1. Introduction
An investigation of the electron transport in
metals began over a hundred years ago when the free electrons were postulated
by Drude [1] to explain the conductivity of
metals. At the same time, the properties of the electrons in metals have been
analyzed by using the classical statistical methods of the gases. It was assumed
that the density of the free electrons is equal to the density of atoms, and
the mean free path of the electrons can be determined from comparison with an
experiment. The Drude theory failed to explain why the electron in a pure metal
can freely travel over a hundred atomic distances without being scattered.
Later a mental imagination that all metal valence electrons become delocalized
and are shared by all atoms in the solid forming a “sea” of negative charge [2,3], and are no longer associated with a given
atom [4]. The nuclei with their core electrons
form ions, which are immersed in this sea of valence electrons. Such ungrounded
imagination about free electron transport in metals has continued for nearly
the last century.
Usually, metal atoms are arranged in regular
three-dimensional periodic lattices. Hard spheres of metal atoms are packed in
such crystal structures: face-centered cubic (FCC), body-centered cubic (BCC),
hexagonal close-packed (HCP), orthorhombic (ORC), and tetragonal (TET). The dependences
of the metal atoms to various lattice structures and their distribution on the
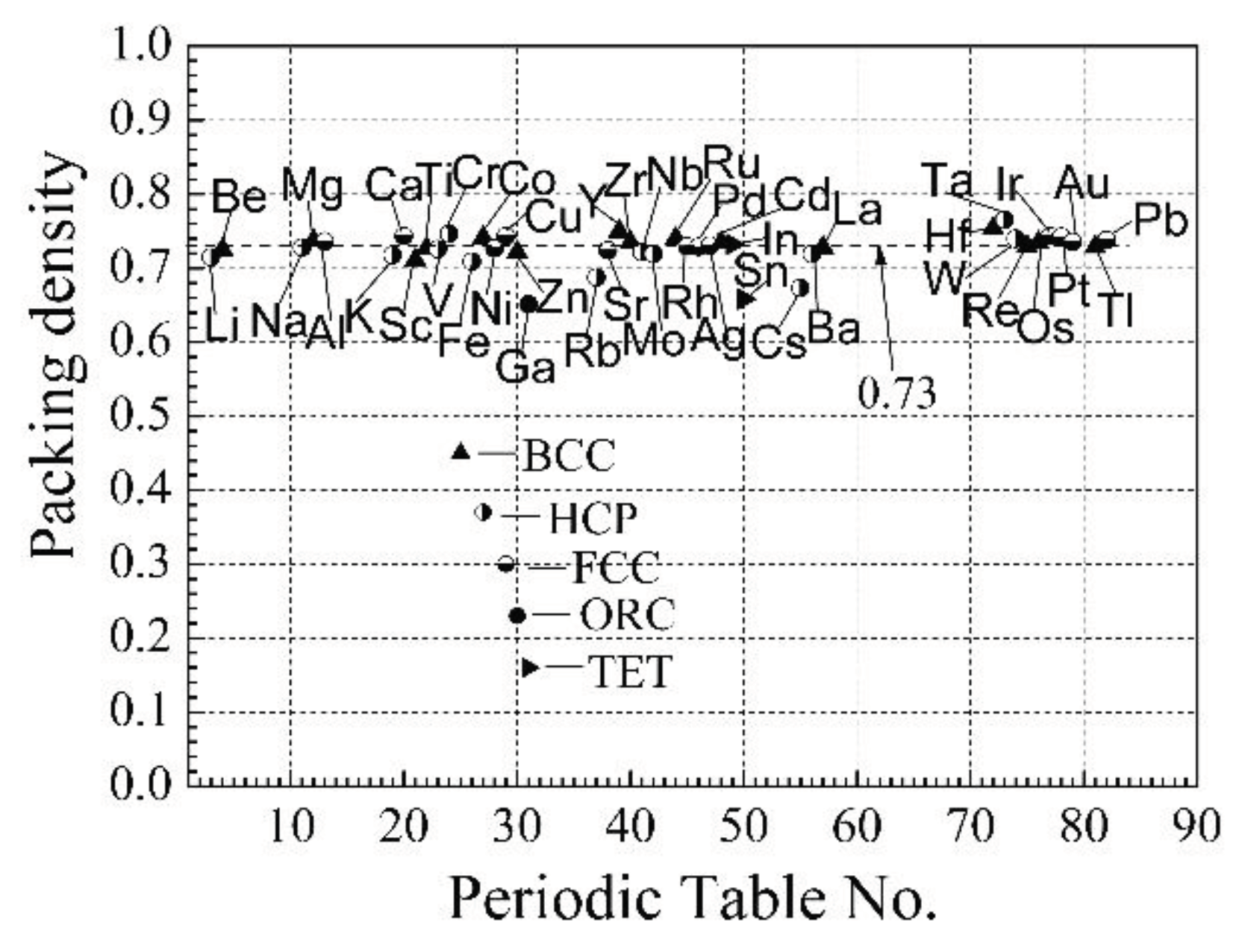
periodic table are presented in
Figure 1 [5,6].
As can be seen from
Figure 1, almost all metal atom packing density is close to the ideal
close-packing value of 0.73, and it does not depend on the lattice structure,
only for Ga, Rb, Cs, and Sn the packing density is smaller than the ideal close
packing density value.
The interaction of the valence electrons with the
ion cores produces the main contribution to the binding energy and lowering of
the energy of the valence electrons in metal compared with that of the free
atom causes the metallic binding [7–9]. The
total number of bonds in metal is considerably large, and not all valence
electron bonds have the same binding energy.
Some difficulties in the explanation of the
electron transport in metals by classical statistics have been understood by
quantum mechanics: the Pauli exclusion principle and Fermi-Dirac statistics. According
to quantum mechanics the free electrons as the Bloch waves can freely move in
the ideal periodic lattice of the metal without any scattering by lattice ions [3,7–10]. The scattering of the free electrons can
be only in spots where there are distortions of the periodicity of the
potential energy of the ideal lattice structure.
The electrons in an ideal periodicity crystal are
arranged in energy bands (allowed zones) separated by forbidden regions in
energy (energy gaps). Each primitive cell exactly contributes only one
independent value of the wave vector
k to each energy band [10–12]. The valence electrons occupy the lowest
energy levels in the conduction band below the Fermi level energy, and their
distribution on energy
E is described by Fermi distribution function
f(
E).
Though the valence electron wave functions overlap with that of neighbor atoms [6–8], they remain associated with native ion cores.
Only valence electrons, which have energies close to the Fermi level energy due
to lattice atom vibrations can be released, and can randomly move in metal [13,14]. The effective density
neff
of the free randomly moving (RM) electrons in metals and other materials has
been defined in [15] as
where
is the density of states (DOS) in the conduction
band. It let to show that the effective density
neff of the
free RM electrons in metals can be described as
, where
at
E=
EF. It also lets us
show that the Einstein′s relation between the diffusion coefficient
D and the drift mobility
of the free RM charge carriers
is valid in all cases with one type of charge
carriers. In [16], there is at the first time
presented the probability density function of the free RM electron distribution
on energy:
, which meets all requirements of the probability
theory [17,18].
Though the value of the
neff
reaches only a few per cents of the total density
n of the valence
electrons of metals at room temperature, the total density of the valence
electrons
n has been traditionally used for description of the
electrical conductivity
σ in
metals and superconductors in the normal state by
where
q,
τ, and
are, respectively, the electron charge, the average electron relaxation time, and its effective mass. From a comparison of the Drude and Sommerfeld conductivity formulae, it has been stated that there is only a difference in the definition of the relaxation time of the free electrons [
10]. The Drude conductivity formula can be used for descriptions of the conductivity of weakly doped semiconductors, but it is completely not applicable to describe the conductivity of metals and other materials with degenerate electron gas.
