Submitted:
20 March 2024
Posted:
20 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
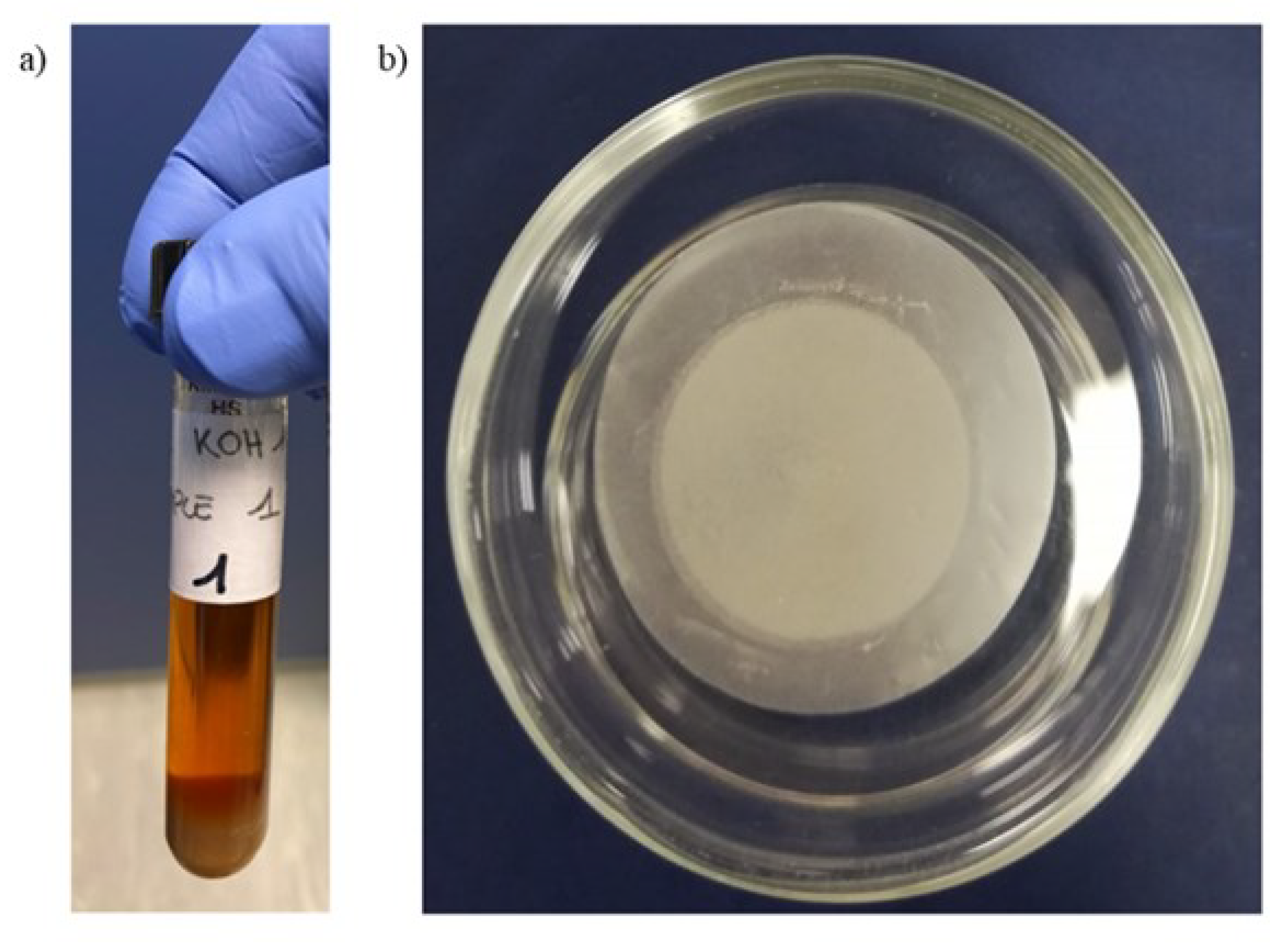
2.1. Chemical Digestion with KOH
2.2. Filtration of Sample
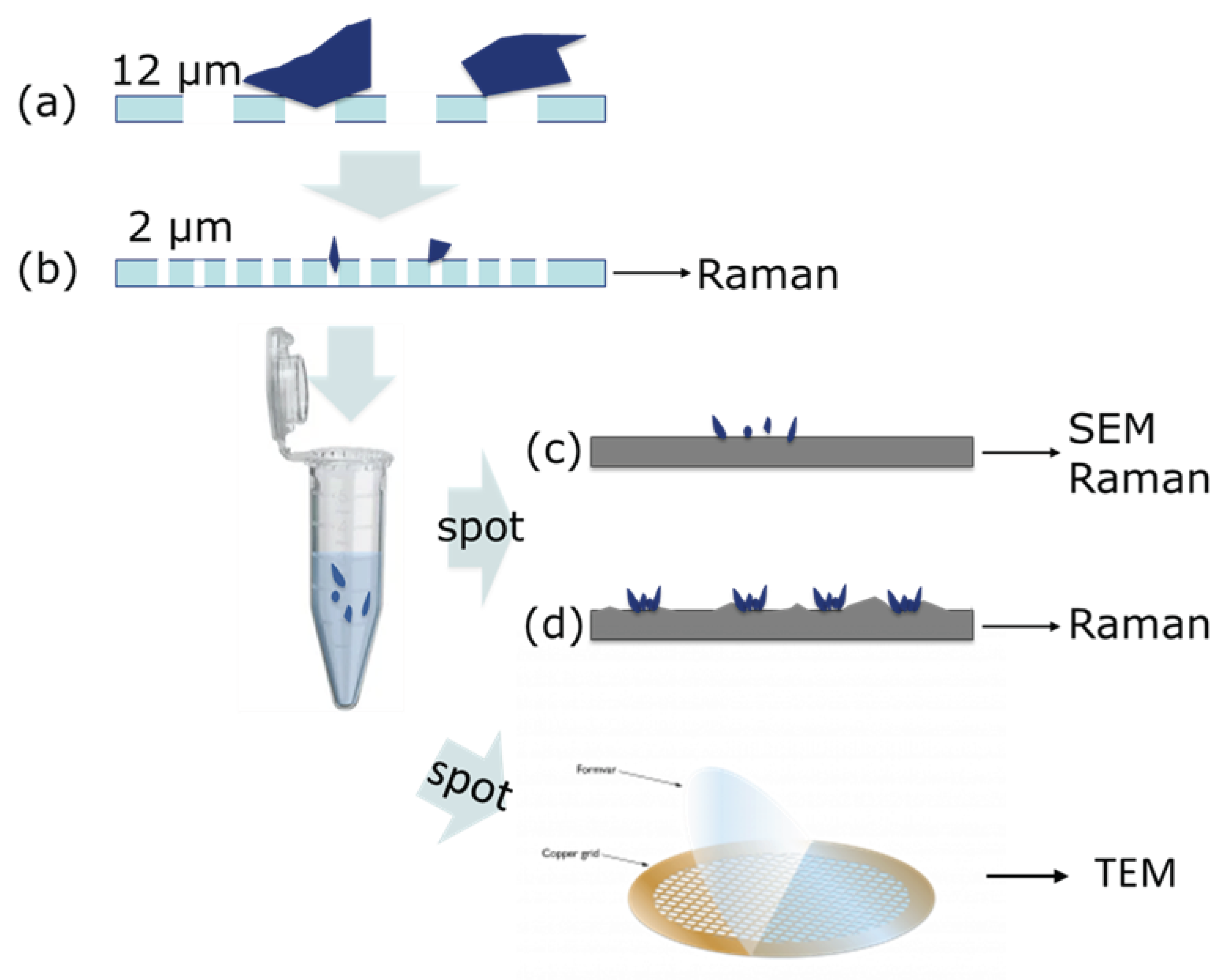
2.3. Confocal Raman Microscopy (CRM) Analysis of 2-12 µm Plastic Fraction
2.4. Scanning Electron Microscopy (SEM) Analysis of < 12 µm and < 2 µm Plastic Fractions
2.5. Transmission Electron Microscopy (TEM) Analysis of < 2 µm Plastic Fraction
2.6. Microplastics Integrity Test
3. Results
3.1. KOH Digestion
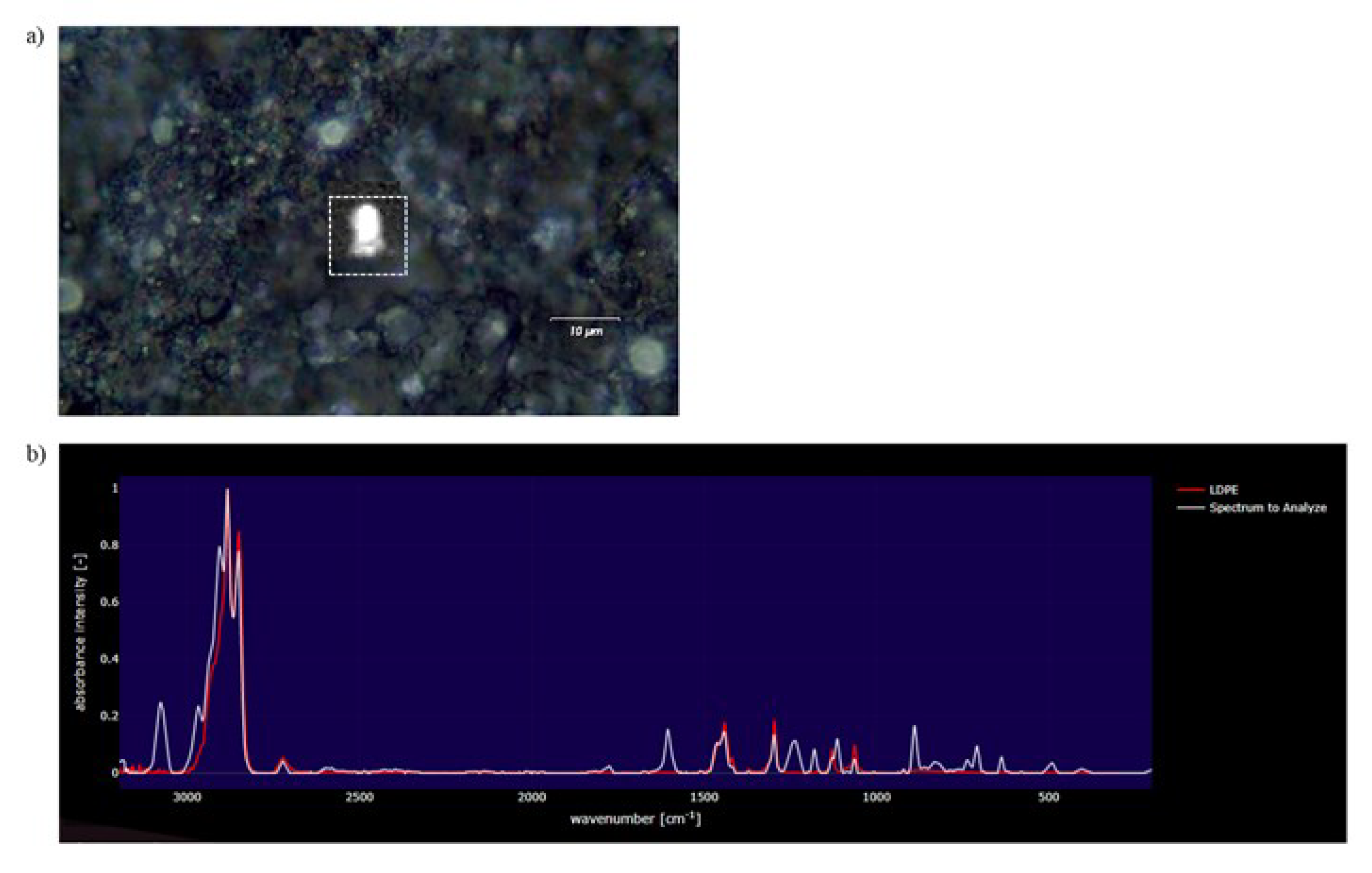
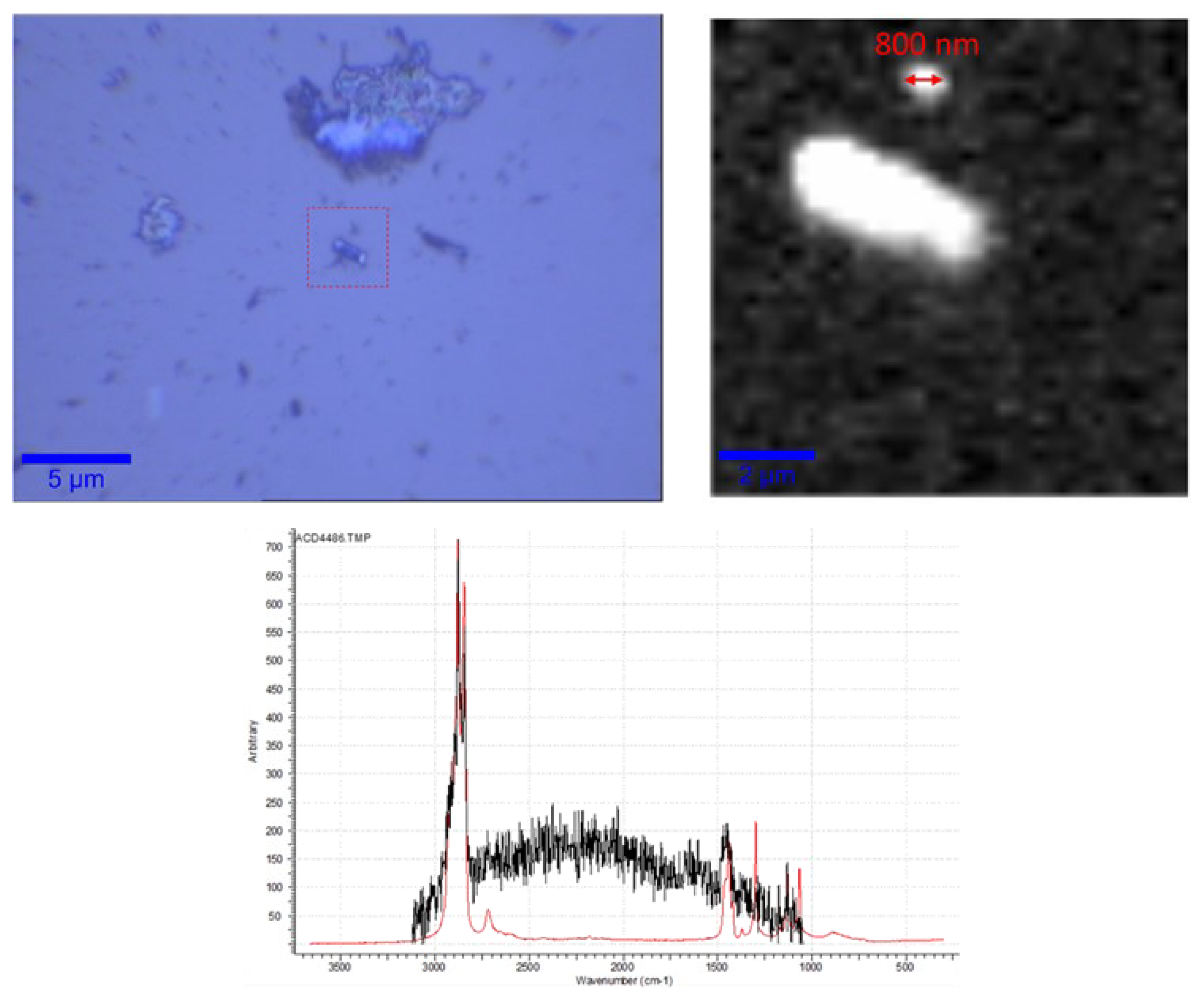
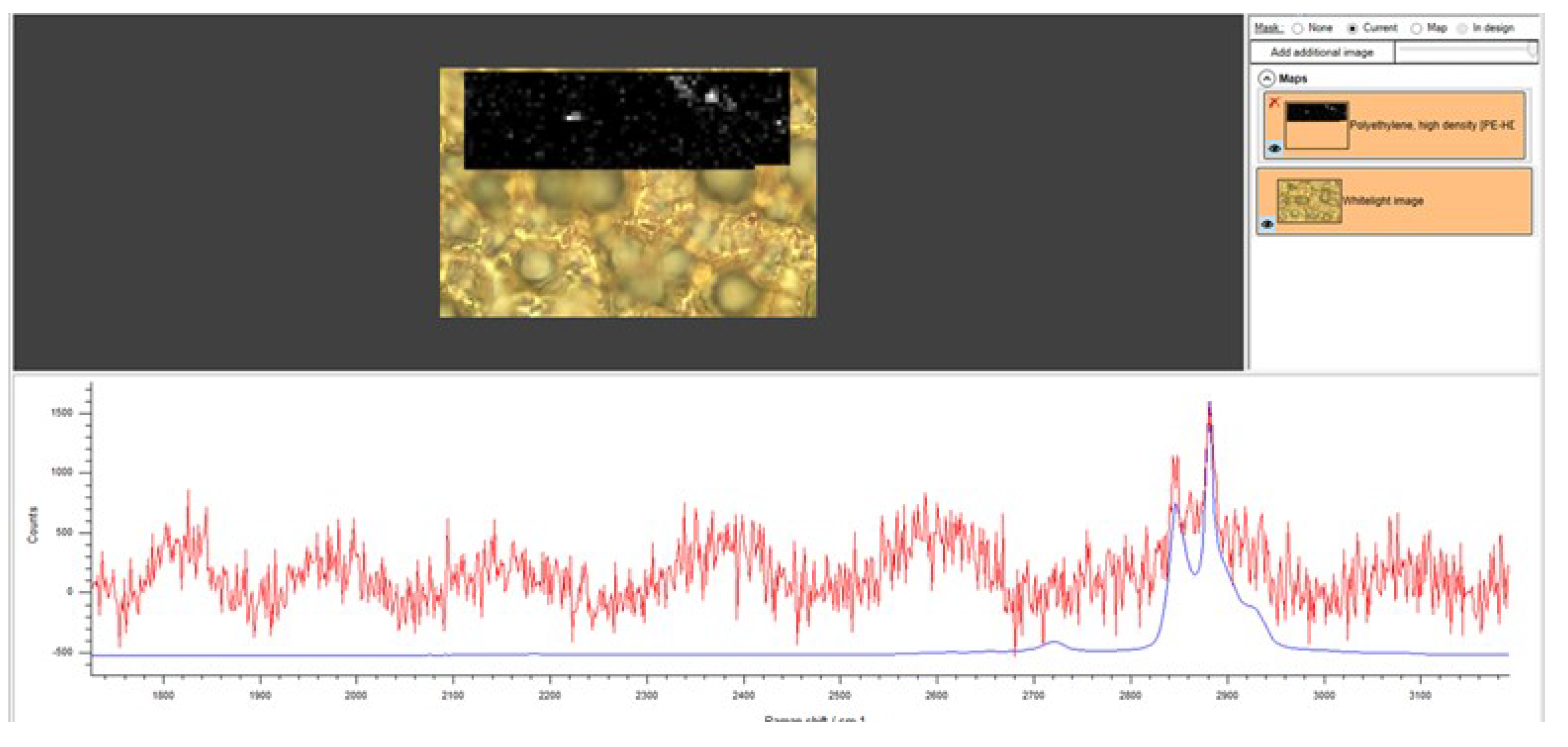
3.2. Raman Mapping and Analysis of Measured Spectra of the 2 µm Filter
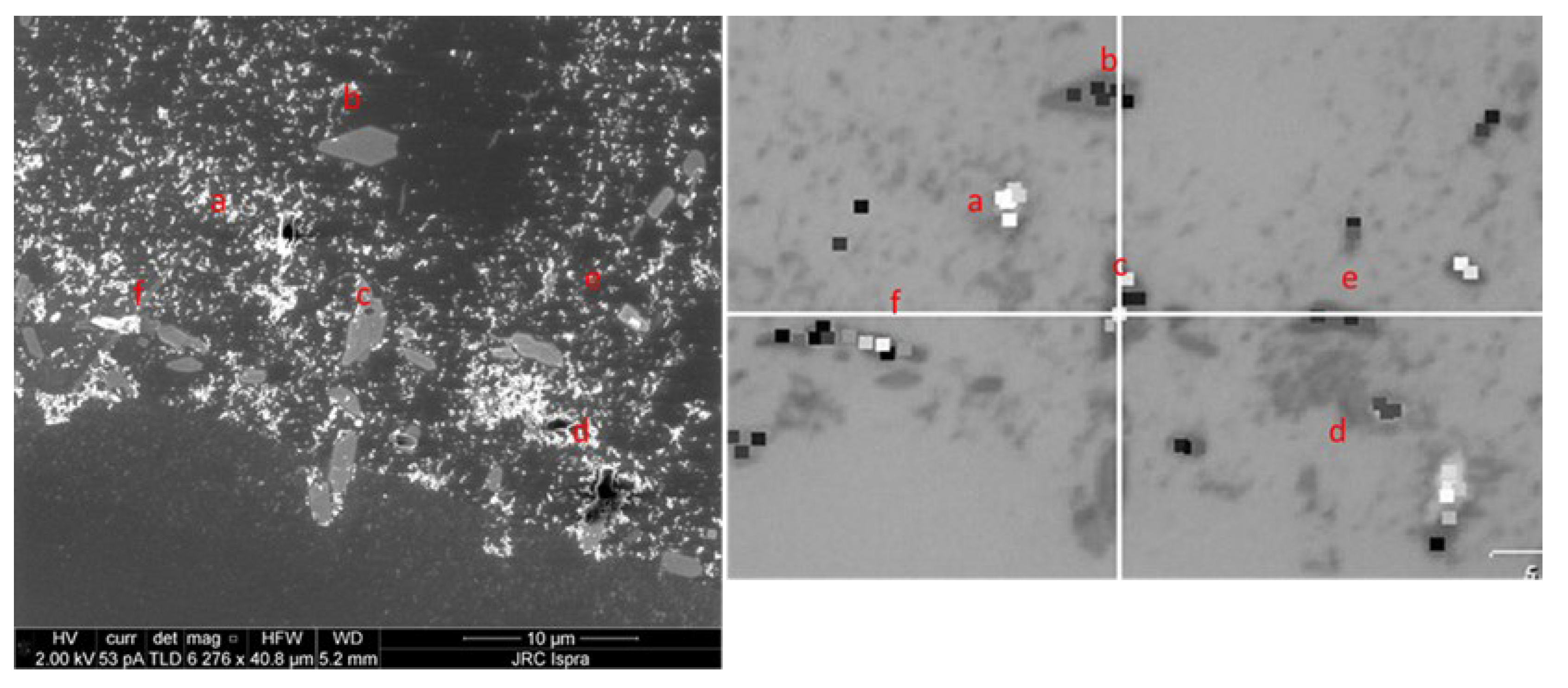
3.3. Correlative Raman and SEM Mapping and Analysis of Measured Spectra of the Particulate < 2 µm on a Positively Charged Surface
3.4. Raman Mapping and Analysis of Measured Spectra of the Particulate < 2 µm Spoted on a Superhydrophobic Surface
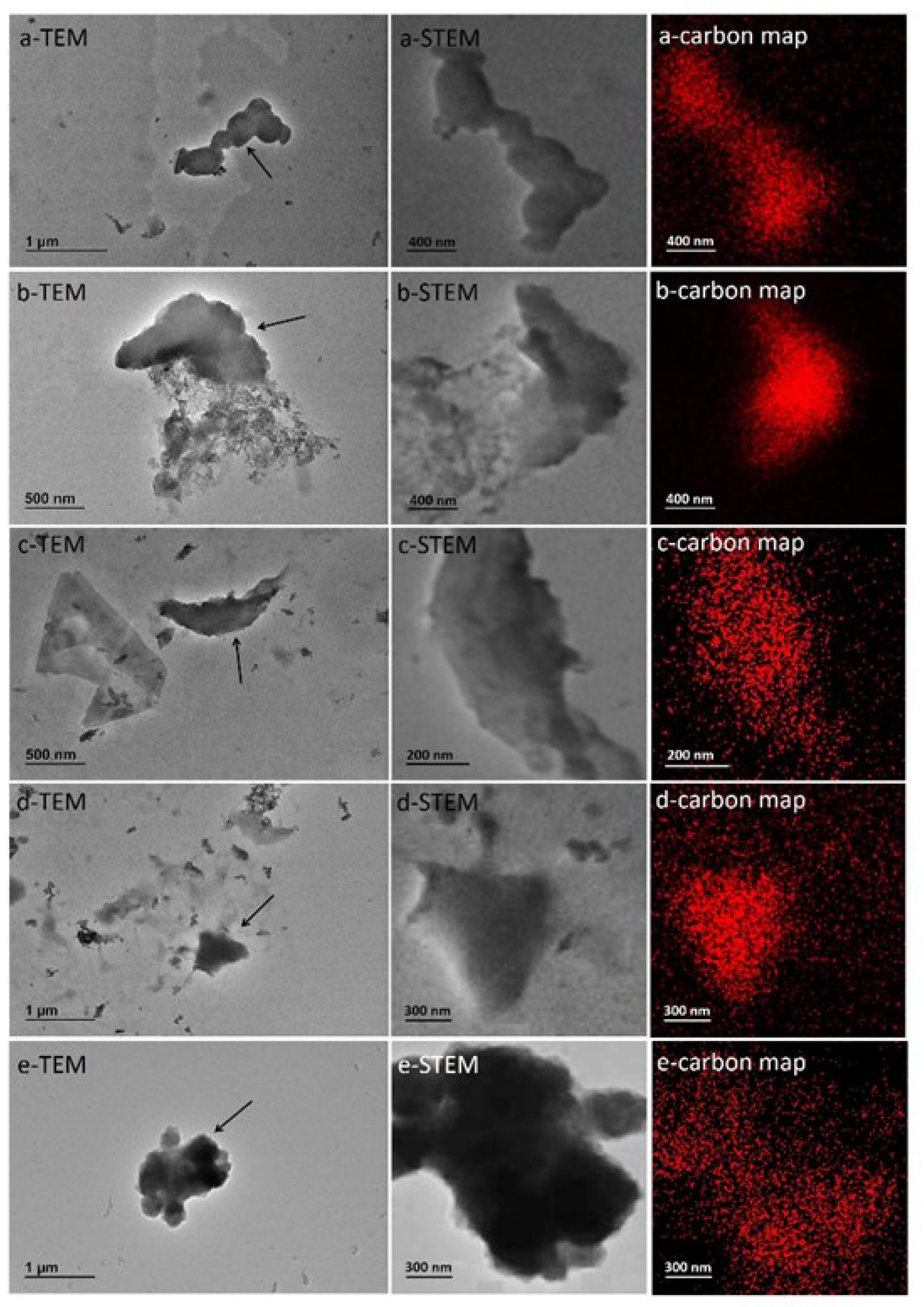
3.5. TEM Characterization of MPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.; Wang, J. Characterization and environmental impacts of microplastics. Gondwana Res. 2021, 98, 63–75. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of plastic polymer types in the marine environment; A meta-analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of plastic pollution in the environment: A review. Bull. Environ. Contam. Toxicol. 