1. Introduction
MPM is an aggressive cancer characterized by a high clinical latency and often diagnosed at an advanced stage when not amenable to curative surgery [
1,
2]. According to the histology, MPM can be classified as epithelioid (the most frequent subtype, accounting for 60-80% of cases), sarcomatoid (~10%) and biphasic (10-15%)[
1]. Since 2003, the front-line treatment for unresectable MPM has been chemotherapy with platinum+pemetrexed [
3]. Only recently, the USFDA has approved the immune checkpoint inhibitor nivolumab+ipilimumab combination, which shows significant yet limited gain in survival [
4,
5]. Many different classes of compounds with various mechanism of action have been tested, with rather disappointing results [
6]. This is apparently due to a very pronounced resistance of MPM cells to anticancer agents [
7]. Further, therapy-induced stress remodels the MPM milieu. We and others have shown that, under stress-induced chemotherapy, profound rearrangements take place in MPM cultures [
8]. These include genomic, epigenetic, proteomic, metabolomic and secretome changes, all concurring to accelerate intra-tumor heterogeneity and the emergence of progenitor-like, EMT-driven, chemoresistant cell subpopulations [
8,
9,
10]. To further complicate this scenario, preclinical studies have often been performed in models that partially failed to recapitulate the disease complexity.
The development of Patient Derived Organoid (PDO) cultures emerged a few years ago as a promising tool within this regard. Patient-derived tumor organoids (PDOs) are three-dimensional, self-assembling structures of cancer cells isolated from surgical specimens or biological fluids [
11]. PDOs were shown to recapitulate the cyto-architecture and, to a significant degree, the heterogeneity of the originating tumor [
12]. PDOs can indeed accurately represent the genomic landscape of their source, in terms of mutation rates, DNA methylation patterns, gene expression signatures and copy number variations [
13]. This makes PDOs clinically relevant tools for disease modeling toward predictive drug screening [
14,
15]. Cocultures of PDOs with components of the tumor microenvironment (TME) (i.e. cancer associated fibroblasts, CAFs) may provide important information, because the CAFs represent a large cell subpopulation of the TME, actively participating in tumor progression/resistance to therapy[
16]. For example, cytokines released by CAFs exposed to 5-FU elicited pro-tumorigenic changes in colorectal cancer organoid-derived cells [
17]. Recently, MAFs were isolated and shown to express a set of markers partially overlapping with the CAFs of other tumors [
18,
19,
20].
Here, we have successfully set up mPDO cultures (n=8). Organoid cultures were obtained from eight MPM patients by using both pleural effusion- and solid specimen- derived cells. Primary MPM specimens and serially passaged (p3) mPDOs were cytologically similar with regard to the expression of mesothelial markers (MSLN; CALB2; KRT5/6; PDPN). As expected from a clinically relevant model, size and number of mPDOs were altered, in a patient-specific way, when pharmacologically relevant doses of chemotherapy drugs were added. We have derived mesothelioma- associated fibroblasts (MAFs) for co-culturing these latter with the mPDOs. The addition of MAFs altered the response of the mPDOs to pemetrexed + cisplatin, toward increased resistance. Conditioned medium by chemotherapy treated MAFs increased the expression of cancer stem cell markers in the recipient mPDOs. We found increased levels of IL-6 in the supernatant of the treated cocultures and demonstrated that this latter was derived from the MAFs. This increased secretion of IL-6 by mesothelioma-associated fibroblasts (MAFs) was functionally relevant, since pretreatment of conditioned media with IL-6 neutralizing antibodies strongly attenuated the response of the mPDOs to chemotherapy.
2. Results
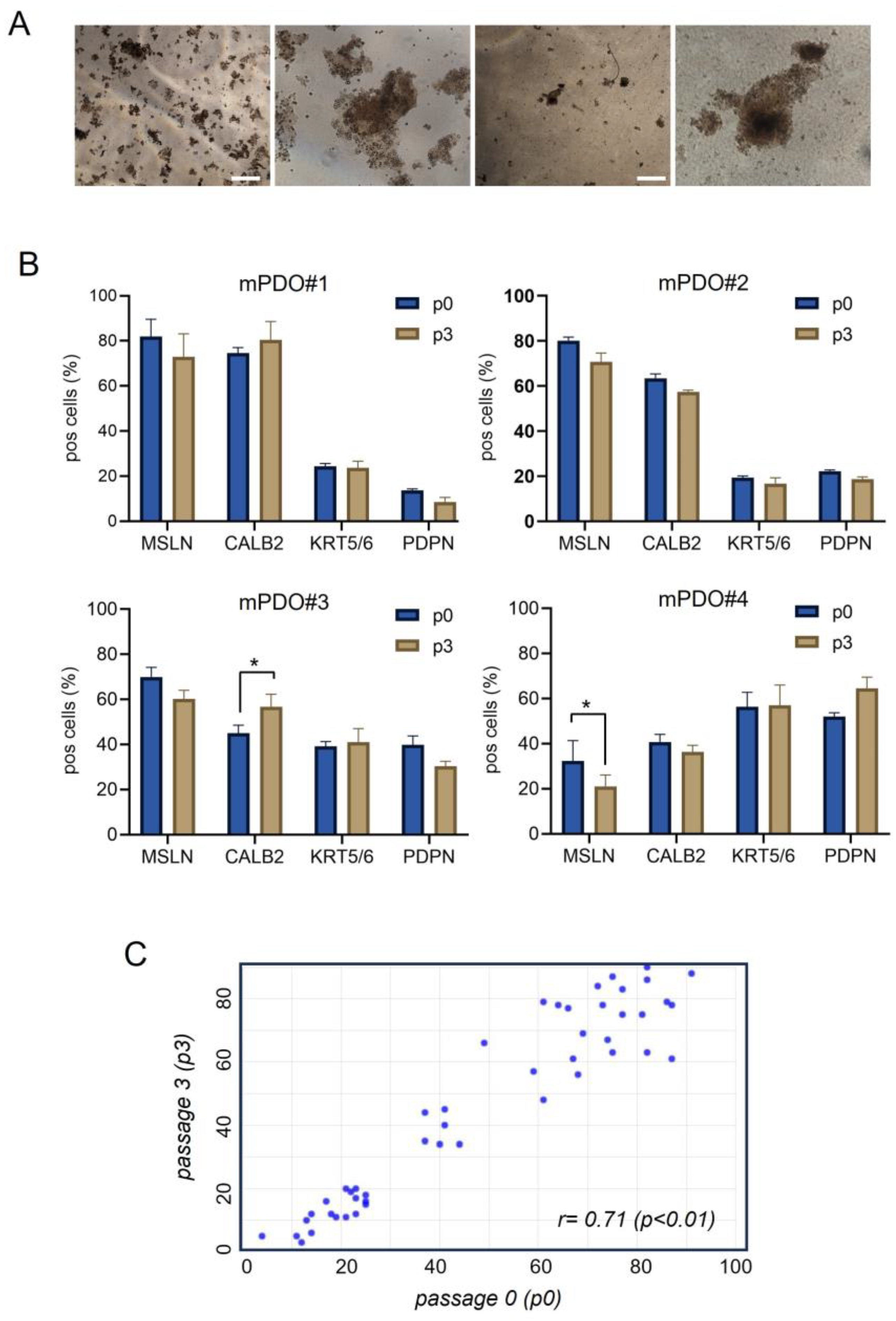
Establishment of mPDO cultures. We set-up patient derived tumor organoids (mPDOs)(n=8) from MPM patients by employing specific growth media and conditions to generate 3D structures from pleural effusion derived cells (
Figure 1a). The medium composition was defined starting from a basic medium thereby adopting a “matrix-like” screening approach. Of note, addition of FGF2 and FGF9 together with B27, as well as the systematic use of conditioned media during serial passaging, proved to be key for the establishment and propagation of the mPDOs (see methods). The establishment rate for this small cohort of samples was 66% (8/12) and the propagation rate up to passage 5 was 60%. Most of the mPDOs exhibited irregular morphology, with clusters of mesothelial cells of varying complexity (
Figure 1a). Additionally, the number of formed organoids was variable in time and the timing of organoid formation was variable as well, ranging from several hours to several days).
