1. Introduction
Hydrophobic drugs and nucleic acid vectors used in therapeutics face significant challenges, including low stability, in vivo degradation, and difficulties in reaching the target site [
1]. Consequently, a robust delivery system is essential to transport these agents safely and efficiently. In recent years, polymeric micelles have emerged as highly effective cargo carriers, owing to their biocompatibility, facile preparation, tunable size and shape, prolonged stability in blood circulation, and enhanced in vivo retention at the target site [
2,
3,
4,
5,
6]. Typically, polymeric micelles consist of pre-synthesized diblock copolymer chains, comprising a hydrophilic and a hydrophobic block, or a mixture of double hydrophilic diblock copolymers with neutral and oppositely charged blocks, that become hydrophobic upon complexation [
7,
8]. The hydrophobic segments form the micelle core, while the hydrophilic ones create the surrounding corona. These micelles are derived from dilute polymer concentrations exceeding the critical micelle concentration (CMC). Recently, innovative assembly models (PISA and PIESA) have allowed micellization at high monomer concentrations, combining diblock copolymer synthesis and micellization in a single process [
9,
10]. The coassembly of cargo molecules with copolymer chains results in the encapsulation of hydrophobic cargo within the micelle core due to the hydrophobic interactions with the solvent.
Smart polymeric micelles, responsive to the microenvironment of the target site, can be designed by incorporating stimuli-responsive switches either assembled into micelles or covalently grafted onto copolymer chains. These switches, responsive to pH, light, heat, redox reactions, and enzymes, facilitate efficient drug delivery [
11,
12,
13]. However, challenges may arise in achieving complete cargo release due to the incomplete dissociation of the micelle. Polymeric micelle carriers with degradable copolymer chains have gained attention for offering controlled yet complete release of cargo molecules and preventing the accumulation of micelles at the target point [
14,
15,
16].
In a noteworthy study Kim et. al. designed amphiphiles based on poly(benzyl-ether) capable of selective demicellization through head-to-tail depolymerization triggered by hydrophobic fluolid molecules [
17]. A labile end-capping unit attached to the end of hydrophobic block is buried inside the core of the micelle, preventing detachment of the labile units and rendering the demicellization signal specific. Fluolid molecules trigger the detachment of the end-capping unit in the hydrophobic block, after which the entire chain spontaneously and continuously depolymerizes in a head-to-tail manner without the need for additional stimuli. Loading doxorubicin inside the micelles led to their molecular-level degradation, resulting in controlled and complete release of the cargo molecules.
The depolymerization-induced disassembly mechanism has primarily been explored for amphiphiles with a linear chain architecture carrying one labile end cap unit. The design of amphiphiles with a different architecture, bearing more labile end cap units, can further enhance the control of depolymerization and the cargo release, which is useful for biological and environmental applications. Due to the lack of such studies, we conducted coarse-grained molecular dynamics simulations of micelles formed by a) Linear AB diblock copolymer with one hydrophilic (A type) and one hydrophobic (B type) block, featuring one end cap unit. b) Miktoarm star copolymer A(B)2 with one hydrophilic and two hydrophobic branches, equipped with two end cap units. c) Miktoarm star copolymer A(B)3 with three hydrophobic branches and three end cap units. We calculated the mass distributions of micelles formed by different copolymers before and after cargo loading, the kinetics of the head-to-tail depolymerization of hydrophobic moieties and the kinetics of cargo release triggered by hydrophobic small molecules.
4. Conclusions
To elucidate the impact of copolymer architecture on demicellization and cargo release through head-to-tail depolymerization triggered by specific stimuli, we conducted comprehensive molecular dynamics simulations. The depolymerization reaction involved two distinct steps: (a) the rapture of bonds between the hydrophobic end cap and B-type beads, initiated by the presence of T-type molecules with a predefined reaction probability (RPT); (b) depolymerization in the absence of external stimuli, with reaction probability (RPB). Our investigation focused on linear A30B30 and miktoarm star A30(B15)2 and A30(B10)3 copolymers, featuring one, two and three hydrophobic arms (and end caps) respectively. We explored these copolymers under constant and stoichiometric concentrations of trigger molecules relative to end cap beads, examining a range of RPT and RPB values. Our findings revealed that the preferential aggregation number of micelles (Np) was higher for linear copolymers and decreased as the number of arms in miktoarm copolymers increased. Interestingly, copolymer architecture did not impact the loading capacity of micelles (LC). However, for LC=7.6%, loaded micelles exhibited an increase of approximately 16%, 30%, and 35% in Np, and a 9%, 16%, and 16% rise in the radius of gyration for the A30B30, A30(B15)2, and A30(B10)3 copolymers respectively. The rate of depolymerization of hydrophobic beads under stoichiometric T-type molecules concentration was consistently faster in A30(B10)3 followed by A30(B15)2 and A30B30 mixtures. For constant trigger molecule concentrations, the depolymerization rate depended on two opposite factors: the excess of trigger molecules, which increased as the number of hydrophobic branches or blocks decreased, and simultaneous head-to-tail depolymerization, which intensified with an increasing number of branches. Initially, at low break fractions, A30B30 exhibited faster depolymerization, followed by A30(B15)2 and A30(B10)3 mixtures. For higher break fractions, the order is reversed, depending on RPT and RPB values. Generally, cargo release rates mirrored copolymer depolymerization rates. However, the fraction of cargo molecules released was not directly proportional to the fraction of depolymerized B-type beads. For a 0.60 degradation of hydrophobic beads, the fraction of cargo release was approximately 0.34, 0.39 and 0.43 for A30B30, A30(B15)2 and A30(B10)3, respectively, at both stoichiometric and constant trigger molecule concentrations with RPT=10-4 and RPB=10-3. Utilizing the non-linear Korsmeyer-Peppas equation, our results strongly suggest that the release of cargo molecules from degradable micelles follows a non-Fickian pattern, where both diffusion and corrosion mechanisms contribute to the release process. These insights may inform the design of copolymers chains with more triggering groups, proving valuable in the development of delivery vehicles for bio and environmental applications.
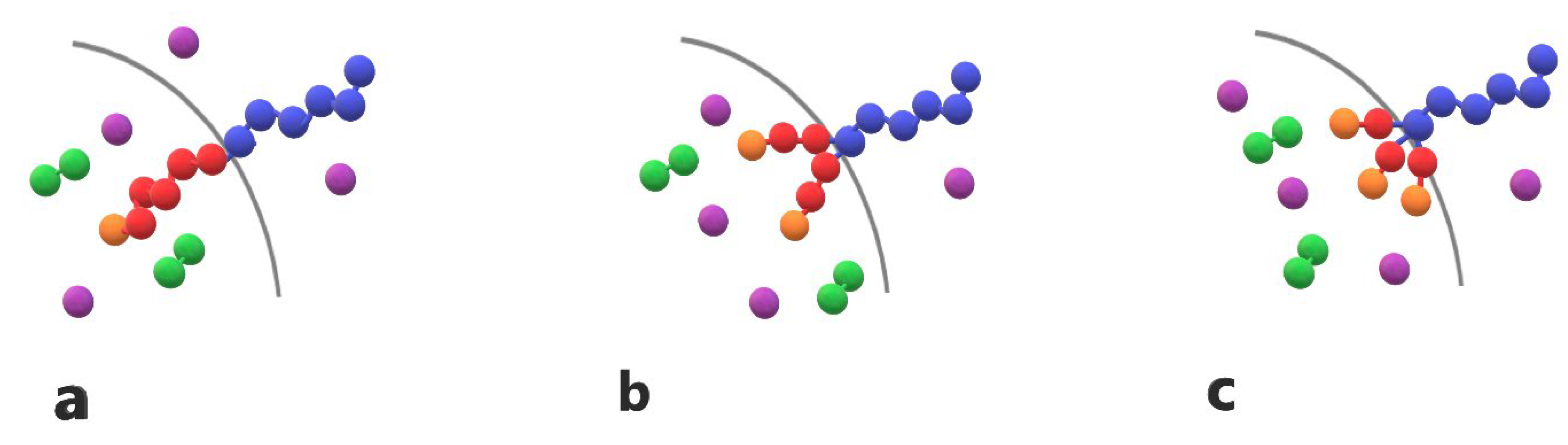
Figure 1.
Cartoon representation of (a) linear diblock copolymer A6B6 (b) miktoarm star copolymer A6(B3)2 (c) miktoarm star copolymer A6(B2)3 with one, two and three end cap beads (orange). T-type trigger (purple) and cargo (green) C2 molecules are also presented.
Figure 1.
Cartoon representation of (a) linear diblock copolymer A6B6 (b) miktoarm star copolymer A6(B3)2 (c) miktoarm star copolymer A6(B2)3 with one, two and three end cap beads (orange). T-type trigger (purple) and cargo (green) C2 molecules are also presented.
