Introduction:
Annually, there are 14000 new rectal carcinoma diagnosed in the UK and nearly half a million cases globally, and about 10-20% are locally-advanced with invasion into adjacent viscera at presentation [
1]. Intraoperatively, it is difficult to differentiate whether the adhesions between the tumor and adjacent viscera is due to malignant infiltration or desmoplasia. To achieve R0 resection, the standard practice is to perform en-bloc resection [
2]. Nonetheless, resection of any locally advanced rectal carcinoma is technically difficult, which is associated with higher treatment failure rates and significant morbidity. This can be partially avoided by input from a well-coordinated multidisciplinary team, including surgeons, radiologist, pathologist, radiation and medical oncologists. The surgical aim in these patients is a complete resection of the tumor and rectum en-bloc with any involved viscera (R0 resection) with preservation of quality of life.
An open procedure has been mostly used in vast majority of cases. The laparoscopic rectal surgery for rectal carcinoma is developed into a safe and effective treatment option. The laparoscopic rectal resection with total meso-rectum (i.e. TME) is matching open resection in terms of quality of surgical specimen (i.e. resection margin) and long-term oncologic outcomes; however, it is technically demanding and still not established as the gold standard surgical procedure for rectal carcinoma [
3,
4,
5]. The technical difficulties are attributed to the narrow anatomical space of the lower pelvis, restricted movements of the rigid laparoscopic instruments, obesity and bulky tumors [
5,
6,
7]. Due to these technical difficulties, for locally advanced rectal carcinoma, laparoscopic surgery may result in incomplete resection and incomplete TME [
8].
Robotic platforms (da Vinci
® Si/X/Xi, Intuitive Surgical, California, USA) offer advantages in overcoming some of these technical difficulties and allow precise dissection in the narrow pelvis through motion scaling and intuitive manipulation, high-definition three-dimensional images (with up to 10x magnification) from a steady camera, endowrist instruments, and stable traction provided by the robotic arms [
4,
5,
6,
7,
9]. With these potential benefits, robotic rectal surgeons have reported oncologic outcomes equivalent to laparoscopic surgery [
10,
11], and improved outcome in difficult clinical situations like obesity, male sex, post chemoradiotherapy, and cancers of the lower 2/3 of the rectum [
5,
12].
Our unit has been performing Robotic colorectal surgery for the last 7 years and more than 500 colorectal cancer resections have been performed. As experience grew we have attempted robotic en-bloc resection of locally advanced rectal carcinoma, which we are presenting in this study. Our primary end-point was to assess the feasibility and oncological safety. The secondary end points included were operative duration, amount of blood loss, hospital course of stay and postoperative adverse events. To our knowledge this is one of the first reports looking at the outcomes of robotic assisted en-bloc resection of stage T4 rectal carcinomas.
Methods:
This study was conducted at the Queen Alexandra Hospital, Portsmouth, UK, from June 2015 to July 2018. The patients were retrospectively identified from an ethics approved prospective database kept at an associated teaching hospital for mandatory national audit.
All patients who underwent total robotic resection of stage T4 rectal cancers en-bloc with involved adjacent visceras were eligible for inclusion and enrolled in the study. The multi-visceral, en-bloc resection was performed on the basis of radiological or intraoperative indication of adjacent visceral invasion. The exclusion criteria included recurrent cancers, incomplete resection, resection in separate pieces, emergency resection, and patients receiving palliative treatment. Written, informed consent was obtained and patients were counseled about their overall physical status, disease condition, the potential risks and benefits of the operation, and other management options.
Preoperative Workup
Preoperative work-up included rectal examination, endoanal ultrasound, pelvic MRI and a staging CT scan of chest, abdomen and pelvis. In some cases, positron emission tomography (PET-CT) was also performed to rule out occult metastasis. All patients were discussed in a complex cancer MDT and planning X-ray meeting before surgery. Urological and gynecological advice was sought in relevant cases. The decision of neoadjuvant treatment was made after the MDT discussion. Tumors at a favorable location (upper rectum), anteriorly-based with uterine or peritoneal reflection involvement without the presence of large vessel extramural venous invasion (EMVI) and good probability of R0 resection were directly scheduled for surgery. Others received neoadjuvant chemoradiotherapy with a view to downstage. Surgery in such patients was scheduled at 10-12 weeks post-chemoradiotherapy. All patients received mechanical bowel preparation with two sachets of picolax.
