1. Introduction
Fomites refer to non-living objects or substances capable of harboring infectious organisms, facilitating their transmission from one person to another. The degree of fomite contamination is influenced by factors such as moisture presence, frequency of usage, and hygiene or cleanliness. Fomites are notorious for being a major source of hospital-acquired infections and serving as a potential pathway for pathogens to spread between patients. Common fomites include door handles, showers, toilet seats and faucets, sinks, lockers, chairs, and tables. They are prevalent in various public spaces like hospitals, hotels, restaurants, and restrooms [
1].
In general, the risk of infection spread via fomites depends on various factors: how often one comes into contact with the contaminated area, the amount of microbes released by the infected individual, the likelihood of passing the infection to someone susceptible, the virulence and potency of the microorganisms, the effectiveness of the immune system of those in contact, and the implementation of preventive measures such as using sanitizers/disinfectants and maintaining personal hygiene. As a result, people who rarely wash their hands after using the restroom may gain community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA), which can cause an outbreak, particularly in places that are highly prevalent. In addition, by aerosolization and direct transmission from hands to the fomite surface, fomites can act as a reservoir for bacteria and viruses [
1].
The regular usage of public restrooms could have a substantial impact on the spread and transmission of infectious diseases and other bacterial contamination. Because many individuals use public restrooms or washbasins and touch doorknobs numerous times a day, contamination and pathogenic infectious disease can spread. As a result, the significance of toilets and washbasins as a source of bacterial contamination and infections becomes clearer. It is self-evident that raising people's awareness of transitory contamination and related diseases can benefit the social health and prevent the spread of infections [
2].
Many species of Gram-negative bacteria such as Escherichia and Salmonella and Gram-negative bacteria such as Staphylococcus, particularly MRSA and Streptococcus, can all be found in public restrooms [
3]. They gain access to restrooms through human excrements (urine and faces) [
4] or through human body as many Gram-positive bacteria reside in many parts in human body such as skin, conjunctiva, nose, pharynx, mouth, lower gastrointestinal tract, anterior urethra, vagina, etc. [
5]. Inadequate toilet cleanliness and improper toilet use might allow bacteria to spread from toilets to other areas. Toilet users' contaminated hands can spread bacteria to the flushing handles, door handles, and faucets of toilets. The large amount of toilet flush aerosols produced while flushing can contaminate toilet seats and lids, adjacent floors, and neighboring surfaces. The pathogen's capacity to live on many surfaces in the toilets provides a significant danger of infection to toilet users. The length of time a pathogen may survive on a surface varies depending on the pathogen. The majority of pathogens, such as Shigella species, Escherichia species, Clostridium species, severe acute respiratory syndrome (SARS) coronavirus, and norovirus, can remain on the surfaces for weeks or even months [
4].
Coliform bacteria are rod-shaped Gram-negative that do not have the ability to form spores and they are facultative anaerobes that ferment lactose quickly into acid and gas. In general, coliform bacteria belong to
Citrobacter freundii,
Enterobacter cloacae,
Enterobacter aerogenes,
E. coli, and
Klebsiella pneumoniae which are bacterial genera that belong to Enterobacteriaceae family of bacteria. Some coliform bacteria are called fecal coliforms and are found in the intestine (colon) of warm-blooded animals, whereas others are found in plants [
4]. If coliform bacteria are found in food, this means that the conditions are favorable for the existence of enteric pathogens, and it may indicate that the sanitary precautions are insufficient. That is why coliform bacteria are used as a sanitation and hygiene indicator (fecal contamination indicator) microorganism [
6,
7]. Normally, coliforms do not cause major sicknesses or diseases, but they can grow easily, and their presence can be a sign of the existence of other pathogenic organisms of fecal origin. Disease-causing bacteria, viruses, and protozoa, as well as many multicellular parasites, are examples of these pathogenic organisms [
8].
Bacteria from public restrooms are a major problem to public health when they enter the body through hand-to-mouth or hand-to-food contact, causing illnesses [
9]. Boils and food borne diseases caused by Staphylococcus aureus and Escherichia coli, urinary tract infections (UTI) and diarrhea caused by Escherichia coli and Pseudomonas aeruginosa, and sore throat caused by Streptococcus pyogenes are among the bacterial diseases that can be transmitted through the use of restrooms [
4].
