Submitted:
19 March 2024
Posted:
19 March 2024
You are already at the latest version
Abstract
Keywords:
Graphical Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism, Culture Conditions and Eu-Adapted Strains
2.2. Growth Kinetics and LC50 Calculation by Flow Cytometry
2.3. Oxidative Stress Detection
2.4. Transmission Electron Microscopy (TEM) and Microanalysis (TEM-XEDS)
2.5. Total RNA Isolation and cDNA Synthesis
2.6. Quantitative RT-PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
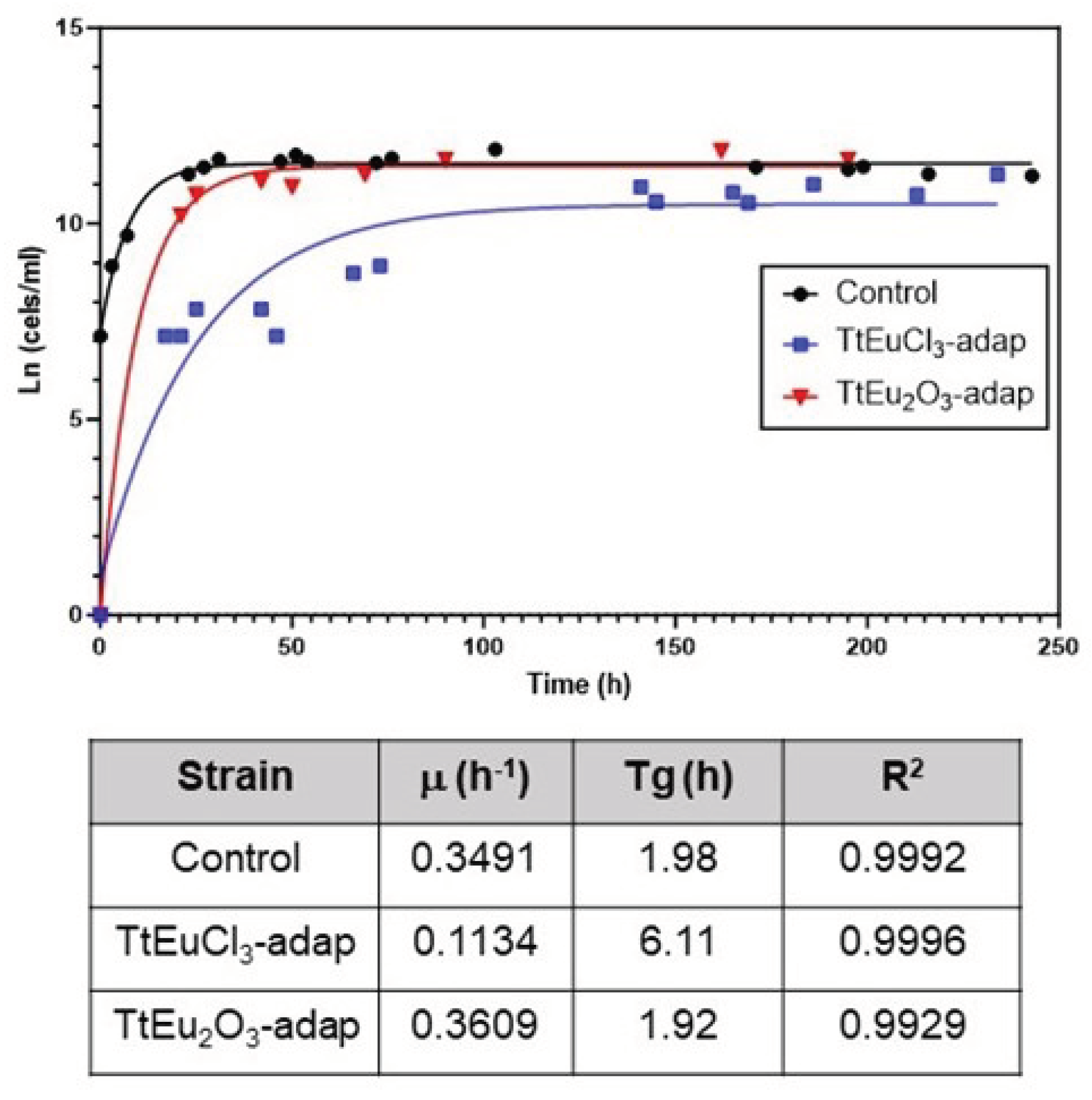
3.1. Ecotoxicological Parameters
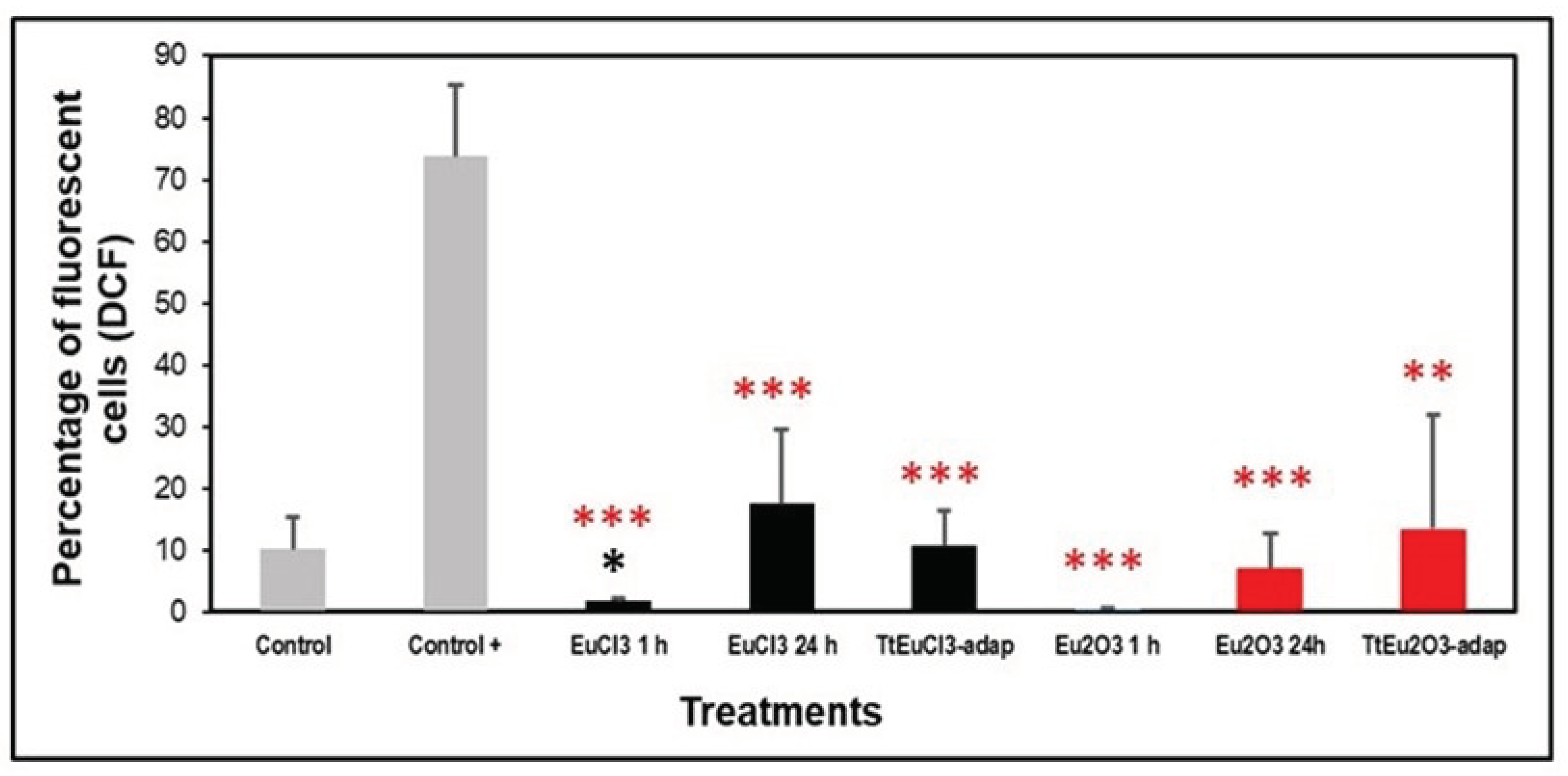
3.2. Oxidative Stress Assessment
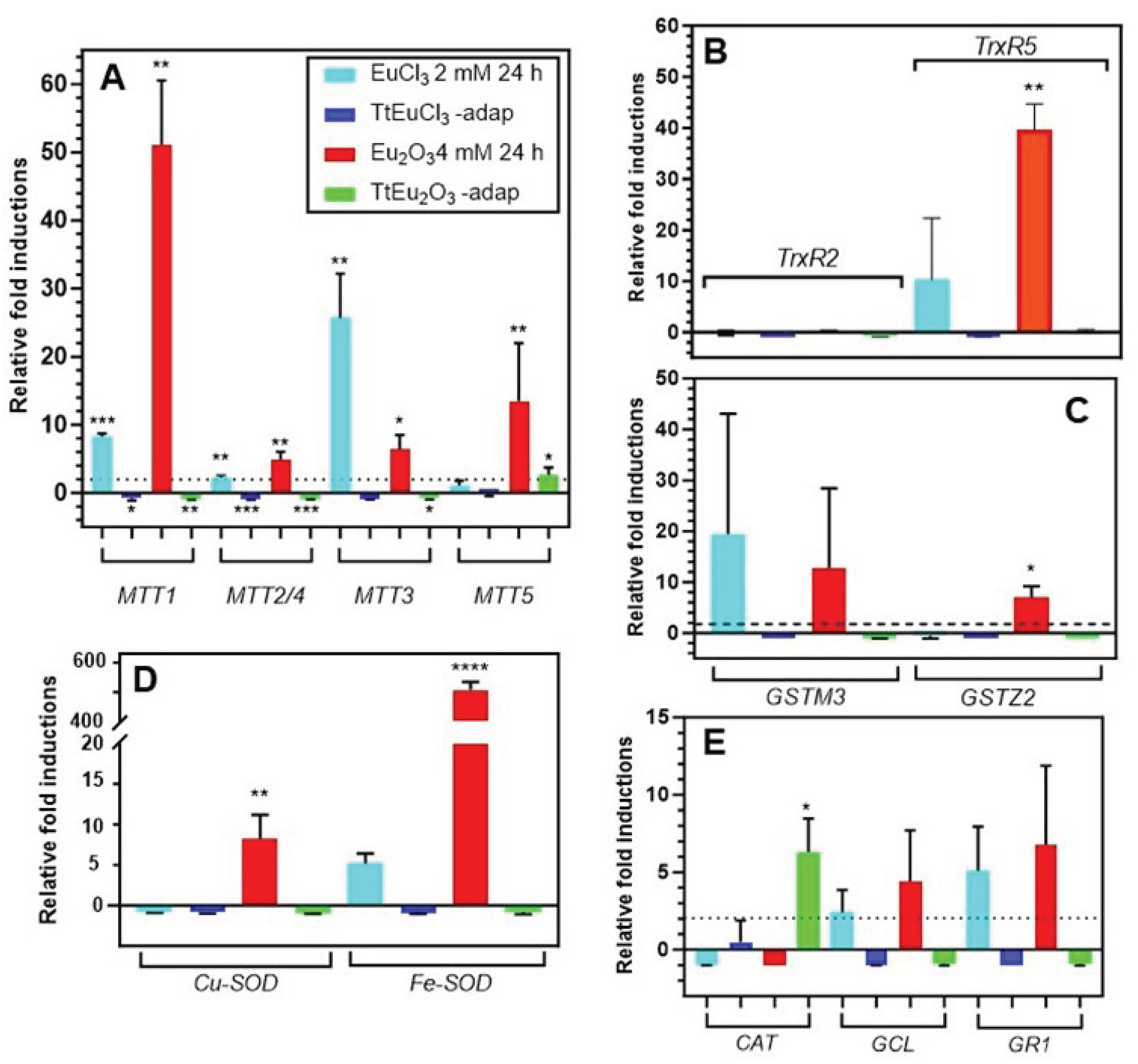
3.3. Comparative Quantitative Expression Analysis of Several Genes Involved in Oxidative and/or General Stress
