1. Introduction
Fluid balance, the net difference between intake and output, traditionally guides undifferentiated fluid management decisions in critically ill patients; however, fluid management in mechanically ventilated patients is complex. In general, positive fluid balance is associated with weaning challenges and adverse outcomes such as extubation failure and higher mortality rates [
1,
2]. According to a recent study, a one-liter surplus on day 3 in the ICU can elevate the mortality risk by 19%[
3]. Conversely, negative fluid balance is associated with improved survival[
1,
4,
5].
Nevertheless, even in the presence of fluid accumulation, some patients do not exhibit detrimental effects, which may be attributable to a condition known as fluid tolerance. This recently defined concept describes the degree to which a patient can tolerate fluid administration without causing organ dysfunction[
6]. Thus, a positive fluid balance alone may not warrant reversal; its clinical impact is significant when associated with fluid overload, a condition that precedes organ dysfunction[
5,
7] and correlates with elevated morbidity and mortality[
8,
9,
10,
11]. Regrettably, most characterizations of fluid overload only define it arithmetically as a gain of 5-10% from admission weight without functional considerations[
5,
7]. Nevertheless, the pathophysiology of fluid overload is intricate and includes a net volume increase, redistribution of fluid from the peripheral to central veins, diminished fluid elimination due to renal impairment, and endothelial dysfunction[
12], all of which may result in organ failure.
This complexity underscores the need for personalized diagnostic approaches that not only quantify fluid balance but also assess the physiological impact on individual cardiovascular function. The classic paradigm of fluid management, primarily guided by aggregate metrics and standardized protocols, often overlooks nuanced physiological variances among individuals. In this context, passive leg raising (PLR) appears to be a helpful noninvasive strategy via the assessment of fluid responsiveness to bridge the gap between the theoretical understanding of the consequences of fluid overload and practical, personalized bedside decision-making regarding volume management. PLR is a simple and reversible maneuver that mimics rapid fluid loading by shifting venous blood from the legs towards the intrathoracic compartment, increasing ventricular preloads, and thereby, stroke volume and cardiac output[
13]. A positive PLR maneuver is a strong indicator of fluid responsiveness in mechanically ventilated patients[
13,
14], revealing a ventricular systolic function operating along the plateau phase of the Frank-Starling curve[
15].
A negative PLR maneuver, indicating fluid unresponsiveness, prompts the safe removal of excess fluid[
16]; similarly, when performed before a spontaneous breathing trial (SBT) in nonsurgical patients[
17], it is predictive of weaning failure of cardiac origin[
18,
19]. During the weaning phase, withdrawal of positive pressure increases venous return. Still, a state of fluid unresponsiveness can impede an appropriate increase in cardiac output[
17,
20,
21], essential for matching the increased V ̇O2 that occurs during the transition to spontaneous ventilation[
20]. However, the predictive value of fluid responsiveness, assessed using PLR before weaning, has not yet been explored in surgical patients[
17].
In the dynamic and critical setting of intensive care following liver transplant surgery, fluid balance management remains a cornerstone of patient care. These patients face critical fluid management challenges owing to their previously altered fluid homeostasis physiology[
22] and surgery-induced fluid shifts, which increase the risk of post-transplant complications[
22,
23,
24,
25]. These facts make liver transplant patients a distinctively informative group for studying the predictive value of PLR, which could potentially guide more precise fluid therapy and improve the weaning process through fluid responsiveness determination.
This study aimed to explore whether fluid responsiveness, assessed using a PLR maneuver before a weaning trial, was associated with favorable ventilatory outcomes in a cohort of mechanically ventilated patients admitted to the ICU after liver transplantation, all of whom presented high postoperative fluid balance. We postulated that a personalized approach to fluid management considering cardiovascular responses using PLR could expedite the weaning process in these patients. In addition, such an approach could be helpful in critical care practice for postoperative patients undergoing great abdominal surgery.
2. Materials and Methods
We conducted a prospective observational study on postoperative liver transplant patients at an academic tertiary care center ICU in 2023. This was an ancillary study of the FLOW protocol (NCT04496583 registration in clinicaltrials.gov), and patients were recruited after obtaining approval from the Institutional Review Board. The study was exempt from informed consent owing to its observational nature, as approved by our Ethics Committee (ID 201015001-2021). The FLOW study was funded by FONDECYT grant Nº 1200248-2020 from the Agencia Nacional de Investigación y Desarrollo (ANID), Chile.