Operative Procedure
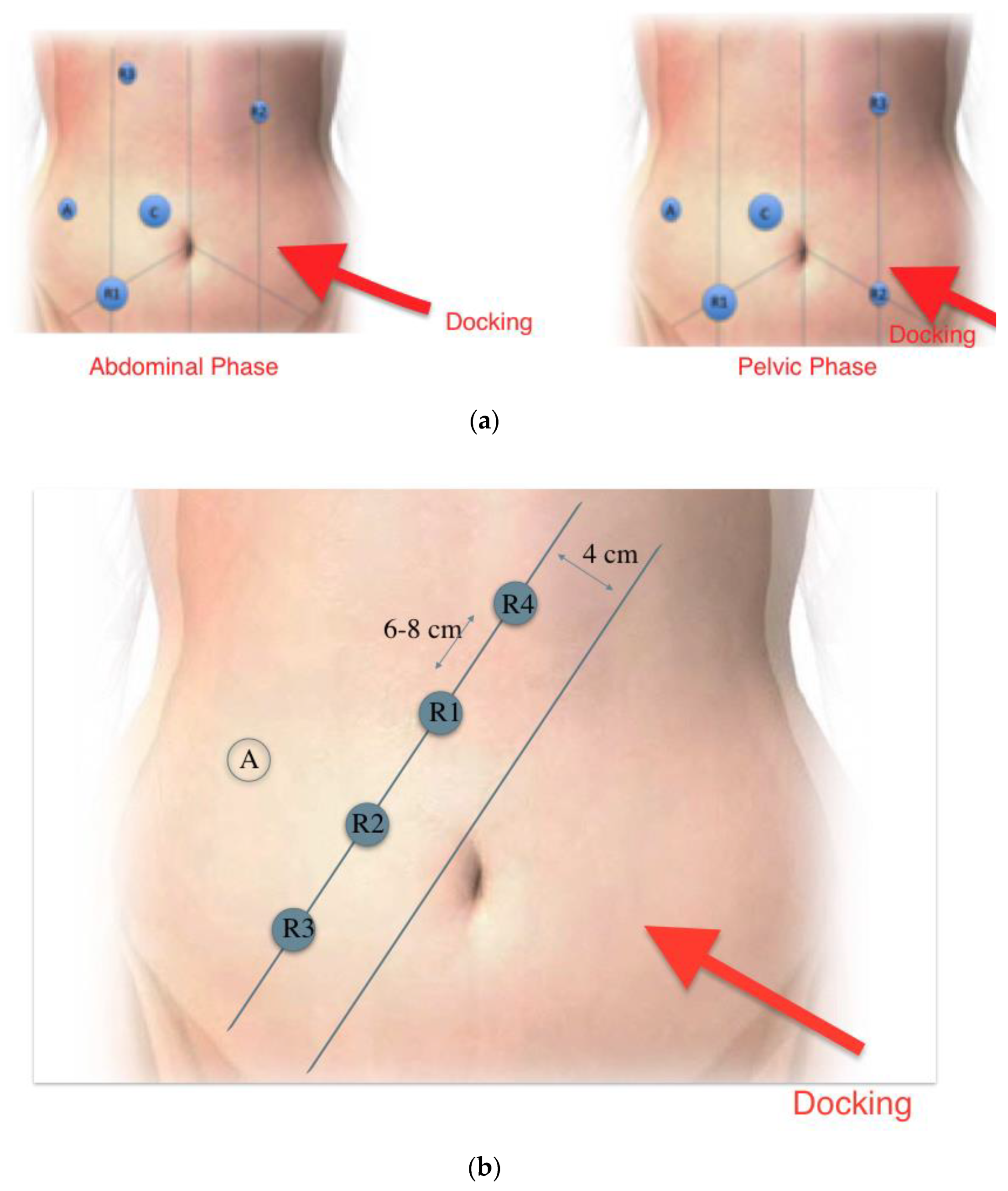
Surgery was carried out using Da Vinci Si and X systems (Intuitive Surgical California, USA). A standardized technique was used, with single docking from the left hip. All patients were operated in modified lithotomy position with 10° trendelenberg and 20° right tilt. Port placement for Si system is shown in figure 1a. These included 4 8-mm DaVinci ports at the right upper and lower, and left upper and lower abdomen, and another 12-mm port laterally on right side (used as the assistant port for Si system). With the X system all robotic ports were placed on a straight line (
Figure 1b)
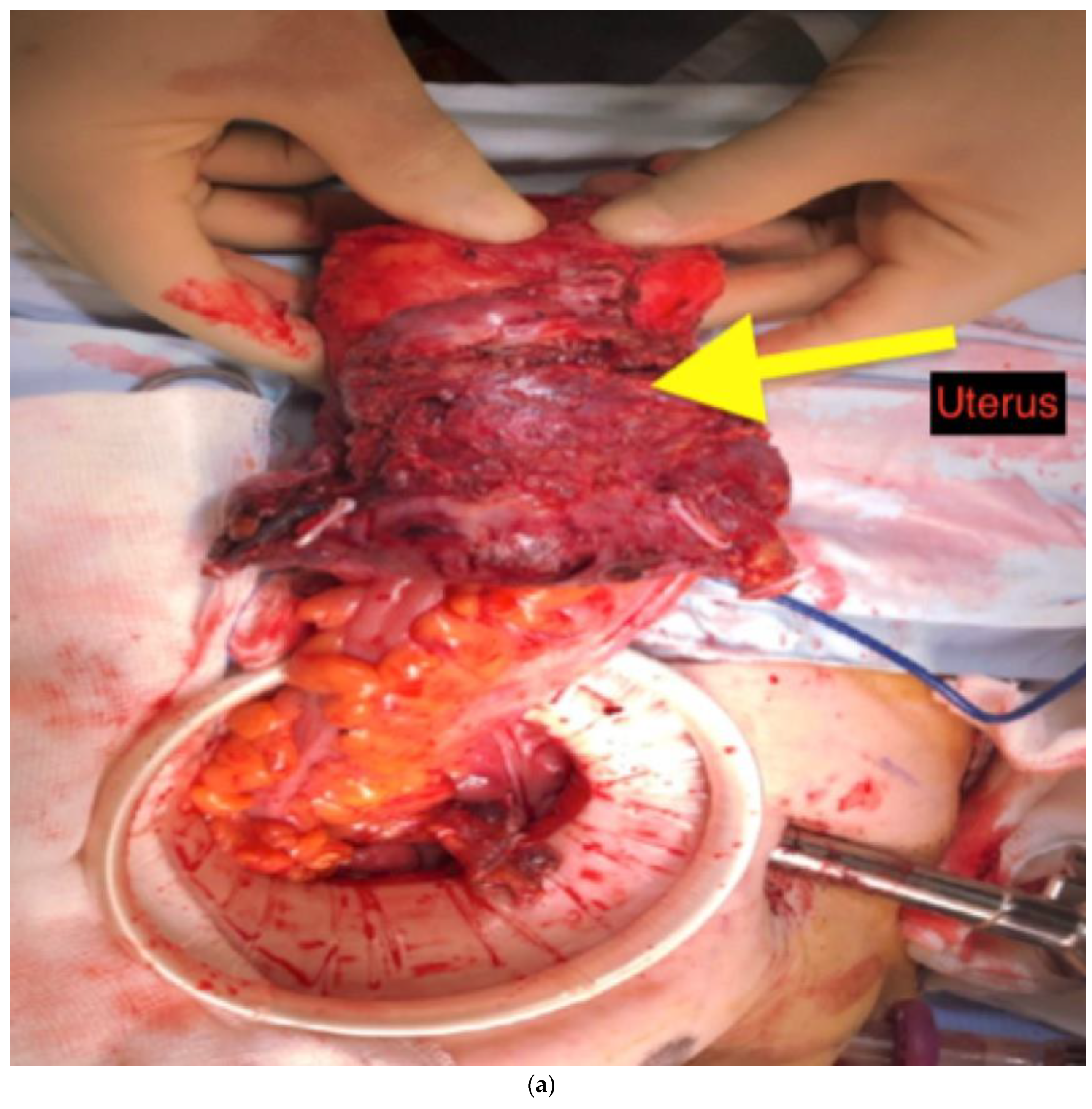
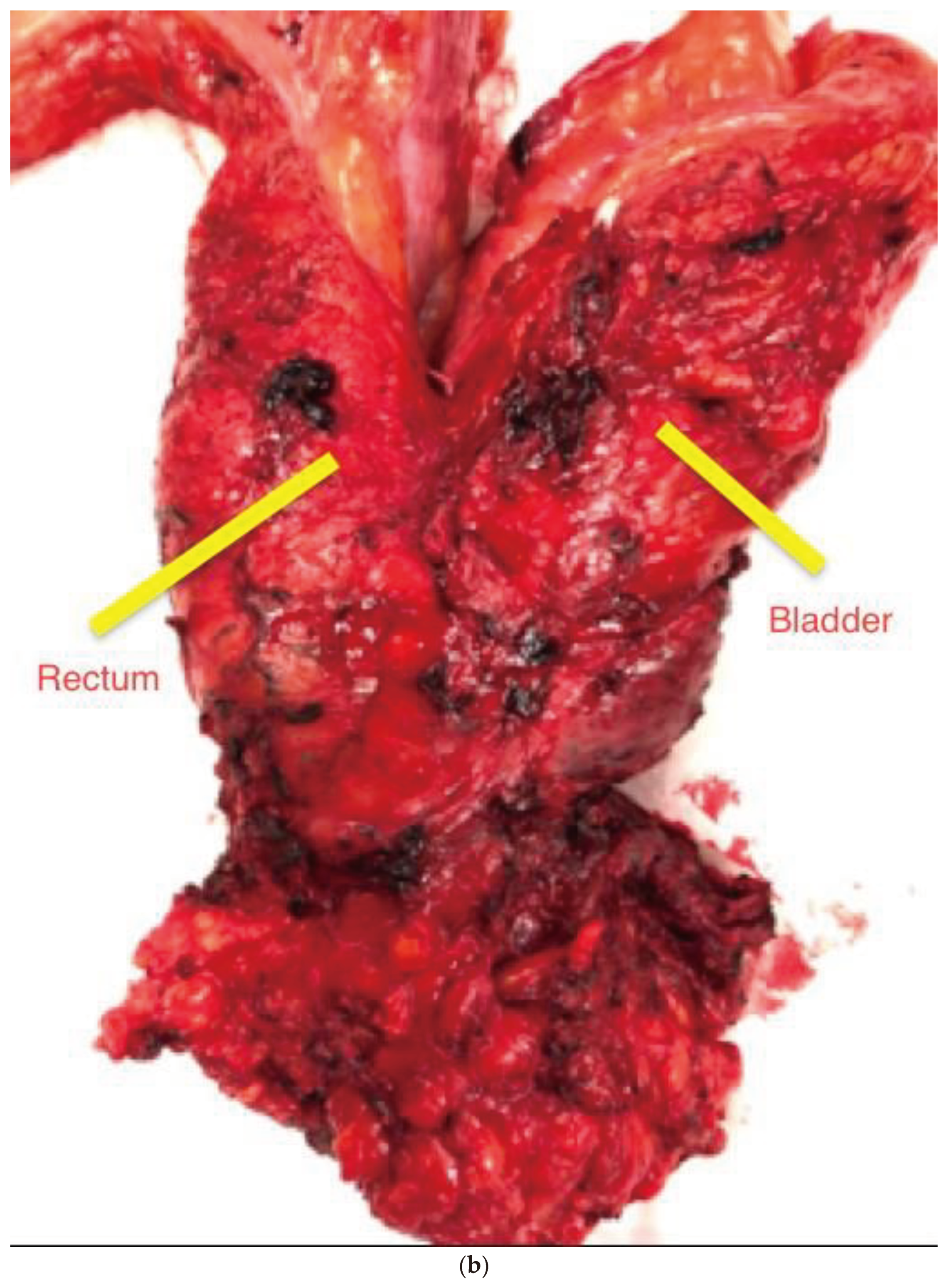
After opening the retroperitoneal space at the sacral promontory, dissection continued in the midline to identify the inferior mesenteric artery, which was divided at its origin. The inferior mesenteric vein was divided below the inferior pancreatic border. We routinely mobilized splenic flexure to facilitate a tension-free anastomosis in the pelvis. The pelvic dissection was performed according to the TME principles. Any densely adherent adjacent viscera were included in the resection. Finally, resection of the cancerous segment was done via endoscopic stapling or inter-sphincteric resection, with restoration