1. Introduction
Tomato (
Solanum lycopersicum L.), a flowering plant belonging to the nightshade family (
Solanaceae), is cultivated extensively in temperate, subtropical, and some tropical regions for edible fruits. As the most consumed vegetable crop worldwide, the global tomato production is over 187 million tons with a total value over
$190 billion [
1]. Although most tomatoes are grown traditionally in open fields, recent years have seen an ever-expanding share of greenhouse-tomatoes in the market (35% of the entire tomato category), as the farming undergoes a profound shift to a capital and production infrastructure intensive model [
2].
Tomato brown rugose fruit virus (ToBRFV) is a relative new member in the genus
Tobamovirus, family
Virgaviridae. Since its first discovery in the Middle East during the 2014-2015 growing season [
3,
4], the virus has been reported in approximately 40 countries [
5,
6], including Asia [
7,
8,
9,
10], Europe [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22], North America [
23,
24,
25,
26], South America [
27], Africa [
28] and Oceania [
29]. The distribution of the virus is likely far greater than the official report given that tomato and pepper seeds exported from Australia, Thailand, India, Japan, Peru, Ethiopia, Lithuania, and Slovakia were also found to be contaminated [
6,
30]. Like other tobamoviruses, ToBRFV is a seed-borne virus, with a low rate of seed transmission [
31,
32]. The extreme robust ToBRFV particle is resilient to harsh environmental conditions and could survive for an extended period in contaminated seeds, fruits and tissue debris, as well as on worker’s hands, clothing, tools and machineries, all of which could become effective inoculum sources for disease dissemination in a production greenhouse [
33,
34,
35]. Although insects are generally not considered as a part of the transmission equation for tobamoviruses, Levitzky et al. [
36] suggested bumblebee, a common pollinator in tomato production, could spread the virus through buzz pollination, and the infected bumblebee hives may serve as the primary inoculum for further virus dissemination in the greenhouse. Under intensive greenhouse conditions, one single ToBRFV-infected plant in the beginning of a production is sufficient to infect nearly every plant within a single cropping season [
37]. Although the virus affects mainly protected production due to frequent hands-on activities for plant growth and crop production, ToBRFV outbreaks have also been recorded in the field [
38]. In two studies, ToBRFV reduced yield by 15-55% in protected tomato production depending on commercial tomato variety [
4,
39].
The striking difference between ToBRFV and other known tobamoviruses is its ability to break resistance of commercial tomato cultivars rendered by
Tm-1, Tm-2, or
Tm-22 resistance genes, which are routinely used to battle with tobamoviruses [
4,
30,
34]. Although great progress has been made to screen tomato germplasm for resistance to ToBRFV given that several genetic materials with promising tolerance or resistance were identified [
40,
41,
42,
43,
44]. Breeding is a time-consuming process, which will take years to develop a new tomato cultivar with ToBRFV resistance. On the other hand, growers are in desperate needs of curative and preventative measures against the virus to maintain crop productivity. Several chemical disinfectants have been shown to be effective to prevent ToBRFV dissemination in the greenhouse through seed treatment [
31,
32,
45], soil disinfection [
46], laundry cleaning and shoe soles [
47,
48], total greenhouse cleaning and tool dipping [
34,
49,
50], and surface material cleaning [
51]. Despite these efforts, the virus continues encroaching upon new territories which triggers the question of whether other means of transmission are involved in greenhouse production. The fact that the sequence of ToBRFV genome has been detected in wastewater samples in Slovenia [
52], the United States [
53,
54,
55], and Canada [
56] suggests possible widespread prevalence of the virus in wastewater systems. A recent report by Mehle et al. [
57] demonstrated the transmissibility of ToBRFV through contaminated irrigation water. However, to our knowledge, the technology to effectively disinfect the ToBRFV in contaminated water has not been reported up to date.
To cope with potential water-mediated transmission of ToBRFV, we employed cold plasma-generated ozone treatment on virus-contaminated water. Ozone (O
3) is a highly reactive oxidant that has been widely used as an antimicrobial agent in food industry, dental and medical field, and wastewater disinfection [
58,
59,
60]. As a flexible antimicrobial process, ozone treatment has a broad-spectrum effect on various viral pathogens [
61,
62] including but not limited to severe acute respiratory syndrome coronavirus (SARS-CoV) [
63] and SARS-CoV-2, the causal agent of the global pandemic coronavirus disease 2019 (COVID-19) [
64]. The cold plasma-generated ozone has gained an increasing attention thanks to its effectiveness in disinfecting viral and other pathogenic microorganisms [
65,
66,
67,
68,
69,
70], holding great promise as a novel broad spectrum disinfecting approach particularly for aqueous solution. In the present study, we aimed to confirm the infectivity of ToBRFV released to the recirculating hydroponic nutrient solutions in commercial greenhouses. Once it was determined, we were interested in investigating the possibility of utilizing the cold plasma-generated ozone treatment to disinfect ToBRFV residing in virus-contaminated solutions. Here we document cold plasma-generated ozone treatment successfully rendered ToBRFV existing in water solution inactive, demonstrating this technology could offer a practical solution to prevent ToBRFV contamination through a recirculating hydroponic system in commercial greenhouse tomato production.
2. Materials and Methods
2.1. Plant Material and ToBRFV Inoculum Source
Tomato seeds of the cultivar ‘Moneymaker’ were germinated on Metro-Mix potting soil (SunGro Horticulture, USA). Upon gemination, seedlings were maintained in a greenhouse with temperature of 25°C (± 1°C) in days and 20°C (± 1°C) at nights, and with natural sunlight in approximately 14h daily. The ToBRFV culture was prepared using the U.S. isolate ‘CA18-01′ originally described by Ling et al. [
25] with its pure culture generated through a serial passage [
33] and maintained on tomato plants in an insect rearing cage inside a greenhouse at the U.S. Vegetable Laboratory in Charleston, South Carolina.
To assess the relative virus concentration in the virus inoculum prepared from the infected tomato plants, we conducted a bioassay [
33] to determine the end-dilution point that could trigger in a positive infection on the inoculated tomato seedlings (4 leaf stage), in comparison to those end-dilution points detected by enzyme-linked immunosorbent assay (ELISA) and quantitative reverse transcription polymerase chain reaction (qRT-PCR), respectively, using a serial 10 × dilution (up to 1:10
12). Inoculated tomato plants were monitored weekly post-inoculation in a greenhouse for symptom development at both four- and eight- weeks post inoculation (WPI), which was followed by a confirmation test for the presence of ToBRFV using both ELISA and qRT-PCR. The results from this end-dilution point experiment were also used to estimate the ToBRFV concentration that could trigger a positive infection on inoculated tomato plants from greenhouse-collected runoff water samples.
