Submitted:
22 March 2024
Posted:
22 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Aphids Rearing, Acetamiprid Exposure and Toxicity Assays
2.2. Transcriptomic Sequencing and Analysis
2.3. Metatranscriptomic Sequencing and Analysis
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Data Analysis
3. Results
3.1. Acetamiprid-Resistant Aphids
3.1. Transcriptomic Profiling Associated with Acetamiprid Resistance
3.1. Metatranscriptomic Profiling Associated with Acetamiprid Resistance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.L.; Qiao, G.X. Research status and trends in Aphidology. Acta Entomol. Sin. 2006, 1017–1026. [Google Scholar] [CrossRef]
- Guerrieria, E.; Digilio, M.C. Aphid-plant interactions: a review. J. Plant Inter. 2008, 3(4), 223–232. [Google Scholar] [CrossRef]
- Phogat, A.; Singh, J.; Kumar, V.; Malik, V. Toxicity of the acetamiprid insecticide for mammals: a review. Environ. Chem. Lett. 2022, 20, 1453–1478. [Google Scholar] [CrossRef]
- Ijaz, M.; Afzal, M.B.S.; Shad, S.A. Resistance risk analysis to acetamiprid and other insecticides in Acetamiprid-Selected population of Phenacoccus solenopsis. Phytoparasitica 2016, 44, 177–186. [Google Scholar] [CrossRef]
- Simon, J.-C.; Peccoud, J. Rapid evolution of aphid pests in agricultural environments. Curr. Opin. Insect Sci. 2018, 26, 17–24. [Google Scholar] [CrossRef]
- Li, R.; Cheng, S.; Chen, Z.; Guo, T.; Liang, P.; Zhen, C.; Wang, J.; Zhang, L.; Liang, P.; Gao, X. Establishment of toxicity and susceptibility baseline of broflanilide for aphis Gossypii glove. Insects 2022, 13, 1033. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Nauen, R. The molecular mechanisms of insecticide resistance in aphid crop pests. Insect Biochem. Mol. Biol. 2023, 156, 103937. [Google Scholar] [CrossRef] [PubMed]
- Kaleem Ullah, R.M.; Gao, F.; Sikandar, A.; Wu, H. Insights into the effects of insecticides on aphids (Hemiptera: Aphididae): resistance mechanisms and molecular basis. Inter. J Mol. Sci. 2023, 24(7), 6750. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Denholm, I. Resistance of insect pests to neonicotinoid insecticides: Current status and future prospects. Arch. Insect Biochem. Physiol. 2005, 58, 200–215. [Google Scholar] [CrossRef]
- Hirata, K.; Kiyota, R.; Matsuura, A.; Toda, S.; Yamamoto, A.; Iwasa, T. Association between the R81T mutation in the nicotinic acetylcholine receptor β1 subunit of Aphis gossypii and the differential resistance to acetamiprid and imidacloprid. J. Pestic. Sci. 2015, 40, 25–31. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, L.; Wang, H.; Qiao, K.; Wang, D.; Wang, K. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid, Aphis gossypii. Pest Manag. Sci. 2011, 67, 1528–1533. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Functional analysis of cytochrome P450 genes linked with acetamiprid resistance in melon aphid, Aphis gossypii. Pesticide Biochem. Physiol. 2020, 170, 104687. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Acetamiprid resistance and fitness costs of melon aphid, Aphis gossypii: An age-stage, two-sex life table study. Pesticide Biochem. Physiol. 2021, 171, 104729. [Google Scholar] [CrossRef]
