Submitted:
20 March 2024
Posted:
21 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
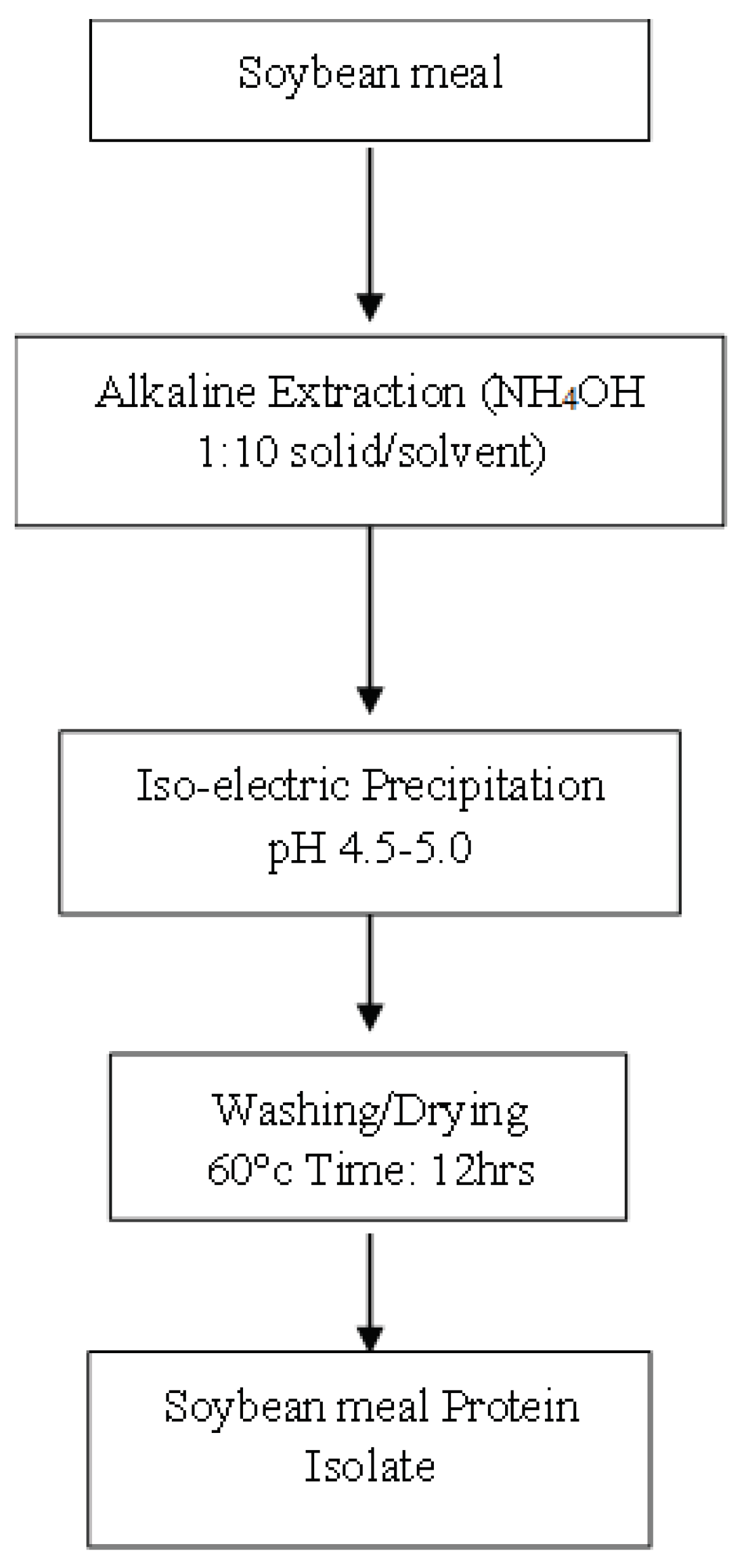
2.2. Extraction of Soybean Meal Protein
2.3. Proximate Analysis
2.3.1. Moisture Content
2.3.2. Ash Content
2.3.3. Fat Content
2.3.4. Total protein