2020, 107, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science (80-. ). 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Rochman, C.M.; Liboiron, M.; Bond, A.L.; Lusher, A.; Bradshaw, H.; Provencher, J.F. Why we need an international agreement on marine plastic pollution. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 9994–9997. [Google Scholar] [CrossRef]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Takada, H.; Mizukawa, K.; Hirai, H.; Iwasa, S.; Endo, S.; Mato, Y.; Saha, M.; Okuda, K.; Nakashima, A.; et al. International Pellet Watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009, 58, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Van, A.; Rochman, C.M.; Flores, E.M.; Hill, K.L.; Vargas, E.; Vargas, S.A.; Hoh, E. Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere 2012, 86, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, B. Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integr. Environ. Assess. Manag. 2017, 13, 510–515. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? 2017. [CrossRef]
- Yan, W.; Hamid, N.; Deng, S.; Jia, P.P.; Pei, D.S. Individual and combined toxicogenetic effects of microplastics and heavy metals (Cd, Pb, and Zn) perturb gut microbiota homeostasis and gonadal development in marine medaka (Oryzias melastigma). J. Hazard. Mater. 2020, 397, 122795. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Rimoldi, S.; Torrecillas, S.; Rapp, J.; Moroni, F.; Herrera, A.; Gómez, M.; Fernández-Montero, Á.; Terova, G. Impact of polypropylene microplastics and chemical pollutants on European sea bass (Dicentrarchus labrax) gut microbiota and health. Sci. Total Environ. 2022, 805, 150402. [Google Scholar] [CrossRef] [PubMed]
