1. Introduction
Fresh fruit and vegetables still contain high amount of water after being harvested, leading to metabolic process, such as ripening and senescence. This relatively high water content is likely to play a key role in increasing the susceptibility of fruit to the fungi responsible for decomposition and the often-high levels of losses and waste [
1]. Injuries on fruit during harvesting, postharvest handling, and commercialization are the primary cause of fungal infections [
2]. Postharvest control of fruit rot has been relied on synthetic fungicides. However, resistant strains and increased residues in treated fruit are main factors limiting their use [
3,
4].
Alternatives are increasingly required to control postharvest diseases including the use of safe low-toxicity compounds, such as natural products with low environmental impact. Organic and inorganic salts, generally recognized as safe (GRAS) ingredients, are considered antifungal products possessing properties as postharvest fruit preservatives [
5]. For that, GRAS salts were approved by the United States Food and Drug Administration (USFDA) and by the European Food Safety Authority (EFSA) allowing its use in food as conservatives [
2,
6].
GRAS salts such as carbonates, bicarbonates, sorbates, benzoates, parabens and metabisulfites, used as aqueous solutions or ingredients of edible coating are widely cited for controlling postharvest diseases [
7,
8,
9,
10,
11,
12]. Sulfites group, including sulfur dioxide (SO
2) (E -numbers E 220-228), are currently approved for some uses on entire fresh fruit. Sulfur dioxide and sulfites are authorized as food additives in the EU [
13]. Recent literature suggests that sulfur-containing salts, such as metabisulfites are effective in controlling important postharvest fruit diseases [
14,
15,
16,
17]. For instance, the GRAS salts sodium carbonate and sodium bicarbonate were reported fungistatic compounds able to reduce temporary postharvest decay without detectible phytotoxic effect on treated fruit [
18]. These salts applied singly on fruit were effective against green mold on lemons and oranges but their activity was least on infected clementine and mandarins [
12,
19]. Moreover, application of hydrogen peroxide (20 g L
−1) followed by potassium phosphite (20 g L
−1) controlled both blue and green molds on ‘Eureka’ lemon fruit [
20].
The objectives of the study were: (i) Evaluation of the in vitro antifungal activity of four GRAS salts against A. alternata, B. cinerea, P. digitatum and P. italicum, (ii) Assessment of the in vivo preventive and curative activity of the most promising salt to control decay on apple fruit, (iii) Identification of the signs of phytotoxicity in treated apples and oranges, for which a quantitative assessment was performed.
2. Materials and Methods
2.1. Fungal Species
Isolates of A. alternata, B. cinerea, P. digitatum and P. italicum were obtained from decayed fruit (mandarin and apple) from local markets ‘Le Kram’ and ‘Grombalia’ (Tunisia). Isolates were identified at the level of species and conserved in PDA medium at room temperature (around 20 °C for 7-14 d in obscurity) for further inoculation.
2.2. GRAS Salts Tested In Vitro
Sodium metabisulfite (SMB), ammonium bicarbonate (AMB), sodium bicarbonate (SB) and potassium dihydrogen orthophosphate (PDO) were firstly tested in vitro (PDA) against the main fungal species of postharvest fruit decay above cited. PDA medium was amended with the respective salt at 0.2 % before autoclaving at 120 °C during 20 min. PDA without salt was served as control. Mycelial plug (10 mm diameter) from 7 to 14 days-old cultures of each isolate was placed in the center of the medium in 90-mm plastic Petri dishes, then incubated at 20 ± 2 °C in obscurity.
Radial mycelial growth was determined in each Petri dish by measuring two perpendicular diameters of the fungal colony. These measurements were taken after 4, 7 and 9 days of incubation depending on the mycelial growth rate of the fungal species. Three replicates (3 Petri dishes) were used for each salt and fungal pathogen. The results were expressed as a percentage inhibition of mycelial growth using the following formula:
where dc = average diameter of the fungal colony on control plates (mm)and dt = average diameter of the fungal colony (mm) on Petri dishes amended with each salt.
2.3. GRAS Salt Tested on Fruit
The fruit used in the experiments were apples (Malus domestica. L) var. 'Golden' and oranges (Citrus sinesnsis. L) var. 'Maltaise' purchased from a supermarket in Ariana (Tunisia). The fruits were selected without wounds, with a healthy appearance, randomized, and surface disinfected by immersing them for 4 minutes in 0.72% sodium hypochlorite, rinsed twice with sterilized distilled water, and left to air dry under aseptic conditions.
Preliminary trial was undertaken in order to determine the appropriate kind of wound on fruit that allows rapid fungal progression. The fruit wounds were either 10 mm in diameter using a cork-borer, or five adjacent small punctures (within a 10 mm diameter circle) using a sterilized needle, with the control fruit intact.
Figure 1 shows that a 10 mm diameter cork-borer wound was the most appropriate, as the larger the wound, the greater the diameter of the rot and its certainty of development. This type of wound was used in subsequent trials.
Accordingly, fruit were wounded twice in the equatorial diameter in opposite positions using a cork-borer (10 mm diameter, 2 mm depth) immediately before treatment or inoculation. The inoculation consists of a fungal mycelium 10 mm in diameter from the edge of a growing colony, which is placed upside down in each wound. The most effective salt in the in vitro experiment, sodium metabisulphite, was used in the following bioassays at different concentrations. For the curative treatment, apples inoculated with B. cinerea were incubated in room ambient (temperature around 16°C) for 24 h, then dipped for 1 min in SMB solutions at concentrations of 0.5, 1, 2 or 3%, and incubated at room temperature for 7 days. For the preventive treatment, the fruits were first treated with SMB at the concentrations mentioned above and kept at room temperature for 24 hours, then inoculated with B. cinerea and incubated for 7 days at the same room ambient. In all cases, the fruits were completely soaked for 1 min in a precise dose of SMB solution in recipients of 1 L each. Fruit soaked for 1 min in distilled water served as the positive control and the negative control was fruit soaked in a solution (4 mL/L) of the commercial fungicide 'Celest extra'.