- Berber, G.; Demirci, B.; Toprak, U.; Inak, E.; Yorulmaz, S. Acetamiprid resistance in the green peach aphid Myzus persicae (Sulzer) (Hemiptera: Aphididae): selection, cross-resistance, biochemical and molecular resistance mechanisms. JAFAG 2022, 39(3), 136–142. [Google Scholar] [CrossRef]
- Jaffar, S.; Ahmad, S.; Lu, Y. Contribution of insect gut microbiota and their associated enzymes in insect physiology and biodegradation of pesticides. Front. Microbiol. 2022, 13, 979383. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I.; Xu, Y. Role of insect gut microbiota in pesticide degradation: a review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.T.; Guo, J.Y.; Long, C.Y.; Liu, H.; Wan, F.H. Advances in endosymbionts and their functions in insects. Acta Entomol. Sin. 2014, 57, 111–122. [Google Scholar] [CrossRef]
- Douglas, A.E. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 1998, 43, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, X.L.; Qiao, G.X. Trends in research on the primary endosymbiont of aphids, Buchnera aphidicola. Chin. J. Appl. Entomol. 2013, 50, 1419–1427. [Google Scholar] [CrossRef]
- Tian, P.-P.; Zhang, Y.-L.; Huang, J.-L.; Li, W.-Y.; Liu, X.-D. Arsenophonus interacts with Buchnera to improve growth performance of aphids under amino acid stress. Microbiol. Spectr. 2023, 11, e01792–23. [Google Scholar] [CrossRef] [PubMed]
- Sl, D.; K, B.; Hr, B.; Bf, J.; W, Z.; F, R.; Sl, O. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 1999, 29. [Google Scholar] [CrossRef]
- Gong, P.; Shen, Z.R.; Li, Z.H. Wolbachia endosymbionts and their manipulation of reproduction of arthropod hosts. Acta Entomol. Sin. 2002, 241–252. [Google Scholar] [CrossRef]
- Jiang, X.F.; Wang, L.; Zhang, L.; Luo, L.Z. Molecular detection of Wolbachia in three species of vegetable aphids collected from Beijing suburb. Plant Prot. 2009, 35, 63–65. [Google Scholar] [CrossRef]
- Bi, J.; Wang, Y.-F. The effect of the endosymbiont Wolbachia on the behavior of insect hosts. Insect Sci. 2020, 27, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Fukatsu, T.; Nikoh, N.; Kawai, R.; Koga, R. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 2000, 66, 2748–2758. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.B.; Zhang, J.; Guo, J.Y.; Guo, J.Y.; Liu, H.; Wan, F.H. Advances of the secondary endosymbionts in sap-feeding insects. J. Biosaf 2016, 25, 92-98+122. [Google Scholar] [CrossRef]
- Tsuchida, T.; Koga, R.; Horikawa, M.; Tsunoda, T.; Maoka, T.; Matsumoto, S.; Simon, J.-C.; Fukatsu, T. Symbiotic bacterium modifies aphid body color. Science 2010, 330, 1102–1104. [Google Scholar] [CrossRef]
- Bressan, A.; Terlizzi, F.; Credi, R. Independent origins of vectored plant pathogenic bacteria from arthropod-associated Arsenophonus endosymbionts. Microb. Ecol. 2012, 63, 628–638. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.S.; Zhu, X.Z.; Wang, L.; Li, D.Y.; Zhang, K.X.; Ji, J.C.; Luo, J.Y. The relationship between symbiotic bacteria and wing dymorphism in Rhopalosiphum padi. Chin. J. Biol. Control. 2022, 38, 205–214. [Google Scholar] [CrossRef]
- Wu, T.; Monnin, D.; Lee, R.A.R.; Henry, L.M. Local adaptation to hosts and parasitoids shape Hamiltonella defensa genotypes across aphid species. Proc. R. Soc. B Biol. Sci. 2022, 289, 20221269. [Google Scholar] [CrossRef]
