1. Introduction
Rhinosinusitis (RS) is an inflammatory disease of the nose and sinuses that can be distinguished into an acute and a chronic form depending on whether the symptoms persist beyond 12 weeks.
In accordance with the definition provided by the European document on CRS (EPOS), the cardinal symptoms that characterize chronic rhinosinusitis (CRS) are:
- -
nasal respiratory obstruction, likewise, understood as the sensation of nasal congestion.
- -
intense nasal discharge, which can be referred to either anteriorly as the need to incessantly blow the nose, or posteriorly, otherwise known as post-nasal drip, understood as retro nasal drainage of secretions [
1].
These manifestations are associated with a plethora of symptoms of varying intensity and number which are often referred as the patient’s main affliction given their strong impact on quality of life; these include a sense of facial pain or heaviness, frontal and/or supraorbital headache, auricular muffling, hyposmia and hypogeusia.
To get a diagnosis of CRS, the symptomatologic picture must be associated with objective data, which can be represented by the presence of at least one among:
- -
endoscopic endonasal signs of chronic inflammation such as the presence of polyps, mucopurulent secretions originating from the meatuses and mucosal edema.
- -
radiological signs that provide an indirect view of inflammation in the sinus and sinuses.
With the introduction of the EPOS symptom-based diagnostic criteria, it was possible to perform regional estimation and comparison of the prevalence of CRS in large populations in a standardized manner[
2,
3].
Patients with nasal polyps, although a minority, tend to have clinically more severe and difficult-to-treat disease. The variant with nasal polyps is mainly an adult-onset disease, with the average age of onset being around 40-50 years; CRS without nasal polyps is prevalent in individuals younger than 40 years of age.
CRS is classically distinguished into a form with nasal polyps (CRSwNP) and a form without nasal polyps (CRSsNP), based on endoscopic evidence. The finding or absence of such an ordinary and easily identifiable feature as nasal polyposis makes it possible to make a clear and immediate phenotypic distinction of patients with CRS thus delineating two macro groups for which different symptoms, radiologic findings, comorbidities, treatments, and risks of recurrence can be recognized. Nasal obstruction and impaired sense of smell and taste are both the most severe and prevalent symptoms in CRSwNP, while in CRSsNP, facial pain, nasal discharge and again nasal obstruction are reported as equally severe.
The understanding of the pathophysiology of CRS has undergone a radical evolution in recent decades: from a purely anatomical view related to impaired ventilation and drainage of the sinus cavities to a more evolved and refined concept that, based on the most current knowledge regarding cellular and molecular mechanisms, focuses on the immunology of the sinus mucosa and the imbalance of its activity.
Inflammatory responses in general can be classified into 3 different types based on a profile composed of immune cells and specific inflammatory mediators. Characterizing and understanding these variations helps explaining the clinical heterogeneity of the disease, defining the best therapeutic strategy, and predicting outcomes and relapse rates.
The attempt by several authors to therefore classify CRS based on the underlying immunopathologic profile has allowed the delineation of several homogeneous subgroups by immunologic activity. Matching these findings with the clinical phenotype of CRS revealed that the three types of inflammation are co-expressed and variously combined in both CRSwNP and CRSsNP, with the understanding that within CRSwNP, type 2 inflammation constitutes the most represented variant, confirmed by the microscopic and immunohistochemical finding at the level of the polypoid tissue of a high concentration of eosinophilic granulocytes, interleukin 5 (IL-5), interleukin 4 (IL-4) and interleukin 13 (IL-13), typical molecular markers of type 2, while the non-type 2 inflammatory forms, i.e., type 1 and type 3, are those most represented in CRSsNP.
The present study aims to demonstrate how the use of a product of natural origin as preventive treatment can help with prevention of recurrences. The study involves the recruitment of 100 patients, randomly divided into two groups, one of which was left untreated, while the other underwent treatment with Pistacia lentiscus for 30 days. Outcomes were evaluated by assessing variables of cell function, presence of biofilm, and quality of life of patients.
2. Materials and Methods
2.1. Inclusion Criteria
100 patients were selected (57 males and 43 females), over 18 years old with a diagnosis of Chronic Rhinosinusitis, with a nasal polypoid score ≤1. All patients came to our attention complaining with more than 5 recurrences per year, determining low quality of life.