Three replicates (3 fruit) per treatment were used and the decay diameter was measured after 7 days of incubation. Fruit rot diameter (mm) was measured twice perpendicularly (to maintain an average measurement per fruit) using a ruler. The percentage of mycelial inhibition was calculated as described below.
2.4. GRAS Salt and Phytotoxicity
‘Golden' apples and 'Maltaise' oranges were wounded equatorially on two diametrically opposite sides. A first lot of fruit was inoculated by depositing a plug of mycelium (10 mm in diameter and 2 mm deep) of B. cinerea for the apples and of P. digitatum for the oranges, placed in the wound of the same size as the plug to completely cover the wound. After two hours, the inoculated fruit were immersed for 1 min in solutions each containing 0.5, 1, 2, 3 or 4% SMB. The second lot (non-inoculated) was wounded in the same way and then dipped for 1 minute in the SMB solutions mentioned above. The wounded fruits of the control were dipped in distilled water only. The fruits were incubated at an ambient temperature of 16 ± 2°C. Each treatment contained three fruits, one fruit being a replicate. The rot diameter of each fruit was measured (in mm using a ruler) twice perpendicularly to determine an average measurement per fruit.
The phytotoxicity caused by the application of SMB to the fruit was assessed at the end of the incubation period when rots reached the apical and/or the peduncle fruit surface. Oranges and apple were incubated for 6 days and 8 days respectively. The phytotoxicity of SMB on wounded fruit was estimated on the basis of the rot diameter. For that, we first calculated:
where ɸ
SMBI is the decay diameter of wounded, inoculated and treated fruit. ɸ
c+ is the diameter of wounded and inoculated but untreated fruit (positive control).
To distinguish between the fungal rot and that induced by the salt, the following approach was pursued. As the fruit rot diameter is the sum of the wound diameter and the rot size itself, it is more accurate to calculate only the rot size for wounded, inoculated and treated fruit (named: A) and the rot size for wounded and treated fruit only (named: B).
A (mm) = (ɸSMBI - ɸC-), where ɸSMBI is the decay diameter of wounded, inoculated and treated oranges or apples, ɸc- is the diameter of only wounded fruit (negative control).
B (mm) = (ɸSMB - ɸC-), where ɸSMB is the decay diameter of wounded and treated fruit.
The size of the rot caused by the fungus is therefore calculated as (A-B).
2.5. Statistical Analyses
Data from all experiments were subjected to analysis of variance (ANOVA). The main factors were GRAS salts and fungal species for the in vitro experiment, and treatments (preventive or curative) and salt concentrations for the in vivo experiment. For the phytotoxicity test, the main factors were SMB concentrations and inoculated or non-inoculated fruit.
4. Discussion
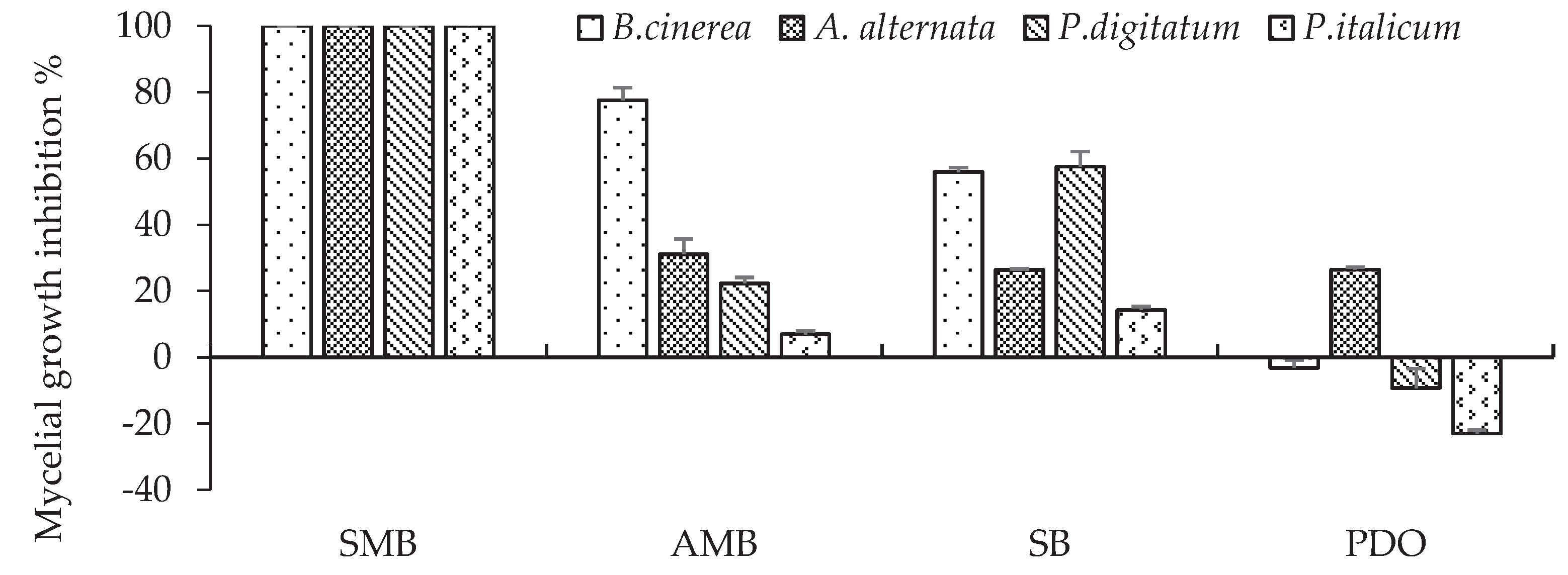
Our results from
in vitro trials using GRAS salts showed that 0.2% AMB induced 77.6% inhibition against
B. cinerea, but showed limited activity against other fungal species. SB was less effective than AMB, with inhibition of 56 and 57.6% against
B. cinerea and
P. digitatum respectively. The PDO was not only ineffective but also induced mycelial growth that was greater than that of the control, particularly in the case of
P. digitatum and
P. italicum. Previous results indicated that at a concentration of 0.16 - 0.50 %, ammonium, potassium and sodium bicarbonates inhibited approximately 95% of the
in vitro growth of
B. cinerea colonies [
21]. This inhibition is explained by the buffering capacity of the carbonate, which alkalinises the environment of pathogens such as
B. cinerea, which needs an acidic medium to grow properly [
22]. Zhao et al. [
23] evaluated
in vitro the antifungal activity of seventeen GRAS salts against four postharvest fungal diseases of citrus fruit. Their results, based on inhibition rates, pointed to differential reactions of fungi to the salts, in agreement with our results. Thus, sodium silicate (1%) was 100% inhibitory against all tested fungi. In contrast, sodium carbonate (1%) was 100% effective against
P. digitatum and
P. italicum, but had no activity against
G. citri-aurantii or
G. gloeosporioides. In this respect, Lyousfi et al. [
24] evaluated
in vitro some organic and inorganic salts (food additives) as antifungal agents against
Monilinia fructigena. Most additives showed significant inhibition of mycelial growth, but the extent of the inhibition varied according to the additive and its concentration (sodium bicarbonate, sodium carbonate, copper sulfate being the most effective, while ammonium carbonate and citric acid were the least effective).