- Li, Q.; Sun, J.; Qin, Y.; Fan, J.; Zhang, Y.; Tan, X.; Hou, M.; Chen, J. Reduced insecticide susceptibility of the wheat aphid Sitobion miscanthi after infection by the secondary bacterial symbiont Hamiltonella defensa. Pest Manag. Sci. 2021, 77, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yuan, M.L. Biological and ecological characteristics of Acyrthosiphon pisum. Pratacultural Sci. 2017, 34, 1727–1740. [Google Scholar] [CrossRef]
- Sandhi, R.K.; Reddy, G.V.P. Biology, ecology, and management strategies for pea aphid (Hemiptera: Aphididae) in pulse crops. J. Integr. Pest Manag. 2020, 11(1), 18. [Google Scholar] [CrossRef]
- Müller, V.; Benjamin, B.; Lueke, B.; Mazzoni, E.; Pym, A.; Bass, C.; Nauen, R. Molecular characterization of pyrethroid resistance in field-collected populations of the pea aphid, Acyrthosiphon pisum. Entomol. Gen. 2023. [Google Scholar] [CrossRef]
- Chang, M.; Cheng, H.; Cai, Z.; Qian, Y.; Zhang, K.; Yang, L.; Ma, N.; Li, D. miR-92a-1-P5 modulated expression of the flightin gene regulates flight muscle formation and wing extension in the Pea Aphid, Acyrthosiphon pisum (Hemiptera: Aphidoidea). J. Insect Sci. 2022, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Moores, G.D.; Gao, X.; Denholm, I.; Devonshire, A.L. Characterisation of insensitive acetylcholinesterase in insecticide-resistant cotton aphids, Aphis gossypii Glover (Homoptera: Aphididae). Pestic. Biochem. Physiol. 1996, 56, 102–110. [Google Scholar] [CrossRef]
- Wu, B.; Huang, L.; Qiu, W.J.; Liu, X.; Shen, Y.W.; Liu, Y.P.; Yang, Z.L.; Li, X.M.; Cui, B.; Xu, S.D.; et al. Small nucleolar RNA Sf-15 regulates proliferation and apoptosis of Spodoptera frugiperda Sf9 cells. BMC Mol. Biol. 2019, 20. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L.; et al. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef]
- Audic, S.; Claverie, J.-M. The significance of digital gene expression profiles. Genome Res. 1997, 7, 986–995. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.R.; Nielsen, J. Uncovering Transcriptional Regulation of Metabolism by Using Metabolic Network Topology. Proc. Natl. Acad. Sci. USA 2005, 102, 2685–2689. [Google Scholar] [CrossRef] [PubMed]
- Matsouaka, R.A.; Singhal, A.B.; Betensky, R.A. An Optimal Wilcoxon–Mann–Whitney Test of Mortality and a Continuous Outcome. Stat. Methods Med. Res. 2018, 27, 2384–2400. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2024. Available online: http://www.R-project.org/ (accessed on 12 March 2024).
- Yang, L.; Sun, Y.; Chang, M.; Zhang, Y.; Qiao, H.; Huang, S.; Kan, Y.; Yao, L.; Li, D.; Ayra-Pardo, C. RNA interference-mediated knockdown of Bombyx mori haemocyte-specific cathepsin l (Cat l)-like cysteine protease gene increases Bacillus thuringiensis kurstaki toxicity and reproduction in insect cadavers. Toxins 2022, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Crawley, M.J. The R Book, John Wiley and Sons Ltd: Chichester, West Sussex, UK, 2007; pp 1-877.
- The International Aphid Genomics Consortium. Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010, 8(2), e1000313. [Google Scholar] [CrossRef]
- Arthropod Pesticide Resistance Database. Available online: https://www.pesticideresistance.org/ (accessed on 11 March 2024).
