1. Introduction
As a terminal stage of peripheral arterial disease (PAD), critical limb ischaemia (CLI), which is characterised by chronic ischemic rest pain, ischemic ulcerations, or gangrene, is associated with a significant risk of amputation of the affected limb [
1,
2]. Revascularisations by percutaneous transluminal angioplasty or bypass graft surgery are essential rescue methods for wound healing and limb salvage. However, up to 30% of patients with CLI are unsuitable for or have failed previous endovascular or surgical revascularisation treatments [
3]. Therefore, in these high-risk patients, limb amputation is the only therapeutic option to relieve pain and stop the spread of wound infection. These patients are called „no-option“ CLI patients, with an annual mortality of 10% to 40% [
4,
5].
Several pre-clinical studies of CLI treatment were conducted in recent years, using novel approaches with bone marrow cells (BMCs). The most used stem cells include bone marrow-derived mononuclear cells (BM-MNCs), CD34
+ bone marrow cells, or bone marrow-derived mesenchymal stem cells. The implanted stem cells can improve blood circulation and increase blood flow to the transplantation site in patients by promoting ischemic angiogenesis and neovascularisation [
6,
7]. Notably, several clinical trials have demonstrated the positive therapeutic efficacy of stem cell transplantation and validated its safety and feasibility [
8,
9,
10]. At the same time, other studies have shown an insignificant moderate prognosis following stem cell therapy, comparable to the placebo group or conservative treatment [
11,
12]. The heterogeneity of studied populations and differences in specific features of transplants might cause these controversial observations. Another shortcoming of the cells used in therapy is their poor survival and retention of transplants
in vivo, caused by the specific properties of individual cell lines and the hostile microenvironment in the ischemic region. For these reasons, attention has been focused on improving stem cell tolerance to the implantation site, which could increase therapeutic efficacy [
13]. In addition, it is necessary to consider previous long-term treatment of CLI patients, which could modify the response to the cell therapy. Recommended therapy in all patients with PAD includes statins, widely used as a cholesterol-lowering agent to prevent cardiovascular disease with a favourable safety profile [
14,
16]. It has been shown that statins can act through various signalling pathways, particularly PI3K/Akt, thus influencing the fate and properties of transplanted bone marrow cells or affecting the implantation site of stem cells [
17]. RAS-acting agents (angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) should be considered as first-line therapy in patients with PAD and hypertension [
14]. A study on 31245 patients recently concluded that statin therapy positively decreased CRP levels and reduced atherosclerotic risk [
15] .
This study explores the clinical background features, prior pharmacotherapy, and transplants of CLI patients treated with bone marrow cells. It examines the characteristics of both responders and non-responders to stem cell transplantation and develops and clarifies the criteria and prognostic factors for positive clinical outcomes.
2. Materials and Methods
Patients
Between May 2016 and November 2018, 33 patients (age 65 ± 10 years, 31 males) with advanced CLI (Rutherford category 5 or 6) after failed or impossible revascularisation were treated with 40 ml of bone marrow nucleated cells via the local intramuscular route. The aetiology of arterial obliteration was atherosclerosis in 30 patients and thromboangiitis obliterans (Buerger disease) in three patients. The patients included in this study met the following criteria: (1) at least 18 years of age with ischemic skin lesions (ulcers or gangrene) with a CLI Rutherford category of 4, 5 or 6 according to the Transatlantic Inter-Society Consensus (TASC) classification (minor or major tissue loss), defined as necrosis or gangrene extending proximal to the metatarsal line or as extensive deep heel gangrene [
2]; (2) CLI defined by ankle-brachial index (ABI)≤0.4 or ankle systolic pressure<50 mmHg, or toe systolic pressure<30 mmHg, and transcutaneous oxygen pressure (TcPO
2)<30 mmHg; (3) no option for endovascular or surgical revascularisation as judged by vascular surgeon and interventionist; (4) failed revascularisation, defined as no change of clinical status with the best standard care four weeks after endovascular or surgical revascularisation. Patients who, at the start of the cell therapy, met any of the following criteria: (1) life expectancy of fewer than six months; (2) presence of malignancy during the last five years; (3) critical coronary artery disease or unstable angina pectoris; (4) patients with end-stage kidney disease and patient on dialysis; (5) bone marrow disease (e.g., severe anaemia, leucopenia, thrombocytopenia, myelodysplastic syndrome) were excluded from the study. The local ethical committee of the National Institute of Cardiovascular Diseases, Bratislava, approved the study design. This study was carried out by the Code of Ethics of the World Medical Association, Declaration of Helsinki (WMA Declaration of Helsinki, 2013).
