Submitted:
20 March 2024
Posted:
21 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
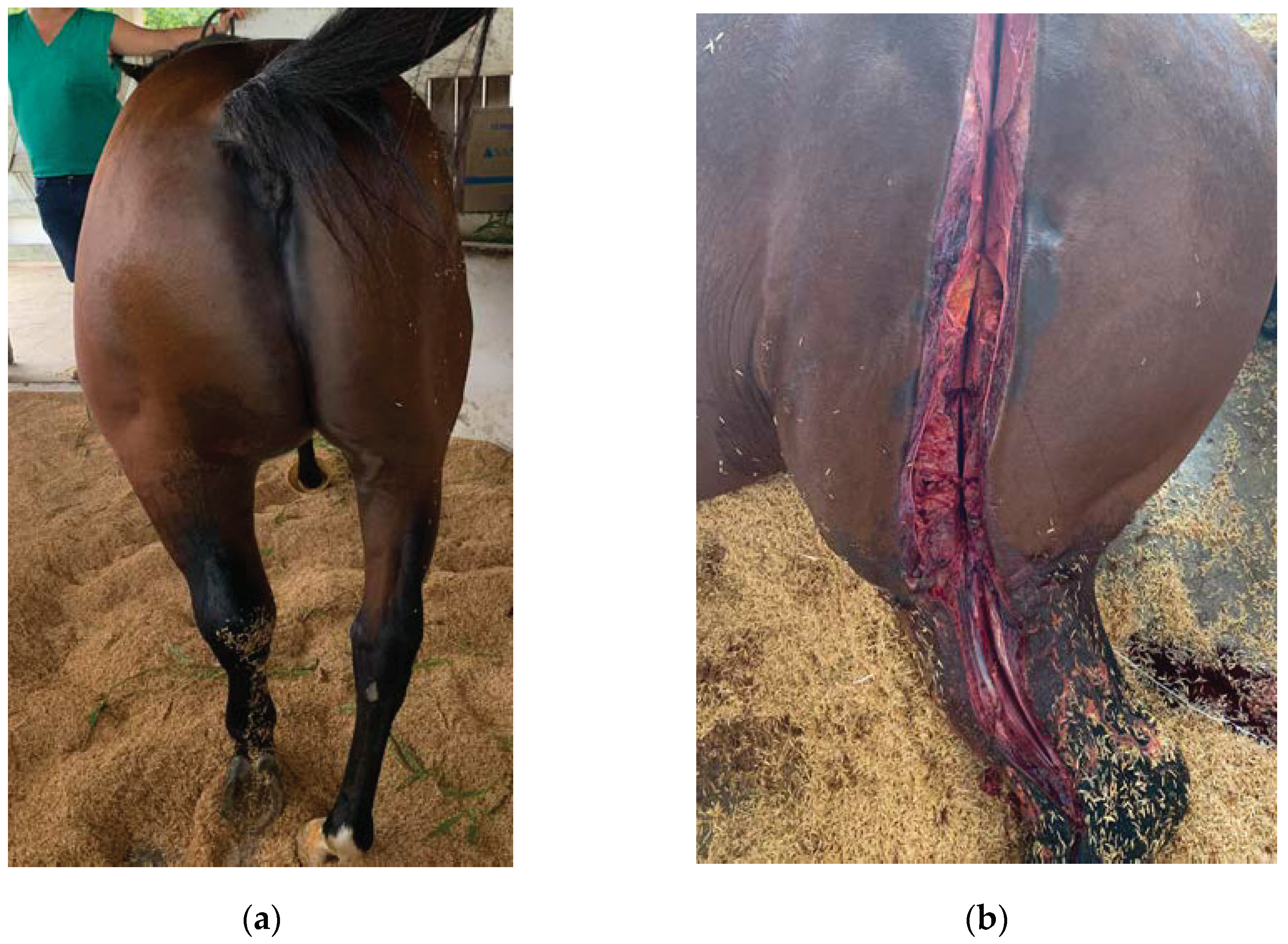
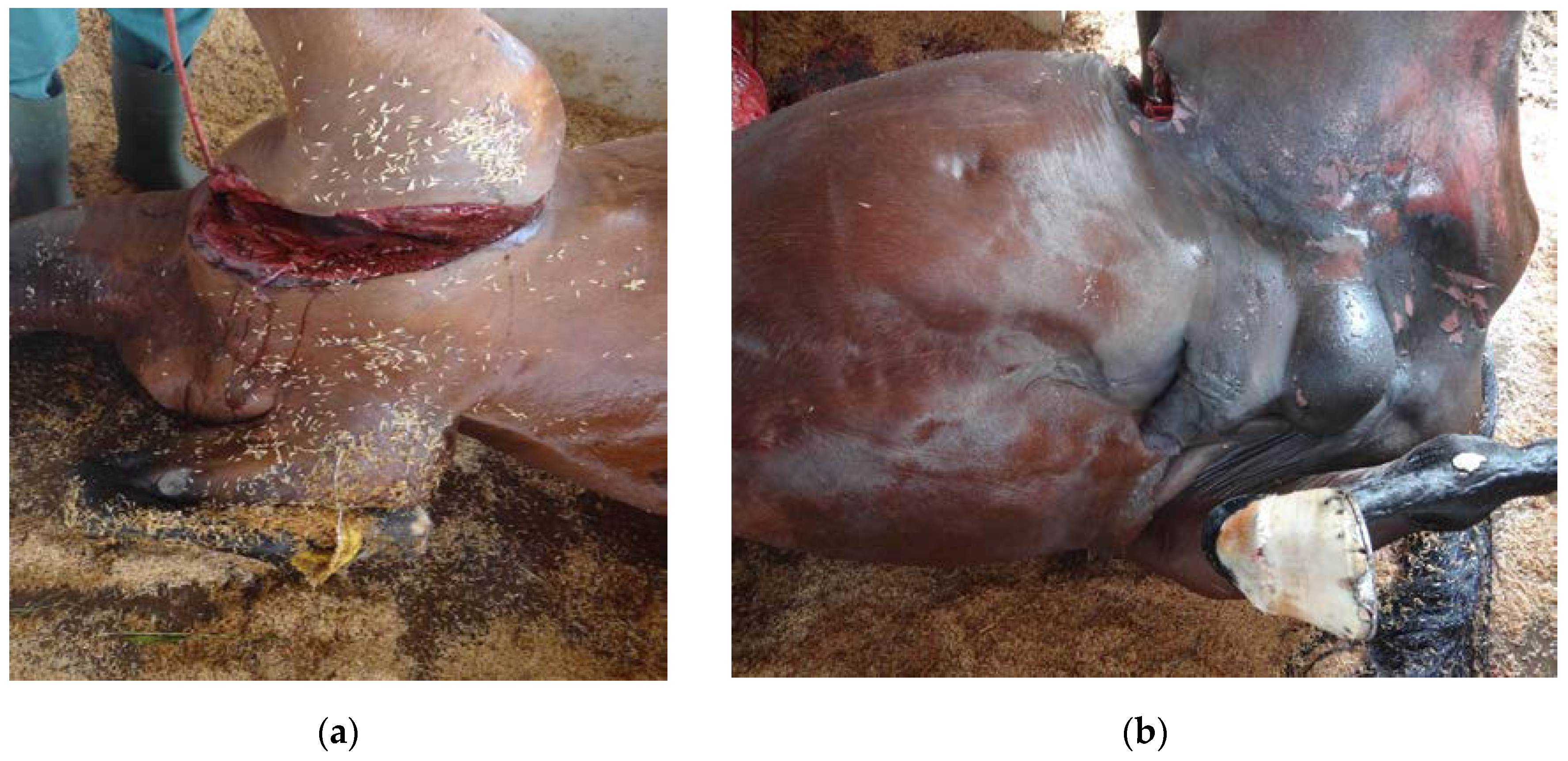
