Submitted:
18 March 2024
Posted:
21 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
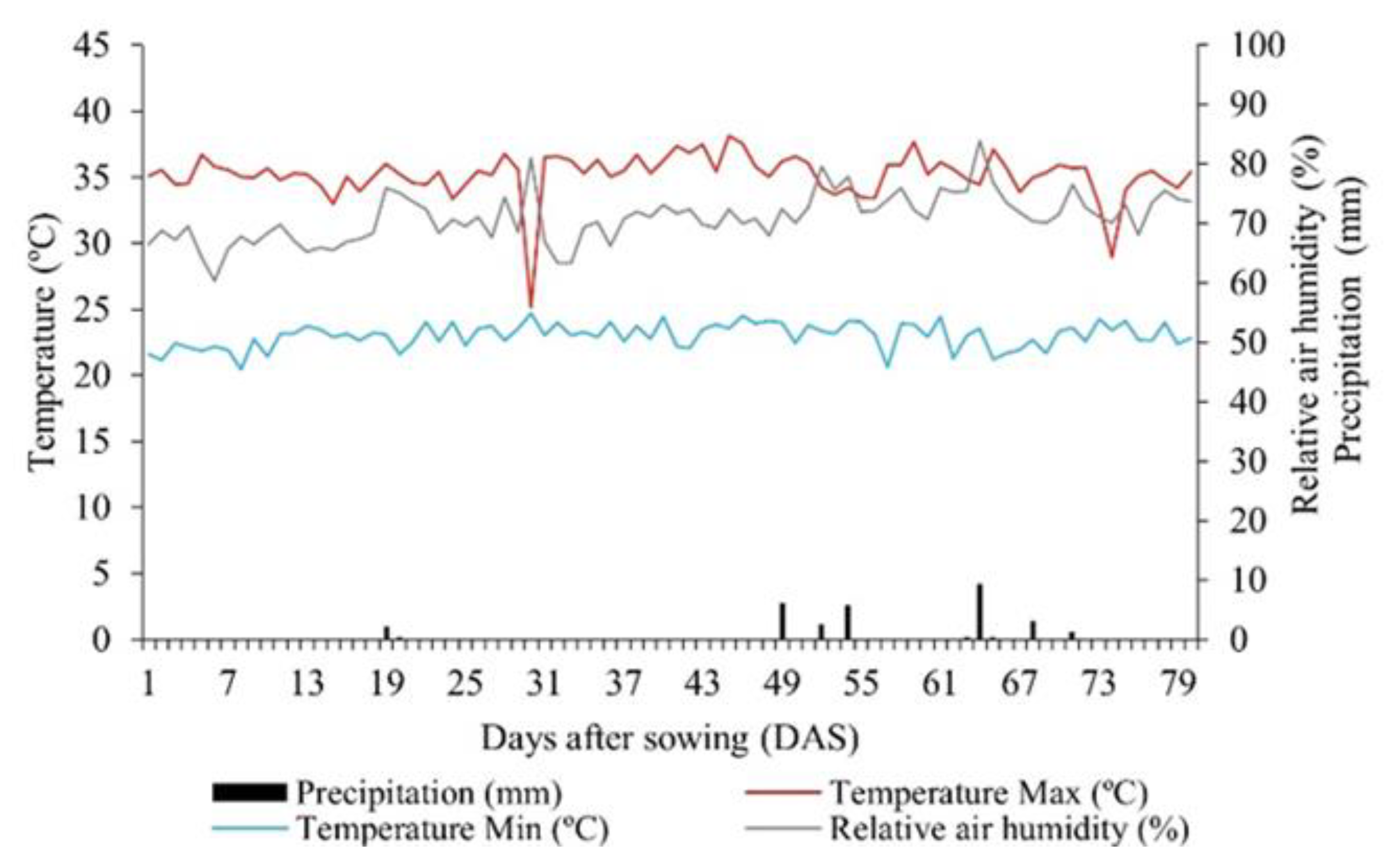
2.1. Location and Characterization of the Experimental Area
2.2. Experimental Design and Treatments
2.3. Plant Material and Fertilization
2.4. Irrigation Management
2.5. Gas Exchange and Chlorophyll Index