Bone Marrow Cell Isolation and Administration
On the day of the transplantation, bone marrow was aspirated from both anterior superior iliac crests under analgosedation with propofol. A total volume of 240 ml of bone marrow was harvested using a standard disposable needle for aspiration. Bone Marrow Aspirate Concentrate System (Harvest, Plymouth, MA, USA), which uses gradient density centrifugation and provides 40 ml of bone marrow-rich product for all blood elements within 15 min, was used to process obtained bone marrow aspirate. After the harvesting and centrifugation of stem cells, 40 ml of bone marrow cells (BMCs) were administered by intramuscular methods using deep injections with a 23-G needle into the muscles of the affected limb along the crural arteries, with each injection being approximately 1 ml. The duration of this procedure was 1 hour. A bone marrow cell sample was immediately analysed using a MACS Quant analyser (Miltenyi Biotec, BergischGladbach, Germany). The BM-MNCs concentration, MSCs concentration and viability of cells were determined using an MSC Enumerating kit and propidium iodide solution (Miltenyi Biotec, BergischGladbach, Germany), according to manufacturer’s instructions.
Pre-Procedure Assessment and Follow-Up
All patients were examined before the administration of BMCs, as well as 90 days and six months after the delivery, along with a peripheral blood test to determine basal serological parameters (including C-reactive protein, CRP). Resting ABI was measured according to the validated standards [
18]. It was performed by measuring the systolic blood pressure from both brachial arteries and dorsalis pedis and posterior tibial arteries after the patient had rested in the supine position for 10 min (normal values, 0.95-1.2). TcPO
2 of the affected limb was assessed using a TCM400 Mk2 monitor (Radiometer Medical ApS, Copenhagen, Denmark). It was measured at the forefoot in the supine position with an electrode temperature of 44°C. Wound healing was assessed by two independent physicians and documented by digital photography. A visual analogue scale (VAS) was used to measure the pain on a scale graded from 0 to 10.
Endpoints
Primary endpoints were limb salvage and improvement in wound healing without major limb amputation at the six months of follow-up. The patients who achieved CLI remission were considered responders to BMCs therapy. Patients requiring major limb amputation or patients without signs of wound healing were regarded as non-responders. A group of patients with limb salvage and complete ischemic wound healing were defined as super-responders to BMCs therapy. Patients who underwent major limb amputation were considered as super-non-responders. In addition, patients treated with atorvastatin for a long time (at least six months before BMCs therapy) were included in the atorvastatin group. The other patients were regarded as a non-atorvastatin group. Similarly, patients treated with RAS-acting agents were included in the RAS group, while patients without RAS treatment were considered in the NON-RAS group. Patients treated with atorvastatin or RAS-acting agents were included in the ATV and RAS group; patients without such therapy were regarded as NON-ATV and NON-RAS group. The secondary endpoints included changes in TcPO2, ABI, pain scale and Rutherford category after BMCs transplantation. The local ethical committee of the National Institute of Cardiovascular Diseases, Bratislava, approved the study design. All patients in the study were informed about the nature of the study and gave their written informed consent.