If bacteria isolated from public restrooms demonstrate resistance to antibiotics, the issue will escalate, worsening the antibiotic resistance crisis. This is because the drug resistant bacteria in this case are found in the publicly shared areas like public restrooms surfaces which makes their transmittance easier. Antibiotic resistance is a worldwide public health concern as antibiotic resistant bacteria are becoming increasingly common. Antibiotic resistance makes some curable bacterial infections incurable as the ability to cure the bacterial infections in human or even animals and plants will be decreased. This leads to higher human illness, suffering, and even death, as well as increased treatment costs and duration, in addition to the increased side effects resulted from the usage of many and stronger medicines [
10]. As few new antibiotics are being developed, antibiotics should be used carefully and only in the urgent cases [
11].
The misuse and overuse of antibiotics leads to the development of antibiotic resistance by bacteria to be able to survive. The main and common antibiotic resistance mechanisms are the antibiotics accumulation prevention through decreasing the uptake or increasing the efflux, alteration of the antibiotic target (i.e., ribosome subunits, cell wall penicillin binding proteins (PBPs), or DNA gyrase and topoisomerase IV), and antibiotic inactivation through the enzymatic modification or degradation [
12].
Regular hand washing, and disinfectant cleaning of the public restrooms at least two times a day are all suggested in the programs designed to control infections and decrease the risk caused by bacterial infections. Sensor-operated paper towel dispensers and touch-free-electric hand dryers are two new technologies used to reduce the infections caused by the usage of public restrooms. The microorganisms (bacteria) number that are emitted into the air can be reduced by closing the toilet seat after usage [
4].
Shopping malls are one of the most heavily frequented public spaces. The economic quality of these facilities varies as some are high class luxurious malls which attract wealthy high socioeconomical individuals. Other malls are of a poorer quality, with poor maintenance and services and thus they are visited by the lower socioeconomic individuals or classes. As a result, due to its economic class, contamination in restrooms is predicted to be influenced by factors affecting the building quality, maintenance, and service excellence.
The aim of this study is to establish a baseline study about the degree of bacterial contamination and variety in the public restrooms in shopping malls, based on their economic status and health-care quality. Particularly, to provide a qualitative and quantitative assessment about the prevalence of Gram positive and Gram-negative bacteria in the public restrooms using several tests, as well as their susceptibility and resistance patterns to a variety of antibiotics classes.
2. Materials and Methods
2.1. Sample Collection and Transportation
In this cross-sectional observational study, dry sterile cotton swabs (Puritan Medical Products) were used to collect samples from public restrooms in 10 selected shopping malls, 5 lower-end and 5 upper-end and from 5 spots which are seat (S), water sprayer (W), tap (T), inner door handle (ID), and outdoor handle (OD). They were opened in the restroom, dipped in the conical tube containing peptone water medium, and rubbed across the surface (spot) of interest. Then, the swabs were returned to the conical tubes and the tubes were labeled according to shopping mall number, restroom category (♂/♀) and economic status (lower-end restroom (LR) or upper-end restroom (UR)), spot, and replicate number. All tubes were placed on ice and taken to the lab for further plating and processing. For standardization, a weekend day was chosen for the sample collection. The standardization was done by observing the bacterial growth at rush and non-rush hours and accordingly a time in-between was chosen for the rest of collections.
2.2. Dilution, Plating, and Incubation
In less than 24 hours, the samples were processed in the lab. Firstly, the tubes were vortexed for 40 seconds (if needed) to disrupt the precipitation at the bottom of the tubes. For the samples targeting Gram-positive bacteria, a serial dilution up to 10-5 dilution was done for all samples for standardization purposes. For each sample, both the original sample as well as the 5 diluted samples were plated. Each sample was aseptically plated in two nutrient agar (NA) plates. The plates were incubated upside down at 37ºC incubator for 48 hours. Regarding the samples targeting Gram-negative bacteria, the plating process was done as mentioned before but the plates used were MacConkey agar (MAC) plates instead of the NA plates.
2.3. Counting and Characterization
After 48 hours, the plates were taken from the incubator to count the colonies and record their number in order to calculate the colony forming unit (CFU) for the samples targeting Gram-positive bacteria and the total coliform count for the samples targeting Gram-negative bacteria. For the samples targeting Gram-positive bacteria, the most dominant isolates (24 isolates) were sub-cultured on new NA plates and incubated upside down at 37ºC incubator for 24 hours. However, for the samples targeting Gram-negative bacteria, the colonies were not sub-cultured. The bacterial isolates were characterized morphologically by identifying the form or shape, surface, color, margins, and/or elevation.
