Submitted:
16 March 2024
Posted:
18 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
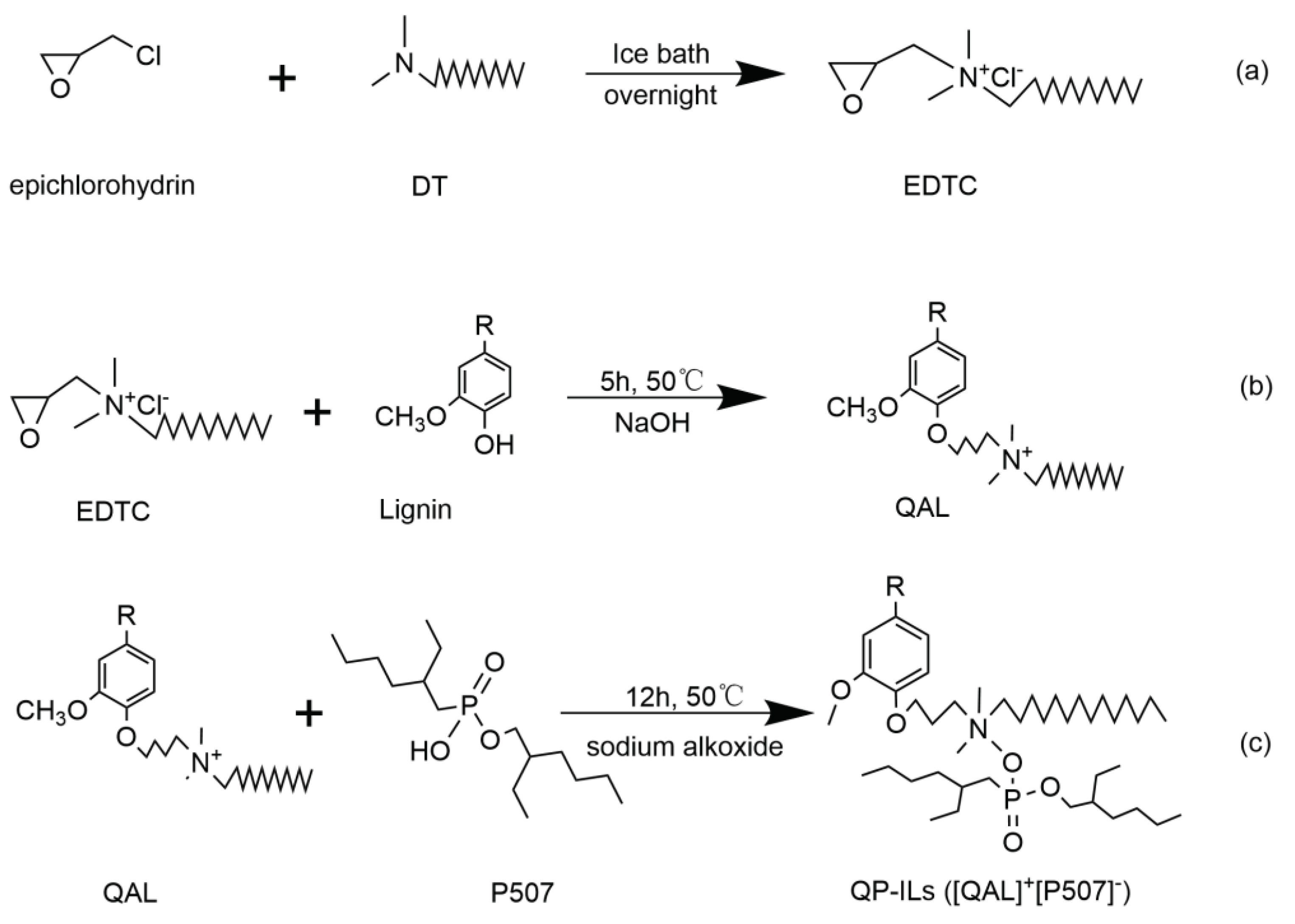
2.1. Synthesis of QP-ILs
2.1.1. Synthesis of Quaternary Ammonium Lignin (QAL)
2.1.2. Synthesis of Quaternary Ammonium Lignin (QAL)
2.3. General Procedure for Co and Ni Extraction
2.4. Characterization
3. Results
3.1. FT IR Spectra of QP-ILs
3.2. NMR Spectroscopic Characterization of QP-ILS
3.3. Effect of Different Factors on Extraction for Co and Ni
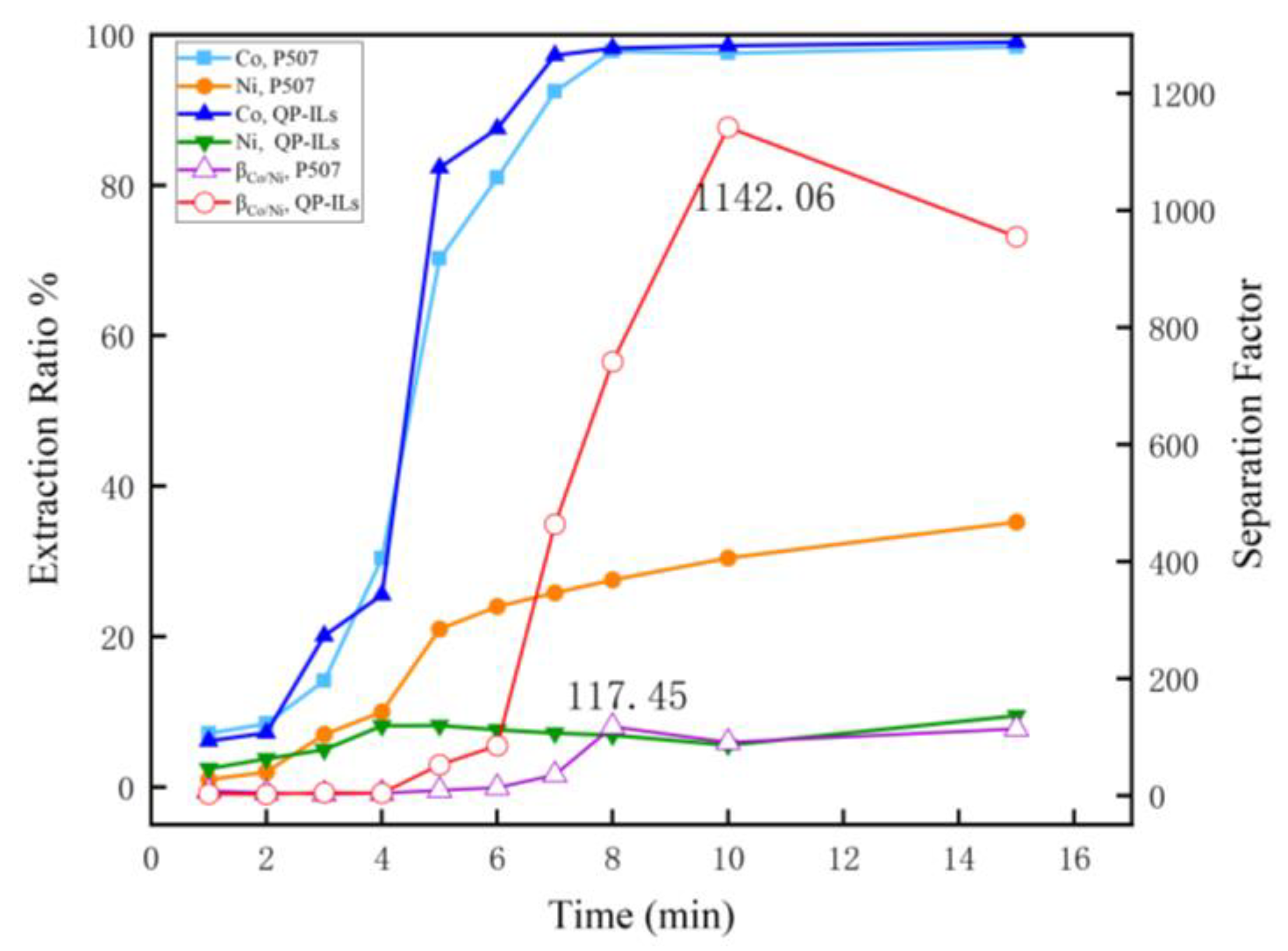
3.1.1. Influence of Time on the Extraction
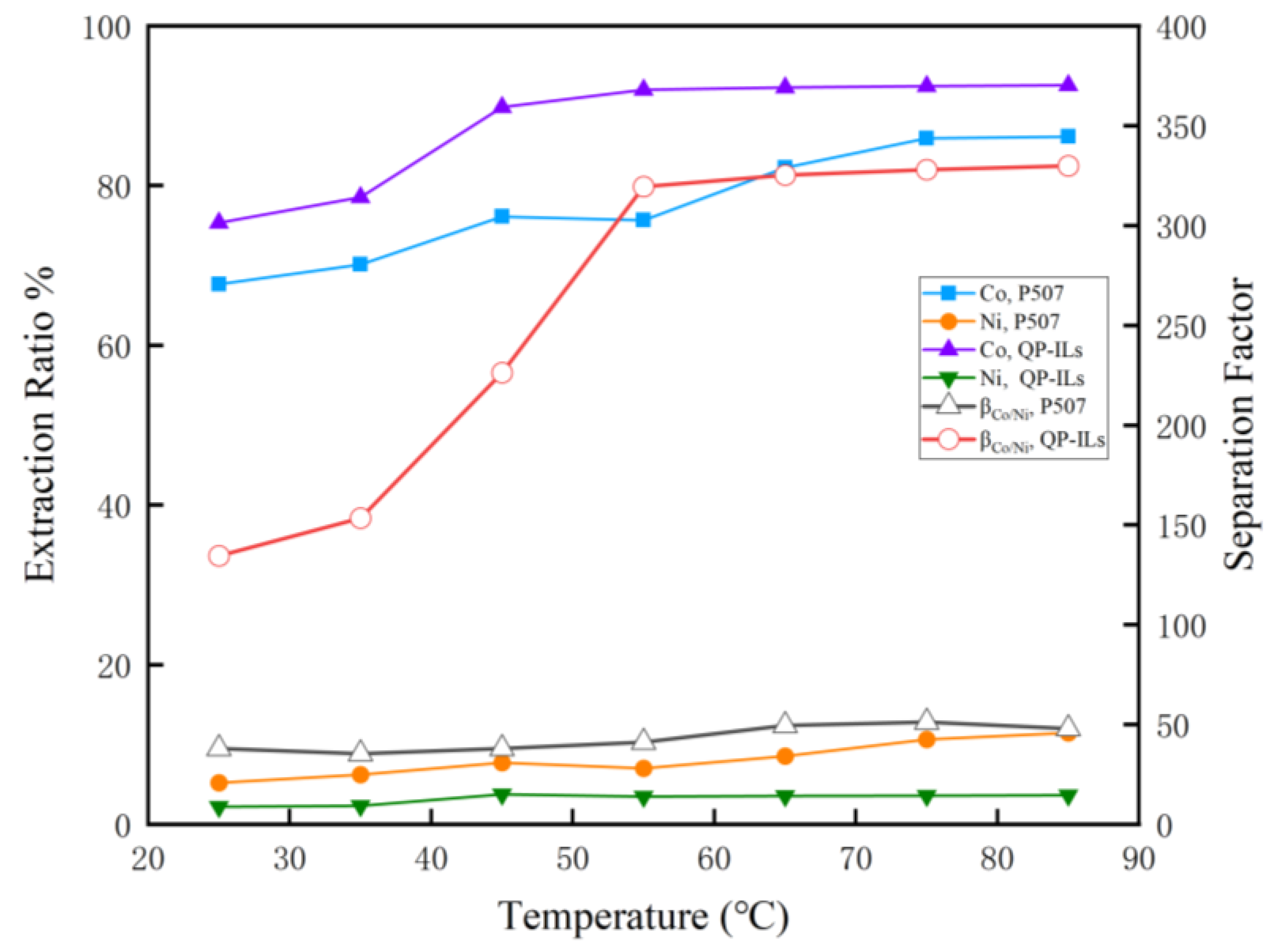
3.1.2. Effect of Temperature on Extraction
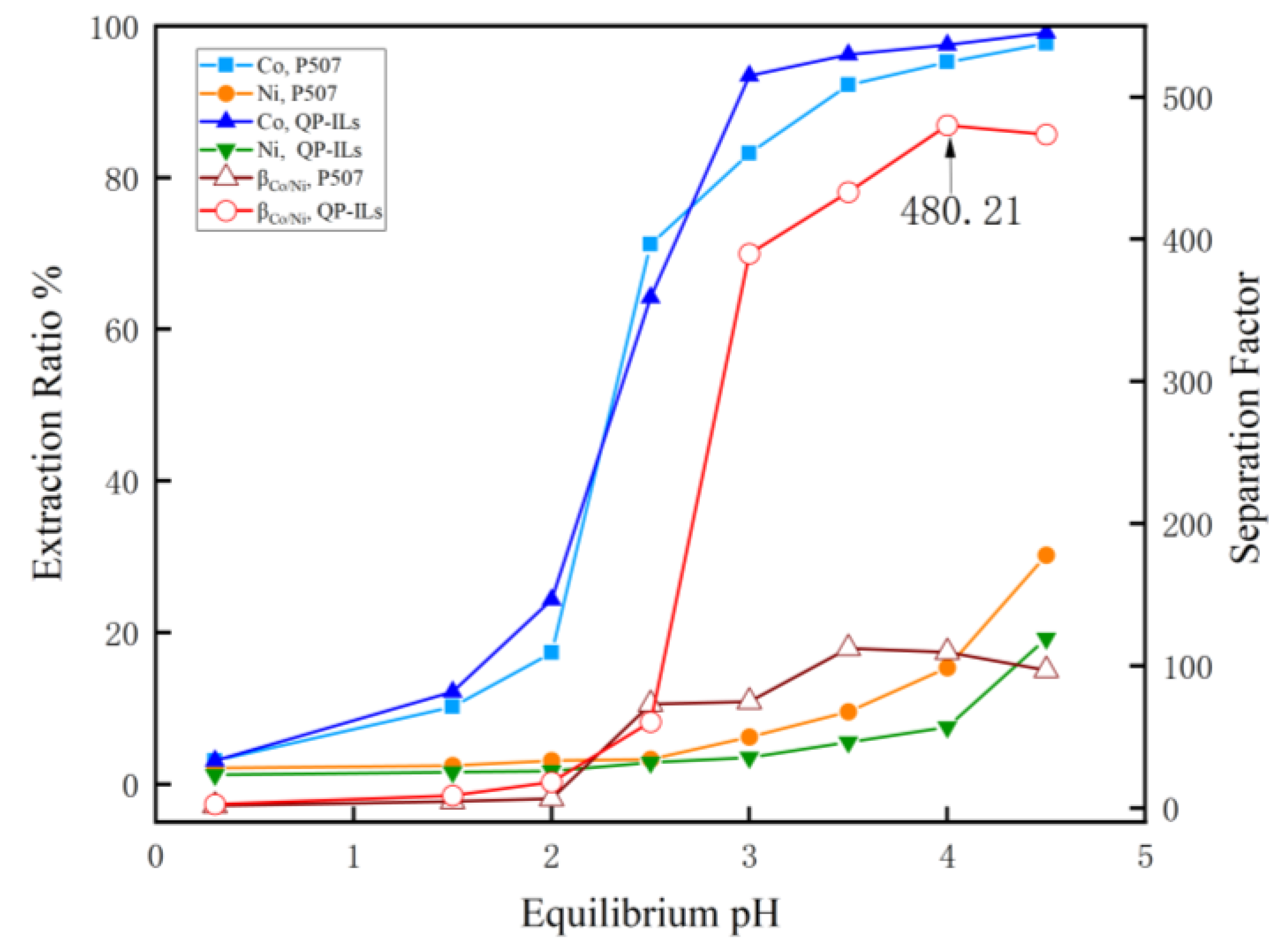
3.1.3. Effect of Equilibrium on Extraction Behavior
3.1.4. Effect of Concentration on Extraction of Co and Ni
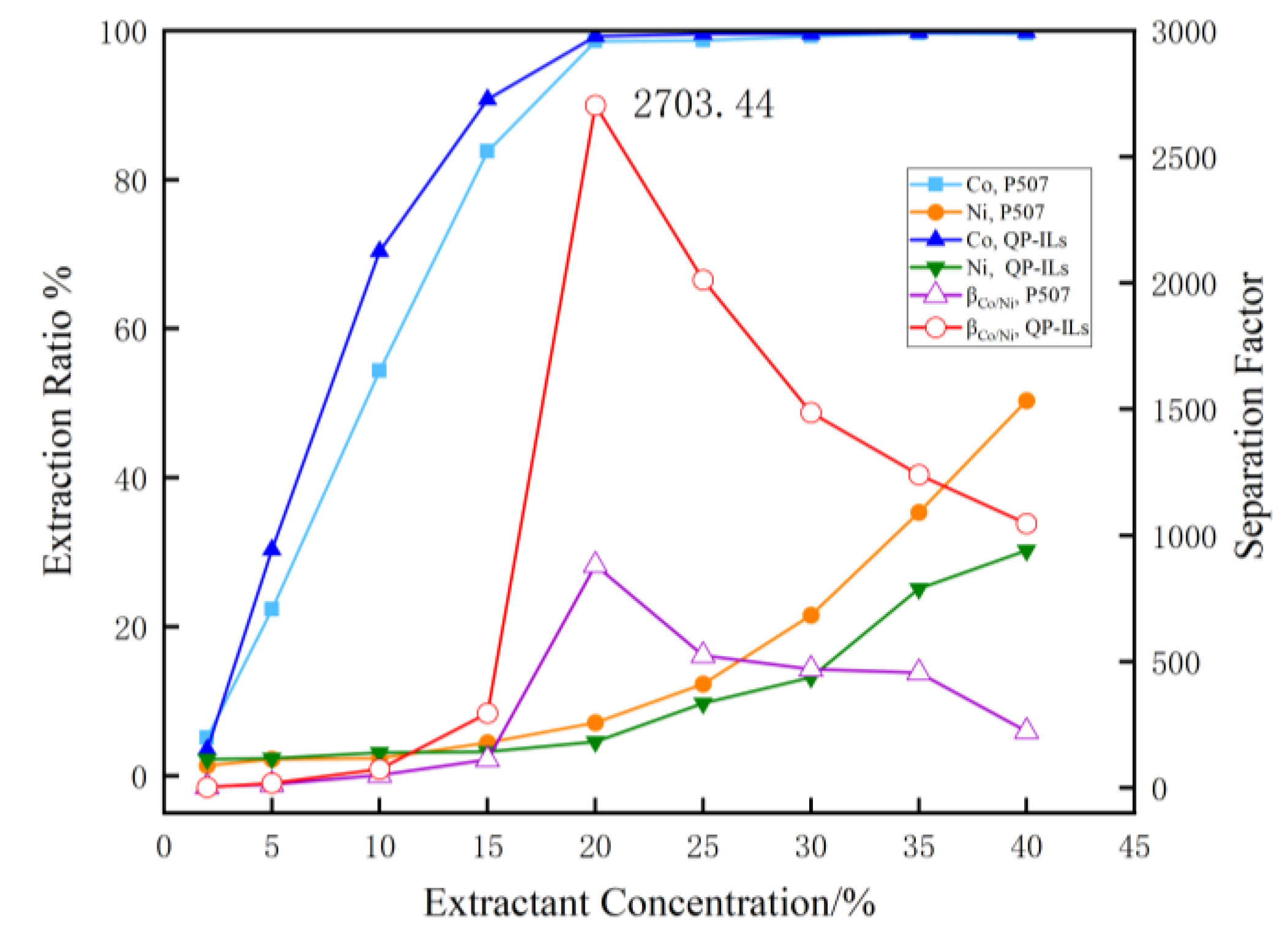
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, S.K. Ionic liquids and lignin interaction: An overview. Bioresource Technology Reports 2022, 17, 100958. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; et al. Progresses in polysaccharide and lignin-based ionic liquids: Catalytic applications and environmental remediation. Journal of Molecular Liquids 2021, 342. [Google Scholar] [CrossRef]
- Sun, S.; Bai, R.; Gu, Y. From waste biomass to solid support: lignosulfonate as a cost-effective and renewable supporting material for catalysis. Chemistry 2014, 20, 549–58. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; et al. Recent progresses in the application of lignin derived (nano)catalysts in oxidation reactions. Molecular Catalysis 2020, 489, 110942. [Google Scholar] [CrossRef]