- Pope, R.D. Some aphid waxes, their form and function (Homoptera: Aphididae). J. Nat. Hist. 1983, 17, 489–506. [Google Scholar] [CrossRef]
- Ammar, E.D.; Alessandro, R.T.; Hall, D.G. Ultrastructural and chemical studies on waxy secretions and wax-producing structures on the integument of the woolly oak aphid Stegophylla brevirostris Quednau (Hemiptera: Aphididae). J. Microsc. Ultrastruct. 2013, 1, 43–50. [Google Scholar] [CrossRef]
- Kanturski, M.; Świątek, P.; Trela, J.; Borowiak-Sobkowiak, B.; Wieczorek, K. Micromorphology of the model species pea aphid Acyrthosiphon pisum (Hemiptera, Aphididae) with special emphasis on the sensilla structure. Eur. Zoo. J. 2020, 87(1), 336–356. [Google Scholar] [CrossRef]
- Tong, H.; Wang, Y.; Wang, S.; Omar, M.A.A.; Li, Z.; Li, Z.; Ding, S.; Ao, Y.; Wang, Y.; Li, F.; Jiang, M. Fatty acyl-CoA reductase influences wax biosynthesis in the cotton mealybug, Phenacoccus solenopsis Tinsley. Commun. Biol. 2022, 5(1), 1108. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, W.J.; Liao, J.M.; Liao, P.; Lu, H. Ribosomal proteins: functions beyond the ribosome. J. Mol. Cell. Biol. 2015, 7(2), 92–104. [Google Scholar] [CrossRef]
