Submitted:
20 March 2024
Posted:
20 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
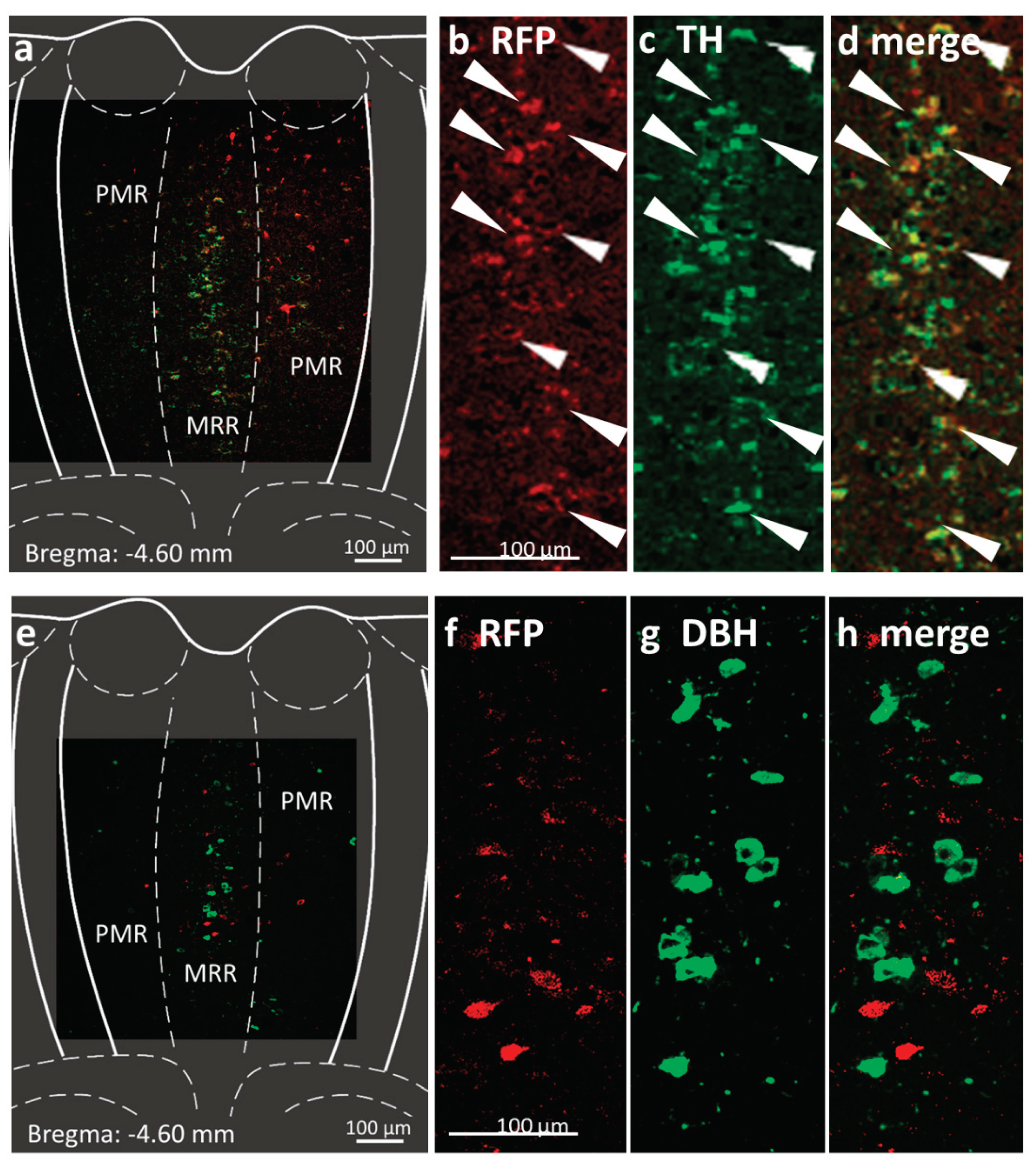
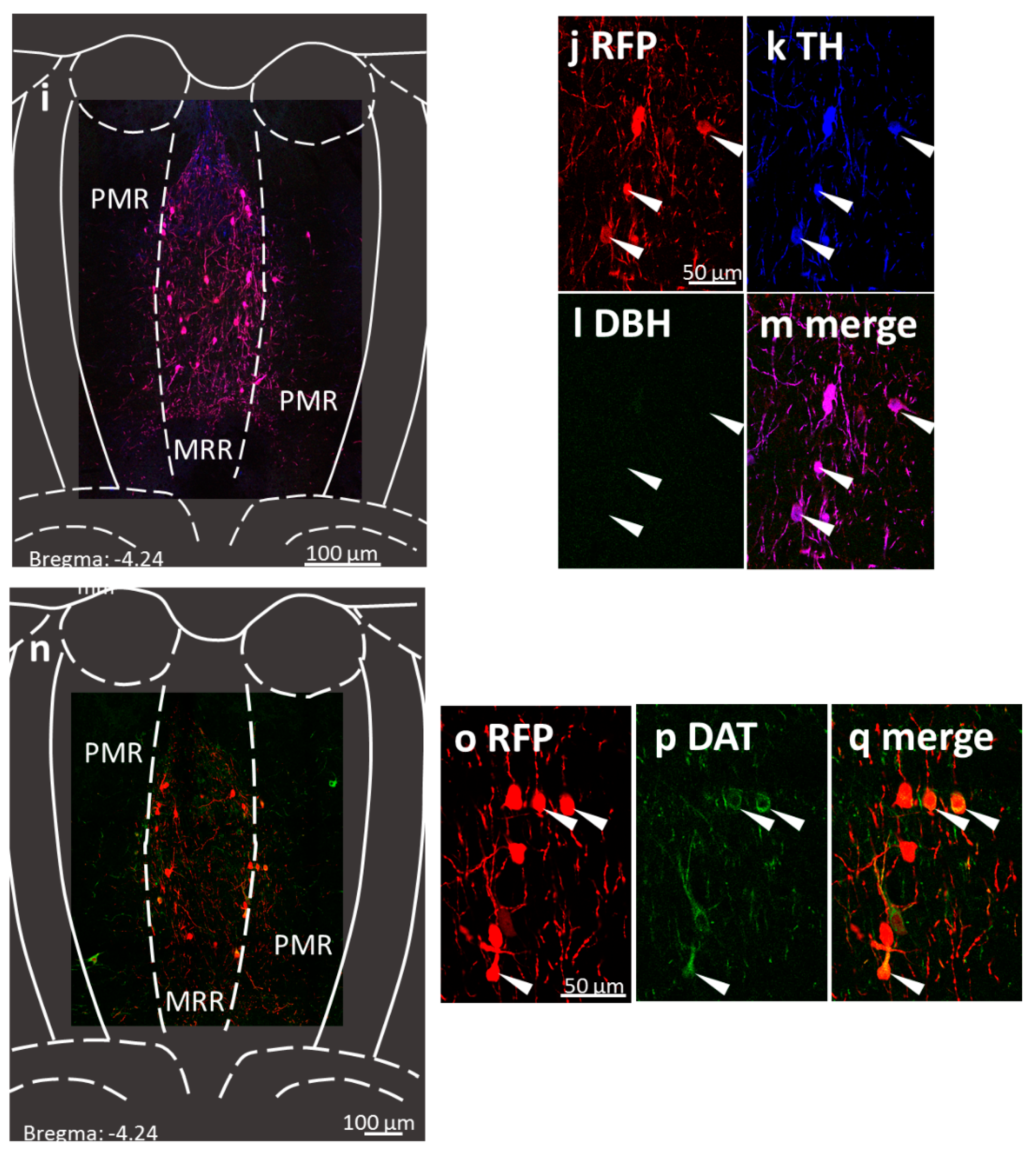
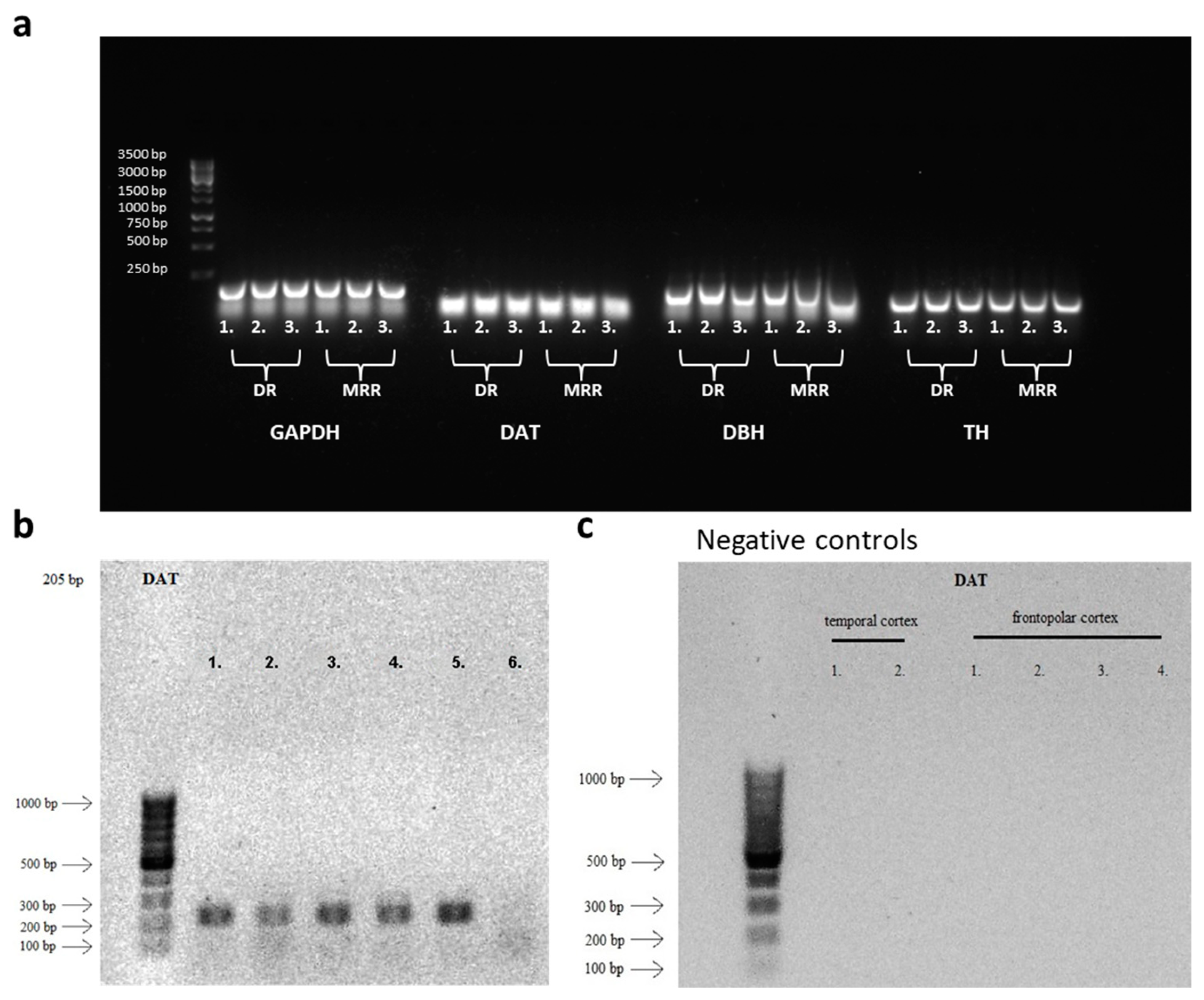
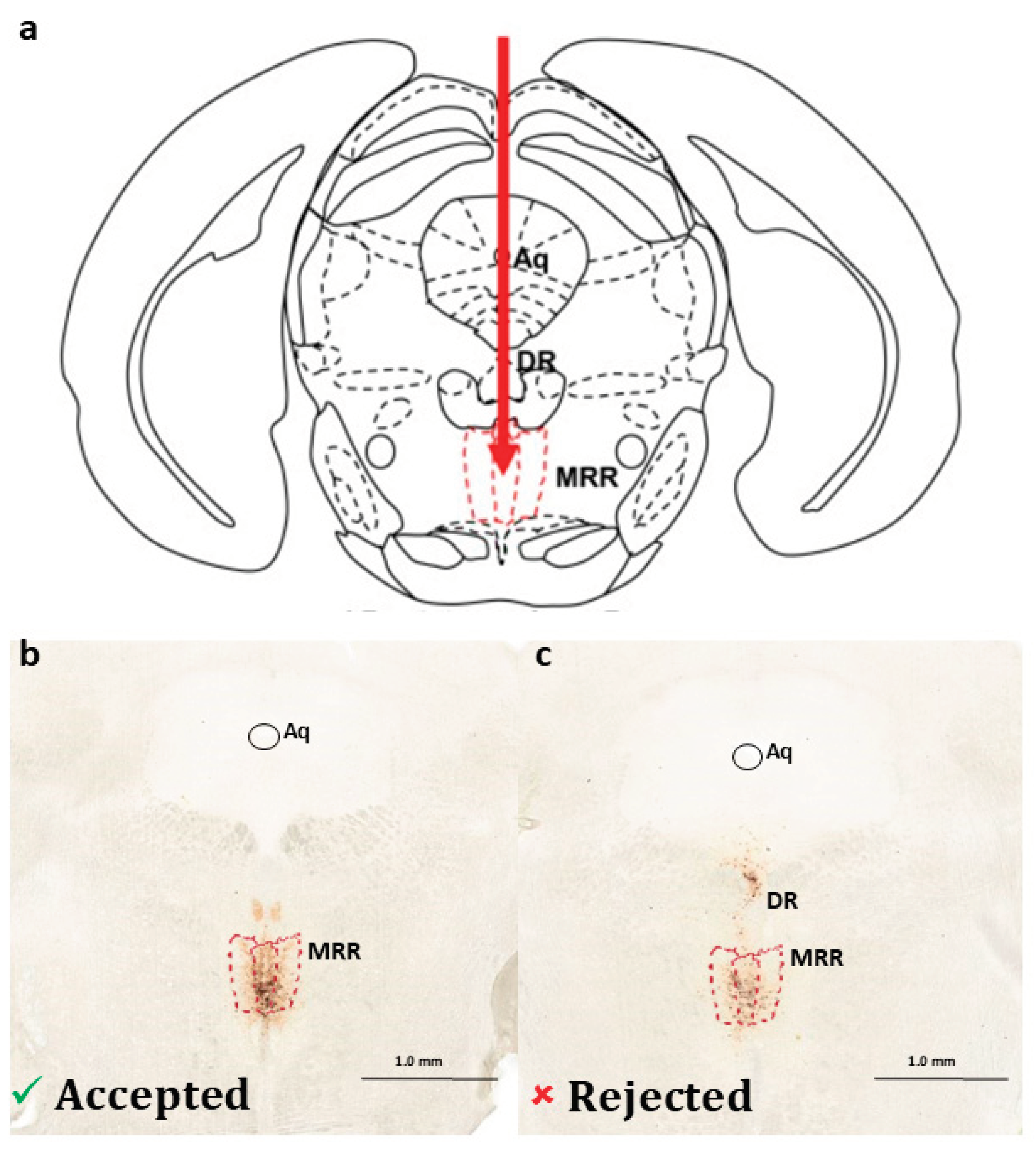
2.1. Presence of Dopaminergic Cells in the MRR
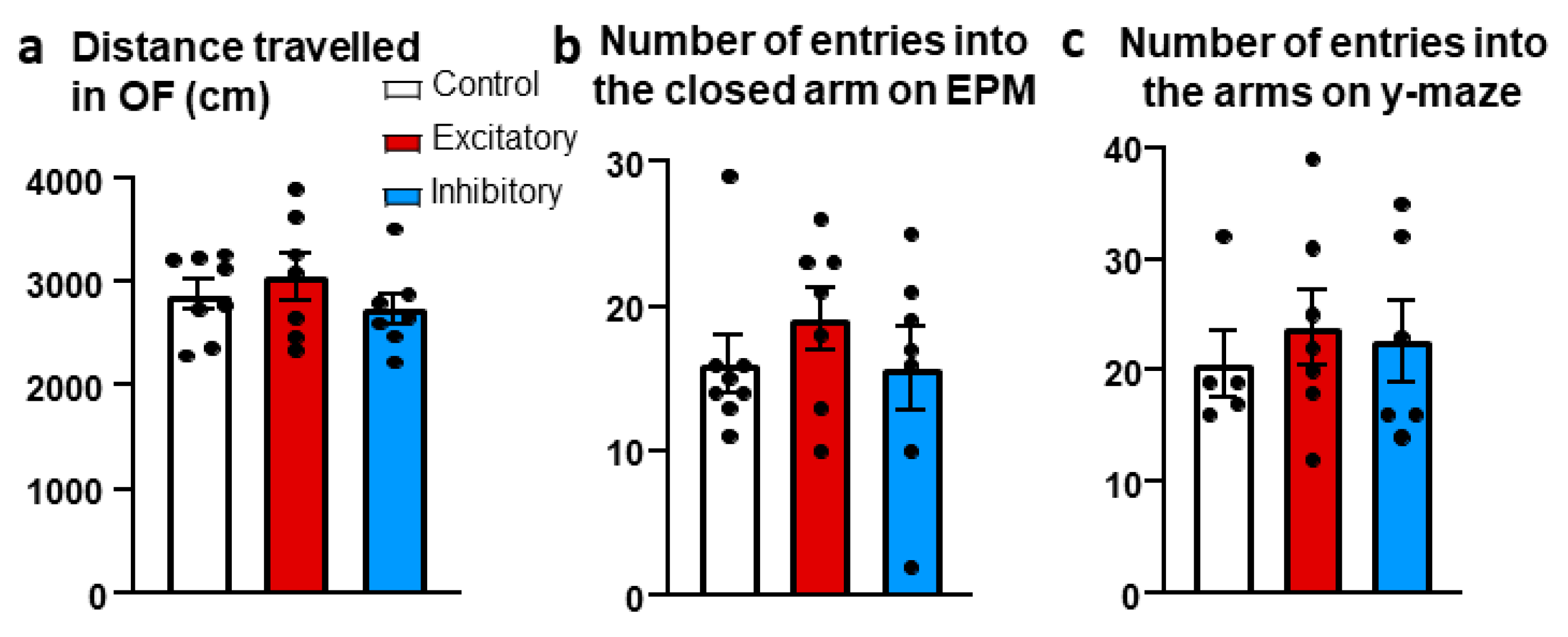
2.2. Locomotion
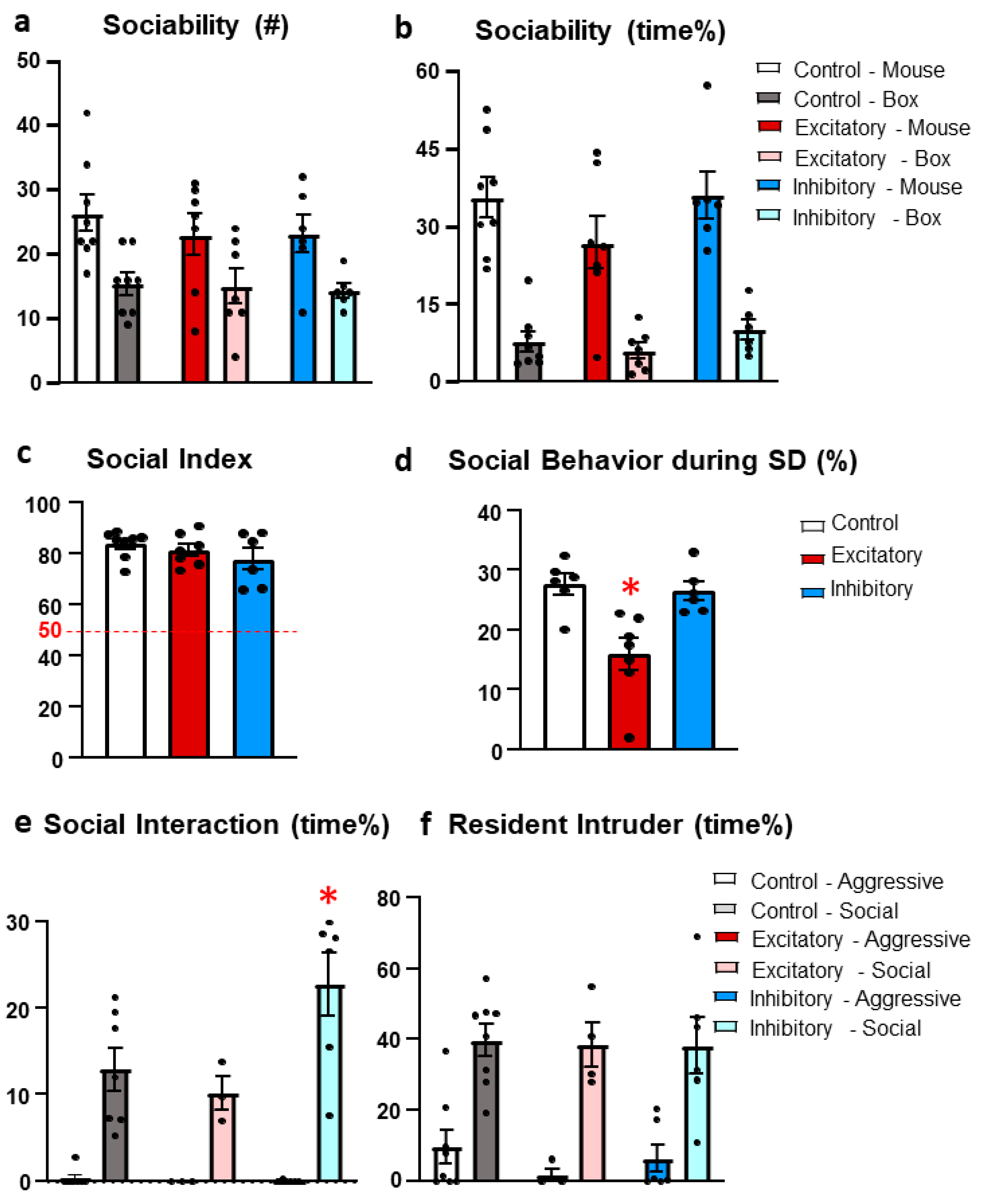
2.3. Social Behavior
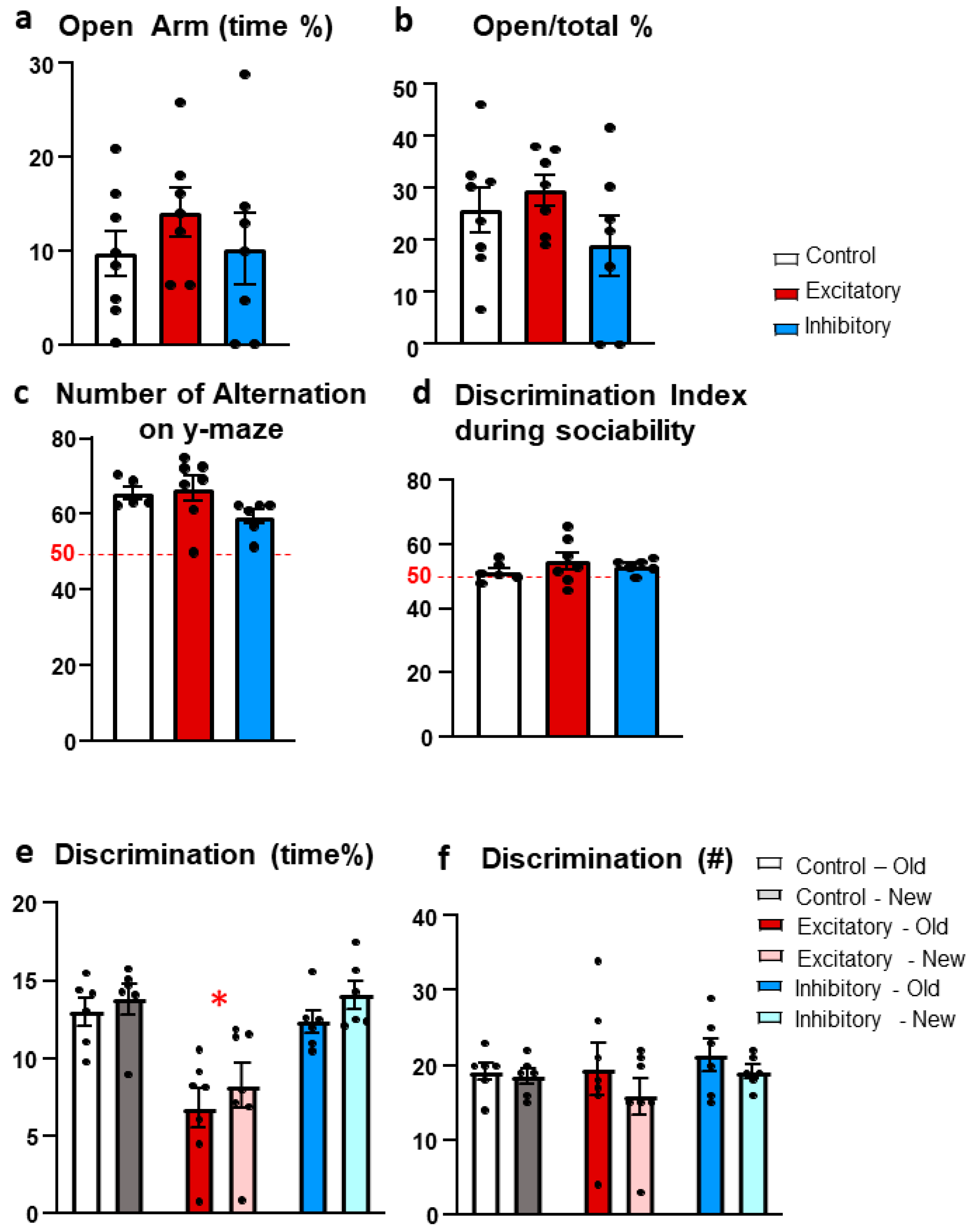
2.4. Anxiety
2.5. Memory
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgery
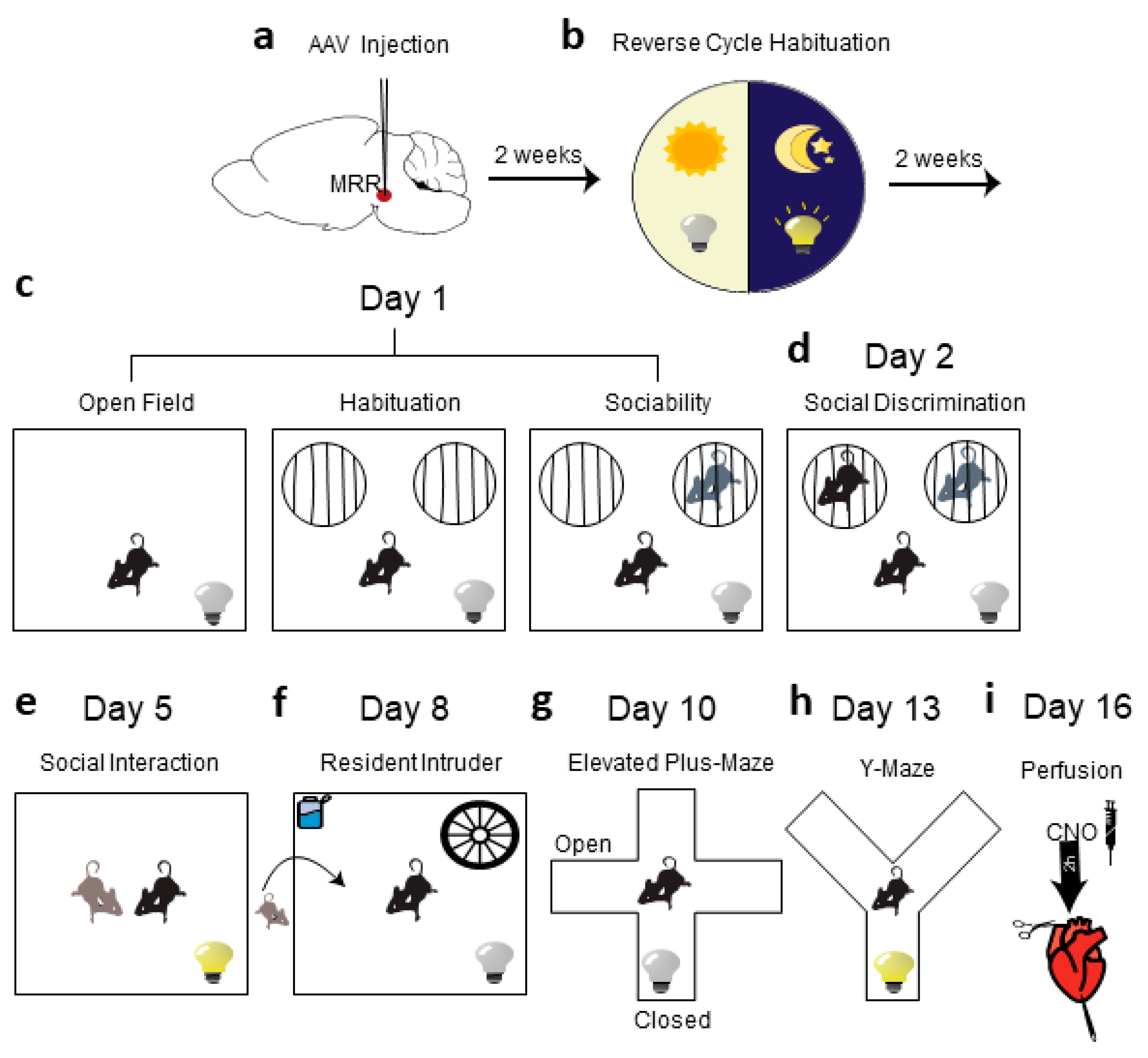
4.3. Behavioral Analysis
4.3.1. Open Field Test (OF)
4.3.2. Sociability Test
4.3.3. Social Interaction Test (SIT)
4.3.4. Resident Intruder Test (RIT)
4.3.5. Elevated Plus Maze Test (EPM)
4.3.6. Y-Maze Test
4.4. Immunohistochemistry
4.4.1. Confirmation of Dopaminergic Cells in the MRR
4.4.2. Verification of the Virus Injection
4.5. Verification of the Presence of DA in the Raphe by RT-PCR
4.5.1. RNA Extraction and cDNA Synthesis
4.5.2. Agarose Gel Electrophoresis
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, A.H.; Bland, R.C. Social dysfunction and mental illness in a community sample. Can J Psychiatry 1995, 40, 15–20. [Google Scholar] [CrossRef]
- Klin, A.; Volkmar, F.R.; Sparrow, S.S. Autistic social dysfunction: some limitations of the theory of mind hypothesis. J Child Psychol Psychiatry 1992, 33, 861–876. [Google Scholar] [CrossRef]
- Seivewright, H.; Tyrer, P.; Johnson, T. Persistent social dysfunction in anxious and depressed patients with personality disorder. Acta Psychiatr Scand 2004, 109, 104–109. [Google Scholar] [CrossRef]