- Sun, S.; Bai, R.; Gu, Y. From Waste Biomass to Solid Support: Lignosulfonate as a Cost-Effective and Renewable Supporting Material for Catalysis. Chemistry – A European Journal 2014, 20, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Burzynska, L.; Gumowska, W. Hydrometallurgical recovery of copper and cobalt from reduction-roasted copper converter slag. Minerals Engineering 2009, 22, 8p. [Google Scholar]
- Prakash, U.; et al. Processing of Waste Copper Converter Slag Using Organic Acids for Extraction of Copper, Nickel, and Cobalt. Minerals 2020, 10, 1p. [Google Scholar]
- Ziyadanogullari, B. , Recovery of Copper and Cobalt from Concentrate and Converter Slag. Separation Science & Technology 2000, 35, 9p. [Google Scholar]
- Arslan, F. Recovery of copper, cobalt, and zinc from copper smelter and converter slags. Hydrometallurgy 2002. 67, 7p. [CrossRef]
- Gumowska, W.; Rudnik, E.; Partyka, J. Mechanism of the anodic dissolution of Cu70-Co4-Fe14-Pb7 alloy originated from reduced copper converter slag in an ammoniacal solution: Recovery of copper and cobalt. Hydrometallurgy 2008, 92, 8p. [Google Scholar]
- Das, C.; Pandey, B.D. Leaching of copper, nickel and cobalt from Indian Ocean manganese nodules by Aspergillus niger. Hydrometallurgy 2010, 105, 7p. [Google Scholar]
- Verma, J.K. Extraction of copper, nickel and cobalt from the leach liquor of manganese-bearing sea nodules using LIX 984N and ACORGA M5640. Minerals Engineering 2011, 24, 4p. [Google Scholar]
- Dreano, A.; Fouvry, S.; Guillonneau, G. Understanding and formalization of the fretting-wear behavior of a cobalt-based alloy at high temperature. Wear 2020, 452. [Google Scholar] [CrossRef]
- Amsarajan, S.; Jagirdar, B.R. Air-stable magnetic cobalt-iron (Co7Fe3) bimetallic alloy nanostructures via co-digestive ripening of cobalt and iron colloids. Journal of Alloys and Compounds 2020, 816. [Google Scholar] [CrossRef]
- Agarwal, A.; et al. Chlorinated polyvinyl chloride (CPVC) assisted leaching of lithium and cobalt from spent lithium-ion battery in subcritical water. Journal of Hazardous Materials 2020, 393, 1p. [Google Scholar]