of bowel continuity with intracorporeal double stapling. A covering loop ileostomy was carried out in patients who had restorative anterior resection. Depending on the pattern of organ involvement, we extend our surgical resections beyond the normal anatomical planes. The adherent visceras were completely resected in an en-bloc fashion (
Figure 2a and
Figure 2b). Working together with the urologists on a dual console platform was very helpful in some of these challenging cases.
T4 rectal cancer invading the bladder trigone received an exenterative procedure (total pelvic exentrtation). Pararectal spaces were created on both sides after mobilizing and medializing ureters. Subsequently the bladder was mobilized from anterior abdominal wall and space of Retzius was dissected and connected with paravesical spaces. Puboprostatic ligament was then divided and the dorsal veins were suture ligated and divided followed by the division of the urethra. The ureters were the divided distally and a bladder reconstruction was done with an ileal conduit. In women, tumors that involved the uterus/cervix we performed a posterior pelvic exenteration (rectum, uterus and partial vagina) with the robotic platform.
Postoperative Care
All patients received adjuvant chemotherapy 6-8 weeks after rectal resection. In cases of diverting stoma, restoration of bowel continuity was completed after adjuvant chemotherapy. The procedure related postoperative adverse events that occurred within 90 days were recorded.
Outcome Measures and Statistical Analysis
A prospective database was maintained for the evaluation of outcomes, both clinical and oncological. Our primary outcome end-point was to assess the oncological safety and technical feasibility assessed by CRM involvement and conversions to open procedure. The secondary end-points were operative duration, amount of blood loss, hospital course of stay and procedure related postoperative adverse events. The histology of adjacent resected viscera was reviewed and cross-referenced with operative notes. Other secondary outcome variables were patient’s survival, disease-free survival and recurrence, all obtained from cancer clinic surveillance records. Other variables noted and analyzed included patient demographics, co-morbidities, colonoscopy findings and preoperative imaging (CT, PET, MRI).
IBM SPSS Statistics software (v 23) was used for statistical analysis. Categorical variables data are presented as frequencies and percentages, while median (range) or mean (standard deviation) was used to assess continuous variables. Inferential statistics were calculated using the chi-square and t test, and a p-value of < 0.05 was considered significant.