Statistical Analysis
The baseline characteristics of the responders and non-responders were compared first. Continuous variables were presented as the mean ± standard deviation (SD). Categorical variables were presented as numbers with percentages, and Fisher's exact test was used to analyse the significance of the differences. Gaussian distribution of data was tested by the Shapiro-Wilk test. The independent Student t-test or Mann-Whitney test was used to analyse the significance of the differences between responders and non-responders. The paired t-test or Wilcoxon signed-rank test was used to analyse the longitudinal changes from baseline to 6 months post-transplantation. The candidate prognostic factors of the responders and super-responders were screened through a univariate binary logistic regression analysis. The correlations between the candidate predictors or the variables at different time points were analysed with linear regression or Spearman nonparametric correlation. Receiver operating characteristics analysis was used to study predictors of clinical response after bone marrow transplantation. The Kaplan–Meier method estimated the survival curve and compared using the log-rank test. P values < 0.05 were considered statistically significant. All statistical analyses were performed using Microsoft Office Excel (2007), XLSTAT statistical and data analysis solution (Addinsoft 2021, New York, USA) or GraphPad Prism version 9.0 (GraphPad Software, San Diego, California, USA).
3. Results
Characteristics of Responders and NON-Responders
The baseline information of patients before the BMCs therapy is presented in
Table 1. The mean age of the responders was higher than that of the non-responders; the difference was significant (67.4 ± 9.3 vs. 59.8 ± 10.7,
P=0.014). No significant variations regarding limb ischaemia risk factors were observed between the responders and non-responders. When the baseline blood parameters were evaluated, the responders had significantly lower CRP levels than the patients in the non-responders (
P=0.013).
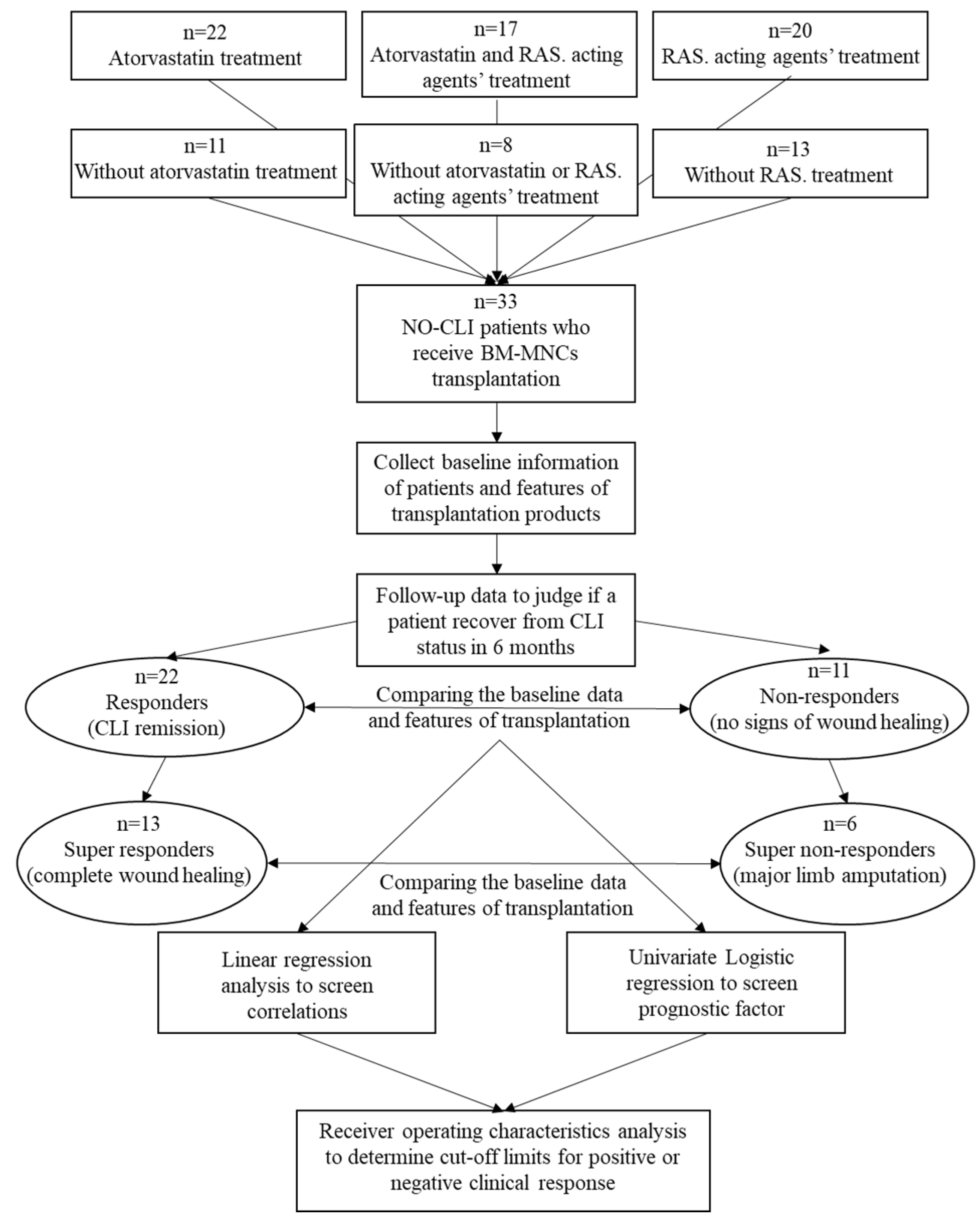
Regarding the treated limbs, the responders were characterised by fewer patients with baseline TcPO2<10 mmHg than the non-responders (
P=0.278). Based on the available complete longitudinal data (n=28), the mean TcPO
2 of the responders increased from 12.5 ± 14.0 mmHg at baseline to 22.7 ± 15.2 mmHg at six months post-transplantation (
P=0.008). At the same time, that of the non-responders did not improve significantly, from 2.4 ± 0.63 mmHg at baseline to 3.18 ± 8.4 mmHg at six months post-transplantation (
P=0.573) (
Table 2,
Figure 2A). TcPO
2 at baseline correlated significantly with that at six months (R