2.2. Initial Screening of Water Samples Collected from Greenhouses and Bioassay Assessing ToBRFV Infectivity on Tomato Plants
Despite the implementation of strict hygiene and sanitation procedures, some ToBRFV infection was detected in several commercial greenhouse farms producing tomatoes in hydroponics. These greenhouse farms were located at three different localities from two states in the U.S. and were all equipped with the closed-loop fertigation system. ToBRFV infection on tomato plants in each of those greenhouses were confirmed by ToBRFV-specific qRT-PCR. We were interested in assessing the presence of ToBRFV and its infectivity in the collected leaching water solutions from individual greenhouses. A total of 134 water samples were collected from these three facilities. Specifically, water samples were collected by collaborating growers at the end of each hydroponic channel using a 50 ml sterile conical tube. The collected water samples were shipped under a USDA-APHIS (United States Department of Agriculture - Animal & Plant Health Inspection Service) permit with a secondary container and kept in a refrigerator (4°C) until test. Initial assessment for the presence of ToBRFV in each of the collected water samples was conducted directly using virus-specific qRT-PCR [
33] on a small aliquot of the solution without RNA extraction step. The infectivity of ToBRFV existing in individual collected water sample was tested using bioassay. Briefly, each water sample was directly rub-inoculated onto tomato leaves lightly dusted with carborundum using a cotton swab saturated with individual water solution. Inoculated plants were maintained in a greenhouse and monitored weekly for symptom development, and the presence of ToBRFV on each individual plant (symptomatic or asymptomatic) was confirmed using ELISA at four to eight WPI.
2.3. Secondary Test to Assess ToBRFV Infectivity in Water Samples under Long Term Storage
Tobamoviruses are typically extremely stable even under hush environmental conditions. Could the ToBRFV infectivity in the water samples collected from the commercial greenhouses remain active after a long storage in a refrigerator at 4°C? To answer this question, we further assessed the infectivity of ToBRFV existing in the four water samples selected from the initial screening. Briefly, a serial dilution (undiluted, 1:10, 1:100 and 1:1000-dilutions) of individual samples were inoculated onto tomato seedlings. All water samples were stored in a refrigerator at 4°C for two months between the initial screening and the secondary test. Three biological replicates were included for each concentration tested and the experiment was repeated twice. In each experiment, tomato seedlings inoculated with a known ToBRFV inoculum was included as the positive control, whereas the negative control was mock-inoculated seedlings using inoculation buffer. Following mechanical inoculation, plants were maintained in a greenhouse for symptom expression between 4 and 8 WPI, and leaf samples located right below the growing tip of individual plant were collected at 8 WPI and tested for the presence of ToBRFV using both ELISA and qRT-PCR.
2.4. Serological Test Using Enzyme-Linked Immunosorbent Assay (ELISA)
Double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) was conducted using a ToBRFV-specific polyclonal antibody following the manufacturer’s instructions (Agdia, Elkhart, IN, U.S.A.). Briefly, after coating with a properly diluted antibody, ELISA plate was loaded with leaf tissue extract (1:20 w/v or other specified concentrations) prepared by mechanically homogenization of freshly collected leaves in each individual meshed plastic sample bag using a HOMEX-6 tissue homogenizer (Biobeba, Reinach, Switzerland). ELISA results were considered positive when the optical density (OD) absorbance value at 405nm was at least three times as that of the healthy control [
35]. The OD value was obtained by averaging the readings of three technical replicates of individual sample using a SpectraMax microplate reader (Molecular Devices, San Jose, USA).
2.5. Quantitative Real-Time PCR
The Taqman
® quantitative RT-PCR assay specific for ToBRFV developed in our laboratory [
33] was used in the present study to make specific detection to confirm the presence of ToBRFV sequence. Briefly, 20 µl reaction was assembled using SuperScript III Platinum One-Step RT-qPCR kit (ThermoFisher, Whaltham, MA, USA) according to the manual with the final concentration of ToBRFV-F1: ToBRFV-P1: ToBRFV-R1 at 0.4 µM: 0.2 µM: 0.4 µM, respectively. Three technical replicates were included for each sample when conducting qRT-PCR using the AriaMX real-time PCR system (Agilent Technologies, Santa Clara, CA, USA). The program was initiated at 50°C for 15 min then at 95°C for 2 min, followed by 40 cycles each consisting of 95°C for 15s and 50°C for 30s.
2.6. Quantitative Immunocapture Real-Time PCR
One drawback in using qRT-PCR for virus detection is the need to use purified RNA preparations, which is laborious and costly for many samples. To circumvent this drawback, we evaluated the possibility of using immunocapture for sample preparation followed by qRT-PCR, herein the quantitative immunocapture real-time PCR (IC-qRT-PCR) was developed and optimized. Briefly, 100 µl low profile strip tubes were coated with 25 µl of ToBRFV-specific polyclonal antibody (1:400 diluted in ELISA coating buffer) and incubated overnight at 4°C. After thorough wash with phosphate washing buffer, 25 µl plant tissue extract (1:100, w/v) and its serial dilutions were then loaded in the anti-ToBRFV antibody coated PCR tubes and incubated at 37°C for 2h. Following the binding of the antibody and potential virus antigen in the sample, plant sap was thoroughly washed using phosphate buffer with three changes. In the washed clean tubes, the qRT-PCR reaction reagents were assembled and followed by the thermal cycling scheme as mentioned above.
2.7. Cold Plasma-Generated Ozone Treatment on ToBRFV Inoculum Prepared from Freshly Collected ToBRFV-Infected Tomato Tissue
Confirmation of ToBRFV infectivity in runoff water solutions collected from looped hydroponic systems demonstrated a high possibility that the virus could be transmitted through contaminated nutrient solution to other parts of a greenhouse under the same irrigation line. We were interested in developing a water treatment method to inactivate ToBRFV infectivity in a contaminated nutrient solution. Because the current laboratory testing methods (i.e., ELISA and qRT-PCR) could not differentiate between live and infectious versus degraded and non-infectious virions, it is necessary to perform bioassay on tomato plants to assess the efficacy of different treatment conditions. ToBRFV-infected plant homogenates were prepared to mimic some potential contamination levels of the virus in the runoff water solutions collected from the commercial greenhouses.
Virus inoculum preparations were processed using leaf tissue samples collected from the ToBRFV-infected tomato plants expressing typical mosaic symptoms and confirmed for the presence of ToBRFV by qRT-PCR. Each individual sample bag containing 0.8 g, 8 g, or 80 g of ToBRFV-infected tomato leave tissue was first processed in a small volume of the inoculation buffer [
33] at 1:10 ratio (w/v) using a Homex-6 tissue homogenizer. Prior to water treatment, individual homogenate was then blended into 8 L of water in a large glass vase, generating ToBRFV inocula containing different dilutions of ToBRFV-infected tissue extract (1:100, 1:10
3, or 1:10
4) (w/v), respectively. Because of the need to assess efficacy using three different concentrations of cold plasma ozone for water treatment, it was necessary to prepare the same concentrations of individual virus inoculum in three separate glass vases for their respective ozone treatments.