We proved that primary MPM specimens and passaged mPDOs were cytologically similar. Indeed, the obtained PDMOs expressed mesothelial markers (including mesothelin, calretinin, cytokeratin 5/6, podoplanin) when serially passaged (p3), very similar to the originating specimen, immediately after thawing and disaggregation (p0) (
Figure 1b). High correlation was shown between the percentage of positive cells (for all the mesothelial markers) at passage 0, and the percentage of positive cells at passage 3 (
Figure 1c). Thus, the percentage of cells expressing the mentioned markers was maintained and persisted over time and passaging.
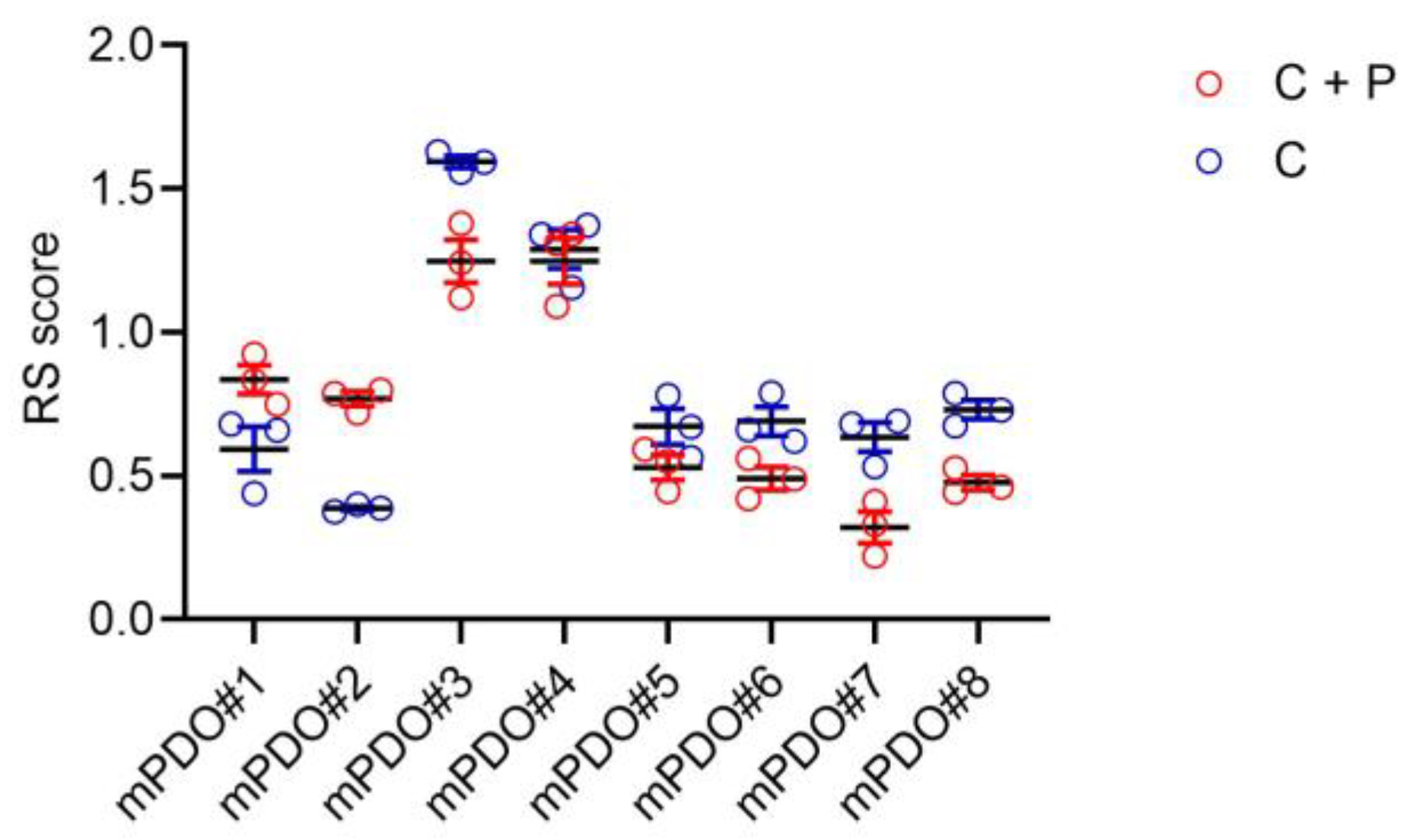
Response of the mPDOs to pemetrexed + cisplatin. Next, we evaluated the response of the obtained mPDOs to chemotherapy, administered with cisplatin (2ugr/ml) (C) or with cisplatin + pemetrexed (213 ng/ml) (C+P) for 96 hrs (with drug washout at 24hrs). When challenged with C or C+P, the mPDO cultures responded heterogeneously, as expected in a clinically relevant setting. We measured the effect of C+P treatment through a response score (RS), taking in account number of formed organoids, average diameter and number of live cells after treatment. Size and number of mPDOs were altered, in a patient-specific way, upon treatment with pharmacologically relevant doses of chemotherapy (
Figure 2). We found that six out of eight mPDO cultures were resistant to C+P, thus showing a RS ≤ 1 (with the exception of the mPDO#3 and mPDO#4). In most of the cases (with the exception of the mPDO#5) we observed a relative stronger effect of the C+P combined versus C alone (
Figure 2). We choose four of the mPDOs belonging to the C+P resistant subgroup to investigate their resistance to therapy and its modulation.
