1. Introduction
Breathing and the perception of breathing impairment (dyspnoea) are important not only in respiratory and cardiac diseases but also in neurological disorders such as amyotrophic lateral sclerosis and myasthenia gravis. Although the brainstem's respiratory centres orchestrate automatic respiratory rhythms [
1], the brain regions associated with respiratory motor control and respiratory perception have only recently been described [
2]. Recent advances in neuroimaging have provided new insights into functional impairments in the human brain [
3]. Several studies using a box-car design, task-based positron emission tomography (PET), and conventional functional magnetic resonance imaging (fMRI) have identified brain regions relevant to respiratory motor control and dyspnoea perception [
2].
In fMRI research, the focus has shifted from identifying regions specialised for specific cognitive tasks (conventional fMRI) to comprehending broader interactions among multiple brain regions. This concept is known as functional connectivity (FC), which refers to the synchronisation of neural activity among regions [
4]. Using resting-state fMRI techniques, a growing body of research has revealed altered FC in brain disorders such as Alzheimer's disease [
5], depression [
6], and schizophrenia [
7]. Although FC has been explored in respiratory disorders such as obstructive sleep apnoea [
8] and chronic obstructive pulmonary disease [
9], these findings could stem from prolonged neuronal damage induced by extended hypoxia and hypercapnia. A network-based approach to task-related FC linked specific areas (the anterior insular (AInsula) and pre-supplementary motor area (SMA)) to tasks involving breathing restriction and craving regulation [
10]. Consequently, our comprehension of altered FC during acute respiratory impairment remains limited.
Analysing FC requires 100–300 whole-brain volumes and a 5–10 min acquisition time [
11]. Previous respiratory-focused neuroimaging studies often employed tasks such as breath-holding, which are challenging to sustain for the required data acquisition period [
12,
13,
14]. To address this, we designed mouthpiece devices that resist breathing flow through the mouth, enabling investigations lasting over 5 min [
15]. Here, we used these devices to examine the FC associated with the processing of dyspnoea induced by a resistive load (effort breathing) through resting-state fMRI.
Conventional fMRI studies have reported that effort breathing activates motor and sensory processes tied to dyspnoea, including the sensory-motor cortex, premotor cortex, SMA, insular cortex, anterior cingulate cortex (ACC), amygdala, thalamus, basal ganglia, cerebellar hemisphere, cerebellar vermis, and brainstem (midbrain, pons, and medulla) regions [
16,
17,
18,
19,
20,
21]. We hypothesised that effortful breathing modulates FC within these brain regions. Therefore, in this exploratory study, we aimed to investigate the effects of effort breathing on resting-state connectivity changes and clinical scores (modified Borg scale), focusing on the mentioned brain regions [
16,
17,
18,
19,
20,
21].
2. Materials and Methods
2.1. Participants
The study enrolled never-smokers aged 20–50 years with stable health, normal lung function, no respiratory symptoms, and no medication use. Exclusion criteria included a history of lung surgery, severe diseases of other organs, pregnancy or nursing status, dementia, or drug or alcohol abuse.
2.2. Induction and Measurement of Respiratory Impairment
The artificial dyspnoea was induced during resting breathing by using a cylindrical polyethylene device (24 mm inner diameter, 26 mm outer diameter, and 900 mm length), which contained a mouthpiece at one full-opened end with 6.3% of corresponding aperture ratio opened 6.0 mm-diameter sizes at the opposite side (Fukuda Denshi, Tokyo, Japan) (
Figure S1) [
15]. In a previous study, the device induced mild artificial dyspnoea without hypoxia during resting breathing by increasing mouth pressure [
15]. Dyspnoea levels were assessed using the self-reported modified Borg scale for each participant [
22,
23].
2.3. Experimental Design
Data were collected during three separate visits. On the first day, the participants provided written informed consent and underwent a medical history assessment. The participants then underwent vital sign measurements, including cognitive levels using the modified Hasegawa dementia scale [
24], respiratory rate, heart rate, blood pressure, body temperature, modified Borg scale score, and resting SpO
2. Spirometry and blood tests were also performed. Enrolment criteria encompassed meeting the following thresholds: 30 points on the modified Hasegawa dementia scale, respiratory rates <20 breaths/min, systolic blood pressure <150 mmHg, heart rate between 40 and 80 beats/min, body temperature <37.0 °C, modified Borg scale <0.5, SpO
2 >96%, and normal lung function (% forced vital capacity (%FVC) prediction >80%, % forced expiratory volume in 1 s (%FEV
1.0) prediction >80% of prediction, and the FEV
1.0/FVC ratio >0.7). On the second day, vital signs, SpO
2, and the modified Borg scale [
22,
23] were monitored every minute over a 14-min period. The first 7 min involved breathing through the mouthpiece alone, followed by 7 min of continuous breathing using a dyspnoea-inducing device. The average modified Borg scale scores were calculated over 1-min intervals during both device-assisted and mouthpiece breathing. Given the challenge of measuring the Borg scale in the MRI scanner and its repeatability within 7 days, we considered the data from day 2 to be equivalent to those from day 3. Within 7 days of day 2, fMRI was conducted under identical conditions to the breathing training on day 2 (day 3). During the fMRI tests, two consecutive imaging series (one for normal respiration and another for mild dyspnoea (effortful breathing)) spanning 400 s were performed for both respiratory conditions using both the mouthpiece alone and the device in each participant. The sequence of normal respiration and effort breathing was counterbalanced among the participants. Vital signs, modified Borg scale values, and SpO
2 were compared between normal respiration and effort breathing states on each time course using Student’s
t-tests and between baseline and subsequent time points using one-way analysis of variance (ANOVA) with the nonparametric Kruskal–Wallis test (day 2). The average modified Borg scale score during the 7-min training period was correlated with the fMRI data and re-evaluated immediately after the fMRI.
2.4. MRI Data Acquisition
Images were acquired using a 3T GE Discover MR 750 W whole-body MRI system. Each session consisted of acquiring 200 echo-planner imaging multi-slice datasets (repetition time, 2,000 ms; echo time, 30 ms; flip angle, 80°; acquisition time, 400 s) while the participants were instructed to relax with their eyes closed. Each multi-slice dataset comprised 34 transverse slices (slice thickness, 4 mm; voxel size, 3.5 × 3.5 × 4 mm; field of view (FOV), 220 mm). High-resolution T1-weighted structural brain images were obtained using a 3D SPGR protocol (repetition time, 8.472 ms; echo time, 3.268 ms; inversion time, 45 ms; FOV, 240 mm; acquisition matrix, 512 × 512; 144 contiguous axial slices; slice thickness, 1.2 mm; and total scan time, 4 min, 3 s).
2.5. Data Pre-Processing
The CONN FC toolbox (version 22a) [
25], coupled with Statistical Parametric Mapping (SPM) 12, was used to execute the CONN default pre-processing pipeline. This encompassed the removal of initial functional scans (10 scans); functional realignment and unwarp (subject motion estimation and correction); functional centring to (0, 0, 0) coordinates (translation); functional slice-timing correction; functional outlier detection (identifying outlier scans exceeding the standard deviation (SD) of the average signal or >0.5 mm frame-wise displacement using the ART toolbox); functional direct segmentation & normalisation (simultaneous Grey/White/Cerebrospinal fluid (CSF) segmentation and Montreal Neurological Institute (MNI) normalisation); structural centring to (0, 0, 0) coordinates (translation); structural segmentation and normalisation (simultaneous Grey/White/CSF segmentation and MNI normalisation); and functional smoothing (8 mm full-width half maximum Gaussian kernel filter). Subsequently, denoising steps were implemented, involving CompCor and bandpass filtering in the range of 0.008–0.09 Hz [
26,
27]. To ensure data quality, an exclusion criterion was set with a threshold of 30% for invalid/outlier scans detected by ART.