Water treatment was conducted using cold plasma ozone generated using a PMOSafe system (Clear Path Holdings Corp, Morganville, NJ, U.S.). Briefly, ozone was introduced into virus inoculum prepared in individual 8 L glass vases in the form of tiny air bubbles to produce O3 concentrations at 0.1 mg/L, 0.6 mg/L, and 1.0 mg/L, respectively. Different concentrations of the prepared stock virus inoculum (at 1:10 ratio (w/v)) were then added to generate water reservoir containing different ToBRFV dilutions (1:100, 1:103, and 1:104) that had been balanced with specific concentration of the cold plasma ozone, which were incubated for pre-designated exposure timeframes (0 min, 24 min, 48 min, and 72 min). The 0 min-treatment was a water sample (3 ml) taken from the reservoir immediately after the virus inoculum was introduced into the ozone-activated water solution with no time for ozone exposure. This treatment served as a positive control for the experiment, establishing a baseline to determine the virus infectivity prior to the ozone treatment. At the end of each ozone exposure timeframe, a small volume of treated water solution (3 ml) was withdrawn from each reservoir with a sterile pipette and then used to assess the efficacy of ozone treatment on ToBRFV infectivity through bioassay. Briefly, for each treatment with a combination of specific ozone concentration and exposure timeframe, six tomato seedlings at four-leaf stage were rub-inoculated with collected water solution. At four and eight WPI, inoculated plants were observed for disease symptoms and the presence of ToBRFV was confirmed using ELISA and qRT-PCR as described above. The percentage of virus infection (number of plants tested positive for ToBRFV/number of plants inoculated) for each treatment was analyzed to reach a conclusion on their respective efficacies.
3. Results
3.1. ToBRFV Detected in Runoff Water Solutions Collected from Commercial Greenhouses Induce Virus Infection on Inoculated Tomato Seedlings
To assess whether ToBRFV exists in the runoff water solution collected from commercial greenhouses and whether the existing ToBRFV is infectious, we conducted an initial screening on a total of 134 water samples. We first performed ToBRFV-specific qRT-PCR assay to detect presence of the virus followed by conducting bioassay on tomato seedlings to determine whether the detected ToBRFV could induce virus infection. Results (
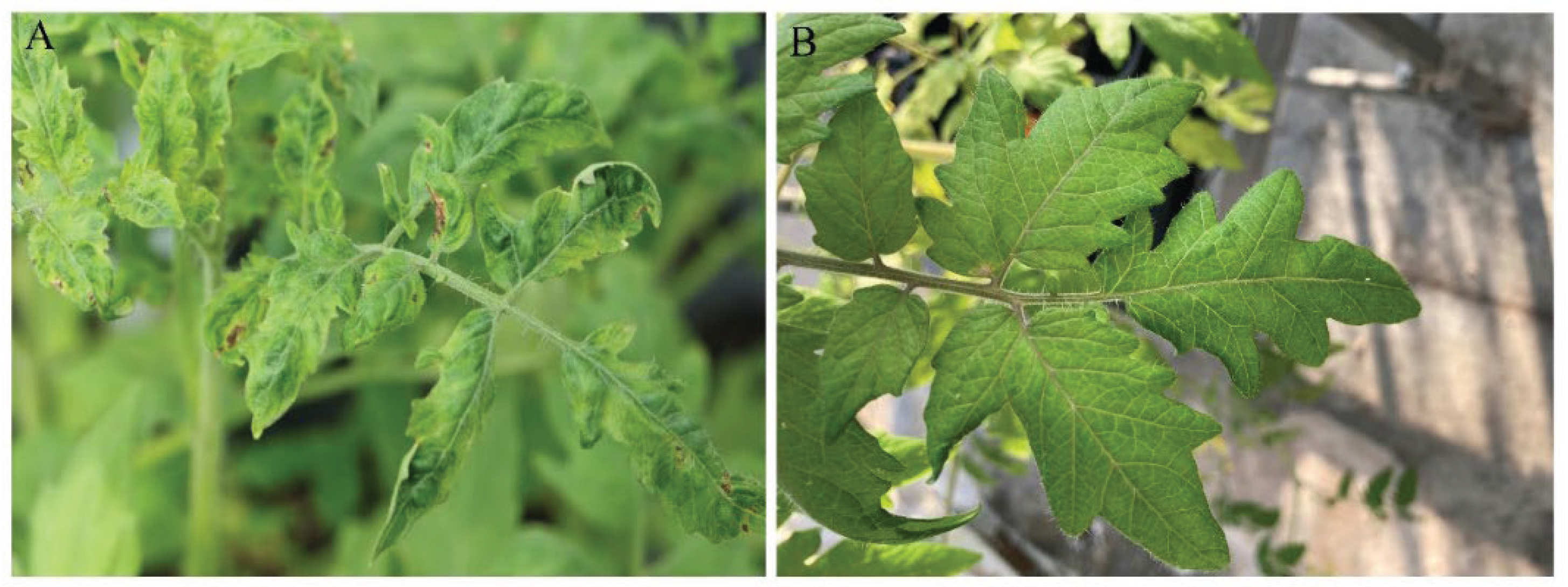
Table 1) showed that both samples collected from the Farm #1 were not only tested positive for the virus but also triggered ToBRFV infection on inoculated tomato plants in bioassay, exhibiting typical mosaic symptoms (
Figure 1) and the virus infection was further confirmed by ELISA. In the Farm #2, although 32 of 35 water samples were tested positive for ToBRFV based on qRT-PCR results, only 2 out of the 32 samples with relatively low Ct values (19.90 and 22.97 respectively) resulted in virus infection on the inoculated tomato plants which was confirmed for the presence of ToBRFV in ELISA (
Table 1). On the other hand, none of 97 samples collected from the Farm #3 resulted in a positive infection on inoculated tomato plants although nearly 50% of them (48 of 97) were tested positive for ToBRFV in qRT-PCR, with most of the Ct readings in water samples near the cut-off value (Ct 30.00) (
Table 1,
Supplementary Table S1). Overall, individual water samples were considered as ToBRFV-infected when they met the following criteria: should be tested positive by qRT-PCR and also induce ToBRFV infection in bioassay. Based on this, four samples (V22-14, V22-15, V22-29, and V22-43) were considered positive and selected for further evaluation.