- Stanghellini, G.; Ballerini, M. Dis-sociality: the phenomenological approach to social dysfunction in schizophrenia. World Psychiatry 2002, 1, 102–106. [Google Scholar] [PubMed]
- JP, H. The Neuronatomy of the Serotonergic System. Handbook of Behavioral Neuroscience 2010, 21, 51–64. [Google Scholar]
- Sos, K.E.; Mayer, M.I.; Cserep, C.; Takacs, F.S.; Szonyi, A.; Freund, T.F.; Nyiri, G. Cellular architecture and transmitter phenotypes of neurons of the mouse median raphe region. Brain Struct Funct 2017, 222, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Commons, K.G. Ascending serotonin neuron diversity under two umbrellas. Brain Struct Funct 2016, 221, 3347–3360. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.A.; Braff, D.L. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull 1987, 13, 643–668. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, Y.; Tanaka, K.F.; Tsunematsu, T.; Yamanaka, A.; Yoshioka, M. Optogenetic activation of serotonergic neurons enhances anxiety-like behaviour in mice. Int J Neuropsychopharmacol 2014, 17, 1777–1783. [Google Scholar] [CrossRef]
- Graeff, F.G.; Guimaraes, F.S.; De Andrade, T.G.; Deakin, J.F. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav 1996, 54, 129–141. [Google Scholar] [CrossRef]
- Dos Santos, L.; de Andrade, T.G.; Zangrossi, H. Jr. Serotonergic neurons in the median raphe nucleus regulate inhibitory avoidance but not escape behavior in the rat elevated T-maze test of anxiety. Psychopharmacology (Berl) 2005, 179, 733–741. [Google Scholar] [CrossRef]