2.6. ROI-to-ROI Analysis
In the first-level ROI-to-ROI analysis, we used whole-brain ROIs (164 ROIs) sourced from the Harvard–Oxford atlas [
28,
29,
30,
31] and cerebellar ROI according to the Automated Anatomical Labelling Atlas [
32]. Both atlases were integrated into the CONN toolbox [
25]. For each ROI, the mean resting-state BOLD time course was extracted to generate individual correlation maps encompassing the entire brain. Correlation coefficients were computed between each ROI's BOLD time course and those of other ROIs. Fisher’s transformation converts these coefficients into normally distributed scores, facilitating a second-level general linear model analysis [
25]. FC measures were computed between the seed regions in the ROI-to-ROI analysis, revealing ROI-to-ROI connectivity patterns through bivariate correlations [
25].
Second-level ROI-to-ROI analyses used paired
t-tests to compare connectivity differences between the normal respiration (mouthpiece alone) and effort-breathing (plus a cylindrical device) groups using paired
t-tests. Cluster-level analysis, accessible through the CONN toolbox (
https://web.conn-toolbox.org/ fmri-methods/cluster-level-inferences), was employed to evaluate inter-condition disparities using parametric statistics based on functional network connectivity [
33] (The cluster threshold was set at a false discovery rate-corrected (FDR)
p <0.05 and an uncorrected connection threshold
p <0.001).
2.7. Seed-to-Voxel Analysis
Seeds for first-level seed-to-voxel analysis were selected from the results of our ROI-to-ROI analysis within the regions reported in prior studies [
16,
17,
18,
19,
20,
21] using CONN’s default atlas for the definition of ROIs, referred to as the Harvard–Oxford atlas [
28,
29,
30,
31]. The mean BOLD time series from these seed regions was extracted and correlated with the time course of each voxel of the brain, resulting in a three-dimensional correlation coefficient (
r) map for each subject and seed. Normalised Fisher-transformed correlation maps were used for group analysis (normal respiration vs. breathing effort). In the second-level analysis of Conn’s pipeline, the differences in connectivity between normal respiration and effortful breathing were measured using paired
t-rests. For each seed ROI, voxel-wise statistics throughout the brain were calculated. The voxel-level threshold was a
p-uncorrected value of <0.001, which was then corrected for whole-brain comparisons by FDR to
p <0.05.
2.8. Graph Theoretical Analysis
To identify network hubs, we conducted a graph theoretical analysis using the CONN-fMRI Toolbox. This entailed examining network centrality measures, focusing on degree and betweenness centralities. The degree of a node is the number of connections that a node has with other nodes, whereas the betweenness centrality of a node is the number of times that a node is included in the shortest path from each node to every other node [
34,
35]. The node included all 164 ROIs, and the threshold of the network edge (adjacency matrix threshold) was set to 0.25 in the correlation coefficient (
r). Within the regions detected by ROI-to-ROI with cluster analysis, a significantly weighted degree and betweenness centrality were identified (uncorrected,
p < 0.05). We also measured the rank of centrality among all the ROIs.
2.9. Correlation with the Modified Borg Scale
To determine correlations with the modified Borg scale, we extracted ROIs in which significant group differences were found between effortful and normal respiration in the ROI-to-ROI analysis. For each participant, the mean FC values across all voxels in each ROI were computed using seed-to-voxel analysis. Spearman’s rank correlation analysis was conducted to evaluate the relationship between the z-scores of the identified FCs and the modified Borg scales using SPSS (version 26; IBM Corp., Armonk, NY, USA). The statistical significance level was p <0.05.
3. Results
3.1. Participant Selection
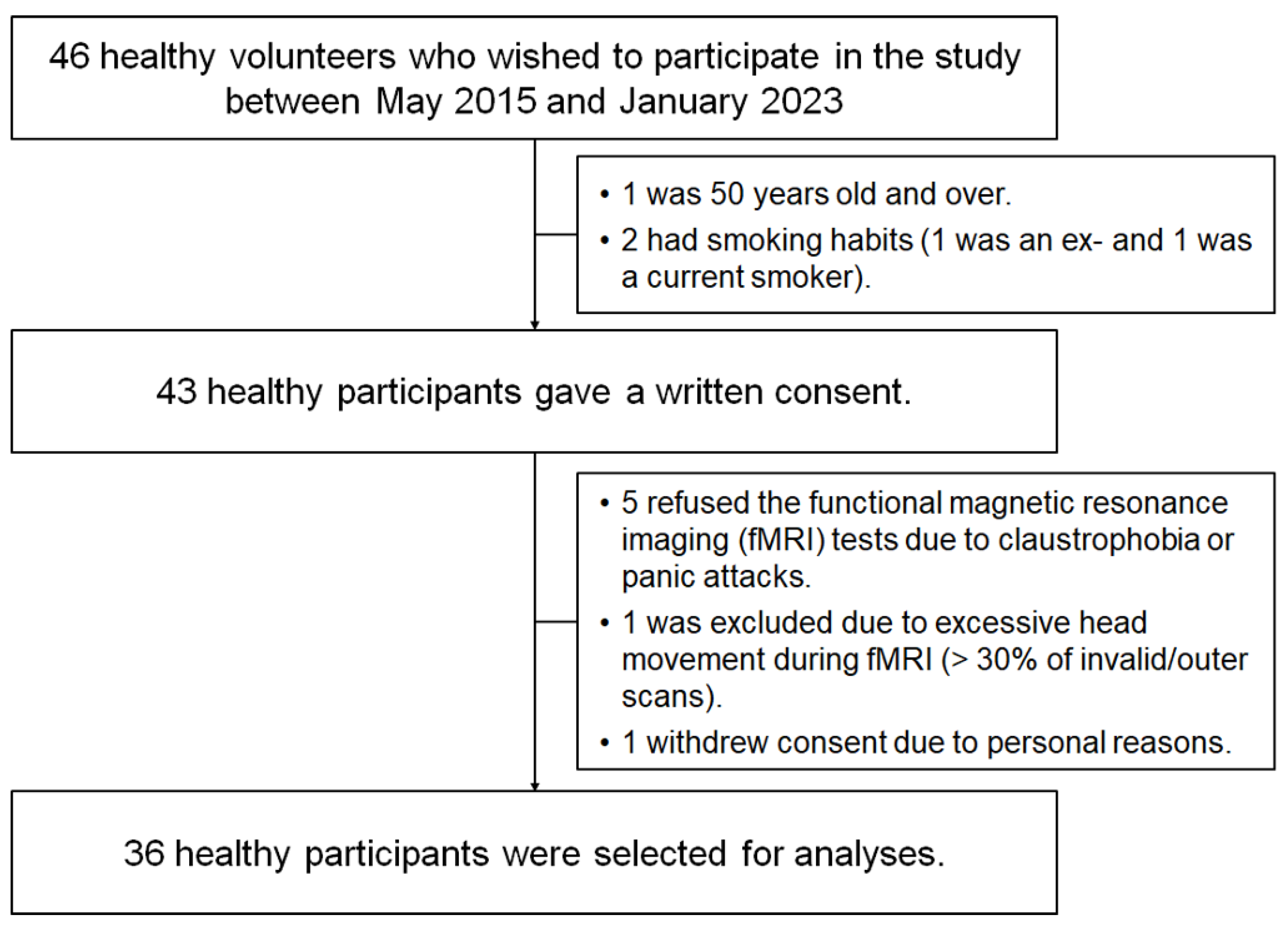
Forty-six healthy volunteers participated in the study between May 2015 and January 2023. Our selection criteria included individuals aged 20–50 years who had never smoked (see Methods and Participants). Candidates aged >50 years (n = 1) and current or former smokers (n = 2) were excluded. Furthermore, seven applicants were excluded owing to MRI refusal due to claustrophobia or panic attack (n = 5), excessive head movement during fMRI (>30% of invalid/outlier scans detected by Artefact Detection Tools (ART)) (n = 1), and withdrawal of consent for personal reasons (n = 1). The remaining 36 participants (18 women and 18 men) were selected for further analysis (
Figure 1,
Table 1). Notably, all participants reported dyspnoea levels of 0 at rest on the modified Borg scale and scored 30 points on the revised Hasegawa dementia scale [
24]. None of the participants showed abnormal pathological findings on anatomical T1- or T2-weighted MRI.