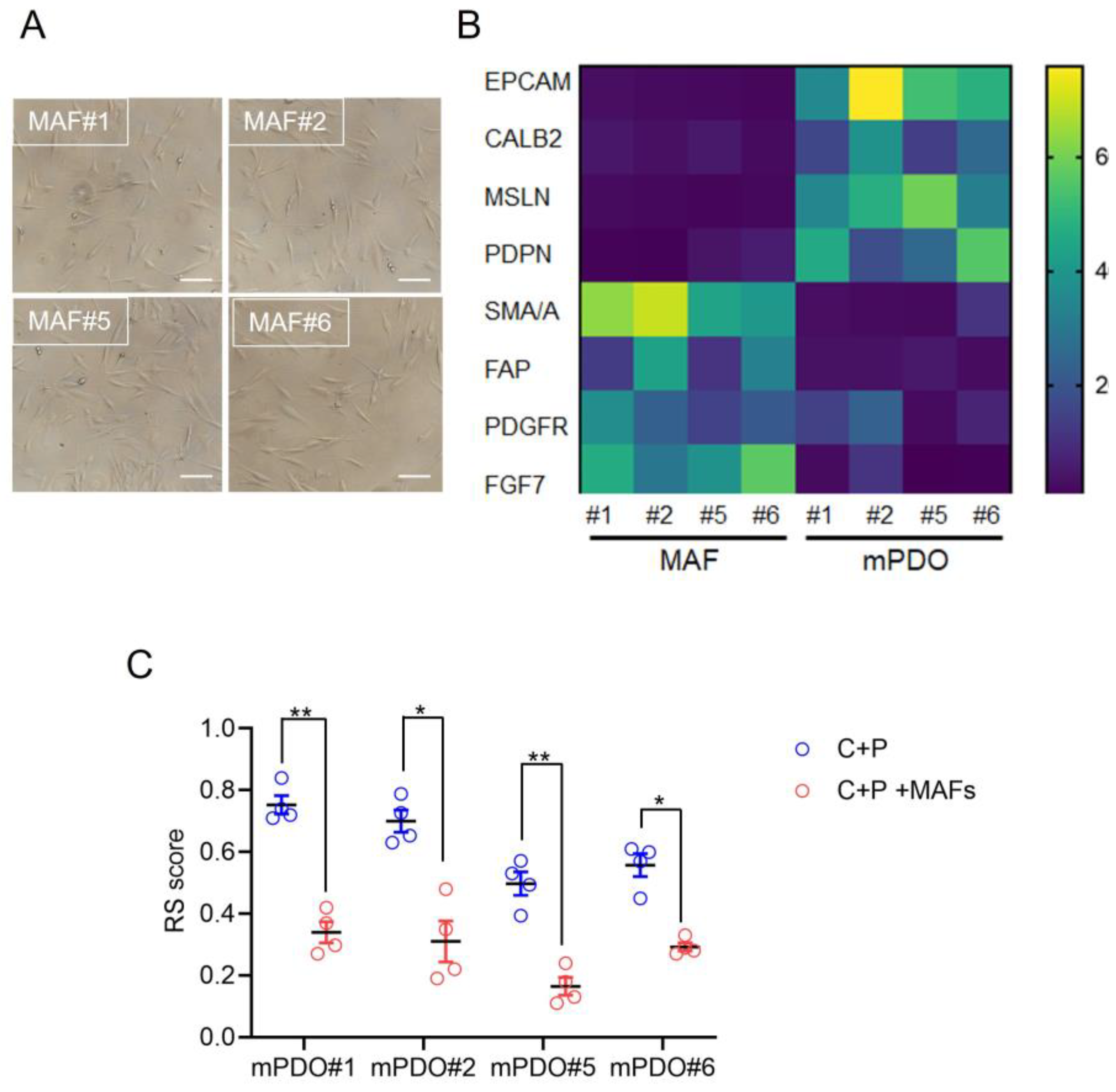
Establishment of Mesothelioma Associated Fibroblast (MAF) cultures. Cancer associated fibroblasts (CAFs) are known to mediate protumorigenic properties, including the resistance to therapy, in many cancer. Thus we explored the consequence of co-culturing organoid and matched MAFs (Mesothelioma Associated Fibroblasts) on the response to C+P. Firstly, MAFs were derived from the same specimen used for obtaining PDOs (
Figure 3a) and the relative enrichment of the obtained cultures was validated by qRT-PCR at passage two. When compared to the mPDOs, the MAF cultures were negative for the expression of mesothelin, calretinin and podoplanin at the mRNA level (
Figure 3b).The MAFs were also negative for the expression of EpCAM mRNA, while exhibiting variable yet significant higher expression levels of PDGFR, FAP, SMA/A and FGF7 (
Figure 3b). Thus, MAF cultures exhibited expression of genes typical of CAFs and negligible expression of mesothelial markers.
Effect of mPDO + MAF co-culturing on the C+P resistance. When challenged with C+P, the mPDO+MAF cocultures exhibited a different response as compared to the mPDOs alone (
Figure 3c), in that an increased resistance (higher RS) was observed for all the four cocultures tested (
Figure 3c). In detail, the response score was generally higher when compared to the single cultured mPDOs (
Figure 3c, compared to
Figure 2).