Results:
Thirty-one patients were enrolled in this study having stage T4 rectal carcinoma invading adjacent viscera. Six of them had metastatic disease at presentation and were managed in a palliative setting. Two patients had a laparoscopic resection and 2 patients had an open procedure based on the operating surgeons’ preference, and were excluded from the final analysis.
Twenty-one patients remained in the study for final analysis; thirteen (62%) of them were females, with a mean age of 74 years. Patient demographics are shown in
Table 1. Most patients were overweight, with a median body mass index of 28.5 kg/m
2. Fifty-seven percent of tumors were located in the lower two-thirds of rectum. Preoperative radiotherapy was used in 10 patients for down staging (7 long-course, 3 short-course). The involved visceras that were resected en-bloc included small bowel, urinary bladder, ovaries, uterus, vagina, seminal vesicles and prostate. Permanent stoma was made in 7 patients (
Table 2).
The median hospital course of stay was 6 days (range 4-25), with 2 patients requiring ITU admission after the operation. In the postoperative follow-up period, there were 3 re-admissions mainly due to nausea and vomiting, high output from stoma and poorly controlled pain. The mean per-operative loss of blood was 20 ml (range 5-200), and the mean operative duration was 280 min (range 240-430).
We achieved R0 resection in 19 out of 21 patients (
Table 3). One positive margin patient had local recurrence during the follow-up period, while the other remained disease-free at the 21-months follow-up. Both patients with R1 resection received neo-adjuvant long course chemoradiotherapy. Our 90-day mortality was zero; postoperative complications are shown in
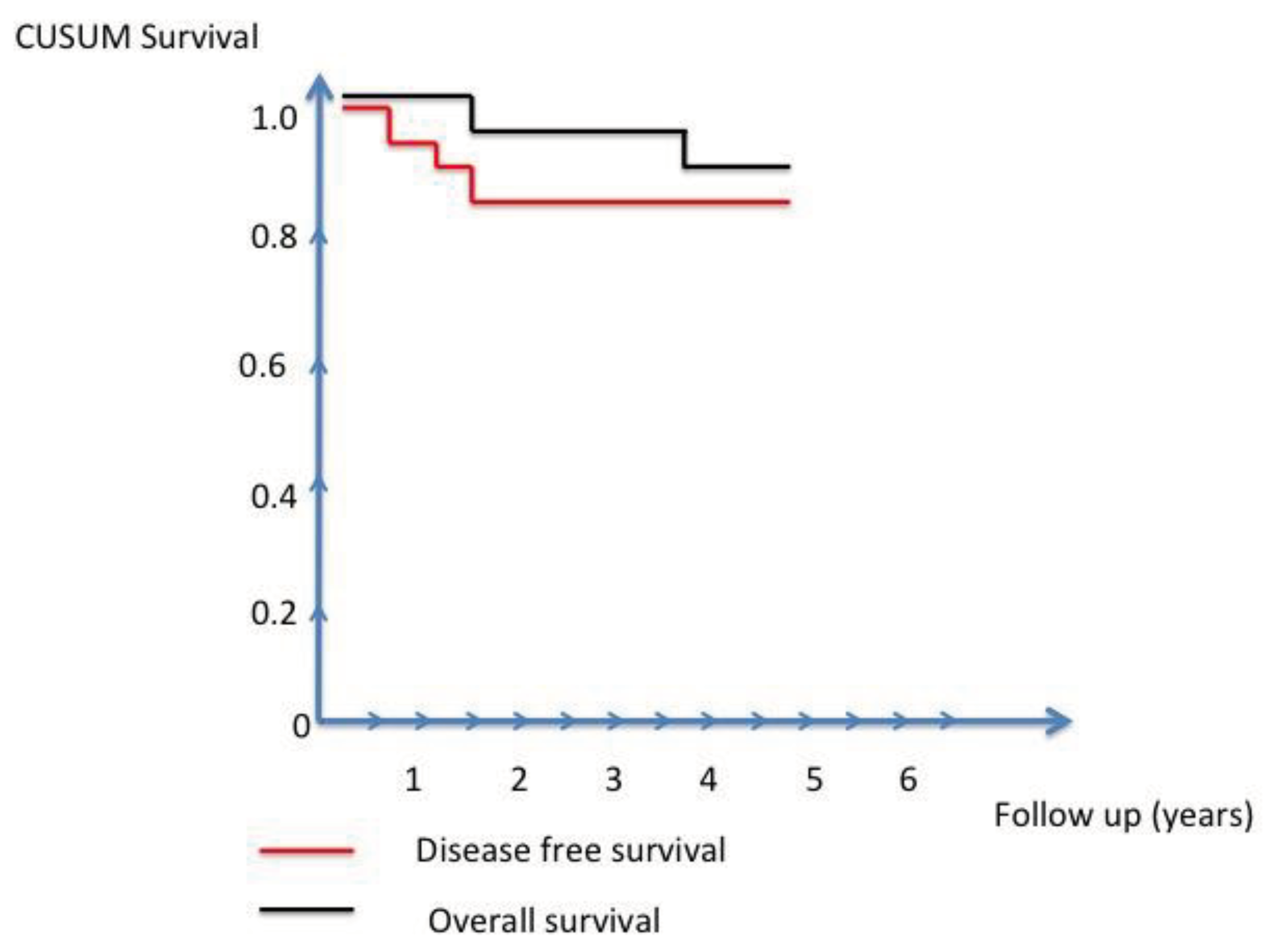
Table 4. With a median follow-up of 36 months, the overall survival at 3 years was 96% and the disease free survival was 84% (
Figure 3). All recurrences occurred within 18 months after surgery.