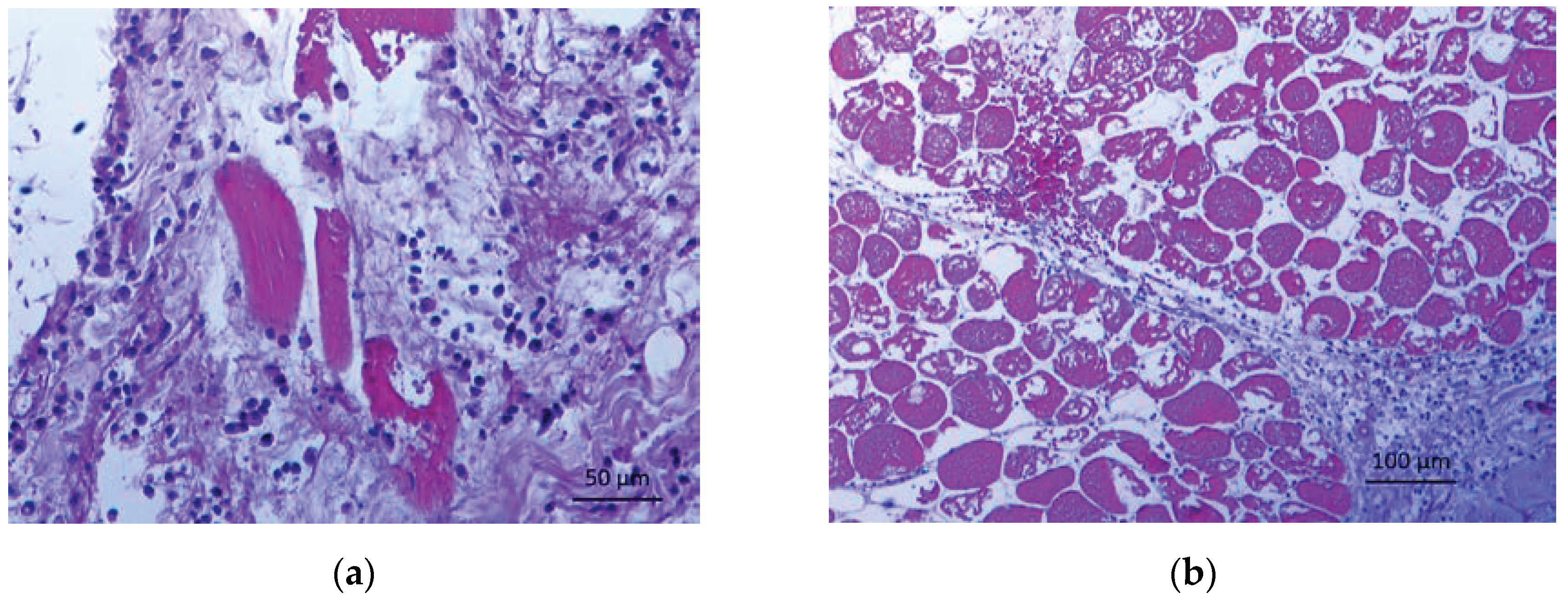
2. Case Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MAPA - Ministério da Agricultura, pecuária e abastecimento - Brasil – 2021. Accessed: https://www.gov.br/agricultura/pt-br/assuntos/politica-agricola/todas-publicacoes-de-politica-agricola/agropecuaria-brasileira-em-numeros/abn-02-2021.pdf.