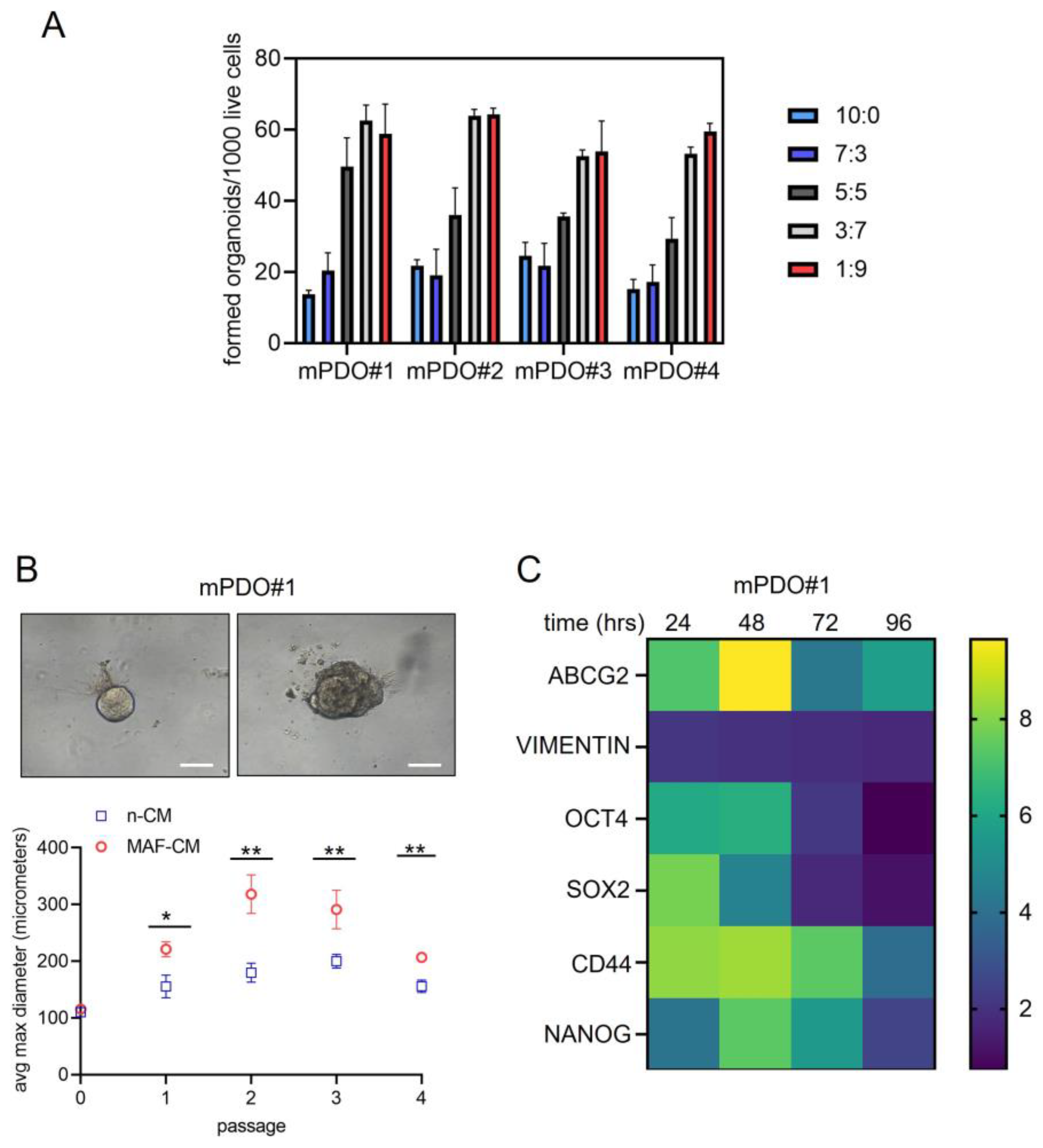
Conditioned medium from C+P -treated MAFs affected organoid number and size of the recipient mPDOs. We tested whether the conditioned medium from C+P treated MAFs could mediate the observed effect on the RS score and we tested different ratios of non-conditioned medium (n-CM) and MAF-conditioned medium (MAF-CM) (MAFs were treated for 24 hrs with C+P and medium collected for 24 hrs after drug washout) on the organoid forming ability (
Figure 4a) and on the size of the treated mPDOs (
Figure 4b). We found that organoid forming ability was increased, dose-dependently, by the MAF-conditioned medium, with a maximum reached at the 3:7 ratio (
Figure 4a) for three out of the four cultures. Similarly, when treating passage two mPDOs with MAF-CM we found a significant effect on their size (
Figure 4b, upper panel). In detail, the MAF-CM consistently increased the size of the treated mPDOs over passaging (
Figure 4b, lower panel and
Suppl. Figure S1).
MAF-CM modulated the expression of cancer stem cell markers and chemoresistance genes. Organoid Forming Ability (OFA) is deemed to be due to activation of pluripotent , tissue resident stem cells [
21]. This may be accompanied by upregulation of stem cell markers. Therefore, we assessed, by qRT-PCR, if the MAF-CM could increase the expression of cancer stem cell markers in the recipient mPDOs (
Figure 4c). This showed an early increase of OCT4, SOX2 and CD44, while NANOG showed an increase at later times and persisted over time. We also evaluated the expression of vimentin, an epithelial to mesenchymal transition gene product. We tested the levels of ABCG2 mRNA, because of the involvement of this latter gene product at mediating cisplatin resistance [
22]. ABCG2 exhibited an early increase, similar to OCT4, ABCG2 and CD44 after MAF-CM, while vimentin was only slightly increased by MAF-CM (
Figure 4c). Such a pattern was variably observed in the remaining three mPDOs (
Suppl. Figure S2). Altogether, we found that conditioned media from C+P treated MAFs could affect OFA and size of the mPDOs, and this correlated with a modulation of cancer stem cell markers and chemoresistance genes.
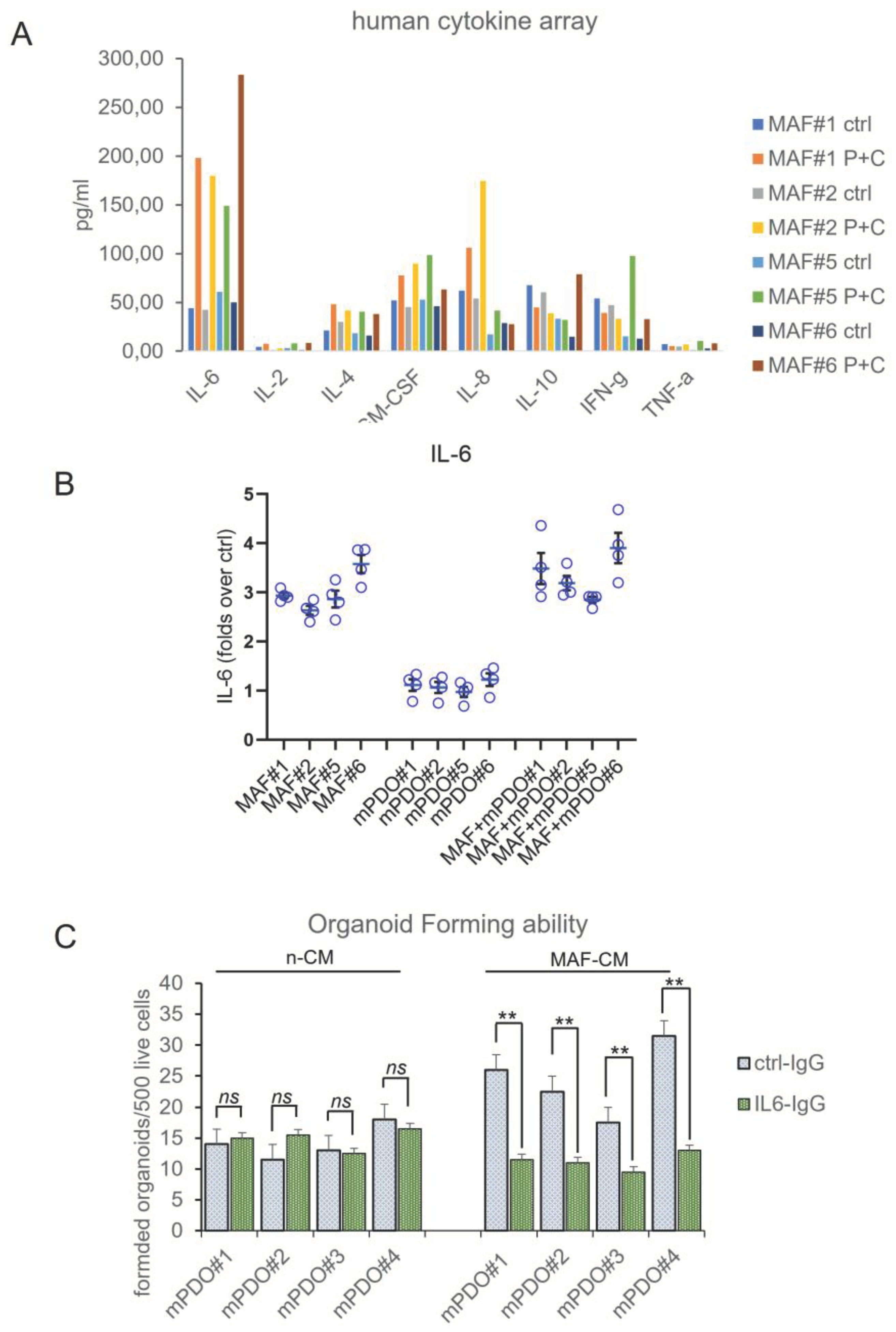
Interleukin-6 released by C+P treated MAFs contributes to chemoresistance of mPDOs. In order to deepen the previous observations, we evaluated the composition of the media conditioned for 24hrs mPDOs + MAFs, after C+P challenge. We evaluated the cytokine levels by means of an 8-plex human cytokine array, both at steady state (ctrl) and after C+P challenge (48hrs). The interleukin-6 (IL-6) stood out as the most consistently elevated cytokine in all the cocultures after C+P treatment (
Figure 5a). Thus, IL-6 was investigated further for its known role at mediating resistance to chemotherapy in MPM and other cancer settings [
23,
24]. Therefore, we investigated whether this was the case for MAFs as well. We measured the amount of secreted IL-6 in the conditioned medium of mPDOs and MAFs either single- or co-cultured at steady state and after P=C treatment, by ELISA assay (
Figure 5b). This firstly, revealed a steady increase of this cytokine starting at 24 hrs after treatment (
Figure 5b) in both MAFs and mPDO co-cultures. Very little IL-6 was secreted by passage three mPDOs (matching the fact that at p3 the mPDOs are devoid of TME components) when compared to MAFs. Notably, the levels of IL-6 secreted were higher in the co-cultures than in the MAFs, thereby suggesting a paracrine contribution of mPDO-derived cells at stimulating IL-6 release by the MAFs (see discussion). Altogether, this suggested that MAFs released IL-6 after C+P treatment.
To evaluate the functional relevance of the released IL-6 toward the changes in the mPDO response to C+P, we employed an IL-6 inhibiting antibody (
Figure 5c). We assessed the effect of this latter on the ability of the MAF-CM to stimulate the OFA in the recipient mPDOs. As expected, medium conditioned by ctrl (vehicle)-treated MAFs was unable to elicit changes in the OFA when administered to recipient mPDOs. Further, addition of the lL-6 neutralizing antibody (10ugr/ml) did not change OFA when compared to the ctrl IgG. On the other hand, when medium conditioned by MAFs pretreated with C+P was administered to mPDOs, a significant increase of the OFA was recorded, in the presence of ctrl IgG. Addition of the IL-6 neutralizing antibody strongly attenuated such effect (