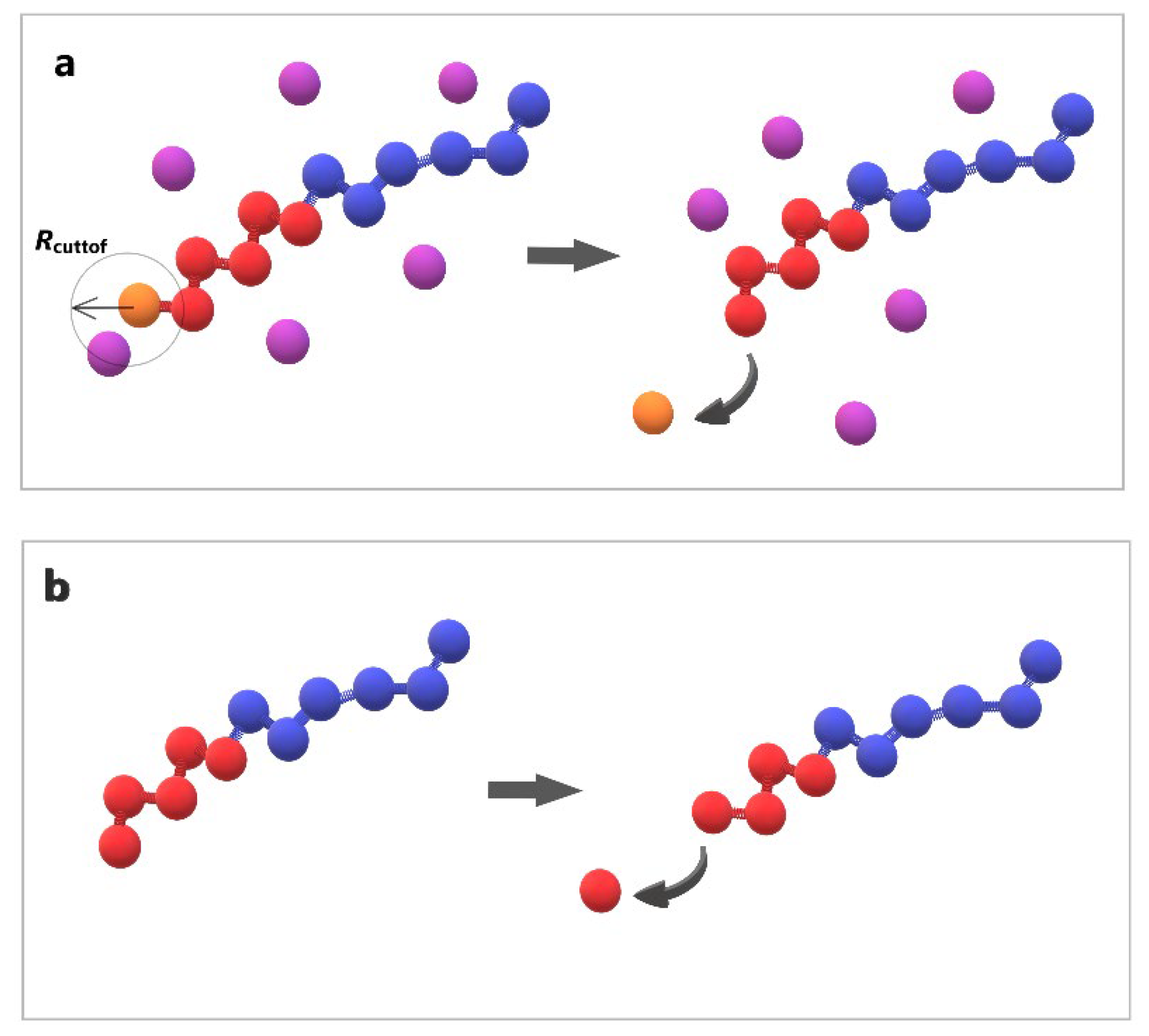
Figure 2.
Cartoon representation of depolymerization algorithm (a) the breaking of bond between end cap (orange) and B-type bead (red) triggered by T-type molecule (purple) (b) the head-to-tail breaking of bond between two B-type beads (red) without stimuli.
Figure 2.
Cartoon representation of depolymerization algorithm (a) the breaking of bond between end cap (orange) and B-type bead (red) triggered by T-type molecule (purple) (b) the head-to-tail breaking of bond between two B-type beads (red) without stimuli.
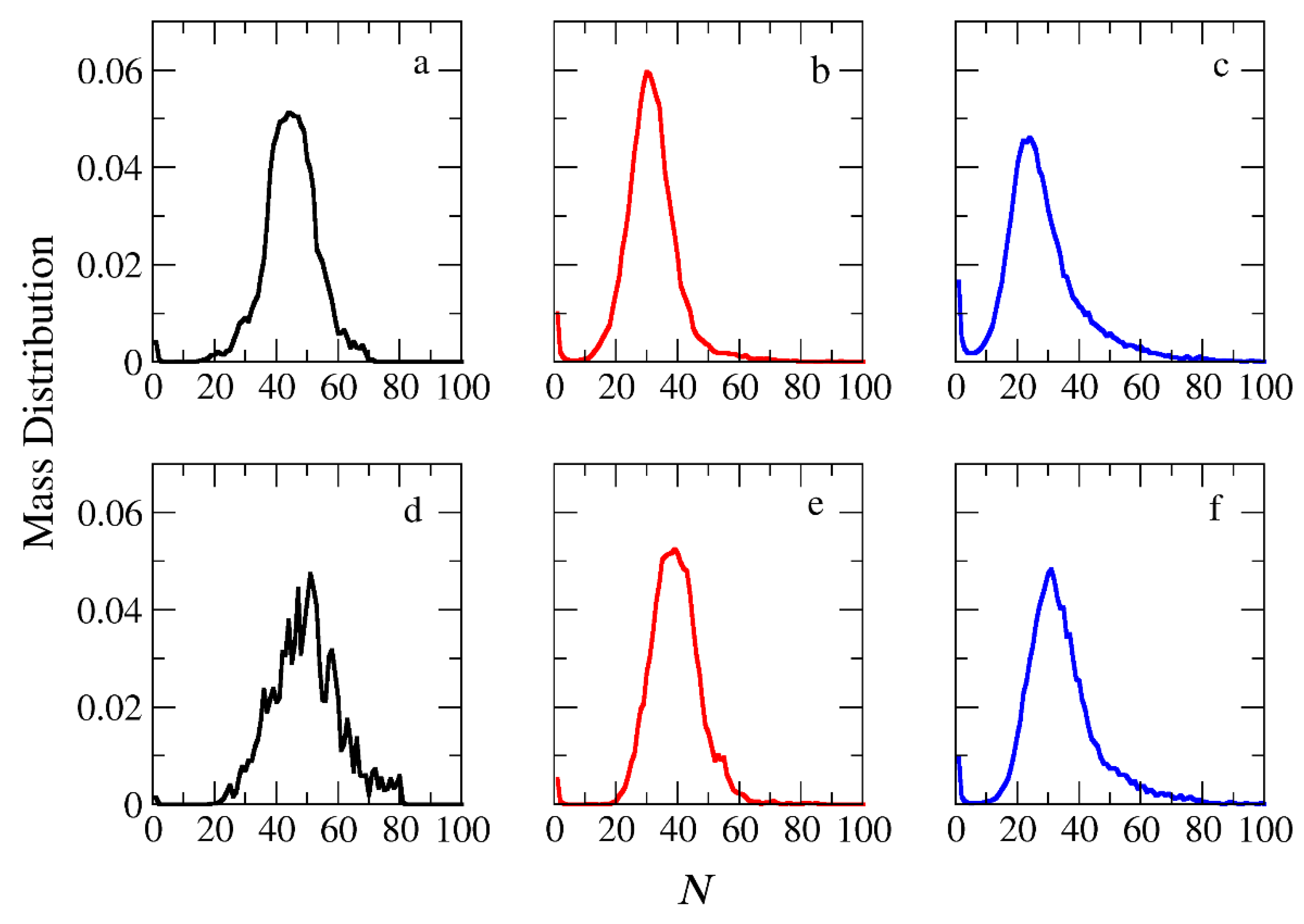
Figure 3.
Mass distribution of micelles as a function of the aggregation number N formed by (a) A30B30, (b) A30(B15)2, and (c) A30(B10)3 copolymers. ibid for mixtures: (d) A30B30 + C3, (e) A30(B15)2 + C3 and (f) A30(B10)3 + C3. In all simulations, [Φ]=0.12.
Figure 3.
Mass distribution of micelles as a function of the aggregation number N formed by (a) A30B30, (b) A30(B15)2, and (c) A30(B10)3 copolymers. ibid for mixtures: (d) A30B30 + C3, (e) A30(B15)2 + C3 and (f) A30(B10)3 + C3. In all simulations, [Φ]=0.12.