2.2. Exclusion Criteria
We excluded patients with a Nasal Polypoid score > 2, with less than 5 episodes of recurrence per year and a SNOT 22 score lower than 10. Patients affected by immunity, neoplastic, neurological and/or psychiatric diseases and/or with inability to undergo therapy were not included into the study.
2.3. Methods
100 Patients with chronic rhinosinusitis were enrolled and divided into two groups by randomization methods using the random number table.
All patients underwent nasal endoscopy, assessment of their SNOT 22 questionnaire, cytological specimen examination, and ciliary motility evaluation at T0 (enrollment visit) and T1 (30 days after). During both visits all these criteria were evaluated. The control group was treated for 30 days with nasal wash with a specific probe and 10 drops of placebo twice daily. The treated group was treated for a 30-day period with nasal wash with a specific probe and 10 drops of lentiscus Pistacia twice a day using Bactorinol® drops made by Pharmaextracta Factory, Piacenza.
At the end of treatment, all patients underwent nasal endoscopy, snot 22, cytological examination and ciliary motility evaluation. The Rinowash® probe is a specific terminal for aerosol therapy of the upper airways. Connected to the traditional device for aerosol therapy with a compressor, it allows a complete and accurate treatment of the upper airways in 1-3 minutes, for the treatment of allergic and non-allergic rhinitis, rhinosinusitis, nasal polyposis. Producing particles with a diameter greater than 10 microns, it acts exclusively at the level of the upper airways, nebulizing 5 ml of solution in one minute. The Technical specifications is Operating pressure from 1 to 3.5 bar, Operating flow from 5 to 20 l / min with a MMAD 18 µm.
2.4. Data Groups
The patients enrolled were aged 21-73 (median 45.8 years). The two groups were of similar age.
The sample was made of 53 males and 47 females; the males in the control group were 25 and 28 in the treated group. (
Table 1) The average number of relapse episodes was 4.8 (range 4-7) in the control group and 4.8 (range 4-7) in the treated group. The average SNOT 22 questionnaire was 33,39 in the control group with a range from 21 to 54 and 36 with a range from 21 to 43 in the treated group. (
Table 2)
Secretions were present in 81 patients (81%) in both groups, of which 42 in the control group (84%) patients and 39 in the treated group (78%). The ciliary motility study carried out in all patients showed presence of a ciliary beat in 61 patients (61%), of which 29 (58%) in the control gropu and 32 (64%) in the treated group. Ciliary motility was observed in vivo with a phase-contrast light microscope and was considered normal in patients who had a ciliary beat for a time longer than 10 minutes. (
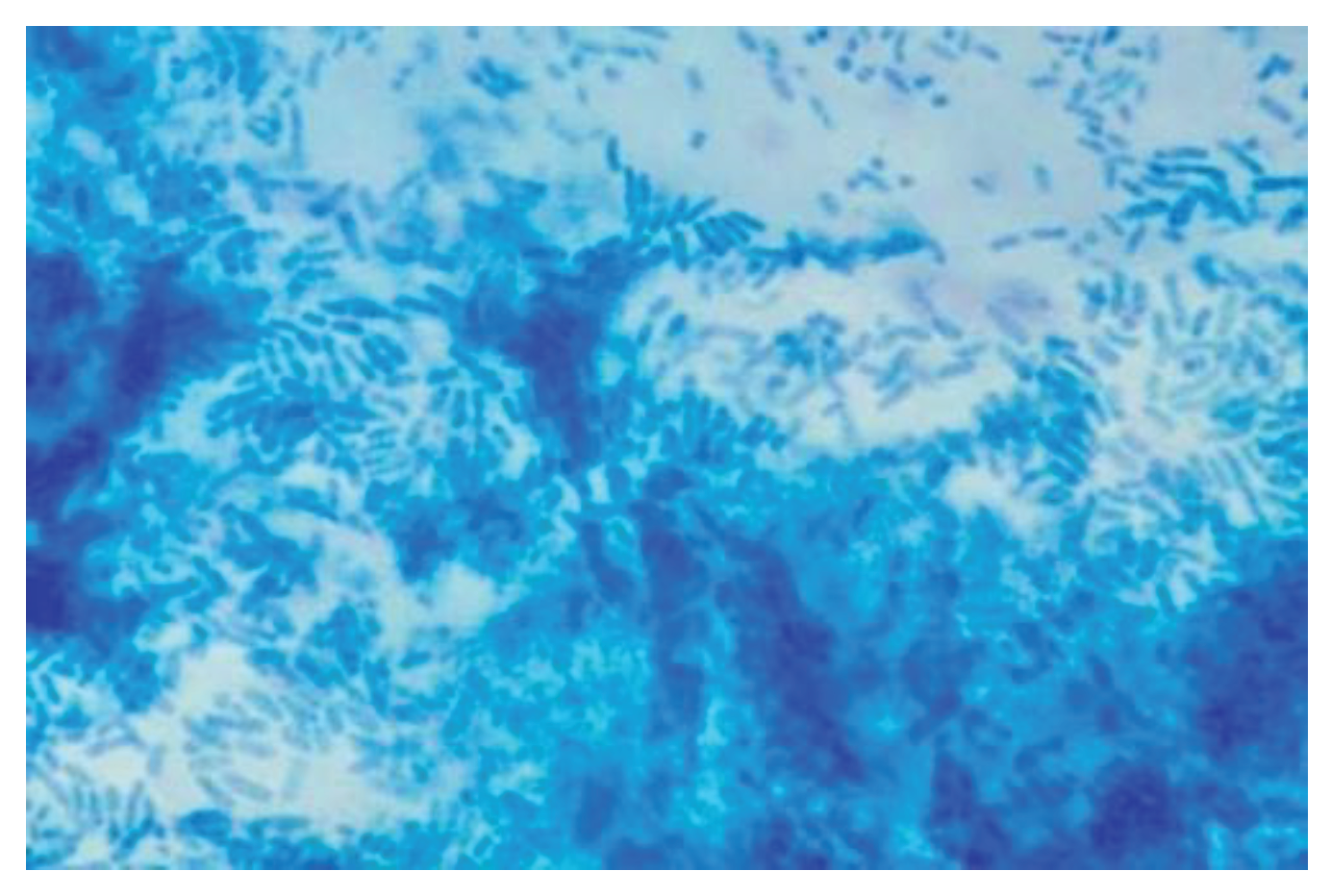
Table 3). In all enrolled patients, cytological sampling was performed at the level of the mucosa of the middle third of the inferior face of the inferior turbinate. The sampling was air-fixed and stained by the May Grunewald Giemsa method. The presence of biofilm is characterized by the presence of cyan-colored areas.