2=0.371,
P=0.001) (
Figure 2B).
No differences were observed between the responders and the non-responders in baseline ABI (
P=0.183), and there was no significant improvement during the 6-month follow-up. Despite this, a significant ABI decrease was observed in the non-responder group (
P=0.043). Moreover, we did not find a significant variation in the pain scale between groups at the baseline (
P=0.366). Still, a significant pain reduction was observed in the responder group during the 6-month follow-up (
P<0.001). We have not seen any significant changes in the non-responder group regarding the pain scale (
P=0.445). In addition, no difference was revealed between groups in terms of the Rutherford class at the baseline (
P=0.208). Still, significant improvement was observed at the 6-month follow-up in the responder group (
P=0.008), with no difference in the non-responder group (
P=0.059) (
Table 2).
Characteristics of Super Responders and Super Non-Responders
Table 3 presents baseline information of patients before BMCs therapy. The mean age of super-responders tended to be lower than that of the super-non-responders, though the difference was not significant 64.8 ± 10.4 vs 66.8 ± 4.0,
P=0.690). Comparing blood parameters at baseline demonstrated lower CRP levels in the super-responder group than patients in the super non-responder group (
P<0.001).
The super responders were characterised by higher baseline TcPO
2 (14.17 ± 14.69 mmHg) and ABI (0.57 ± 0.37) than the super non-responders (TcPO
2 3.0 ± 2.65 mmHg,
P=0.536, ABI 0.18 ± 0.36,
P=0.101) however these differences were not statistically significant (
Table 3). Based on the available complete longitudinal data, the mean TcPO
2 of the super-responders increased from 14.17 ± 14.69 mmHg at baseline to 25.92 ± 14.92 mmHg at six months post-transplantation (
P=0.012). In contrast, that of the super non-responders did not improve because of the deteriorating health and amputation of the affected limb. There was no significant improvement in the case of ABI of the super-responders (0.57 ± 0.37 at baseline, 0.57 ± 0.29 at six months,
P=0.983), while significant improvement of pain scale was demonstrated in the super-responder’s group (5.61 ± 1.71 at baseline, 1.46 ± 1.2 at six months post-transplantation, P<0.0001), comparing to the worsen pain scale in the super-non-responder group (6.33 ± 0.58 at baseline, 9.0 at six months,
P=0.102). Regarding the Rutherford scale, the super-responder group has significantly improved from 5.0 at baseline to 3.92 ± 1.04 at six months post-transplantation (
P=0.008). In comparison, the Rutherford scale decreased from 4.83 ± 0.75 at baseline to 6.0 (
P=0.038) at six months post-transplantation.