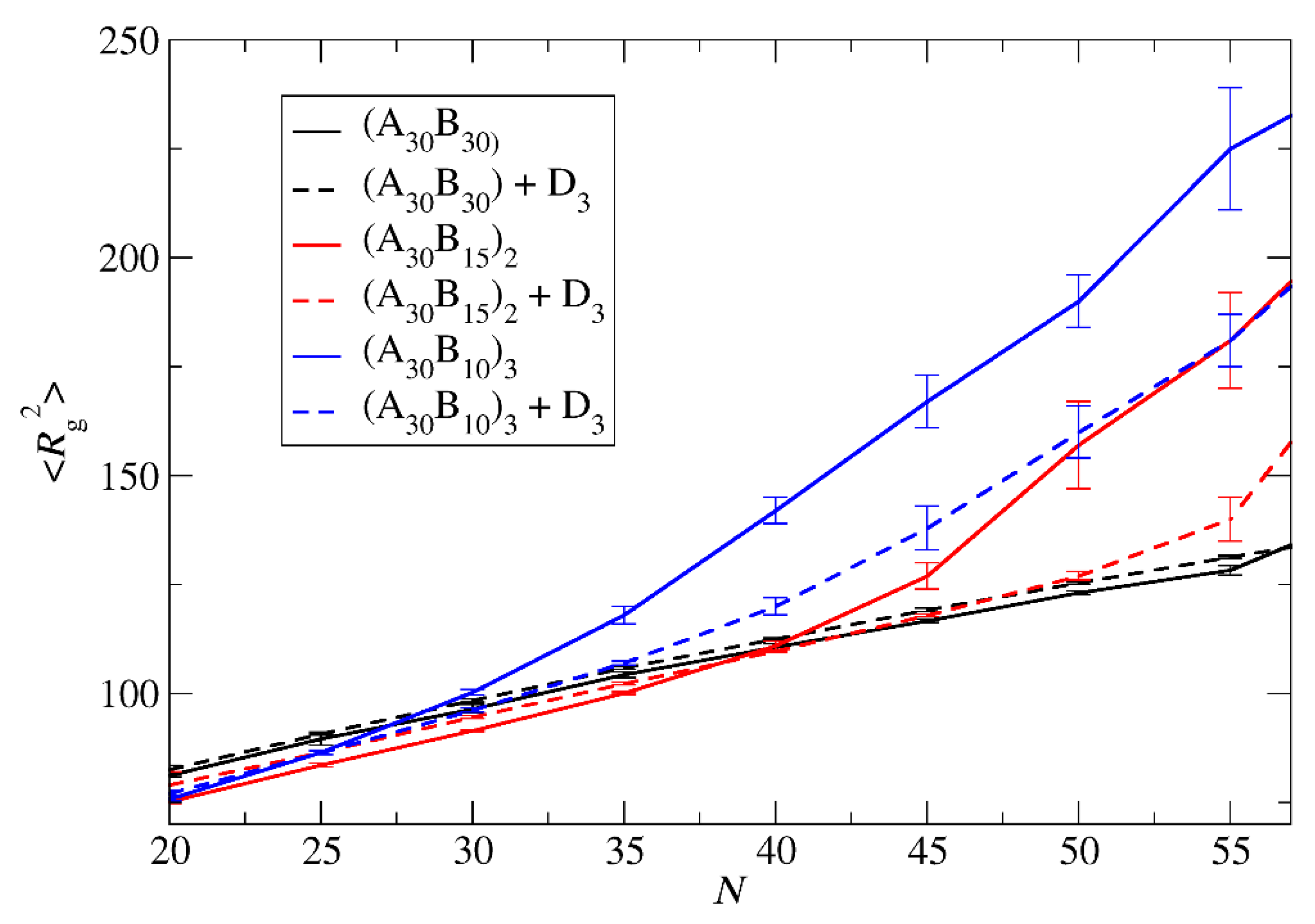
Figure 4.
The mean squared radius of gyration a) for ‘empty’ micelles formed by A30B30, A30(B15)2, and A30(B10)3 copolymers and b) for micelles formed by mixtures with 2000 C3 cargo molecules, plotted against N. Standard deviation is indicated by the error bars.
Figure 4.
The mean squared radius of gyration a) for ‘empty’ micelles formed by A30B30, A30(B15)2, and A30(B10)3 copolymers and b) for micelles formed by mixtures with 2000 C3 cargo molecules, plotted against N. Standard deviation is indicated by the error bars.
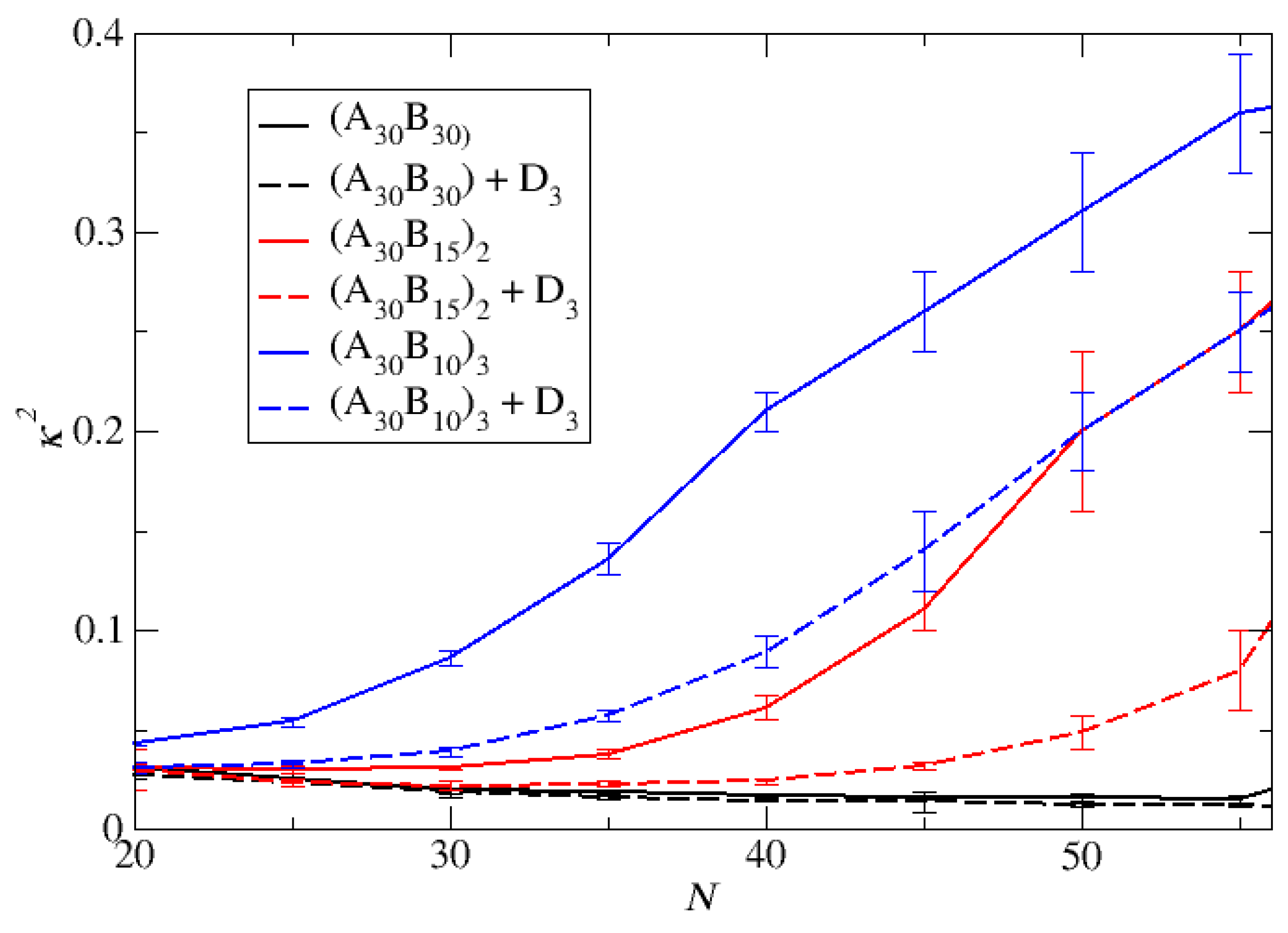
Figure 5.
The mean shape anisotropy parameter κ2 a) for ‘empty’ micelles formed by A30B30, A30(B15)2, and A30(B10)3 copolymers and b) for micelles formed by mixtures with 2000 C3 cargo molecules, plotted against N. Standard deviation is indicated by the error bars.
Figure 5.
The mean shape anisotropy parameter κ2 a) for ‘empty’ micelles formed by A30B30, A30(B15)2, and A30(B10)3 copolymers and b) for micelles formed by mixtures with 2000 C3 cargo molecules, plotted against N. Standard deviation is indicated by the error bars.
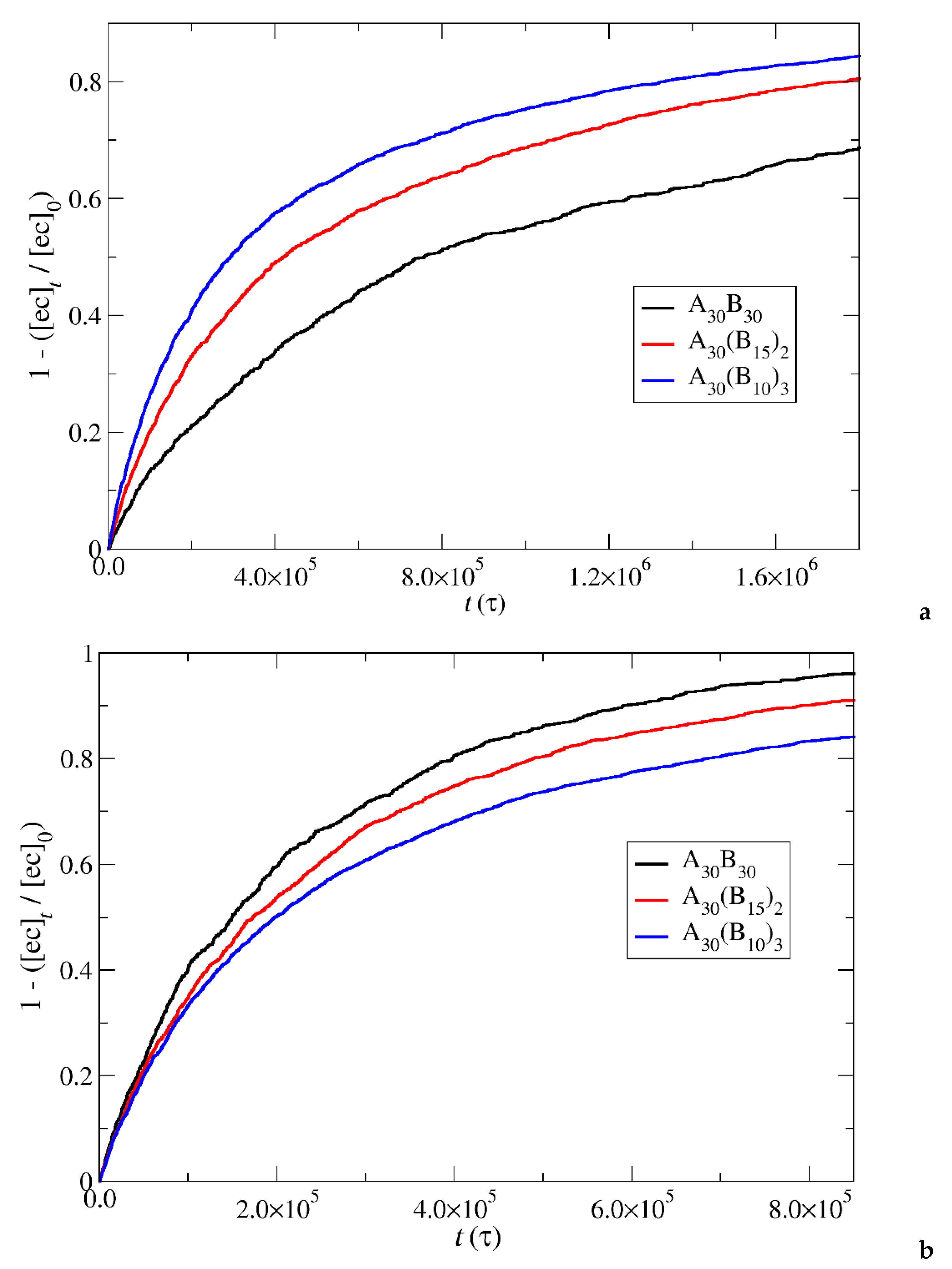
Figure 6.