- Ahrendt, C.; Perez-Venegas, D.J.; Urbina, M.; Gonzalez, C.; Echeveste, P.; Aldana, M.; Pulgar, J.; Galbán-Malagón, C. Microplastic ingestion cause intestinal lesions in the intertidal fish Girella laevifrons. Mar. Pollut. Bull. 2020, 151, 110795. [Google Scholar] [CrossRef]
- Pedà, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef]
- Bonfanti, P.; Colombo, A.; Saibene, M.; Motta, G.; Saliu, F.; Catelani, T.; Mehn, D.; La Spina, R.; Ponti, J.; Cella, C.; et al. Microplastics from miscellaneous plastic wastes: Physico-chemical characterization and impact on fish and amphibian development. Ecotoxicol. Environ. Saf. 2021, 225. [Google Scholar] [CrossRef]
- Jacob, H.; Besson, M.; Oberhaensli, F.; Taylor, A.; Gillet, B.; Hughes, S.; Melvin, S.D.; Bustamante, P.; Swarzenski, P.W.; Lecchini, D.; et al. A multifaceted assessment of the effects of polyethylene microplastics on juvenile gilthead seabreams (Sparus aurata). Aquat. Toxicol. 2021, 241, 106004. [Google Scholar] [CrossRef]
- Jovanović, B.; Gökdağ, K.; Güven, O.; Emre, Y.; Whitley, E.M.; Kideys, A.E. Virgin microplastics are not causing imminent harm to fish after dietary exposure. Mar. Pollut. Bull. 2018, 130, 123–131. [Google Scholar] [CrossRef]