- Fu, Y.; et al. Enhancement in leaching process of lithium and cobalt from spent lithium-ion batteries using benzenesulfonic acid system. Waste Manag 2019, 88, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; et al. Efficient and economical recovery of lithium, cobalt, nickel, manganese from cathode scrap of spent lithium-ion batteries. Journal of Cleaner Production 2018, 204, 10p. [Google Scholar]
- Kumari, A.; et al. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Management 2013, 33, 8p. [Google Scholar]
- Bhanvase, B.A.; Sonawane, S.H. Investigation on liquid emulsion membrane (LEM) prepared with hydrodynamic cavitation process for cobalt (II) extraction from wastewater. Separation & Purification Technology 2020, 237, 1p. [Google Scholar]
- Wang, W.-Y.; et al. Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. Journal of Material Cycles and Waste Management 2019, 21, 300–307. [Google Scholar] [CrossRef]
- Biswal, B.K.; et al. Biological Leaching and Chemical Precipitation Methods for Recovery of Co and Li from Spent Lithium-Ion Batteries. ACS Sustainable Chemistry & Engineering 2018, 6, 12343–12352. [Google Scholar]
- Feng, X.N.; et al. Characterization of large format lithium ion battery exposed to extremely high temperature. Journal of Power Sources 2014, 272, 457–467. [Google Scholar] [CrossRef]
- Fu, G.; Wang, Z.; Hall, P. Recovering lithium cobalt oxide, aluminium, and copper from spent lithium-ion battery via attrition scrubbing. Journal of Cleaner Production 2020, 260, 1p. [Google Scholar]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review. Resources, Conservation and Recycling 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Hu, M.; et al. Separation of cobalt from Ni(OH)2 positive materials by a reduction and dissolution process in alkali solution. Separation and Purification Technology 2013, 120, 198–205. [Google Scholar] [CrossRef]