Discussion:
The laparotomy still considers as the standard procedure for stage T4 rectal carcinoma, and the laparoscopic resection may result in incomplete resection and incomplete TME [
13]. Due to technical difficulties, the adoption of laparoscopy in locally advanced rectal cancer remains limited. In patients requiring pelvic exenteration, a laparoscopic vesico-urethral anastomosis or ileal conduit formation is technically demanding. Robotic platforms not only can make these challenging steps relatively easier but also can overcome many of the other limitations of laparoscopy mentioned earlier. In the current literature, there is limited experience about robotic assisted T4 rectal cancer resections.
Intraoperative differentiation of malignant infiltration from inflammatory adhesion is difficult; confirmation can only be made by histopathological examination. Hence, the standard operative strategy for locally-advanced colorectal cancers abutting other viscera is to perform en-bloc multi-visceral resection to achieve R0 resection margin. Achieving a clear resection margin reduces the local recurrence risks. Conversely, the rate of local recurrence can be high when an attempt is made to dissect the adherent viscera, as reported in literature [
14]. In addition, the 5-year survival is lowered in cases of inadvertent dissection or tumor rupture after en-bloc resections [
15]. In our case series, we achieved R0 resection in 90% of cases with locally advanced T4 tumors.
In this series, the malignant infiltration was histologically confirmed in 65% of rectal cancers, which were found adherent to adjacent viscera. Nishikawa et al reported 60.9% malignant infiltration to adjacent viscera in a series consisting of open, laparoscopic, and robotic resections [
2], whereas other studies reported 25-40% malignant infiltration [
16,
17,
18]. A clear resection margin (R0) is one of the most important prognostic factors in the management of stage T4 carcinomas of colorectum [
19]. However, even en-bloc multi-visceral resection fails to guarantee clear resection margin, varying between 40-90% [
2,
20,
21,
22]. With a higher percentage of R0 resections in our series of robotically-treated patients, we expect better survival outcomes.
There is increased risk of morbidity and mortality with en-bloc multi-visceral resections for stage T4 rectal carcinomas. Previous studies reported very high morbidity (28-43%) and mortality rates with open and laparoscopic approach (>13%) [
18,
22,
23,
24,
25]. Using the robotic system, we had no 90-day mortalities, and relatively low morbidity rates (
Table 4). This reflects the careful selection of cases after discussion in the MDT meeting, the experience of the principal operating surgeon, experienced trainee fellows, and well-trained operation theater and intensive care unit staff. Furthermore, to achieve low risk of anastomotic leaks and reoperation rates in these cases, we used triple assessment of colorectal anastomosis, the so-called Portsmouth protocol; this protocol consists of indigocyanine green (ICG) in combination with Firefly technology (DaVinci), flexible sigmoidoscopy and underwater-leak test [
26]. Intra-operative blood loss is usually significant in pelvic exenterative surgery. In our study the reported blood loss ranged from 5 to 300 ml hence this translates in less physiological derangements for patients. The benefit of the robotic platform has also demonstrated a reduced length of stay.
The other benefit of the robotic platform in these patients is the ability to offer a dual console and allow the second surgeon to take active part in the operation and this can minimize the effects of surgical fatigue due to long hours of concentration. As opposed to open pelvic exenteration where there are limited views for the team other than the primary surgeon, the robotic platform offers equal and excellent 3D views to both surgeons adding a further element of safety. Endowrist instruments and a stable camera can provide the surgeon with optimum resources to perform the complex procedures of resecting the prostate, bladder, and seminal vesicles en bloc with the rectal primary.