Sulphur-containing food additives act effectively
in vitro against various postharvest fungal diseases. This was observed in our in vitro tests with SMB at 0.2%, which was the most effective, irrespective of the fungal species, as mycelial growth was completely inhibited at this concentration. Kim et al. [
25] highlighted that sodium metabisulfite was one of the most effective salts for controlling microbial growth. Tian et al. [
26] demonstrated the strong
in vitro antifungal activity of potassium metabisulfite and sodium hyposulfite (0.1%) against four kiwifruit soft rot fungi, inhibiting mycelial growth in a concentration-dependent manner. SMB completely inhibited mycelial growth of
A. alternata and
B. cinerea when used at a concentration of 0.2 M (3.8 %) [
6]. The sulphur salt completely inhibited the mycelial growth of
P. italicum at 0.4 % [
9] and of
P. digitatum at 2 % [
27]. Inhibition of
P. digitatum and
P. italicum by potassium and sodium metabisulfite in Petri Dish at concentrations of 0.19-1.9 % has also been reported [
5]. Sodium metabisulfite at a concentration of 0.19 % completely inhibited mycelial growth of
A. solani [
6]. In another study, 0.01% SMB caused total inhibition of conidial germination and germ-tube elongation in
Venturia inaequalis [
28], such concentration, according to our results on mycelial growth, seems to be a very low dose to ensure total inhibition
in vitro. Our in vitro results showed that 0.2% SMB is effective in ensuring total inhibition of fungi in vitro, but that 0.1% is not sufficient to achieve the same result and is therefore considered a limiting concentration for in vitro efficacy.
According to Talibi et al. [
29], the ability of GRAS salts to control postharvest diseases can be estimated by
in vitro experiments, but