Study Population
We recruited patients aged >18 years who were admitted to the ICU after liver transplantation for postoperative management. The exclusion criteria were acute postoperative, hemorrhagic, or vascular complications such as bleeding or hepatic artery or portal vein thrombosis. Patients were included when the research team was available (business days from 8:00 am to 12:00 pm). All patients had a central venous catheter and an arterial line at ICU entry.
Demographic and clinical profiles, along with standard ICU monitoring and fluid balance metrics, were systematically documented. In addition to fluid balance, we determined the estimated plasma volume (ePV) using the Strauss-derived[
26] and precision-adjusted Duarte formula[
27] to calculate the plasma volume status (PVS) using the Kaplan-Hakim formula[
28]. PVS indicates the actual versus ideal plasma volume disparity calculated and was determined after ICU admission and before SBT for comparison with fluid balance and responsiveness status. The PVS offers a percentage-based evaluation of plasma volume that correlates well with plasma volume estimation when measured using a radio labeled albumin assay[
26,
29]. We used the suggested cut-off of 6.3%for this parameter[
30].
Bioreactance Monitoring
We used a non-invasive bioreactance monitor (Cheetah-Starling SV©, Baxter. USA) because it provided continuous real-time data on cardiovascular function, inexistent risk of complications, and increased patient comfort, which is especially important in the postoperative setting. Bioreactance analyses the relative phase shift of an oscillating current passing through the thoracic cavity[
31]. The device automatically recorded all data every 8 s to an exportable spreadsheet file.
Study Procedures
Hemodynamic Monitoring
After recruitment, a bioreactance-monitoring device was placed on each patient. We recorded the cardiac index (CI), stroke volume index (SVI), stroke volume variation (SVV), and thoracic fluid content (TFC) as the main hemodynamic variables. We assessed the absolute and relative positional variations in hemodynamic measures across baseline and passive leg raise at T1 and T2, as well as before and after SBT, in addition to the monitor’s automated data output. The default 10% fluid responsiveness threshold of the device was utilized. A dual-investigator review of patient charts ensured a detailed capture of the impact of the PLR maneuver on hemodynamic parameters.
Spontaneous Breathing Trial
After the patient’s condition stabilized following liver transplant surgery, sedation was withdrawn, and the process of gradually reducing mechanical ventilation support was initiated to transition from controlled to spontaneous ventilation. A protocolized weaning program was implemented to prepare patients for extubation, which involved assessing hemodynamic stability, peripheral perfusion, and neurological function, including consciousness and cough reflex. Once patients could tolerate a reduced applied airway pressure support of 10 cmH2O, SBT was conducted for 30 min using a standardized protocol that included inspiratory pressure augmentation of 7 cm H2O and zero positive end-expiratory pressure. No SBTs were performed using the T-piece.
Upon successful completion of SBT, the patient was extubated if deemed eligible by the attending physician. The patient was monitored for 48 h to ensure that reintubation was not required, and the maintenance of spontaneous ventilation by day 7 was considered consolidated extubation. A standardized post-extubation respiratory support protocol, including an oxygen mask, a high-flow nasal cannula (HFNC), and non-invasive ventilation, was available if needed. The respiratory therapy team managed the entire weaning process, which provided airway secretion clearance, bronchodilators, or other necessary interventions under physician supervision. Additionally, a post-extubation swallowing screening assessment was performed on all patients.