3.2. Changes in the Modified Borg Scale and Vital Signs during Respiratory Training between Normal Breathing and Effortful Breathing
Figure S2 illustrates the time course of the modified Borg scale [
22,
23], percutaneous oxygen saturation (SpO
2, %), respiratory rate (breaths/min), blood pressure (mmHg), and heart rate (beats/min). After the MRI, the re-evaluated mean modified Borg scale with effort breathing (0.90 ± 0.84,
p <0.0001 using a non-paired
t-test) was significantly higher than that of normal breathing (0.21 ± 0.44). Notably, no significant differences in SpO
2 and respiratory rates were observed between effort breathing and normal respiration across time courses or between baseline and subsequent time courses.
3.3. Adverse Events
Four participants experienced mild adverse events. All mild adverse events occurred during respiratory training and resolved after training. Transient adverse effects were observed, including tachycardia (n = 1), transient hypopnoea (n = 1), and transient hypertension (n = 1), using the device. Oxygen saturation dropped from 97% to 94% a minute after using the mouthpiece alone but recovered to 95% a minute later. None of the participants experienced any subjective symptoms.
3.4. ROI-to-ROI Results
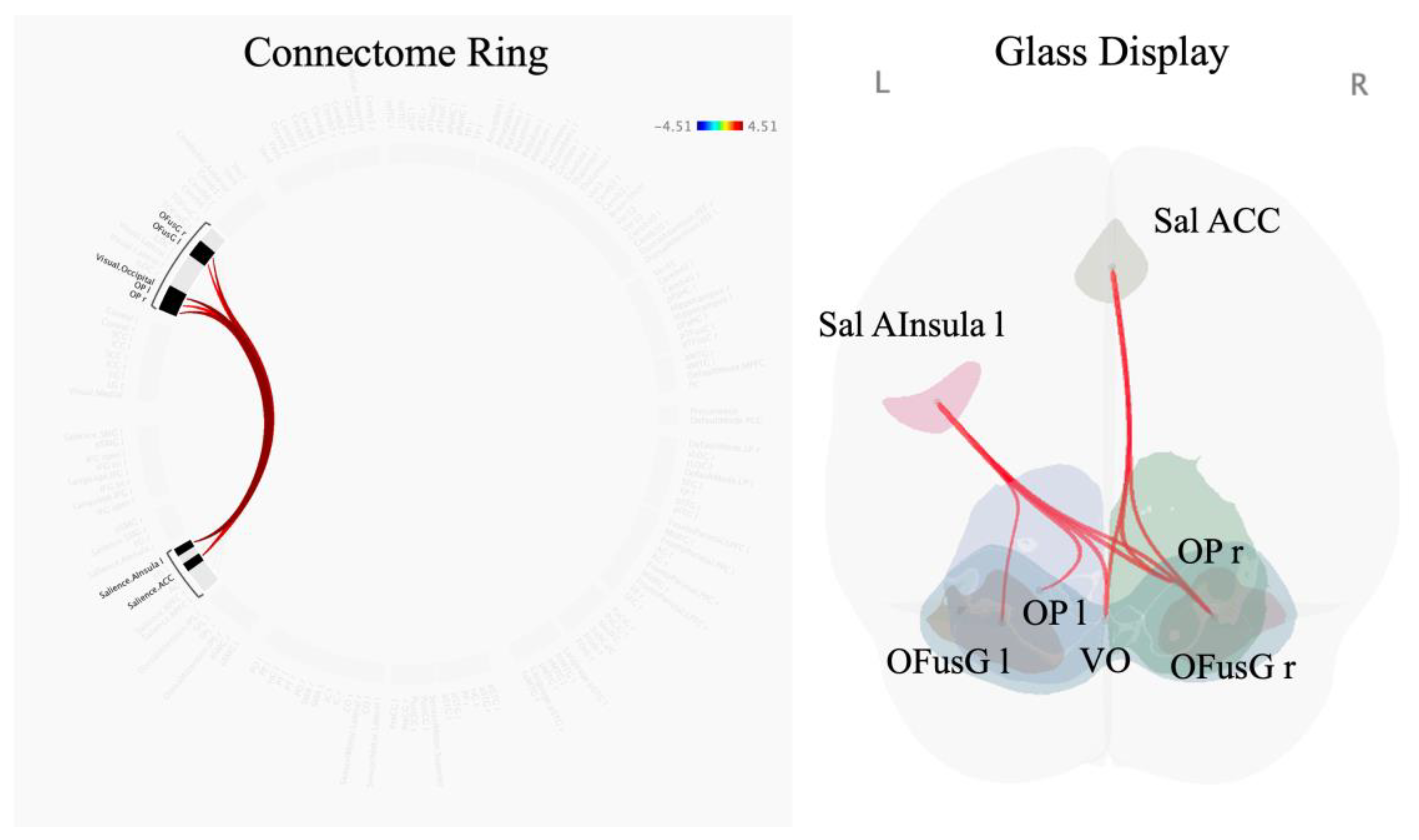
Compared with normal respiration, effort breathing activated a cluster of FCs between the salience network (including the ACC, and left anterior insular (AInsula)) and secondary visual network (including visual occipital (VO), bilateral occipital pole (OP), and right occipital fusiform gyrus (OFusG)) (F (3, 68) = 9.16,
p-FDR = 0.011618) including 8 positive connectivity changes (between the left salience (Sal) AInsula and VO,
t = 4.51,
p-FDR = 0.004139; between the left Sal AInsula and right OP,
t = 4.26,
p-FDR = 0.004326; between the left Sal AInsula and right OFusG,
t = 4.19,
p-FDR = 0.004326; between the Sal ACC and right OP,
t = 3.92,
p-FDR = 0.019694; between the Sal ACC and right OFusG,
t = 3.78,
p-FDR = 0.019694; between the Sal ACC and VO,
t = 3.75,
p-FDR = 0.019608; between the left Sal AInsula and left OP,
t = 3.52,
p-FDR = 0.028116; between the left Sal AInsula and left OFusG,
t = 3.48,
p-FDR = 0.028116) (
Figure 2,
Table 2). Only the ACC and left AInsula belong to the regions reported in prior studies [
16,
17,
18,
19,
20,
21]; therefore, both were selected as seeds for subsequent analysis.