Depolymerization fraction of end cap beads of A30B30, A30(B15)2, and A30(B10)3 copolymers as a function of time for a) stoichiometric trigger molecules concentration, b) constant trigger molecules concentration. RPT=10-4, RPB=10-3. [ec]0 is the initial end cap beads concentration, [ec]t is the end cap concentration.
Figure 6.
Depolymerization fraction of end cap beads of A30B30, A30(B15)2, and A30(B10)3 copolymers as a function of time for a) stoichiometric trigger molecules concentration, b) constant trigger molecules concentration. RPT=10-4, RPB=10-3. [ec]0 is the initial end cap beads concentration, [ec]t is the end cap concentration.
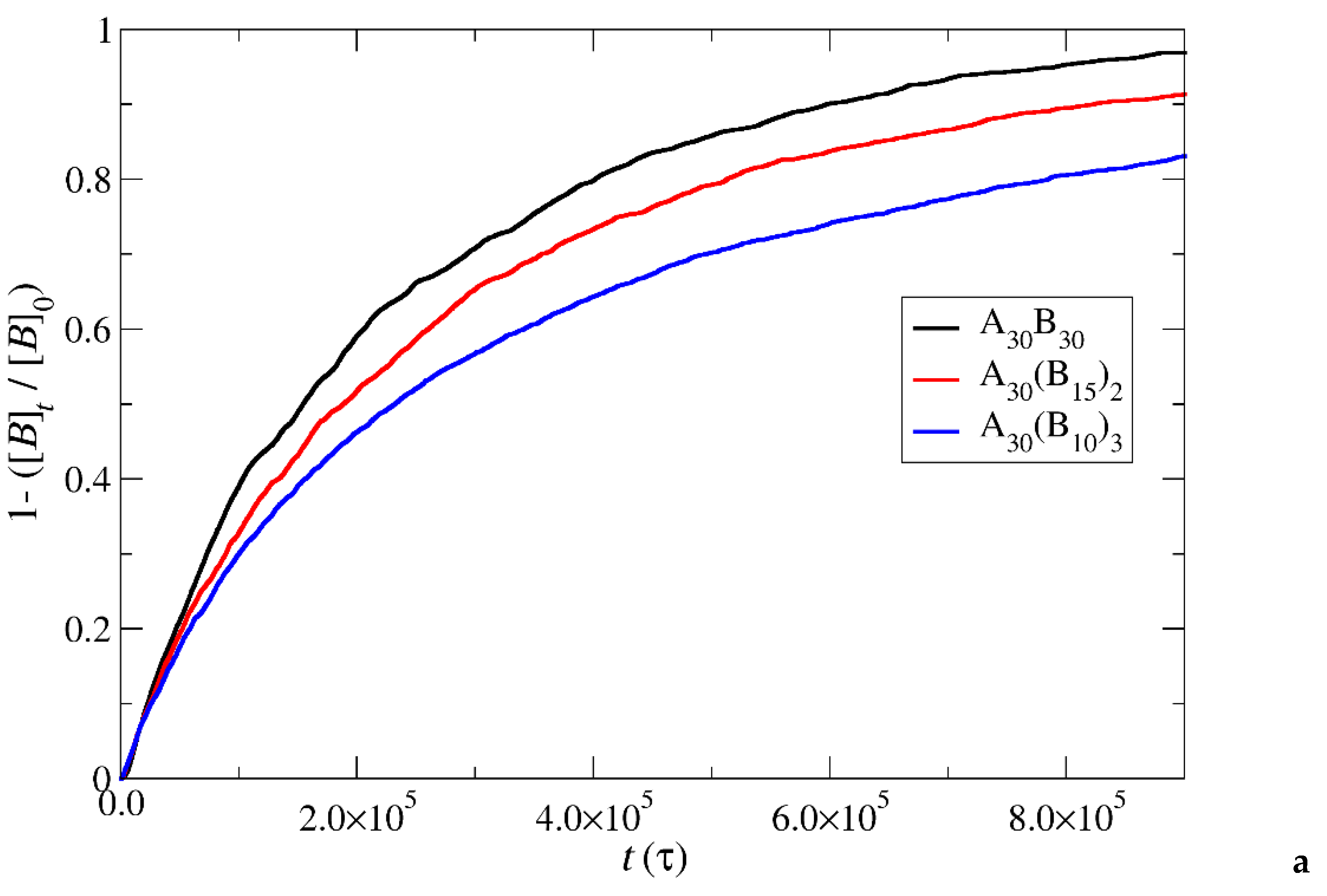
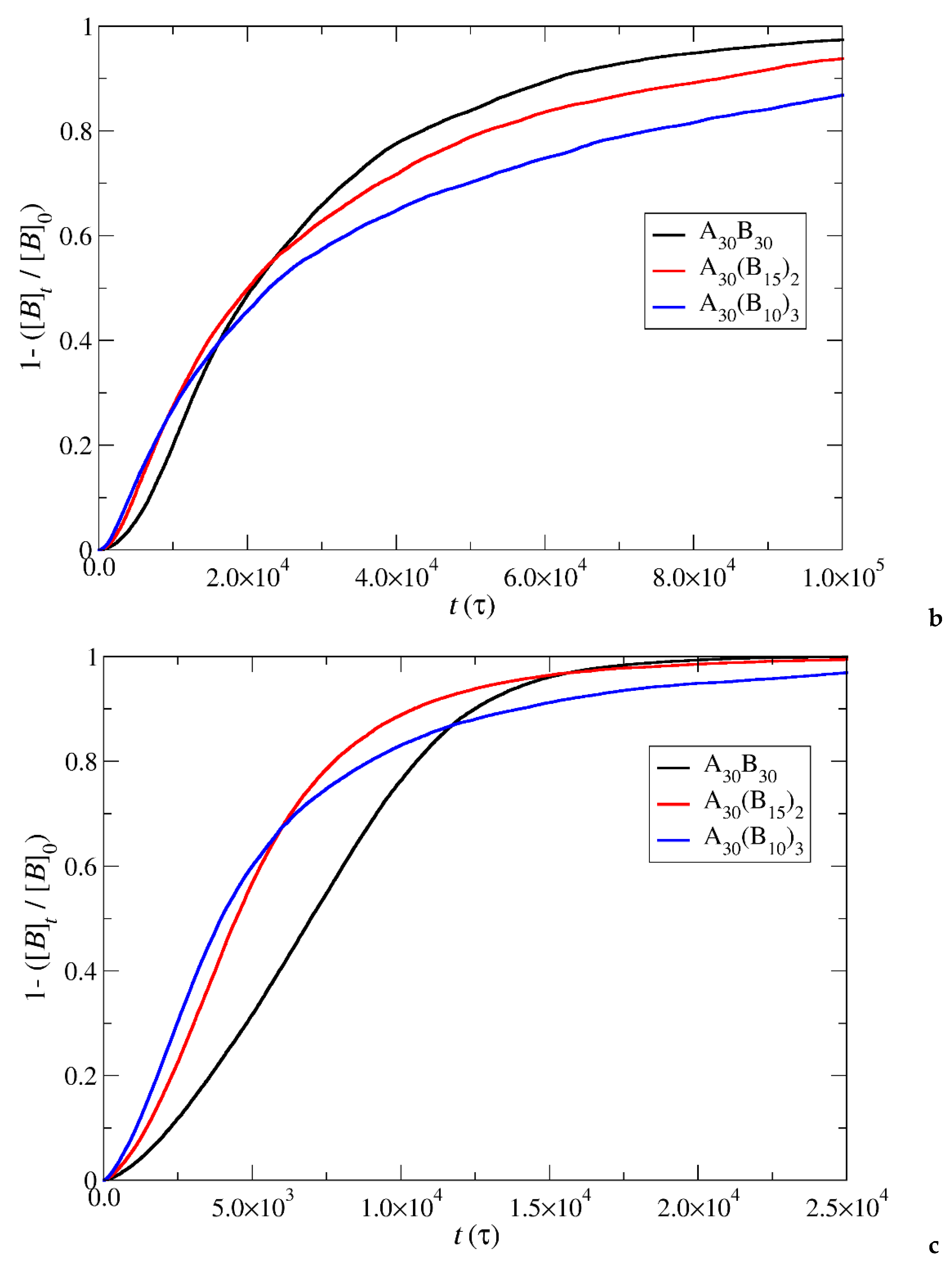
Figure 7.