- Freitas, N.F.Q.R; Otaka, D.Y.; Galvão, C.C.; de Almeida, D M. ; Ferreira, M.R.; Júnior, C.M.; Hidalgo, M.M.M.H.; Conceição, F.R.; Salvarani, F.M. Humoral immune response evaluation in horses vaccinated with recombinant Clostridium perfringens toxoids alpha and beta for 12 months. Toxins 2021, 13, 566. [Google Scholar] [CrossRef]
- Farias, L.D.; Azevedo, M.D.S.; Trost, M.E.; De La Côrte, F.D.; Irigoyen, L.F.; de Vargas, A.C. Acute Myonecrosis in Horse Caused by Clostridium novyi Type A. Braz. J. Microbiol. 2014, 45, 221–224. [Google Scholar] [CrossRef]
- Freitas, N.F.Q.R; Barbosa, J.D.; Otaka, D.Y.; Ferreira, M.R.A.; Rodrigues, R.R.; Junior, C.M.; Conceição, F.R.; Salvarani, F.M. Clostridium perfringens α and β recombinant toxoids in equine immunization. Pesq. Vet. Bras. 2020, 40, 776–780. [Google Scholar] [CrossRef]
- Amorim, R.M.; Rino, A.S.; Dal-Pai-Silva, M.; Borges, A.S.; Oliveira Filho, J.P.; Freitas, N.P.P.; Maia, L.; Rezende, L.A.L. Aspectos morfológicos de biópsias musculares em equinos com miopatia sob forma de surto. Pesq. Vet. Bras 2011, 31, 479–585. [Google Scholar] [CrossRef]
- Uzal, F.A; Navarro, M.A; Asin, J.; Henderson, E.E. Clostridial Diseases of Horses: A Review. Vaccines 2022, 10, 318. [Google Scholar] [CrossRef]
- Junior, C.A.O.; Silva, R.O.; Lobato, F.C.; Navarro, M.A.; Uzal, F.A. Gas gangrene in mammals: a review. J. Vet. Diag. Invest. 2020, 32, 175–183. [Google Scholar] [CrossRef]
- Ferreira, M.R.; Moreira, G.M.; Cunha, C.E.; Mendonça, M.; Salvarani, F.M. Moreira, A.N.; Conceição, F.R. Recombinant alpha, beta, and epsilon toxins of Clostridium perfringens: production strategies and applications as veterinary vaccines. Toxins 2019, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, N.E.; Orellana, C.A.; Moonen, G.A.; Moutafis, G.; Marcellin, E. Vaccine Production to protect animals against pathogenic Clostridia. Toxins 2019, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.G.; Silva, R.O.S.; Pires, P.S.; Martinho, A.P.V.; Lucas, T.M.; Teixeira, A.I.P.; Paes, A.C; Barros, C.B.; Lobato, F.C.F. Myonecrosis by Clostridium septicum in a dog, diagnosed by a new multiplex-PCR. Anaerobe 2012, 8, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Prophet, E.B.; Mills, B.; Arrington, J.B.; Sobin, L.H. Laboratory methods in histotechnology. 1st ed. American Registry of Pathology: Washington, EUA, 1991, pp. 221-222.
- Ferreira Junior, J.A.; Silveira, R.O.S; Lobato, F.C.F; Teixeira Neto, A.R.; Nascimento, K.A.; Almeida e Macedo, J.T.S.; Pedroso, P.M.O. Malignant Edema in Horse by Clostridium perfringens Type A. Acta Scien. Vet., 2020, 48. [Google Scholar] [CrossRef]
- Sacco, S.C.; Ortega, J.; Navarro, M.A.; Fresneda, K.C.; Anderson, M.; Woods, L.W.; Moore, J.; Uzal, F.A. Clostridium sordellii-associated gas gangrene in 8 horses, 1998-2019. J. Vet. Diag. Invest. 2020, 32, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Musher, D.M.; Watson, D.A.; Eddy, H.; Hamill, R.J.; Gyorkey, F.; Rosen, H.; Mader, J. Spontaneous, nontraumatic gas gangrene due to Clostridium septicum. Rev. Inf. Dis. 1990, 12, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Elkbuli, A.; Diaz, B.; Ehrhardt, J.D.; Hai, S.; Kaufman, S.; McKenney, M.; Boneva, D. Survival from Clostridium toxic shock syndrome: Case report and review of the literature. Int. J. Surg. Case. Rep. 2018, 50, 64–67. [Google Scholar] [CrossRef]
- Breauhaus, B.A.; Brown, C.M.; Scott, E.A.; Ainsworth, D.M.; Taylor, R.F. Clostridial muscle infections following intramuscular injections in the horse. J. Equine Vet Sci. 1983, 3, 42–46. [Google Scholar] [CrossRef]