Figure 5c). This matches the observation that IL-6 was released by MAFs after C+P challenge and suggest that its effect on the mPDOs was observed under stress conditions. Altogether, this evokes an important role for IL-6 secreted by MAFs at mediating the response of the co-cultures to the chemotherapy agents, thus establishing a key role for the C+P elicited release of IL-6 by the MAFs.
3. Discussion
To tackle MPM resistance to therapy, by identifying targetable determinants of resistance, down to a “single patient” level, seems more feasible thank to the setup of patient derived organoids[
25]. Here, we have applied the organoid technology to the study of MPM resistance to cisplatin and pemetrexed.
We were not the first to attempt mPDO generation and propagation[
26]. Pleural mesothelioma organoids from two patients have been setup by Mazzocchi and colleagues, by using a microfluidic device and conventional media[
27] . Additionally, some important examples from peritoneal mesothelioma have been recently published[
25]. We noted that the protocol we have setup here is similar to what published for peritoneal mesothelioma organoids [
25], with some key differences. In detail, we found that including FGF9 could significantly increase the yield and stability of the formed mPDOs. Notably, FGF9 is prognostic in MPM patients, toward overall survival (
Suppl. Figure S3). We also recorded a very important contribution of using the conditioned medium from previous passages. We believe that one possibility for explaining such an effect of conditioned medium is that keeping it during passaging may provide factor secreted by stromal TME cells which is lost during serial passaging, since organoid formation is intrinsically biased toward formation of enriched epithelial structures[
28,
29]. Another important novelty of our protocol is the addition of low doses of arachidonic acid (ARA). Beside ARA being a polyunsaturated fatty acid essential for normal health, we and others have shown that arachidonic acid may contribute survival properties to MPM cells[
30]. Additionally, in ovarian cancer ascites, it represents a prognostic determinant [
31]. Arachidonic acid, in absence of stressors like chemotherapy (when a cPLA2- mediated release is acted by MPM cells)[
30] , is produced by TME components, such as endothelial cells, monocytes and platelets[
32]. We believe that addition of ARA at low doses may indeed suffice for the absence of TME producing cells factors which may be, once again, lost during mPDO passaging. Finally, the doses of ARA used in the OGM medium here formulated , are compatible with those found, in many human tissues, in physiological conditions [
33]. We challenged our mPDO with the currently approved first-line chemotherapy regimen for MPM (cisplatin-pemetrexed combination) and we obtained a heterogeneous response. We believe this is expected from an experimental model of clinical relevance, such as PDOs. Here, we have identified Mesothelioma Associated fibroblasts as determinant of chemoresistance. Our evidence adds to a long story of relationship between CAF (MAF) presence and tumor progression [
18,
20]. When obtaining MAF cultures, we have not investigated the entire repertoire of MAF expression markers. However, we found the MAFs express SMA/A, FGF7, FAP and PDGFR, this partially echoing recently published work (18). We recorded a heterogeneous expression of the mentioned markers which does correlate with the demonstrated heterogeneity of such cell subpopulations [
34,
35,
36].
We were able to obtain eight mPDO cultures out of twelve pleural effusions. One sample was technically lost and three did not form organoids. All eight mPDO cultures were derived from epithelioid MPM, therefore it is possible that the conditions we have setup here may favor propagation of epithelioid MPM lineages. However, the small size of our casuistry (only one biphasic and one sarcomatoid MPM among the three non PDO-forming ones) does not allow us to reach conclusions and further study are warranted on biphasic and sarcomatoid MPMs.
IL-6 is produced by many cell types including endothelial cells, macrophages, epithelial cells, monocytes, and fibroblasts [
37]: we found that in MAFs, IL-6 levels were induced by C+P treatment. This is echoing what recently published on the crosstalk between mesothelioma cells and lung fibroblasts, with the former capable of modulating the activation state of the lung fibroblasts [
19].