- Zeytin, S.; Wagner, G.; Mackay-Roberts, N.; Gerdts, G.; Schuirmann, E.; Klockmann, S.; Slater, M. Quantifying microplastic translocation from feed to the fillet in European sea bass Dicentrarchus labrax. Mar. Pollut. Bull. 2020, 156. [Google Scholar] [CrossRef] [PubMed]
- Primpke, S.; Christiansen, S.H.; Cowger, W.; De Frond, H.; Deshpande, A.; Fischer, M.; Holland, E.B.; Meyns, M.; O’Donnell, B.A.; Ossmann, B.E.; et al. Critical Assessment of Analytical Methods for the Harmonized and Cost-Efficient Analysis of Microplastics; 2020; Vol. 74; ISBN 0003702820921.
- Fu, W.; Min, J.; Jiang, W.; Li, Y.; Zhang, W. Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci. Total Environ. 2020, 721, 137561. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Francischini, D.S.; Arruda, M.A.Z. When a picture is worth a thousand words: Molecular and elemental imaging applied to environmental analysis – A review. Microchem. J. 2021, 169, 106526. [Google Scholar] [CrossRef]
- Phuong, N.N.; Fauvelle, V.; Grenz, C.; Ourgaud, M.; Schmidt, N.; Strady, E.; Sempéré, R. Highlights from a review of microplastics in marine sediments. Sci. Total Environ. 2021, 777. [Google Scholar] [CrossRef]
- Valsesia, A.; Quarato, M.; Ponti, J.; Fumagalli, F.; Gilliland, D.; Colpo, P. Combining microcavity size selection with Raman microscopy for the characterization of Nanoplastics in complex matrices. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Vogel, R.; Savage, J.; Vella, G.; Law, A.; Della Camera, G.; Hannon, G.; Peacock, B.; Mehn, D.; Ponti, J.; et al. Measuring particle size distribution and mass concentration of nanoplastics and microplastics: addressing some analytical challenges in the sub-micron size range. J. Colloid Interface Sci. 2021, 588, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.A.; Corcoran, P.L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Pollut. Bull. 2010, 60, 650–654. [Google Scholar] [CrossRef]
- Mahamud, A.G.M.S.U.; Anu, M.S.; Baroi, A.; Datta, A.; Khan, M.S.U.; Rahman, M.; Tabassum, T.; Tanwi, J.T.; Rahman, T. Microplastics in fishmeal: A threatening issue for sustainable aquaculture and human health. Aquac. Reports 2022, 25, 101205. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in fish and fishmeal: an emerging environmental challenge? Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Rimoldi, S.; Antonini, M.; Gasco, L.; Moroni, F.; Terova, G. Intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) may be improved by feeding a Hermetia illucens meal/low-fishmeal diet. Fish Physiol. Biochem. 2021, 47, 365–380. [Google Scholar] [CrossRef]
- Rimoldi, S.; Terova, G.; Ascione, C.; Giannico, R.; Brambilla, F. Next generation sequencing for gut microbiome characterization in rainbow trout (Oncorhynchus mykiss) fed animal by-product meals as an alternative to fishmeal protein sources. PLoS One 2018, 13, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Terova, G.; Rimoldi, S.; Ascione, C.; Gini, E.; Ceccotti, C.; Gasco, L. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev. Fish Biol. Fish. 2019, 29, 465–486. [Google Scholar] [CrossRef]