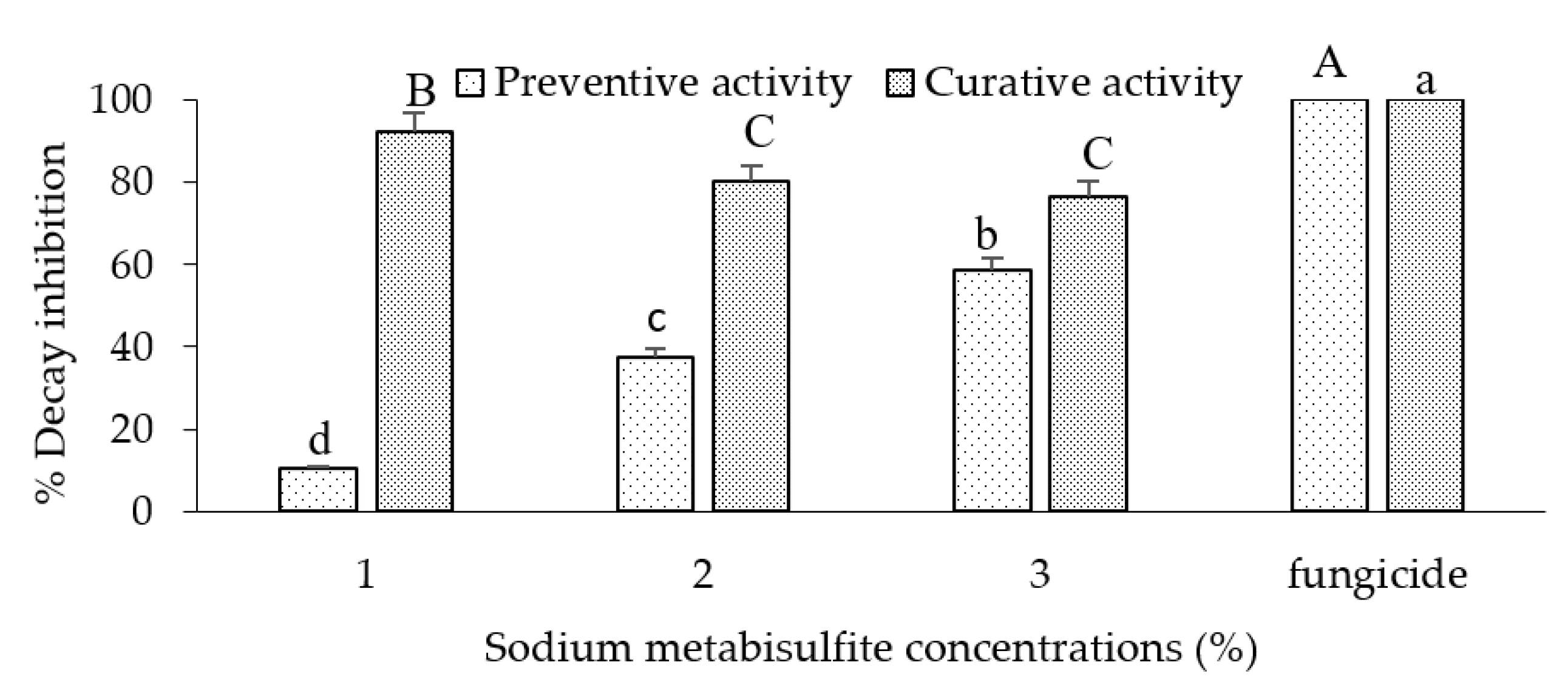
in vivo bioassays are still needed to confirm the in vitro results. In the first part of our
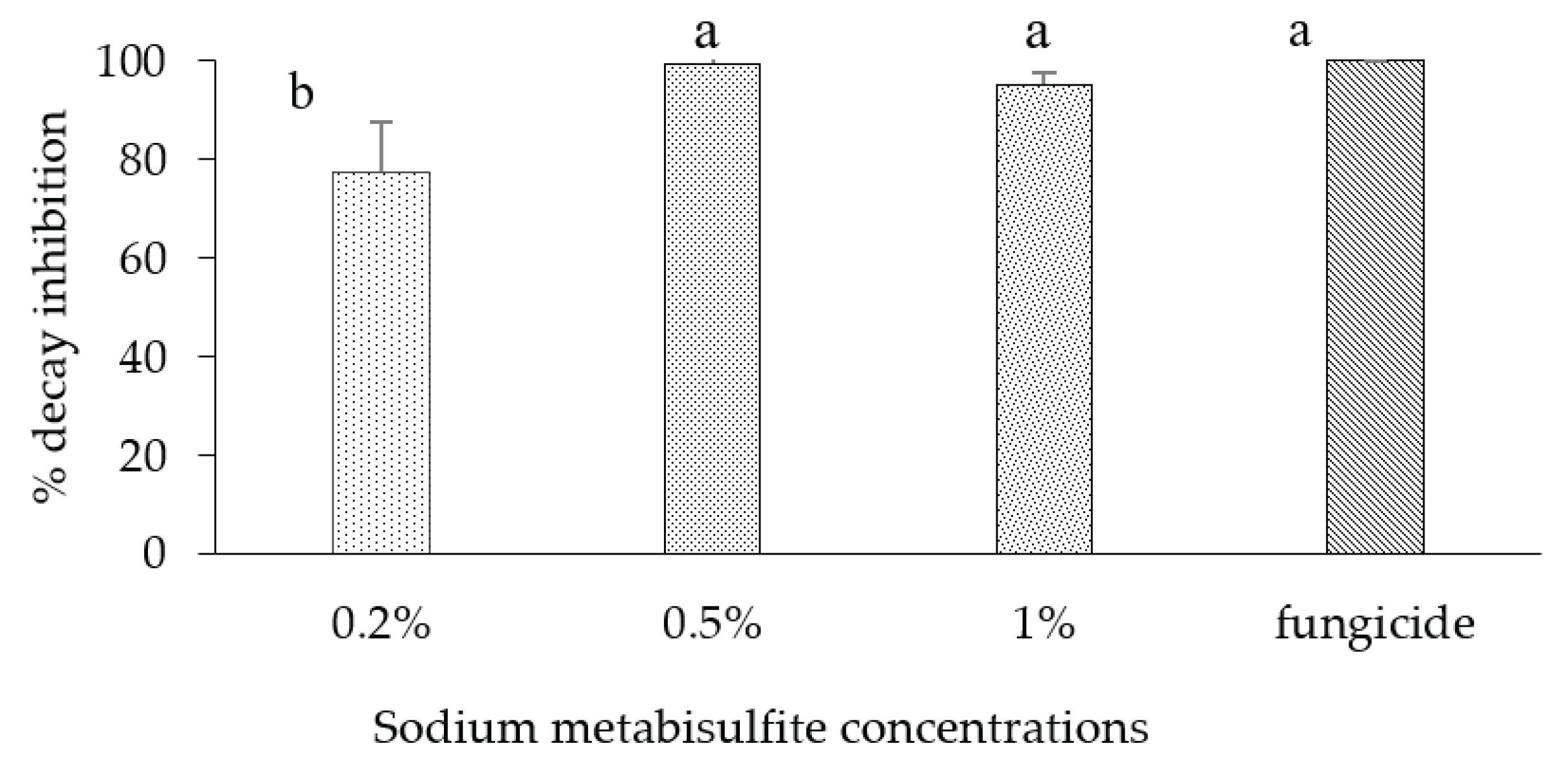
in vivo study, inoculated apples were dipped in different concentrations of SMB (1 %, 2 % and 3 %) as preventive and curative treatments. Results showed that SMB was ineffective when used preventively at all concentrations. However, the 1 % curative dip showed high efficacy (92.4%) compared with the commercial fungicide (100 %). In the second part of the study, we tested SMB curatively on apples at concentrations of 0.2 %, 0.5 % and 1%. The results showed that SMB, when used curatively at 0.5%, was effective in controlling rot on apples inoculated with
B. cinerea.
Sulphite salts dissolved in aqueous solutions are released as sulphur dioxide (SO
2) (67.4%) [
13,
30]. It has been reported that the mode of action of sulphite salts depends on pH [
31]. Contact between the salt solution and the fruit pericarp can modify the pH of the wound in which the fungus develops [
5]. The antifungal activity of sodium metabisulphite may increase because the pH at the point of inoculation is lower than that of the surrounding fruit tissue. The sulphur dioxide released by metabisulphites alters the permeability of the fungal membrane [
31] and accumulates in the cytoplasm, inhibiting mycelial growth through intercellular reactions [
13,
30]. Moreover, other factors may influence the mode of action of sulfur-containing fungistatic salts. Accordingly, it has been reported that pathogenicity of some fungal pathogens increases, and are able to alkalinize the medium in the peel infection wound, such as
Alternaria. Whereas other species, such as
Penicillium acidify the ambient [
32,
33,
34].
