholar]
- RUAN, J.; MA, L.; YI, X.; SHI, Y.; NI, K.; LIU, M.; ZHANG, Q. Integrated Nutrient Management in Tea Plantation to Reduce Chemical Fertilizer and Increase Nutrient Use Efficiency. Journal of Tea Science 2020, 85–95. (in Chinese). [Google Scholar]
- Ruan, J.; Wong, M.H. Accumulation of Fluoride and Aluminium Related to Different Varieties of Tea Plant. Environmental Geochemistry and Health 2001, 23, 53–63. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.-K.; Wang, N.; Li, X.-H. Soil Acidification of Alfisols as Influenced by Tea Cultivation in Eastern China. Pedosphere 2010, 20, 799–806. [Google Scholar] [CrossRef]
- Fung, K.F.; Carr, H.P.; Zhang, J.; Wong, M.H. Growth and nutrient uptake of tea under different aluminium concentrations. Journal of the Science of Food and Agriculture 2008, 88, 1582–1591. [Google Scholar] [CrossRef]
- De Vries, W.; Posch, M.; Kämäri, J. Simulation of the long-term soil response to acid deposition in various buffer ranges. Water, Air, and Soil Pollution 1989, 48, 349–39. [Google Scholar] [CrossRef]
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Wenyan, H. Soil acidification in Chinese tea plantations. Science of The Total Environment 2020, 715, 136963. [Google Scholar] [CrossRef]
- Yang, X.-d.; Ni, K.; Shi, Y.-z.; Yi, X.-y.; Zhang, Q.-f.; Fang, L.; Ma, L.-f.; Ruan, J. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agriculture, Ecosystems & Environment 2018, 252, 74–82. [Google Scholar]
- Rajonandraina, T.; Ueda, Y.; Wissuwa, M.; Kirk, G.J.D.; Rakotoson, T.; Manwaring, H.; Andriamananjara, A.; Razafimbelo, T. Magnesium supply alleviates iron toxicity-induced leaf bronzing in rice through exclusion and tissue-tolerance mechanisms. Frontiers in plant science 2023, 14, 1213456. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. 2008.
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. The Crop Journal 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Farhat, N.; Khouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiologiae Plantarum 2016, 38, 1–10. [Google Scholar] [CrossRef]
- Andersson, I. Catalysis and regulation in Rubisco. Journal of Experimental Botany 2008, 59, 1555–1568. [Google Scholar] [CrossRef]
- Fink, S. Microscopic Criteria for the Diagnosis of Abiotic Injuries to Conifer Needles. In: Huettl, R.F., Mueller-Dombois, D. (eds) Forest Decline in the Atlantic and Pacific Region. Springer, Berlin, Heidelberg, 1993; pp.175-188.
- Puech, L.; Mehne-Jakobs, B. Histology of magnesium-deficient Norway spruce needles influenced by nitrogen source. Tree Physiology 1997, 17, 301–310. [Google Scholar] [CrossRef]
- Ogura, T.; Kobayashi, N.I.; Hermans, C.; Ichihashi, Y.; Shibata, A.; Shirasu, K.; Aoki, N.; Sugita, R.; Ogawa, T.; Suzuki, H.; et al. Short-Term Magnesium Deficiency Triggers Nutrient Retranslocation in Arabidopsis thaliana. Frontiers in Plant Science 2020, 11, 563. [Google Scholar] [CrossRef]
- Hermans, C.; Verbruggen, N. Physiological characterization of Mg deficiency in Arabidopsis thaliana. Journal of Experimental Botany 2005, 56, 2153–2161. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Marutani, Y.; Yamauchi, Y.; Kimura, Y.; Mizutani, M.; Sugimoto, Y. Damage to photosystem II due to heat stress without light-driven electron flow: involvement of enhanced introduction of reducing power into thylakoid membranes. Planta 2012, 236, 753–76. [Google Scholar] [CrossRef]
- Queval, G.; Foyer, C.H. Redox regulation of photosynthetic gene expression. Proceedings of The Royal Society B-biological Sciences 2012, 367, 3475–3485. [Google Scholar] [CrossRef]
- Quiles, M.J.C.; López, N.I. Photoinhibition of photosystems I and II induced by exposure to high light intensity during oat plant growth: Effects on the chloroplast NADH dehydrogenase complex. Plant Science 2004, 166, 815–823. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, Navdeep S. ROS Function in Redox Signaling and Oxidative Stress. Current Biology 2014, 24, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Diversity 2019, 42, 33–43. [Google Scholar] [CrossRef]