Characteristics of Transplanted Bone Marrow Cells
According to the characteristics of BMCs transplant, several variables differed between groups (
Table 4). The concentration of BM-MNCs appeared to be higher in the responders (3.53 ± 1.5 x 10
9) than in the non-responders (2.86 ± 1.13 x 10
9), although the difference was not statistically significant (
P=0.213). However, in the case of subgroup analysis of super-responders and super-non-responders, we found a significantly higher number of BM-MNCs in the group of super-responders compared to super-non-responders (3.68 ± 1.51 x 10
9 vs. 2.26 ± 0.74 x 10
9,
P=0.049).
Prognostic Factors and Predictors of BMCs Treatment Outcomes
According to the results of the univariate logistic regression, two variables were screened out for responders: CRP [OR 0.955, 95% CI 0.901-1.012,
P=0.044] and TcPO
2 [OR 1.149, 95% CI 0.949-1.392,
P=0.021] (
Table 5). The results of univariate logistic regression for super-responders showed that CRP [OR 0.544, 95% CI 0.221-1.341,
P<0.0001], ABI [OR 56.140, 95% CI 0.531-5932.9,
P=0.035] and BM-MNCs concentration [OR 1.0, 95% CI 0.999-1.0,
P=0.035] were screened out for super-responders (
Table 5).
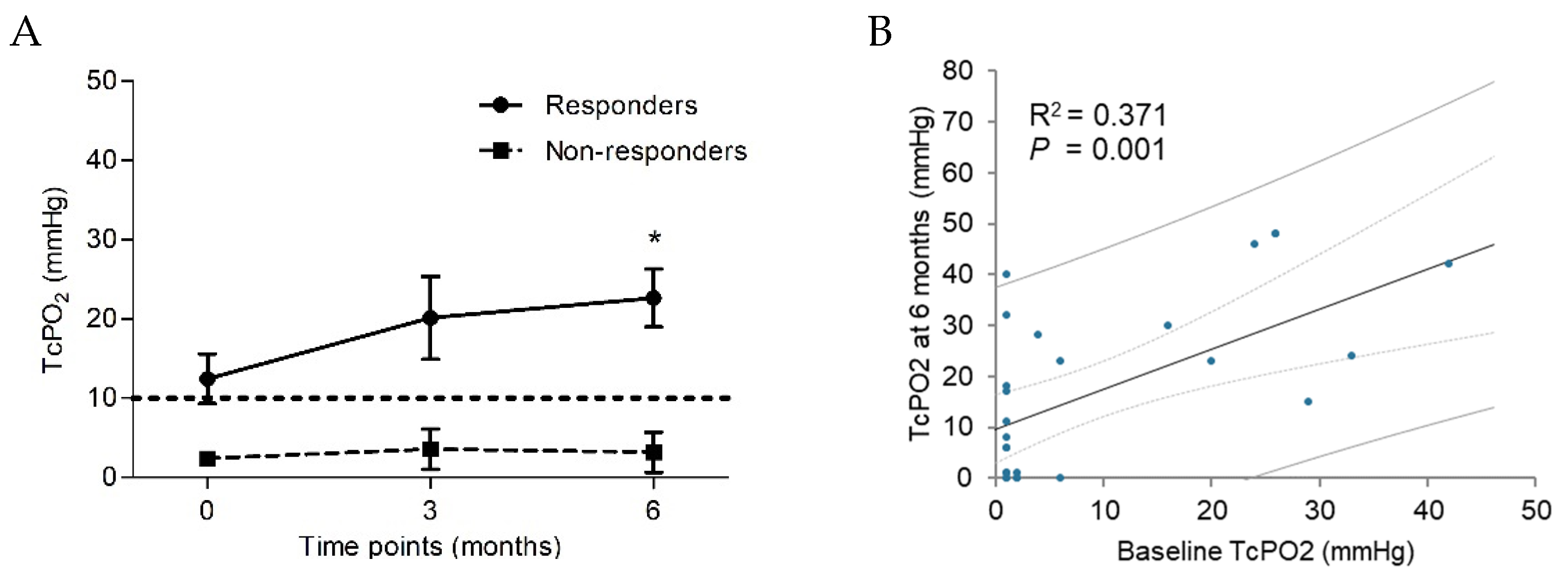
The receiver operating characteristics (ROC) analysis (
Figure 3) was made based on the univariate logistic regression results for the responder. A ROC curve was generated to address the sensitivity and specificity of CRP. The area under the ROC curve for CRP was 0.768 (95% CI 0.572-0.96,
P=0.014), indicating good discrimination for non-responders. Analysis showed a cut-off limit of baseline CRP > 8.1 mg/L before transplantation was predictive for negative clinical response with 81% specificity and 82% sensitivity (
Figure 3A). Analysis of linear regression showed a significant dependence between the levels of baseline CRP and the concentration of BM-MNCs in transplanted bone marrow (R
2=0.162,
P=0.03) (
Figure 3B).