3.5. Seed-to-Voxel Results
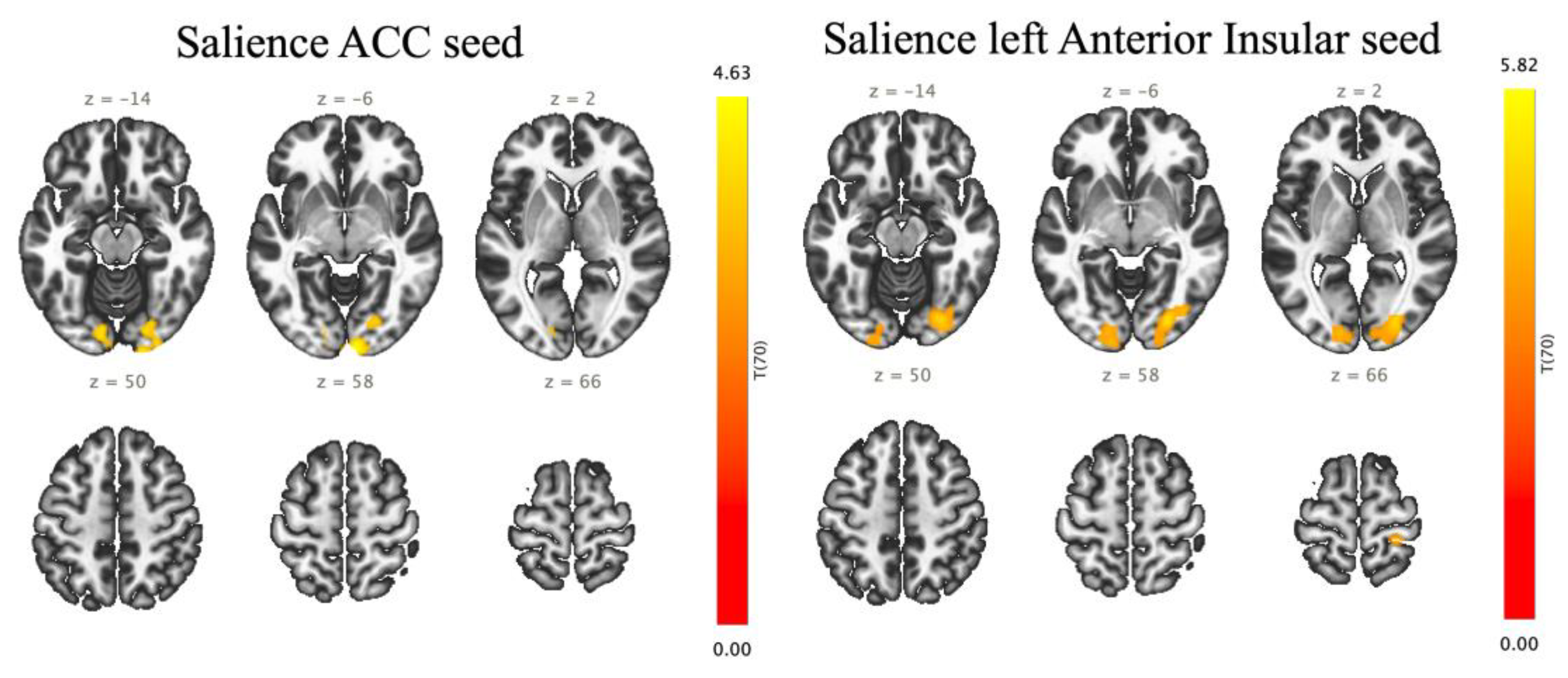
The seed-to-voxel analysis represents that effort breathing increased FCs between the salience ACC and the right OP (303 voxels (35%) covering 12% of OP r) and right OFusG (194 voxels (23%) covering 22% of OFusG r) (size of contiguous voxels = 858;
p-FDR = 0.000018) (
Figure 3,
Table 3). Effort breathing significantly activated FCs between the seeds (the left A-Insula) with three different clusters, named A, B, and C. In cluster A (size of contiguous voxels = 1,145;
p-FDR = 0.000001), the left AInsula showed increased connectivity with the right OP (349 voxels (30%) covering 14% of OP r) and right OFusG (317 voxels (28%) covering 36% of OFusG r); in cluster B (size of contiguous voxels = 572;
p-FDR = 0.000291), the left AInsula disclosed increased connectivity with the left OP (329 voxels (58%) covering 12% of OP l); in cluster C (size of contiguous voxels = 219;
p-FDR = 0.031177), the left AInsula represents increased connectivity with the right postcentral gyrus (PostCG) (185 voxels (84%) covering 6% of PostCG r).
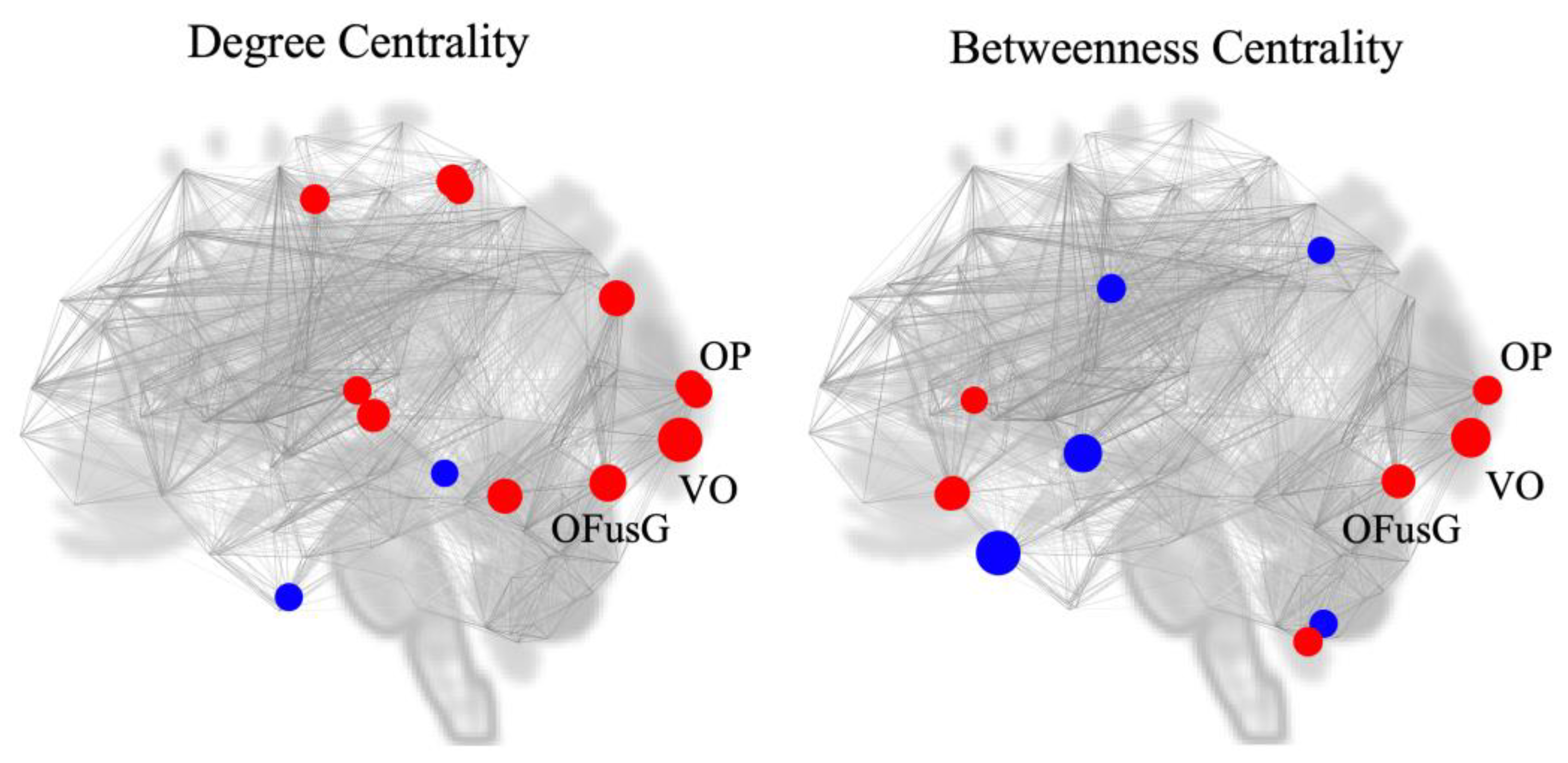
3.6. Measurement of Nodal Centrality
To investigate the potential of the visual cortex as a network hub, we performed two measures of nodal importance (degree and betweenness centrality) using graph theoretical analysis. During effort breathing, significant values of degree centrality were observed at the visual networks (VO,
t = 3.26,
p = 0.001706, first place in all ROIs; left OFusG,
t = 2.71,
p = 0.008518, second place; left OP,
t = 2.26,
p = 0.026699, 7
th place; right OP,
t = 2.20,
p = 0.031157, 8
th place) and in-betweenness centrality (VO,
t = 2.93,
p = 0.004589, first place in all ROIs; left OFusG,
t = 2.49,
p = 0.015310, fifth place; left OP,
t = 2.14,
p = 0.035570, 8
th place) (
Figure 4,
Table 4).