3.2. Secondary Test of ToBRFV Infectivity in Selected Water Samples through Serial Dilution
To further confirm the infectivity of ToBRFV in the four selected water samples identified in the preliminary screening, we conducted an extensive bioassay using their serial dilutions to assess the potential infectivity of ToBRFV remained in them after stored at 4°C for two months. Results showed that virus infection was confirmed on tomato plants inoculated with two out of the four water samples (V22-15 from the Farm #1 and V22-43 from the Farm #2), and only from their undiluted groups. In comparison, none of the plants inoculated using further their dilutions (1:10, 1:100 or 1:1,000) triggered the infection (
Table 2), suggesting the concentration of infectious ToBRFV in the undiluted water samples approached the lowest virus titer to trigger ToBRFV infection after two-month storage. For the water sample V22-15 from the Farm #1, only one of the six inoculated tomato plants was infected and expressed typical mosaic symptoms at 8 WPI, which was confirmed for the presence of ToBRFV using ELISA and IC-qRT-PCR. For the water sample V22-43 from Farm #2, two of the inoculated tomato plants expressed mosaic symptoms at 4 WPI, while a third one at 8 WPI (
Table 2). All four infected plants (one in V22-15 and three from V22-43) exhibited typical mosaic symptoms for ToBRFV at the time when also tested positive for the virus (
Figure 1). This experiment further validated that bioassay is a powerful tool to evaluate the level of infectious virus in a given sample.
3.3. Assessing the Dilution Endpoint of the Inoculum Prepared from ToBRFV-Infected Tomato Tissue for Its Ability to Trigger Virus Infection on Tomato Plants
Given that only undiluted runoff water samples caused ToBRFV infection, we were interested in assessing the level of infectious ToBRFV in these water samples compared to that in the serial dilutions of the ToBRFV inoculum we used in this experiment. Understanding the dilution end point that could trigger virus infection using the ToBRFV inoculum we generated, which reflects the level of infectious ToBRFV in the runoff water could help us determine appropriate parameters used for the water treatment. Through testing serial dilutions (1: 100 – 1: 10
12) of the virus inoculum prepared from ToBRFV-infected leaf tissue, ToBRFV was detectable up to 1: 10
6 based on the absorbance readings at OD
405nm in DAS-ELISA and from Ct values in IC-qRT-PCR (
Table 3). However, in bioassay, a successful infection on the inoculated tomato plants was detectable up to 1: 10
5-dilution (
Table 3).
3.4. Efficacy of Cold Plasma-Generated Ozone Treatment against the Infectivity of ToBRFV
Upon proving that ToBRFV existing in the runoff water solutions from commercial greenhouses could trigger virus infection in bioassay through direct inoculating the water solution onto tomato plants, which indicates that ToBRFV could potentially be transmitted through a hydroponic irrigation line to other parts of a greenhouse, we were interested in developing a water treatment using cold plasma ozone against ToBRFV infection. To evaluate the efficacy of the treatment, we conducted bioassay to assess the infectivity of ToBRFV inocula prepared in different dilutions after being exposed to different ozone concentrations for pre-selected timeframes. With a combination of three ozone concentrations and four treatment timeframes, 15 different treatments including a mock inoculation were evaluated for three dilutions of virus inoculum (1:100-, 1:1000-, and 1:10,000-dilutions) (
Table 4). Results showed that under the highest concentration of ToBRFV inoculum (1:100-dilution), a prolonged exposure (72 min) to the two higher ozone concentrations input (0.6 mg/L and 1.0 mg/L) resulted in lower percentage of the test plants (33.3% and 16.7%) developing virus infection (
Figure 1), although the infectivity of ToBRFV was not completely aborted. Under a medium-concentration of the virus inoculum (1:1000-dilution), although an exposure for 24 min at two higher ozone concentrations (0.6 mg/L and 1.0 mg/L) only reduced the virus infection rate to 16.7%, longer exposure for 48 min did completely abort virus infectivity demonstrated by 0% infection on inoculated plants. The same effect was achieved using even the lowest concentration of ozone input (0.1 mg/L). Furthermore, under the lowest concentration of virus inoculum (1:10,000-dilution), the virus was completely inactivated at even a shorter exposure time (24 min) in any of the three ozone concentrations (0.1 mg/L – 1.0 mg/L). Given that the level of infectious ToBRFV in the greenhouse-collected runoff water samples, as determined above, was comparable to the lowest concentration of the virus inoculum (1:10,000-dilution) used here which can be fully inactivated using minimal ozone input within the shortest treatment timeframe selected, results from this study show great promise in inactivating ToBRFV residing in the runoff water solutions from greenhouses.
4. Discussion
The existence of plant viruses in water environments has been described for many years with a primary concern that the contaminated water could serve as an inoculum to initiate a secondary virus infection, exacerbating the disease problem [
57,
71,
72]. However, virus concentration from water samples collected from rivers, creeks, stream, drainage, and sea water was often too low to directly work on, therefore they were often concentrated by ultracentrifugation or polyethylene glycol (PEG) precipitation before any in-depth test such as RT-PCR could be performed [
73,
74]. Agricultural manufacturing facilities such as large-scale hydroponic farms equipped with closed recycling fertigation water systems, on the other hand, aid in accumulating water-borne pathogens. Previous studies did reveal that some highly transmissible viruses such as tomato mosaic virus (ToMV) and cucumber green mottle mosaic virus (CGMMV) could spread easily from one single infected plant to the entire hydroponic system in greenhouses [
75,
76].
In the present study, recirculating nutrient water solution was collected from tomato hydroponic farms where ToBRFV infection occurred, which was directly used to inoculate tomato seedlings without any further concentration step. Successful ToBRFV infection became established from four out of 134 samples (~3%) collected from 3 greenhouses in the initial screening. In the secondary test using water samples that were stored for two months at 4°C, virions in two of the above four positive samples remained infectious. Although sample V22-29 contained higher virus titer compared with V22-15 and V22-43 as measured by qRT-PCR and DAS-ELISA and were able to establish ToBRFV infection in the initial screening (
Table 1), it failed to trigger ToBRFV infection in the secondary test. This is likely due to virions existing in the V22-29 degraded under a prolonged storage, leading to the loss of virus infectivity. Up to this point, we proved that ToBRFV detected in the runoff solution collected from hydroponic farms was infectious, which coincides with a recent report where infectious ToBRFV was detected in drainage water collected from commercial greenhouses [
57]. The fact that ToBRFV residing in the runoff water solutions remained infectious for at least two months substantiates the robust structure the virus has, which greatly increases its possibility of circulating through the hydroponic irrigation system in the commercial greenhouses.