Passive Leg Raising
Stable data for the baseline SVI were obtained after 3 min in a semi-recumbent position at 45°. The first PLR maneuver (T1) was initiated by placing the patient in the supine position with the motorized ICU bed system and simultaneously raising the legs to 45° by two operators. The legs were secured using a rigid-cushioned frame. The results were automatically displayed on the screen 3 min after starting the test. After 6 minutes, the patient was returned to the previous position. A second PLR maneuver was performed before SBT, as described for the first maneuver (T2). Before each PLR maneuver, the patients were informed of the test to avoid stressful triggers that could hinder the results. In addition, the same hemodynamic parameters were recorded before and after SBT (T3), at least 10-min lapse after T2 (
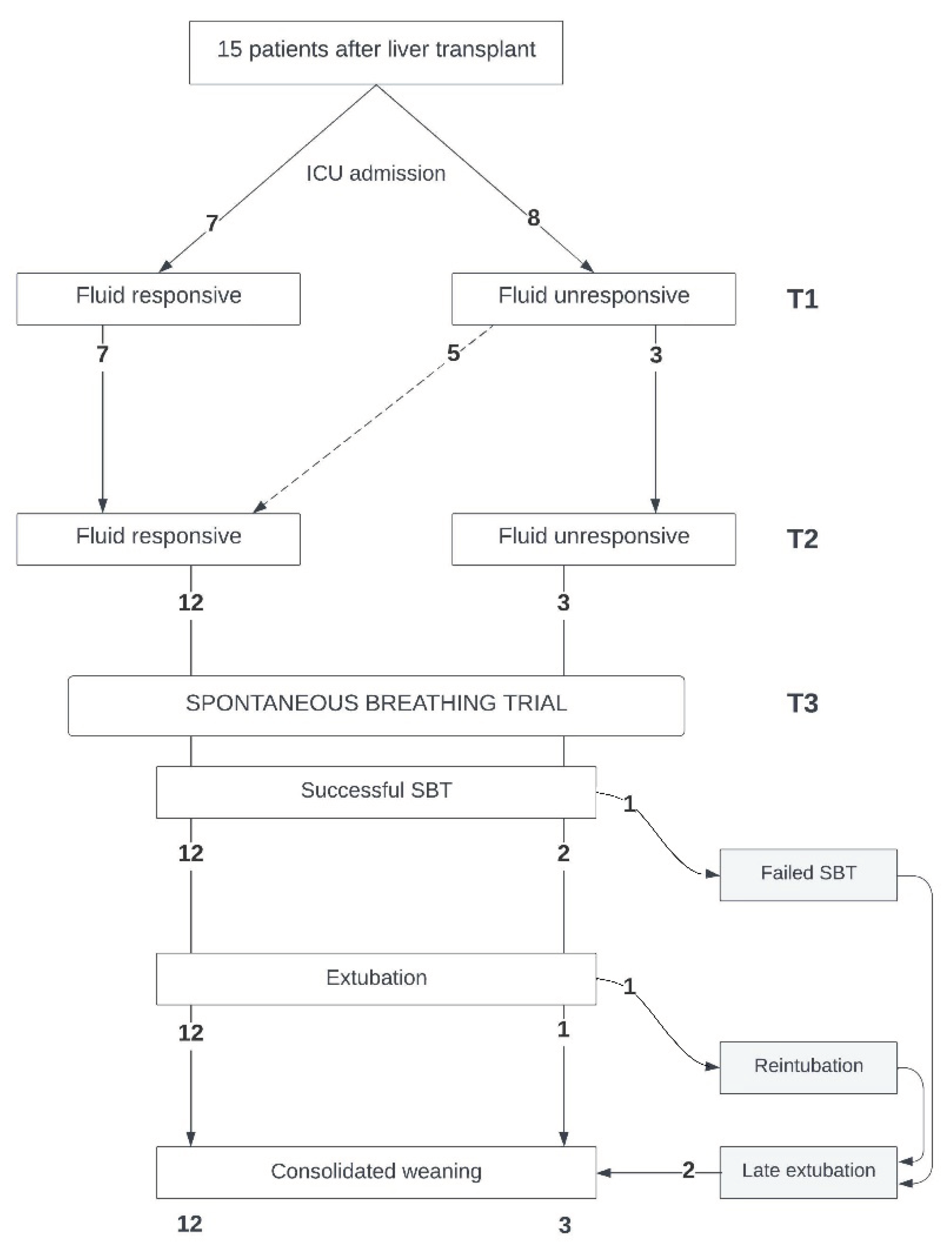
Figure 1). At all times, attending physicians were unaware of the fluid responsiveness state.