- Ichlas, Z.T.; et al. Processing mixed nickel-cobalt hydroxide precipitate by sulfuric acid leaching followed by selective oxidative precipitation of cobalt and manganese. Hydrometallurgy 2020, 191. [Google Scholar] [CrossRef]
- Mubarok, M.Z.; et al. Processing mixed nickel‑cobalt hydroxide precipitate by sulfuric acid leaching followed by selective oxidative precipitation of cobalt and manganese. Hydrometallurgy 2020, 191, 1p. [Google Scholar]
- Wen, J.; Tran, T.T.; Lee, M.S. Comparison of separation of Mn(II), Co(II), and Ni(II) by oxidative precipitation between chloride and sulfate solutions. Physicochemical Problems of Mineral Processing 2024. [CrossRef]
- Dhiman, S.; Gupta, B. Partition studies on cobalt and recycling of valuable metals from waste Li-ion batteries via solvent extraction and chemical precipitation. Journal of Cleaner Production 2019, 225, 820–832. [Google Scholar] [CrossRef]
- Han, B.; et al. Efficient phenyl phosphate ester extractant synthesis and solvent extraction performance evaluation for transition metals. Separation and Purification Technology 2024, 336. [Google Scholar] [CrossRef]
- Abo Atia, T.; et al. Solvent Extraction Process for Refining Cobalt and Nickel from a “Bulk Hydroxide Precipitate” Obtained by Bioleaching of Sulfidic Mine Tailings. Industrial & Engineering Chemistry Research 2023, 62, 17947–17958. [Google Scholar]
- Rodrigues, I.R.; et al. Separation of cobalt and nickel via solvent extraction with Cyanex-272: Batch experiments and comparison of mixer-settlers and an agitated column as contactors for continuous counter-current extraction. Separation and Purification Technology 2022, 296. [Google Scholar] [CrossRef]