Bioassay is still the most reliable approach to assess the infectivity of virus existing in a given sample even though DAS-ELISA and qRT-PCR may have higher sensitivity in detecting the presence of the virus (
Table 1 and
Table 3). It is noteworthy that neither of the two methods could differentiate between infectious and non-infectious virion in a particular sample considering the molecular detection assay only target specific regions of virus genetic material, and in the case of ToBRFV it is a 98-nucleotide segment of the movement protein gene, whereas the serological assay detects the presence of structural components of the virion, for instance, viral capsid protein. These components could be present in samples containing either intact virus particles or remnants of degraded virions [
77,
78], and latter could still yield positive results in molecular and/or serological detection but would fail in triggering virus infection in bioassay. For this reason, bioassay was utilized in the current study to evaluate the infectivity of collected runoff water solutions and the efficacy of cold plasma-generated ozone treatment against ToBRFV. In conjunction with the results of bioassay, none of the further diluted runoff water solutions led to ToBRFV infection indicating the approximate active virus titer in samples collected from the Farms #1 and #2 approached 1: 10
5- dilution of ToBRFV-infected tissue (
Table 2 and
Table 3). This number is at an alarmingly high level considering that these runoff water solutions are uncondensed environmental samples. On the other hand, none of the 97 runoff water sample collected from the Farm #3 could trigger ToBRFV infection suggesting the active virus titer there was below this threshold, despite a number of samples tested positive in qRT-PCR. In addition, Mehle and colleagues proved that ToBRFV residing in the nutrient solution or irrigation water could infect healthy plants through roots [
57], further substantiating that the water-borne nature of ToBRFV and the fact that virus-contaminated hydroponic irrigation system is a potential high-risk factor in disseminating the virus and hence should be targeted as a vulnerable stage in the ToBRFV disease cycle. To minimize potential disease dispersal, water solution circulating in the hydroponic system should be treated properly to inactivate ToBRFV infectivity prior to recirculate within the same hydroponic system.
Although chemical disinfectants have been shown to be effective against ToBRFV through various treatments [
31,
32,
34,
45,
46,
47,
48,
49,
50,
51], offering practical solutions to manage both vertical and horizontal transmission of the virus in the greenhouse, applying chemotherapy such as using these chemicals to hydroponic water systems can be challenging due to the concern of phytotoxicity and chemical residues. It is therefore imperative to explore other environmental-friendly methods that can be safely applied in water treatment. Different non-chemical methods such as heat, sonication, media and membrane filtrations, etc. have also been used to control viruses [
79]. Ultraviolet (UV) radiation, another commonly used sanitation measure in hydroponic production systems, was shown to greatly reduce tobacco mosaic virus (TMV) and confine the spread of lettuce ring necrosis virus (an ophiovirus transmitted by
Olpidium brassicae), respectively [
80,
81]. However, the disinfection effect of UV radiation on many other targeted viruses in water environments is generally considered to be minimal [
66]. To cope with this challenge, we treated virus-contaminated water reservoir with ozone generated by cold plasma and subsequently evaluated the efficacy of this approach in inactivating the ToBRFV infectivity. Results showed that the effectiveness of ozone treatment is affected by a combination of virus titer in contaminated water, input ozone concentration and the timeframe exposed to ozone treatment. When the virus inoculum was 1:100-dilution of ToBRFV, virus infectivity was not affected until the water was exposed to higher ozone concentrations for 72 min (
Table 4). As the relative virus titer reduced to 1:1000-dilution, 48 min exposure at the lowest ozone level (0.1 mg/L) is sufficient to inactivate the virus. Furthermore, minimal exposure time (24 min) of the lowest level of ozone input was adequate to inactivate the virus when the virus inoculum drops to 1:10,000-diution, which is equivalent to the active virus titer detected from the greenhouse-collected runoff water samples. Taken together, our results suggest cold plasma-generated ozone treatment is effective in inactivating ToBRFV infectivity in water if appropriate ozone concentration and exposure timeframe were applied. Given that the lowest active virus titer tested in this experiment (1:10
4- dilution of ToBRFV inoculum), which can be completely inactivated by the lowest ozone input with 24 min exposure, is 10-time higher than that in the runoff water solutions collected from commercial greenhouse farms (approximately 1:10
5- dilution of ToBRFV inoculum), the cold plasma ozone treatment could offer a promising solution to prevent potential ToBRFV outbreaks in a hydroponic greenhouse.
5. Conclusions
ToBRFV has become a most troubling plant pathogen threatening global tomato production in recent years, with the highly mechanical transmissibility and seed-borne characteristics serving as the driving forces for its quick expansion worldwide. Apart from known transmission pathways, here we proved the waterborne nature of the virus and added a new element to its epidemiology. Although additional studies are needed to thoroughly assess water-mediated ToBRFV transmission to answer questions on whether virus-infected water is the disease trigger directly causing outbreaks, the fact that ToBRFV-contaminated nutrient solution and irrigation water could infect healthy plants through their roots presents an alarming new sign for vegetable growers [
57], while raising special concerns for hydroponic farms as virus outbreaks continue to occur even when strict sanitation measures have been implemented. Although the number of water sample tested positive in this study is relatively low (4/134), in the closed-loop fertigation system where nutrient solution is captured and recirculated, virus infection occurring in only one spot could be readily spread to the entire system [
37]. With the proven efficacy of ozone treatment in disinfecting ToBRFV in nutrient solution reported here, we are one step closer to conquering this devastating virus through minimizing its spread in greenhouse tomato production.
Supplementary Materials
Supplementary Table S1. Preliminary screening results of ToBRFV in runoff water samples collected from three commercial greenhouses.
Author Contributions
Conceptualization, K.L.; methodology, K.L., J.Z. and A.G.; validation, K.L., J.Z. and A.G.; formal analysis, K.L. and J.Z.; investigation, J.Z., K.L. and A.G.; resources, K.L.; data curation, K.L., J.Z. and A.G.; writing—original draft preparation, J.Z.; writing—review and editing, K.L. and A.G.; visualization, K.L. and J.Z; supervision, K.L.; project administration, K.L.; funding acquisition, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the USDA-ARS National Plant Disease Recovery System (grant number: 6080-22000-032-000D) and the USDA-NIFA Crop Protection and Pest Management program, grant number: 2023-70006-40603 to KSL.
Data Availability Statement
Data are presented in the supplementary materials.