Statistical Methods
The study participants’ baseline demographic and general hemodynamic parameters are presented as median and 25-75 interquartile ranges (IQR 25,75) and proportions. Comparisons between fluid-responsive and fluid-unresponsive patients were performed using the Wilcoxon rank-sum test for continuous variables, owing to the nonparametric distribution of data. Multiple linear regression analyses were performed to examine the influence of MELD score, fluid responsiveness status, fluid balance, and CI on time to SBT and time to extubation. The significance level was set at p < 0.05. Data analysis and graphical representation were performed using the DATAtab Online Statistics Calculator (DATAtab e.U. Graz, Austria.
https://datatab.net).
3. Results
Fifteen patients were recruited (general characteristics:
Table 1; patient flow:
Figure 2). At ICU admission, all patients presented with weight increase (9.3 [8.4,10.5] kg since hospital admission, 15% increase), high fluid balance (4480 [3698,5723] mL), and high PVS (13 [8,17] %).
At ICU admission, seven patients were fluid-responsive, whereas eight were fluid-unresponsive. At T2, of the eight initially fluid-unresponsive patients, five became fluid-responsive (
Figure 2). General hemodynamic and respiratory parameters were similar in fluid-responsive and fluid-unresponsive patients at T1 and T2, and maintained similar values during SBT (T3) (
Table 2A). Heart rate was comparable between fluid-responsive and fluid-unresponsive patients at T1 and T2, and continued alike during SBT (
Table 2B). Fluid-responsive patients started SBT earlier 14 [12,27] hours versus 35.5 [20;112] hours; p = 0.06) and achieved successful extubation sooner (20 [15;40] hours versus 45 [42;121] hours; p = 0.045) than their fluid-unresponsive counterparts. All fluid-responsive patients at T2 passed the SBT successfully and were extubated without complications. Conversely, two of the three fluid-unresponsive patients at T1 who remained in that state until SBT had weaning problems: one failed SBT, and the other had to be reintubated (
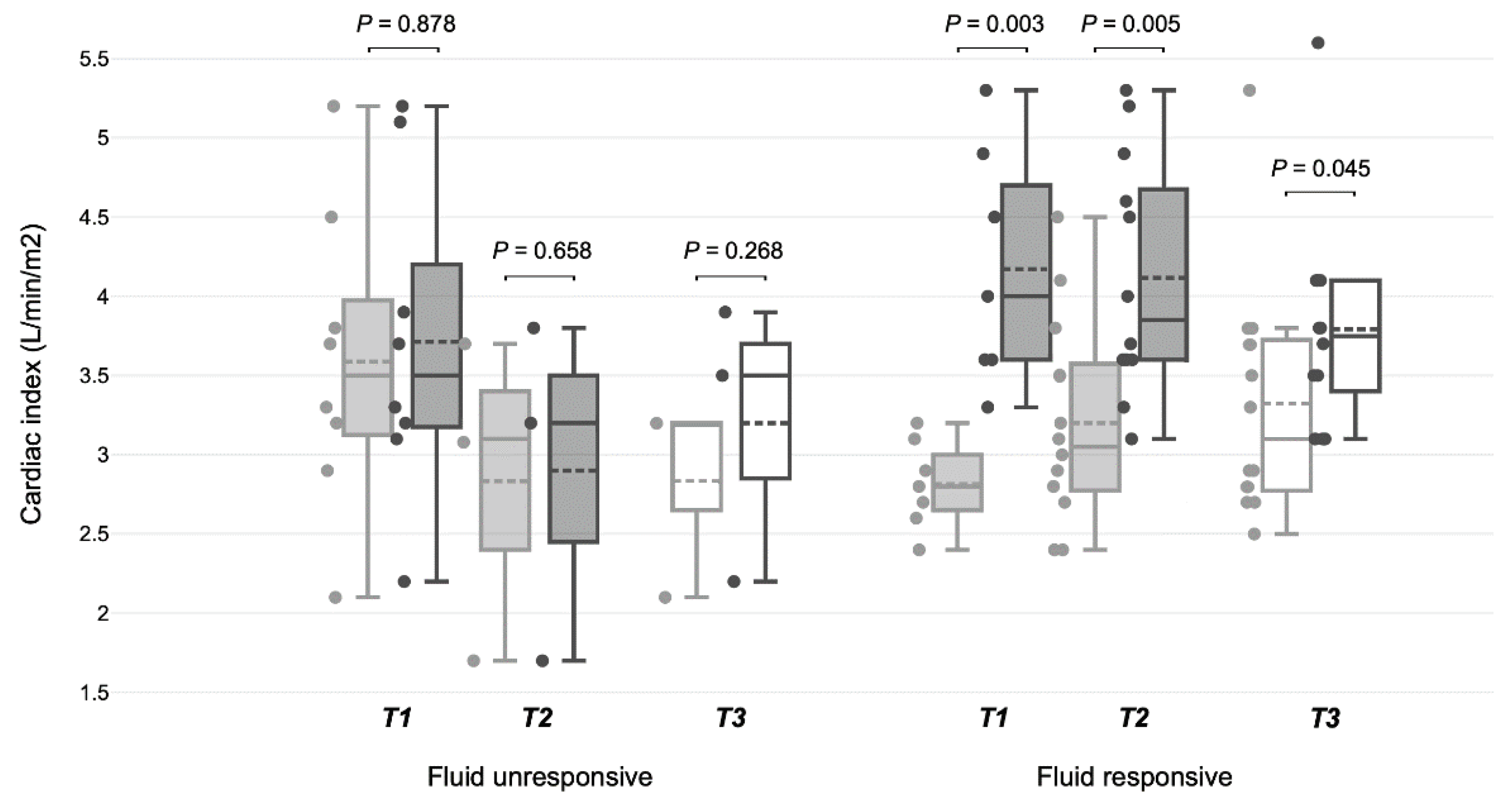
Figure 2). The SVI increased significantly in fluid-responsive patients at T1 and T2, unlike in fluid-unresponsive patients (
Table 2B,
Figure 3). During SBT, significant increases in CI and SVI were observed in patients previously identified as fluid-responsive at T1 and T2. There were no statistically significant changes in CVP, SvO2, pCO2, dCO2, or TFC in any group at T1, T2, or T3.
Fluid balance and PVS were similar between the fluid-responsive and fluid-unresponsive patients at T1 and T2 (
Table 3A-B). Fluid balance and PVS were not associated with fluid responsiveness at T1 or T2 (
Table 3A-B).