Depolymerization fraction of all hydrophobic beads of A30B30, A30(B15)2, and A30(B10)3 copolymers plotted against time for constant trigger molecule concentration: a) RPT=10-4, RPB=10-3, b) RPT=10-2, RPB=10-3 and c) RPT=10-3, RPB=10-3. [B]0 is the initial hydrophobic beads concentration, [B]t is the hydrophobic beads concentration.
Figure 7.
Depolymerization fraction of all hydrophobic beads of A30B30, A30(B15)2, and A30(B10)3 copolymers plotted against time for constant trigger molecule concentration: a) RPT=10-4, RPB=10-3, b) RPT=10-2, RPB=10-3 and c) RPT=10-3, RPB=10-3. [B]0 is the initial hydrophobic beads concentration, [B]t is the hydrophobic beads concentration.
Figure 8.
Depolymerization fraction of all hydrophobic beads of A30B30, A30(B15)2, and A30(B10)3 copolymers plotted against time for stoichiometric trigger molecules concentration. RPT=10-4, RPB=10-3. [B]0 is the initial hydrophobic beads concentration, [B]t is the hydrophobic beads concentration.
Figure 8.
Depolymerization fraction of all hydrophobic beads of A30B30, A30(B15)2, and A30(B10)3 copolymers plotted against time for stoichiometric trigger molecules concentration. RPT=10-4, RPB=10-3. [B]0 is the initial hydrophobic beads concentration, [B]t is the hydrophobic beads concentration.
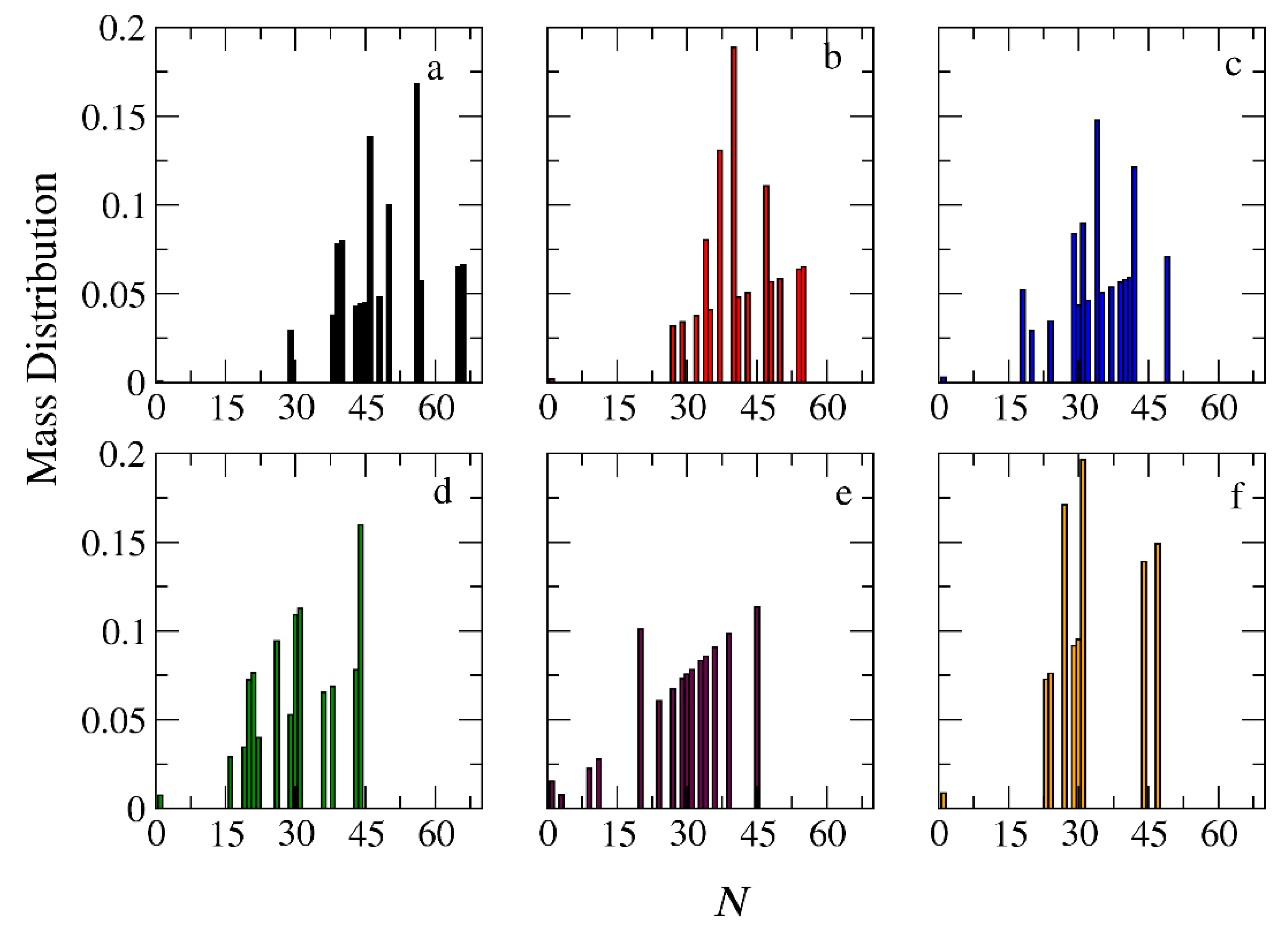
Figure 9.
Mass distribution of micelles formed by linear A30B30 copolymers across various time points and depolymerization fractions of all hydrophobic beads: a) t=0, 0, b) t=135000τ, 0.15, c) t=360000τ, 0.31, d) t=630000τ, 0.45, e) t= 1260000τ, 0.60, f) t= 1800000τ, 0.68. The trigger molecule concentration is maintained stoichiometric to end cap beads in all cases. RPT=10-4 and RPB=10-3.
Figure 9.
Mass distribution of micelles formed by linear A30B30 copolymers across various time points and depolymerization fractions of all hydrophobic beads: a) t=0, 0, b) t=135000τ, 0.15, c) t=360000τ, 0.31, d) t=630000τ, 0.45, e) t= 1260000τ, 0.60, f) t= 1800000τ, 0.68. The trigger molecule concentration is maintained stoichiometric to end cap beads in all cases. RPT=10-4 and RPB=10-3.
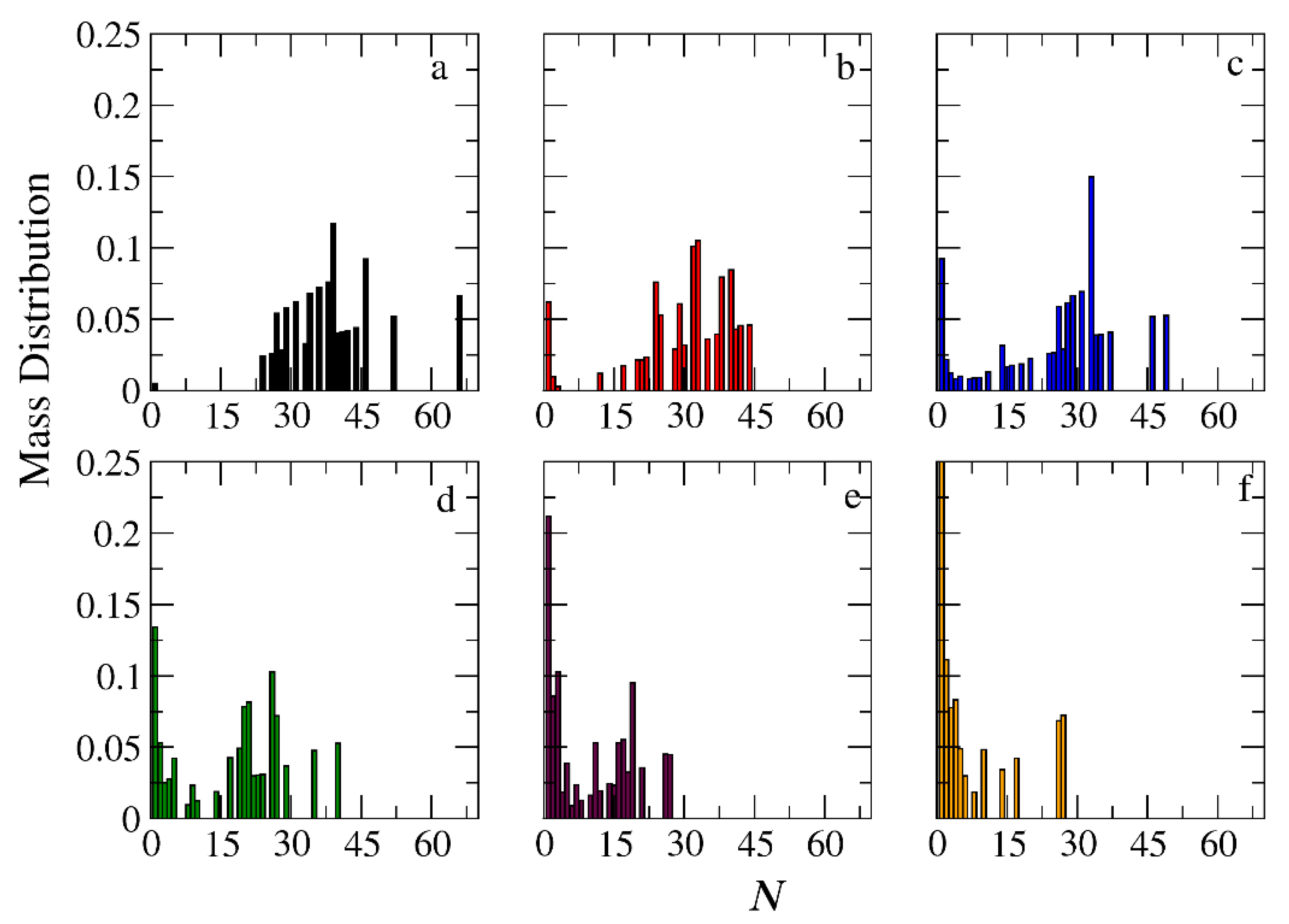
Figure 10.