- Li, K.; et al. Kinetic studies of gold leaching from a gold concentrate calcine by thiosulfate with cobalt-ammonia catalysis and gold recovery by resin adsorption from its pregnant solution. Separation & Purification Technology 2019, 213, 10p. [Google Scholar]
- Zioui, D.; et al. Membranes based on polymer miscibility for selective transport and separation of metallic ions. J Hazard Mater 2017, 336, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; et al. Biorecovery of cobalt and nickel using biomass-free culture supernatants from Aspergillus niger. Appl Microbiol Biotechnol 2020, 104, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; et al. Extraction/Separation of Cobalt by Solvent Extraction: A Review. Applied Chemistry for Engineering 2015, 26, 631–639. [Google Scholar] [CrossRef]
- Kumari, A.; et al. Extraction of rare earth metals (REMs) from chloride medium by organo-metallic complexation using D2EHPA. Separation and Purification Technology 2019, 227. [Google Scholar] [CrossRef]
- Pavon, S.; et al. Solvent extraction modeling of Ce/Eu/Y from chloride media using D2EHPA. Aiche Journal 2019, 65. [Google Scholar] [CrossRef]
- Yin, S.; et al. Microfluidic solvent extraction of La (III) with 2-ethylhexyl phosphoric acid-2-ethylhexyl ester (P507) by a microreactor. Chemical Engineering and Processing: Process Intensification 2015, 91, 1–6. [Google Scholar] [CrossRef]
- Zhang, L.; et al. Co and Ni extraction and separation in segmented micro-flow using a coiled flow inverter. Chemical Engineering Journal 2017, 307, 1–8. [Google Scholar] [CrossRef]
- Irannajad, M.; Haghighi, H.K.; Nasirpour, Z. New Solvent Extraction Process of Nickel and Copper by D2EHPA in the Presence of Carboxylates. Transactions of the Indian Institute of Metals 2020, 73, 1053–1063. [Google Scholar] [CrossRef]