Additionally, we found that the amount of IL-6 produced when mPDO -derived cells were co-cultured with MAFs was significantly higher when compared to the MAF single cultured. This may unravel a more complex mechanism of action whereby factors produced by mPDOs may facilitate IL-6 secretion by MAFs under C+P challenge. One possible candidate for this would be IL-1 alpha [
38]. For example, IL-1α activates IL-6/IL-8 cytokine network through both NF-κB and C/EBPβ during oncogenic SASP [
8,
39]. The limited cytokine array - has not allowed us to investigate this latter possibility, which will be the subject of further investigation in the future.
An interesting question is what lies downstream of IL-6 action on the OFA of the mPDOs? We and others have shown, both in MPM and in additional cancer settings, that modulation of the number and activity of chemoresistant ALDHbright cells may take place through IL-6 stimulated-STAT3 in CRC[
17]. Another possibility, not mutually exclusive, is that NFkB activation may follow IL-6 binding to mPDO-derived cells. This would match our previous observations that a dual STAT3 and NFKB inhibitor, butein, may attenuate resistance of MPM to pemetrexed and cisplatin both in vitro and in vivo [
40,
41].
One additional limitation of this study is the number of samples employed and, within the eight mPDO cultures established, the choice of those exhibiting resistance to C+P. This may have biased our investigation toward stress -activated mechanisms. We plan to expand our mPDO bank. Another limitation is that we have not studied whether other stimuli, different in nature from pemetrexed and cisplatin, may elicit a similar increase of IL-6. This broader possibility is currently investigated in our lab.
4. Materials and Methods
Source of MPM specimens. MPM pleural exudates and their corresponding cell pellets (n=12) were obtained from Mesobank, a Research Ethics Committee approved Research Tissue Bank. All patients provided written informed consent and samples were anonymized before they were released. Mesobank is supported by Asthma and Lung UK, The Victor Dahdaleh Foundation and the June Hancock Mesothelioma Research Fund.
Reagents. Pemetrexed and Cisplatin were from Sellekchem (Houston, TX, USA).
Flow cytometry. mPDOs were mechanically and enzymatically freed of BME, disaggregated, and filtered through a 70um filter mesh before staining. The following antibodies were employed, in separate tubes, each antibody matched to its isotype specific-related control antibody, in PBS1X-0.2% BSA, for 45 min at 4 °C, light protected. For the KRT5/6 and CALB2 staining, cell permeabilization was performed before staining with the Cell Fixation & Cell Permeabilization Kit (ThermoFisher, Waltham, MA, USA). For viability assay, the disaggregated PDOs were stained with Sytox Blue Helix NP Blue (Biolegend, CA, USA) for 5 min on ice before flow cytometry. Data were acquired with CytoFLEX Flow Cytometer (Beckman Coulter, IN, USA) and analyzed with the provided companion software. The following antibodies were employed, from Abcam (Cambridge, UK) and ThermoFisher (Waltham, MA, USA): ABCAM ab315357 Alexa Fluor® 488 Anti-Mesothelin antibody rabbit monoclonal; ABCAM ab303715 Alexa Fluor® 647 Anti-Podoplanin antibody rabbit monoclonal; ABCAM ab210633 PE Anti-Calretinin antibody rabbit monoclonal; ThermoFisher PA5-116450; Cytokeratin 5/6 Antibody (PA5-116450) rabbit polyclonal.
PDO cultures. Mesothelioma Patient Derived Organoids (mPDOs) cultures were obtained as follows. Cell aggregates were collected from pleural effusions of diagnosed patients after mild centrifugation (300 g x10 minutes at RT). All the MPM (n=12) were of mainly epithelioid histology except for one biphasic and one sarcomatoid MPM. Cell aggregates were washed three times in washing medium: Advanced DMEM-F12 (Thermofisher, Waltham, MA, USA), 0.2% BSA, Amphotericin B, Ciprofloxacin 2ugr/ml, insulin 1ugr/ml) and then resuspended in Dispase II solution (Stem Cell Technology, Vancouver, CA) for 15 minutes at 37C in slow agitation. After that, the cells were filtered through a 100uM strainer (CORNING, NY, USA) and resuspended in Organoid Growing Medium (OGM) composed of: Advanced DMEM-F12, 0.5% BSA, 50ng/ml EGF, 10ng/ml FGF2, 10ng /ml FGF9 (Cedarlane labs, Burlington, CA), 1X B27(Thermofisher, Waltham, MA, USA), Insulin 10ugr/ml (St. Louis, MO, USA) recombinant human R-spondin1 50 ng/ml (R&D, Minneapolis, MN) and recombinant human Noggin 50 ng/ml (Thermofisher, Waltham, MA, USA), 1ugr and seeded for 24 hrs in BIOFLOAT™ 24-well plate (FaCellitate, Mannheim, Germany), at a density of 5000 cells for well. After 24hrs, cells were collected without further filtering and resuspended in BME (R&D Systems, Minneapolis, MN, USA) drops (500 live cells /35ul drop) with a ratio of cell pellet to BME of 1:4 on ice and plated in a preheated 24well dish. After 30 minutes in the 37C incubator, warm OGM was added on the side of the dish (to avoid dislodging the BME drops), mixed to 30% of conditioned medium from the previous pre-aggregation step.
Validation of PDO cultures. Flow cytometry was performed on both MPM specimens and passage 3 mPDOs immediately after mechanical and enzymatic disaggregation and staining for mesothelin (MSLN), cytokeratin 57 (CK5/7), Podoplanin (PDPN) and Calretinin (CALB2).
Cytokine array. Briefly, PDO culture supernatants were analyzed for cytokine production with a Bio-Plex Pro Human Cytokine 8-plex Assay (BIORAD Hercules, CA 94547, USA), according to the manufacturer’s instructions. Plates were read using a Luminex-200 plate reader, MFIs were normalized to absolute values with provided standard curve as per manufacturer’s instructions. A minimum of 6 x 24wells (containing one BME drop each) were collected for each assay.
mPDO Treatment. mPDO (or mPDO + MAFs) were mechanically and enzymatically disaggregated to single cells and 500–1000 live cells were plated into BME drops in 24-well plates 24 h before starting treatments. mPDO cultures were treated with cisplatin (2ugr/ml) (C) or with cisplatin + pemetrexed (213 ng/ml) (C+P) for 96hrs (with drug washout at 24hrs). We classified the PDOs as resistant or sensitive based on an empirically defined response score (RS), according to the formula: number of formed organoids x average max diameter x viable cells (%) at time 0 day / number of organoids x average max diameter x viable cells (%) after 96 h. Please note that an RS score of 1 denoted no effect and a RS ≤ 1 indicated resistance to the treatment.