The resistivity of metals linear increase with a temperature above Debye's temperature was usually explained by charge carriers scattering due to lattice atom vibrations: due to an increase of the electron scattering cross-section [6,9–12,19–22]. As shown in [
13,
14], the electron scattering cross-section at a temperature above the Debye temperature does not depend on temperature, and the lattice vibrations play another role: they produce the free RM electrons, and at the same time they produce the electronic defects (weakly screened ions, which lost valence electrons). The effective density of the electronic defects
. The rest part
N –
Neff of atoms which have the valence electrons with energy lower than the Fermi level energy does not have sufficient thermal vibration energy to excite their valence electrons. As it is pointed in [
13], the rest part
n –
neff of the valence electrons, which energy is lower by a few
kT than Fermi level energy, due to the Pauli exclusion principle and Fermi-Dirac statistics can not change their energy, and they cannot scatter the free RM electrons.
3. The Study of the Electrical Resistivity Law AT 2 of Metals at very Low Temperatures
The idea that electron-electron scattering contributes to electrical and thermal resistivities of metals was proposed by Landau and Pomeranchuk [
23], but about 20 years the resistivity dependence
has not been observed in simple metal. On the base of the conservation of the quasi-momentum and energy of free electrons, it has been proposed that the electron-electron scattering rate changes as
T2, and as a consequence it must give the law
T2 of the electrical resistivity at very low temperatures. According to [
9], the electron-electron scattering rate
can be described as
where
A0 is the non-dimensional quantity. On the base of this relation, it is believed that relaxation time changes as 1/
T2, and that it can take part only at very low temperatures in very pure metals. From it also follows that temperature dependence of resistivity
for a three-dimensional metal due to electron-electron scattering can be described as
.
A detailed analysis of the electron-electron scattering and its contribution to the electrical resistivity for conducting materials is presented in [
24]. The problem of electron-electron scattering in metals also have been solved by using the Boltzmann kinetic equation [
25] Recently [
26], it has been shown that Eq. (12) is correct with
A0 =
Nimp/4
π2, where
Nimp is the number of impurities (or scattering centers) in a given sample. The effects of electron-electron scattering in metals also have been investigated theoretically in both a simple tight-binding model and in a transition metal tungsten by calculating the electron scattering rate at the Fermi surface [
27].
Considering that the contribution of the term
to resistivity at very low temperatures is very small, it was needed the extremely precise measurement technique, better than p.p.m. relative accuracy in the measurement of the low-temperature electrical resistivity, which appeared only a half-century ago [
24,
28,
29,
30]. High-resolution measurements of the electrical resistivity of simple metals at low temperatures have yielded new data that were in contradiction to the generally accepted theories. It has been found that the electrical resistivity does not follow the expected
law for any metal at very low temperatures.
A detailed analysis of the electrical resistivity of the alkali metals at low temperatures is presented in [
24,
31,
32]. It is shown that for potassium at low temperatures, the phonon drag can play an important role leading to an exponential decrease with temperature [
33,
34]. It has been believed that the experimental parameter
Aexp would be the same for all samples of the same metal, but the values
Aexp are different about ten times. For lithium, it has been expected that values
Aexp would be similar to K, but the observed values are more than an order larger [
24,
35,
36].
The electrical resistivity of noble metals at low temperatures has been investigated by many authors [
37,
38,
39,
40]. It has been shown that the electrical resistivity of copper and silver at low temperatures has approximate
T 4 behavior, and the term
T 2 appears only below 2 K [
37]. From the measurement of the electrical resistivity of high-quality copper whiskers taking account of the surface scattering at different magnetic fields, it has been estimated the parameter
A for the electrical resistivity of bulk copper [
38]. Temperature dependence of the scattering rate in copper also has been investigated by radio frequency size effect to extract the contribution of electron-electron collisions [
39]. The observed
T 4 behavior for the low-temperature electrical resistivity of Ag is related to the simultaneous presence of both electron-phonon and electron-electron scattering [
40]. To observe the term
T 2 in noble metals it is needed very low temperatures and very pure metals.
Aluminum is a simple polyvalent metal, which electron-electron scattering has been intensively investigated [
41,
42,
43,
44]. In [
41], it has been presented that the electrical resistivity of aluminum at low temperatures has the term
AT 2. The surface scattering contribution for electrical resistivity of high-purity aluminum samples in the thickness range from 0.1 μm to 7 mm has been investigated in [
42]. It is shown that surface scattering of the conduction electrons affects the temperature dependence of the resistivity for samples less than 0.5 mm in thickness. The electrical resistivity term
AT 2is attributed to electron-electron scattering, and it is shown that coefficient
A practically does not depend on the residual resistivity value [
43]. The influence of the electron-phonon interaction on electron-electron scattering is described within the framework of the Landau Fermi-liquid theory, and electron-electron scattering contribution to the low-temperature resistivity of aluminum can be enhanced by a factor of ~20 due to electron-phonon interaction [
44].
Due to that transition group metals differ from simple metals, which have partially filled
d- and
s-band electrons it has been believed that for transition metals the major contribution to resistivity
should be caused by highly mobile
s-electrons being scattered by the “heavy”
d-electrons, i. e. the so-called
sd-scattering. It is observed that coefficient
A for transition metals is often one or two orders of magnitude larger than for simple metals. Quadratic temperature dependence for
for many transition metals has been demonstrated at low temperatures [
45,
46,
47,
48,
49,
50], but interpretations are different. In [
45], it is shown that there is some correlation between coefficient
Aexp and the electronic heat capacity parameter γ. The electron-electron scattering in tungsten has been investigated by using radio-frequency size-effect for electron scattering rate estimation [
46]. The term
AT 2 has been found over the temperature range 1.5–5 K for molybdenum, it was concluded that the quadratic term in the temperature dependence of the scattering rate in molybdenum is due to the electron-electron scattering [
47]. The electrical resistivity of a single crystal of vanadium at low temperatures has been analyzed in terms of electron-impurity, electron-electron, and electron-phonon scatterings [
48]. They have obtained the term
AT 2 with coefficient
A≈(1.6 – 0.2)⋅10
-11 Ωcm/K
2. The effects of electron-electron scattering in vanadium have been investigated theoretically, and the calculated scattering rates agree with experimental measurement results [
49]. The electron-electron scattering in high-purity single crystals of molybdenum has been measured at low temperatures [
50], it is shown that when approaching an ideal metallic crystal structure, there have been observed the deviations from the Ohm’s law.
The traditional explanation [
10] for the large magnitude of
Aexp due to
sd-scattering in transition metals is based on the prepositions that the effective mass of the
d-electron is very high, implying that its velocity
vd is very low compared with the velocity
vs of the
s-electron. These arguments are very questionable because the drift mobility and drift velocity of metals (see Eqs. (2) and (3)) do not depend on the electron mass. All electrons at the Fermi surface have the same Fermi level energy and are randomly moving with the same Fermi velocity. Often in many cases there is confused the large density of states at the Fermi surface with the effective mass of electrons.
There we want to point attention to the fact that conservation of the quasi-momentum and energy of free electrons due to electron-electron scattering do not produce energy losses, and consequently, this process is elastic and has no affect on material resistivity. It is confirmed by the thermal noise relation with the material resistivity [
51], and by the fluctuation-dissipation theorem [
52,
53]. Therefore, almost for all elemental metals the electrical resistivity at low temperatures has the term
AT 2, but its explanation by electron-electron scattering is doubtful.