Biofilms were observed in 70 (70%) enrolled patients, of which 34 (68%) in the control group and 36 (72%) in the group of treated patients. (
Table 4). Analysis of cytological preparations showed the presence of the supranuclear stria in 32 specimens, of which 18 were in the control group and 21 in the treated group. Supranuclear stria is an important element in defining cell viability, as its absence represents an index of disease at the cellular level related to bacterial or viral infections.In the cytological preparation it is possible to find the presence of bacterial elements, however, these elements are difficult to identify morphologically, therefore, a diagnosis of the type of bacteria present is not possible. In this case series, bacteria were evidenced in 38 (76%) patients including 20 (40%) in the control group and 25 (50%) in the treated group. (
Table 5) The cellular study of the cytological collection did not show any variability in cell morphological types, the only notable feature being the presence of neutrophils in most of the samples. Neutrophil and mast cells are indicative of a local inflammatory process. In the healthy patient, the presence of neutrophils, eosinophils and mast cells usually turns out to be irrelevant because of the low presence of these cellular type. (
Table 6,
Table 7 and
Table 8)
3. Results
The results of the study showed that quality of life improved in the treated group compared with the control group; in fact, there was a 39.2 % increase in SNOT 22 values in the treated group compared with 7.1 % in the control group with a statistical significance of the value (
Table 9)
3.1. Ciliar Motility
The study of ciliary motility showed an increase only in the treated group of 8.5% from baseline. (
Table 10)
3.2. Nasal Secretion
The presence of nasal secretions radically decreased in the treated group by 84.6% compared to the control group with a statistically significant finding. (
Table 11)
3.3. Biofilm
Detection of biofilms on the nasal specimen saw a 12.8% increase in presence in the control group, while it decreased by 66.75% in the treated group. (
Table 12) (
Figure 2)
3.4. Supranuclear stria
The increased presence of supranuclear stria in 67.4% of patients in the treated group represents the increase in cell viability at the mucosal level, indicative of improved cell function. While the decrease in the control group demonstrates how the lack of adequate treatment at the mucosal level leads to a worsening of mucosal cellularity. (
Table 13)
3.5. Bacteria
Bacteria in the treated group of patients significantly decreased from 50% to 32% of patients, which can be explained by the decrease of bacterial forms organized in biofilms and by improvement in cell function and integrity. (
Table 14)
The study of cellularity at the sample level did not demonstrate a significant change in cell populations other than a 20% reduction in neutrophil numbers from baseline in the treated group, compared with an almost unchanged cellularity at the control group level.