2.3.5. Total Carbohydrate
2.4. Enzymatic Hydrolysis of Soybean Meal Protein Isolates
2.4.1. Amine Quantification
2.5. FTIR
2.6. GEL ELECTROPHORESIS
3. Results and Discussion
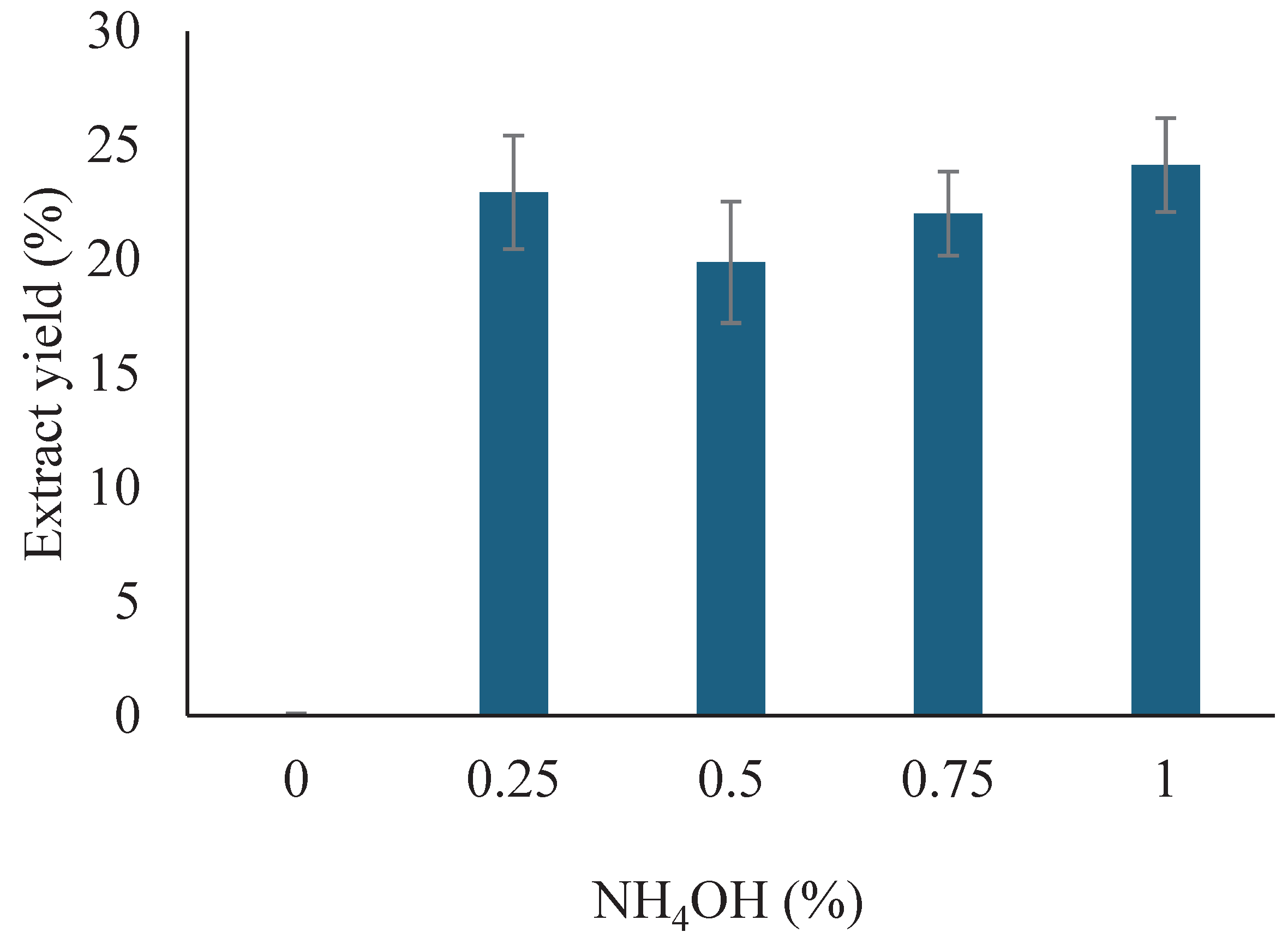
3.1. Extractability of Soybean Meal Protein
3.2. Proximate Composition