In [
13,
14], it has been shown that the lattice atom vibrations produce not only the effective density of free randomly moving electrons
(here
is the density of states at the Fermi level energy), but also the same density of electronic defects (not completely screened ions) is produced
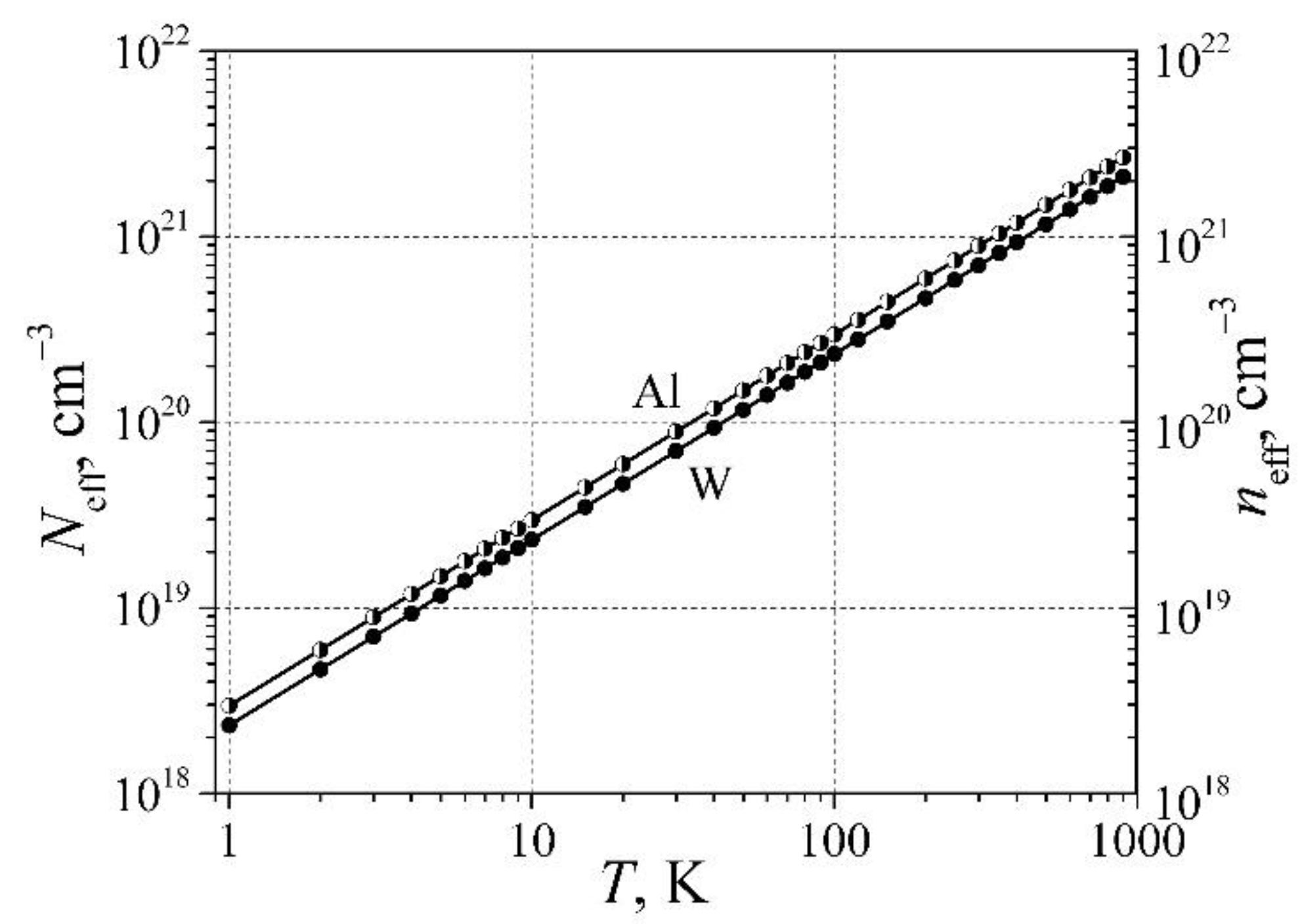
. In
Figure 2, there are demonstrated the electronic defect dependence on temperature for aluminum, and for transition group metal tungsten. As can be seen, the effective density of the electronic defects even at temperature 1 K is higher than 10
18 cm
-3, and these electronic defects cannot be, in principle, decreased as it can be done with the residual defects.
As it is shown in [
13,
14], the resistivity of the elemental metals dependence on temperature can be described as
where
Θ is the Debye temperature,
is the phonon mediation factor accounting the free RM electron scattering by electronic defects:
The resistivity
at
T=
T0 is described as
here we used
, where
is the effective scattering cross-section estimated at temperature
T0. The value of the temperature
T0 must be taken from the linear resistivity dependence range on temperature. Then the effective scattering cross-section is estimated as
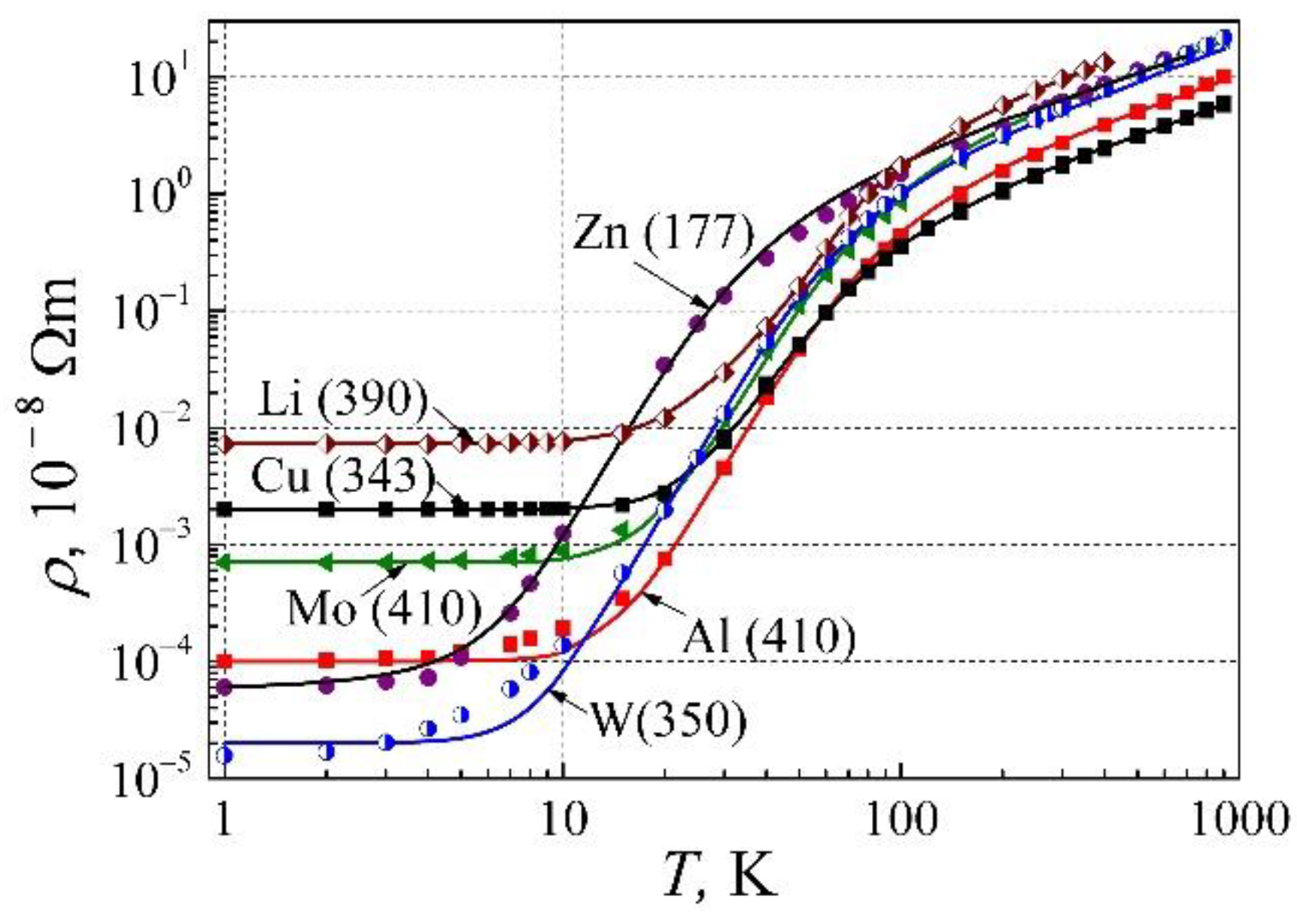
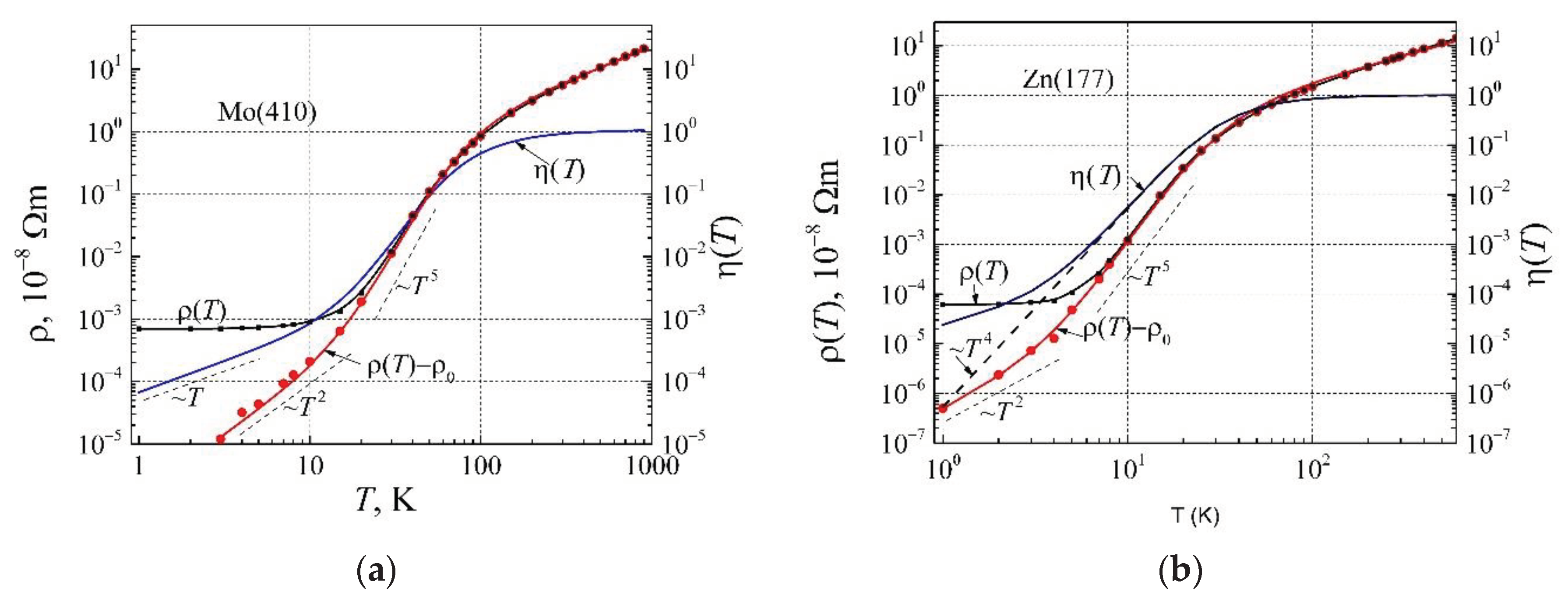