The reduction in neutrophil numbers in the treated group can be explained by the improved mucosal conditions, reduced local inflammation related to the presence of nasal secretions, bacteria, biofilms and increased cell viability, supranuclear stria, and increased mucociliary clearance.
4. Discussion
Ultimately, CRSsNP non-type 2 and CRSwNP type 2 are the two ends of a spectrum of disease that has a broad combination of the three types of inflammatory response.
Framing of patients involves looking for particular frequently associated inflammatory conditions such as bronchial asthma, allergic rhinitis, hypersensitivity to NSAIDs, atopic dermatitis. In subjects with polyposis, the presence of atopy appears to be high but there is insufficient evidence to conclude that it plays a causal role in pathogenesis. At present, the only "strong" correlations are observed between rhinitis and asthma and between severe asthma and polyposis, but not between polyposis and rhinitis.
Asthma is a more common comorbidity in patients with CRSwNP than those with CRSsNP and is often late onset, nonatopic (nonallergic) and refractory to standard medical treatment. Of note, late-onset asthma often has type 2 inflammatory signs, suggesting common etiologic processes and therapeutic targets with CRSwNP.
Clinically CRSwNP has a higher morbidity due to a greater severity of the disease associated with the peculiar tendency for recurrence that leads the patient to undergo repeated surgery and a higher rate of exposure to medications, primarily antibiotics and oral corticosteroids.
One of the most typical shrubs in the Mediterranean maquis (shrubland) of Europe, Morocco, Turkey, Iraq and Iran is PIL. In Italy, it is characteristic of the sensitive ecosystem, like that of Sardinia [
18] where it grows along the coast up to 700 m above sea level. It is well-adapted to harsh growing conditions, dryness and a warm environment, which all exercise an influence on the genotype and richness of secondary metabolites [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. The plant is dioecious, where male and female flowers are on independent trees.
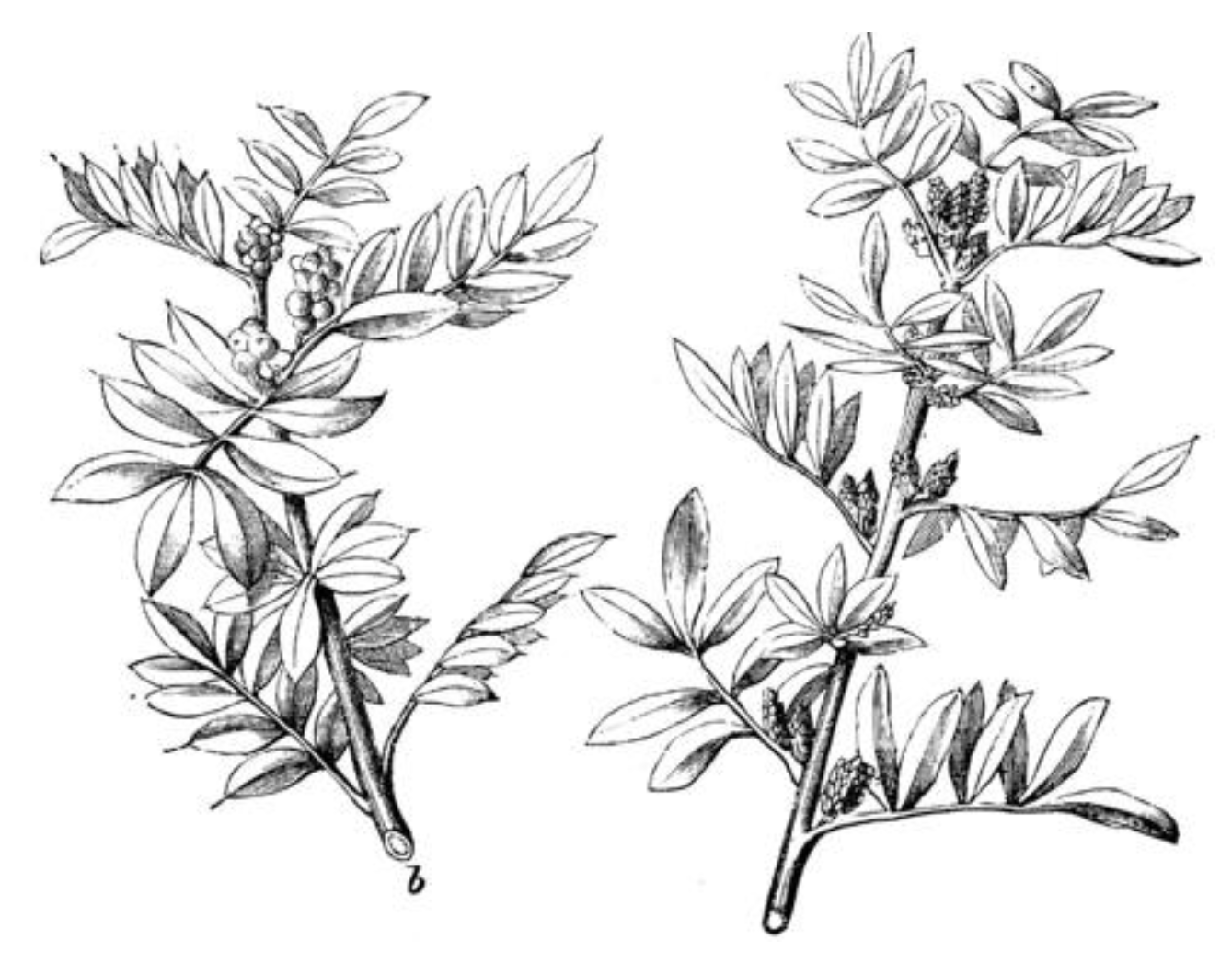
Figure 1. The leaves are leathery, bright green and alternate. They are arranged in compound, pinnate whorls. The unisexual flowers are grouped in clusters. The globular fruit is a fleshy drupe, which ripens in August and ranges in color from red to brown in view of the different degrees of maturity [
1]
. PlL can develop leaf galls due to insect attack, particularly aphid attacks[