3.7. Correlation with the Modified Borg Scale
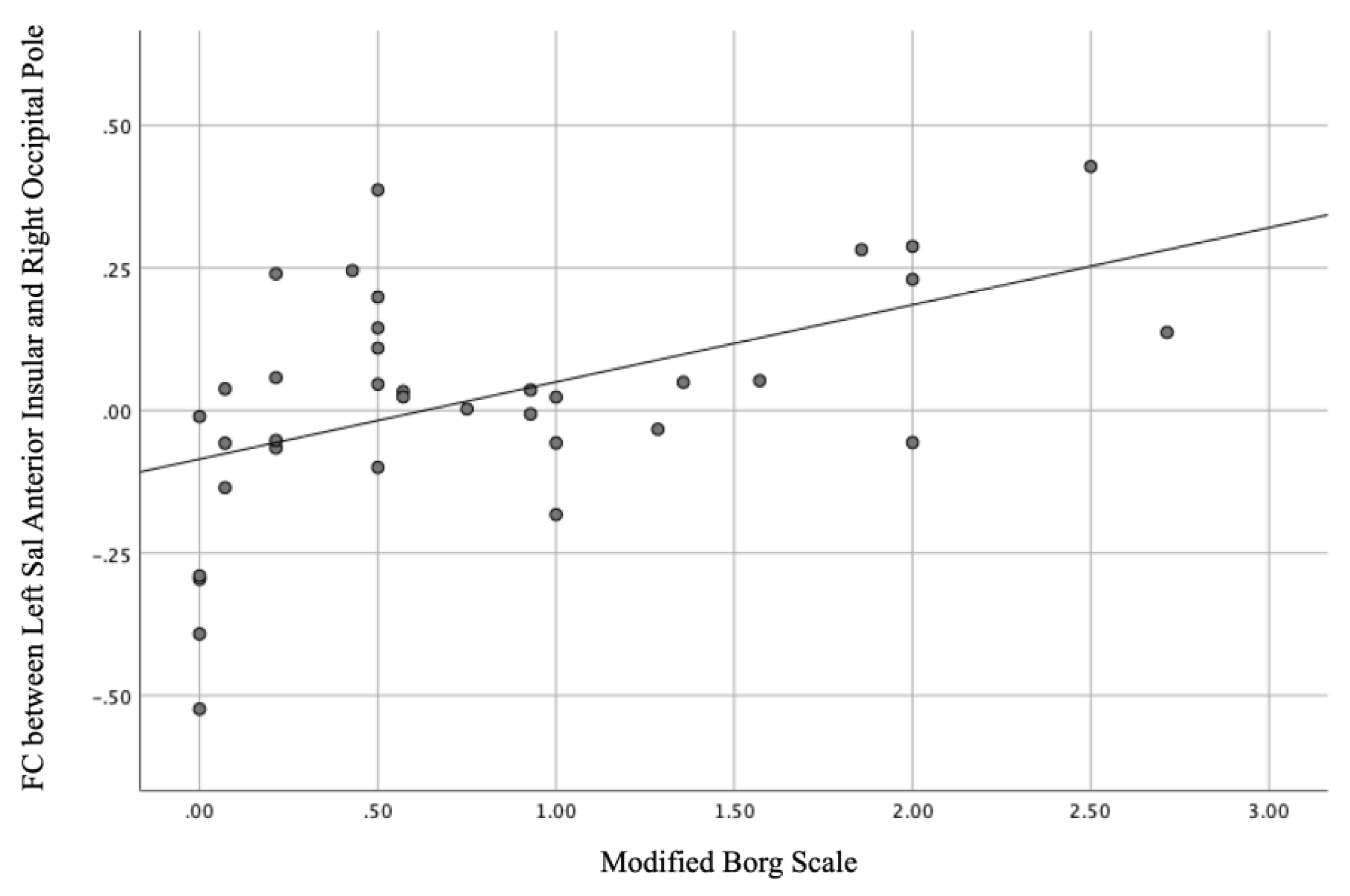
Eight regions were tested against the modified Borg scale based on networks that showed significant differences between groups in our ROI-to-ROI analysis. Strong positive correlations were observed between the mean scores of the modified Borg scale and the standardised intensity of the FC between the left AInsula and right OP (
r = 0.489,
p = 0.002) in the group with effortful breathing (
Figure 5).
4. Discussion
In this study, we examined altered FCs associated with transient mild effortful breathing by employing cluster-level ROI-to-ROI, seed-to-voxel, and graph theory analyses. Our novel findings can be summarised as follows: 1) We successfully induced mild respiratory impairment for nearly 7 min in all participants without any discernible change in SpO2. 2) In ROI-to-ROI analysis, effort breathing potentiates the FCs between the salience networks and the secondary visual network. 3) Seed-to-voxel analysis revealed altered FC between the left AInsula and right postCG. 4) Graph theory analysis revealed significant nodal centrality within the visual network. 5) A significant positive relationship was found between the FC (between the left AInsula and right OP) and the clinical score (modified Borg scale).
4.1. Unique Point of Our Resistive Load
Mild respiratory impairment was induced for approximately 7 min. Recent research has highlighted that moderate breathlessness (modified Borg scale score exceeding 3) affected SpO
2 [
36] and oxy-haemoglobin concentration [
37]. Moreover, the potential implications of moderate-to-severe dyspnoea on blood oxygen level-dependent (BOLD) contrast, a core element of fMRI [
38], highlight the significance of understanding its influence. Notably, our utilisation of a “grade II” cylindrical device to induce mild dyspnoea while maintaining the SpO
2 unaffected represents a unique aspect of our study.