2.6. Biomass Production
2.7. Mineral Element Concentration
2.8. Data Analysis
3. Results e Discussion
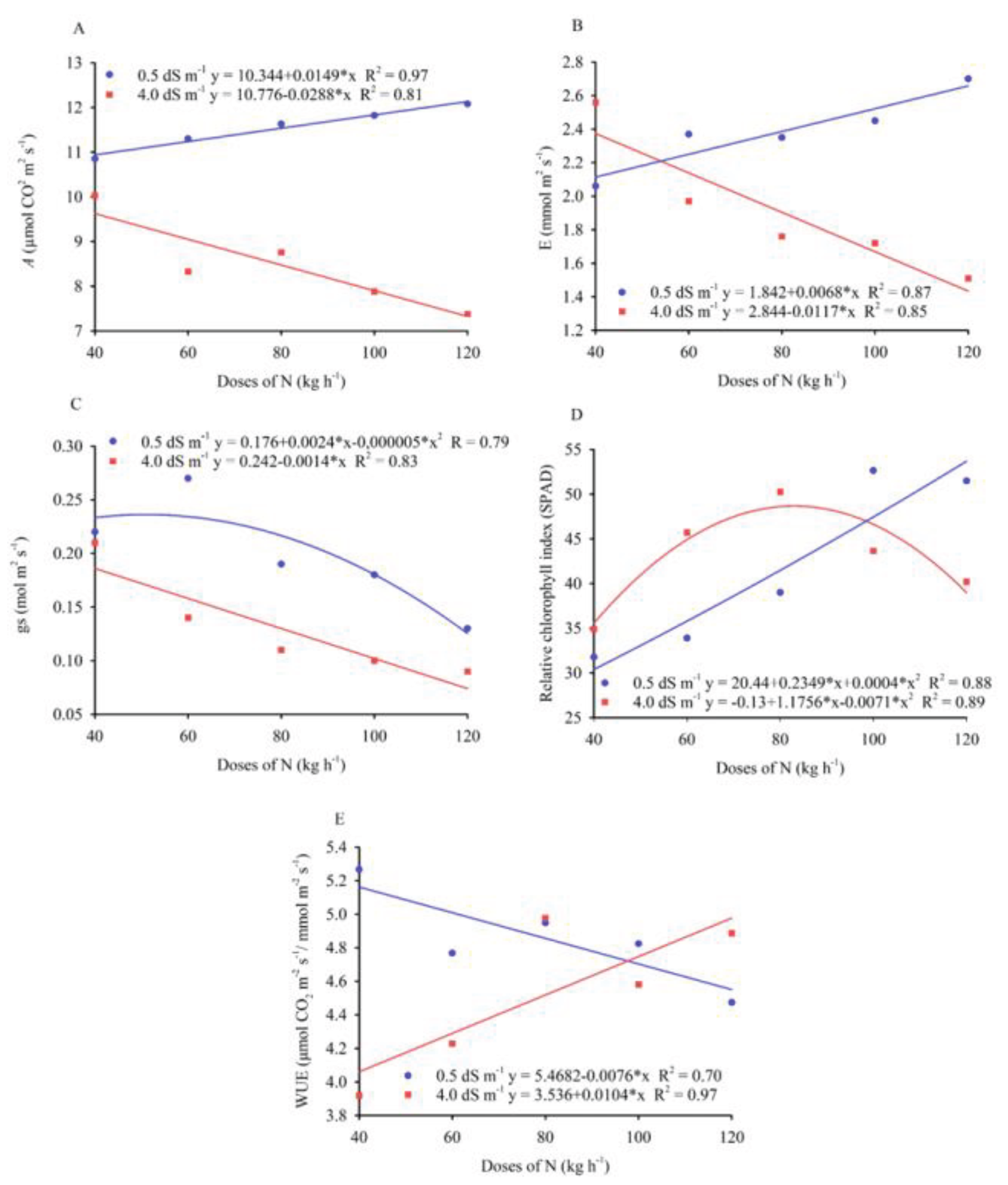
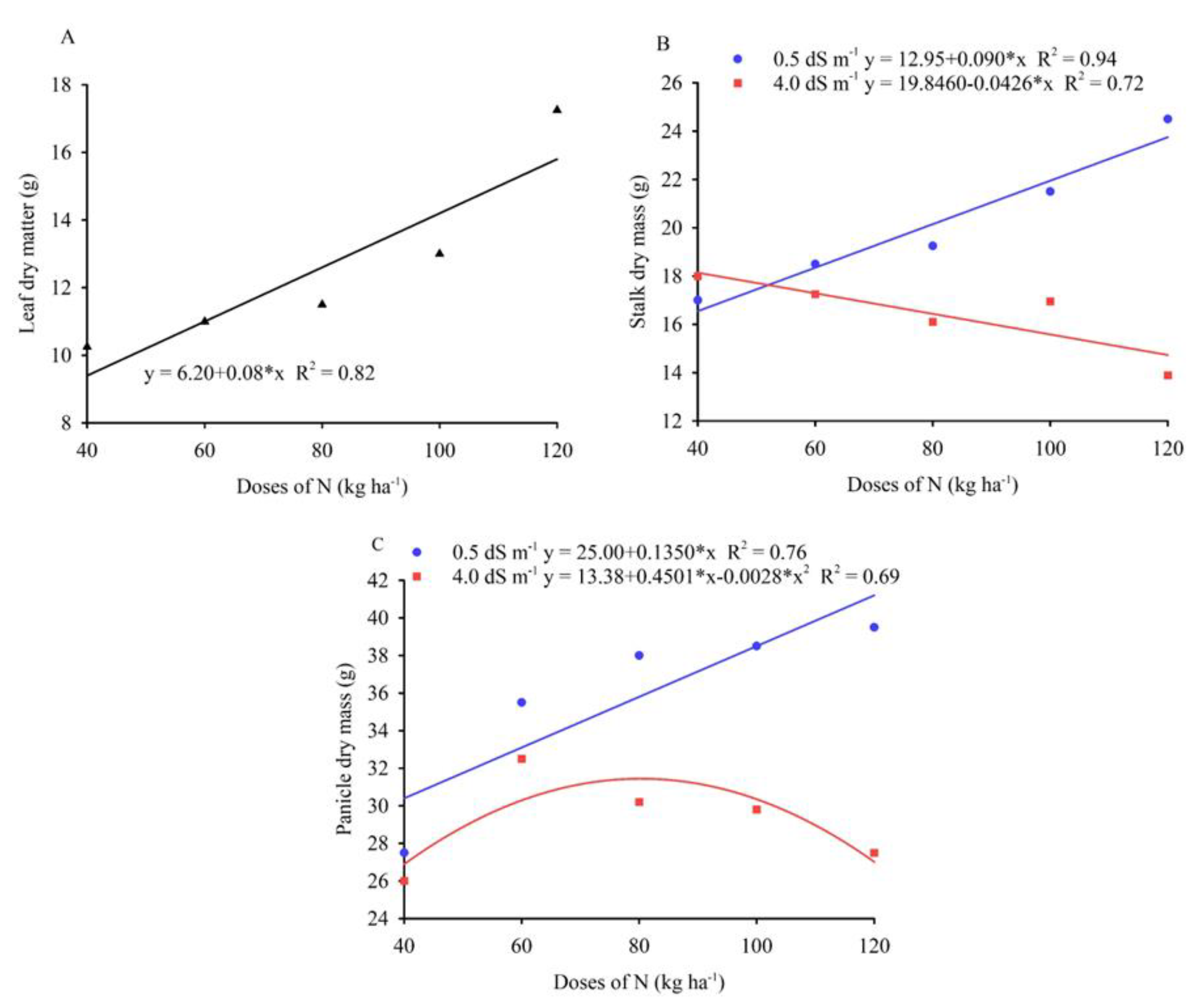
3.1. Leaf Gas Exchange and Biomass Production
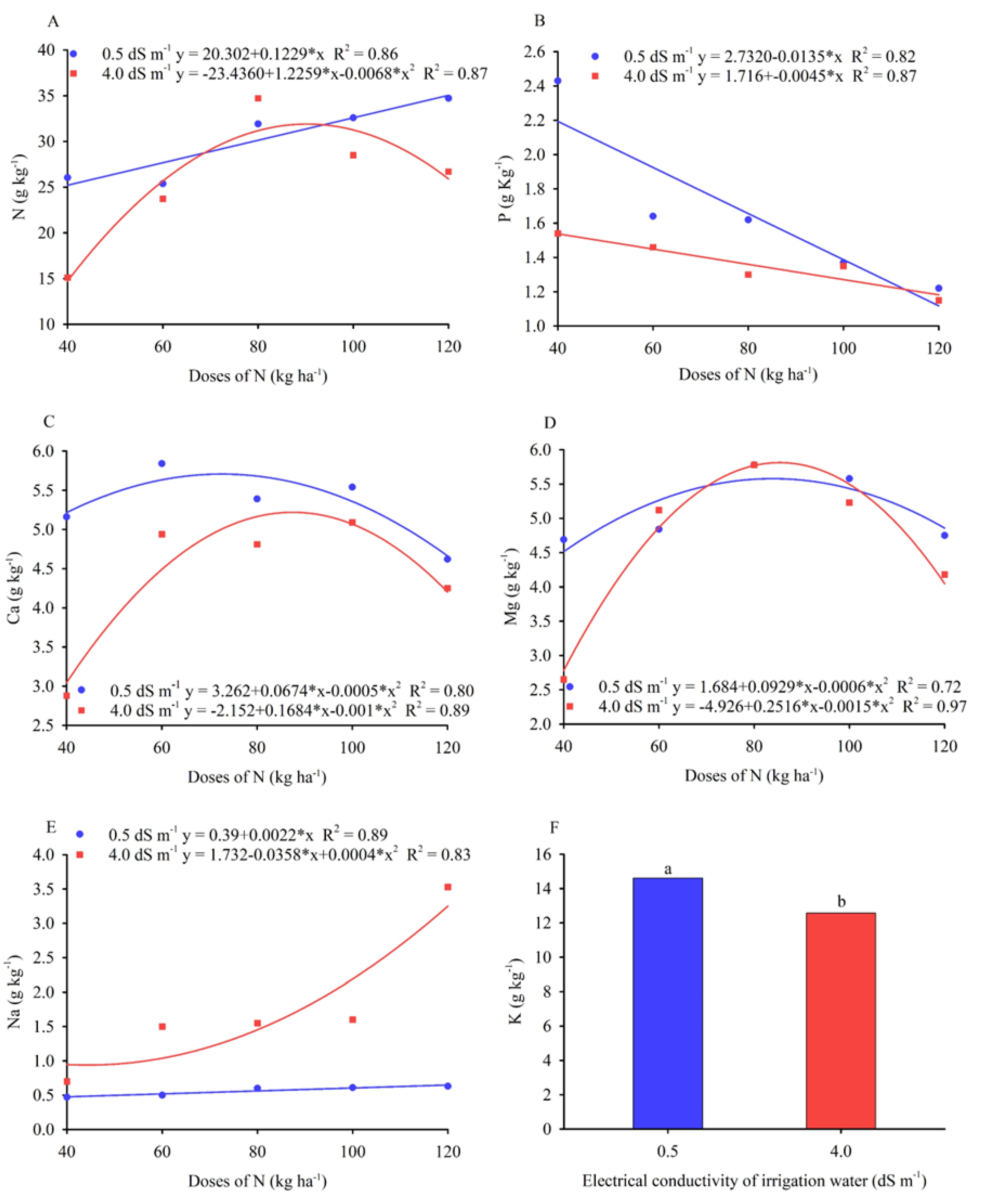
3.2. Leaf Concentration of Nutrients
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Marcante, N. C. , Camacho, M. A., & Junior, F. P. P. Teores de nutrientes no milheto como cobertura de solo. P. P. Teores de nutrientes no milheto como cobertura de solo. J. Biosci. 2011, 27, 196–204. [Google Scholar]
- Jacovetti, R.; França, A. F. S.; Carnevalli, R. A.; Miyagi, E. S.; Brunes, L. C.; Corrêa, D. C. Milheto como silagem comparado a gramíneas tradicionais: aspectos quantitativos, qualitativos e econômicos. Ciênc. anim. Bras 2018, 265–239. [Google Scholar] [CrossRef]
- Ferreira, F. N.; Oliveira, I. C. M.; Andrade, C. L. T.; Simeão, R. M.; Souza, I. R. P. Produção de silagem de milheto sob diferentes lâminas de irrigação. Sete Lagoas, MG: Embrapa Milho e Sorgo, 2020. 25 p.