For further study of the resistivity dependence on temperature, we shall investigate the elemental metals from various sites of the periodic tables: the alkali group metal Li, the noble metal Cu, the second group IIB metal Zn, the third group IIIA metal Al, and the transition group metals Mo and W. The resistivity dependences on temperature for these metals are presented in
Figure 3: dots are the experimental results, and the solid lines are calculated by Eq. (13). As can be seen, the resistivity
ρ(
T) of metals has three clearly expressed parts: i) at low-temperature so-named residual resistivity caused by impurity and structural imperfections of metal, ii) so-named phonon-assisted part with ρ ~
T 5, which at higher temperatures crosses to iii) the linear resistivity dependence on temperature.
Even though the entire description of the resistivity of metals by Eq. (13) is sufficiently good, at temperatures below 20 K there is a small inadequacy for metals Al, Zn, Mo, and W. Considering the earlier presented experimental results of the electrical resistivity term AT 2, we shall see further, what causes such electrical resistivity dependence on very low temperatures.
Considering the so-named law
ρ=
AT 2 at low temperatures, we shall carefully investigate the resistivity dependence on low temperatures on the sample of Al. For this aim, we shall find what characteristics cause such resistivity (
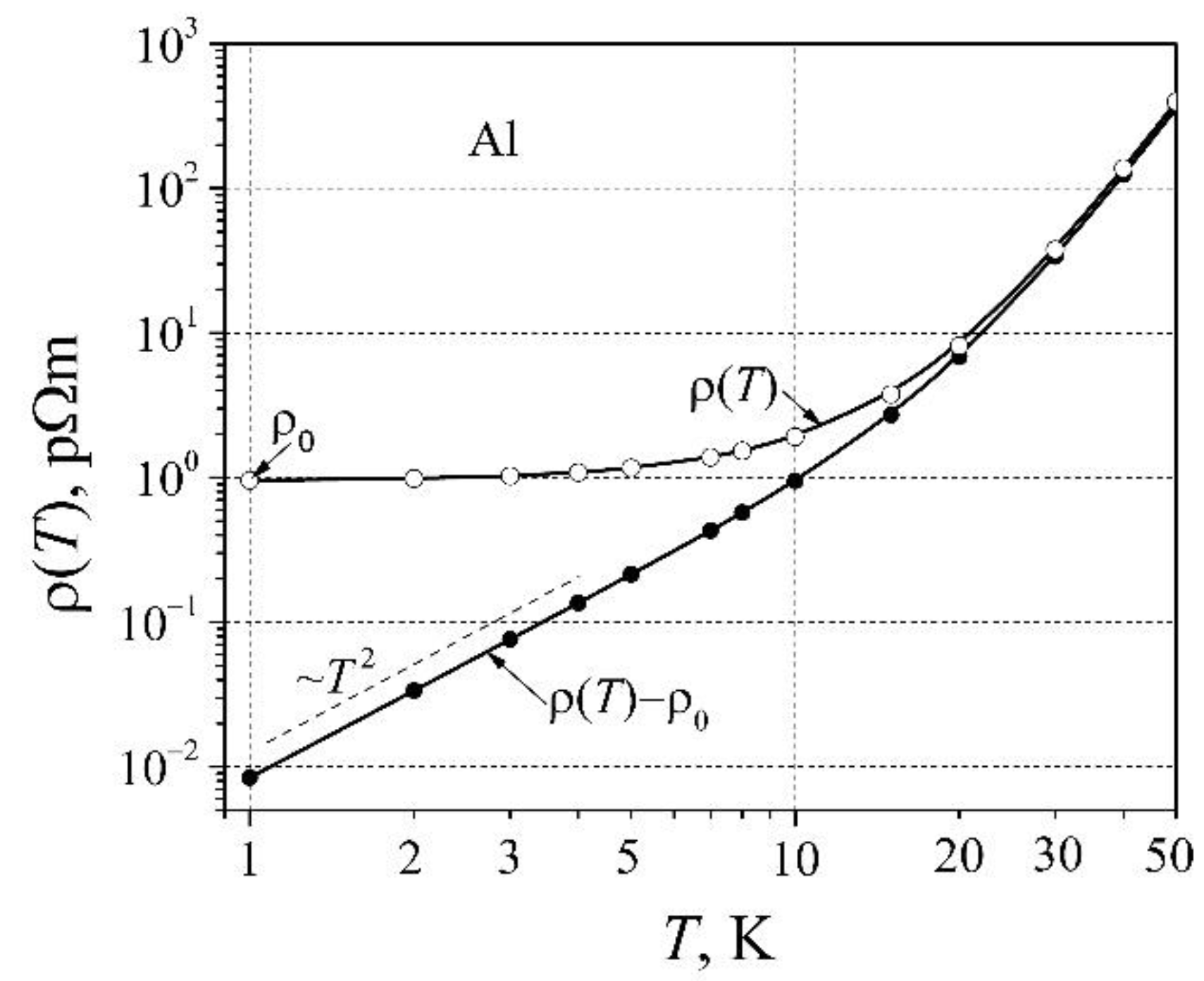
dependence on temperature. In
Figure 4 there are presented the resistivity
and
dependences on low temperatures for aluminum.