4.2. Functional Connectivity within the Respiratory-Associated Area
Our ROI-to-ROI and seed-to-voxel analyses showed that effort breathing prompted heightened FCs between the salience networks (ACC and AIusula) and secondary visual networks (VO, OP, and OFusG) compared with normal respiration, as expected. The ACC is interconnected with the primary sensory cortex [
39] and plays a pivotal role in coordinating autonomic, visceromotor, and endocrine functions associated with emotion [
40]. Meanwhile, the insular cortex is instrumental in the perception of various unpleasant sensations, such as pain [
41], hunger [
42], and negative emotions [
43]. AInsular is typically associated with social and affective tasks involving pain, empathy, disgust, and introspective processes [
44,
45], whereas ACC is closely associated with response selection, conflict resolution, and cognitive control [
46]. Together, they shape the salience network’s role in integrating sensory, emotional, and cognitive information [
44,
47]. Furthermore, anatomical, and electrophysiological studies have demonstrated reciprocal connections between insular and medullary respiratory neurons [
48]. Our seed-to-voxel analysis also found increased FC between the left AInsular and right PostCG. Earlier reports focusing on acute models of respiratory impairment have demonstrated activation of the ACC, AInsula, and PostCG during classic fMRI investigations of the respiratory sensory area (employing methods such as hypercapnia and air hunger) [
2,
49,
50].
4.3. Functional Connectivity Outside of the Respiratory-Associated Area
We observed augmented FCs within the secondary visual cortex, including the VO, OP, and OFusG, due to effort breathing. Previous neuroimaging studies on respiratory, sensory, and motor processing have reported no involvement of the visual cortex, although these studies did not explore the FC [
2]. Interestingly, a recent neuroimaging investigation suggested that OP is activated during the anticipation of severe dyspnoea caused by effort breathing [
19]. Thus, increased FC between the salience network and secondary visual cortex may suggest an increase in dyspnoea perception and anticipation of its severity. The secondary visual cortex has been proposed as a “network hub” that plays a significant role in diverse cognitive tasks and the dynamic coupling of functional networks [
34]. The default mode network (which includes the superior parietal and superior frontal cortices and posterior cingulate gyrus) and the salience-processing network (ACC and AInsula) are multimodal and functional hubs [
51]. Additionally, primary cortical regions, such as the primary motor and visual cortices, have been identified as hubs within single or limited functional networks [
52]. To explore the likelihood of the secondary visual cortex functioning as a hub, we analysed the degree and betweenness centralities using graph theoretical analysis [
53]. We identified the potential of the secondary visual cortex (VO, OP, and OFusG) to serve as a hub for FC. Collectively, our study signified that effortful breathing activates FCs during the sensory processing of respiration [
2,
49,
50], primarily through network hubs within the secondary visual cortex.
4.4. Correlation with Clinical Score (Modified Borg Scale)
We found a positive correlation between the modified Borg scale score and the values between the left AInsula and left OP FC during effort breathing. The modified Borg scale is a 12-point scoring system with ratings ranging from 0 to 10 with verbal anchors that are more convenient for interindividual comparisons and correlate well with physiological parameters during exercise testing [
54,
55]. The modified Borg scale is widely used to evaluate dyspnoea intensity in healthy and cardiopulmonary populations [
36] and acute bronchospasms, such as obstructive pulmonary disease (COPD) and asthma [
54]. A strong correlation has been reported between the intensity of breathlessness as described by the modified Borg scale and the amount of work performed during exercise [
56,
57]. A significant negative correlation was observed between the change in the modified Borg scale and the peak expiratory flow rate (PEFR) [
55], which might be affected by effortful breathing. Taken together, the modified Borg scale scores could be associated with the FC values between the sensory processing of respiration and the network hub.
5. Limitations
Our study had a few limitations. First, the relatively small sample size and inclusion of relatively young participants may limit generalisability. Second, we did not investigate the participants’ precise psychological states. Third, we focused solely on resistive load-induced respiratory impairment, which causes the sensation of increased work and mild dyspnoea. Thus, our results cannot be generalised to other qualities of respiratory impairment, such as air hunger and chest tightness. Fourth, sole reliance on the modified Borg scale to assess respiratory impairment may have limited the assessment. Correlating findings with other clinical scales could offer insights into the differentiation of FCs linked to motor control, perception, or anticipation. Lastly, the acute model of dyspnoea used cannot be applied to chronic diseases such as asthma, chronic obstructive pulmonary disease, obstructive sleep apnoea, and panic disorder.
6. Conclusions
Our study demonstrated that mild transient effort breathing altered FCs within the sensory area of respiration (salience network and PostCG) via a network hub (secondary visual cortex). One FC positively correlated with the modified Borg scale score. The study focused on effort breathing; therefore, future research may explore the mechanisms of other respiratory impairments, such as hypercapnia, hyperpnea, and the urge to cough.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: The structure of the device and the results from the preliminary study using the devices in healthy volunteers.
(A) Front and top views of the devices. The polyethylene device (inner and outer diameter: 24 mm and 26 mm, respectively; length: 900 mm) is cylindrical, with an oval, fully open mouthpiece at one end. On the other end, a round aperture is present in the centre. The device is produced in four different types, from grade I to IV, for increasing breathing restriction. The opening sizes (diameter) of grade I, II, III, and IV devices are 8.2, 6.0, 4.5, and 3.0mm, respectively, with corresponding aperture ratios of 11.7, 6.3, 3.5, and 1.6%, respectively [
15].
(B) The device is fitted with a filter and disposable paper mouthpiece for spirometry and forced oscillation technique. A disposable paper mouthpiece (Mouthpiece 100; Fukuda Denshi, Tokyo, Japan) with a bacteria-trapping filter (Hybrid Filter; Fukuda Denshi) was used for the measurements of mouth pressure, forced oscillation technique (FOT) [
58], and spirometry [
15]. In a preliminary study of healthy volunteers
1, we measured the mean peak mouth pressure during the expiratory to inspiratory phase at the mouthpiece alone, and with the grade I, II, III, and IV devices, it was 0.01 to 0.00, 0.01 to −0.01, 0.02 to −0.02, 0.02 to −0.03, and 0.03 to −0.05 kPa/L/s, respectively, using the flow-pressure metre (SP-370COPDhaiPer; Fukuda Denshi) (n = 5). Compared to the baseline control (0.25 ± 0.05; mean ± SD), the mean respiratory resistance at a frequency of 5 Hz was significantly higher for grade II (1.04 ± 0.27 hPa/L/s,
p < 0.01), III (2.16 ± 0.71,
p < 0.0001), and IV (4.72 ± 1.71,
p < 0.0001) devices, but not for the grade I device, using the FOT [
58] (Master Screen IOS, Jaeger GmbH, Germany) (n = 32).; Figure S2. Changes in modified Borg scale and vital signs during respiratory training between normal breathing (mouthpiece alone) and resistive load (device). The modified Borg scale, a saturation of percutaneous oxygen (SpO
2, %), respiratory rates (/min), blood pressure (mmHg), and heartbeats (/min) were assessed every 1 min with the mouthpiece alone for 7 min and with the device for 7 min (total of 14 min) by a veteran technician. Each participant tried to breathe with the mouthpiece first, then continuously switched to the device and kept breathing. However, the modified Borg scale was confirmed as 0 at the baseline. Closed circles and bars were expressed as the average and standard deviation, respectively. min: minute.