- Andrews, N.; File, S.E.; Fernandes, C.; Gonzalez, L.E.; Barnes, N.M. Evidence that the median raphe nucleus--dorsal hippocampal pathway mediates diazepam withdrawal-induced anxiety. Psychopharmacology (Berl) 1997, 130, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.G.; Zangrossi, H. Jr.; Graeff, F.G. The median raphe nucleus in anxiety revisited. J Psychopharmacol 2013, 27, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, Y.; Tsutsui-Kimura, I.; Sasamori, H.; Nebuka, M.; Nishitani, N.; Tanaka, K.F.; Yamanaka, A.; Yoshioka, M. Different roles of distinct serotonergic pathways in anxiety-like behavior, antidepressant-like, and anti-impulsive effects. Neuropharmacology 2020, 167, 107703. [Google Scholar] [CrossRef] [PubMed]
- Teissier, A.; Chemiakine, A.; Inbar, B.; Bagchi, S.; Ray, R.S.; Palmiter, R.D.; Dymecki, S.M.; Moore, H.; Ansorge, M.S. Activity of Raphe Serotonergic Neurons Controls Emotional Behaviors. Cell Rep 2015, 13, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, C.L.; Bellardie, M.; Torok, B.; Sipos, E.; Toth, B.; Baranyi, M.; Sperlagh, B.; Dobos-Kovacs, M.; Chaillou, E.; Zelena, D. Pharmacogenetic excitation of the median raphe region affects social and depressive-like behavior and core body temperature in male mice. Life Sci 2021, 286, 120037. [Google Scholar] [CrossRef] [PubMed]
- Chaves, T.; Torok, B.; Fazekas, C.L.; Correia, P.; Sipos, E.; Varkonyi, D.; Hellinger, A.; Erk, D.; Zelena, D. Median raphe region GABAergic neurons contribute to social interest in mouse. Life Sci 2022, 289, 120223. [Google Scholar] [CrossRef]