As can be seen, the resistivity (
without residual resistivity
has a well-expressed dependence on temperature term
AT 2, where proportionality coefficient
A≈6.5 fΩm K
-2. Accounting that the resistivity of metals is described as
, where
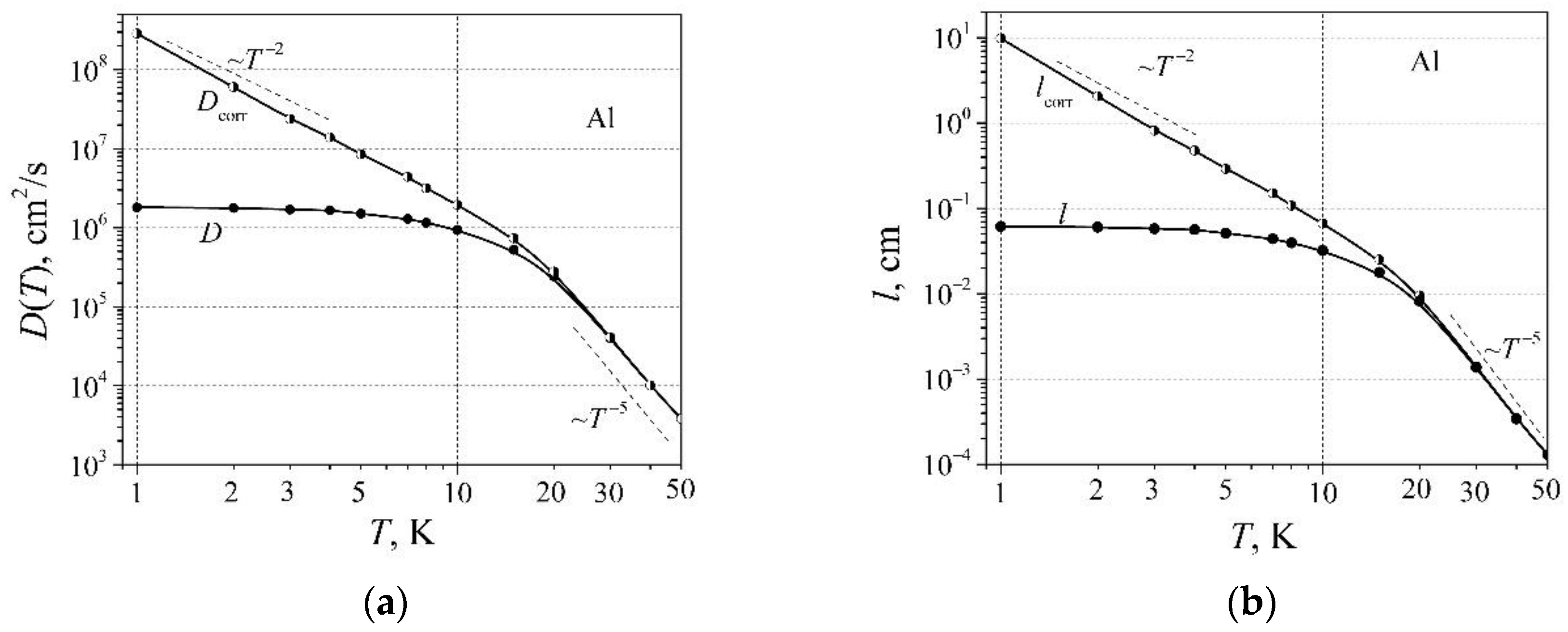
D is the diffusion coefficient of free RM charge carriers. The diffusion coefficient of free RM charge carrier dependence on temperature is shown in
Figure 5a. As can be seen, the diffusion coefficient at very low temperatures changes as
T -2, and then crosses to the proportionality
T -5 as the temperature is over 20 K.
The mean free path of charge carriers has been estimated as
, and
, which are presented in
Figure 5b. As can be seen, the mean free path
at very low temperatures changes as
T -2, and exceeds several centimeters. It seems that precise resistivity measurements should be done with a very large sample that the transverse dimensions which should greatly exceed the mean path of the charge carriers in order to vanish the charge carrier scattering from the sample boundaries.