In the case of PLR maneuvers, Set 1 corresponds to baseline parameters and Set 2 for parameters at the end of the test. In the case of SBT, Set 1 corresponds to the hemodynamic parameters recorded for comparative analyses included CI, SVI, SVV, CVP, and TFC (see main text for details) before starting SBT and Set 2 to the recording of the same parameters at the end of the trial.
4. Discussion
The findings of our observational study, albeit small-scale, highlight the profound impact that personalized fluid management may have on post-liver transplant recovery. Our data suggest that identifying the condition of fluid responsiveness after surgery is potentially beneficial for achieving earlier weaning and successful extubation, independent of fluid balance. Notably, we observed that a high fluid balance was not equivalent to a fluid unresponsiveness state. Fluid responsiveness, assessed using PLR maneuvers, delineates a subgroup of patients who significantly benefit from tailored fluid management strategies, manifesting in more successful weaning and extubation outcomes. This correlation not only proposes fluid responsiveness as a critical determinant of patient-specific care but also underscores the limitations of conventional, one-size-fits-all fluid management and removal protocols.
All patients who emerged fluid-responsive after liver transplant surgery maintained this state. This differs from other ICU contexts, such as sepsis, where fluid responsiveness is inconstant [
32,
33]. In our patients, the tendency towards early fluid responsiveness post-transplantation may suggest a subset of individuals better adapted from a cardiovascular perspective to significant surgical stress, hence displaying readiness for weaning. These patients can adequately accommodate the intrathoracic positive pressure loss inherent to an SBT, the concurrent increase in venous return, and, owing to their fluid-responsiveness state, respond to increasing their cardiac index, allowing for an uncomplicated extubation. Conversely, fluid-responsiveness assessment by performing a PLR maneuver may serve as a precautionary measure for fluid-unresponsive patients post-surgery, signaling a need for fluid removal that can be performed without risking hemodynamic stability [
14,
34].
It is crucial to recognize that the measured post-surgery fluid balance, often an imprecise estimate [
35], could reflect less critical conditions such as fluid redistribution or capillary leak syndrome, which do not necessarily induce cardiac or other organs’ dysfunction. Therefore, our findings support the consideration of fluid responsiveness over immediate fluid balance correction during the weaning process of postoperative liver transplant patients. In any case, the potential benefit of transitioning patients from fluid unresponsiveness to responsiveness before weaning warrants further investigation.