- Numasawa, Y.; Hattori, T.; Ishiai, S.; Kobayashi, Z.; Kamata, T.; Kotera, M.; Ishibashi, S.; Sanjo, N.; Mizusawa, H.; Yokota, T. Depressive disorder may be associated with raphe nuclei lesions in patients with brainstem infarction. J Affect Disord 2017, 213, 191–198. [Google Scholar] [CrossRef]
- Morin, L.P. Neuroanatomy of the extended circadian rhythm system. Exp Neurol 2013, 243, 4–20. [Google Scholar] [CrossRef]
- Sorman, E.; Wang, D.; Hajos, M.; Kocsis, B. Control of hippocampal theta rhythm by serotonin: role of 5-HT2c receptors. Neuropharmacology 2011, 61, 489–494. [Google Scholar] [CrossRef]
- Jackson, J.; Dickson, C.T.; Bland, B.H. Median raphe stimulation disrupts hippocampal theta via rapid inhibition and state-dependent phase reset of theta-related neural circuitry. J Neurophysiol 2008, 99, 3009–3026. [Google Scholar] [CrossRef]
- Szonyi, A.; Zicho, K.; Barth, A.M.; Gonczi, R.T.; Schlingloff, D.; Torok, B.; Sipos, E.; Major, A.; Bardoczi, Z.; Sos, K.E.; Gulyas, A.I.; Varga, V.; Zelena, D.; Freund, T.F.; Nyiri, G. Median raphe controls acquisition of negative experience in the mouse. Science 2019, 366, 6469. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.M.; Grobecker, H.; Zivin, J. Catecholamines in the raphe nuclei of the rat. Brain Res 1976, 114, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Loullis, C.C.; Felten, D.L.; Shea, P.A. HPLC determination of biogenic amines in discrete brain areas in food deprived rats. Pharmacol Biochem Behav 1979, 11, 89–93. [Google Scholar] [CrossRef]