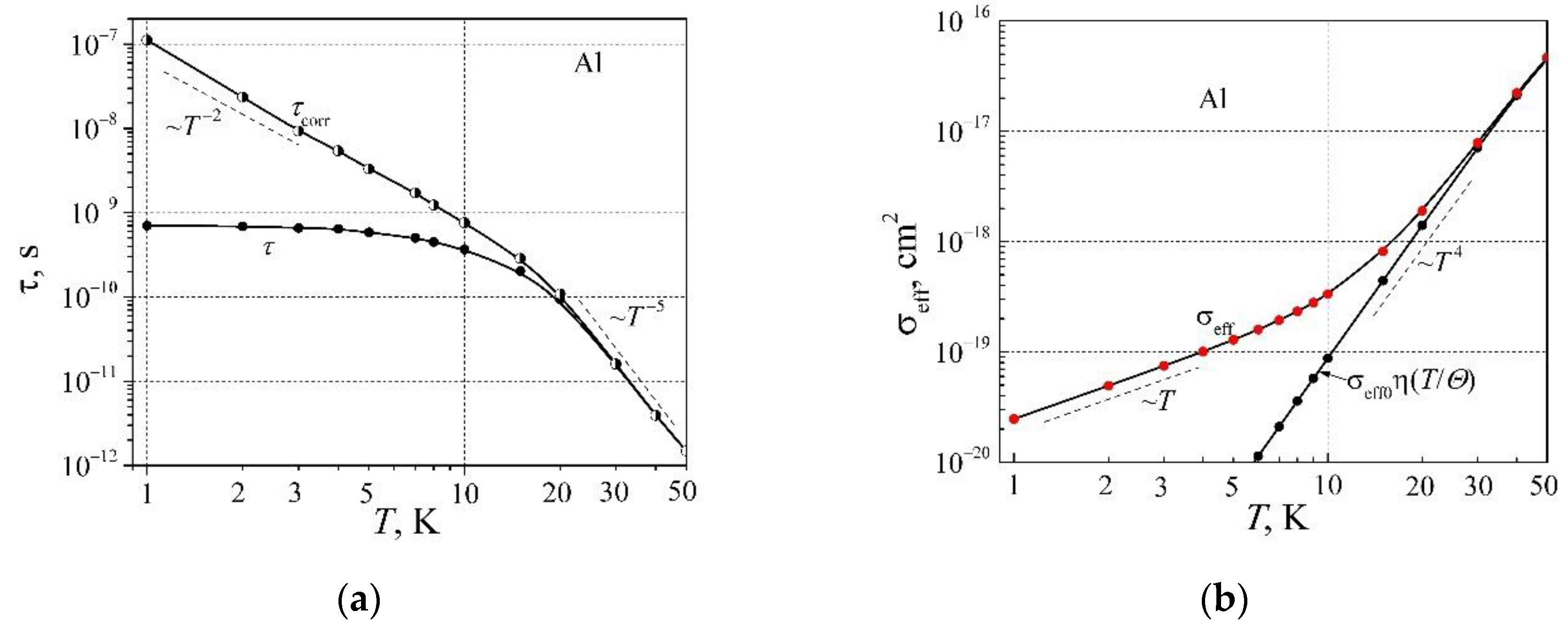
The relaxation time of charge carriers has been evaluated as
and
, which are presented in
Figure 6a. It is believed, that the relaxation time
changes with temperature as
T -2 at low temperatures. Now let's estimate the effective scattering cross-section of free RM electrons by electronic defects:
where
Neff is the electronic defect density. The effective scattering cross-section dependence on temperature is presented in
Figure 6b. As can be seen, the effective scattering cross-section at very low temperatures is proportional to temperature
T, and at higher temperatures coincides with
, and is proportional to
T 4. It seems that the resistivity term
AT 2 at low temperatures, the solution key is related to the linear effective scattering cross-section dependence on temperature.
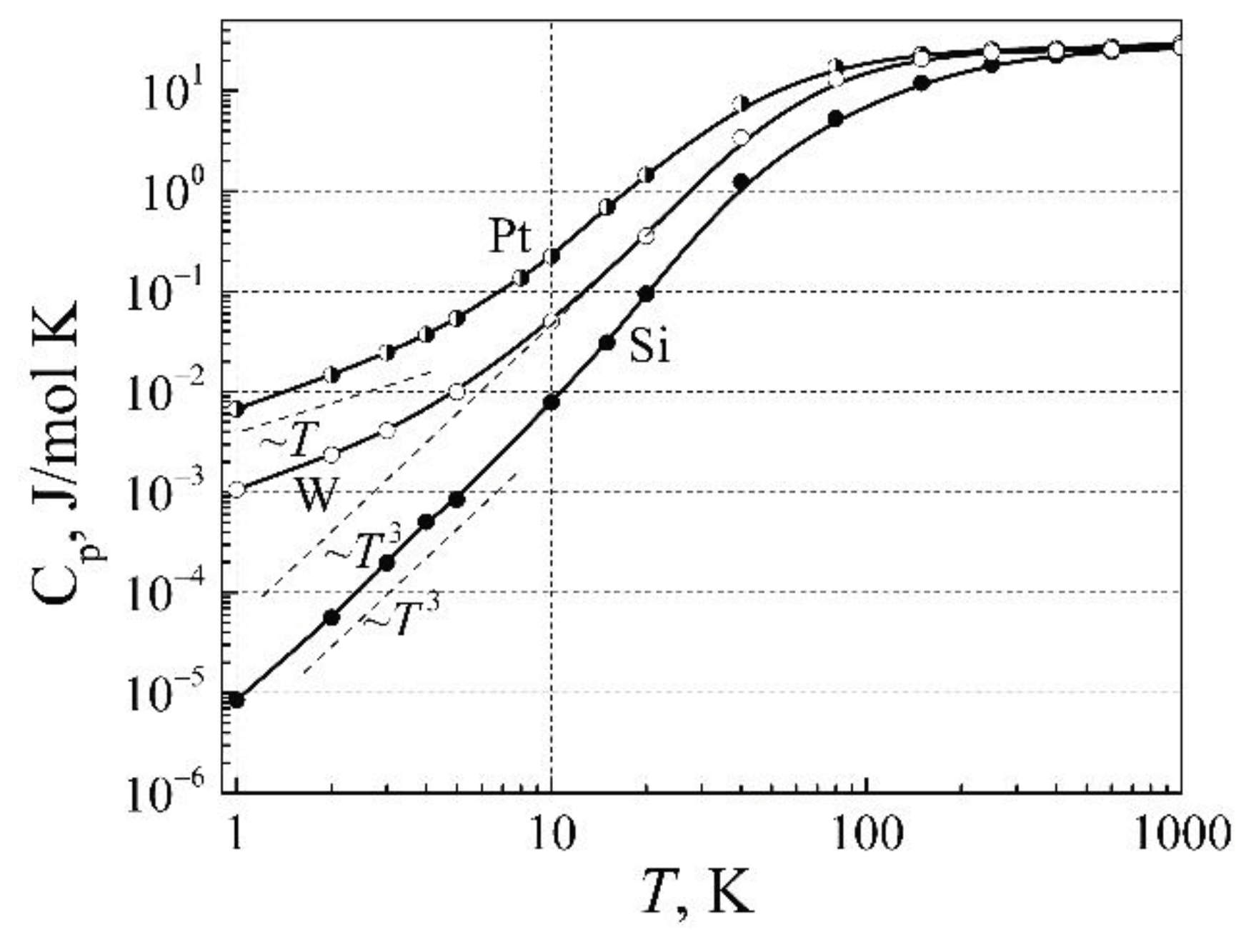
In
Figure 7, there are presented the heat capacity dependence on temperature for conductors (Pt and W), and pure Si, which at low temperatures is an insulator. The heat capacity of the insulator is described by the Debye′s model, for which the heat capacity at low temperatures increases as
, or the thermal energy (quantity of heat) increases as
. In the case of metals, the heat capacity at low temperatures changes slower, approximately linearly, and thermal energy changes as
T 2. As can be seen, to increase the metal of the one mole mass from 1 K to 5 K, it is needed a larger amount of heat, then it is needed for the insulator. This difference is related to the free electrons random moving, and due to their scattering by electronic defects. In equilibrium, the thermal energies between free electrons and lattice atoms are changing by the interaction of free electrons with the electronic defects.
Earlier for estimation of the phonon mediation factor
(Eq. 14) has been accounted only for the lattice thermal energy based on the Debye model. Additional thermal energy for conductors is caused by free randomly moving charge carriers, which are scattered by electronic defects. It gives a correction to phonon mediation factor
:
where the correction factor
is equal to the ratio between the ratio of the thermal (phonon) energy increase Δ
Eph1 of the metal atom caused by free RM electron interaction with electronic defects and the average free RM electron energy
Ee1=1.64
kT at low temperatures. The variation of the thermal energy of the metal atom due to interaction with free randomly moving electrons can be estimated as
where
NA is the Avogadro constant. The Eq. (20) can be rewritten as
where
γ is the electronic heat capacity parameter. Then the correction factor can be described in the following form:
where
R=
kNA=8.31 J/ (mol K) is the universal gas constant.