- Terova, G.; Ceccotti, C.; Ascione, C.; Gasco, L.; Rimoldi, S. Effects of partially defatted Hermetia illucens meal in rainbow trout diet on hepatic methionine metabolism. Animals 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Gasco, L.; Acuti, G.; Bani, P.; Dalle Zotte, A.; Danieli, P.P.; De Angelis, A.; Fortina, R.; Marino, R.; Parisi, G.; Piccolo, G.; et al. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital. J. Anim. Sci. 2020, 19, 360–372. [Google Scholar] [CrossRef]
- Facchetti, S. V.; La Spina, R.; Fumagalli, F.; Riccardi, N.; Gilliland, D.; Ponti, J. Detection of metal-doped fluorescent pvc microplastics in freshwater mussels. Nanomaterials 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Desmet, C.; Valsesia, A.; Oddo, A.; Ceccone, G.; Spampinato, V.; Rossi, F.; Colpo, P. Characterisation of nanomaterial hydrophobicity using engineered surfaces. J. Nanoparticle Res. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Liu, S.; Hu, T.; Zheng, K.; Wang, Y.; Long, H. Recent advances in the research on effects of micro/nanoplastics on carbon conversion and carbon cycle: A review. J. Environ. Manage. 2023, 334, 117529. [Google Scholar] [CrossRef]
- Parenti, C.C.; Binelli, A.; Caccia, S.; Della Torre, C.; Magni, S.; Pirovano, G.; Casartelli, M. Ingestion and effects of polystyrene nanoparticles in the silkworm Bombyx mori. Chemosphere 2020, 257, 127203. [Google Scholar] [CrossRef]