Author Contributions
All authors listed in the manuscript contributed sufficiently to qualify for authorship: Conceptualisation, A.Y., To.K., T.H., and T.Tan.; collected the respiratory and behavioural data, To.K., Ta.K., H.O., Y.T., and T.H.; collected the fMRI data, A.Y., M.I., T.Tat., Y.S., N.U., T.A., and T.Tan.; analysed the data, To.K., T.Tat. and T.Tan.; created the figures, To.K. and T. Tan.; writing original drafts, A.Y., To.K., Ta.K., and T.Tat. and T. Tan.; writing, review, and editing, M.I., Y.S., N.U., T.A., and T.H. All authors have read and agreed to the final version of the manuscript.
Funding
This study was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan (15K09365) and (18K08195).
Institutional Review Board Statement
This study was conducted in accordance with Good Clinical Practice guidelines and was approved by the Ethics Board of Kurume University (Approved No. 15-072) on May 18, 2015. Additionally, it was registered at the University Hospital Medical Information Network (UMIN) Centre (UMIN No. R000019888) in Japan on April 16, 2015.
Informed Consent Statement
Written informed consent was obtained from each participant prior to participation.
Data Availability Statement
The datasets generated and/or analysed in the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Mrs. Kyoko Yamaguchi, BSc, for providing respiratory training and data management. The authors are also grateful to Mr. Yoshinori Tobigaya and Mr. Akira Kudo, Fukuda Sangyo Corporation Ltd., for their assistance with the data and providing the devices. We thank Honyaku Center Inc. for English language editing.
Conflicts of Interest
The authors declare that there is no competing interest.
References
- Feldman, J.L.; Del Negro, C.A. Looking for inspiration: new perspectives on respiratory rhythm. Nat. Rev. Neurosci. 2006, 7, 232–242. [Google Scholar] [CrossRef]
- Evans, K.C. Cortico-limbic circuitry and the airways: insights from functional neuroimaging of respiratory afferents and efferents. Biol. Psych. 2010, 84, 13–25. [Google Scholar] [CrossRef]
- Gore, J.C. Principles and practice of functional MRI of the human brain. J. Clin. Invest. 2003, 112, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Rosazza, C.; Minati, L. Resting-state brain networks: literature review and clinical applications. Neurol. Sci. 2011, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Srivastava, G.; Reiss, A.L.; Menon, V. Default mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. USA 2004, 101, 4637–4642. [Google Scholar] [CrossRef]
- Greicius, M.D.; Flores, B.H.; Menon, V.; Glover, G.H.; Solvason, H. B.; Kenna, H. , Reiss, A.L.; Schatzberg, A.F. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 2007, 62, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Garrity, A.G.; Pearlson, G.D.; McKiernan, K.; Lloyd, D.; Kiehl, K.A.; Calhoun, V.D. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry 2007, 164, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, D.; Qin, W.; Li, Q.; Chen, B.; Zhang, Y.; Yu, C. Altered resting-state brain activity in obstructive sleep apnea. Sleep 2013, 36, 651–659B. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xin, H.; Yu, J.; Yu, H.; Zhang, J.; Wang, W.; Peng, D. Abnormal intrinsic functional hubs and connectivity in stable patients with COPD: a resting-state MRI study. Brain Imaging Behav. 2020, 14, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.; Kausch,A. ; Dorfschmidt, L.; Waller, L.; Chinichian, N.; Veer, I.; Hilbert, K.; Lüken, U.; Paulus, M.P.; Goschke, T.; Kruschwitz, J.D. Self-control and interoception: linking the neural substrates of craving regulation and the prediction of aversive interoceptive states induced by inspiratory breathing restriction. Neuroimage 2020, 215, 116841. [Google Scholar]
- Barkhof, F.; Haller, S; Rombouts, S. A.R.B. Resting-state functional MR imaging: a new window to the brain. Radiology 2014, 272, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Macefield, V.G.; Gandevia, S.C.; Henderson, L.A. Neural sites involved in the sustained increase in muscle sympathetic nerve activity induced by inspiratory capacity apnea: a fMRI study. J. Appl. Physiol. 2006, 100, 266–273. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.C.; Adams, L.; Frackowiak, R.S.; Corfield, D.R. A bilateral corticobulbar network associated with breath holding in humans, determined by functional magnetic resonance imaging. NeuroImage 2008, 40, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Pattinson, K.T.S.; Mitsis, G.D.; Harvey, A.K.; Jbabdi, S.; Dirckx, S.; Mayhew, S.D.; Rogers, R.; Tracey, I.; Wise, R.G. Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imaging. NeuroImage. 2009, 44, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Yorita, A.; Tokunaga, Y.; Kinoshita, T.; Nakakura, A.; Oda, H.; Imaoka, H.; Matsunaga, K.; Kakuma, T.; Hoshino, T.; Kawayama, T. Usefulness of a 4-grade novel mouthpiece device for increased mouth pressure reproducing artificial difficulty in breathing. Kurume Med. J. 2023, 68, 229–238. [Google Scholar] [CrossRef]
- Fink, G.R.; Corfield, D.R.; Murphy, K.; Kobayashi, I.; Dettmers, C.; Adams, L.; Frackowiak, R.S.; Guz, A. Human cerebral activity with increasing inspiratory force: a study using positron emission tomography. J. Appl. Physiol. 1996, 81, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, C.; Poline, J.-B.; Thivard, L.; Aubier, M.; Samson, Y. Neural substrates for the perception of acutely induced dyspnea. Am. J. Respr. Crit. Care Med. 2001, 163, 951–957. [Google Scholar] [CrossRef]
- Ramsay, S.C.; Adams, L.; Murphy, K.; Corfield, D.R.; Grootoonk, S.; Bailey, D.L.; Frackowiak, R.S.J.; Guz, A. Regional cerebral blood flow during volitional expiration in man: a comparison with volitional inspiration. J. Physiol. 1993, 461, 85–101. [Google Scholar] [CrossRef]
- Stoeckel, M.C.; Esser, R.W.; Gamer. M.; Buchel, C.; von Leupolt, A. Brain responses during the anticipation of dyspnea. Neural Plast. 2016, 2016, 6434987. [Google Scholar] [CrossRef]
- von Leupoldt, A.; Sommer, T. , Kegat, S., Baumann, H.J., Klose, H., Dahme, B., Buchel, C. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am. J. Respir. Crit. Care Med. 2008, 177, 1026–1032. [Google Scholar] [CrossRef]
- von Leupoldt, A.; Sommer, T.; Kegat, S.; Baumann, H.J.; Klose, H.; Dahme, B.; Buchel, C. Dyspnea and pain share emotion-related brain network. NeuroImage. 2009, 48, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Subjective effort and physical activities. Scand. J. Rehab. Med. 1978, 6, 105–113. [Google Scholar]