- Yu, J.; Hu, S.; Ma, K.; Sun, L.; Hu, H.; Zou, F.; Guo, Q.; Lei, Z.; Zhou, D.; Sun, Y.; et al. Ribosomal protein S29 regulates metabolic insecticide resistance through binding and degradation of CYP6N3. PLOS ONE 2014, 9, e94611. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Sachdev, B.; Sharma, N.; Seth, R.; Bhatnagar, R.K. Interaction of Bacillus thuringiensis vegetative insecticidal protein with ribosomal S2 protein triggers larvicidal activity in Spodoptera frugiperda. Appl. Environ. Microbiol. 2010, 76(21), 7202–7209. [Google Scholar] [CrossRef] [PubMed]
- Ayra-Pardo, C.; Ochagavía, M.E.; Raymond, B.; Gulzar, A.; Rodríguez-Cabrera, L.; Rodríguez de la Noval, C.; Morán Bertot, I.; Terauchi, R.; Yoshida, K.; Matsumura, H.; Téllez Rodríguez, P.; Hernández Hernández, D.; Borrás-Hidalgo, O.; Wright, D.J. HT-SuperSAGE of the gut tissue of a Vip3Aa-resistant Heliothis virescens (Lepidoptera: Noctuidae) strain provides insights into the basis of resistance. Insect Sci. 2019, 26(3), 479–498. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Guo, X.X.; Lou, Z.W.; Wang, H.Y.; Cheng, P. Correlation research between Serratia oryzae and insecticide-resistance of Aedes albopictus. J. Med. Pest Control 2023, 39, 205–210. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, J.; Wang, L.; Zhang, L.; Zhu, X.; Jiang, W.; Cui, J. Bacterial communities in natural versus pesticide-treated Aphis gossypii populations in North China. MicrobiologyOpen 2019, 8, e00652. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, Y.; Zheng, C.; Gao, X.; Wei, X.; Xi, J.; Peng, T.; Shang, Q. Rapid evolution of symbiotic bacteria populations in spirotetramat-resistant aphis Gossypii glover revealed by pyrosequencing. Comp. Biochem. Physiol. Part D Genomics Proteomics 2016, 20, 151–158. [Google Scholar] [CrossRef]
- Zytynska, S.E.; Tighiouart, K.; Frago, E. Benefits and costs of hosting facultative symbionts in plant-sucking insects: A meta-analysis. Mol. Ecol. 2021, 30, 2483–2494. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Zhang, T.; Bai, S.; He, K.; Zhang, Y.; Francis, F.; Wang, Z. Secondary symbionts affect aphid fitness and the titer of primary symbiont. Front. Plant Sci. 2023, 14, 1096750. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Spragg, C.J.; Altincicek, B.; Gerardo, N.M. Symbiont-mediated protection against fungal pathogens in pea aphids: a role for pathogen specificity? Appl. Environ. Microbiol. 2013, 79, 2455–2458. [Google Scholar] [CrossRef] [PubMed]
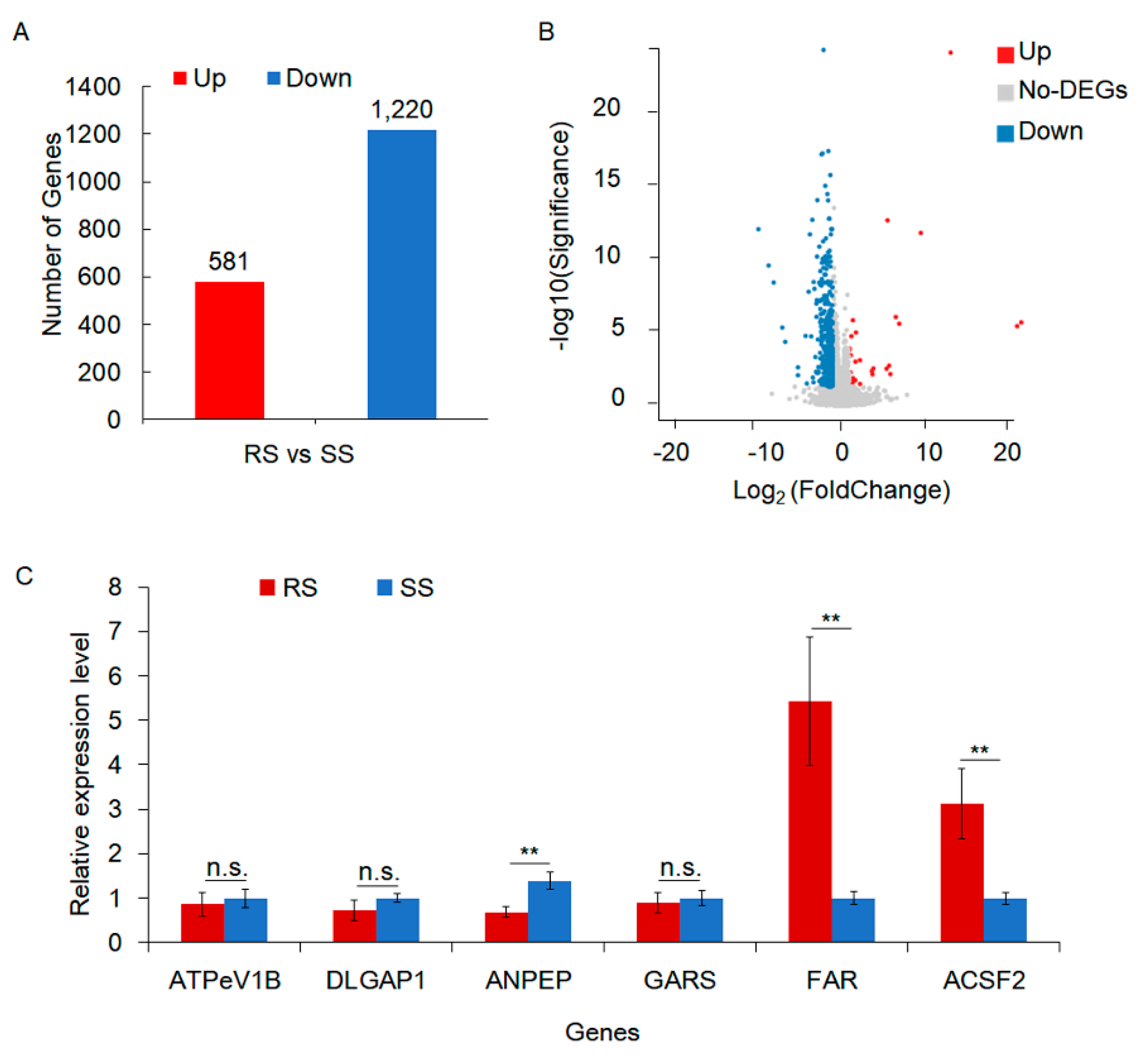
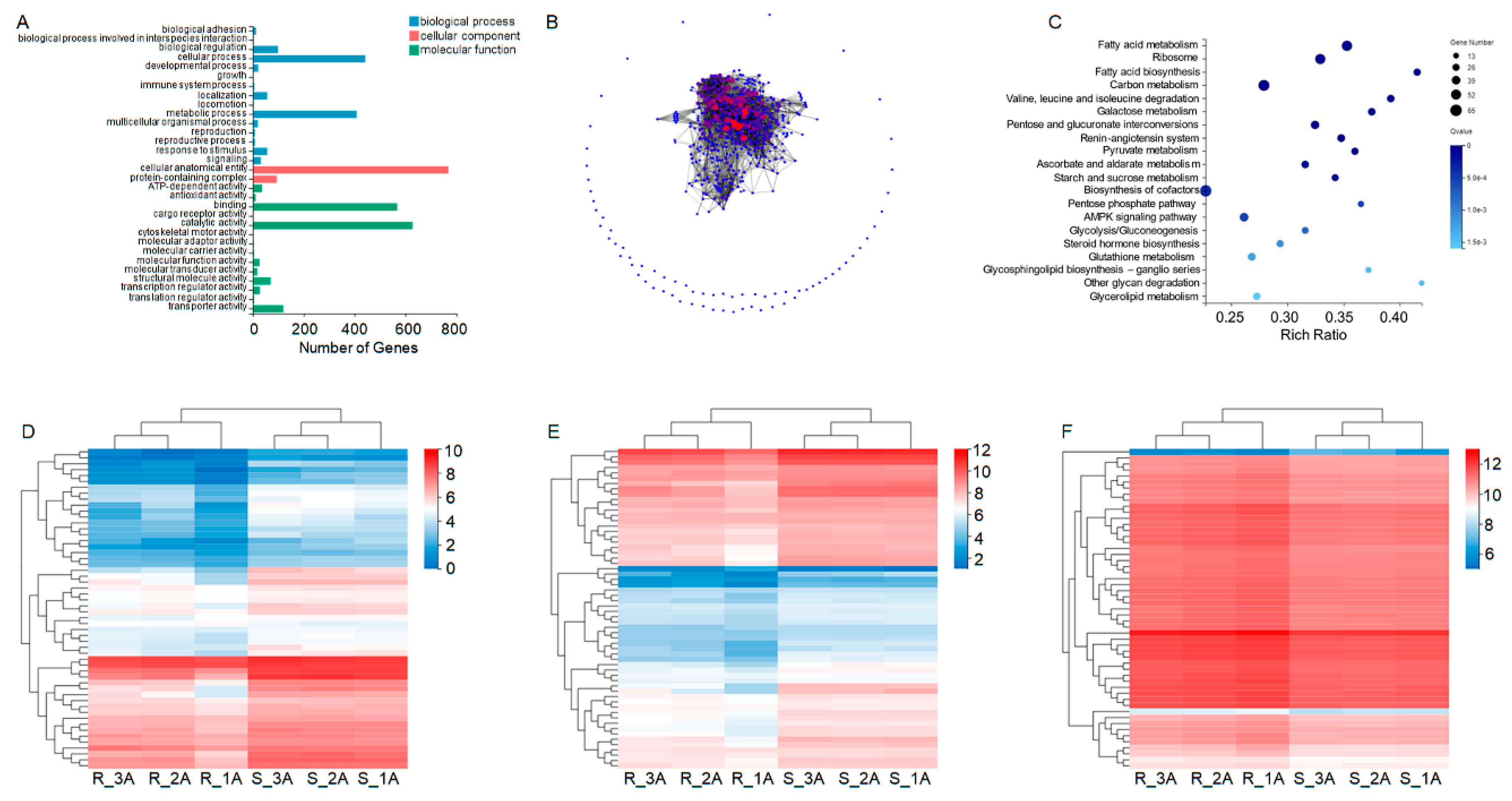
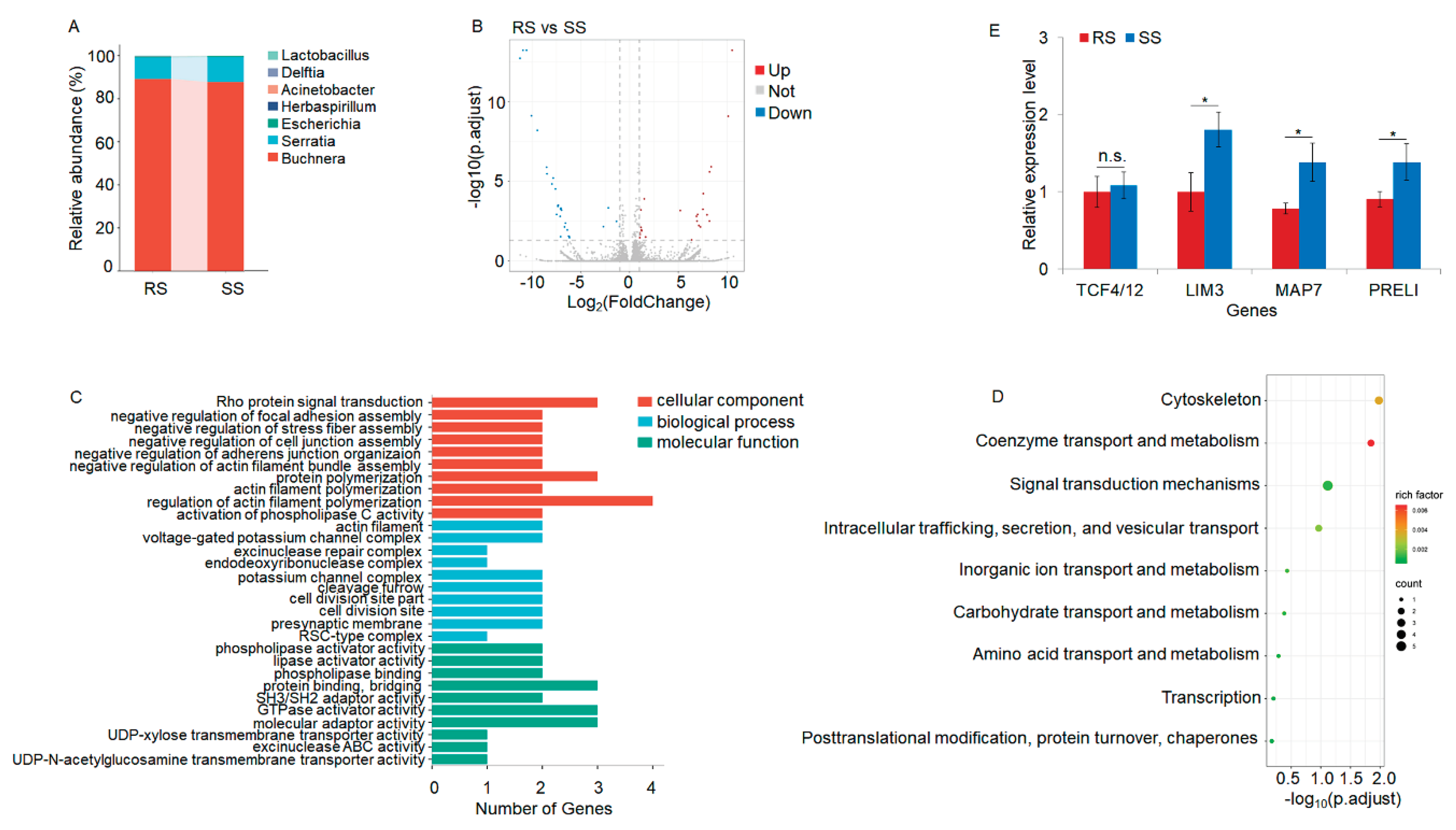
| Genes | Forward primer (5'-3') | Reverse primer (5'-3') |
|---|---|---|
| ATP6V1 (NM_001293544) | TCGTCAAATCTATCCACCAA | AATGCCTCTTCTCCCACAAC |
| DLGAP1 (XM_029490798) | AATTCCTCGGTTTATGTGAG | ATTGCCTTGCGTTGTTCTTC |
| ANPEP (XM_001950011) | TTGGATGGGCATTGTTTCTA | ATAGTCCATATCACCGACCT |