Effect of Atorvastatin therapy before BMCs Treatment on the Outcomes of Stem Cell Treatment
The patient's baseline characteristics before BMCs therapy are presented in
Table 6. No significant differences were observed between the atorvastatin and non-atorvastatin groups regarding the basic characteristics, previous blood examination or parameters of limb ischaemia at baseline (
Table 6).
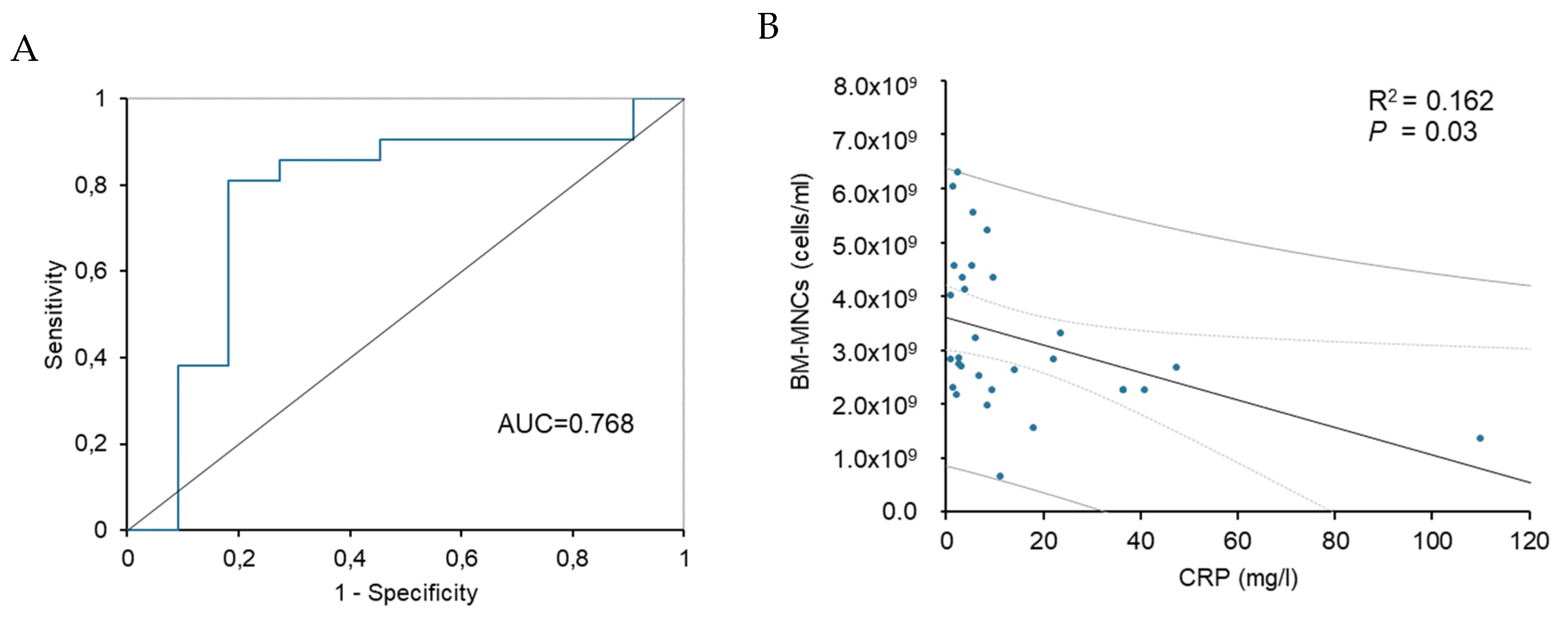
Parameters of Limb Ischaemia after BMCs Delivery in Atorvastatin or Non-Atorvastatin Group
Based on the functional outcomes after BMCs treatment, significant improvement of TcPO
2 was observed in the atorvastatin group (
P=0.015), compared to nonsignificant improvement in the non-atorvastatin group (
P=0.611) (
Table 7). Reduction in pain scoring was statistically significant in the atorvastatin group (
P=0.004), while no difference was observed in the non-atorvastatin group (
P=0.202). Amputation-free survival was achieved in 18 patients (82%) in the ATV group, while in the NON-ATV group was achieved in 8 patients (73%,
P=0.661). The risk of major amputation was decreased in those prescribed statins (Hazard ratio (HR) 0.44, 95% CI 0.08-2.5,
P=0.36) but did not differ significantly (
Figure 4C).
Characteristics of Transplanted Bone Marrow Cells in ATV and NON-ATV Group
Table 8 compares the characteristics of BMCs transplants administered to the affected limb. The concentration of BM-MNCs was significantly higher in the atorvastatin group (3.64 ± 1.53 x 10