- Holanda, J.S., Amorim, J.R.A., Ferreira Neto, M., Holanda, A.C., Sá, F.V.S. Qualidade da água para irrigação. In Manejo da salinidade na agricultura: estudos básicos e aplicados, 2. ed; Gheyi, H.R., Dias, N.S., Lacerda, C.F., Gomes Filho, E., Eds.; INCTSal: Fortaleza, Brazil, 2016; pp. 35–50. [Google Scholar]
- Lima, G. S.; Moreira, B. L.; Silva, A. G.; Diniz Neto, M. L.; Oliveira, D. S.; Cavalcante, A. P. Crescimento e produtividade de algodão de fibra colorida cultivado sob estresse salino e adubação nitrogenada. Rev. Bras. Eng. Agric. Ambient. 2017, 21, 415–420. [Google Scholar] [CrossRef]
- Sousa, G. G. D. , Sousa, H. C., Lessa, C. I., Goes, G. F., Freire, M. H. D. C., de Souza, M. V., & Schneider, F. Production of watermelon seedlings in different substrates under salt stress. Rev. Bras. Eng. Agric. Ambient. 2023, 27, 343–351. [Google Scholar]
- FAO. Global Map of Salt Affected Soils. FAO, 2021.
- Rai, A.K.; Basak, N.; Sundha, P. Saline And Sodic Ecosystems In The Changing World. In Soil Science: Fundamentals to Recent Advances; Rakshit, A., Singh, S., Abhilash, P., Biswas, A., Eds.; Springer: Singapore, 2021. [Google Scholar]
- Cavalcante, Í. H., Oliveira, F. A. D., Cavalcante, L. F., Beckmann, M. Z., Campos, M. C., & Gondim, S. C. Growth and production of two cotton cultivars irrigated with saline water. Rev. Bras. Eng. Agric. Ambient. 2021, 9, 108–111. [Google Scholar]
- Li, Y.; Xu, X.; Hu, Min; Chen, Z.; Tan, J.; Liu, Liu; Xiong, Y.; Huang, Q.; Huang, G. Modeling water−salt−nitrogen dynamics and crop growth of saline maize farmland in Northwest China: Searching for appropriate irrigation and N fertilization strategies. Agric. Water Manag. 2023, 282, 108271. [Google Scholar] [CrossRef]
- Li, T.; Xie, Y.; Gao, Z.; Hong, J.; Li, L.; Meng, H.; Ma, H.; Jia, J. Year-round film mulching system with monitored fertilization management improve grain yield and water and nitrogen use efficiencies of winter wheat in the dryland of the Loess Plateau, China. Environ. Sci. Pollut. Res. Int. 2019, 26, 9524–9535. [Google Scholar] [CrossRef]
- Sousa, H. C.; Sousa, G. G.; Lessa, C. I. N.; Lima, A. F. S. Ribeiro, R. M. R.; Rodrigues, F. H. C. Growth and gas exchange of corn under salt stress and nitrogen doses. Rev. Bras. Eng. Agric. Ambient. 2021, 23, 907–913. [Google Scholar] [CrossRef]
- Prado, Renato de Mello. Nutrição de plantas. 2. ed. São Paulo: Editora Unesp, 2020. v. 1. 414p.
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef]
- Zheng, C., Liu, C., Liu, L., Tan, Y., Sheng, X., Yu, D.,... & Duan, M. Effect of salinity stress on rice yield and grain quality: A meta-analysis. Eur. J. Agron. 2023, 144, 126765. [Google Scholar] [CrossRef]
- Sousa, G. G.; Lacerda, C. F.; Cavalcante, L. F.; Guimarães, F. V. A.; Bezerra, M. E. J.; Silva, G. L. Nutrição mineral e extração de nutrientes de planta de milho irrigada com água salina. Rev. Bras. Eng. Agric. Ambient. 2010, 14, 1143–1151. [Google Scholar] [CrossRef]
- Ibrahim, M. E. H.; Zhu, X.; Zhou, G.; Ali, A. Y. A.; Ahmad, I.; Elsiddig, A. M. I. Fertilizante nitrogenado reduz o impacto do cloreto de sódio no rendimento de trigo. J. Agron. 2018, 110, 1731–1737. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, C.; Hu, Y.; Shao, K.; Tang, X.; Zhang, L.; Gao, G.; Qin, B. Climate-induced salinization may lead to increased lake nitrogen retention. Water Res. 2023, 228, 119354. [Google Scholar] [CrossRef]
- Pereira Filho., I. A.; Pereira, A. S.; Coelho, A. M.; Casela, C. R.; Karam, D.; Rodrigues, J. A. S.; Cruz, J. C.; Waquil, J. M. Manejo da cultura do milheto. Sete Lagoas: Embrapa-CNPMS, 2003. 17 p. (Embrapa-CNPMS. Circular Técnica, 29).