- Cuthbert, R.N.; Al-Jaibachi, R.; Dalu, T.; Dick, J.T.A.; Callaghan, A. The influence of microplastics on trophic interaction strengths and oviposition preferences of dipterans. Sci. Total Environ. 2019, 651, 2420–2423. [Google Scholar] [CrossRef]
- Scherer, C.; Wolf, R.; Völker, J.; Stock, F.; Brennhold, N.; Reifferscheid, G.; Wagner, M. Toxicity of microplastics and natural particles in the freshwater dipteran Chironomus riparius: same same but different? Sci. Total Environ. 2020, 711, 134604. [Google Scholar] [CrossRef]
- van Oss, C.J. The Extended DLVO Theory. Interface Sci. Technol. 2008, 16, 31–48. [Google Scholar] [CrossRef]
- Rahman, T.; Mustakima, S.; Ferdous, Z.; Tabassum, T.; Sofi Uddin Mahamud, A.G.M.; Siddika, M.; Akter, M.; Alam, M.S.; Haque, M.N. Properties and abundance of microplastics found in fish feed, tissues, and culture water of catfish (Heteropneustes fossilis). Int. J. Aquat. Biol. 2022, 10, 1–10. [Google Scholar] [CrossRef]
- Matias, R.S.; Gomes, S.; Barboza, L.G.A.; Salazar-Gutierrez, D.; Guilhermino, L.; Valente, L.M.P. Microplastics in water, feed and tissues of European seabass reared in a recirculation aquaculture system (RAS). Chemosphere 2023, 335. [Google Scholar] [CrossRef]
- Muhib, M.I.; Rahman, M.M. Microplastics contamination in fish feeds: Characterization and potential exposure risk assessment for cultivated fish of Bangladesh. Heliyon 2023, 9, e19789. [Google Scholar] [CrossRef] [PubMed]