9) than in the non-atorvastatin group (2.58 ± 0.73 x 10
9,
P=0.038) (
Table 8). No other statistically significant differences were observed between groups in terms of cell viability and count of MSCs in the transplant.
Effect of RAS-Acting Agents’ Therapy Prior to BMCs Treatment on the Outcomes of Stem Cell Treatment
Table 9 presents the baseline characteristics of patients before BMCs therapy. Patients with RAS-acting agents’ treatment were characterised by higher age (
P=0.006) and higher rate of arterial hypertension (
P=0.025).
Parameters of Limb Ischaemia after BMCs Delivery in RAS. or NON-RAS Group
Based on the functional outcomes after BMCs treatment, significant improvement in pain scale was observed in the RAS group (
P=0.005), compared to nonsignificant improvement in the NON-RAS group (
P=0.139). We have not seen any significant changes in the RAS or NON-RAS group comparing Rutherford class, TcPO
2 or ABI during the 6-month follow-up (
Table 10).
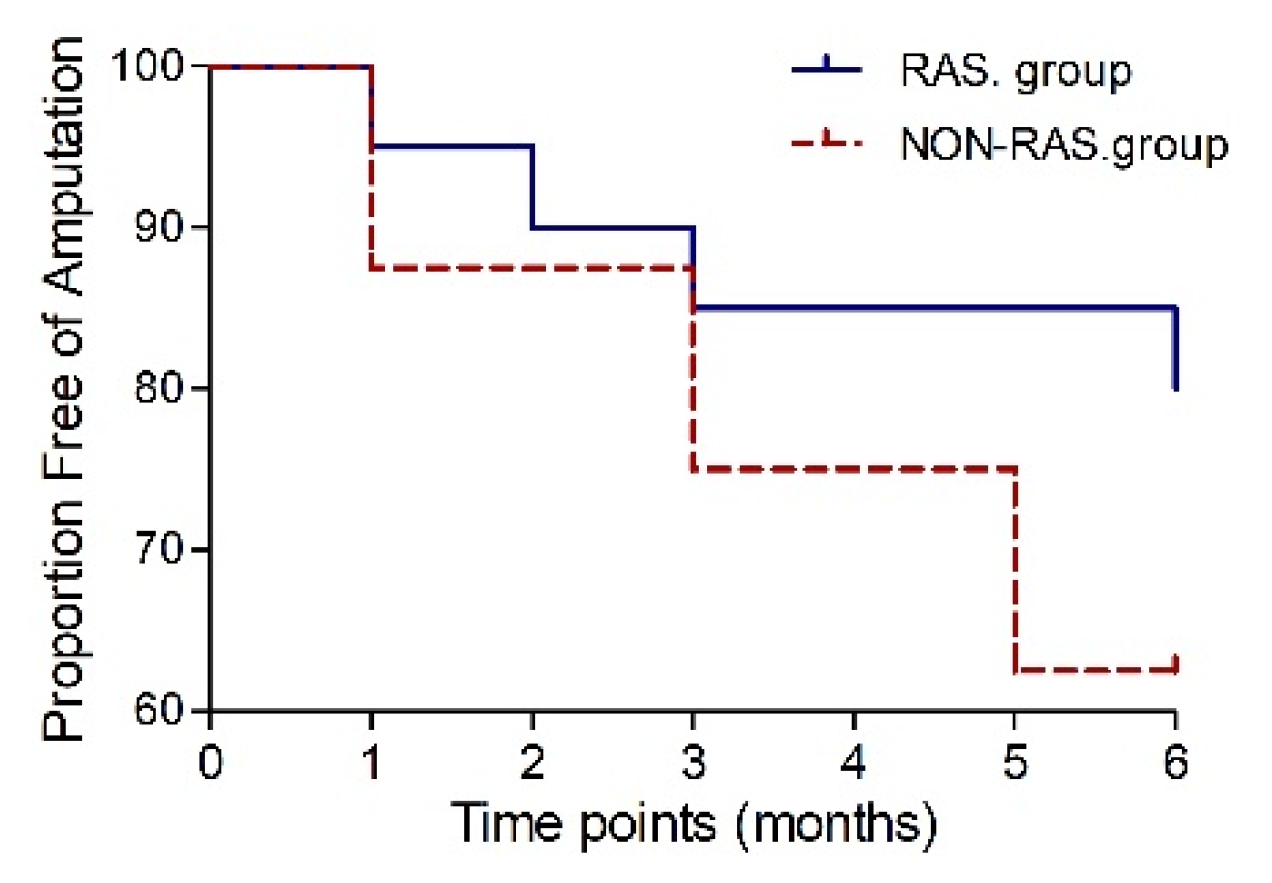
In the RAS group, 16 patients (80%) achieved major amputation-free survival six months post-transplantation, while in the NON-RAS group was, amputation-free survival achieved in 10 patients (77%,
P=1.0). The risk of major amputation was decreased in those prescribed RAS-acting agents (HR 0.438, 95% CI 0.08-2.4,
P=0.34) but did not differ significantly (
Figure 5).
Characteristics of Transplanted Bone Marrow Cells in RAS. or NON-RAS Group