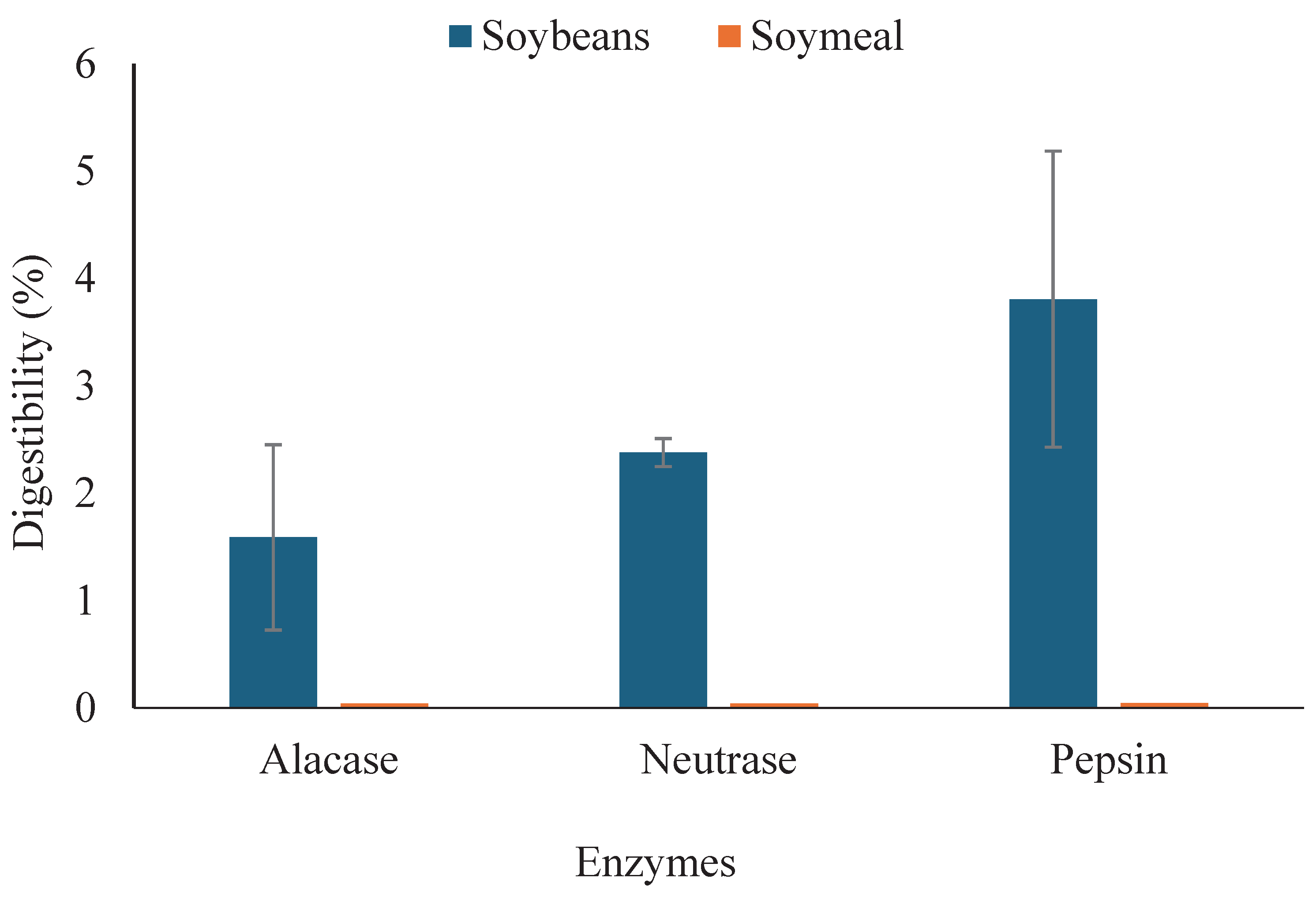
3.3. Digestibility of Soybean Meal Protein by Different Industrial Proteases
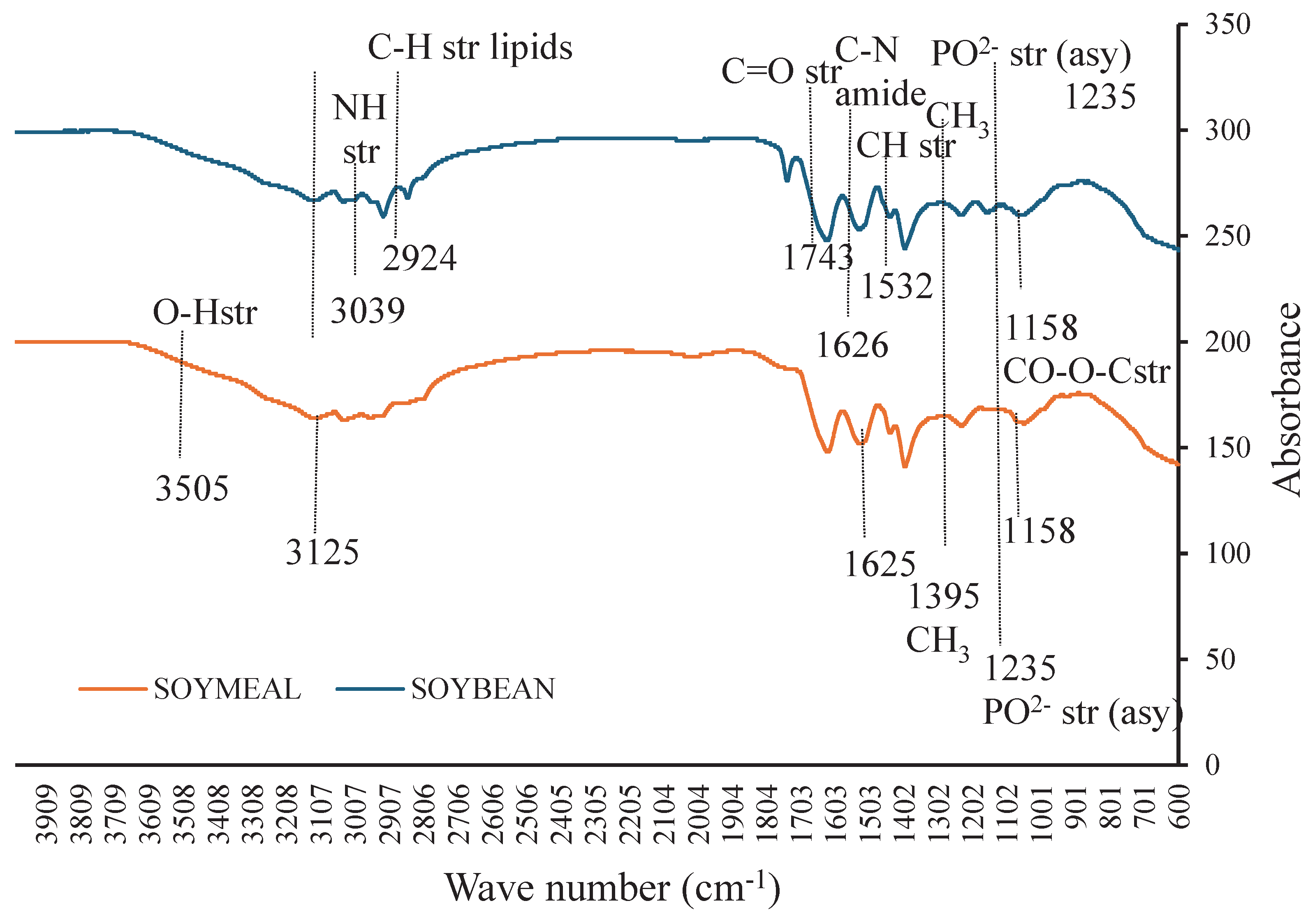
3.4. FTIR Spectroscopy
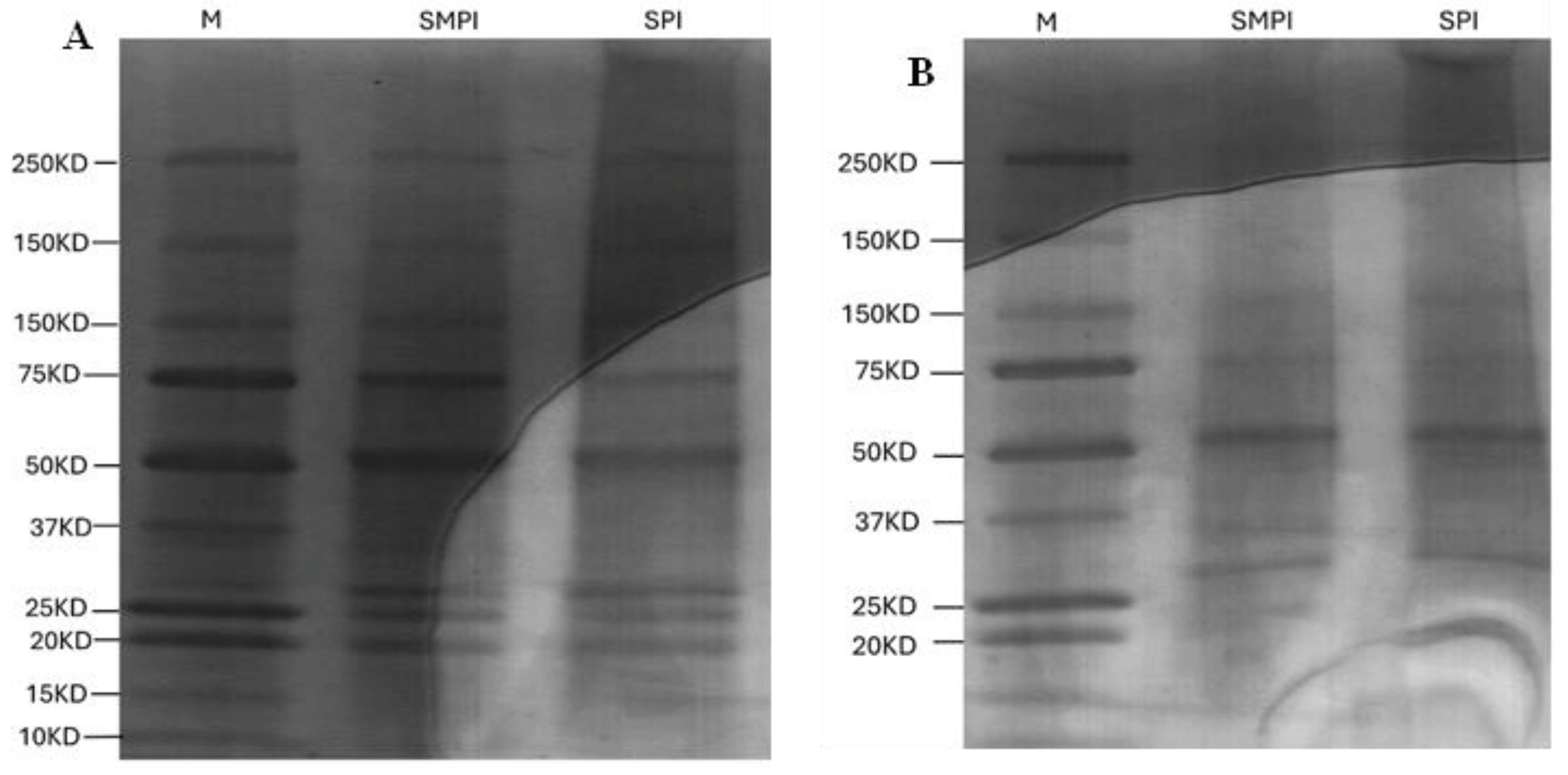
3.5. SDS-PAGE Banding Pattern of Soybean and Soybean Meal Protein Isolates
4. Conclusion
References
- Ruiz, N.; Parsons, C.M.; Stein, H.H.; Coon, C.N.; Eys, J.; Miles, R.D. A review: 100 years of soybean meal. ADM 2020. [Google Scholar]
- USDA ERS–Oil Crops Sector at a Glance”. Available online: https://www.ers.usda.gov/topics/crops/soybeans-and-oil-crops/oil-crops-sector-at-a-glance/ (accessed on 22 February 2024).
- Challenges Ahead For Soybean-Based Renewable Fuels Demand | Biodiesel Magazine”. Available online: https://biodieselmagazine.com//articles/challenges-ahead-for-soybean-based-renewable-fuels-demand-2518696 (accessed on 22 February 2024).