- Ayers, R. S.; Westcot, D. W. A qualidade da água na agricultura. 2.ed. Campina Grande: UFPB, 1999. 153p. Estudos FAO: Irrigação e Drenagem.
- Bernardo, S. , Mantovani, E. C., Silva, D. D., & Soares, A. A.Irrigation Manual. 2019. 545p.
- Rhoades, J. D.; Kandiah, A.; Mashali, A. M. Uso de águas salinas para produção agrícola. Campina Grande: UFPB. 2000, 117p. Estudos da FAO, Irrigação e Drenagem.
- Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Embrapa Comunicação para Transferência de Tecnologia:.
- Brasília, Brazil, 1999; p. 370.Richards, L. A. Diagnosis and improvement of saline and alkali soils. Washington: US Department of Agriculture, 1954. 160p. USDA Agriculture Handbook, 60.
- Miyazawa, M.; Pavan, M. A.; Muraoka, T.; Carmo, C. A.; Melo, W. J. D. Análise química de tecido vegetal. In: Silva, F. C. Manual de análises químicas de solos, plantas e fertilizantes. Brasília: Embrapa Informação Tecnológica, 2009. Cap.2, p.193-233.
- Silva, F. C. , & DA SILVA, F. C Manual de métodos de análise de solo. 3.ed. Brasília: Embrapa, 2009. 573p.
- Silva, F. A. S.; Azevedo, C. A. V. The Assistat Software version 7.7 and its use in the analysis of experimental data. Afr. J. Agric. Res. 2016, 11, 733–3740. [Google Scholar]
- Zhang, M. , Wang, Z. J., Huang, J. C., Sun, S., Cui, X., Zhou, W., & He, S. (2021). Salinity-driven nitrogen removal and its quatitative molecular mechanisms in artificial tidal wetlands. Water Res. 2021, 202, 202–117446. [Google Scholar]
- Ribeiro, R. M. R. , de Sousa, G. G., Barbosa, A. S., de Lacerda, C. F., Freire, M. H. D. C., & Moraes, J. G. L Irrigation strategies with saline water and phosphate fertilization in cowpea culture. Rev Bras. de Ciên. Agra. 2022, 17, 1–12. [Google Scholar]
- Roque, I. A. , Soares, L. A. D. A., LIMA, G. S. D., Lopes, I. A. P., Silva, L. D. A., & Fernandes, P. D. Biomass, gas exchange and production of cherry tomato cultivated under saline water and nitrogen fertilization. Rev. Caatinga 2022, 20, 686–696. [Google Scholar]
- Có, E. G. , de Sousa, G. G., Gomes, S. P., Freire, M. H. D. C., & da Silva, F. D. Strategies for the management of irrigation with saline water and nitrogen fertilization in millet crop. Rev. Caatinga 2023, 2023, 424–431. [Google Scholar]
- Melo, H. F. D. , Souza, E. R. D., Duarte, H. H., Cunha, J. C., & Santos, H. R. Gas exchange and photosynthetic pigments in bell pepper irrigated with saline water. Rev. Bras. Eng. Agric. Ambient 2017, 21, 38–43. [Google Scholar]
- Li, Z. , Zhu, L., Zhao, F., Li, J., Zhang, X., Kong, X., & Zhang, Z. Plant salinity stress response and nano-enabled plant salt tolerance. Front. Plant Sci 2022, 13, 843994. [Google Scholar]
- Zhou, H.; Kang, S.; Li, F.; Du, T. , Shukla, M. K.; Li, X. Nitrogen application modified the effect of deficit irrigation on tomato transpiration, and water use efficiency in different growth stages. Sci. Hortic 2020, 263, 09112. [Google Scholar] [CrossRef]
- Adhikari, B. , Dhungana, S. K., Kim, I. D., & Shin, D. H. Effect of foliar application of potassium fertilizers on soybean plants under salinity stress. J. Saudi Soc. Agric. Sci. 2020, 19, 261–269. [Google Scholar]
- Morales, F. , Ancín, M., Fakhet, D., González-Torralba, J., Gámez, A. L., Seminario, A., & Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 80–88. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I. M.; Murphy, A. Fisiologia e desenvolvimento vegetal. 6.ed. Porto Alegre: ArtMed, 2017. 888p.