- Candan, N.; Tarhan, L. Relationship among chlorophyll-carotenoid content, antioxidant enzyme activities and lipid peroxidation levels by Mg2+ deficiency in the Mentha pulegium leaves. Plant Physiology and Biochemistry 2003, 41, 35–40. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, X.; Dong, Y.; Xu, L.; Zhang, X.; Kong, J.; Liu, S. Effects of Exogenous Salicylic Acid and Nitric Oxide on Physiological Characteristics of Perennial Ryegrass Under Cadmium Stress. Journal of Plant Growth Regulation 2013, 32, 721–731. [Google Scholar] [CrossRef]
- Beale, S.I. Enzymes of chlorophyll biosynthesis. Photosynthesis Research 1999, 60, 43–73. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.-H.; Zhang, X.-Y.; Zhang, L.-Y.; Zhao, P.-L.; Wen, T.; Zhang, J.-Q.; Xu, W.-L.; Guo, F.; Zhao, H.; et al. Exploring the Effects of Magnesium Deficiency on the Quality Constituents of Hydroponic-Cultivated Tea (Camellia sinensis L.) Leaves. Journal of agricultural and food chemistry 2021, 69, 14278–14286. [Google Scholar] [CrossRef]
- Tanoi, K.; Kobayashi, N.I. Leaf Senescence by Magnesium Deficiency. Plants 2015, 4, 756–772. [Google Scholar] [CrossRef]
- Wang; Hua, f.; Zhu; Yi, h.; Sun; Hai, j. Determination of drought tolerance using root activities in Robinia pseudoacacia ‘Idaho' transformed with mtl-D gene. Forest Ecosystems 2006, 8, 75–81. [Google Scholar]
- Bhadoria, P.S.; El Dessougi, H.; Liebersbach, H.; Claassen, N. Phosphorus uptake kinetics, size of root system and growth of maize and groundnut in solution culture. Plant and Soil 2004, 262, 327–33. [Google Scholar] [CrossRef]
- Bing-Sheng, L.; Xiao-Wei, L.; Hong-Yuan, M.; Yang, S.; Li-Xing, W.; Chang-Jie, J.; Zheng-Wei, L. Differences in Growth and Physiology of Rice in Response to Different Saline-Alkaline Stress Factors. Agronomy journal 2013, 105, 1119–1128. [Google Scholar]
- Dai, J.; Duan, L.; Dong, H. Comparative Effect of Nitrogen Forms on Nitrogen Uptake and Cotton Growth Under Salinity Stress. Journal of Plant Nutrition 2015, 38, 1530–1543. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Fang, Z. Effects of different pH on the growth and development of Mimosa pudica in hydroponics. ournal of Capital Normal University(Nat.Sci.Edi.) 2008, 43-45(in Chinese).
- Deng, N.; Zhu, H.; Xiong, J.; Gong, S.; Xie, K.; Shang, Q.; Yang, X. Magnesium deficiency stress in rice can be alleviated by partial nitrate nutrition supply. Plant Physiology and Biochemistry 2023, 196, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Meng-r, Z. Generation of Reactive Oxygen Species and Their Functions and Deleterious Effects in Plants. Acta Botanica Boreali-Occidentalia Sinica 2014, 34, 1916–1926. (in Chinese). [Google Scholar]
- Foyer, C.H.; Noctor, G. Oxygen processing in photosynthesis: regulation and signalling. New Phytologist 2000, 146, 359–388. [Google Scholar] [CrossRef]
- Zhang, F.; Du, P.; Song, C.; Wu, Q.-S. Alleviation of Mycorrhiza to Magnesium Deficiency in Trifoliate Orange: Changes in Physiological Activity. Emirates Journal of Food and Agriculture 2015, 27, 763–769. [Google Scholar] [CrossRef]
- Rehman, H.u.; Alharby, H.F.; Alzahrani, Y.; Rady, M.M. Magnesium and organic biostimulant integrative application induces physiological and biochemical changes in sunflower plants and its harvested progeny on sandy soil. Plant Physiology and Biochemistry 2018, 126, 97–105. [Google Scholar] [CrossRef]
- Ze, Y.; Yin, S.; Ji, Z.; Luo, L.; Liu, C.; Hong, F. Influences of magnesium deficiency and cerium on antioxidant system of spinach chloroplasts. BioMetals 2009, 22, 941–949. [Google Scholar] [CrossRef]
- Tang, N.; Li, Y.; Chen, L.-S. Magnesium deficiency–induced impairment of photosynthesis in leaves of fruiting Citrus reticulata trees accompanied by up-regulation of antioxidant metabolism to avoid photo-oxidative damage. Journal of Plant Nutrition and Soil Science 2012, 175, 784–793. [Google Scholar] [CrossRef]
- Kong, X.; Peng, Z.; Li, D.; Ma, W.; An, R.; Khan, D.; Wang, X.; Liu, Y.; Yang, E.; He, Y.; et al. Magnesium decreases aluminum accumulation and plays a role in protecting maize from aluminum-induced oxidative stress. Plant and Soil 2020, 457, 71–81. [Google Scholar] [CrossRef]
- Cakmak, I. Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced in magnesium- and potassium-deficient leaves, but not in phosphorus-deficient leaves. Journal of Experimental Botany 1994, 45, 1259–1266. [Google Scholar] [CrossRef]