The ineffectiveness of SMB when applied as a preventive measure could be attributed to the gradual dissipation of the sulphur component, so that the subsequent fungal infection lost its full activity. However, there are reports on other GRAS salts without a sulphur component, such as potassium sorbate, showing that they are also ineffective as a preventative measure. For example, Olmedo et al. [
35] reported that potassium sorbate was tested against green and blue mould on lemon fruit inoculated and treated preventively and curatively. They pointed out that no efficacy was observed as a preventive measure, whereas curative treatment was effective. The same observation was underlined when thermal treatments were used. In fact, Lydakis and Aked [
36] reported that ‘Sultanina' table grape berries inoculated with
B. cinerea after vapour heat treatment (preventive treatment) were more susceptible to the fungal disease than the control. Nevertheless, curative heat treatments applied after inoculation significantly reduced infection compared to control. In a review, Palou et al. [
37] considered that the lack of preventive activity is one of the main handicaps of GRAS salts. Our results on the ineffectiveness of preventive treatment of fruit with SMB are in accordance with the previous findings using other means of treatments.
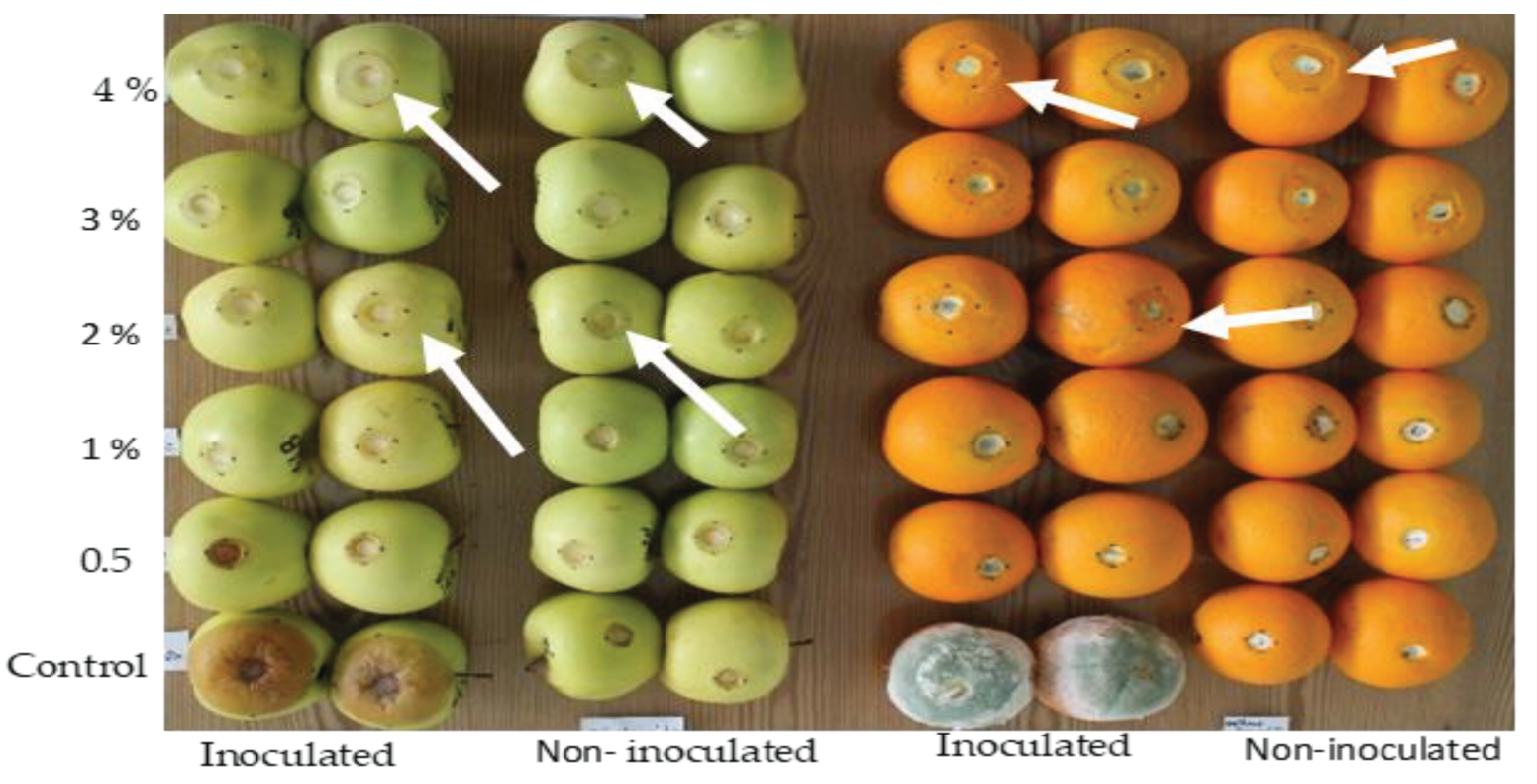
Phytotoxicity of sodium metabisulfite was assessed at concentrations from 0.5 to 4 % on apples and oranges. Necrosis was developed on fruit peel around the point of injury even though on un-inoculated fruit. This confirms that necrosis is not only produced by pathogenic fungi, but also by SMB at concentrations above 0.5 %. An increase of the necrosis area was registered even in healthy fruit (fruit wounded and treated but not inoculated). In literature, salts inducing phytotoxicity on fruit pericarp was described. For example, the application of copper sulfate has shown phytotoxic effects on fruit rinds, leading to visible darkening, and sinking at the inoculation point [
24]. Martínez-Blay et al. [
5] pointed out the phytotoxicity of sodium and potassium metabisulfite as well as aluminum and potassium sulfate on citrus fruit.