Mesothelioma Associated Fibroblasts (MAFs) isolation and propagation . MAFs were isolated as described in Cioce et al, 2023 with some modifications. Briefly, one-third of the MPM cells obtained from the pleural effusions after centrifugation were cultured in plastic dishes in 20% human serum containing OGM for 72 h, to enrich for adherent cell subpopulations. After that, the growth medium was shifted to a 20% human serum -containing advanced DMEM-F12 supplemented with non-essential-aminoacids (NEAA) (ThermoFisher, Waltham, MA, USA) and cells in suspension were removed at each passage by PBS 1X washing. MAFs samples were then tested positive for mRNA expression of fibroblast markers and negative for the expression of mesothelial markers, as indicated, within passage five from the isolation. MAFs + PDO cocultures. Disaggregated mPDO-derived cells were mixed to a variable ratio (1:1 to 1:5 live cells) with CAFs and included into BME drops, as previously described, in complete OGM, to start treatment 24 h later. Harvesting of conditioned media. MAFs were treated with either vehicle (Ctrl) or C+P for 24hrs and then media were substituted with OGM for additional 24hrs, to allow conditioning of the medium. Conditioned media were collected and centrifuged at 3000rpm for 10 minutes before being used or cryopreserved (-80C). We did not record significant proliferative effects on the growth of the MAFs when grown with OGM for 24hrs, to allow conditioning of the medium.
Detection of IL-6 by ELISA. The amount of IL-6 secreted in the medium of PDO cultures was quantified with Human IL-6 Quantikine ELISA Kit (R&D,Minneapolis, MN USA). PDO and mPDO + MAF culture supernatants were centrifuged at 4C and diluted appropriately before detection.
Treatment with IL-6 antibody. Neutralizing monoclonal antibody against human interleukin 6 and its biologically inactive isotype ctrl ab Mouse IgG1, kappa were from Invivogen (Invivogen, San Diego, CA, USA) and were used as indicated in the main text.
mRNA extraction and expression analysts. For RNA extraction, organoid- containing BME drops were collected and mechanically disaggregated on ice. The pellet was washed twice with ice cold PBS1X and then resuspended in RNA extraction reagent. Please note that no enzymatic disaggregation of the organoids was performed when subsequently extracting the RNA. Total RNA was extracted using the Trizol Reagent (Thermofisher Waltham, MA, USA).).
qRT-PCR analysis. The first-strand cDNA was synthesized according to manufacturer’s instructions (M-MLV-RT kit, Thermofisher Waltham, MA, USA). Gene expression was measured by Real-time PCR using the Sybr Green assay (Thermofisher Waltham, MA, USA) on a 7900HT instrument Applied Biosystems. All the primers used were from Origene (Rockwille, MA, USA) and sequences will be made available upon request.
Statistics. GraphPad Prism (Version 9.0) was used to perform the data analysis. The data, except where indicated, were from at least three independent experiments and were presented as mean ± SEM. Kaplan Meier analysis was performed with the Xena platform [
42].
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Figure S1; Supplementary Figure S2, Supplementary Figure S3.
Author Contributions
Conceptualization, MC, VG, VMF, FP; methodology, FN, NMG; formal analysis, GP, AF; investigation, MC,VG.; data curation, MC, VG; writing—original draft preparation, MC, VG.; writing—review and editing, AM,MC,VMF,FP.; funding acquisition, MC,,VMF. All authors have read and agreed to the published version of the manuscript.
Funding
MC, VG and VMF were supported by a grant from the Italian Ministry of Health (PSC SALUTE 2014-2020-POS2 “Cal-Hub-Ria”). MC and VMF were supported by a grant CNR IFT DBA.AD005.225 -NUTRAGE- FOE2021.
Institutional Review Board Statement
MPM pleural exudates and their corresponding cell pellets (n=12) were obtained from Mesobank, a Research Ethics Committee approved Research Tissue Bank. All patients provided written informed consent and samples were anonymized before they were released. Mesobank is supported by Asthma and Lung UK, the Victor Dahdaleh Foundation and the June Hancock Mesothelioma Research Fund.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Any source or data deemed as needed is available upon request.
Acknowledgments
We thankfully acknowledge Daniela Rutigliano, Claudio Pulito and Sara Donzelli (Regina Elena Cancer Institute, Rome) for sharing reagents and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khan, A.M.H.; Anwer, S.H.; Sayed, S.; Mansha, M.A.; Kamran, Y.B.; Khursheed, A.; et al. Comprehensive clinical overview of malignant pleural mesothelioma. Respir Med. 2024, 222, 107511. [Google Scholar] [CrossRef]
- Baas, P.; Daumont, M.J.; Lacoin, L.; Penrod, J.R.; Carroll, R.; Venkatesan, S.; et al. Treatment patterns and outcomes for patients with malignant pleural mesothelioma in England in 2013-2017: A nationwide CAS registry analysis from the I-O Optimise initiative. Lung Cancer. 2021, 162, 185–193. [Google Scholar] [CrossRef]
- Meirson, T.; Pentimalli, F.; Cerza, F.; Baglio, G.; Gray, S.G.; Correale, P.; et al. Comparison of 3 Randomized Clinical Trials of Frontline Therapies for Malignant Pleural Mesothelioma. JAMA Netw Open. 2022, 5, e221490. [Google Scholar] [CrossRef]
- Correale, P.; Pentimalli, F.; Nardone, V.; Giordano, A.; Mutti, L. CONFIRM trial: What is the real efficacy of second-line immunotherapy in mesothelioma? Lancet Oncol. 2022, 23, e13. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; Ewings, S.; Ottensmeier, C.; Califano, R.; Hanna, G.G.; Hill, K.; et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): A multicentre, double-blind, randomised, phase 3 trial. Lancet Oncology. 2021, 22, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Hiddinga, B.I.; Rolfo, C.; van Meerbeeck, J.P. Mesothelioma treatment: Are we on target? A review. J Adv Res. 2015, 6, 319–330. [Google Scholar] [CrossRef]
- Mujoomdar, A.A.; Tilleman, T.R.; Richards, W.G.; Bueno, R.; Sugarbaker, D.J. Prevalence of in vitro chemotherapeutic drug resistance in primary malignant pleural mesothelioma: Result in a cohort of 203 resection specimens. J Thorac Cardiovasc Surg. 2010, 140, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Canino, C.; Mori, F.; Cambria, A.; Diamantini, A.; Germoni, S.; Alessandrini, G.; et al. SASP mediates chemoresistance and tumor-initiating-activity of mesothelioma cells. Oncogene. 2012, 31, 3148–3163. [Google Scholar] [CrossRef]
- Lin, Y.; Burt, B.M.; Lee, H.S.; Nguyen, T.T.; Jang, H.J.; Lee, C.; et al. Clonal gene signatures predict prognosis in mesothelioma and lung adenocarcinoma. NPJ Precis Oncol. 2024, 8, 47. [Google Scholar] [CrossRef]