2.4. Identification of the Isolates
For the samples targeting Gram-positive bacteria, the most 24 dominant types of colonies or isolates undergone Gram staining to select the Gram-positive isolates for further processing and identification. Then, Gram-positive isolates were sub-cultured and purified on NA plates and incubated upside down at 37ºC incubator for 24 hours. After that, a series of identification methods were performed to identify the types of Gram-positive bacteria in the samples. Three identification methods were done which are the primary identification by the conventional and biochemical tests, matrix-assisted laser desorption ionization time-flight mass-spectroscopy (MALDI-TOF-MS) rapid identification, and the confirmatory identification by the BD Phoenix™ automated microbiology system. The identification process for the samples targeting Gram-negative bacteria was done using MALDI-TOF-MS after selecting 5 bacterial isolates and sub-culturing them on NA plates.
Conventional and Biochemical Tests. These tests include Gram staining, catalase production test, carbohydrate (glucose/arabinose) fermentation test, and selective media (Mannitol salt agar (MSA) and MAC) tests.
MALDI-TOF-MS Analysis. The most dominant isolates were purified in NA plates one day before and analyzed using MALDI-TOF-MS. The purified isolates were transferred to MALDI target plate and a matrix solution, an energy absorbent organic substance, was added to the samples to be analyzed by MALDI-TOF-MS. The resulting spectrum for each sample was analyzed by MALDI Biotyper (MBT) Compass Software and a molecular fingerprint peptide mass fingerprint (PMF) was generated from the mass spectrum giving a species-specific pattern. This software assesses each spectrum compared to a reference spectrum in the database to determine the best match for each sample. A score (QI) between 0 and 3 was given to each sample to compare the level of similarity between the pattern given by the unknown sample and the database where the higher similarity is represented by a higher score (closer to 3). This test was repeated twice for confirmation.
BD Phoenix™ Identification Test. The samples tested using MALDI-TOF-MS were double tested by the BD Phoenix™ automated microbiology system as a confirmatory identification test.
2.5. Antimicrobial Susceptibility Testing (AST)
Antibiotic susceptibility and resistance patterns of the isolates were studied using BD PhoenixTM automated microbiology system. This system gives susceptible, intermediate, and resistant (SIR) based interpretations. Selected samples from the samples targeting Gram-positive bacteria were tested by two resistance markers which are the phoenix methicillin-resistance in Staphylococci (MRS) and the beta lactamase producing bacteria (BLACT). Staphylococci spp., which are frequently isolated from restrooms were chosen to be tested against 23 different antibiotics. In the case of samples targeting Gram-negative bacteria, 21 antibiotics were used.
2.6. Statistical Analysis
The statistically significant differences were determined using students unpaired t test using 95% confidence level (significance level (α) = 0.05). The software used to analyze the data was GraphPad Prism 9. In all cases, a P-value less than 0.05 (*P˂0.05) was considered significant and the values was expressed as “mean ± SD”.