We can assume that dipping healthy fruit in high concentrations of SMB would affect the cellular cohesion of the fruit pericarp, which would then allow the fruit to be infected more rapidly. In addition, since the natural antagonists of the microbiome, which are normally latent on the pericarp, would be disrupted after treatment of the fruit, the pathogen would be able to multiply again without mechanical/antagonistic barriers. For this reason, using a higher level of sodium metabisulphite will not improve the inhibition of rot size, but will increase the sensitivity of the fruit skin to the side effect of this salt. Our analyses highlighted that the appropriate concentration was 0.5% in the curative treatment without showing any noticeable phytotoxicity on the fruit. Thus, Martinez-Blay et al. [
5] reported that the application of SMB at a concentration of 100 mM was phytotoxic on 'Valencia' oranges, but that 50 mM caused no apparent damage to the rind of the treated fruit and was very effective against mould. The 50 mM concentration (0.85 %) is very close to 0.50 %, showing in our trials efficacy with no apparent phytotoxic reaction in treated fruit.
To our knowledge, the evaluation of salt phytotoxicity was mainly based on the qualitative visual description of the symptoms appearing after treatment of the fruit. For example, in dip treatments of stone fruit to control fungal pathogens after harvest using food additives, phytotoxicity on the fruit skin caused by the salts was assessed visually on a scale of 1 to 4, depending on the surface area covered by fungal lesions [
38]. Delisle-Houde et al. [
39] used also visual scale of 0-4 to assess the severity of phytotoxicity of different salts on foliar surface of lettuce infected with the bacteria ‘
Pseudomonas cichorii’ causing lettuce varnish spot. Our analyses on severity of phytotoxicity used a quantitative procedure by relating the size of necrosis measured on treated fruit to the corresponding concentration of salt. We suggest using the protocol/methodology applied here as a model for a quantitative analysis of the phytotoxicity of compounds used to treat fruit. This suggestion may become valid after other tests including different compounds on various fruit (apples are the best candidates thanks to the obvious necrosis margin).
Figure 1.
Importance of wound type allowing a better growth of B. cinerea on apple fruit.
Figure 1.
Importance of wound type allowing a better growth of B. cinerea on apple fruit.
Figure 2.
Percentage inhibition of mycelial growth of B. cinerea, A. alternata, P. digitatum and P. italicum on PDA amended with one of the four 0.2% GRAS salts, incubated at 20 °C. Bars with standard error are averages of three replicates. SMB: sodium metabisulphite, AMB: ammonium bicarbonate, SB: sodium bicarbonate PDO: potassium dihydrogen orthophosphate. For B. cinerea, mycelial growth was determined after 4 days, for P. digitatum after 7 days and for A. alternata and P.italicum after 9 days.
Figure 2.
Percentage inhibition of mycelial growth of B. cinerea, A. alternata, P. digitatum and P. italicum on PDA amended with one of the four 0.2% GRAS salts, incubated at 20 °C. Bars with standard error are averages of three replicates. SMB: sodium metabisulphite, AMB: ammonium bicarbonate, SB: sodium bicarbonate PDO: potassium dihydrogen orthophosphate. For B. cinerea, mycelial growth was determined after 4 days, for P. digitatum after 7 days and for A. alternata and P.italicum after 9 days.
Figure 3.
Percentage of rot inhibition by sodium metabisulfite treatment (1-3 %) of 'Golden' apples inoculated with Botrytis cinerea. Fruits were dipped preventively or curatively in different concentrations of sodium metabisulphite and then incubated at room temperature for 7 days. The bars represent the standard error on the mean of three replicates. Means with different letters are significantly different at p ≤ 0.05. ANOVA summary: F ratios: Treatment (152.9**), SMB concentration (5.6*) and interaction Treatment × SMB concentration (22.4**).
Figure 3.
Percentage of rot inhibition by sodium metabisulfite treatment (1-3 %) of 'Golden' apples inoculated with Botrytis cinerea. Fruits were dipped preventively or curatively in different concentrations of sodium metabisulphite and then incubated at room temperature for 7 days. The bars represent the standard error on the mean of three replicates. Means with different letters are significantly different at p ≤ 0.05. ANOVA summary: F ratios: Treatment (152.9**), SMB concentration (5.6*) and interaction Treatment × SMB concentration (22.4**).
Figure 4.
Percentage of rot inhibition in apple fruit var. 'Golden' inoculated with B. cinerea and curatively dipped in different concentrations (0.2-1%) of sodium metabisulphite then incubated at room temperature for 7 d. Bars represent the standard error on the mean of three replicates. Means with different letters are significantly different at p ≤ 0.05. ANOVA summary: F-ratio of SMB concentrations (14.23*).
Figure 4.
Percentage of rot inhibition in apple fruit var. 'Golden' inoculated with B. cinerea and curatively dipped in different concentrations (0.2-1%) of sodium metabisulphite then incubated at room temperature for 7 d. Bars represent the standard error on the mean of three replicates. Means with different letters are significantly different at p ≤ 0.05. ANOVA summary: F-ratio of SMB concentrations (14.23*).
Figure 5.
Phytotoxicity (as rot size) of sodium metabisulphite in relation to increasing concentrations from 0.5 to 4% on apples inoculated/un-inoculated with B. cinerea (left) and on oranges inoculated/un-inoculated with P. digitatum (right). ANOVA Sumary of the phtytoxicity in apple fruit: Factor SMB concentration: F value = (11.6*), Factor pathogen: F value = (2.6 ns), Interaction SMB concentration×pathogen: F value = (2.6)*. ANOVA Sumary of the phtytoxicity in orange fruit: Factor SMB concentration: F value = (22.4*), Factor pathogen: F value = (0.2 ns), Interaction SMB concentration×pathogen: F value = (0.6 ns). * Significance at p ≤ 0.05, ns not significant.