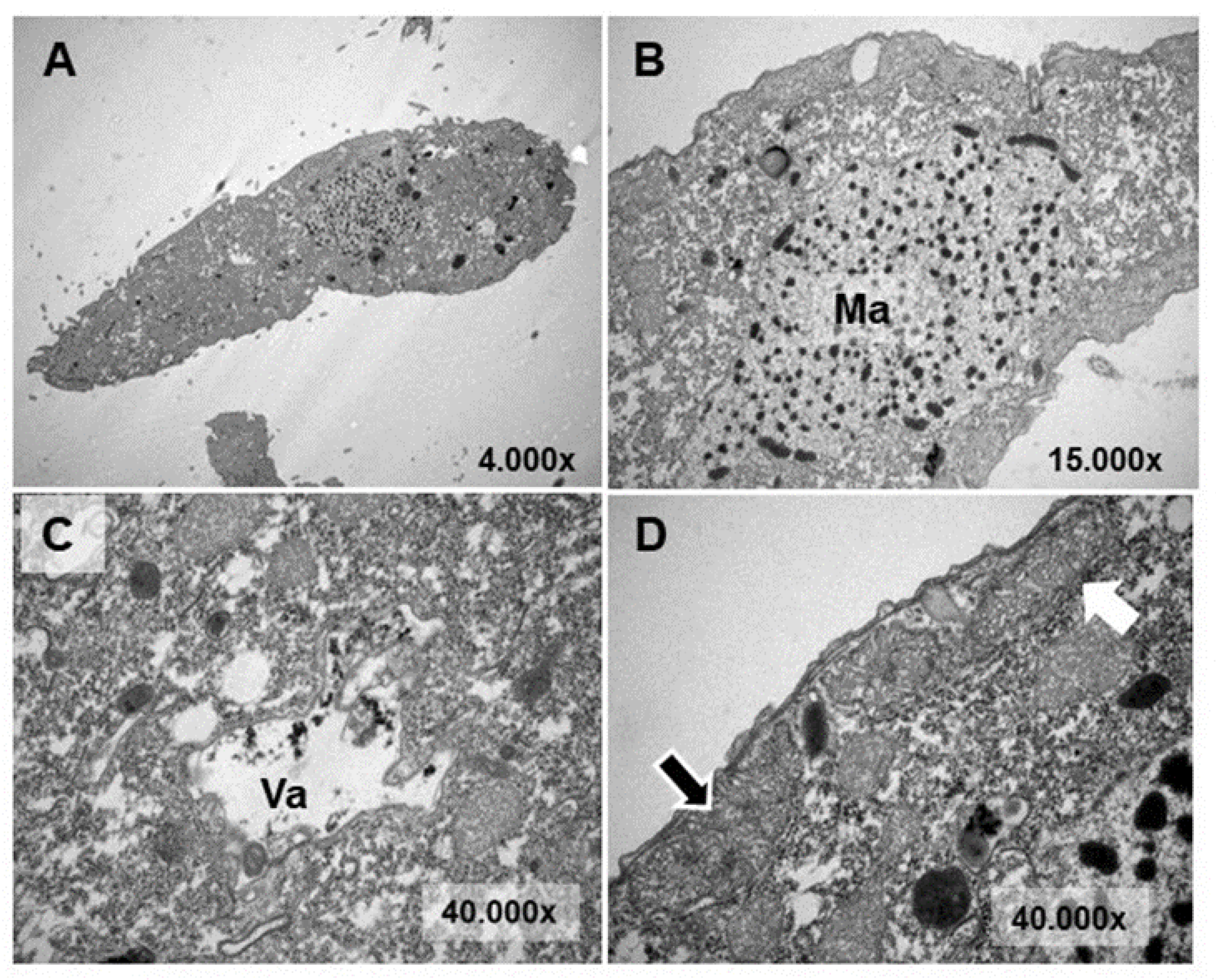
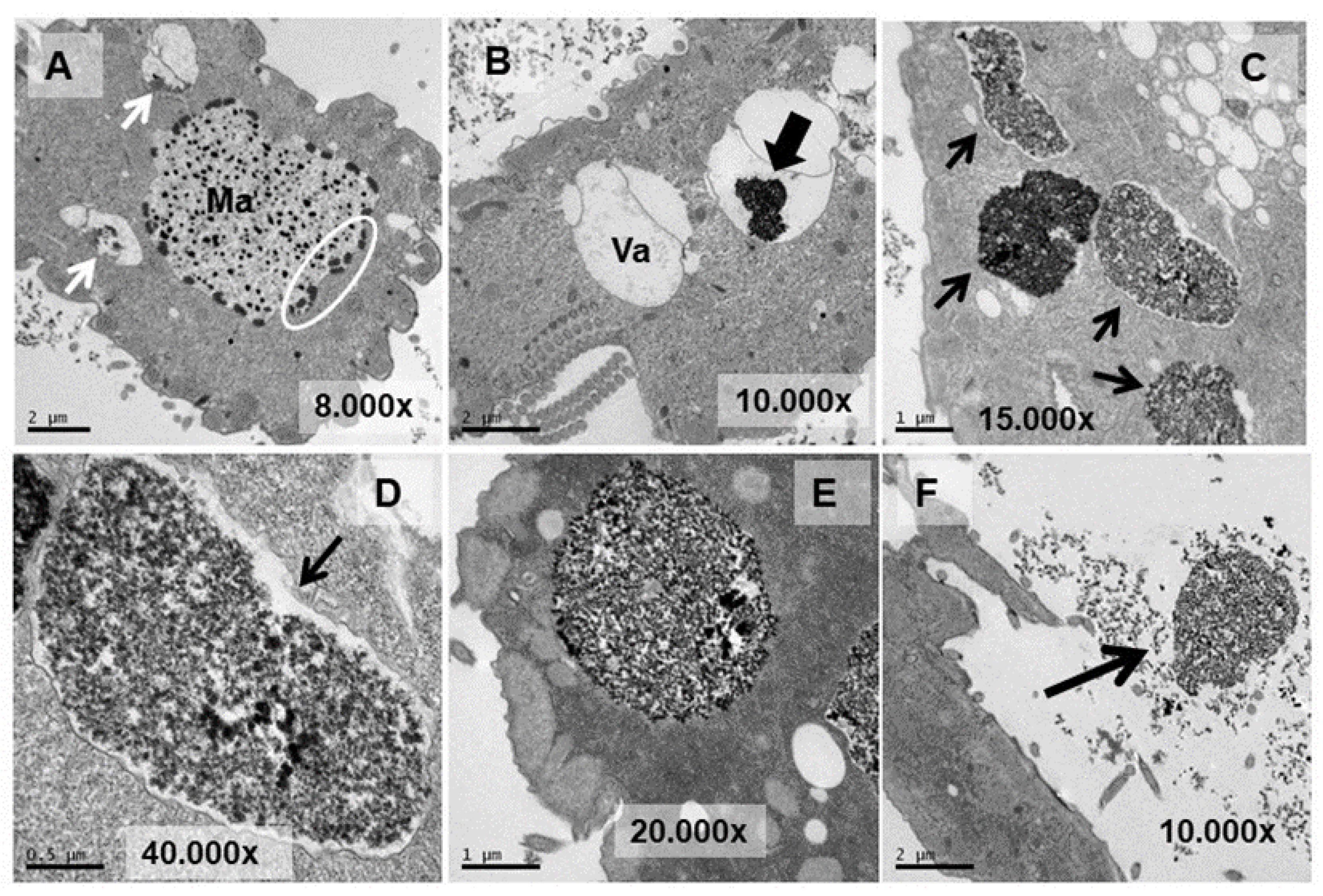
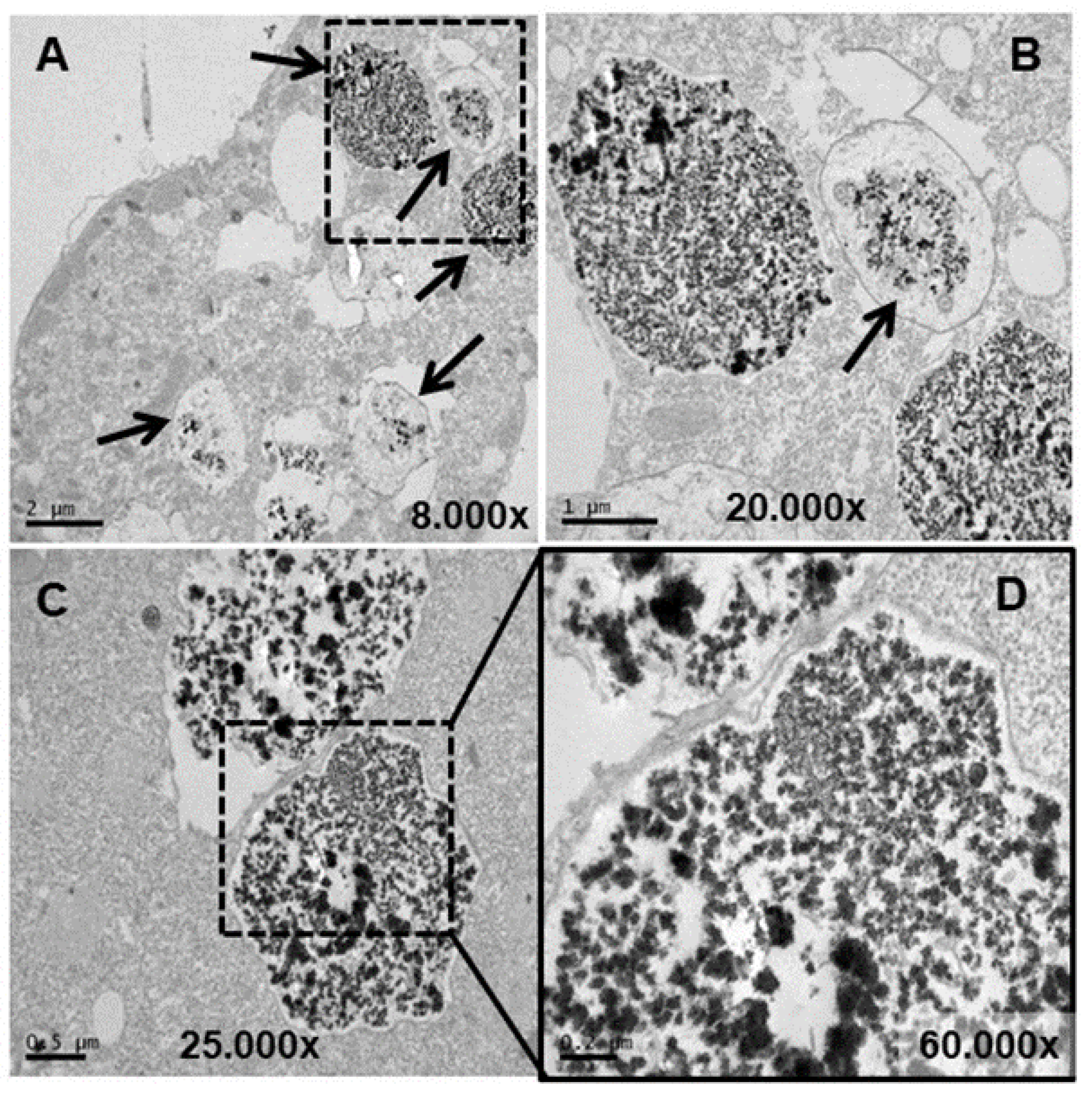
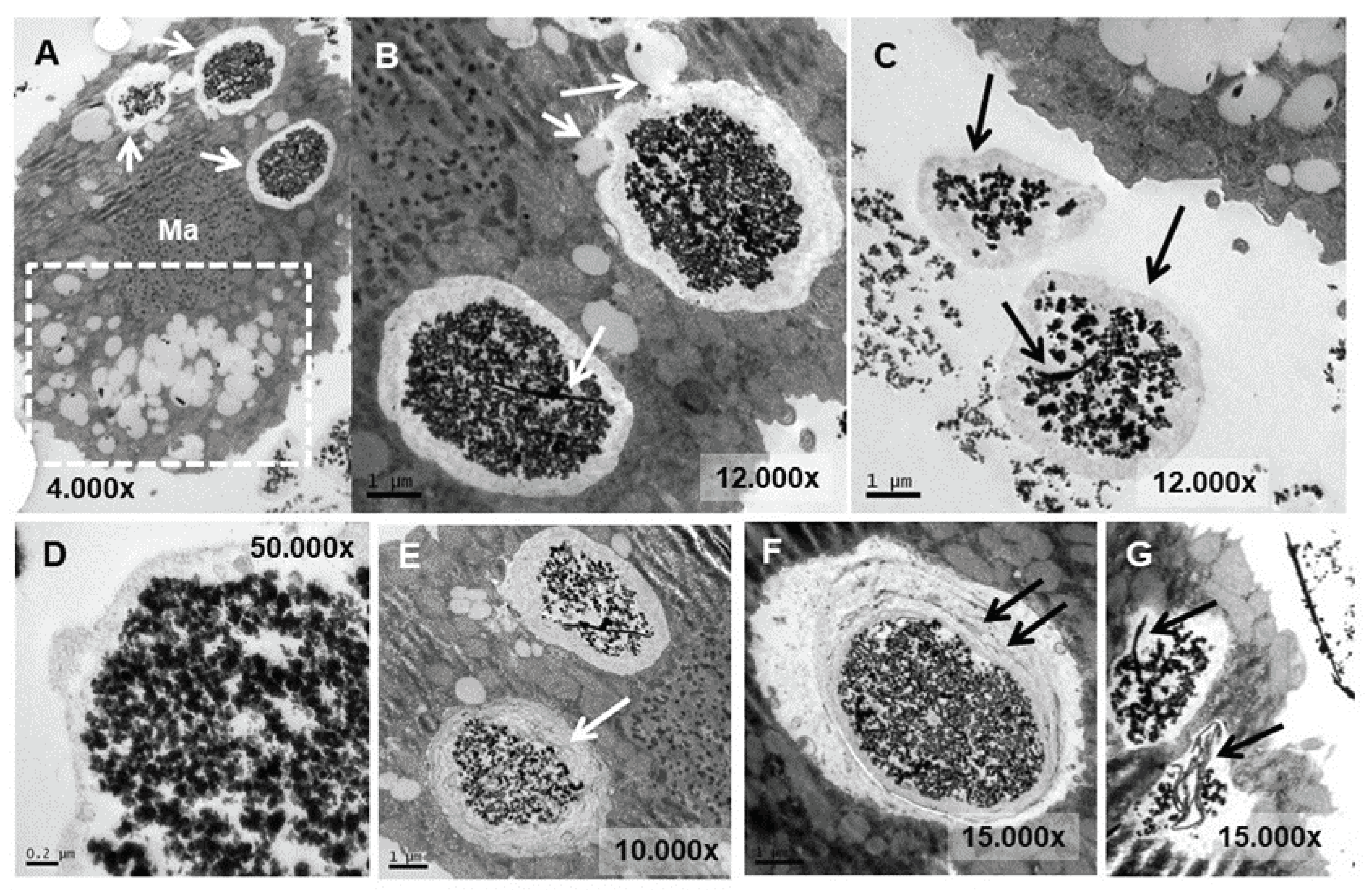
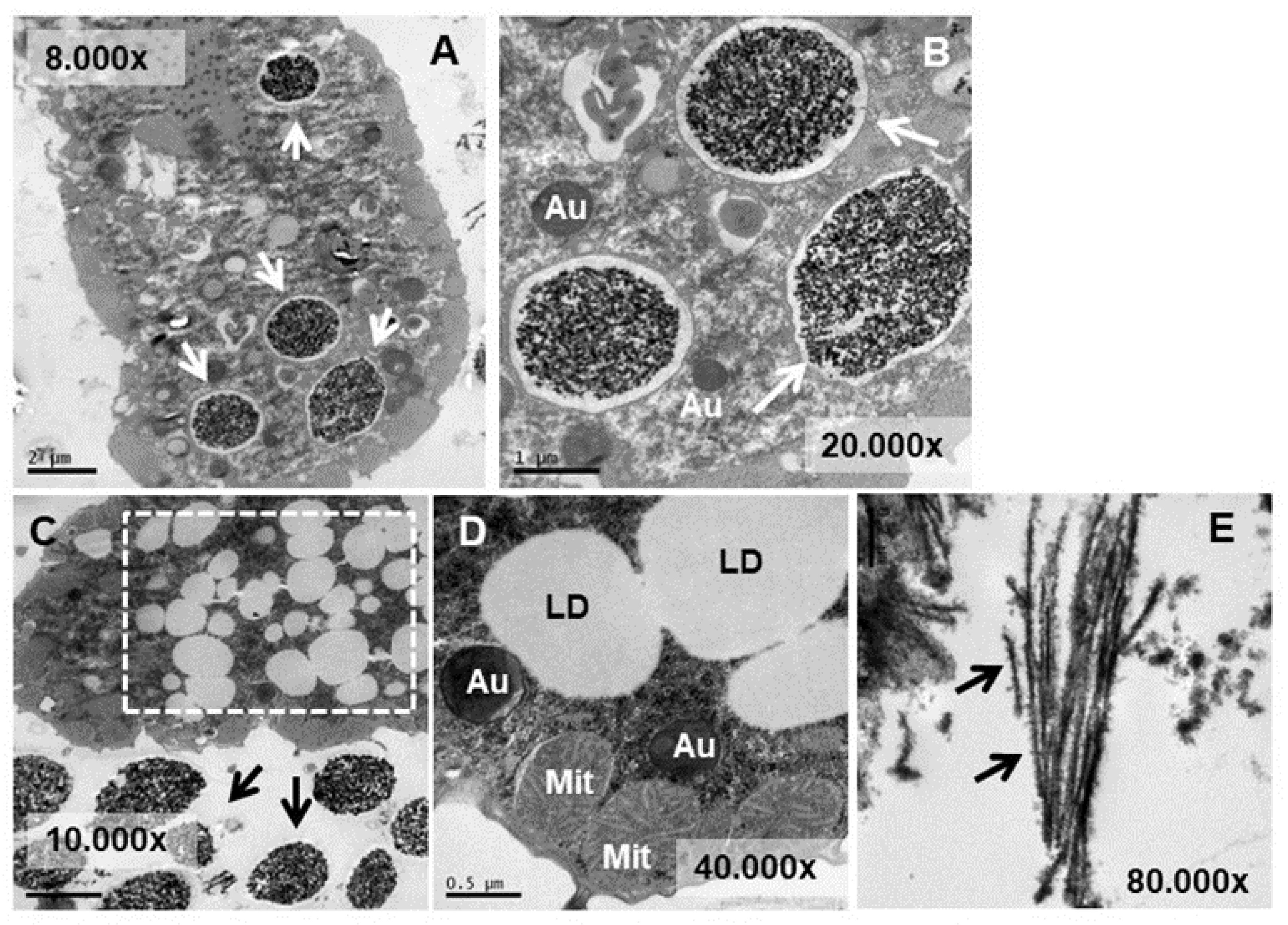
3.4. Ultrastructural Analysis
3.5. Microanalysis (TEM-XEDS)
4. Discussion
4.1. Toxicological and Growth Kinetics Parameters
4.2. Oxidative Stress Assessment
4.3. Expression Analysis of Genes Involved in General and/or Oxidative Stress Cell Response
4.4. Ultrastructural Modifications and Microanalysis
5. Conclusions
- -
- In T. thermophila, EuCl3 is more toxic than Eu2O3. Regardless of it, this microorganism seems to be more resistant to europium compounds than shown by other reported eukaryotic microorganisms.
- -
- Cell adaptation to EuCl3 affects the growth rate of the adapted strain, but does not affect the growth of the Eu2O3-adapted strain.