- Wilson, R.C.; Jones, P.W. A comparison of the visual analogue scale and modified Borg scale for the measurement of dyspnoea during exercise. Clin. Sci. (Lond) 1989, 76, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Kim, K.W.; Lee, D.Y.; Lee, S.B.; Park, J.H.; Choi, E.A.; Choe, J.Y.; Do, Y.J.; Ryang, J.S.; Roh, H.A.; Park, Y.S.; Choi, Y.; Woo, J.I. A normative study of the Revised Hasegawa Dementia Scale: comparison of demographic influences between the Revised Hasegawa Dementia Scale and the Mini-MentalStatus Examination. Dement. Geriatr. Cogn. Disord. 2007, 24, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Porcu, M.; Craboledda, D.; Garofalo, P.; Barberini, L.; Sanfilippo, R.; Zaccagna, F.; Wintermark, M.; Montisci, R.; Saba, L. Reorganization of brain networks following carotid endarterectomy: an exploratory study using resting state functional connectivity with a focus on the changes in default mode network connectivity. Eur. J. Radiol. 2019, 110, 233–241. [Google Scholar] [CrossRef]
- Podgorski, P.; Waliszewska-Prosol, M.; Zimny, A.; Sasiadek, M.; Bladowska, J. Resting-State functional connectivity of the ageing female brain-differences between young and elderly female adults on multislice short TR rs-fMRI. Front. Neurol. 2021, 12, 645974. [Google Scholar] [CrossRef]
- Desikan, R. S.; Segonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; Albert, M.S.; Killiany, R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Frazier, J.A.; Chiu, S.; Breeze, J.L.; Makris, N.; Lange, N.; Kennedy, D.N.; Herbert, M.R.; Bent, E.K.; Koneru, V.K.; Dieterich, M.E.; Hodge, S.M.; Rauch, S.L.; Grant, P.E.; Cohen, B.M.; Seidman, L.J.; Caviness, V.S.; Biederman, J. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am. J. Psychiatry 2005, 162, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.M.; Seidman, L.J.; Makris, N.; Ahern, T.; O’Brien, L.M.; Caviness Jr, V.S.; Kennedy, D.N.; Faraone, S.V.; Tsuang, M.T. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol. Psychiatry 2007, 61, 935–945. [Google Scholar] [CrossRef]
- Makris, N.; Goldstein, J.M.; Kennedy, D.; Hodge, S.M.; Caviness, V.S.; Faraone, S.V.; Tsuang, M.T.; Seidman, L.J. Decreased volume of left and total anterior insular lobule in schizophrenia, Schizophr. Res. 2006, 83, 155–171. [Google Scholar]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard,O. ; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Jafri, M.J.; Perlson, G.D.; Stevens, M.; Calhoun, V.D. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage. 2008, 39, 1666–1681. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. 2013, 17, 683–696. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010, 54, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Malagutti, N.; Di Laora, A.; Barbetta, C.; Groppo, E.; Tugnoli, V.; Sette, E.; Astolfi, L.; Beswick, W.; Borin, M.; Ciorba, A.; Pelucchi, S.; Stomeo, F.; Contoli, M. Is peripheral oxygen saturation a reliable predictor of upper airways air-flow limitation? J. Emer. Med. 2018, 55, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, Y.; Sano, A.; Nishiyama, O.; Sano, H.; Iwanaga, T.; Haraguchi, R.; Chiba, Y.; Fukuda, K.; Tohda, Y. Prefrontal cortex activation is associated with dyspnea during methacholine bronchial provocation tests in patients with bronchial asthma. Allergol. Int. 2020, 69, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef]
- Baker, C.M.; Burks, J.D.; Briggs, R.G.; Stafford, J.; Conner, A.K.; Glenn, C.A.; Sali, G.; McCoy, T.M.; Battiste, J.D.; O'Donoghue, D.L.; Sughrue, M.E. A connectomic atlas of the human cerebrum-Chapter 4: the medial frontal lobe, anterior cingulate gyrus, and orbitofrontal cortex. Oper. Neurosurg. (Hagerstown) 2018, 15 (suppl_1), S122–S174.
- Sturm, V.E.; Sollberger, M.; Seeley, W.W.; Rankin, K.P.; Ascher, E. A.; Rosen, H.J.; Miller, B. L.; Levenson, R. W. Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc. Cogn. Affect. Neurosci. 2013, 8, 468–474. [Google Scholar] [CrossRef]
- Price, D.D. Psychological and neural mechanisms of the affective dimension of pain. Science 2000, 288, 1769–1772. [Google Scholar] [CrossRef]
- Tataranni, P.A.; Gautier, J.F.; Chen, K.; Uecker, A.; Bandy, D.; Salbe, A.D.; Pratley, R.E.; Lawson, M.; Reiman, E.M.; Ravussin, E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc. Natl. Acad. Sci. USA 1999, 96, 4569–4574. [Google Scholar] [CrossRef]
- Phan, K.L.; Wager, T.; Taylor, S.F.; Liberzon, I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002, 16, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Craig, A. D. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.; Critchley, H.D.; Preuschoff, K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 2009, 13, 334–340. [Google Scholar] [CrossRef]
- Botvinick, M. M.; Cohen, J. D.; Carter, C. S. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 2004, 8, 539–546. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Gaytan, S.P.; Pasaro, R. Connections of the rostral ventral respiratory neuronal cell group: an anterograde and retrograde tracing study in the rat. Brain Res. Bull. 1998, 47, 625–642. [Google Scholar] [CrossRef]
- Corfield, D.R.; Fink, G.R.; Ramsay, S.C.; Murphy, K.; Harty, H.R.; Watson, J.D.G.; Adams, L.; Frackowiak, R.S.; Guz, A. Evidence for limbic system activation during CO2-stimulated breathing in man. J. Physiol. 1995, 488, 77–84. [Google Scholar] [CrossRef]
- Evans, K.C.; Banzett, R.B.; Adams, L.; McKay, L.; Frackowiak, R.S.; Corfield, D.R. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J. Neurophysiol. 2002, 88, 1500–1511. [Google Scholar] [CrossRef]
- Sepulcre, J.; Sabuncu, M.R.; Yeo, T.B.; Liu, H.; Johnson, K.A. Stepwise connectivity of the modal cortex reveals the multimodal organization of the human brain. J. Neurosci. 2012, 32, 10649–10661. [Google Scholar] [CrossRef]
- Leech, R.; Braga, R.; Sharp, D. J. Echoes of the brain within the posterior cingulate cortex. J. Neurosci. 2012, 32, 215–222. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, M.I.; Turk, E.; Manning, J.H.; Hect, J.; Hernandez-Andrate, E.; Hassan, S.S.; Romero, R.; van den Heuvel, M.P.; Thomason, M.E. Hubs in the human fetal brain network. Dev. Cogn. Neurosci. 2018, 30, 108–115. [Google Scholar] [CrossRef]
- Kendrick, K.R.; Baxi, S.C.; Smith, R.M. Usefullness of the modified 0-10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthoma. J. Emerg. Nurs. 2000, 26, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Mancini, I.; Body, J.J. Assessment of dyspnea in advanced cancer patients. Support Care Cancer. 1999, 7, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Mador, M.J.; Rodis, A.; Magalang, U.J. Reproducibility of Borg scale measurements of dyspnea during exercise in patients with COPD. Chest 1995, 107, 1590–1597. [Google Scholar] [CrossRef]
- Wilson, R.C.; Jones, P.W. A comparison of the visual analogue scale and modified Borg scale for the measurement of dyspnea during exercise. Clin. Sci. 1989, 76, 277–282. [Google Scholar] [CrossRef]
- Oostveen, E.; MacLeod, D.; Lorino, H.; Farre, R.; Hantos, Z.; Desager, K.; Marchal, F. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur. Respir. J. 2003, 22, 1026–1041. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).