- Zangen, A.; Overstreet, D.H.; Yadid, G. Increased catecholamine levels in specific brain regions of a rat model of depression: normalization by chronic antidepressant treatment. Brain Res 1999, 824, 243–250. [Google Scholar] [CrossRef]
- Trulson, M.E.; Cannon, M.S.; Raese, J.D. Identification of dopamine-containing cell bodies in the dorsal and median raphe nuclei of the rat brain using tyrosine hydroxylase immunochemistry. Brain Res Bull 1985, 15, 229–234. [Google Scholar] [CrossRef]
- Ochi, J.; Shimizu, K. Occurrence of dopamine-containing neurons in the midbrain raphe nuclei of the rat. Neurosci Lett 1978, 8, 317–320. [Google Scholar] [CrossRef]
- Jahanshahi, A.; Steinbusch, H.W.; Temel, Y. Distribution of dopaminergic cell bodies in the median raphe nucleus of the rat brain. J Chem Neuroanat 2013, 53, 60–63. [Google Scholar] [CrossRef]
- Kocabicak, E.; Jahanshahi, A.; Schonfeld, L.; Hescham, S.A.; Temel, Y.; Tan, S. Deep Brain Stimulation of the Rat Subthalamic Nucleus Induced Inhibition of Median Raphe Serotonergic and Dopaminergic Neurotransmission. Turk Neurosurg 2015, 25, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Liesi, P.; Paetau, A.; Rechardt, L.; Dahl, D. Glial uptake of monoamines in primary cultures of rat median raphe nucleus and cerebellum. A combined monoamine fluorescence and glial fibrillary acidic protein immunofluorescence study. Histochemistry 1981, 73, 239–250. [Google Scholar] [CrossRef]