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20]. Common aphid species, such as Slavum wertheimae and Baizongia pistaciae L., manipulate the leaves to form tumorous galls for the safety and nutriment of their larva [
20]. The galls are rich in volatile-like terpenes with an abundance of monoterpenes, α -pinene and limonene
29. Their chemical composition differs from that of the healthy leaves, which have in general a higher content of sesquiterpenes [
22].
The anti-inflammatory effect of PlL is of high relevance in ethnopharmacology. The presence of important anti-inflammatory terpenes in the composition of PlL can explain its efficacy. It is well-demonstrated that terpenes are capable of inhibiting several inflammatory molecules, e.g., IL-1β, IL-6, TNFα and COX-2 [
6,
7,
8,
9,
10], thus disrupting the amplification of inflammatory mechanisms. Meanwhile, the anti-inflammatory properties of PlL extracts can be related to the richness of polyphenols, the interplay of which, in the inflammatory cascade, is mainly demonstrated toward macrophages by inhibiting multiple key regulators of the inflammatory response. Additionally, polyphenols reduce the release of arachidonic acid, prostaglandins and leukotrienes directly related to the inhibition of COX and LOX [
9].Other considerations regard several flavonoids in polyphenols, which can directly modulate the expression of pro-inflammatory cytokines and chemokines. The ability of these natural compounds to modify the expression of several pro-inflammatory genes in addition to their antioxidant characteristics, such as reactive oxygen species (ROS) scavenging, contributes to the regulation of inflammatory signaling [
23,
24,
25]
Several studies reported the antimicrobial activity of PlL oils and extracts, trying to clarify scientifically their popular use in infectious diseases. Commonly studied pathogens comprise bacteria known for antibiotic resistance (Staphylococcus aureus incl. methicillin-resistant strains (MRSA), Escherichia coli and Pseudomonas aeruginosa), and other bacteria associated with oral diseases, as well as yeasts, with regard to Candida albicans. [
16]. Different antimicrobial activity was reported in the studies testing PlL EO and extracts. A high capacity against both bacteria and yeasts were demonstrated regarding the leaves EO from plants growing in different regions. Additionally, the leaves ethanol or methanol extracts showed activity against several Gram-positive and Gram-negative bacteria [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18].