Mass distribution of micelles formed by miktoarm A30(B15)2 copolymers across various time points and depolymerization fractions of all hydrophobic beads: a) t=0, 0, b) t=90000τ, 0.17, c) t=180000τ, 0.29, d) t=360000τ, 0.44, e) t= 720000τ, 0.60, f) t= 1440000τ, 0.75. The trigger molecule concentration is maintained stoichiometric to end cap beads in all cases. RPT=10-4 and RPB=10-3.
Figure 10.
Mass distribution of micelles formed by miktoarm A30(B15)2 copolymers across various time points and depolymerization fractions of all hydrophobic beads: a) t=0, 0, b) t=90000τ, 0.17, c) t=180000τ, 0.29, d) t=360000τ, 0.44, e) t= 720000τ, 0.60, f) t= 1440000τ, 0.75. The trigger molecule concentration is maintained stoichiometric to end cap beads in all cases. RPT=10-4 and RPB=10-3.
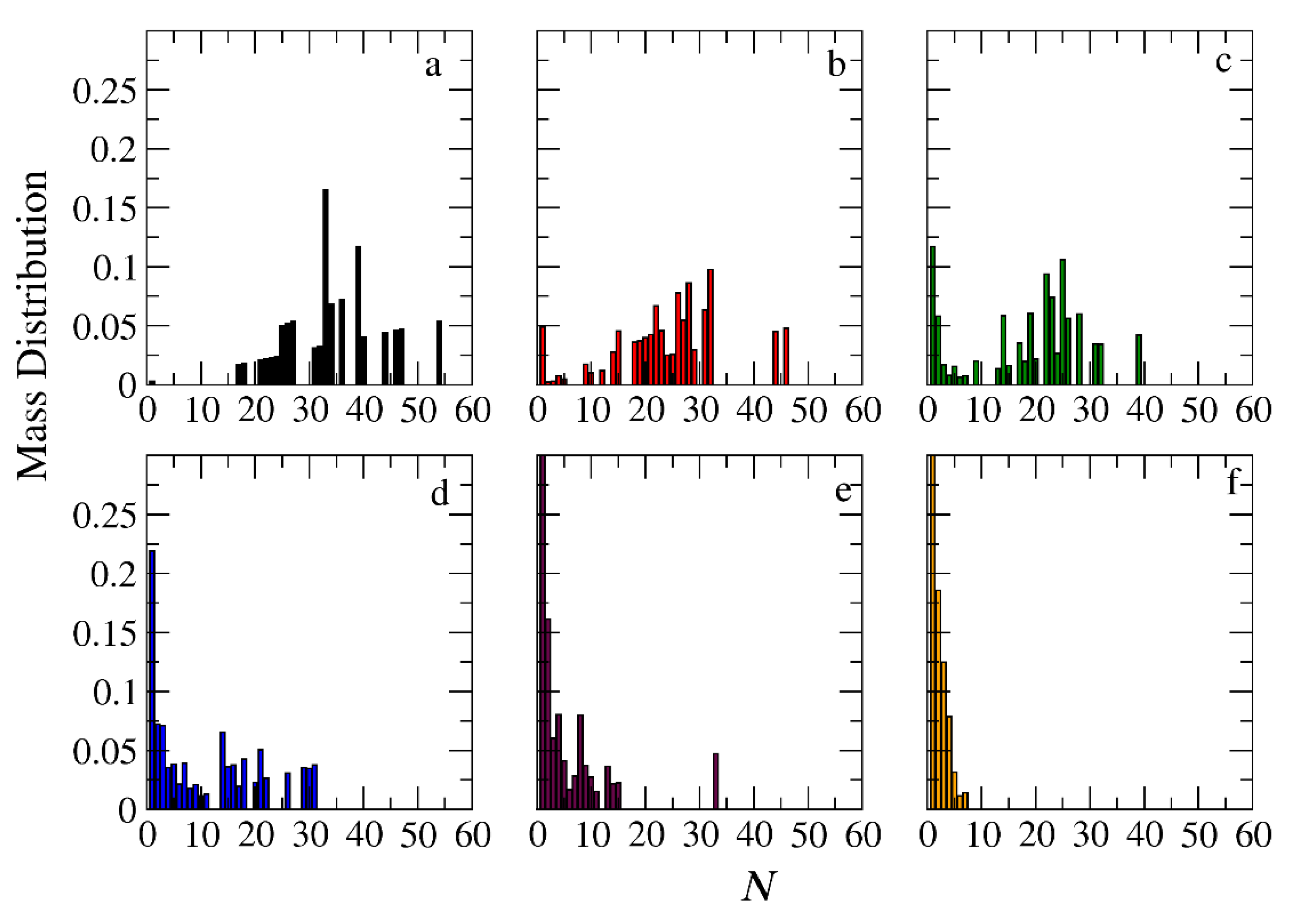
Figure 11.
Mass distribution of micelles formed by miktoarm A30(B10)3 copolymers across various time points and depolymerization fractions of all hydrophobic beads: a) t=0, 0, b) t=45000τ, 0.13, c) t=135000τ, 0.29, d) t=270000τ, 0.44, e) t= 540000τ, 0.59, f) t= 1170000τ, 0.75. The trigger molecule concentration is maintained stoichiometric to end cap beads in all cases. RPT=10-4 and RPB=10-3.
Figure 11.
Mass distribution of micelles formed by miktoarm A30(B10)3 copolymers across various time points and depolymerization fractions of all hydrophobic beads: a) t=0, 0, b) t=45000τ, 0.13, c) t=135000τ, 0.29, d) t=270000τ, 0.44, e) t= 540000τ, 0.59, f) t= 1170000τ, 0.75. The trigger molecule concentration is maintained stoichiometric to end cap beads in all cases. RPT=10-4 and RPB=10-3.
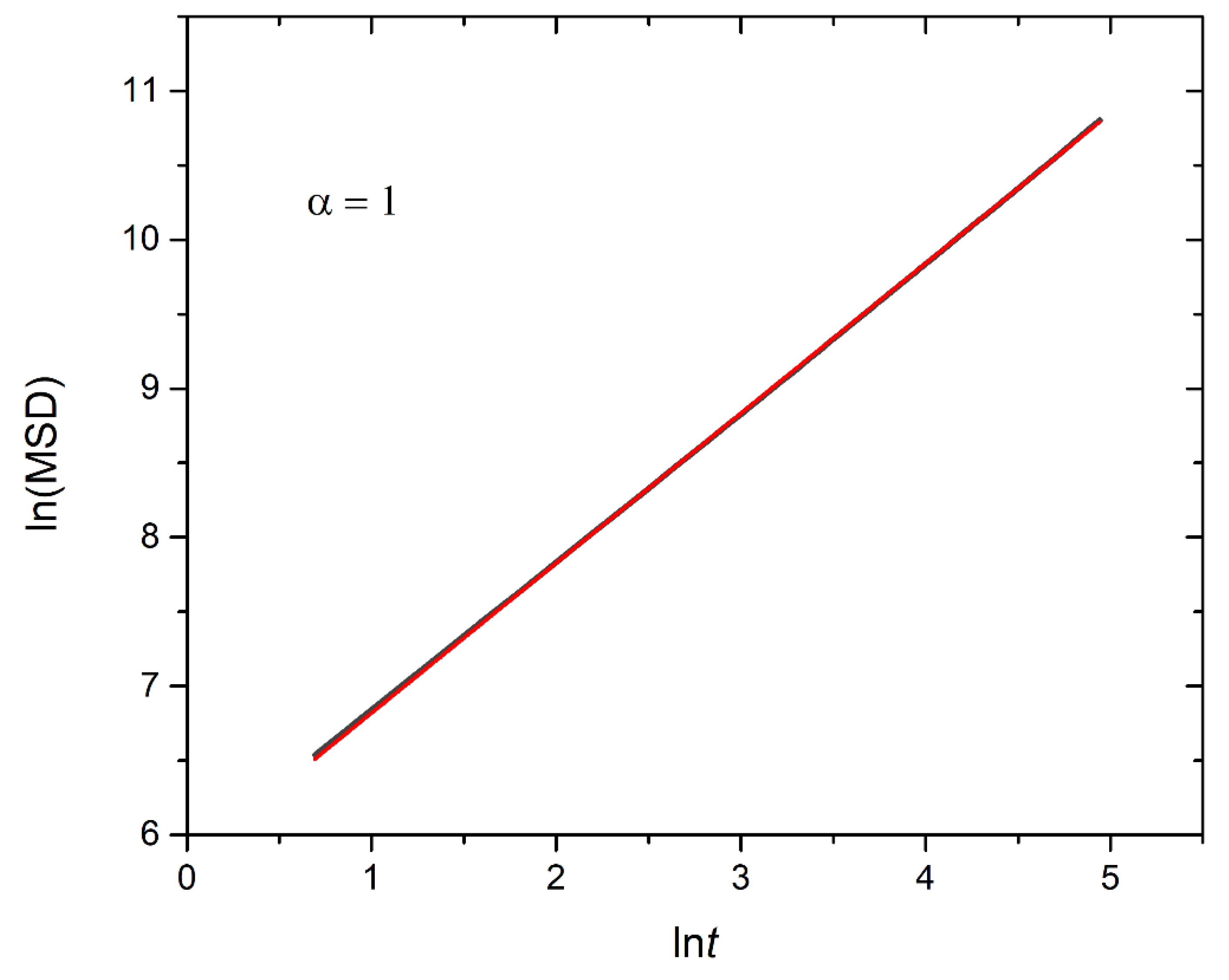
Figure 12.