- Sá, A.G.A.; Laurindo, J.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Influence of Emerging Technologies on the Utilization of Plant Proteins. Front. Nutr. 2022, 9, 809058. [Google Scholar] [CrossRef] [PubMed]
- Gerliani, N.; Hammami, R.; Aïder, M. Extraction of protein and carbohydrates from soybean meal using acidic and alkaline solutions produced by electro-activation. Food Sci. Nutr. 2020, 8, 1125–1138. [Google Scholar] [CrossRef]
- Colletti, A.; Attrovio, A.; Boffa, L.; Mantegna, S.; Cravotto, G. Valorisation of By-Products from Soybean (Glycine max (L.) Merr.) Processing. Molecules 2020, 25, 2129. [Google Scholar] [CrossRef]
- Scott, E.; Peter, F.; Sanders, J. Biomass in the manufacture of industrial products—the use of proteins and amino acids. Appl. Microbiol. Biotechnol. 2007, 75, 751–762. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and Edible Uses of Soy Protein Products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Detzel, A.; Krüger, M.; Busch, M.; Blanco-Gutiérrez, I.; Varela, C.; Manners, R.; Bez, J.; Zannini, E. Life cycle assessment of animal-based foods and plant-based protein-rich alternatives: an environmental perspective. J. Sci. Food Agric. 2021, 102, 5098–5110. [Google Scholar] [CrossRef]
- Grieshop, C.M.; Kadzere, C.T.; Clapper, G.M.; Flickinger, E.A.; Bauer, L.L.; Frazier, R.L.; Fahey, G.C. Chemical and Nutritional Characteristics of United States Soybeans and Soybean Meals. J. Agric. Food Chem. 2003, 51, 7684–7691. [Google Scholar] [CrossRef]
- Karr-Lilienthal, L.K.; Grieshop, C.M.; Spears, J.K.; Fahey, G.C. Amino Acid, Carbohydrate, and Fat Composition of Soybean Meals Prepared at 55 Commercial U.S. Soybean Processing Plants. J. Agric. Food Chem. 2005, 53, 2146–2150. [Google Scholar] [CrossRef]
- Thakur, M.; Hurburgh, C.R. Quality of US Soybean Meal Compared to the Quality of Soybean Meal from Other Origins. J. Am. Oil Chem. Soc. 2007, 84, 835–843. [Google Scholar] [CrossRef]
- Stein, H.H.; Berger, L.L.; Drackley, J.K.; Fahey, G.C.; Hernot, D.C.; Parsons, C.M. 18–Nutritional Properties and Feeding Values of Soybeans and Their Coproducts. In Soybeans, edited by Lawrence A. Johnson, Pamela J. White, and Richard Galloway, 613–60. AOCS Press, 2008. [CrossRef]
- Sriperm, N.; Pesti, G.; Tillman, P. The distribution of crude protein and amino acid content in maize grain and soybean meal. Anim. Feed. Sci. Technol. 2010, 159, 131–137. [Google Scholar] [CrossRef]
- Banaszkiewicz, T. Nutritional Value of Soybean Meal. In H. El-Shemy (Ed.), Soybean and Nutrition. InTech, 2011. [CrossRef]
- Feng, J.; Liu, X.; Xu, Z.R.; Wang, Y.Z.; Liu, J.X. Effects of Fermented Soybean Meal on Digestive Enzyme Activities and Intestinal Morphology in Broilers. Poult. Sci. 2007, 86, 1149–1154. [Google Scholar] [CrossRef]
- Li, H.; Yin, J.; He, X.; Li, Z.; Tan, B.; Jiang, Q.; Chen, J.; Ma, X. Enzyme-Treated Soybean Meal Replacing Extruded Full-Fat Soybean Affects Nitrogen Digestibility, Cecal Fermentation Characteristics and Bacterial Community of Newly Weaned Piglets. Front. Veter- Sci. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raheem, S.M.; Mohammed, E.S.Y.; Mahmoud, R.E.; El Gamal, M.F.; Nada, H.S.; El-Ghareeb, W.R.; Marzok, M.; Meligy, A.M.A.; Abdulmohsen, M.; Ismail, H.; et al. Double-Fermented Soybean Meal Totally Replaces Soybean Meal in Broiler Rations with Favorable Impact on Performance, Digestibility, Amino Acids Transporters and Meat Nutritional Value. Animals 2023, 13, 1030. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, S.; Kalashnikova, O.; Ivanova, S.; Prosekov, A.; Krol, O.; Kriger, O.; Fedovskikh, N.; Babich, O. Evaluating the Influence of Microbial Fermentation on the Nutritional Value of Soybean Meal. Fermentation 2022, 8, 458. [Google Scholar] [CrossRef]
- Bello, I.; Adeniyi, A.; Mukaila, T.; Hammed, A. Developing and modelling of sustainable protein extraction using ammonium hydroxide – A recoverable and reusable solvent. Food Bioprod. Process. 2023, 140, 16–28. [Google Scholar] [CrossRef]