4. Discussion
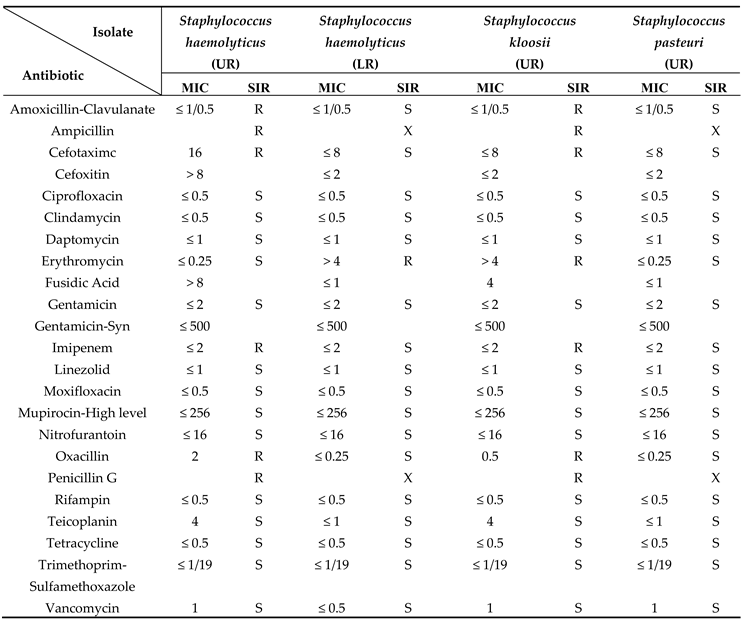
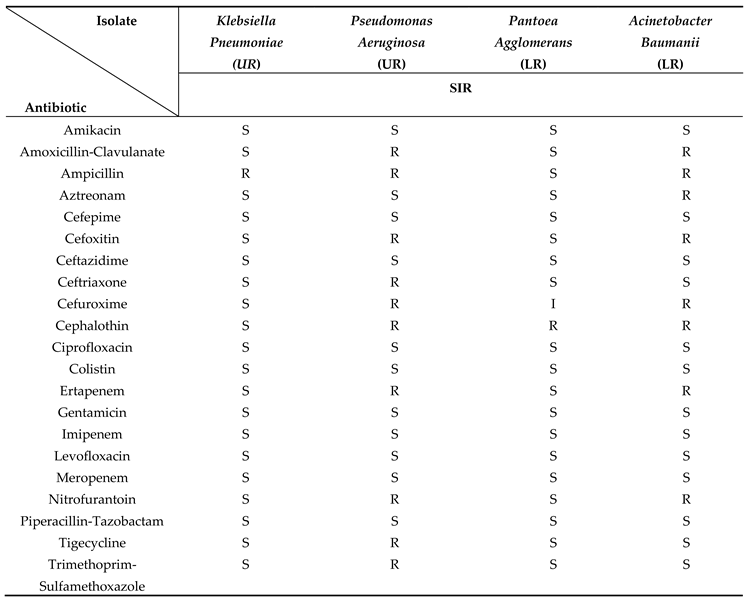
Identification of selected isolates using matrix-assisted laser desorption ionization time-flight mass-spectroscopy (MALDI-TOF-MS) and other techniques revealed that the coagulase-negative staphylococci (CNS) species including Staphylococcus haemolyticus, Staphylococcus kloosi, and Staphylococcus pasteuri represented 86% of the identified Gram-positive isolates with the Staphylococcus haemolyticus being the most dominant strain among them (75%). The Micrococcus leuteus and Bacillus clausii, represented 9% and 1% of the identified Gram-positive isolates, respectively. The most dominant identified Gram-negative bacteria were Klebsiella pneumoniae (87.35%), Pseudomonas aeruginosa (8.74%), Pantoea agglomerants (0.26%), Acinetobacter baumanii (2.15%), and Acinetobacter lwoffii/haemolyticus (1.50%).
The results of the antibiotic sensitivity test (AST) using BD Phoenix™ automated microbiology system revealed that there are some multi-drug resistant (MDR) bacteria among the Gram-positive and Gram-negative isolates which are Staphylococcus haemolyticus, Staphylococcus kloosi, Pseudomonas aeruginosa, and Acinetobacter baumanii. Some of the isolated bacteria are part of the human microbiota, but they are pathogenic and cause many infectious diseases and that is why their resistance pattern as MDR bacteria can affect the future of treatment and transmission of infectious diseases.