Furthermore, a high inhibition of C. albicans was reported when using PlL extracts. The activity of water or ethanol extracts has been attributed to the flavonoid contents. Notably, the phenolic compound tannic acid was reported as more active against the yeast than the antifungals nystatin and amphotericin.
The chemistry shows that PlL EO is composed of up to 64 molecules, while 46 constituents have been identified in the extracts. Further minor fractions were determined in the reports analyzing both the EO and the extracts even if they could not be quantified. The more recurrent chemical components in the EO from plants growing in the Mediterranean area are represented by α-pinene, terpinenes, caryophyllene, limonene and myrcene. Important properties in antagonizing immune-mediated and autoimmunity, neuro-inflammatory, neurological and neurodegenerative diseases, in addition to infections and cancer have been addressed to these molecules. However, the biological character of PlL cannot be entirely focused on one of the main concentrated molecules. The abilities should be attributed to the whole mixture of the terpenes working in synergy, or in addition independently by their concentration in the agent. It is remarkable to note that concentrations of non-cannabinoid terpenoids equal to or above 0.05% increase the pharmacological potency of PlL oil.
2 Regarding the antimicrobial activity, the capacity of the oil and extracts against periodontal bacteria has been largely documented. This evidence can prove scientifically the popular use of PlL in the relief of gingival bleeding and tooth ache. The ability against periodontal bacteria, further ameliorated by the anti-inflammatory potency, is attractive with regards to a possible use of the EO to antagonize gingivitis, as a primary strategy to prevent periodontitis and as a secondary preventive strategy to prevent recurrent periodontitis after periodontal surgery. The possibility to formulate PlL derivates as potential oral health care products or therapeutics in periodontal disease is further strengthened by the largely documented biocompatibility and antioxidant capacity [
11,
12,
13,
14].
Furthermore, PIL inhibits the growth of C. albicans and C. glabrata with low MICs. In addition, the prevention of arachidonic acid oxidation by COX-2 and LOX antagonism by PlL oil should be of interest for inhibiting the development of Candida biofilm and disseminations. Subsequently, PlL could act directly against the yeast and indirectly against its virulence, with no oral cytotoxicity [
3].
The main feature of CRS is, in many patients, disease recurrence due to multiple factors. Prevention of recurrences is the best method to avoid worsening in patients’ quality of life. Recurrences are mainly related to local inflammatory factors, alterations in mucociliary clearance, anatomical changes in rhinosinusal structures, and the patient's immune system [
4].
An important role in the establishment of persistent bacterial infections is played by the formation of bacterial biofilms, implicated in at least 60 % of all chronic and/or relapsing infections. The scientific literature continues, moreover, to report the formation of biofilms by an increasingly wide range of microbial species. A biofilm is a structured community of bacterial cells enclosed in a self-produced polymer matrix and adhered to a non-intact surface. The main characteristic of biofilms is their ability to make microorganisms more resistant to attack by antibiotic therapies. The organization of sessile bacteria into this sort of functional consortium not only allows for a considerable supply of oxygen and nutrients, but also gives them an important defense against antibiotic agents and the host's immune system both because of the chemical-mechanical barrier offered by the polymer matrix and because of the low efficacy of antibiotics. Typically, antibiotic therapy resolves the symptoms caused by the planktonic cells released by the biofilm but fails to eradicate and eliminate the biofilm completely. For this reason, infections with biofilm-producing bacteria are recurrent, making cycles of antibiotic therapy necessary until the sessile population is completely removed from the organism. Planktonic bacterial cells are released from biofilms [
15,
16].Therefore, biofilms may act as "hotbeds" of acute infection if the host-mobilized defenses fail to eliminate the planktonic cells that are released at any time during biofilm infection. Therefore, biofilm would play a crucial role in the etiopathogenesis of recurrent upper airway infections. More specifically, infections that various microorganisms contribute through biofilm production, to become chronic as in chronic rhinosinusitis. For this reason, actions aimed at disrupting the polysaccharide matrix represent an important complement in preventing the biofilms. This action carried out by topical treatment of
Pistacia lentiscus may be a valuable aid in chronic rhinosinusitis [
5].
In the study performed, the action at the cellular level was demonstrated with a statistically significant 66.75% reduction in the presence of biofilms (p<0.001) compared to the increase in the control group, which can also be demonstrated by the fact that the number of bacteria present in the treatment group compared to the control group decreased by 68% demonstrating the action on biofilm but also the ability to have an antibacterial action. Action that is both related to the reduction of biofilm but also to the improvement of the barrier capacity of the nasal mucosa, which not subjected to inflammatory stimuli restored proper functioning.