Logarithmic plot illustrating the mean squared displacement calculated from 90 cargo molecules within an A30B30 micelle with N=50, against time for stoichiometric trigger molecule concentration with end cap beads and RPT=10-4 and RPB=10-3. The exponent α corresponds to the scaling behavior of t.
Figure 12.
Logarithmic plot illustrating the mean squared displacement calculated from 90 cargo molecules within an A30B30 micelle with N=50, against time for stoichiometric trigger molecule concentration with end cap beads and RPT=10-4 and RPB=10-3. The exponent α corresponds to the scaling behavior of t.
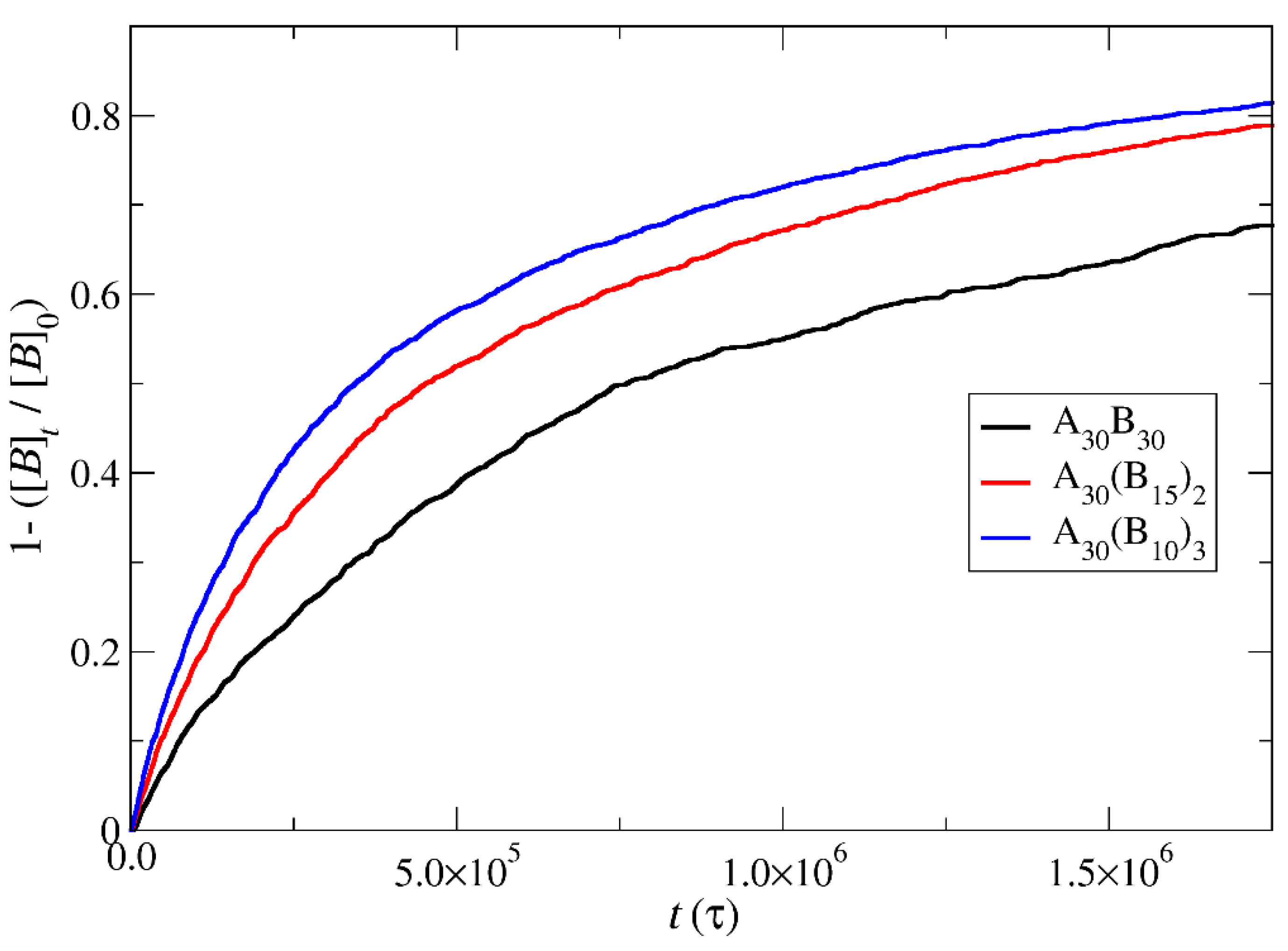
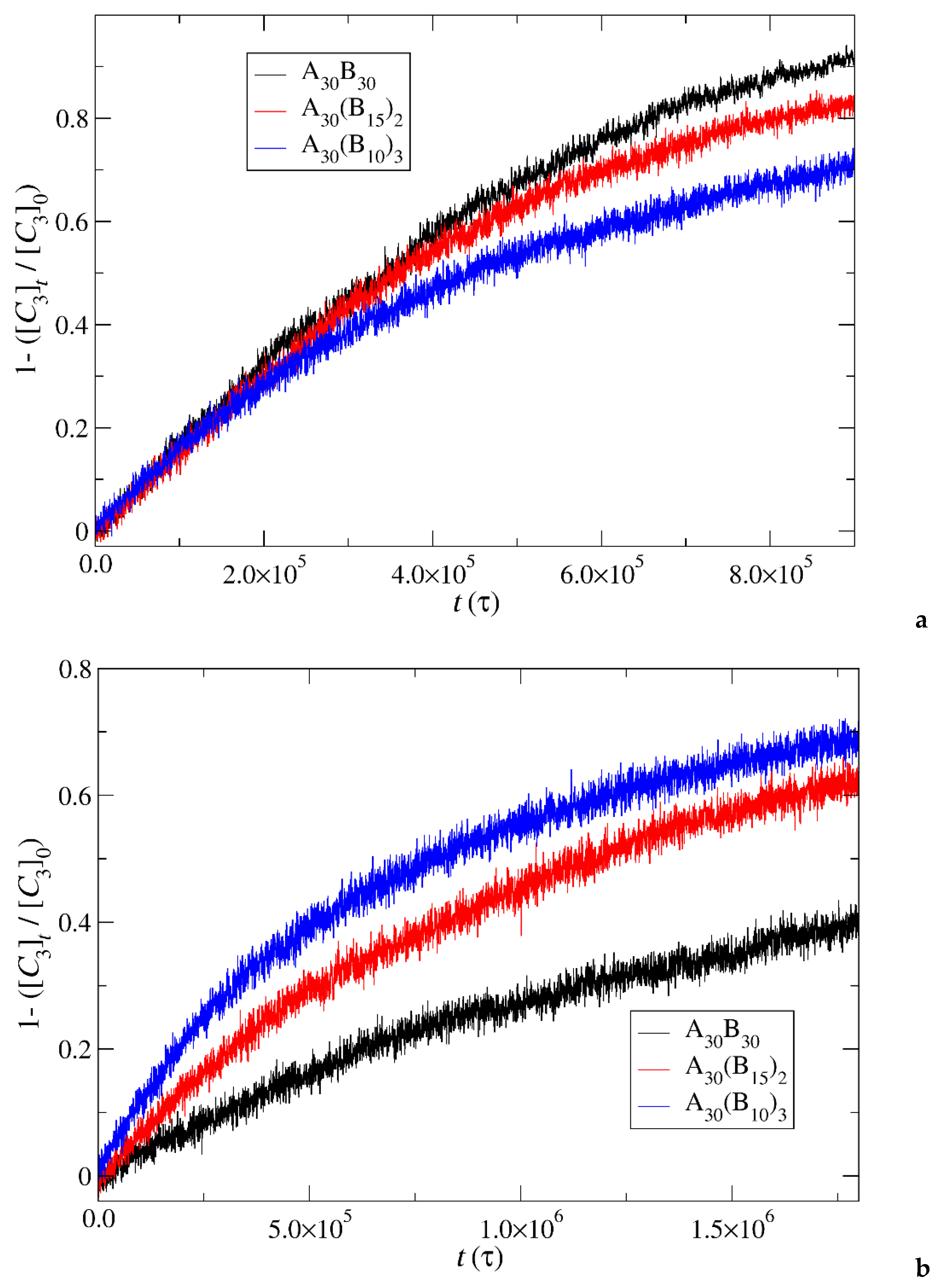
Figure 13.