- Peek, S.F.; Semrad, S.D.; Perkins, G.A. Clostridial myonecrosis in horses (37 cases 1985-2000). Equine Vet. J. 2003, 35, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Vengust, M.; Arroyo, L.G.; Weese, J.S.; Baird, J.D. Preliminary evidence for dormant clostridial spores in equine skeletal muscle. Equine Vet. J. 2003, 35, 514–516. [Google Scholar] [CrossRef]
- Ray, D.; Cohle, S.D.; Lamb, P. Spontaneous clostridial myonecrosis. J. For. Sci. 1992, 37, 1428–1432. [Google Scholar] [CrossRef]
- Songer, J.G. Clostridial diseases of animals. The Clostridia: Molecular Biology and Pathogenesis 1st ed. 98 Academic Press, San Diego, EUA. 1997, 153–180. [Google Scholar] [CrossRef]
- Seeger, M. G.; Silva, G.B.; Machado, C.S.; Silva, D.R.P.D.; Corte, F.D.D.L.; Vogel, F.S.F.; Cargnelutti, J.F. Myonecrosis caused by Clostridium septicum in a horse from Southern Brazil. Ciên. Rur. 2022, 52, e20210736. [Google Scholar] [CrossRef]
- Kennedy, C.L.; Lyras, D.; Cheung, J.K.; Hiscox, T.J.; Emmins, J.J.; Rood, J.I. Cross-complementation of Clostridium perfringens PLC and Clostridium septicum a-toxin mutants reveals PLC is sufficient to mediate gas gangrene. Mic. Infec. 2009. 11, 413–418. [CrossRef]
- Alves, M.L.F.; Ferreira, M.R.A.; Donassolo, R.A.; Rodrigues, R.R.; Conceição, F.R. Clostridium septicum: A review in the light of alpha-toxin and development of vaccines. Vaccine 2021, 39, 4949–4956. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kang, M.S.; Yoo, H.S.; Lee, D.Y.; Lee, H.C.; Kim, D.Y. Clostridium perfringens Type A Myonecrosis in a Horse in Korea. J. Vet. Med. Sci. 2003, 65, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Souza, R. A. P. R. Tétano em equinos: Uma revisão narrativa. PhD Sci. Rev. 2021, 1, 20–28. [Google Scholar] [CrossRef]
- Mora, Z.V.D.L.; Macías-Rodríguez, M.E.; Arratia-Quijada, J.; Gonzalez-Torres, Y.S.; Nuño, K.; Villarruel-López, A.; Villarruel-López, A. Clostridium perfringens as foodborne pathogen in broiler production: pathophysiology and potential strategies for controlling necrotic enteritis. Animals 2020, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R. Tetanus in animals. J. Vet.Diag. Invest. 2020, 32, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Posthaus, H.; Kittl, S.; Tarek, B.; Bruggisser, J. Clostridium perfringens type C necrotic enteritis in pigs: diagnosis, pathogenesis, and prevention. J. Vet. Diag.Invest. 2020, 32, 203–212. [Google Scholar] [CrossRef]
- Carvalho, A.L.M.A.; Rodrigues, L.H.A.; Chalfun, L.H.L. Carbúnculo sintomático em bovino: evolução clínica e terapêutica. Ciên Anim. 2023, 33, 175–186. [Google Scholar]
- Lemfers, T.R.; VON AH, R.M.L.; Gagliardi, B.; Pucci, L.R.; Stanciar, B.P.; Dércoli, T.E.; Calefi, A.S. Systemic Clostridiosis due to Clostridium perfringens infection in Equus Asinus – Case Report. Ars Vet. 2022, 38, 180–184. [Google Scholar] [CrossRef]
- Hustá, M.; Ducatelle, R.; Haesebrouck, F.; Van Immerseel, F.; Goossens, E. A comparative study on the use of selective media for the enumeration of Clostridium perfringens in poultry faeces. Anaerobe 2020, 63, 102205. [Google Scholar] [CrossRef]
- Raymundo, D.L.; Pavarini, S.P.; Bezerra Junior, P.S.; Antoniassi, N.A.B.; Bandarra, P.M.; Bercht, B.S.; Gomes, M.J.P; Driemeier, D. Mionecrose aguda por Clostridium septicum em equinos. Pesq. Vet. Bras. 2010, 30, 637–640. [Google Scholar] [CrossRef]
- Morris, W.E.; Uzal, F.A.; Fattorini, F.R.; Terzolo, H. Malignant oedema associated with blood-sampling in sheep. Aust. Vet. J. 2002, 5, 280–281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