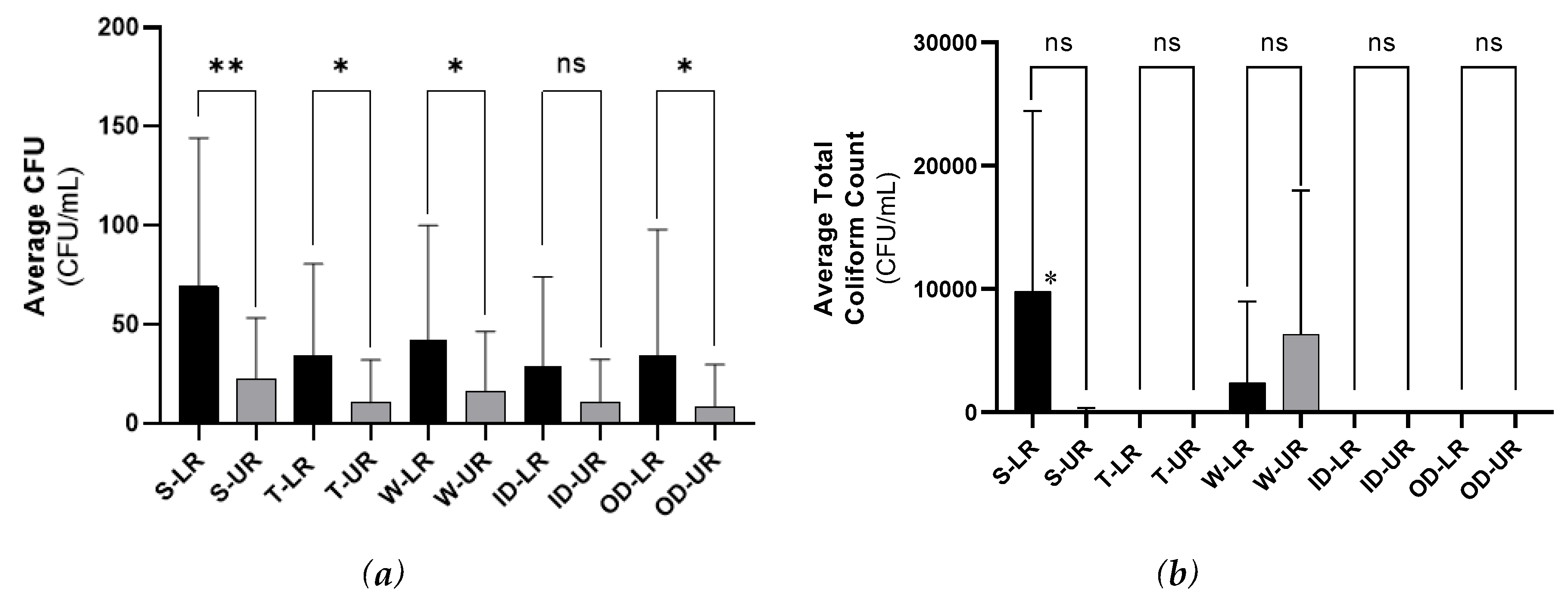
Due to their warm and humid atmosphere, public restrooms are shown to have the ideal conditions for the accumulation of pathogenic or non-pathogenic microorganisms, especially bacteria. In general, the LR category of the restrooms contains higher contamination levels compared to the UR category. Some factors can influence the contamination degree in the public restrooms which are the frequency of cleaning shifts, especially during rush hours, and the quality of cleaning products. In contrast to the LR category, the UR restrooms are cleaned after every single use. Moreover, the building design can have a huge effect and it differs between UR and LR categories. Unlike the LR category, it was noticed that most restrooms in high class shopping malls do not have outer doors, instead, they have halls leading to the restroom area, and the sink taps open automatically by laser sensors, instead of manual tap handles. Consequently, the designs of the UR category lowered the number of touched surfaces in the restrooms, thus less bacterial contamination was observed. In general, these observations about restroom design differences regarding the “T” and “OD” spots might explain the reason why the contamination level in these spots was low especially in the UR category as these spots/surfaces are not touched frequently. This agrees with a study done by the National Research Council that links the spread of infections to the features of buildings. The study has addressed a number of factors that contributes to the contamination transmission in school buildings and their restrooms, and these factors include surfaces’ sanitizing, and the number and availability of touched surfaces like sinks and toilets. They suggested the replacement of traditional designs of door handles, flushers, soap, and towel dispensers by an alternative hands-free design in order to eliminate contamination spread which might help the infectious disease transmission [
13]. Furthermore, the “S” spot especially in the LR category was found to be hugely contaminated with both Gram positive and Gram-negative bacteria as indicated by the uncountable growth and this is possibly because this spot has a high contact frequency by the restroom users.
An observational study looked at the sanitation of the public restrooms and tested the facilities' (i.e., handwashing and hand-drying facilities) microbes. This study found that high and/or middle-category restrooms were significantly more likely than low-category restrooms to have toilet seat disinfectants as well as a cleaner environment in the toilet/urinal area, floor areas, walls, and sinks [
9]. Another study examined the bacterial contamination of regularly handled surfaces, such as toilet surfaces, in four shopping malls in the United Arab Emirates. According to this study, a lot of people of diverse ages, cultures, social classes, and, of course, different hygiene habits use the public restrooms in UAE shopping malls, making them significantly more likely to be contaminated with bacteria than restrooms elsewhere. Additionally, the results show that mall cleaning and sanitization procedures must be changed to accommodate visitor density, with weekend and holiday cleaning efforts necessitating more frequent efforts than during regular weekdays [
14].