Therefore, the corrected phonon mediation factor can be presented as
and the resistivity can be described as
and
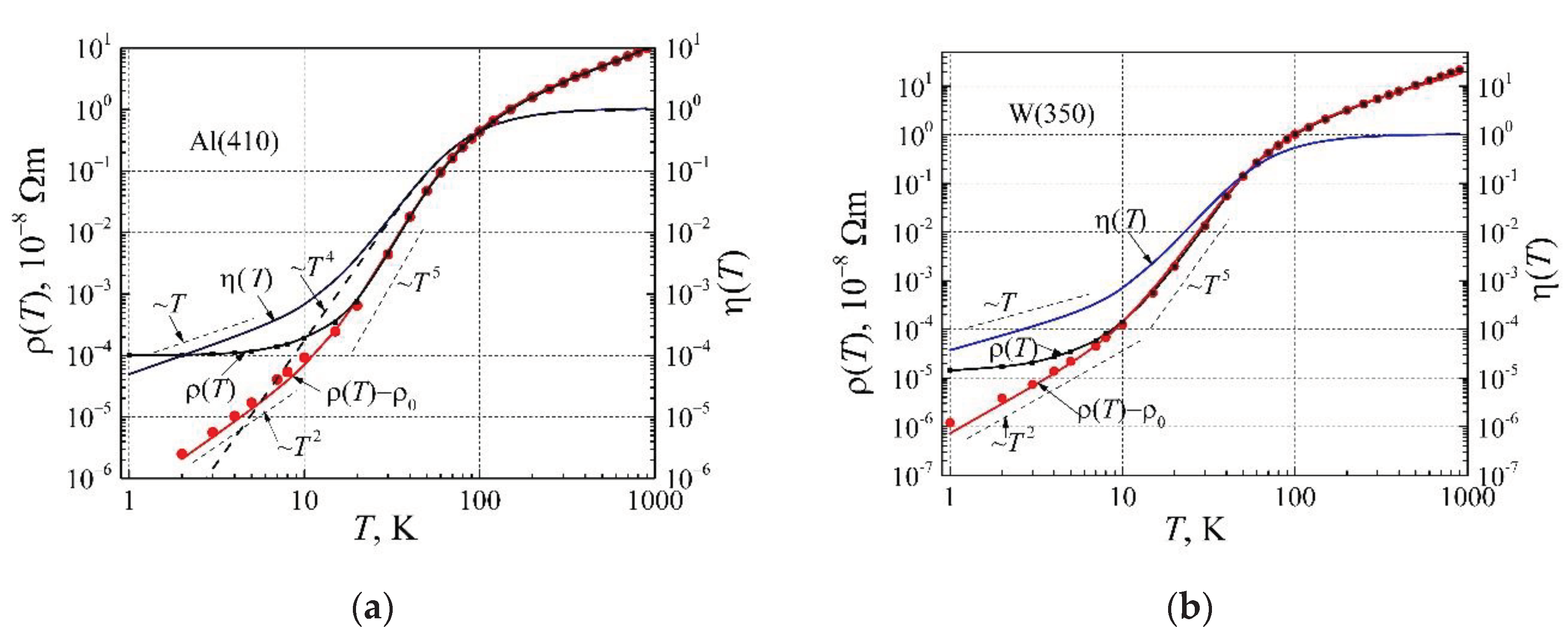
Now let us describe the elemental metals from different columns of the periodic table Al, W, Mo, Zn, Li, and Cu resistivity dependences on a very wide temperature range accounting the obtained correction factor. In
Figure 8a, there are presented resistivity and corrected phonon mediation factor dependences on temperature for aluminum, and in
Figure 8b for wolfram. In
Figure 8, the small black dots are the experimental dots of the resistivity [
54], and the solid by the black line calculated by Eq. (24); the red dots are experimental data of (
, the red solid line is calculated by Eq. (25), the corrected phonon mediation factor
is calculated by Eq. (23), the dash line proportional to
is estimated by Eq. (14) the phonon mediation factor without correction. From the comparison of the resistivity (
curves in
Figure 2 and
Figure 8a, it is seen that Eq. (24) very well describes the experimental results below 25 K temperature. As can be seen from
Figure 8a, at low temperatures the resistivity (
, where
A≈6 fΩm/K
2.
The similar results to aluminum shown in
Figure 8a, in
Figure 8b there are presented the investigation results for wolfram. Though the wolfram has a two times larger number of valence electrons than aluminum, the DOS g(
EF) of wolfram is about 1.3 times smaller than that of aluminum. The residual resistivity of a given sample of wolfram is about ten times smaller than that of aluminum. The difference between resistances
and (
at low temperatures for aluminum begins at
T<20 K, while for wolfram at T<8 K, and the coefficient
A≈8 fΩm/K
2. The phonon mediation factor
η(
T) dependences on temperature are almost the same for both metals.
In
Figure 9a, there are presented resistivity and corrected phonon mediation factor dependences on temperature for molybdenum, and in
Figure 9b for zinc. The Debye′s temperature obtained from resistivity dependence on temperature for Mo and Al is the same 410 K, and corrected phonon mediation factors are close one to other, while the residual resistivity of a given sample of molybdenum is about 10 times larger than that of aluminum. The resistivity (
proportionality to
T 2 for molybdenum is obtained at
T<10 K. The characteristics of the two-valent zinc (
Figure 9b) differ from those of molybdenum (
Figure 9a). It is related to the Debye′s temperature of zinc 177 K, which is over two times smaller than that of Mo 410 K shifts the resistivity characteristic to the lower temperatures. Besides, the DOS g(
EF) of zinc is about two times smaller than that of molybdenum. As a consequence, the phonon mediation factor linear part begins at a lower temperature, which causes the resistivity (
proportionality to
T 2 would be observed only at
T<4 K.
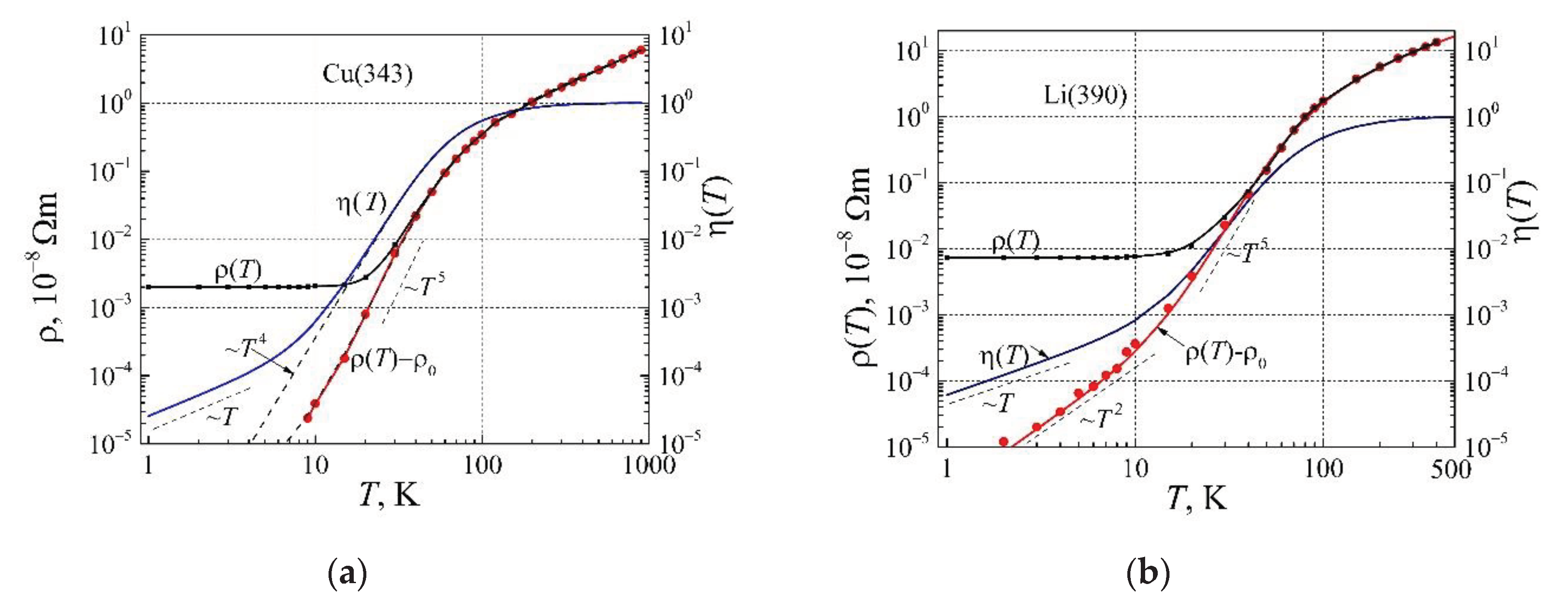
In
Figure 10, there are presented the resistivity and the corrected phonon mediation factor dependences on temperature for copper and lithium. Considering that the presented sample of the copper resistivity data has sufficiently high residual resistivity, there was not capable to observe the
T 2 dependence on temperature (
Figure 10a). As it is seen from this Figure, the resistivity (
changes at low temperatures approximately as
T4, the similar behavior has been observed for other noble metals by [
37,
40]. On the other hand, the linear part of the corrected phonon mediation factor begins only below 4 K, from this follows that the resistivity law
AT 2 can be obtained at resistivity values smaller than 10 fΩm. Maybe, it explains that the resistivity law
AT 2 for noble metals is difficult to obtain. In
Figure 10b, there are presented the resistivity and corrected phonon mediation factor dependences for lithium. As can be seen, the linear part of the corrected phonon mediation factor begins below 7 K, the square resistivity dependence on temperature is observed at a temperature below 7 K, and the proportionality coefficient
A is approximately equal to 20 fΩm/K
2.