| GARS (XM_003245009) | TCATTGCCTCCATTAGTAGC | ATTTGTTCCATTGAATCCCT |
| FAR (XM_003242260) | ACTACGAGTCACCACCTTTG | TTTCTGCTTTCGCATACATT |
| ACSF2 (XM_016806965) | CGCCAACTCTACAAGACAAC | CATGACAAGATACCCACGAA |
| TCF4/12 (XM_016808432) | TCGCCCGATGATGATAGTGT | GTGCCGTCCAAGTAATAAGA |
| LIM3 (XM_008188468) | GAACGCAGAACAGTAAAGAA | CTGGTATAATAACGGAGGAA |
| MAP7 (XM_008190631) | AGAGTTGCGGTTGCAGTTGG | TGTTGCTCGGCAGATTCAGT |
| PRELI (XM_029488204) | GAAGAATGTTGGTATGACGA | CATGTTGGATTTGGTGTAAT |
| Aphid strain | LC50 | LC99 | LC5 | Slope (± S.E.) | na | RRb |
|---|---|---|---|---|---|---|
| SS | 2.68 (2.46-2.92) | 32.10 (31.34-32.87) | 0.55 (0.01-1.09) | 6.38 ± 0.37 | 150 | – |
| RS | 5.09 (4.88-5.31) | 44.75 (44.02-45.48) | 1.26 (0.81-1.72) | 8.28 ± 0.56 | 150 | 1.9 |
| Gene ID | log2(R/S)* | Annotation |
|---|---|---|
| LOC115034459 | -10.09 | alpha-N-acetylgalactosaminidase |
| LOC100573163 | -8.84 | glycerol-3-phosphate dehydrogenase (NAD+) |
| LOC103308887 | -8.23 | SRA stem-loop-interacting RNA-binding protein, mitochondrial |
| LOC100168848 | -7.17 | NULL |
| LOC100572322 | -6.82 | polyhomeotic-like protein 1 |
| LOC100570759 | -5.26 | MFS transporter, PAT family, solute carrier family 33 (acetyl-CoA transportor), member 1 |
| LOC100570250 | -5.26 | lysophospholipid acyltransferase 7 |
| LOC100159801 | -4.33 | gamma-glutamyltranspeptidase / glutathione hydrolase / leukotriene-C4 hydrolase |