- Nadimi, H.; Haghshenas Fatmehsari, D.; Firoozi, S. Separation of Ni and Co by D2EHPA in the Presence of Citrate Ion. Metallurgical and Materials Transactions B 2017, 48, 2751–2758. [Google Scholar] [CrossRef]
- Abu Elgoud, E.M.; et al. Separation of cerium(IV) and yttrium(III) from citrate medium by solvent extraction using D2EHPA in kerosene. Chemical Papers 2020, 74, 2461–2469. [Google Scholar] [CrossRef]
- Abu Elgoud, E.M.; et al. Extraction of Some Rare Earth Elements (La, Pr and Er) from Citrate Medium Using D2EHPA in kerosene. Arab Journal of Nuclear Sciences and Applications 2019, 52, 74–85. [Google Scholar]
- Jafari, H.; et al. Solvent extraction of zinc from synthetic Zn-Cd-Mn chloride solution using D2EHPA: Optimization and thermodynamic studies. Separation and Purification Technology 2018, 197, 210–219. [Google Scholar] [CrossRef]
- Habibpour, R.; et al. Comparative study on Ce (III) and La (III) solvent extraction and separation from a nitric acid medium by D2EHPA and Cyanex272, Metallurgical Research & Technology 2018, 115. [Google Scholar]
- Mohapatra, P.K.; Raut, D.R.; Sengupta, A. Extraction of uranyl ion from nitric acid medium using solvent containing TOPO and its mixture with D2EHPA in room temperature ionic liquids. Separation and Purification Technology 2014, 133, 69–75. [Google Scholar] [CrossRef]
- Torkaman, R.; et al. Reactive extraction of cobalt sulfate solution with D2EHPA/TBP extractants in the pilot plant Oldshue-Rushton column. Chemical Engineering Research & Design 2017, 120, 58–68. [Google Scholar]
- Asadollahzadeh, M., M. Torab-Mostaedi, and M. Ghanadi Maragheh, Reactive extraction of cobalt sulfate solution with D2EHPA/TBP extractants in the pilot plant Oldshue-Rushton column. Chemical Engineering Research & Design: Transactions of the Institution of Chemical Engineers Part A 2017, 120, 11p.