Our insights provide a re-evaluation of clinical practice, advocating for integrating simple, personalized management strategies into standard care protocols. While our findings may contribute to the evidence on PLR utility in post-surgical settings, they are yet to be robustly validated. Our results propose an expanded application of PLR for predicting cardiovascular and respiratory responses to SBT among pre-weaning surgical patients, an area previously unreported [
17].
Our study was limited by its small size, single-center setting, potential assessment bias owing to device technology, unblinded fluid management from the attending physicians, and its observational nature. Therefore, these insights should be interpreted as provisional and call for further research to ascertain the predictive value of PLR in broader surgical critical care scenarios.
5. Conclusions
Our preliminary observations suggest that, irrespective of postoperative fluid balance, patients demonstrating fluid responsiveness post-liver transplantation may start weaning earlier and achieve successful extubation. This underscores the potential prognostic value of fluid responsiveness as an indicator of sufficient cardiovascular adaptation for weaning in surgical patients and highlights the profound impact of personalized fluid assessment and management on critical surgical care patient recovery.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Table S1.
Author Contributions
Conceptualization, R.C., P.B. and F.M.; methodology, R.C.; formal analysis, R.C., P.B. and F.M.; investigation, R.C., P.B., F.M. and J.B.; resources, R.C. data curation, R.C., P.B., C.M.; writing—original draft preparation, R.C..; writing—review and editing, R.C., E.K. and G.H.; visualization, R.C..; supervision, G.H. and J.B.; project administration, R.C. and P.B.X.; funding acquisition, R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT grant number 1200248, from the Agencia Nacional de Investigación y Desarrollo (ANID), Chile.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Pontificia Universidad Católica de Chile (ID 201015001-2021). This was an ancillary study of the FLOW protocol (NCT04496583 registration in clinicaltrials.gov). The study was exempt from informed consent.
Informed Consent Statement
Patient consent was waived due to its observational nature as approved by the Ethics Committee of Pontificia Universidad Católica de Chile.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Silversides, J.A.; Fitzgerald, E.; Manickavasagam, U.; Lapinsky, S.E.; Nisenbaum, R.; Hemmings, N.; Nutt, C.; Trinder, T.J.; Pogson, D.; Fan, E.; et al. Deresuscitation of Patients With Iatrogenic Fluid Overload Is Associated With Reduced Mortality in Critical Illness*. Crit Care Med 2018, 46, 1600–1607. [Google Scholar] [CrossRef]
- Wang, T.-J.; Pai, K.-C.; Huang, C.-T.; Wong, L.-T.; Wang, M.-S.; Lai, C.-M.; Chen, C.-H.; Wu, C.-L.; Chao, W.-C. A Positive Fluid Balance in the First Week Was Associated With Increased Long-Term Mortality in Critically Ill Patients: A Retrospective Cohort Study. Front. Med. 2022, 9, 727103. [Google Scholar] [CrossRef]
- Messmer, A.S.; Zingg, C.; Mûller, M.; Gerber, J.; Schefold, J.C.; Pfortmueller, C.A. Fluid Overload and Mortality in Adult Critical Care Patients—A Systematic Review and Meta-Analysis of Observational Studies*. Critical Care Medicine 2020, 48, 1862–1870. [Google Scholar] [CrossRef]