| LOC103310139 | -4.18 | leucine-rich repeat and immunoglobulin-like domain-containing nogo receptor-interacting protein |
| LOC100162019 | -3.96 | lactase-phlorizin hydrolase |
| LOC100169462 | 13.39 | V-type H+-transporting ATPase subunit B |
| LOC100575793 | 9.74 | glycyl-tRNA synthetase |
| LOC100159545 | 7.11 | discs, large-associated protein 1 |
| LOC107882136 | 6.69 | Alanyl aminopeptidase |
| LOC100569077 | 6.02 | NULL |
| LOC100571352 | 5.87 | lysosomal acid phosphatase |
| LOC100570391 | 5.67 | alcohol-forming fatty acyl-CoA reductase |
| LOC103310260 | 5.56 | medium-chain acyl-CoA ligase, mitochondrial |
| LOC100570903 | 3.95 | SWI/SNF related-matrix-associated actin-dependent regulator of chromatin subfamily C |
| LOC115034301 | 3.84 | glutamate receptor, ionotropic, invertebrate |
| Sample | B. aphidicola (%) | S. symbiotica (%) | E. coli (%) | H. huttiense (%) | A. soli (%) | D. acidovorans (%) | L. iners (%) |
|---|---|---|---|---|---|---|---|
| RS_1 | 87.97 | 11.09 | 0.44 | 0.32 | 0.18 | 0.0 | 0.0 |
| RS_2 | 88.78 | 10.74 | 0.28 | 0.0 | 0.04 | 0.13 | 0.04 |
| RS_3 | 90.93 | 8.07 | 0.18 | 0.02 | 0.01 | 0.0 | 0.79 |
| SS_1 | 90.70 | 8.49 | 0.46 | 0.0 | 0.05 | 0.0 | 0.29 |
| SS_2 | 86.42 | 13.34 | 0.24 | 0.0 | 0.0 | 0.0 | 0.0 |
| SS_3 | 86.23 | 13.04 | 0.50 | 0.0 | 0.23 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