Acknowledgments
We thank the anonymous collaborating greenhouse tomato growers who helped collected and shipped runoff water samples for this study. Technical support provided by the Clear Path Holdings Corp in setting up the PMOSafe system is greatly appreciated. This research was supported in part by an appointment to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA). ORISE is managed by ORAU under DOE contract number DE-SC0014664. All opinions expressed in this paper are the author’s and do not necessarily reflect the policies and views of USDA, DOE, or ORAU/ORISE. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ofori, P.A.; Owusu-Nketia, S.; Opoku-Agyemang, F.; Agbleke, D.; Naalamle Amissah, J. Greenhouse tomato production for sustainable food and nutrition security in the tropics. in Tomato – From cultivation to processing technology, ed. Viskelis, P.; Urbonavičienė, D.; Viskelis, J. IntechOpen, 2022. [CrossRef]
- Minor, T.; Baskins, S.; Bond, J.K. Imported greenhouse tomatoes from Mexico illustrate the growing diversity in fresh-market tomatoes. 2019. USDA ERS - Imported Greenhouse Tomatoes From Mexico Illustrate the Growing Diversity in Fresh-Market Tomatoes. (accessed 3/18/2024).
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.W.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol., 2016, 161, 503-506.
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I. et al. A new Israeli tobamovirus isolate infects tomato plants harboring Tm-22 resistance genes. PLoS ONE, 2017, 12, e0170429.
- EPPO global database. Tomato brown rugose fruit virus (TOBRFV)[World distribution]| EPPO Global Database. 2024 (accessed 3/18/2024).
- Salem, N.M.; Jewehan, A.; Aranda, M.A.; Fox, A. Tomato brown rugose fruit virus pandemic. Annu. Rev. Phytopathol. 2023, 61, 137–164. [Google Scholar] [CrossRef] [PubMed]
- Fidan, H.; Sarikaya, P.; Calis, O. First report of Tomato brown rugose fruit virus on tomato in Turkey. New Dis. Rep., 2019, 39, 18.
- Ghorbani, A.; Rostami, A.; Seifi, S.; Izadpanah, K. First report of Tomato brown rugose fruit virus in greenhouse tomato in Iran. New Dis. Rep., 2021, 44, e12040.
- Sabra, A.; Al-Saleh, M.A.; Al-Shahwan, I.M.; Amer, M.A. First report of Tomato brown rugose fruit virus infecting the tomato crop in Saudi Arabia. Plant Dis. 2022, 106, 1310. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-Y.; Ma, H.-Y.; Han, S.-L.; Geng, C.; Tian, Y.-P.; Li, X.-D. First report of Tomato brown rugose fruit virus infection tomato in China. Plant Dis. 2019, 103, 2973. [Google Scholar] [CrossRef]
- Alfaro-Fernández, A.; Castillo, P.; Sanahuja, E.; Rodríguez-Salido, M.C.; Font, M.I. First report of Tomato brown rugose fruit virus in tomato in Spain. Plant Dis., 2021, 105, 515.
- Beris, D.; Malandraki, I.; Kektsidou, O.; Theologidis, I.; Vassilakos, N.; Varveri, C. First report of Tomato brown rugose fruit virus infecting tomato in Greece. Plant Dis., 2020, 104, 2035.
- Eichmeier, A.; Hejlova, M.; Orsagova, H.; Frejilchova, L.; Hakalova, E.; Tomankova, K.; et al. Characterization of tomato brown rugose fruit virus (ToBRFV) detected in Czech Republic. Diversity, 2023, 15, 301.
- Hamborg, Z.; Blystad, D-R. First report of Tomato brown rugose fruit virus in tomato in Norway. Plant Dis., 2022, 106, 2004.
- Hasan, Z.M.; Salem, N.M.; Ismail, I.D.; Akel, E.H.; Ahmad, A.Y. First report of Tomato brown rugose fruit virus on greenhouse tomato in Syria. Plant Dis., 2022, 106, 772.
- Mahillon, M.; Kellenberger, I.; Dubuis, N.; Brodard, J.; Bunter, M.; Weibel, F.; et al. First report of Tomato brown rugose fruit virus in tomato in Switzerland. New Dis. Rep., 2022, 45, 12065.
- Menzel, W.; Knierim, D.; Winter, S.; Hamacher, J.; Heupel, M. First report of Tomato brown rugose fruit virus infecting tomato in Germany. New Dis. Rep., 2019, 39, 1.
- Orfanidou, C.G.; Cara, M.; Merkuri, J.; Papadimitriou, K.; Katis, N.I.; Maliogka, V.I. First report of Tomato brown rugose fruit virus in tomato in Albania. J. Plant Pathol., 2022, 104, 855.
- Panno, S.; Caruso, A.G.; Davino, S. First report of Tomato brown rugose fruit virus on tomato crops in Italy. Plant Dis., 2019, 103, 1443.
- Skelton A.; Buxton-Kirk, A.; Ward, R.; Harju, V.; Frew, L.; Fowkes, A.; et al. First report of Tomato brown rugose fruit virus in tomato in the United Kingdom. New Dis. Rep., 2019, 40, 12.
- Skelton A.; Gentit, P.; Porcher, L.; Visage, M.; Fowkes, A.; Adams, I.P.; et al. First report of Tomato brown rugose fruit virus in tomato in France. New Dis. Rep., 2022, 45, e12061.
- Van de Vossenberg, B.T.K.H.; Visser, M.; Bruinsma, M.; Koenraadt, H.M.S.; Westenberg, M. Real-time tracking of Tomato brown rugose fruit virus outbreaks in the Netherlands using Nextstrain. PLoS ONE, 2020, 15, e0234671.
- Camacho-Beltrán E.; Pérez-Villarreal, A.; Leyva-López, N.E.; Rodríguez-Negrete, E.A.; Ceniceros-Ojeda, E.A.; Méndez-Lozano, J. Occurrence of Tomato brown rugose fruit virus infecting tomato crops in Mexico. Plant Dis., 2019, 103, 1440.
- Cambrón-Crisantos, J.M.; Rodríguez-Mendoza, J.; Valencia-Luna, J.B.; Alcasio-Rangel, S.; García-Ávila, C.J.; López-Buenfil, J.A.; Ochoa-Martínez, D.L. First report of tomato brown rugose fruit virus (ToBRFV) in Michoacan, Mexico. Rev. Mex. Fitopatol., 2018, 37, 185–192.
- Ling, K.-S.; Tian, T.; Gurung, S.; Salati, R.; Gilliard, A. First report of Tomato brown rugose fruit virus infecting tomato in the United States. Plant Dis., 2019, 103, 1439.
- Sarkes, A.; Fu, H.; Geindel, D.; Harding, M.; Feng, J. Development and evaluation of a loop-mediated isothermal amplification (LAMP) assay for the detection of Tomato brown rugose fruit virus (ToBRFV). PLoS ONE, 2020, 15, e0230403.
- Obregón, V.G.; Ibañez, J.M.; Lattar, T.E.; Juszczak, S.; Growth-Helms, D. First report Tomato brown rugose fruit virus in tomato in Argentina. New Dis. Rep., 2023, 48, e12203.
- Amer, M.A.; Mahmoud, S.Y. First report of tomato brown rugose fruit virus on tomato in Egypt. New. Dis. Rep., 2020, 41, 24.