- Sousa, C.D.; Nascimento, M.; Ferreira, I.L.S. Modeling of nickel extraction by D2EHPA in sulfuric media. Rem-Revista Escola De Minas 2011, 64, 447–452. [Google Scholar]
- Lin, L.; et al. Extraction studies of cobalt (II) and nickel (II) from chloride solution using PC88A. Transactions of Nonferrous Metals Society of China 2006, 16, 687–692. [Google Scholar]
- ZHANG, Y.; et al. Extraction of cobalt with P507 and preparation of cobalt oxalate powders by ethane diacid stripping. Journal of Central South University (Science and Technology) 2011, 2. [Google Scholar]
- Flett, D.S. Solvent extraction in hydrometallurgy: the role of organophosphorus extractants. Journal of Organometallic Chemistry 2005, 690, 2426–2438. [Google Scholar] [CrossRef]
- Kang, J.; et al. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
- Tanong, K.; et al. Recovery of Zn (II), Mn (II), Cd (II) and Ni (II) from the unsorted spent batteries using solvent extraction, electrodeposition and precipitation methods. Journal of Cleaner Production 2017, 148, 233–244. [Google Scholar] [CrossRef]
- Qiu, L.N.; et al. Application of a functionalized ionic liquid extractant tributylmethylammonium dibutyldiglycolamate ([A336][BDGA]) in light rare earth extraction and separation. Plos One 2018, 13. [Google Scholar] [CrossRef]
- Wang, Y.; et al. Microcapsules containing ionic liquid [A336][P507] for La3+/Sm3+/Er3+ recovery from dilute aqueous solution. Journal of Rare Earths 2016, 34, 1260–1268. [Google Scholar] [CrossRef]
- Fernandes, A.; Afonso, J.C.; Dutra, A.J.B. Separation of nickel(II), cobalt(II) and lanthanides from spent Ni-MH batteries by hydrochloric acid leaching, solvent extraction and precipitation. Hydrometallurgy 2013, 133, 37–43. [Google Scholar] [CrossRef]
- Fernandes, A.; Afonso, J.C.; Bourdot Dutra, A.J. Hydrometallurgical route to recover nickel, cobalt and cadmium from spent Ni–Cd batteries. Journal of Power Sources 2012, 220, 286–291. [Google Scholar] [CrossRef]