- Cordemans, C.; laet, I.D.; Regenmortel, N.V.; Schoonheydt, K.; Dits, H.; Martin, G.; Huber, W.; Malbrain, M.L. Aiming for a Negative Fluid Balance in Patients with Acute Lung Injury and Increased Intra-Abdominal Pressure: A Pilot Study Looking at the Effects of PAL-Treatment. Ann Intensive Care 2012, 2, S15. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.N.G.; Marik, P.E.; Witters, I.; Cordemans, C.; Kirkpatrick, A.W.; Roberts, D.J.; Regenmortel, N.V. Fluid Overload, de-Resuscitation, and Outcomes in Critically Ill or Injured Patients: A Systematic Review with Suggestions for Clinical Practice. Anaesthesiol Intensive Ther 2014, 46, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Kattan, E.; Castro, R.; Miralles-Aguiar, F.; Hernández, G.; Rola, P. The Emerging Concept of Fluid Tolerance: A Position Paper. Journal of Critical Care 2022, 71, 154070–154070. [Google Scholar] [CrossRef] [PubMed]
- Pfortmueller, C.A.; Dabrowski, W.; Malbrain, M.L.N.G. Fluid De-Resuscitation in Critical Illness – A Journey into Uncertain Territory. J. Crit. Care 2023, 76, 154249. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.N.; Ricci, Z.; Bagshaw, S.M.; Piccinni, P.; Gibney, N.; Ronco, C. Renal Replacement Therapy in Adult Critically Ill Patients: When to Begin and When to Stop. Contrib. Nephrol. 2010, 165, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Villa, G.; Katz, N.; Ronco, C. Extracorporeal Membrane Oxygenation and the Kidney. Cardiorenal Med. 2015, 6, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Granado, R.C.-D.; Mehta, R.L. Fluid Overload in the ICU: Evaluation and Management. BMC Nephrol. 2016, 17, 109. [Google Scholar] [CrossRef]
- Zeuthen, E.; Wichmann, S.; Schønemann-Lund, M.; Järvisalo, M.J.; Wahlin, R.R.; Sigurðsson, M.I.; Holen, E.; Bestle, M.H. Nordic Survey on Assessment and Treatment of Fluid Overload in Intensive Care. Frontiers in Medicine 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Koratala, A.; Ronco, C.; Kazory, A. Diagnosis of Fluid Overload: From Conventional to Contemporary Concepts. Cardiorenal Med. 2022, 12, 141–154. [Google Scholar] [CrossRef]
- Monnet, X.; Rienzo, M.; Osman, D.; Anguel, N.; Richard, C.; Pinsky, M.R.; Teboul, J. Passive Leg Raising Predicts Fluid Responsiveness in the Critically Ill*. Crit Care Med 2006, 34, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Marik, P.; Teboul, J.-L. Passive Leg Raising for Predicting Fluid Responsiveness: A Systematic Review and Meta-Analysis. Intensiv. Care Med. 2016, 42, 1935–1947. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.E.; Teboul, J.-L. Prediction of Fluid Responsiveness: An Update. Ann Intensive Care 2016, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Cipriani, F.; Camous, L.; Sentenac, P.; Dres, M.; Krastinova, E.; Anguel, N.; Richard, C.; Teboul, J.-L. The Passive Leg Raising Test to Guide Fluid Removal in Critically Ill Patients. Ann. Intensiv. Care 2016, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, F.; Teboul, J.; Anguel, N.; Beurton, A.; Bezaz, N.; Richard, C.; Monnet, X. Cardiac Dysfunction Induced by Weaning from Mechanical Ventilation: Incidence, Risk Factors, and Effects of Fluid Removal. Critical Care 2016, 20. [Google Scholar] [CrossRef]
- Dres, M.; Teboul, J.-L.; Anguel, N.; Guerin, L.; Richard, C.; Monnet, X. Passive Leg Raising Performed before a Spontaneous Breathing Trial Predicts Weaning-Induced Cardiac Dysfunction. Intens Care Med 2015, 41, 487–494. [Google Scholar] [CrossRef]
- Lemaire, F.; Teboul, J.-L.; Cinotti, L.; Giotto, G.; Abrouk, S.F.; Steg, G.; Macquin-Mavier, I.; Zapol, W.M. Acute Left Ventricular Dysfunction during Unsuccessful Weaning from Mechanical Ventilation. Anesthesiology 1988, 69, 171–179. [Google Scholar] [CrossRef]