- Anonymous. New Zealand: ToBRFV detected in small seed lot. 2021, www.hortidaily.com/article/9281236/new-zealand-tobrfv-detected-in-small-seed-lot/ (accessed 3/18/2024).
- Zhang, S.; Griffiths, J.S.; Marchand, G.; Bernards, M.A.; Wang, A. Tomato brown rugose fruit virus: an emerging and rapidly spreading plant RNA virus that threatens tomato production worldwide. Mol. Plant. Pathol., 2022, 23, 1262-1277.
- Davino, S.; Caruso, A.G.; Bertacca, S.; Barone, S.; Panno, S. Tomato brown rugose fruit virus: seed transmission rate and efficiency of different seed disinfection treatments. Plants, 2020, 9, 1615.
- Salem, N.M.; Sulaiman, A.; Samarah, N.; Turina, M.; Vallino, M. Location of mechanical transmission of tomato brown rugose fruit virus in tomato seeds. Plant Dis., 2022, 106, 275-281.
- Chanda, B.; Gilliard, A.; Jaiswal, N.; Ling, K.-S. Comparative analysis of host range, ability to infect tomato cultivars with Tm-22 gene and real-time RT-PCR detection of tomato brown rugose fruit virus. Plant Disease, 2021a, 105, 3643-3652.
- Chanda, B.; Shamimuzzaman, M.; Gilliard, A.; Ling, K.-S. Effectiveness of disinfectants against the spread of tobamoviruses: Tomato brown rugose fruit virus and Cucumber green mottle mosaic virus. Virol. J., 2021b, 18, 7.
- Klap, C.; Luria, N.; Smith, E.; Bakelman, E.; Belausov, E.; Laskar, O.; Lachman, O.; Gal-On, A.; Dombrovsky, A. The potential risk of plant-virus disease initiation by infected tomatoes. Plants, 2020, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of Tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE, 2019, 14, e0210871.
- Panno, S.; Caruso, A.G.; Barone, S.; Lo Bosco, G.; Rangel, E.A.; Davino, S. Spread of tomato brown rugose fruit virus in Sicily and evaluation of the spatiotemporal dispersion in experimental conditions. Agronomy, 2020, 10, 834. [Google Scholar] [CrossRef]
- Dey, K.K.; Velez-Climent, M.; Soria, P.; Batuman, O.; Mavrodieva, V.; Wei, G.; et al. First report of Tomato brown rugose fruit virus infecting tomato in Florida, USA. New Dis. Rep., 2021, 44, e12028.
- Avni, B.; Gelbart, D.; Sufrin-Ringwald, T.; Zinger, A.; Chen, L.; Machbash, Z.; Bekelman, I.; et al. Tomato genetic resistance to tobamoviruses is compromised. Acta Hortic., 2021, 1316, 89-98.
- Jewehan, A.; Salem, N.; Tóth, Z.; Salamon, P.; Szabó, Z. Screening of Solanum (sections Lycopersicon and Juglandifolia) germplasm for reactions to the tomato brown rugose fruit virus. J. Plant Dis. & Protect., 2022a, 129, 117–123.
- Jewehan, A.; Salem, N.; Tóth, Z.; Salamon, P.; Szabó, Z. Evaluation of responses to tomato brown rugose fruit virus (ToBRFV) and selection of resistant lines in Solanum habrochaites and Solanum peruvianum germplasm. J. Gen. Plant Pathol., 2022b, 88, 187–196.
- Kabas, A.; Fidan, H.; Kucukaydin, H.; Atan, H.N. Screening of wild tomato species and interspecific hybrids for resistance/tolerance to Tomato brown rugose fruit virus (ToBRFV). Chil. J. of Agric. Res., 2022, 82, 189–196.
- Jaiswal, N.; Chanda, B.; Gilliard, A.; Shi, A.; Ling, K.-S. Evaluation of tomato germplasm against tomato brown rugose fruit virus and identification of resistance in Solanum pimpinellifolium. Plants, 2024, 13, 581. [CrossRef]
- Zinger, A.; Lapidot, M.; Harel, A.; Doron-Faigenboim, A.; Gelbart, D.; Levin, I. Identification and mapping of tomato genome loci controlling tolerance and resistance to tomato brown rugose fruit virus. Plants, 2021, 10, 1–16.
- Samarah, N.; Sulaiman, A.; Salem, N.M.; Turina, M. Disinfection treatments eliminated tomato brown rugose fruit virus in tomato seeds. Eur. J. Plant Pathol., 2021, 159, 153–162. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Mor, N.; Gantz, S.; Lachman, O.; Smith, E. Disinfection efficacy of tobamovirus-contaminated soil in greenhouse-grown crops. Horticulturae, 2022, 8, 563. [Google Scholar] [CrossRef]
- Ehlers, J.; Nourinejhad Zarghani, S.; Kroschewski, B.; Büttner, C.; Bandte, M. Cleaning of tomato brown rugose fruit virus (ToBRFV) from contaminated clothing of greenhouse employees. Horticulturae, 2022a, 8, 751.
- Ehlers, J.; Nourinejhad Zarghani, S.; Kroschewski, B.; Büttner, C.; Bandte, M. Decontamination of tomato brown rugose fruit virus-contaminated shoe soles under practical conditions. Horticulturae, 2022b, 8, 1210.
- Ling, K.-S.; Gilliard, A.C.; Zia, B. Disinfectants useful to manage the emerging tomato brown rugose fruit virus in greenhouse tomato production. Horticulturae, 2022, 8, 1193.
- Rodriguez-Diaz, C. I.; Zamora-Macorra, E. J.; Ochoa-Martinez, D.L.; Gonzalez-Garza, R. Disinfectants effectiveness in tomato brown rugose fruit virus (ToBRFV) transmission in tobacco plants. Rev. Mex. Fitopatol., 2022, 40, 240-253.
- Skelton, A.; Frew, L.; Ward, R.; Hodgson, R.; Forde, S.; McDonough, S.; Webster, G.; Chisnall, K.; Mynett, M.; Buxton-Kirk, A.; Fowkes, A. R.; Weekes, R.; Fox, A. Tomato brown rugose fruit virus: survival and disinfection efficacy on common glasshouse surfaces. Viruses, 2023, 15, 2076.
- Bačnik, K.; Kutnjak, D.; Pecman, A.; Mehle, N.; Žnidarič, M.T.; Aguirre, I.G.; Ravnikar, M. Viromics and infectivity analysis reveal the release of infective plant viruses from wastewater into the environment. Water Res., 2020, 177, 115628.