Selection of patients for minimally invasive procedures is the key for successful surgery in patients with stage T4 carcinomas of rectum. A good radiological report and review of scans with the radiologist at complex cancer MDT is of paramount importance. Centrally based tumors are more favorable for this approach and have a higher R0 resection rate as compared to the lateral or posteriorly based lesions. Experience of the surgical team is another important factor in this context. The learning curve in robotic colorectal surgery was reported, using cumulative sum (CUSUM) method, as being 15-25 cases, with 3 phases of learning; the third phase of mastery can be achieved after 25 cases, when a surgeon can take more advanced cases [
27]. Another study by Sng et al [
28] reported this figure to be as higher as 35 cases. We started attempting locally advanced cancers after having performed 100 robotic standard colorectal cancer cases. We do not recommend surgeons attempting locally advanced rectal cancer cases during their initial phase of learning robotic surgery. The lack of tactile feedback can be a major problem in a narrow pelvic cavity and hence only surgeons who are on mastery level should attempt these cases.
Our study describes early experience of robotic en-bloc resections of locally-advanced rectal tumors and is not without limitations. First, the study is retrospective and single-center, with low patients’ volume. Second, the follow-up time is short to make any final comment on survival benefits. A prospective study with longer follow up may provide more evidence in this context. However all three recurrences in this study were seen in the first 18 months after surgery.
Conclusions:
The robotic en-bloc resection of adjacent involved multiple visceras for stage T4 rectal carcinoma is technically safe and feasible, with acceptable morbidity and mortality rates. Based on careful patient selection and appropriate robotic experience, this minimally invasive approach can represent an alternative treatment for selected T4 rectal cancers.
Acknowledgments
The authors are grateful to Deborah Nock (Medical WriteAway, Norwich, UK) for providing the editorial support.
Ethical Statement
This study was registered and approved by R&D department, Portsmouth Hospitals NHS Trust, registration number 4648.
Conflicts of Interest
MS: RJ, PT, SS, IM and SN have no conflicts of interest JK is a robotic trainer and proctor with Intuitive Surgical Funding: No external or internal funding was provided for this work.
Authors Contribution
Concept MS, JK; Data Acquisition SS, IM, SN; Data Analysis MS, SS, RJ, PT; Writing of draft MS, RJ, JK.
References
- Stefan, S.; Siddiqi, N.; Rutgers, M.; Naqvi, S.; Khan, J. Robotic Multi-Visceral Resection for Locally-Advanced Rectal Cancer Invading Other Viscera. Eur J Surg Oncol. 2019, 45, 2197–2198. [Google Scholar] [CrossRef]
- Nishikawa, T.; Ishihara, S.; Emoto, S.; Kaneko, M.; Murono, K.; Sasaki, K.; et al. Multivisceral resections for locally advanced colorectal cancer after preoperative treatment. Mol Clin Oncol. 2018, 493–498. [Google Scholar] [CrossRef]
- Hu, M.; Miao, C.; Wang, X.; Ma, Y. Robotic surgeries for patients with colorectal cancer who have undergone abdominal procedures: Protocol for meta-analysis. Med (United States) 2018, 97, 12–14. [Google Scholar] [CrossRef]
- Huang, C.-W.; Tsai, H.-L.; Yeh, Y.-S.; Su, W.-C.; Huang, M.-Y.; Huang, C.-M.; et al. Robotic-assisted total mesorectal excision with the single-docking technique for patients with rectal cancer. BMC Surg. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Huang, Y.J.; Wei, P.-L. Outcomes of robotic versus laparoscopic surgery for mid and low rectal cancer after neoadjuvant chemoradiation therapy and the effect of learning curve. Medicine (Baltimore) 2017, 96, e8171. [Google Scholar] [CrossRef]
- Mak, T.W.C.; Lee, J.F.Y.; Futaba, K.; Hon, S.S.F.; Ngo, D.K.Y.; Ng, S.S.M. Robotic surgery for rectal cancer: A systematic review of current practice. World J Gastrointest Oncol. 2014, 6, 184–193. [Google Scholar] [CrossRef]
- Watanabe, T.; Hata, K. Robotic surgery for rectal cancer with lateral lymph node dissection. Br J Surg. 2016, 103, 1755–1757. [Google Scholar] [CrossRef]
- Azman, Z.A.M.; Kim, S.-H. A review on robotic surgery in rectal cancer. Transl Gastroenterol Hepatol. 2016, 1, 1–7. [Google Scholar]