Cargo molecules release fraction from A30B30, A30(B15)2, and A30(B10)3 copolymer mixtures plotted against time for a) constant trigger molecule concentration, and b) stoichiometric trigger molecule concentration with end cap beads. RPT=10-4 and RPB=10-3. [C3]0 is the initial cargo molecules concentration, [C3]t is the cargo molecules concentration.
Figure 13.
Cargo molecules release fraction from A30B30, A30(B15)2, and A30(B10)3 copolymer mixtures plotted against time for a) constant trigger molecule concentration, and b) stoichiometric trigger molecule concentration with end cap beads. RPT=10-4 and RPB=10-3. [C3]0 is the initial cargo molecules concentration, [C3]t is the cargo molecules concentration.
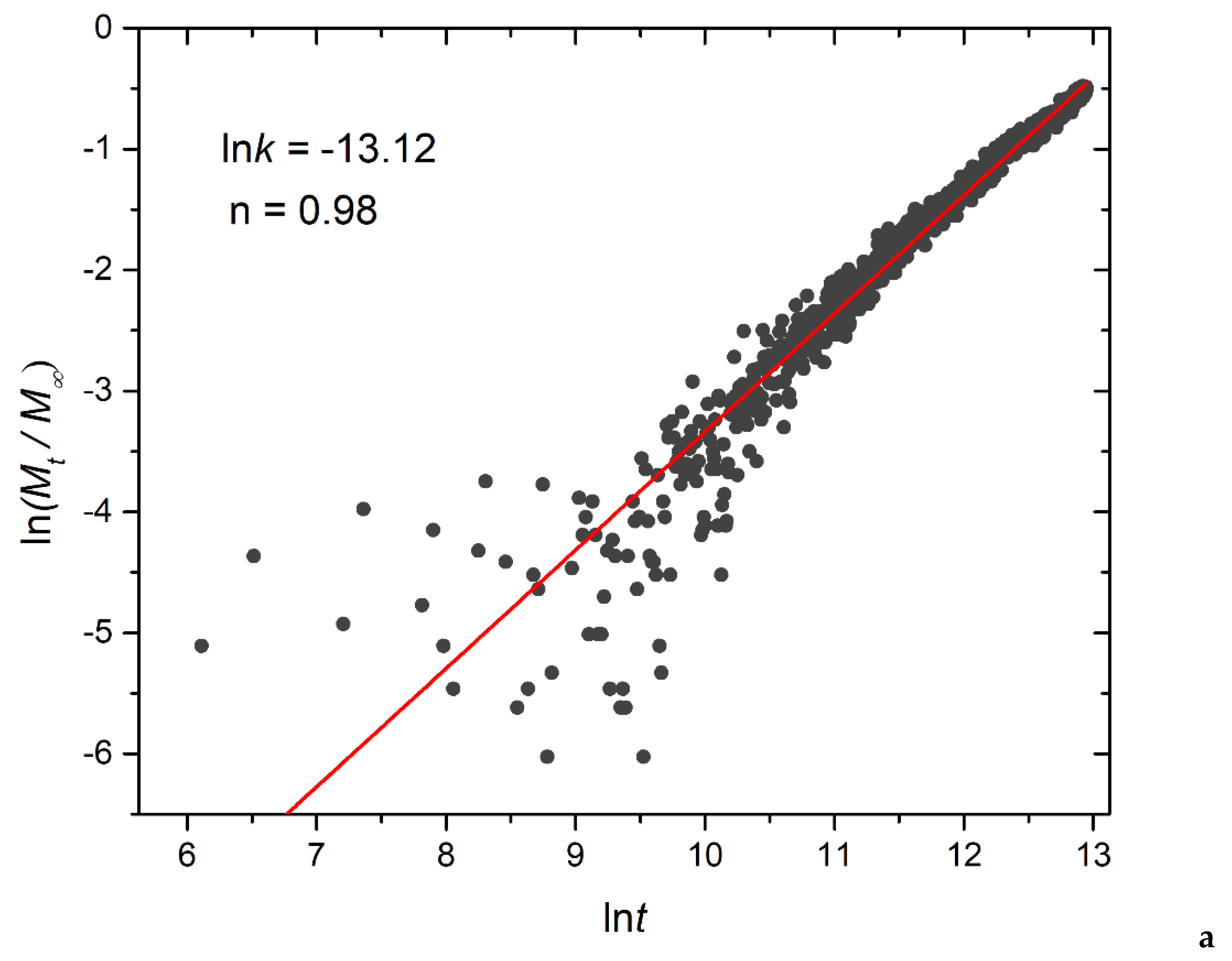
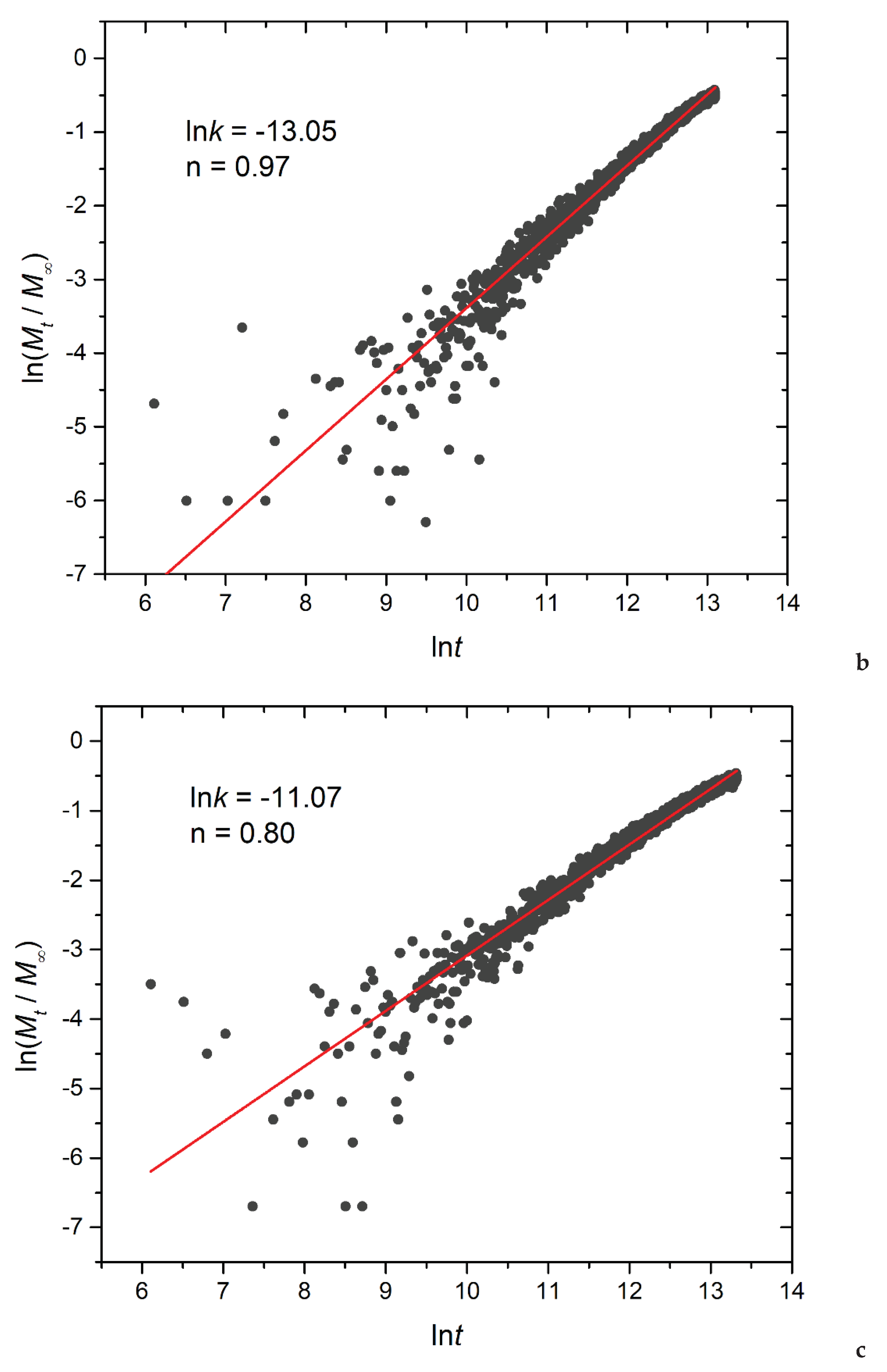
Figure 14.
Logarithmic plot depicting the fraction of released cargo molecules against time for a) linear A30B30, b) miktoarm A30(B15)2, and c) miktoarm A30(B10)3 copolymers. Fitting lines, k and n correspond to the Korsmeyer-Peppas equation. Trigger molecule concentration is maintained constant in all cases. RPT=10-4 and RPB=10-3.
Figure 14.
Logarithmic plot depicting the fraction of released cargo molecules against time for a) linear A30B30, b) miktoarm A30(B15)2, and c) miktoarm A30(B10)3 copolymers. Fitting lines, k and n correspond to the Korsmeyer-Peppas equation. Trigger molecule concentration is maintained constant in all cases. RPT=10-4 and RPB=10-3.