The diversity of the bacterial taxa presents in toilets, which is influenced by the number of different human occupants each day, can generally have an impact on the bacterial pollutants [
15]. Due to physiological differences in the normal microbiota, gender can have an impact on diversity too.
The qualitative analysis of bacterial isolates from the numerous areas swabbed in the current experiment showed the abundance of the typical skin flora. Non-pathogenic bacteria of the skin flora, such as Coagulase-negative Staphylococcus (CNS) species including
Staphylococcus haemolyticus,
Staphylococcus kloosi, and
Staphylococcus pasteuri were identified. The CNS was the most dominant type of species among the identified isolates [
16]. This finding is consistent with research done at Michael Okpara University of Agriculture, Umudike, to isolate, identify, and evaluate the pattern of antibiotic sensitivity of bacterial contaminants recovered from door handles, including restrooms. According to this study, CNS species make up 21.2% of the 130 bacterial isolates in total [
17]. In addition, the UAE study previously cited indicated that 99% of all positive samples included non-pathogenic skin bacteria such Staphylococcus epidermidis and other CNS species [
14].
Given that the majority of the surfaces studied come into close touch with human skin, the presence and dominance of the skin microbiota on toilet surfaces is not unexpected and is predicted. Research has shown that skin-associated bacteria are usually resilient and have the capacity to survive and remain on surfaces for extended periods of time [
18]. Despite having a reputation as skin commensals, CNS species are the most common endemic nosocomial pathogen in newborns. Bloodstream infections, which cause 51% to 78% of newborns with very low birth weights (VLBW), make up the majority of CNS infections. CNS pathogens, however, have a low fatality rate and low pathogenicity [
19].
Staphylococcus aureus was suspected to be among the isolates, as shown by the biochemical tests, because of its traits as a Gram-positive, cocci-shaped, catalase-positive, glucose, mannitol, and lactose fermenter as well as its inability to grow in MAC medium [
16,
20].
At the Sokoine University of Agriculture in Morogoro, Tanzania, a study was conducted to isolate, identify, and ascertain the bacterial loads in the public restrooms of the student residences. This study demonstrated the presence of MRSA, a drug-resistant form of
Staphylococcus aureus, as well as other bacteria, in public restrooms. Through human waste (faces and pee), they gain entry to the restrooms. Bacteria may spread from the toilets to adjacent locations due to poor toilet hygiene and incorrect toilet use. The flushing handles, door handles, and faucets of toilets can become contaminated with bacteria from the hands of toilet users [
4]. Because
Staphylococcus aureus is thought to be a component of human skin and mucosal membranes and because people are their main reservoir, its presence in public restrooms is anticipated. It can spread from person to person through direct touch or fomites. As one of the most common bacterial infections in humans, it is also the root cause of a number of illnesses, including bacteremia, infective endocarditis, skin and soft tissue infections, osteomyelitis, septic arthritis, infections of prosthetic devices, pulmonary infections, etc. [
21]. Additionally, certain locations in the restrooms had Bacillus spp. Because they are spore-forming bacteria, these species can endure extreme temperatures, cold, radiation, desiccation, and chemical disinfectants [
17].
Some of the detected isolates fall into the taxa/phyla linked with the gut. For instance, Staphylococci species are members of the Firmicutes phylum, which together with the Bacteroidetes phylum accounts for 99% of the gut microbiota [
22]. Taxa associated with the gut were more prevalent on toilet surfaces, indicating that feces had been present there. Indirect contact with water splashes (aerosols) from toilet flushing can also result in fecal contamination, as can direct contact with feces or dirty hands. Given that enteropathogenic bacteria may spread similarly to how human commensals do, the high number of gut-associated species found in restrooms is alarming for the general public's health [
23].
Other studies focusing on non-hospital environments with equally diverse bacterial populations have mentioned that restroom environments are a possible harbor for bacterial populations with an antibiotic resistance and suggested that the diverse bacterial populations can provide the favorable conditions and environments to support the development, sustainability, and spread of bacterial antibiotic resistance. In addition to the prevalence of human diseases in restrooms, these studies have also suggested that restroom environments are a possible harbor for the bacterial populations with an antibiotic resistance. Additionally, cells might be able to resist and survive under such conditions even if resources are scarce. Overall, this study indicated that toilets are one of the primary and potential sources of infections and that they may be able to sustain bacterial "resistomes” [
3].