- -
- The absence of peroxides or hydroxyl radicals after treatment with both Eu(III) compounds could be due to the protection system developed by the ciliate with the intracellular increase of antioxidant enzymes, as confirmed, partially, by the overexpression of the genes encoding them.
- -
- The overexpression of metallothioneins under treatment with Eu(III) compounds supports the possibility that this lanthanide may interact with the -SH groups of the cysteine residues from MTs and/or displace essential cations of these proteins during their homeostasis function.
- -
- Both lipid metabolism and autophagy are involved in the cellular response to europium stress.
- -
- Like other microorganisms, in T. thermophila the main detoxification mechanism of Eu(III) compounds is bioaccumulation into vacuoles and subsequent expulsion out of the cell, probably linked to a biomineralization process to europium phosphate.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blinova, I.; Muna, M.; Heinlaan, M.; Lukjanova, A.; Kahru, A. Potential hazard of lanthanides and lanthanide-based nanoparticles to aquatic ecosystems: data gaps, challenges and future research needs derived from bibliometric analysis. Nanomaterials. 2020, 10, 328. [CrossRef]
- Gonzalez, V.; Vignati, D.A.L.; Leyval, C.; Giamberini, L. ; Environmental fate and ecotoxicology of lanthanides: are they a uniform group beyond chemistry? Environ. Int. 2014, 71, 148–157. [Google Scholar] [CrossRef]
- Gonzalez, V.; Vignati, D.A.L. ; Pons, M-N.; Montarges-Pelletier, E.; Bojic, C.; Giamberini, L. Lanthanide ecotoxicity: first attempt to measure environmental risk for aquatic organisms. Environ. Pollut. 2015, 199, 139-147. [CrossRef]
- Martino, C.; Chianese, T.; Chiarelli, R.; Roccheri, M.C.; Scudiero, R. Toxicological impact of rare earth elements (REEs) on the reproduction and development of aquatic organisms using sea urchins as biological models. Int. J. Mol. Sci. 2022, 23, 2876. [Google Scholar] [CrossRef]
- Pagano, G.; Thomas, P.J.; Nunzio, A.D.; Trifuoggi, M. Human exposures to rare earth elements: present knowledge and research prospects. Environ. Res. 2019, 171, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Cotruvo, Jr. J.A. The chemistry of lanthanides in biology: recent discoveries, emerging principles, and technological applications. ACS. Cent. Sci. 2019, 5, 1496-1506. https://doi.101021/acscentsci.9b00642.