Figure 5.
Phytotoxicity (as rot size) of sodium metabisulphite in relation to increasing concentrations from 0.5 to 4% on apples inoculated/un-inoculated with B. cinerea (left) and on oranges inoculated/un-inoculated with P. digitatum (right). ANOVA Sumary of the phtytoxicity in apple fruit: Factor SMB concentration: F value = (11.6*), Factor pathogen: F value = (2.6 ns), Interaction SMB concentration×pathogen: F value = (2.6)*. ANOVA Sumary of the phtytoxicity in orange fruit: Factor SMB concentration: F value = (22.4*), Factor pathogen: F value = (0.2 ns), Interaction SMB concentration×pathogen: F value = (0.6 ns). * Significance at p ≤ 0.05, ns not significant.
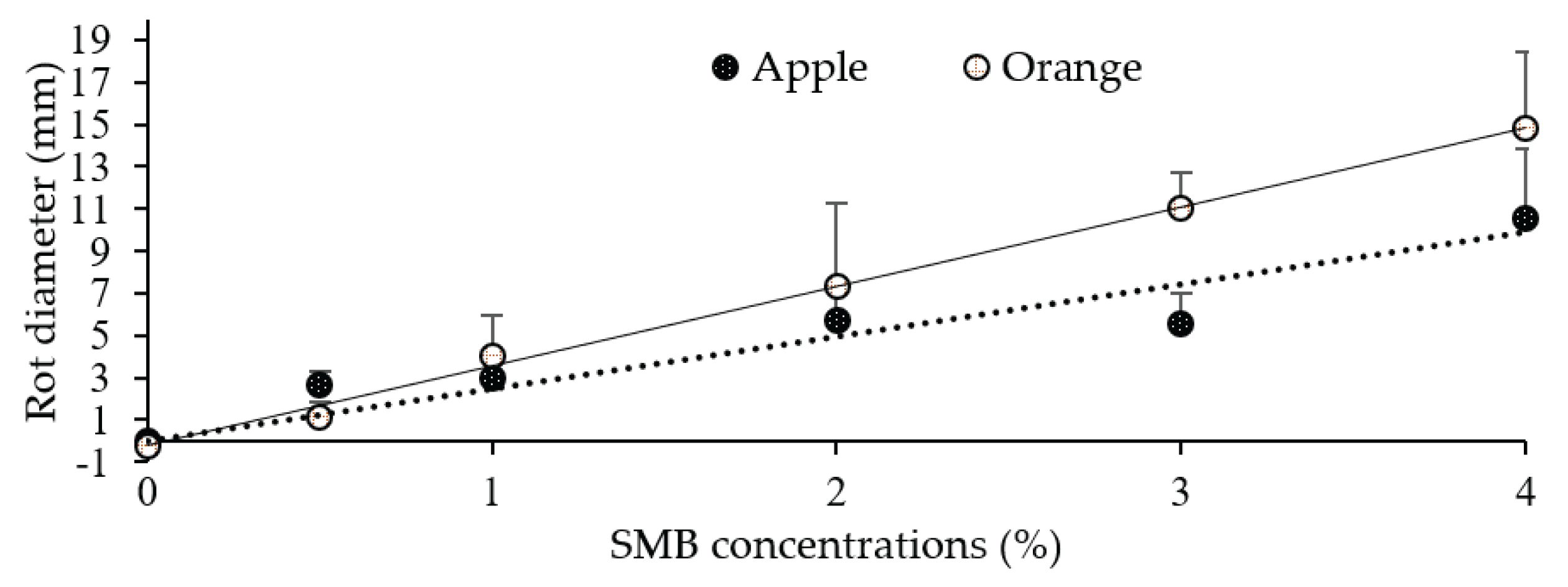
Figure 6.
Linear regression between sodium metabisulphite concentration (x) and rot diameter size (y) measured on oranges (6 dpi) and apples (8 dpi). Each point is the mean diameter of eight replicates (fruit). Vertical bars represent standard deviations.
Figure 6.
Linear regression between sodium metabisulphite concentration (x) and rot diameter size (y) measured on oranges (6 dpi) and apples (8 dpi). Each point is the mean diameter of eight replicates (fruit). Vertical bars represent standard deviations.