- Reymann, K.; Pohle, W.; Muller-Welde, P.; Ott, T. Dopaminergic innervation of the hippocampus: evidence for midbrain raphe neurons as the site of origin. Biomed Biochim Acta 1983, 42, 1247–1255. [Google Scholar]
- Lin, L.H.; Pivorun, E.B. Analysis of serotonin, dopamine and their metabolites in the caudate putamen, the suprachiasmatic nucleus and the median raphe nucleus of euthermic and torpid deermice, Peromyscus maniculatus. Pharmacol Biochem Behav 1989, 33, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Aoki, N.; Mori, C.; Serizawa, S.; Kihara-Negishi, F.; Homma, K.J.; Yamaguchi, S. Dopaminergic nuclei in the chick midbrain express serotonin receptor subfamily genes. Front Physiol 2022, 13, 1030621. [Google Scholar] [CrossRef]
- Stratford, T.R.; Wirtshafter, D. Ascending dopaminergic projections from the dorsal raphe nucleus in the rat. Brain Res 1990, 511, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Nevue, A.A.; Elde, C.J.; Perkel, D.J.; Portfors, C.V. Dopaminergic Input to the Inferior Colliculus in Mice. Front Neuroanat 2015, 9, 168. [Google Scholar] [CrossRef]
- Le Moal, M.; Simon, H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev 1991, 71, 155–234. [Google Scholar] [CrossRef]
- Creese, I.; Iversen, S.D. The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia 1974, 39, 345–357. [Google Scholar] [CrossRef]
- Ryan, J.M. Pharmacologic approach to aggression in neuropsychiatric disorders. Semin Clin Neuropsychiatry 2000, 5, 238–249. [Google Scholar] [CrossRef]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders. 5th ed.; Washington, DC, 2013.