- Sousa, G. G. , de Araújo Viana, T. V., Neto, M. D. O. R., Da Silva, G. L., De Azevedo, B. M., & Costa, F. R. B. Características agronômicas do girassol irrigado com águas salinas em substratos com fertilizantes orgânicos. Rev Agrogeo 2017, 9, 1–8. [Google Scholar]
- Chen, Z. , Tao, X., Khan, A., Tan, D. K., Luo, H. Biomass accumulation, photosynthetic traits and root development of cotton as affected by irrigation and nitrogen-fertilization. Front. Plant Sci 2018, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Hessini, K. , Issaoui, K., Ferchichi, S., Saif, T., Abdelly, C., Siddique, K. H., & Cruz, C. Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiol. Biochem 2019, 139, 171–178. [Google Scholar]
- Chen, J. , Liu, L., Wang, Z., Zhang, Y., Sun, H., Song, S.,... & Li, C. Nitrogen fertilization increases root growth and coordinates the root–shoot relationship in cotton. Front. Plant Sci 2020, 11, 880. [Google Scholar]
- Bianchet, P. , Sangoi, L., Souza, C. A. D., Klauberg Filho, O., & Panison, F. Desenvolvimento vegetativo do arroz irrigado afetado pela inoculação com Azospirillum e aplicação de nitrogênio mineral. Rev. Fac. Agron 2015, 114, 2015. [Google Scholar]
- Sousa, G. G. D. , Rodrigues, V. D. S., Soares, S. D. C., Damasceno, Í. N., Fiusa, J. N., & Saraiva, S. E. Irrigation with saline water in soybean (Glycine max (L.) Merr.) in a soil with bovine biofertilizer. Rev. Bras. Eng. Agric. Ambient. 2018, 22, 604–609. [Google Scholar]
- Costa, F. H. , Sousa, G. G. D., Lima, J. M. D. P., Almeida, M. D. S., Sousa, H. C., Gomes, S. P., Cruz Filho, E. M. & Azevedo, B. M. D. Frequencies of irrigation in millet crop under salt stress. Rev. Bras. Eng. Agric. Ambient. 2024, 28, 272197. [Google Scholar]
- Ashraf, M. , Shahzad, S. M., Imtiaz, M., & Rizwan, M. S. Salinity effects on nitrogen metabolism in plants–focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr 2018, 41, 1065–1081. [Google Scholar]
- Iqbal, N. , Umar, S., & Khan, N. A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol 2015, 178, 84–91. [Google Scholar] [PubMed]
- Sousa, G. G. D. , Sousa, H. C., Santos, M. F. D., Lessa, C. I. N., & Gomes, S. P. Saline water and nitrogen fertilization on leaf composition and yield of corn. Rev. Caatinga 2022, 35, 191–198. [Google Scholar]
- Ueda, Y. , & Yanagisawa, S. Perception, transduction, and integration of nitrogen and phosphorus nutritional signals in the transcriptional regulatory network in plants. J. Exp. Bot 2019, 70, 3709–3717. [Google Scholar] [PubMed]
- Tanveer, K. , Gilani, S., Hussain, Z., Ishaq, R., Adeel, M., & Ilyas, N. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 2020, 43, 28–35. [Google Scholar]
- Esmaili, E. , Kapourchal, S. A., Malakouti, M. J., & Homaee, M. Interactive effect of salinity and two nitrogen fertlizers on growth and composition of sorghum. Plant Soil Environ 2008, 54, 537–546. [Google Scholar]