An additional finding of improved mucosal function is the reduction in the number of neutrophils at the cellular level, although the finding is not statistically significant. Moreover, an increase in supranuclear stria at the cellular level was observed, which is an indication of cell vitality. The antibacterial capacity of the Pil is well demonstrated by the reduction of the number of bacteria in the mucosa specimen.
The increase in the percentage of patients in the treated group with the presence of active ciliary motility (mucociliary clearance) represents an additional factor in improving our body's first line of defense: mucociliary clearance. Ciliary motility was observed with a phase-contrast light microscope and the presence of mobility for more than 10 min of observation was considered a normal value.
The increase in ciliary motility is then to be correlated with the reduction in the presence of nasal secretions. This not only correlated with the improved functioning of ciliary motility but also with the reduction of the local inflammatory process that led to a reduction in secretory production.
This is correlated with the improved functioning of ciliary motility but also with the reduction of the local inflammatory process that led to a reduction in secretory production. Also correlated with the anti-inflammatory action of Pistacia lentiscus at the cellular level.
The anti-inflammatory action of Pil is due to the action IL-1β, IL-6, TNFα and COX-2.
From the quality-of-life point of view, the reduction in SNOT 22 in treated versus untreated patients was seen with a statistically significant 32% improvement over the pretreatment data.
5. Conclusions
The use of Pistacia lentiscus represents a means of supplementing medical therapies for the treatment of chronic rhinosinusitis. The constant use of nasal washes with special nasal douche has permitted to highlight how the action at the level of nasal cellularity, ciliary function and secretions is evidenced by the results of the present study. Importantly, how the improvement of disease control led to the improvement of patients' quality of life.
Cellular action demonstrated through increased cell viability, increased supranuclear striae is to be taken into consideration in improving nasal function and mucosal reactivity. An important factor was the ability to reduce the presence of biofim at the nasal cellular level.
The cyclic use of Pistacia lentiscus derivative represents an attempt to increase patient therapeutic compliance by improving functional activity at the nasal level and to improve the quality of life of the patients, with the decrease of secretion and episode of recurrence.
Author Contributions
Conceptualization, AM ; methodology, GM.; validation, MB ; formal analysis, CC ; investigation, GM ; writing—original draft preparation,CC; writing—review and editing, GM.; All authors have read and agreed to the published version of the manuscript
Funding
This research received no external funding.
Institutional Review Board Statement
the Institutional Review Board Statement and approval number Approved by the ethical committee of the Italian Academy of Rhinology IAR20221123 “The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee Approved by the ethical committee of the Italian Academy of Rhinology IAR20221123.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zrira S., Elamrani A., Benjilali B. Chemical composition of the essential oil of Pistacia lentiscus L. from Morocco. Flavour Frag. J. 2003;18:475–480. [CrossRef]
- Botsaris G., Orphanides A., Yiannakou E. et al. Antioxidant and antimicrobial effects of Pistacia lentiscus L. extracts in pork sausages. Food Technol. Biotechnol. 2015;53:472–478. [CrossRef]
- Tassou C.C., Nychas G.J.E. Antimicrobial activity of the essential oil of mastic gum (Pistacia lentiscus var. chia) on Gram positive and Gram negative bacteria in broth and in Model Food System. Int. Biodeter. Biodegr. 1995;36:411–420. [CrossRef]
- Azaizeh H., Halahleh F., Abbas N.,et al. Polyphenols from Pistacia lentiscus and Phillyrea latifolia impair the exsheathment of gastro-intestinal nematode larvae. Vet. Parasitol. 2013;191:44–50. [CrossRef]
- Gardeli C., Vassiliki P., Athanasios M.et al. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008;107:1120–1130. [CrossRef]
- Romani A., Pinelli P., Galardi C. et al.Identification and quantification of galloyl derivatives, flavonoid glycosides, and anthocyanins in leaves of Pistacia lentiscus L. Phytochem. Anal. 2002;3:79–86. [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis et al. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020; 254, 112485. [CrossRef]
- Elgubbi, H.; Alfageih, L.; Zorab, et al. Pistacia lentiscus tree and its role in riddance of some environmental polluters. EC Nutr. 2017; 10, 8–14.