- Davis, K.L.; Kahn, R.S.; Ko, G.; Davidson, M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991, 148, 1474–1486. [Google Scholar] [PubMed]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Telang, F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A 2011, 108, 15037–15042. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Kollins, S.H.; Wigal, T.L.; Newcorn, J.H.; Telang, F.; Fowler, J.S.; Zhu, W.; Logan, J.; Ma, Y.; Pradhan, K.; Wong, C.; Swanson, J.M. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA 2009, 302, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Pei, Z.J.; Zhang, J.; Vlasov, Y.K.; Davis, T.; Taylor, E.; Weng, F.J.; Van Dort, J.C.; Solt, K.; Brown, N.E. The Role of Glutamatergic and Dopaminergic Neurons in the Periaqueductal Gray/Dorsal Raphe: Separating Analgesia and Anxiety. eNeuro 2019, 6, ENEURO–0018. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.T.; Zhang, Q.; Wen, S.Y.; Chen, F.F.; Zhou, C.Q. Efficacy and safety of non-ergot dopamine-receptor agonists as an adjunct to levodopa in advanced Parkinson’s disease: A network meta-analysis. Eur J Neurol 2022. [Google Scholar] [CrossRef]
- Juza, R.; Musilek, K.; Mezeiova, E.; Soukup, O.; Korabecny, J. Recent advances in dopamine D(2) receptor ligands in the treatment of neuropsychiatric disorders. Med Res Rev 2023, 43, 55–211. [Google Scholar] [CrossRef]
- Novak, G.; Seeman, M.V. Dopamine, Psychosis, and Symptom Fluctuation: A Narrative Review. Healthcare (Basel) 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, B.N.; Li, X.; Pausch, M.H.; Herlitze, S.; Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A 2007, 104, 5163–5168. [Google Scholar] [CrossRef]
- Roth, B.L. DREADDs for Neuroscientists. Neuron 2016, 89, 683–694. [Google Scholar] [CrossRef]
- Lewis, D.A.; Campbell, M.J.; Foote, S.L.; Goldstein, M.; Morrison, J.H. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci 1987, 7, 279–290. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J.; Franklin, K.B.J. The mouse brain in stereotaxic coordinates., 2nd ed.; Academic Press: San Diego, 2001. [Google Scholar]
- Mlynarik, M.; Zelena, D.; Bagdy, G.; Makara, G.B.; Jezova, D. Signs of attenuated depression-like behavior in vasopressin deficient Brattleboro rats. Horm Behav 2007, 51, 395–405. [Google Scholar] [CrossRef]
- Shimada, S.; Kitayama, S.; Walther, D.; Uhl, G. Dopamine transporter mRNA: dense expression in ventral midbrain neurons. Brain Res Mol Brain Res 1992, 13, 359–362. [Google Scholar] [CrossRef]
- Tran, A.H.; Tamura, R.; Uwano, T.; Kobayashi, T.; Katsuki, M.; Ono, T. Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci U S A 2005, 102, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Ryczko, D.; Dubuc, R. Dopamine and the Brainstem Locomotor Networks: From Lamprey to Human. Front Neurosci 2017, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Beninger, R.J. The role of dopamine in locomotor activity and learning. Brain Res 1983, 287, 173–196. [Google Scholar] [CrossRef]
- Shim, I.; Stratford, T.R.; Wirtshafter, D. Dopamine is differentially involved in the locomotor hyperactivity produced by manipulations of opioid, GABA and glutamate receptors in the median raphe nucleus. Behav Brain Res 2014, 261, 65–70. [Google Scholar] [CrossRef]
- Wirtshafter, D.; Klitenick, M.A.; Asin, K.E. Is dopamine involved in the hyperactivity produced by injections of muscimol into the median raphe nucleus? Pharmacol Biochem Behav 1988, 30, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Vertes, R.P.; Martin, G.F. Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J Comp Neurol 1988, 275, 511–541. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Hernandez, X.I.; Vogel, P.; Betz, S.; Kalisch, R.; Sigurdsson, T.; Duvarci, S. Dopamine neurons drive fear extinction learning by signaling the omission of expected aversive outcomes. Elife 2018, 7. [Google Scholar] [CrossRef]
- Kalisch, R.; Gerlicher, A.M.V.; Duvarci, S. A Dopaminergic Basis for Fear Extinction. Trends Cogn Sci 2019, 23, 274–277. [Google Scholar] [CrossRef]
- Stubbendorff, C.; Hale, E.; Cassaday, H.J.; Bast, T.; Stevenson, C.W. Dopamine D1-like receptors in the dorsomedial prefrontal cortex regulate contextual fear conditioning. Psychopharmacology (Berl) 2019, 236, 1771–1782. [Google Scholar] [CrossRef]
- Fyk-Kolodziej, B.E.; Shimano, T.; Gafoor, D.; Mirza, N.; Griffith, R.D.; Gong, T.W.; Holt, A.G. Dopamine in the auditory brainstem and midbrain: co-localization with amino acid neurotransmitters and gene expression following cochlear trauma. Front Neuroanat 2015, 9, 88. [Google Scholar] [CrossRef]
- Szonyi, A.; Mayer, M.I.; Cserep, C.; Takacs, V.T.; Watanabe, M.; Freund, T.F.; Nyiri, G. The ascending median raphe projections are mainly glutamatergic in the mouse forebrain. Brain Struct Funct 2016, 221, 735–751. [Google Scholar] [CrossRef]
- Bariselli, S.; Hornberg, H.; Prevost-Solie, C.; Musardo, S.; Hatstatt-Burkle, L.; Scheiffele, P.; Bellone, C. Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Nat Commun 2018, 9, 3173. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Lin, R.; Wen, T. Dopamine and dopamine receptor D1 associated with decreased social interaction. Behav Brain Res 2017, 324, 51–57. [Google Scholar] [CrossRef]
- de Boer, S.F.; Olivier, B.; Veening, J.; Koolhaas, J.M. The neurobiology of offensive aggression: Revealing a modular view. Physiol Behav 2015, 146, 111–127. [Google Scholar] [CrossRef]
- Haller, J.; Varga, B.; Ledent, C.; Barna, I.; Freund, T.F. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci 2004, 19, 1906–1912. [Google Scholar] [CrossRef]
- Bahi, A.; Dreyer, J.L. Dopamine transporter (DAT) knockdown in the nucleus accumbens improves anxiety- and depression-related behaviors in adult mice. Behav Brain Res 2019, 359, 104–115. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Johnson, N.J.; Carr, J.; Hodgson, T.P. Resistance of experientially-induced changes in murine plus-maze behaviour to altered retest conditions. Behav Brain Res 1997, 86, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Dalvi, A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev 1997, 21, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. What’s wrong with my mouse? : behavioral phenotyping of transgenic and knockout mice., 2nd ed.; Wiley-Interscience: Hoboken, N.J. 2007. [Google Scholar]