- Kumar Tewari, R.; Kumar, P.; Tewari, N.; Srivastava, S.; Sharma, P.N. Macronutrient deficiencies and differential antioxidant responses—influence on the activity and expression of superoxide dismutase in maize. Plant Science 2004, 166, 687–694. [Google Scholar] [CrossRef]
- Ding, Y.-C.; Chang, C.-R.; Luo, W.; Wu, Y.-S.; Ren, X.-L.; Wang, P.; Xu, G.-H. High Potassium Aggravates the Oxidative Stress Inducedy by Magnesium Deflciency in Rice Leaves1 1Project supported by the Dead Sea Works Ltd., Israel. Pedosphere 2008, 18, 316–327. [Google Scholar] [CrossRef]
- Yang, G.-H.; Yang, L.-T.; Jiang, H.-X.; Li, Y.; Wang, P.; Chen, L.-S. Physiological impacts of magnesium-deficiency in Citrus seedlings: photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Wen, B.; Li, R.; Zhao, X.; Ren, S.; Chang, Y.; Zhang, K.; Wang, S.; Guo, G.; Zhu, X.; Papadakis, E.; et al. A Quadratic Regression Model to Quantify Plantation Soil Factors That Affect Tea Quality. Agriculture 2021, 11, 1125. [Google Scholar] [CrossRef]
- Tang, S.; Pan, W.; Tang, R.; Ma, Q.; Zhou, J.; Zheng, N.; Wang, J.; Sun, T.; Wu, L. Effects of balanced and unbalanced fertilisation on tea quality, yield, and soil bacterial community. Applied Soil Ecology 2022, 175, 104442. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Lin, L.; Zhang, Q.; Ding, L.; Guo, W.; Wang, H. Effects of Soil Acidity on Tea Yield and Contents of Leave Quality Components. Chinese Journal of Tropical Crops 2021, 42, 260–266. [Google Scholar]
- Chen, D.; Sun, Z.; Gao, J.; Peng, J.; Wang, Z.; Zhao, Y.; Lin, Z.; Dai, W. Metabolomics combined with proteomics provides a novel interpretation of the compound differences among Chinese tea cultivars (Camellia sinensis var. sinensis) with different manufacturing suitabilities. Food Chemistry 2022, 377, 13197. [Google Scholar] [CrossRef]
- Miyauchi, S.; Yuki, T.; Fuji, H.; Kojima, K.; Yonetani, T.; Tomio, A.; Bamba, T.; Fukusaki, E. High-quality green tea leaf production by artificial cultivation under growth chamber conditions considering amino acids profile. Journal of Bioscience and Bioengineering 2014, 118, 710–715. [Google Scholar] [CrossRef]
- Ye, J.-H.; Wang, H.-B.; Yang, X.-Y.; Zhang, Q.; Li, J.-Y.; Jia, X.-L.; Kong, X.-H.; He, H.-B. Autotoxicity of the soil of consecutively cultured tea plantations on tea (Camellia sinensis) seedlings. Acta Physiologiae Plantarum 2016, 38, 195. [Google Scholar] [CrossRef]
- Aniţei, M.; Schuhfried, G.; Chraif, M. The influence of energy drinks and caffeine on time reaction and cognitive processes in young Romanian students. Procedia - Social and Behavioral Sciences 2011, 30, 662–67. [Google Scholar] [CrossRef]
- Jian, R. Status of Mg Availability and the Effects of Mg Application in Tea Fields of Red Soil Area in China. Chinese Agricultural Science Bulletin 2002, 35, 815–820. (in Chinese). [Google Scholar]
- Penna, E.; Zúñiga, M.; Fuenzalida, R.; López-Planes, R. Caracterización sensorial y química de la calidad de TÉS (Thea sinensis) consumidos en Chile. Archivos Latinoamericanos de Nutrición 2005, 55, 93–100. [Google Scholar]
- Chen, P.-A.; Lin, S.-Y.; Liu, C.-F.; Su, Y.-S.; Cheng, H.-Y.; Shiau, J.-H.; Chen, I.-Z. Correlation between nitrogen application to tea flushes and quality of green and black teas. Scientia Horticulturae 2015, 181, 102–107. [Google Scholar] [CrossRef]
- Chen, M.; Tang, D.; Gong, S.; Yang, J.; Zhang, Y. Quantitative analysis and correlation evaluation on taste quality of green tea. Journal of Zhejiang University (Agriculture and Life Sciences) 2014, 40(6), 670–678. [Google Scholar]
- Maillard, A.; Etienne, P.; Diquélou, S.; Trouverie, J.; Billard, V.; Yvin, J.-C.; Ourry, A. Nutrient deficiencies modify the ionomic composition of plant tissues: a focus on cross-talk between molybdenum and other nutrients in Brassica napus. Journal of Experimental Botany 2016, 67, 5631–5641. [Google Scholar] [CrossRef]
- Hannaway, D.B.; Bush, L.P.; Leggett, J.E. Mineral composition of kenhy tall fescue as affected by nutrient solution concentrations of Mg and K. Journal of Plant Nutrition 1982, 5, 137–151. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kobayashi, Y.; Watanabe, T.; Shaff, J.; Ohta, H.; Kochian, L.; Wagatsuma, T.; Kinraide, T.; Koyama, H. Molecular and Physiological Analysis of Al3+ and H+ Rhizotoxicities at Moderately Acidic Conditions. Plant physiology 2013, 163, 180–192. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).