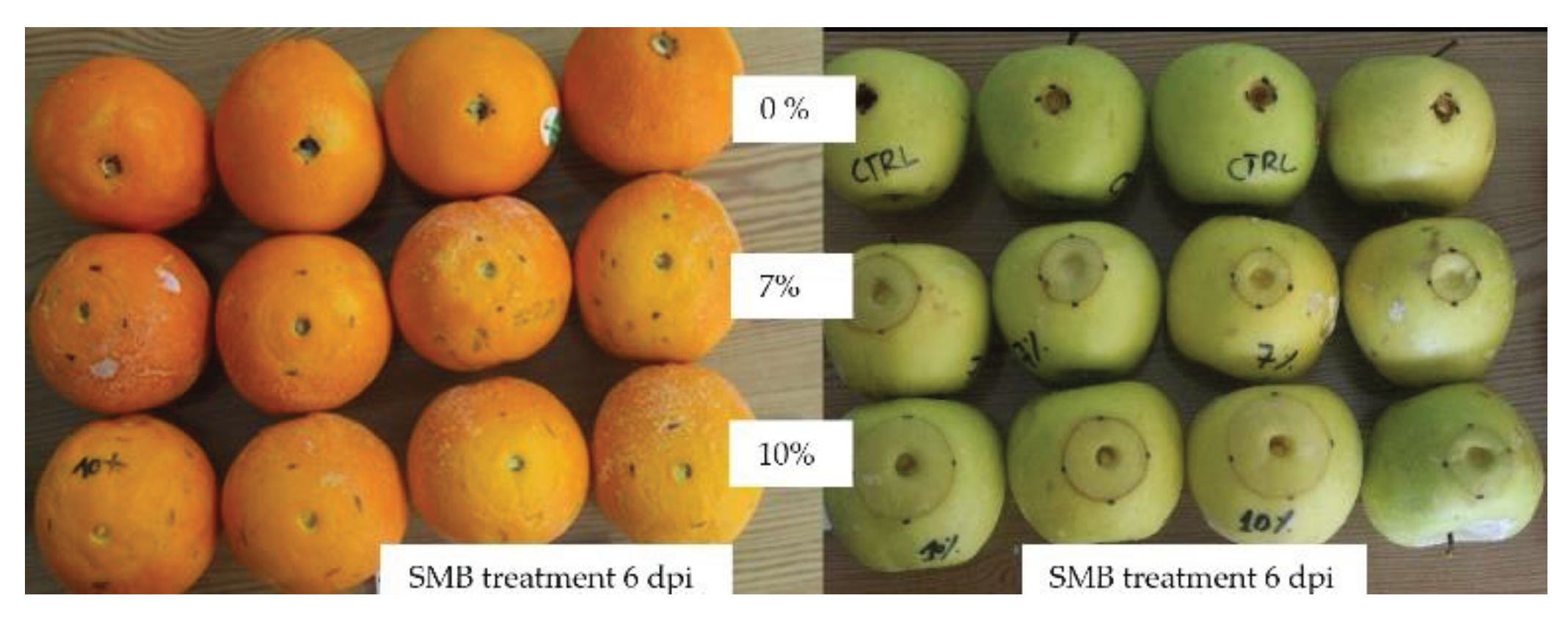
Figure 7.
Evolution of the phytotoxic reaction to SMB in the form of a circular necrosis around the wound on 'Golden' apples and 'Thompson' citrus fruit treated with 7 % and 10 % SMB and incubated at 18 °C for 6 days.
Figure 7.
Evolution of the phytotoxic reaction to SMB in the form of a circular necrosis around the wound on 'Golden' apples and 'Thompson' citrus fruit treated with 7 % and 10 % SMB and incubated at 18 °C for 6 days.
Table 1.
Analysis of variance in the effect of GRAS salts on mycelial inhibition of various fungi.
Table 1.
Analysis of variance in the effect of GRAS salts on mycelial inhibition of various fungi.
| Source of variation |
DF |
F-value |
| Factor A (GRAS salts) |
3 |
230.4** |
| Factor B (fungi) |
3 |
18.2* |
| Interaction A×B |
9 |
10.6* |
Table 2.
Evaluation of the phytotoxicity of SMB by the size of rots on oranges and apples treated with increasing SMB concentrations.
Table 2.
Evaluation of the phytotoxicity of SMB by the size of rots on oranges and apples treated with increasing SMB concentrations.
| SMB concentrations % (x) |
0.5 |
1 |
2 |
3 |
4 |
| Oranges (cv. ‘Maltaise’) |
| Decay inhibition (%)1
|
98.7 |
94.1 |
88.0 |
84.8 |
78.2 |
| A (mm)= ɸSMBI - ɸC-
|
0.9 ± 0. 1 |
4 ± 1.4 |
8.2 ± 5.6 |
10.4 ± 1.1 |
14.9 ± 3.5 |
| B (mm) = ɸSMB - ɸC-
|
1.3 ± 0.3 |
4 ± 2.1 |
6.4 ± 2.3 |
11.7 ± 1.5 |
9.2 ± 2.9 |
| Fungal effect (A-B) (mm) |
-0.4 |
0 |
1.8 |
-1.3 |
5.7 |
| (A+B) /2, [y observed] (mm) |
1.1 |
4 |
7.3 |
11.05 |
12.05 |
| y = a. x +b, [y calculated] |
y = 3.76.x - 0.2 ; R2 = 0.99 |
| Apples (cv. ‘Golden’) |
| Decay inhibition (%)1
|
93.2 |
91.6 |
84.7 |
87.1 |
71.7 |
| A (mm)= ɸSMBI - ɸC-
|
2.5 ± 0.3 |
2.5 ± 0.6 |
5.1 ± 1.6 |
5.8 ± 1.3 |
9.5± 2.3 |
| B (mm) = ɸSMB - ɸC-
|
2.8 ± 0.9 |
3.5 ± 1.7 |
6.3 ± 1.4 |
5.3 ± 1.6 |
11.7 ± 4.2 |
| Fungal effect (A-B) (mm) |
0.3 |
1.0 |
1.3 |
-0.5 |
2.2 |
| (A+B) /2, [y observed] (mm) |
2.7 |
3.0 |
5.7 |
5.6 |
10.6 |
| y = a. x +b, [y calculated] |
y = 2.47.x ; R2 = 0.96 |
Table 3.
Mean values of rot diameter (mm) measured and those expected from the respective linear regressions of apples and oranges in relation to SMB concentrations and chi-square test comparing the critical and calculated chi-square values.
Table 3.
Mean values of rot diameter (mm) measured and those expected from the respective linear regressions of apples and oranges in relation to SMB concentrations and chi-square test comparing the critical and calculated chi-square values.
| |
SMB (%) |
Observed values |
Expected values |
Chi-square test |
Oranges
cv. ‘Thompson’ |
7 |
26.02 |
26.12 |
χ2c = 0.809
χ2 (0.05, 1) = 3.84
|
| 10 |
31.9 |
37.4 |
| Apples cv. ‘Golden’ |
7 |
16.7 |
17.3 |
χ2 c = 0.23
χ2 (0.05, 1) = 3.84
|
| 10 |
22.4 |
24.7 |