- Cioce, M.; Sacconi, A.; Pass, H.I.; Canino, C.; Strano, S.; Blandino, G.; et al. Insights into Intra-Tumoral Heterogeneity: Transcriptional Profiling of Chemoresistant MPM Cell Subpopulations Reveals Involvement of NFkB and DNA Repair Pathways and Contributes a Prognostic Signature. Int J Mol Sci. 2021, 22. [Google Scholar] [CrossRef]
- Hedayat, S.; Valeri, N. Patient-Derived Organoids: Promises, Hurdles and Potential Clinical Applications. Clin Oncol (R Coll Radiol). 2020, 32, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.J.; Murray, G.I.; McLean, M.H. Current concepts in tumour-derived organoids. Br J Cancer. 2020, 123, 1209–1218. [Google Scholar] [CrossRef]
- Qu, S.; Xu, R.; Yi, G.; Li, Z.; Zhang, H.; Qi, S.; et al. Patient-derived organoids in human cancer: A platform for fundamental research and precision medicine. Mol Biomed. 2024, 5, 6. [Google Scholar] [CrossRef]
- Khorsandi, D.; Yang, J.W.; Foster, S.; Khosravi, S.; Hosseinzadeh Kouchehbaghi, N.; Zarei, F.; et al. Patient-Derived Organoids as Therapy Screening Platforms in Cancer Patients. Adv Healthc Mater. 2024, e2302331. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, S.; Cioce, M.; Sacconi, A.; Zanconato, F.; Daralioti, T.; Goeman, F.; et al. A PIK3CA-mutant breast cancer metastatic patient-derived organoid approach to evaluate alpelisib treatment for multiple secondary lesions. Mol Cancer. 2022, 21, 152. [Google Scholar] [CrossRef]
- Papait, A.; Romoli, J.; Stefani, F.R.; Chiodelli, P.; Montresor, M.C.; Agoni, L.; et al. Fight the Cancer, Hit the CAF! Cancers (Basel). 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Cioce, M.; Fumagalli, M.R.; Donzelli, S.; Goeman, F.; Canu, V.; Rutigliano, D.; et al. Interrogating colorectal cancer metastasis to liver: A search for clinically viable compounds and mechanistic insights in colorectal cancer Patient Derived Organoids. J Exp Clin Cancer Res. 2023, 42, 170. [Google Scholar] [CrossRef]
- Ries, A.; Flehberger, D.; Slany, A.; Pirker, C.; Mader, J.C.; Mohr, T.; et al. Mesothelioma-associated fibroblasts enhance proliferation and migration of pleural mesothelioma cells via c-Met/PI3K and WNT signaling but do not protect against cisplatin. J Exp Clin Cancer Res. 2023, 42, 27. [Google Scholar] [CrossRef]
- Chrisochoidou, Y.; Roy, R.; Farahmand, P.; Gonzalez, G.; Doig, J.; Krasny, L.; et al. Crosstalk with lung fibroblasts shapes the growth and therapeutic response of mesothelioma cells. Cell Death Dis. 2023, 14, 725. [Google Scholar] [CrossRef]
- Borchert, S.; Mathilakathu, A.; Nath, A.; Wessolly, M.; Mairinger, E.; Kreidt, D.; et al. Cancer-Associated Fibroblasts Influence Survival in Pleural Mesothelioma: Digital Gene Expression Analysis and Supervised Machine Learning Model. Int J Mol Sci. 2023, 24. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef]
- Noguchi, K.; Katayama, K.; Sugimoto, Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: Basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers Med. 2014, 7, 53–64. [Google Scholar] [CrossRef]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 trans-signalling: Past, present and future prospects. Nat Rev Immunol. 2023, 23, 666–681. [Google Scholar] [CrossRef]
- Johnson, D.E.; O'Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Fang, X.; Shu, L.; Chen, T.; Zhao, X.; Yang, L.; Dou, T.; et al. Organoids derived from patients provide a new opportunity for research and individualized treatment of malignant peritoneal mesothelioma. Mol Cancer. 2024, 23, 12. [Google Scholar] [CrossRef]
- Gao, Y.; Kruithof-de Julio, M.; Peng, R.W.; Dorn, P. Organoids as a Model for Precision Medicine in Malignant Pleural Mesothelioma: Where Are We Today? Cancers (Basel). 2022, 14. [Google Scholar] [CrossRef]
- Mazzocchi, A.R.; Rajan, S.A.P.; Votanopoulos, K.I.; Hall, A.R.; Skardal, A. In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Sci Rep. 2018, 8, 2886. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Little, M.H. Organoids are not organs: Sources of variation and misinformation in organoid biology. Stem Cell Reports. 2023, 18, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Cioce, M.; Canino, C.; Pass, H.; Blandino, G.; Strano, S.; Fazio, V.M. Arachidonic acid drives adaptive responses to chemotherapy-induced stress in malignant mesothelioma. J Exp Clin Cancer Res. 2021, 40, 344. [Google Scholar] [CrossRef] [PubMed]
- Dietze, R.; Hammoud, M.K.; Gomez-Serrano, M.; Unger, A.; Bieringer, T.; Finkernagel, F.; et al. Phosphoproteomics identify arachidonic-acid-regulated signal transduction pathways modulating macrophage functions with implications for ovarian cancer. Theranostics. 2021, 11, 1377–1395. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Saavedra, D.; Stanford, K.I. Lipid Mediators in Cardiovascular Physiology anund Disease. In Cardiovascular Signaling in Health and Disease; Parinandi, N.L., Hund, T.J., Eds.; Cham, 2022; pp. 235–258. [Google Scholar]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits - A review. J Adv Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in cancer: Unity in heterogeneity. Cell. 2023, 186, 1580–1609. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, R.; Trono, P.; Di Carlo, A.; Di Modugno, F.; Nistico, P. Transcription factors in fibroblast plasticity and CAF heterogeneity. J Exp Clin Cancer Res. 2023, 42, 347. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, X.; Chen, Z.; Liu, C.; Wu, W.; Zhang, N.; et al. Define cancer-associated fibroblasts (CAFs) in the tumor microenvironment: New opportunities in cancer immunotherapy and advances in clinical trials. Mol Cancer. 2023, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. The biology of interleukin-6 in the 21st century. Semin Immunol. 2014, 26, 1. [Google Scholar] [CrossRef]
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A. 2009, 106, 17031–17036. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008, 133, 1019–1031. [Google Scholar] [CrossRef]
- Canino, C.; Luo, Y.; Marcato, P.; Blandino, G.; Pass, H.I.; Cioce, M. A STAT3-NFkB/DDIT3/CEBPbeta axis modulates ALDH1A3 expression in chemoresistant cell subpopulations. Oncotarget. 2015, 6, 12637–12653. [Google Scholar] [CrossRef]
- Cioce, M.; Canino, C.; Pulito, C.; Muti, P.; Strano, S.; Blandino, G. Butein impairs the protumorigenic activity of malignant pleural mesothelioma cells. Cell Cycle. 2012, 11, 132–140. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repecka, K.; McDade, F.; Kamath, A.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).