- Natarajan, A.; Fremin, B.J.; Schmidtke, D.T.; Wolfe, M.K.; Zlitni, S.; Graham, K.E., et al. The tomato brown rugose fruit virus movement protein gene is a novel microbial source tracking marker. Appl. Environ. Microbiol., 2023, 89, e0058323.
- Rothman, J.; Whiteson, K. Sequencing and variant detection of eight abundant plant-infecting tobamoviruses across southern California wastewater. Microbiol. Spectr., 2022, 10, e0305022.
- Sherchan, S.P.; Malla, B.; Haramoto, E. First quantitative detection of tomato brown rugose fruit virus in wastewater in Louisiana. Sci. Total Environ., 2023, 888, 164001.
- Nash, D.; Ellmen, I.; Knapp, J.J.; Menon, R.; Overton, A.K.; Cheng, J.; Lynch, M.D.J.; Nissimov, J.I.; Charles, T.C. A novel tiled amplicon sequencing assay targeting the tomato brown rugose fruit virus (ToBRFV) genome reveals widespread distribution in municipal wastewater treatment systems in the province of Ontario, Canada. Viruses, 2024, 16, 460. [CrossRef]
- Mehle, N.; Bačnik, K.; Bajde, I.; Brodarič, J.; Fox, A.; Gutiérrez-Aguirre, I., et al. Tomato brown rugose fruit virus in aqueous environments – survival and significance of water-mediated transmission. Front. Plant Sci. 2023, 14, 1187920.
- Baysan, A.; Lynch, E. The use of ozone in dentistry and medicine. Prim. Dent. J., 2005, 12, 47-52.
- Khadre, M.A.; Yousef, A.E.; Kim, J.-G. Microbiological aspects of ozone applications in food: a review. J. Food Sci., 2001, 66, 1242-1252.
- Seridou, P.; Kalogerakis, N. Disinfection application of ozone micro- and nanobubbles. Environ. Sci.: Nano, 2021, 8, 3493-3510.
- Sato, H.; Wananabe, Y.; Miyata, H. Virucidal effect of ozone treatment of laboratory animal viruses. Jikken Dobutsu., 1990, 39, 223–229.
- Boast, N.; Heselton, D.; Hudson, J. Apparatus and method for using ozone as a disinfectant. International Publication Number WO2005087278A1. World Intellectual Property Organization. 2008. [Google Scholar]
- Zhang J.; Zheng C.; Xiao G.; Zhou Y.; Gao R. Examination of the efficacy of ozone solution disinfectant in in-activating SARS virus. Chin. J. Disinfect., 2004, 21, 27–28.
- Hu, X.; Chen, Z.; Su, Z.; Deng, F.; Chen, X.; Yang, Q.; et al. Ozone water is an effective disinfectant for SARS-CoV-2. Virol. Sin., 2021, 36, 1066-1068.
- Filipić, A.; Primc, G.; Zaplotnik R.; Mehle, N.; Gutierrez Aguirre, I.; Ravnikar, M.; Mozetič, M.; Žel, J.; Dobnik, D. Cold atmospheric plasma as a novel method for inactivation of potato virus Y in water samples. Food Environ. Virol., 2019, 11, 220-228.
- Filipić, A.; Dobnik, D.; Tušek Žnidarič, M.; Žegura, B.; Štern, A.; Primc, G.; et al. Inactivation of pepper mild mottle virus in water by cold atmospheric plasma. Front. Microbiol. 2021, 12, 618209. [Google Scholar] [CrossRef] [PubMed]
- Mahnot, N.K.; Mahanta, C.L.; Keener, K.M.; Misra, N.N. Strategy to achieve a 5-log Salmonella inactivation in tender coconut water using high voltage atmospheric cold plasma (HVACP). Food Chem., 2019, 284, 303-311.
- Nguyen, D.V.; Ho, P.Q.; Pham, T.V.; Nguyen, T.V.; Kim L. Treatment of surface water using cold plasma for domestic water supply. Environ. Eng. Res., 2019, 24, 412-417.
- Patange, A.; Lu, P.; Boehm, D.; Cullen, P.J.; Bourke, P. Efficacy of cold plasma functionalized water for improving microbiological safety for fresh produce and wash water recycling. Food Microbiol. 2019, 84, 103226. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Qiu, H.; He, S.; Hong, B.; Liu, K.; Lou, F., et al. Efficient disinfection of SARS-CoV-2-like coronavirus, pseudotyped SARS-CoV-2 and other coronaviruses using cold plasms induces spike protein damage. J. Hazard. Mater., 2022, 430, 128414.
- Büttner, C.; Nienhaus, F. Virus contamination of soils in forest ecosystems of the Federal Republic of Germany. Eur. J. For. Path., 1989, 19, 47-53.
- Koenig, R. Plant viruses in rivers and lakes. Adv. Virus Res., 1986, 31, 321-333.
- Gosalvez, B.; Navarro, J.A.; Norca, A.; Botella, R.; Sánchez-Pina, M.A.; Pallas, V. Detection of melon necrotic spot virus in water samples and melon plants molecular methods. J. Virol. Methods., 2003, 113, 87-93.
- Mehle, N.; Ravnikar, M. Plant viruses in aqueous environment – survival, water mediated transmission and detection. Water Res. 2012, 46, 4902–4917. [Google Scholar] [CrossRef] [PubMed]
- Paludan, N. Spread of viruses by recirculated nutrient solutions in soilless cultures. Tidsskr. Planteavl, 1985, 89, 467-474.
- Pategas, K.G.; Schuerger, A.C.; Wetter, C. Management of tomato mosaic virus in hydroponically grown pepper (Capsicum annuum). Plant Dis., 1989, 73, 570-573.
- Girt, G.C.; Lakshminarayanan, A.; Huo, J.; Dormon, J.; Norman, C.; Afrough, B., et al. The use of nanobodies in a sensitive ELISA test for SARS-CoV-2 spike 1 protein. R. Soc. Open. Sci., 2021, 8, 211016.
- Motley, M.P.; Bennett-Guerrero, E.; Fries, B.C.; Spitzer, E.D. Review of viral testing (Polymerase chain reaction) and antibody/serology testing for severe acute respiratory syndrome-coronavirus-2 for the intensivist. Crit Care Explor., 2020, 2, e0154.
- Mori, J.; Smith, R. Transmission of waterborne fish and plant pathogens in aquaponics, and their control with physical disinfection and filtration: a systematized review. Aquaculture, 2019, 504, 380-395.
- Gharbi, S.; Verhoyen, M. Sterilization UV irradiation of nutrients solution with view to avoiding viral infections transmitted by O. brassicae in hydroponic culture of lettuce. HortScience, 1993, 58, 873-874.
- Runia, W. Elimination of root-infecting pathogens in recirculation water from closed cultivation systems by ultra-violet radiation. Acta Hortic., 1994, 361, 361-371.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).