CNS species are clinically significant, firstly due to their nosocomial pathogenicity and secondly because many strains of CNS species are methicillin-resistant and are developing more antibiotic resistance which make CNS a serious problem [
24], especially their resistance to consistently and commonly used antibiotics [
25]. The findings of this investigation support the notion that
Staphylococcus haemolyticus is a multidrug-resistant (MDR) bacterium because it displayed resistance to 7 different drugs. In the current investigation,
Staphylococcus kloosi also demonstrated resistance to 7 different antibiotics. This finding partially accords with a study that examined the antibiotic resistance profile of
Staphylococcus kloosi isolated from a blood culture of a patient with sepsis and an intracranial hemorrhage using the disc diffusion method. Penicillin, oxacillin, erythromycin, clindamycin, cotrimoxazole, ofloxacin, and linezolid were all ineffective against
Staphylococcus kloosi [
26]. The
Staphylococcus pasteuri isolate used in this investigation did not exhibit resistance to any of the examined antibiotics. A different study, however, found that
Staphylococcus pasteuri was resistant to a variety of antibiotics, including Chloramphenicol, Streptomycin, Fosfomycin, Macrolides, Lincosamides, Streptogramins, and Tetracyclines [
27].
Regarding Gram-negative bacteria, the study conducted in Tanzania revealed that
Pseudomonas aeruginosa was more common (13.3%) than
Klebsiella pneumonia (11.6%) in public bathrooms. These results are consistent with and comparable to those of this study. The isolates discovered in this study can also spread or cause a variety of illnesses. For instance,
Klebsiella pneumonia produces pneumonia while
Pseudomonas aeruginosa causes UTI [
4]. A Gram-negative bacteria that is connected with plants called
Pantoea agglomerans has the potential to infect people through open wounds and, in severe circumstances, can lead to septic arthritis. It is not always an infectious agent in people. It could, however, be a source of opportunistic human infections, mainly in immunocompromised people, through wound infection with plant material or as a hospital-acquired infection [
28].
Pantoea agglomerans was the least prevalent isolate in this study (0.26%) which is most likely due to it being a plant-associated bacteria.
Acinetobacter baumanii is a Gram-negative bacterium that can cause bacteremia, a disease when germs are present in the patient's circulation, as well as a number of infections affecting the urinary, gastrointestinal, and respiratory tracts. This isolate displayed penicillin and cephalosporin resistance characteristics [
29]. We found a similar antibiotic resistance pattern with this isolate in this study. Similarly,
Acinetobacter lowffii/haemolyticus are also Gram-negative isolates that can cause bacteremia [
30]. Globally,
Klebsiella pneumoniae is a common cause of MDR infections. Recent research has revealed that there is a MDR
Klebsiella pneumoniae strain that is resistant to the last-line antibiotic colistin [
31]. However, the findings of this study showed that the isolated strain of
Klebsiella pneumonia is only resistant to ampicillin.
In recent years, there has been increasing concern about the ability of MDR bacteria to evade the killing effect of disinfectants, which are commonly used to control the spread of infectious pathogens. One of the most notable discoveries in this study is the identification of highly multi-drug resistant (MDR) bacteria in an upscale restroom where a designated individual regularly disinfects the toilet after each use. This highlights the ability of MDR bacteria to withstand the disinfectant's killing effect. Despite the significantly lower bacterial count in high-end restrooms compared to low-end ones, they are not entirely free from MDR bacteria. This underscores the importance of public awareness and the necessity of maintaining good hygiene practices when using public restrooms. Continued research and vigilance are essential to address this growing threat and develop effective strategies to combat disinfectant-resistant MDR bacteria.
The current study encountered some limitations. Due to the enormous number of bacterial colonies that were recovered from numerous locations, not every isolated colony was identified. In contrast, a small number of colonies were picked for identification based on their frequency and shape. It's possible that the bacterial species found in the current study don't accurately represent the species distribution in the sites examined. Additionally, due to a lack of resources, antibiotic sensitivity patterns were only performed on a small number of chosen isolates. Additionally, the layout of the facilities as well as the placement of the paper towel and hand soap dispensers were not monitored. This is crucial because, despite the fact that hand soaps, detergents, and paper towels are provided for bathroom users, they may occasionally be hidden from view or positioned incorrectly.