- Adeniyi, A.; Bello, I.; Mukaila, T.; Monono, E.; Hammed, A. Enzyme-driven bioprocessing for enhanced bio-ammonia production from soybean meal protein isolate. Biomass- Convers. Biorefinery 2023, 1–10. [Google Scholar] [CrossRef]
- Bello, I.; Adeniyi, A.; Mukaila, T.; Hammed, A. Optimization of Soybean Protein Extraction with Ammonium Hydroxide (NH4OH) Using Response Surface Methodology. Foods 2023, 12, 1515. [Google Scholar] [CrossRef]
- Verfaillie, D.; Janssen, F.; Van Royen, G.; Wouters, A.G. A systematic study of the impact of the isoelectric precipitation process on the physical properties and protein composition of soy protein isolates. Food Res. Int. 2023, 163, 112177. [Google Scholar] [CrossRef]
- Beard, E.G. Moisture Measurement. Manufacturing Confectioner 2001, 73. [Google Scholar]
- Official Methods of Analysis of AOAC INTERNATIONAL. Oxford University Press, 2023. [CrossRef]
- He, Z.; Zhang, H.; Olk, D.C.; Shankle, M.; Way, T.R.; Tewolde, H. Protein and Fiber Profiles of Cottonseed from Upland Cotton with Different Fertilizations. Mod. Appl. Sci. 2014, 8, p97. [Google Scholar] [CrossRef]
- de Conto, L.C.; Gragnani, M.A.L.; Maus, D.; Ambiel, H.C.I.; Chiu, M.C.; Grimaldi, R.; Gonçalves, L.A.G. Characterization of Crude Watermelon Seed Oil by Two Different Extractions Methods. J. Am. Oil Chem. Soc. 2011, 88, 1709–1714. [Google Scholar] [CrossRef]
- Hunsakul, K.; Laokuldilok, T.; Sakdatorn, V.; Klangpetch, W.; Brennan, C.S.; Utama-Ang, N. Optimization of enzymatic hydrolysis by alcalase and flavourzyme to enhance the antioxidant properties of jasmine rice bran protein hydrolysate. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Tömösközi, S.; Lásztity, R.; Haraszi, R.; Baticz, O. Isolation and study of the functional properties of pea proteins. Mol. Nutr. Food Res. 2001, 45, 399–401. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Enhanced alkaline extraction techniques for isolating and modifying plant-based proteins. Food Hydrocoll. 2023, 145. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Castro, E. Protein extraction from agri-food residues for integration in biorefinery: Potential techniques and current status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y.W.; Mulder, W.J.; Sanders, J.P.M.; Bruins, M.E. Towards plant protein refinery: Review on protein extraction using alkali and potential enzymatic assistance. Biotechnol. J. 2015, 10, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Deleu, L.J.; Lambrecht, M.A.; Van de Vondel, J.; Delcour, J.A. The impact of alkaline conditions on storage proteins of cereals and pseudo-cereals. Curr. Opin. Food Sci. 2019, 25, 98–103. [Google Scholar] [CrossRef]
- Hou, F.; Ding, W.; Qu, W.; Oladejo, A.O.; Xiong, F.; Zhang, W.; He, R.; Ma, H. Alkali solution extraction of rice residue protein isolates: Influence of alkali concentration on protein functional, structural properties and lysinoalanine formation. Food Chem. 2017, 218, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dossou, S.; Wang, W.; Zhang, X.; Shadrack, R.S.; Mzengereza, K.; Zhu, K.; et al. Optimization of Soybean Meal Fermentation for Aqua-Feed with Bacillus subtilis natto Using the Response Surface Methodology. Fermentation 2021, 7, 306. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Russin, T.A.; Arcand, Y.; Boye, J.I. PARTICLE SIZE EFFECT ON SOY PROTEIN ISOLATE EXTRACTION. J. Food Process. Preserv. 2007, 31, 308–319. [Google Scholar] [CrossRef]
- Etiosa, O.; Chika, N.; Benedicta, A. Mineral and Proximate Composition of Soya Bean. Asian Journal of Physical and Chemical Sciences 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Sun, X.-F.; Xu, F.; Sun, R.C.; Fowler, P.; Baird, M.S. Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr. Res. 2005, 340, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Ogawa, Y. In vitro protein digestibility and biochemical characteristics of soaked, boiled and fermented soybeans. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liener, I.E. Factors affecting the nutritional quality of soya products. J. Am. Oil Chem. Soc. 1981, 58, 406–415. [Google Scholar] [CrossRef]