- Bünzli, J-C. G. Lanthanide light for biology and medical diagnosis. J. Lumin. 2016, 170, 866–878. [CrossRef]
- Qin, X.; Wang, J.; Yuan, Q. ; Synthesis and biomedical applications of lanthanides-doped persistent luminescence phosphors with NIR emissions. Front. Chem. 2020, 8: 608578. [CrossRef]
- Syamchand, S.S.; Sony, G. Europium enabled luminescent nanoparticles for biomedical applications. J. Lumin. 2015, 165, 190–215. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, S.; Wang, L. ; Kung, H-T.; Wang, Y.; Hu, A.; Ding, S. Environmental biogeochemical behaviors of rare earth elements in soil–plant systems. Environ. Geochem. Health. 2005, 27, 301–311. [CrossRef]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.A.; Shafiq, M.; Yi, H. , Jilani, G. ; Javed, R.; Horton, R.; Rui, Y.; Tsang, D.C.W.; Xing, B. Cryptic footprints of rare earth elements on natural resources and living organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef]
- Turra, C. Sustainability of rare earth elements chain: from production to food-a review. Int. J. Environ. Health. Res. 2017, 28, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyadoe, E. Sources, behaviour, and environmental and human health risks of high technology rare earth elements as emerging contaminants. Sci. Total Env. 2018, 636, 289–313. [Google Scholar] [CrossRef]
- Tommasi, F.; Thomas, P. J.; Pagano, G.; Perono, G.A.; Oral, R.; Lyons, D.M.; Toscanesi, M.; Trifuoggi, M. Review of rare earth elements as fertilizers and feed additives: a knowledge gap analysis. Arch. Environ. Contam. Toxicol. 2021, 81:531–540. [CrossRef]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological evaluations of rare earths and their health impacts to workers: a literature review. Saf. Health Work. 2013, 4, 12–26. [Google Scholar] [CrossRef]
- Malhotra, N. ; Hsu, H-S.; Liang, S-T.; Roldan, M.J.M.; Lee, J-S.; Ger, T-R.; Hsiao, C-D. An update review of toxicity effect of the rare earth elements (REEs) on aquatic organisms. Animals. 2020, 10, 1663. [CrossRef]
- Kumar, S.; Prakash, R.; Singh, V.K. Synthesis, characterization, and applications of europium oxide: a review. Rev. Adv. Sci. Eng. 2015, 4, 247-257. [CrossRef]
- Silva, R.; Chojnacki, J.; Falcão, E.H.; Alves, S. New coordination polymers based on a V-shaped ligand and lanthanides: Structural description and symmetry-luminescence correlation using europium as a probe. J. Lumin. 2017, 182, 29–38. [Google Scholar] [CrossRef]
- Avila, J.N.; Ireland, T.R.; Lugaro, M.; Gyngard, F.; Zinner, E.; Cristallo, S.; Holden, P.; Rauscher, T. Europium s-process signature at close-to-solar metallicity in stardust sic grains from asymptotic giant branch stars. Astrophysic. J. Lett. 2013, 768:L18. [CrossRef]
- Trifuoggi, M.; Pagano, G.; Guida, M.; Palumbo, A.; Siciliano, A.; Gravina, M.; lyons, D.M.; Burié, P.; Levak, M.; Thomas, P.J.; Giarra, A.; Oral, R. Comparative toxicity of seven rare earth elements in sea urchin early life stages. Enviro. Sci. Poll. Res. 2017, 24, 20803-20810. http://doi.10. 1007. [Google Scholar]
- Bollu, V.S.; Nethi, S.K.; Dasari, R. K.; Rao, S.S.N.; Misra, S.; Patra, C.R. Evaluation of in vivo cytogenetic toxicity of europium hydroxide nanorods (EHNs) in male and female Swiss albino mice. Nanotoxicol. 2016, 10, 413-425. [CrossRef]
- Zhuang, W. Q.; Fitts, J. P.; Ajo-Franklin, C. M.; Maes, S.; Alvarez-Cohen, L.; Hennebel, T. Recovery of critical metals using biometallurgy. Curr. Opin. Biotechnol. 2015, 33, 327-335. [CrossRef]
- Maleke, M.; Valverde, A.; Vermeulen, J. G.; Cason, E.; Gomez-Arias, A.; Moloantoa, K.; Coetsee-Hugo, L.; Swart, H.; Heerden, E.; Castillo, J. Biomineralization and bioaccumulation of europium by a thermophilic metal resistant bacterium. Front. Microbiol. 2019, 10, 1-10. [CrossRef]
- Das, N.; Das, D. Recovery of rare earth metals through biosorption: an overview. J. Rar. Eart. 2013, 31, 933–943. [Google Scholar] [CrossRef]
- Furuhashi, Y.; Honda, R.; Noguchi, M.; Hara-Yamamura, H.; Kobayashi, S.; Higashimine, K.; Hasegawa, H. Optimum conditions of pH, temperatura and preculture for biosorption of europiu by microalgae Acutodesmus acuminatus. Biochen. Engin. J. 2019, 143, 58–64. [Google Scholar] [CrossRef]
- Jena, A.; Pradhan, S.; Mishra, S.; Sahoo, N.K. Evaluation of europium biosorption using Deinococcus radiodurans. Environ. Process. 2021, 8, 251–265. [Google Scholar] [CrossRef]
- Philip, L.; Iyengar, L.; Venkobachar, C. Biosorption of U, La, Pr, Nd, Eu, and Dy by Pseudomonas aeruginosa. J. Ind. Microbiol. Biotechmol. 2000, 25, 1–7. [Google Scholar] [CrossRef]
- Liang, J.; Li, L.; Song, W. Improved Eu(III) immobilization by Cladosporium sphaerospermum induced by low-temperature plasma. J. Radioanal. Nuc. Chem. 2018, 316, 963–970. [Google Scholar] [CrossRef]
- Arunraj, B.; Sathvika, T.; Rajesh, V.; Rajesh, N. Cellulose and Saccharomyces cerevisiae embark to recover europium from phosphor powder. ACS. Omega. 2019, 4, 940-952. [CrossRef]
- Kozai, N.; Skamoto, F.; Tanaka, K.; Ohnuki, T.; Satoh, T.; Kamiya, T.; Grambow, B. Complexation of Eu(III), Pb(II) and U(VI) with a Paramecium glycoprotein: microbial transformation of heavy elements in the aquatic environment. Chemosphere. 2018, 196, 135–144. [Google Scholar] [CrossRef]
- Bonnet, J-L. ; Dusser, M.; Bohatier, J.; Laffosse, J. Cytotoxicity assessment of three therapeutic agents, cyclosporine-A, cisplatin and doxorubicin, with the ciliated protozoan Tetrahymena pyriformis. Res. Microbiol. 2003, 154, 375–385 https://. [CrossRef]
- Diaz, S.; Martin-Gonzalez, A.; Gutierrez, J.C. Evaluation of heavy metal acute toxicity and bioaccumulation in soil ciliated protozoa. Environ. Int. 2006, 32, 711-717. [CrossRef]