As can be seen, the resistivity ( proportionality to T 2 at low temperatures are observed for alkali, noble, simple polyvalent or transition group metals, and temperatures at which it takes place depend on both the Debye temperature, and on the DOS at the Fermi surface of metals.
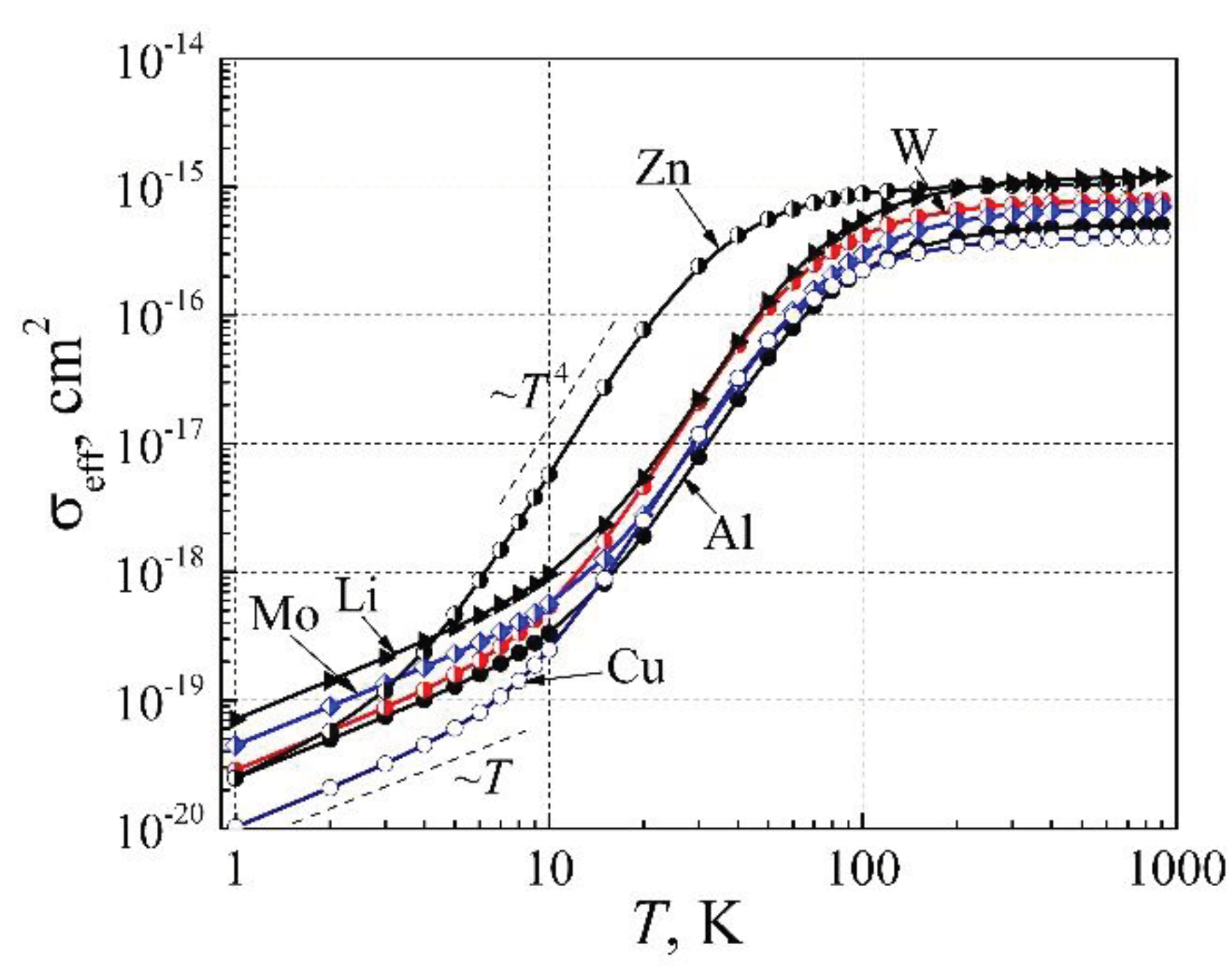
In
Figure 11, there are presented the effective electron scattering cross-section dependences on temperature for the analyzed metals Al, Cu, Li, Mo, W, and Zn. As can be seen, the effective free electron scattering cross-section dependences on temperature have three characteristic parts: (i) the linear ~
T at
T < 10 K; (ii) proportional to
T 4 in the transition temperature range below the Debye′s temperature; and (iii) the constant at
T > 200 K. The constant part of the effective free electron scattering cross-section shows that earlier explanations that free electron scattering cross-section at room and higher temperature increases proportional to temperature
T due to lattice atom vibrations [
7,
8,
9,
10,
11,
19,
20,
21,
22] are unfounded. The linear
η(
T) increase at very low temperatures is caused by the linear heat capacity increase with temperature, which causes the quadratic resistivity of elemental metals dependence on temperature. Therefore, it shows that the earlier explanation of metal resistivity by electron-electron scattering at very low temperatures is not acceptable.
From free RM electron effective scattering cross-section dependence on temperature follows such resistivity of elemental metals dependence on temperature characteristic parts: (i) the quadratic term AT 2at T<10 K (by eliminating the residual resistivity), (ii) the term ~ T 5 in the transition temperature range below the Debye′s temperature, and (iii) the linear term ~T at T>200 K. The whole resistivity (including the residual resistivity) temperature dependence is described by Eq. (24) accounting the corrected phonon mediation factor (Eq. (23)), i.e. the elemental metal resistivity in the overall temperature range can be explained by free randomly electron scattering by electronic defects accounting the phonon mediation factor.