- Delgado-Andrade, C. Maillard reaction products: some considerations on their health effects. cclm 2014, 52, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kohli, V.; Singha, S. Protein digestibility of soybean: how processing affects seed structure, protein and non-protein components. Discov. Food 2024, 4, 1–16. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Rahma, E.H.; El-Bedawy, A.A.; Sobihah, T.Y. Effect of soaking process on nutritional quality and protein solubility of some legume seeds. Mol. Nutr. Food Res. 2000, 44, 339–343. [Google Scholar] [CrossRef]
- Kathuria, D.; Dhiman, A.K.; Attri, S.; Kumar, M. Effect of processing method on quality characteristics of harit soybean (glycine max): in vitro protein digestibility, hplc, ftir analysis. Nutr. Food Sci. 2021, 52, 684–697. [Google Scholar] [CrossRef]
- Shi, L.; Mu, K.; Arntfield, S.D.; Nickerson, M.T. Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Canada. J. Food Sci. Technol. 2017, 54, 1014–1022. [Google Scholar] [CrossRef]
- Kayembe, N.; van Rensburg, C.J. Germination as a processing technique for soybeans in small-scale farming. South Afr. J. Anim. Sci. 2013, 43. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Zhang, C.; Kong, X.; Hua, Y. The Heat-Induced Protein Aggregate Correlated with Trypsin Inhibitor Inactivation in Soymilk Processing. J. Agric. Food Chem. 2012, 60, 8012–8019. [Google Scholar] [CrossRef]
- Gilani, G.S.; A Cockell, K.; Sepehr, E. Effects of Antinutritional Factors on Protein Digestibility and Amino Acid Availability in Foods. J. AOAC Int. 2005, 88, 967–987. [Google Scholar] [CrossRef]
- Zahir, M.; Fogliano, V.; Capuano, E. Effect of soybean processing on cell wall porosity and protein digestibility. Food Funct. 2019, 11, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Oliver, A.E.; Tablin, F.; Crowe, J.H. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr. Res. 2004, 339, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, K.M.; Chandramohan, A.; Babu, G.A.; Ramasamy, P. Synthesis, crystal growth, spectral, optical, thermal and dielectric studies of dichloro (4-hydroxy-l-proline) cadmium (II) single crystals. Optik 2014, 125, 1390–1395. Available online: https://www.sciencedirect.com/science/article/pii/S0030402613011637?casa_token=elMauyLKqScAAAAA:6aKj-f78eHj8aAdtNKMV3oAdOJgU-w6vSf04KJgAnppyRWVW3XwrKHAZq0583w7o7TXnKuxgxg. [CrossRef]
- Hoffmann, D.H. Assessment of soy cake protein quality for broiler feeding through infrared spectroscopy calibration validated by in-vitro feed analyses and in-vivo feeding studies. Ph.D. Thesis, Technische Universität München, 2019. Available online: https://mediatum.ub.tum.de/1468716.
- Dixit, A.K.; Antony, J.; Sharma, N.K.; Tiwari, R.K. 12. Soybean constituents and their functional benefits. Research Signpost 2011, 37, 2. Available online: https://www.researchgate.net/profile/Navin-Sharma-3/publication/304524926_Soybean_constituents_and_their_functional_benefits/links/5772319308ae6219474b1a20/Soybean-constituents-and-their-functional-benefits.pdf.
- Tocher, D.R.; Bendiksen, E.Å.; Campbell, P.J.; Bell, J.G. The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture 2008, 280, 21–34. Available online: https://www.sciencedirect.com/science/article/pii/S0044848608003384?casa_token=XenQEUx_ziIAAAAA:UrKnUgR--9fn2xeSyYWpNR4CUnOf5DcZRds5xohGYd_v0q-0SqreXcIL-wv3GVI0EQIAWX-eQg. [CrossRef]
- Brimas, E.; Raudonis, R.; Kareiva, A. Analytical methods used for the characterisation of specific features of biological tissues related with obesity: A review. Chemija 2022, 33. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Chassenieux, C.; Nicolai, T. Data on the characterization of native soy globulin by SDS-Page, light scattering and titration. Data Brief 2016, 9, 749–752. [Google Scholar] [CrossRef] [PubMed]
| Parameters | SMPI (%) | SPI (%) |
|---|---|---|
| Moisture content | 14 ± 0.58 | 11 ± 1.00 |
| Ash | 2.12 ± 2.47 | 1.66 ± 0.28 |
| Fat | 0.02 ± 0.00 | 8.67± 0.01 |
| Protein | 68 ± 4.50 | 56.8 ± 0.80 |
| Carbohydrate | 15.86 ± 3.01 | 21.87 ± 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