- Diaz, S.; Martin-Gonzalez, A.; Cubas, L.; Ortega, R.; Amaro, F.; Rodriguez-Martin, D.; Gutierrez, J.C. High resistance of Tetrahymena thermophila to paraquat: mitocondrial alterations, oxidative stress and antioxidant genes expression. Chemosphere. 2016, 144, 909–917. [Google Scholar] [CrossRef]
- Gutierrez, J.C.; Martin-Gonzalez, A.; Diaz, S.; Ortega, R. Ciliates as a potential source of cellular and molecular biomarkers/biosensors for heavy metal pollution. Eu. J. Protistol. 2003, 39, 461–467. [Google Scholar] [CrossRef]
- Nilsson, L.R. 1989. Dose- and pH-dependent effects of chloroquine on Tetrahymena. Eu. J. Protistol. 1989, 24, 297–307. [Google Scholar] [CrossRef]
- Romero, I.; De Francisco, P.; Gutierrez, J.C.; Martin-Gonzalez, A. Selenium cytotoxicity in Tetrahymena thermophila: new clues about its biological effects and cellular resistance mechanisms. Sci. Total Environ. 2019, 671, 850–865. [Google Scholar] [CrossRef]
- Sauvant, N.P.; Pepin, D.; Piccinni, E. Tetrahymena pyriformis: a tool for toxicological studies. A review. Chemosphere. 1999, 38, 1631–1669. [Google Scholar] [CrossRef]
- Eisen, J.A.; Coyne, R.S.; Wu, M.; Wu, D.; Thiagarajan, M.; et al. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol. 2006, 4(9): e286. [CrossRef]
- De Francisco, P.; Martin-Gonzalez, A.; Turkewitz, A.P.; Gutierrez, J.C. Genome plasticity in response to stress in Tetrahymena thermophila: selective and reversible chromosome amplification and paralogous expansion of metallothionein genes. Environ. Microbiol. 2018, 20, 2410-2421. [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martin, D.; Murciano, A.; Herraiz, M.; De Francisco, P.; Amaro, F.; Gutierrez, J.C.; Martin-Gonzalez, A.; Diaz, S. Arsenate and arsenite differential toxicity in Tetrahymena thermophila. J. Hazard Mater. 2022, 431, 128532. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Johnson, L.A.; Yue, S.T.; Roth, B.L.; Haugland, R.P. Dual DNA staining assessment of bovine sperm viability using SYBR-14 and propidium iodide. J. Androl. 1994, 15, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flowcytometry. Methods Mol. Biol. Humana Press Inc. 2010, pp. 57-72. [CrossRef]
- Cubas-Gaona, L.L.; De Francisco, P.; Martin-Gonzalez, A.; Gutierrez, J.C. Tetrahymena glutathione peroxidase family: a comparative analysis of these antioxidant enzymes and differential gene expression to metals and oxidizing agents. Microorganisms. 2020, 8, 1008. [CrossRef]
- Dentler, W. Fixation of Tetrahymena cells for electron microscopy. Methods Cell Biol. 2000, 62, 323-331. [CrossRef]
- Borges, A.; Tsai, S.M.; Gomes-Caldas, D.G. Validation of reference genes for RT-qPCR normalization in common bean during biotic and abiotic stresses. Plant Cell Rep. 2012, 31, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Larionov, A.; Krause, A.; Miller, W. A standard curve-based method for relative real time PCR data processing. BMC Bioinf. 2005, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.C.; Amaro, F.; Diaz, S.; De Francisco, P.; Cubas, L.L.; Martin-Gonzalez, A. Ciliate metallothioneins: unique microbial eukaryotic heavy-metal-binder molecules. J. Biol. Inorg. Chem. 2011, 16, 1025-1034. https://doi.10. 1007. [Google Scholar]
- Díaz, S.; Amaro, F.; Rico, D.; Campos, V.; Benítez, L.; Martín-González, A.; Hamilton, E.P.; Orias, E.; Gutiérrez, J.C. Tetrahymena metallothioneins fall into two discrete subfamilies. PLoS One 2007, 2 (3), e291. [CrossRef]
- Gallego, A.; Martin-Gonzalez, A.; Ortega, R.; Gutierrez, J.C. Flow cytometry assessment of cytotoxicity and reactive oxygen species generation by single and binary mixtures of cadmium, zinc and copper on populations of the ciliated protozoon Tetrahymena thermophila. Chemosphere. 2007, 68, 647-661. [CrossRef]
- Pallares, R.M.; An, D.D.; Hebert, S.; Faulkner, D.; Loguinov, A.; Proctor, M.; Villalobos, J.A.; Bjornstad, k.A.; Rosen, C.J.R.; Vulpe, C.; Abergel, R.J. Multidimensional genome-wide screening in yeast provides mechanistic insights into europium toxicity. Metallomics. 2021, 13, mfab061. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Zhao, Q.; Su, D.; Li, P.; Stagnitti, F. Biological toxicity of lanthanide elements on algae. Chemosphere. 2010, 80, 1031-1033. [CrossRef]
- Kurvet, I.; Juganson, K.; Vija, H.; Sihtmae, M.; Blinova, I.; Syvertsen-Wiig, G.; Kahru, A. Toxicity of nine (doped) rare earth metal oxides and respective individual metals to aquatic microorganisms Vibrio fischeri and Tetrahymena thermophila. Materials. 2017, 10, 754. [CrossRef]
- Wang, Y.; Zhang, M.; Wang, X. Population growth responses of Tetrahymena shanghaiensis in exposure to rare earth elements. Biol. Trace Elem. Res. 2000, 75, 265-275. [CrossRef]
- Maleke, M.; Valverde, A.; Gomez-Arias, A.; Cason, E.D. ; Vermeulen, J-G. ; Coetsee-Hugo, L.; Swart, H.; van Heerden, E.; Castillo, J. Anaerobic reduction of europium by a Clostridium strain as a strategy for rare earth biorecovery. Scin. Rep. 2019, 9, 14339. [Google Scholar] [CrossRef]
- Zhao, H.; Osborne, O.J.; Lin, S.; Ji, Z.; Damoiseux, R.; Wang, Y.; Nel, A.E.; Lin, S. Lanthanide hydroxide nanoparticles induce angiogenesis via ROS-sensitive signaling. Small. 2016, 12, 4404-4411. [CrossRef]
- Brown, M.B.; Forsythe, A.B. Robust tests for the equality of variances. J. Amer. Statis. Assoc. 1974, 69, 364-367. [CrossRef]
- Gutierrez, J.C.; Martin-Gonzalez, A.; Diaz, S.; Amaro, F.; Ortega, R.; Gallego, A.; De Lucas, M.P. Ciliates as cellular tools to study the eukaryotic cell-heavy metal interactions. In Heavy Metal Pollution. Nova Science Publishers, Inc. 2008, pp. 1-44. ISBN: 978-1-60456-899-8.