- Walkinshaw, C.; Tolhurst, T.J.; Lindeque, P.K.; Thompson, R.; Cole, M. Detection and characterisation of microplastics and microfibres in fishmeal and soybean meal. Mar. Pollut. Bull. 2022, 185, 114189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Zhu, X.; Sun, C.; Teng, J.; Chen, L.; Shan, E.; Zhao, J. Microplastics in fish meals: An exposure route for aquaculture animals. Sci. Total Environ. 2022, 807, 151049. [Google Scholar] [CrossRef] [PubMed]
- Gündogdu, S.; Eroldoğan, O.T.; Evliyaoğlu, E.; Turchini, G.M.; Wu, X.G. Fish out, plastic in: Global pattern of plastics in commercial fishmeal. 2020. [CrossRef]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef]
- Herrera, A.; Acosta-Dacal, A.; Pérez Luzardo, O.; Martínez, I.; Rapp, J.; Reinold, S.; Montesdeoca-Esponda, S.; Montero, D.; Gómez, M. Bioaccumulation of additives and chemical contaminants from environmental microplastics in European seabass (Dicentrarchus labrax). Sci. Total Environ. 2022, 822. [Google Scholar] [CrossRef] [PubMed]
- Compa, M.; Ventero, A.; Iglesias, M.; Deudero, S. Ingestion of microplastics and natural fibres in Sardina pilchardus (Walbaum, 1792) and Engraulis encrasicolus (Linnaeus, 1758) along the Spanish Mediterranean coast. Mar. Pollut. Bull. 2018, 128, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Legislation on Food Contact Union Guidelines on Regulation ( EU ) No 10 / 2011 on plastic materials and articles intended to come into contact with food as regards information in the supply chain. 2016, Version 1., 1–45.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