- Pinsky, M.R. Breathing as Exercise: The Cardiovascular Response to Weaning from Mechanical Ventilation. Intens Care Med 2000, 26, 1164–1166. [Google Scholar] [CrossRef]
- Ferré, A.; Guillot, M.; Lichtenstein, D.; Mezière, G.; Richard, C.; Teboul, J.-L.; Monnet, X. Lung Ultrasound Allows the Diagnosis of Weaning-Induced Pulmonary Oedema. Intens Care Med 2019, 1–8. [Google Scholar] [CrossRef]
- Kashani, A.; Landaverde, C.; Medici, V.; Rossaro, L. Fluid Retention in Cirrhosis: Pathophysiology and Management. QJM: An International Journal of Medicine 2008, 101, 71–85. [Google Scholar] [CrossRef]
- Carrier, F.M.; Chassé, M.; Wang, H.T.; Aslanian, P.; Bilodeau, M.; Turgeon, A.F. Effects of Perioperative Fluid Management on Postoperative Outcomes in Liver Transplantation: A Systematic Review Protocol. Syst. Rev. 2018, 7, 180. [Google Scholar] [CrossRef]
- Avolio, A.W.; Gaspari, R.; Teofili, L.; Bianco, G.; Spinazzola, G.; Soave, P.M.; Paiano, G.; Francesconi, A.G.; Arcangeli, A.; Nicolotti, N.; et al. Postoperative Respiratory Failure in Liver Transplantation: Risk Factors and Effect on Prognosis. PLoS ONE 2019, 14, e0211678. [Google Scholar] [CrossRef]
- Suphathamwit, A.; Pongraweewan, O.; Lakkam, S.; Tovikkai, C. Predictive Score for Immediate Extubation after Liver Transplantation. Clin. Transplant. 2021, 35, e14212. [Google Scholar] [CrossRef]
- Kobayashi, M.; Huttin, O.; Rossignol, P.; Girerd, N. The Unit of Estimated Plasma Volume in Patients with Heart Failure Using the Strauss-Derived Duarte Formula Is Not Liter but DL/g. J. Card. Fail. 2019, 25, 140–140. [Google Scholar] [CrossRef] [PubMed]
- Duarte, K.; Monnez, J.M.; Albuisson, É.; Pitt, B.; Zannad, F.; Rossignol, P. Prognostic Value of Estimated Plasma Volume in Heart Failure. JACC: Hear. Fail. 2015, 3, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Hakim, R.M. Plasmapheresis; JT, D., PG, B., TS, I., Eds.; 3rd ed.; Lippincott, Williams and Wilkins: Philadelphia, 2001.
- Kim, K.H.; Cho, H.J.; Kim, S.C.; Lee, J. Prognostic Value of Estimated Plasma Volume Status in Patients With Sepsis. Journal of Korean Medical Science 2022, 37. [Google Scholar] [CrossRef]
- Ahlgrim, C.; Birkner, P.; Seiler, F.; Grundmann, S.; Bode, C.; Pottgiesser, T. Estimated Plasma Volume Status Is a Modest Predictor of True Plasma Volume Excess in Compensated Chronic Heart Failure Patients. Sci. Rep. 2021, 11, 24235. [Google Scholar] [CrossRef] [PubMed]
- Keren, H.; Burkhoff, D.; Squara, P. Evaluation of a Noninvasive Continuous Cardiac Output Monitoring System Based on Thoracic Bioreactance. Am J Physiol-heart C 2007, 293, H583–H589. [Google Scholar] [CrossRef] [PubMed]
- Douglas, I.S.; Alapat, P.M.; Corl, K.A.; Exline, M.C.; Forni, L.G.; Holder, A.L.; Kaufman, D.A.; Khan, A.; Levy, M.M.; Martin, G.S.; et al. Fluid Response Evaluation in Sepsis Hypotension and Shock A Randomized Clinical Trial. Chest 2020, 158, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Kattan, E.; Ospina-Tascón, G.A.; Teboul, J.-L.; Castro, R.; Cecconi, M.; Ferri, G.; Bakker, J.; Hernández, G.; Hernandez, G.; Ospina-Tascón, G.; et al. Systematic Assessment of Fluid Responsiveness during Early Septic Shock Resuscitation: Secondary Analysis of the ANDROMEDA-SHOCK Trial. Crit. Care 2020, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Shi, R.; Teboul, J.-L. Prediction of Fluid Responsiveness. What’s New? Ann. Intensiv. Care 2022, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Butti, F.; Pache, B.; Winiker, M.; Grass, F.; Demartines, N.; Hübner, M. Correlation of Postoperative Fluid Balance and Weight and Their Impact on Outcomes. Langenbeck’s Arch. Surg. 2020, 405, 1191–1200. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).