- Trabelsi, H.; Cherif, O.A.; Sakouhi, F. et al. Total lipid content, fatty acids and 4-desmethylsterols accumulation in developing fruit of Pistacia lentiscus L. growing wild in Tunisia. Food Chem. 2012; 131, 434–440. [CrossRef]
- Orru, G.; Demontis, C.; Mameli et al. The Selective Interaction of Pistacia Lentiscus Oil vs. Human Streptococci, an Old Functional Food Revisited with New Tools. Front. Microbiol. 2017;8, 2067. [CrossRef]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.L. et al. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Med. 1999;65, 749–752. [CrossRef]
- Duru, M.E.; Cakir, A.; Kordali, S. et al Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia 2003, 74, 170–176. [CrossRef]
- Dob,T.; Dahmane, D.; Chelghoum, C. Chemical composition of the essential oils of Pistacia lentiscus L. from Algeria. J. Essent. Oil Res. 2006; 18, 335–338. [CrossRef]
- Said, S.A.; Fernandez, C.; Greff, S. et al. Inter-Population Variability of Terpenoid Composition in Leaves of Pistacia lentiscus L. from Algeria: A Chemoecological Approach. Molecules 2011;16, 2646–2657. [CrossRef]
- Aouinti, F.; Imelouane, B.; Tahri, M.et al New study of the essential oil, mineral composition and antibacterial activity of Pistacia lentiscus L. from Eastern Morocco. Res. Chem. Intermed. 2014;40, 2873–2886. [CrossRef]
- Bampouli, A.; Kyriakopoulou, K.; Papaefstathiou, G. et al Comparison of different extraction methods of Pistacia lentiscus var. chia leaves: Yield, antioxidant activity and essential oil chemical composition. J. Appl. Res. Med. Aromat. Plants 2014; 1, 81–91. [CrossRef]
- Aissi, O.; Boussaid, M.; Messaoud, C. Essential oil composition in natural populations of Pistacia lentiscus L. from Tunisia: Effect of ecological factors and incidence on antioxidant and antiacetylcholinesterase activities. Ind. Crop. Prod. 2016;91, 56–65. [CrossRef]
- Ben Khedir, S.; Mzid, M.; Bardaa, S. et al. In vivo evaluation of the anti-inflammatory effect of Pistacia lentiscus fruit oil and its effects on oxidative stress. Evid. Based Complement. Altern. Med. 2016; 2016, 6108203. [CrossRef]
- Buriani, A.; Fortinguerra, S.; Sorrenti, V. et al. Human Adenocarcinoma Cell Line Sensitivity to Essential Oil Phytocomplexes from Pistacia Species: A Multivariate Approach. Molecules 2017; 22, 1336. [CrossRef]
- Marengo, A.; Piras, A.; Falconieri, D. et al. Chemical and biomolecular analyses to discriminate three taxa of Pistacia genus from Sardinia Island (Italy) and their antifungal activity. Nat. Prod. Res. 2018;32, 2766–2774. [CrossRef]
- Ammari, M.; Othman, H.; Hajri, A. et al. Pistacia lentiscus oil attenuates memory dysfunction and decreases levels of biomarkers of oxidative stress induced by lipopolysaccharide in rats. Brain Res. Bull. 2018;140, 140–147. [CrossRef]
- Yosr, Z.; Imen, B.H.Y.; Rym, J. t al. Sex-related differences in essential oil composition, phenol contents and antioxidant activity of aerial parts in Pistacia lentiscus L. during seasons. Ind. Crop. Prod. 2018; 121, 151–159. [CrossRef]
- Bouyahya, A.; Assemian, I.C.C.; Mouzount, H. et al. Could volatile compounds from leaves and fruits of Pistacia lentiscus constitute a novel source of anticancer, antioxidant, antiparasitic and antibacterial drugs? Ind. Crop. Prod. 2019; 128, 62–69. [CrossRef]
- Longo, L.; Scardino, A.; Vasapollo, G. Identification and quantification of anthocyanins in the berries of Pistacia lentiscus L., Phillyrea latifolia L. and Rubia peregrina L. Innov. Food Sci. Emerg. Technol. 2007; 8, 360–364. [CrossRef]
- Benhammou, N.; Bekkara, F.A.; Panovska, T.K. Antioxidant and antimicrobial activities of the Pistacia lentiscus and Pistacia atlantica extracts. Afr. J. Pharm. Pharmacol. 2008; 2, 22–28.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).