- Nathawat, N. S. , Kuhad, M. S., Goswami, C. L., Patel, A. L., & Kumar, R. Interactive effects of nitrogen source and salinity on growth indices and ion content of Indian mustard. J. Plant Nutr. 2007, 30, 569–598. [Google Scholar]
- Slama, I. , M’Rabet, R., Ksouri, R., Talbi, O., Debez, A., & Abdelly, C. Water deficit stress applied only or combined with salinity affects physiological parameters and antioxidant capacity in Sesuvium portulacastrum. Flora: Morphol. Distrib. Funct. Ecol. Plants. 2015, 213, 69–76. [Google Scholar]
- Abbasi, H. , Jamil, M., Haq, A., Ali, S., Ahmad, R., Malik, Z., & Parveen, Z. Salt stress manifestation on plants, mechanism of salt tolerance and potassium role in alleviating it: a review. Zemdirbyste-Agriculture 2016, 103, 229–238. [Google Scholar]
- Rodrigues, V. D. S. , Sousa, G. G. D., Soares, S. D. C., Leite, K. N., Ceita, E. D., & Sousa, J. T. M. D. Rev. Ceres. 2021, 68, 453–459. [Google Scholar]
- Calone, R. , Sanoubar, R., Lambertini, C., Speranza, M., Vittori Antisari, L., Vianello, G., & Barbanti, L. Salt tolerance and Na allocation in Sorghum bicolor under variable soil and water salinity. Plants 2020, 9, 561. [Google Scholar]
| Chemical Characteristics 1 | |||||||||
| O.M | N | P | Mg | K | Ca | Na | pH (in water) | ESP (%) | ECse |
| g kg-1 | mg Kg-1 | cmolc dm-3 | |||||||
| 4.34 | 0.26 | 65 | 1.20 | 0.65 | 1.20 | 0.33 | 6.2 | 7.00 | 1.19 |
| ECw | Ca2+ | Mg2+ | K+ | Na+ | Cl- | HCO3- | pH | SAR | Classification |
| dS m-1 | -----------------mmolc L-1-------------- | ----mmol L-1---- | H2O | (mmolc L-1) | |||||
| 0.3 | 0.6 | 1.4 | 0.2 | 0.4 | 2.5 | 0.1 | 6.9 | 0.4 | C2S1 |
| 4.0 | 8.44 | 10.18 | 2.66 | 20.53 | 33.33 | 1.12 | 7.38 | 7.86 | C4S2 |
| SF | DF | Medium Square | |||||||
| A | gs | E | WUE | RCI | DML | DMS | PDM | ||
| Doses of N (D) | 4 | 5.65** | 0.01158** | 0.597** | 1.51* | 359.44** | 62.15** | 311.15** | 356.35** |
| Salinity (S) | 1 | 40.17** | 0.03600** | 2.148** | 0.083ns | 77.17* | 14.40ns | 286.22** | 184.90ns |
| Interaction (D x S) | 4 | 3.89* | 0.00658** | 0.339** | 2.27** | 164.48** | 35.65ns | 314.22** | 268.65** |
| Residue | 30 | 1.4 | 0.0007 | 0.033 | 0.43 | 12.01 | 14.53 | 28.67 | 57.03 |
| Total | 39 | ||||||||
| CV (%) | - | 11.88 | 15.24 | 8.16 | 14.64 | 8.03 | 30.26 | 23.77 | 19.9 |
| SV | DF | Medium Square | |||||
| N | P | Ca | Mg | Na | K | ||
| Doses of N (D) | 4 | 239,33** | 0,48** | 2,44** | 5,06** | 2,84** | 1,62 ns |
| Salinity (S) | 1 | 101,06** | 1,06** | 6,81** | 2,63** | 11,07** | 41,57** |
| Interaction D x S | 4 | 49,92** | 0,35** | 1,58** | 1,70** | 2,44** | 5,91 ns |
| Residue | 4,63 | 0,81 | 0,32 | 0,15 | 0,13 | 3,35 | |
| CV (%) | 7,65 | 17,12 | 11,52 | 7,98 | 33,97 | 13,48 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