- Sarangi, K. Separation of copper, zinc, cobalt and nickel ions by supported liquid membrane technique using LIX 84I, TOPS-99 and Cyanex 272. Separation & Purification Technology 2008, 59, 6p. [Google Scholar]
- Parija, C.; Reddy, B.; Sarma, P.B. Recovery of nickel from solutions containing ammonium sulphate using LIX 84-I. Hydrometallurgy 1998, 49, 255–261. [Google Scholar] [CrossRef]
- Guimardes, A.S.; et al. Purification of concentrated nickel sulfuric liquors via synergistic solvent extraction of calcium and magnesium using mixtures of D2EHPA and Cyanex 272. Separation and Purification Technology 2020, 239. [Google Scholar]
- Xiong, P.; et al. High-efficient and selective extraction of vanadium (V) with N235-P507 synergistic extraction system. Chemical Engineering Research and Design 2017, 120, 284–290. [Google Scholar] [CrossRef]
- Zhang, F.; et al. Synergistic extraction and separation of lanthanum (III) and cerium (III) using a mixture of 2-ethylhexylphosphonic mono-2-ethylhexyl ester and di-2-ethylhexyl phosphoric acid in the presence of two complexing agents containing lactic acid and citric acid. Hydrometallurgy 2014, 149, 238–243. [Google Scholar] [CrossRef]
- Rao, M.J.; et al. Solvent Extraction of Ni and Co from the Phosphoric Acid Leaching Solution of Laterite Ore by P204 and P507. Metals 2020, 10. [Google Scholar] [CrossRef]
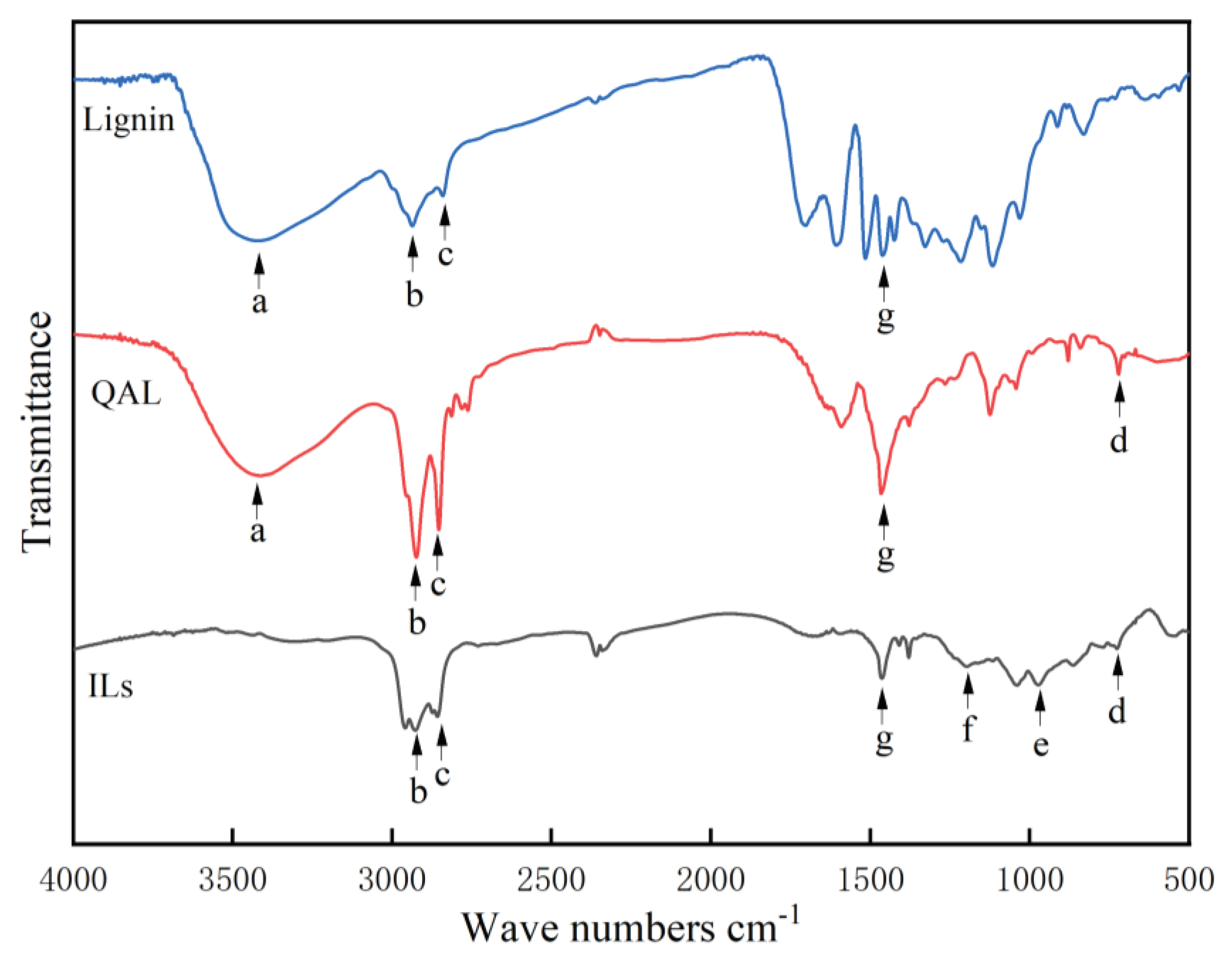

| Wave numbers | Characteristic groups | |
|---|---|---|
| a | 3430 cm-1 | -OH hydroxyl group |
| b c |
2926 cm-1 2856 cm-1 |
C-H of long chain alkyl group |
| d | 721 cm-1 | C-N bond |
| e | 972 cm-1 | P-OH |
| f | 1196 cm-1 | P=O |
| g | 1462 cm-1 | phenolic ring |
| 1000 ~ 910 cm-1 | quaternary ammonium salt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