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J. ; Miller, A-F. ; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, H.; Jiang, C. Spectrofluorimetric determination of superoxide dismutase using a europium-tetracycline probe. Spectrochim. Acta Part A. 2008, 70, 389-393. [CrossRef]
- De Francisco, P.; Melgar, L.M.; Diaz, S.; Martin-Gonzalez, A.; Gutierrez, J.C. The Tetrahymena metallothionein gene family: twenty-one new cDNAs, molecular characterization, phylogenetic study and comparative analysis of the gene expression under different abiotic stressors. BMC Genomics. 2016, 17:346. [CrossRef]
- De Francisco, P.; Martin-Gonzalez, A.; Turkewitz, A.P.; Gutierrez, J.C. Extreme metal adapted, knockout and knockdown strains reveal a coordinated gene expression among different Tetrahymena thermophila metallothionein isoforms. PloS One. 2017, 12(12):e0189076. [CrossRef]
- Wang, Q.; Xu, J.; Zhu, Y.; Chai, B.; Liang, A.; Wang, W. Lanthanum (III) impacts on metallothionein MTT1 and MTT2 from Tetrahymena thermophila. Biol. Trace Elem. Res. 2011, 143, 1808-1818. [CrossRef]
- Geng, M-J.; Liang, S-X.; liu, W.; Jin, Y. Quantification of metallothioneins in the earthworm by lomefloxacin-europium(III) fluorescent probe. Environ. Sci. Processes Impacts. 2014, 16, 1923-1929. [CrossRef]
- Silber, H.B.; Paquette, S.J. Complexes of lanthanide ions with amino acids, nucleotides, and other ligands of biological interest in solution. In Metal ions in biological systems. The lanthanides and their Interrelations with biosystems. 2003, Vol. 40. pp. 799. Ed. Sigel, A, and Sigel, H. CRC Press. 9: ISBN 13, 8247. [Google Scholar]
- Lafita-Navarro, M.C.; Conacci-Sorrell, M. Nucleolar stress: from development to cancer. Semin. Cell Develop. Biol. 2023, 136, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M. New insights into connection of nucleolar functions and cancer. Tanaffos. 2019, 18, 173-179. ISSN: 1735-0344.
- Weeks, S.; Metge, B.J.; Samant, R.S. The nucleolus: a central response hub for the stressors that drive cancer progression. Cell. Mol. Life Sci. 2019, 76, 4511–4524. [Google Scholar] [CrossRef]
- Neuburger, M.; Herget, G.W.; Plaumann, L.; Falk, A.; Schwab, H. ; Adler, C-P. Change in size, number, and morphology of the nucleoli in human hearts as a result of hyperfunction. Pathol. Res. Pract. 1998, 194, 385-390. [CrossRef]
- MacMillan, G.A.; Clayden, M.G.; Chetelat, J.; Richardson, M.C.; Ponton, D.E.; Perron, T.; Amyot, M. Environmental drivers of rare earth element bioaccumulation in freshwater zooplankton. Environ. Sci. Technol. 2019, 53, 1650-1660. [CrossRef]
- Henne, W.M.; Reese, M.L.; Goodman, J.M. 2018. The assembly of lipid droplets and their roles in challenged cells. EMBO J. 2018, 37:e98947. [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. 2009. Autophagy regulates lipid metabolism. Nature. 2009, 458,1131-1135. [CrossRef]
- Cui, L.; Liu, P. Two types of contact between lipid droplets and mitochondria. Front. Cell. Dev. Biol. 2020, 8:618322. [CrossRef]
- Amen, T.; Kaganovich, D. Dynamic droplets: the role of cytoplasmic inclusions in stress, function, and disease. Cell. Mol. Life Sci. 2015, 72, 401-415. [CrossRef]
- Rajakumar, S.; Nachiappan, V. Lipid droplets alleviate cadmium-induced cytotoxicity in Saccharomyces cerevisiae. Toxicol. Res. 2017, 6, 30-41. [CrossRef]
- Kennedy, D.C.; Lyn, R.K.; Pezacki, J.P. Cellular lipid metabolism is influenced by the coordination environment of copper. J. Am. Chem. Soc. 2009, 131, 2444–2445. [Google Scholar] [CrossRef] [PubMed]
- Moron, A.; Martin-Gonzalez, A.; Diaz, S.; Gutierrez, J.C.; Amaro, F. Autophagy and lipid droplets are a defense mechanism against toxic copper oxide nanotubes in the eukaryotic microbial model Tetrahymena thermophila. Sci. Total Environ. 2022, 847, 157580. [Google Scholar] [CrossRef]
- Jiang, M.; Ohnuki, T.; Utsunomiya, S. Biomineralization of middle rare earth element samarium in yeast and bacteria systems. Geomicrobiol. J. 2018, 35, 375–384. [Google Scholar] [CrossRef]
- Nadella, S.; Sahoo, J.; Subramanian, P.S.; Sahu, A.; Mishra, S.; Albrecht, M. Sensing of phosphates by using luminescent Eu(III) and Tb(III) complexes: application to the microalgal cell Chlorella vulgaris. Chem. Eur. J. 2014, 20, 1-8. [CrossRef]
- Jiang, M.; Ohnuki, T.; Kozai, N.; Tanaka, K.; Suzuki, Y.; Sakamoto, F.; Kamiishi, E.; Utsunomiya, S. Biological nano-mineralization of Ce phosphate by Saccharomyces cerevisiae. Chem. Geol. 2010, 277, 61-69. [CrossRef]
- Jiang, M.; Ohnuki, T.; Kozai, N.; Tanaka, K.; Kozai, N.; Kamiishi, E.; Utsunomiya, S. Post-adsorption process of Yb phosphate nano-particle formation by Saccharomyces cerevisiae. Geochim. Cosmochim. Acta. 2012, 93, 30-46. [CrossRef]
- De Francisco, P.; Amaro, F.; Martin-Gonzalez, A.; Serrano, A.; Gutierrez, J.C. Quantitative proteomic analyses of a Pb-adapted Tetrahymena thermophila strain reveal the cellular strategy to Pb(II) stress including lead biomineralization to chloropyromorphite. Sci. Total Environ. 2023, 891, 164252. http://dx.doi.org710.1016/j.scitotenv.2023. 1642. [Google Scholar]

| Medium | EuCl3 (μM) | Eu2O3 (μM) |
|---|---|---|
| Tris-HCl | 127.93 | 173.32 |
| PP210 | 4830.68 | 7916.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