- Grogan, J.; Bogacz, R.; Tsivos, D.; Whone, A.; Coulthard, E. Dopamine and Consolidation of Episodic Memory: Timing is Everything. J Cogn Neurosci 2015, 27, 2035–2050. [Google Scholar] [CrossRef]
- Guzman-Ramos, K.; Moreno-Castilla, P.; Castro-Cruz, M.; McGaugh, J.L.; Martinez-Coria, H.; LaFerla, F.M.; Bermudez-Rattoni, F. Restoration of dopamine release deficits during object recognition memory acquisition attenuates cognitive impairment in a triple transgenic mice model of Alzheimer’s disease. Learn Mem 2012, 19, 453–460. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M.; Venneri, A. Volume and Connectivity of the Ventral Tegmental Area are Linked to Neurocognitive Signatures of Alzheimer’s Disease in Humans. J Alzheimers Dis 2018, 63, 167–180. [Google Scholar] [CrossRef]
- Thor, D.H.; Holloway, W.R. Social Memory of the Male Laboratory Rat. Journal of Comparative and Physiological Psychology 1982, 96, 1000–1006. [Google Scholar] [CrossRef]
- Bluthe, R.M.; Gheusi, G.; Dantzer, R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology 1993, 18, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.; Williams Avram, S.K.; Cymerblit-Sabba, A.; Song, J.; Young, W.S. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol Psychiatry 2016, 21, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Kogan, J.H.; Frankland, P.W.; Silva, A.J. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 2000, 10, 47–56. [Google Scholar] [CrossRef]
- Garrido Zinn, C.; Clairis, N.; Silva Cavalcante, L.E.; Furini, C.R.; de Carvalho Myskiw, J.; Izquierdo, I. Major neurotransmitter systems in dorsal hippocampus and basolateral amygdala control social recognition memory. Proc Natl Acad Sci U S A 2016, 113, E4914–E4919. [Google Scholar] [CrossRef] [PubMed]
- Balazsfi, D.G.; Zelena, D.; Farkas, L.; Demeter, K.; Barna, I.; Cserep, C.; Takacs, V.T.; Nyiri, G.; Goloncser, F.; Sperlagh, B.; Freund, T.F.; Haller, J. Median raphe region stimulation alone generates remote, but not recent fear memory traces. PLoS One 2017, 12, e0181264. [Google Scholar] [CrossRef]
- Foley, P.B. Dopamine in psychiatry: a historical perspective. J Neural Transm (Vienna) 2019, 126, 473–479. [Google Scholar] [CrossRef]
- Calne, D.B.; Sandler, M. L-Dopa and Parkinsonism. Nature 1970, 226, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Stip, E.; Tourjman, V. Aripiprazole in schizophrenia and schizoaffective disorder: A review. Clin Ther 2010, 32 (Suppl. 1), S3–20. [Google Scholar] [CrossRef]
- Muneer, A. The Treatment of Adult Bipolar Disorder with Aripiprazole: A Systematic Review. Cureus 2016, 8, e562. [Google Scholar] [CrossRef] [PubMed]
- Ondo, W.G.; Tintner, R.; Thomas, M.; Jankovic, J. Tetrabenazine treatment for Huntington’s disease-associated chorea. Clin Neuropharmacol 2002, 25, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Winlow, W. Pramipexole in restless legs syndrome: an evidence-based review of its effectiveness on clinical outcomes. Core Evid 2005, 1, 35–42. [Google Scholar]
- Herceg, M.; Nagy, F.; Pal, E.; Janszky, J.; Kesmarky, I.; Komoly, S.; Kovacs, N. Pramipexole may be an effective treatment option in essential tremor. Clin Neuropharmacol 2012, 35, 73–76. [Google Scholar] [CrossRef]
- Sandi, C.; Haller, J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci 2015, 16, 290–304. [Google Scholar] [CrossRef]
- Backman, C.M.; Malik, N.; Zhang, Y.; Shan, L.; Grinberg, A.; Hoffer, B.J.; Westphal, H.; Tomac, A.C. Characterization of a mouse strain expressing Cre recombinase from the 3’ untranslated region of the dopamine transporter locus. Genesis 2006, 44, 383–390. [Google Scholar] [CrossRef]
- Perna, J.C.; Wotjak, C.T.; Stork, O.; Engelmann, M. Timing of presentation and nature of stimuli determine retroactive interference with social recognition memory in mice. Physiol Behav 2015, 143, 10–14. [Google Scholar] [CrossRef]
- Hadicke, J.; Engelmann, M. Social investigation and long-term recognition memory performance in 129S1/SvImJ and C57BL/6JOlaHsd mice and their hybrids. PLoS One 2013, 8, e54427. [Google Scholar] [CrossRef] [PubMed]
- Hut, R.A.; Van der Zee, E.A. The cholinergic system, circadian rhythmicity, and time memory. Behav Brain Res 2011, 221, 466–480. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp 2015, 96, e52434. [Google Scholar]
- Camats Perna, J.; Engelmann, M. Recognizing Others: Rodent’s Social Memories. Curr Top Behav Neurosci 2017, 30, 25–45. [Google Scholar] [PubMed]
- Haller, J.; Halasz, J.; Makara, G.B. Housing conditions and the anxiolytic efficacy of buspirone: the relationship between main and side effects. Behav Pharmacol 2000, 11, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; Coppens, C.M.; de Boer, S.F.; Buwalda, B.; Meerlo, P.; Timmermans, P.J. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp 2013, 77, e4367. [Google Scholar]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated plus maze for mice. J Vis Exp 2008, 22. [Google Scholar]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Varkonyi, D.; Torok, B.; Sipos, E.; Fazekas, C.L.; Banrevi, K.; Correia, P.; Chaves, T.; Farkas, S.; Szabo, A.; Martinez-Bellver, S.; Hangya, B.; Zelena, D. Investigation of Anxiety- and Depressive-like Symptoms in 4- and 8-Month-Old Male Triple Transgenic Mouse Models of Alzheimer’s Disease. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Knoll, J.; Zelena, D.; Timar, J.; Baghy, K.; Mervai, Z.; Miklya, I. Synthetic enhancer compounds, besides acting on biogenic amine system, influence the glutamate transmission and stress response. Behav Brain Res 2020, 378, 112290. [Google Scholar] [CrossRef]
- Balazsfi, D.; Fodor, A.; Torok, B.; Ferenczi, S.; Kovacs, K.J.; Haller, J.; Zelena, D. Enhanced innate fear and altered stress axis regulation in VGluT3 knockout mice. Stress 2018, 21, 151–161. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol Biol 2019, 1916, 105–111. [Google Scholar]
| Function | Test | Excitatory | Inhibitory |
|---|---|---|---|
| Locomotion | OF | ∅ | ∅ |
| EPM | ∅ | ∅ | |
| Y Maze | ∅ | ∅ | |
| Social Behavior |
Sociability | Decreased social interest | ∅ |
| Social Interaction | ∅ | Increased social behavior | |
| Resident Intruder | ∅ | ∅ | |
| Anxiety | EPM | ∅ | ∅ |
| Memory | Y Maze | ∅ | ∅ |
| Social discrimination at 24 h | ∅ | ∅ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