- Feroci, F.; Vannucchi, A.; Bianchi PPietro Cantafio, S.; Garzi, A.; Formisano, G.; et al. Total mesorectal excision for mid and low rectal cancer: Laparoscopic vs robotic surgery. World J Gastroenterol. 2016, 22, 3602–3610. [Google Scholar] [CrossRef]
- Staderini, F.; Foppa, C.; Minuzzo, A.; et al. Robotic rectal surgery: state of the art. World J Gastrointest Oncol. 2016, 8, 757–771. [Google Scholar] [CrossRef]
- de Jesus, J.P.; Valadao, M.; de Castro Araujo, R.O.; et al. The circumferential resection margins status: a comparison of robotic, laparoscopic and open total mesorectal excision for mid and low rectal cancer. Eur J Surg Oncol. 2016, 42, 808–812. [Google Scholar] [CrossRef]
- Ahmed, J.; Cao, H.; Panteleimonitis, S.; Khan, J.; Parvaiz, A. Robotic versus laparoscopic rectal surgery in high-risk patients. Colorectal Dis. 2017, 19, 1092–1099. [Google Scholar] [CrossRef]
- Bonjer, H.J.; Deijen, C.L.; Abis, G.A.; et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015, 372, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.A.; Ryan, J.A., Jr.; Schultz, P. En bloc resection of colon cancer adherent to other organs. Am J Surg. 1987, 154, 67–71. [Google Scholar] [CrossRef]
- Gall, F.P.; Tonak, J.; Altendorf, A. Multivisceral resections in colorectal cancer. Dis Colon Rectum. 1987, 30, 337–341. [Google Scholar] [CrossRef]
- Sökmen, S.; Terzi, C.; Unek, T.; Alanyali, H.; Füzün, M. Multivisceral resections for primary advanced rectal cancer. Int J Colorectal Dis. 1999, 14, 282–285. [Google Scholar] [CrossRef]
- Gezen, C.; Kement, M.; Altuntas, Y.E.; Okkabaz, N.; Seker, M.; Vural, S.; Gumus, M.; Oncel, M. Results after multivisceral resections of locally advanced colorectal cancers: An analysis on clinical and pathological t4 tumors. World J Surg Oncol. 2012, 10, 39. [Google Scholar] [CrossRef]
- Lehnert, T.; Methner, M.; Pollok, A.; Schaible, A.; Hinz, U.; Herfarth, C. Multivisceral resection for locally advanced primary colon and rectal cancer: An analysis of prognostic factors in 201 patients. Ann Surg. 2002, 235, 217–225. [Google Scholar] [CrossRef]
- Smith, J.D.; Nash, G.M.; Weiser, M.R.; Temple, L.K.; Guillem, J.G.; Paty, P.B. Multivisceral resections for rectal cancer. Br J Surg. 2012, 99, 1137–1143. [Google Scholar] [CrossRef]
- Eveno, C.; Lefevre, J.H.; Svrcek, M.; Bennis, M.; Chafai, N.; Tiret, E.; Parc, Y. Oncologic results after multivisceral resection of clinical T4 tumors. Surgery. 2014, 156, 669–675. [Google Scholar] [CrossRef]
- Hallet, J.; Zih, F.S.; Lemke, M.; Milot, L.; Smith, A.J.; Wong, C.S. Neo-adjuvant chemoradiotherapy and multivisceral resection to optimize R0 resection of locally recurrent adherent colon cancer. Eur J Surg Oncol. 2014, 40, 706–712. [Google Scholar] [CrossRef]
- Derici, H.; Unalp, H.R.; Kamer, E.; Bozdag, A.D.; Tansug, T.; Nazli, O.; Kara, C. Multivisceral resections for locally advanced rectal cancer. Colorectal Dis 2008, 10, 453–459. [Google Scholar] [CrossRef]
- Kim, K.Y.; Hwang, D.W.; Park, Y.K.; Lee, H.S. A single surgeon's experience with 54 consecutive cases of multivisceral resection for locally advanced primary colorectal cancer: Can the laparo- scopic approach be performed safely? Surg Endosc. 2012, 26, 493–500. [Google Scholar] [CrossRef]
- Nagasue, Y.; Akiyoshi, T.; Ueno, M.; Fukunaga, Y.; Nagayama, S.; Fujimoto, Y.; Konishi, T.; Nagasaki, T.; Nagata, J.; Mukai, T.; et al. Laparoscopic versus open multivisceral resection for primary colorectal cancer: Comparison of perioperative outcomes. J Gastrointest Surg. 2013, 17, 1299–1305. [Google Scholar] [CrossRef]
- Eisenberg, S.B.; Kraybill, W.G.; Lopez, M.J. Long-term results of surgical resection of locally advanced colorectal carcinoma. Surgery. 1990, 108, 779–786. [Google Scholar]
- Waqas, A.; Stefan, S.; Sagias, F.; Naqvi, S.; Flashman, K.; Khan, J. Portsmouth protocol for triple assessment of colorectal anastomosis in robotic surgery reduces anastomotic leak & reoperation rates in rectal cancer surgery. Eur J Surg Oncol. 2018, 44, 1841. [Google Scholar]
- Bokhari, M.B.; Patel, C.B.; Ramos-Valadez, D.I.; Ragupathi, M.; Haas, E.M. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc. 2011, 25, 855–860. [Google Scholar] [CrossRef]
- Sng, K.K.; Hara, M.; Shin, J.W.; Yoo, B.E.; Yang, K.S.; Kim, S.H. The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc. 2013, 27, 3297–3307. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).