No statistical differences were observed between the RAS and NON-RAS groups in terms of characteristics of BMCs transplants administered to the affected limb (
Table 11.)
Effect of Atorvastatin and RAS-Acting Agents’ Therapy prior to BMCs Treatment on the Outcomes of Stem Cell Treatment
Of the 33 patients, 17 were treated with atorvastatin together with RAS-acting agents before stem cell treatment, while eight patients were without atorvastatin and RAS-acting agents’ treatment. The baseline characteristics of these groups were compared first, with only a significant difference in the mean age of patients (
P=0.026) (
Table 12).
Parameters of Limb Ischaemia after BMCs Delivery in ATV and RAS. Group or NON-ATV. and NON-RAS. Group
According to the results of BMCs treatment after six months, several variables were screened out in the ATV and RAS group. Based on the available complete longitudinal data, the mean TcPO
2 of the RAS and ATV group increased from 11.6 ± 13.5 mmHg at baseline to 16.5 ± 15.0 mmHg at six months post-transplantation (
P=0.033). In contrast, that of the NON-ATV and NON-RAS groups did not improve significantly (
P=0.344). Regarding the results of improvement in the pain scale, the ATV and RAS group was associated with significant improvement of the pain scale from 5.80 ± 1.42 at baseline to 3.50 ± 3.43 at six months (
P=0.009), while assessment of the pain scale did not change significantly in NON-ATV and NON-RAS group (
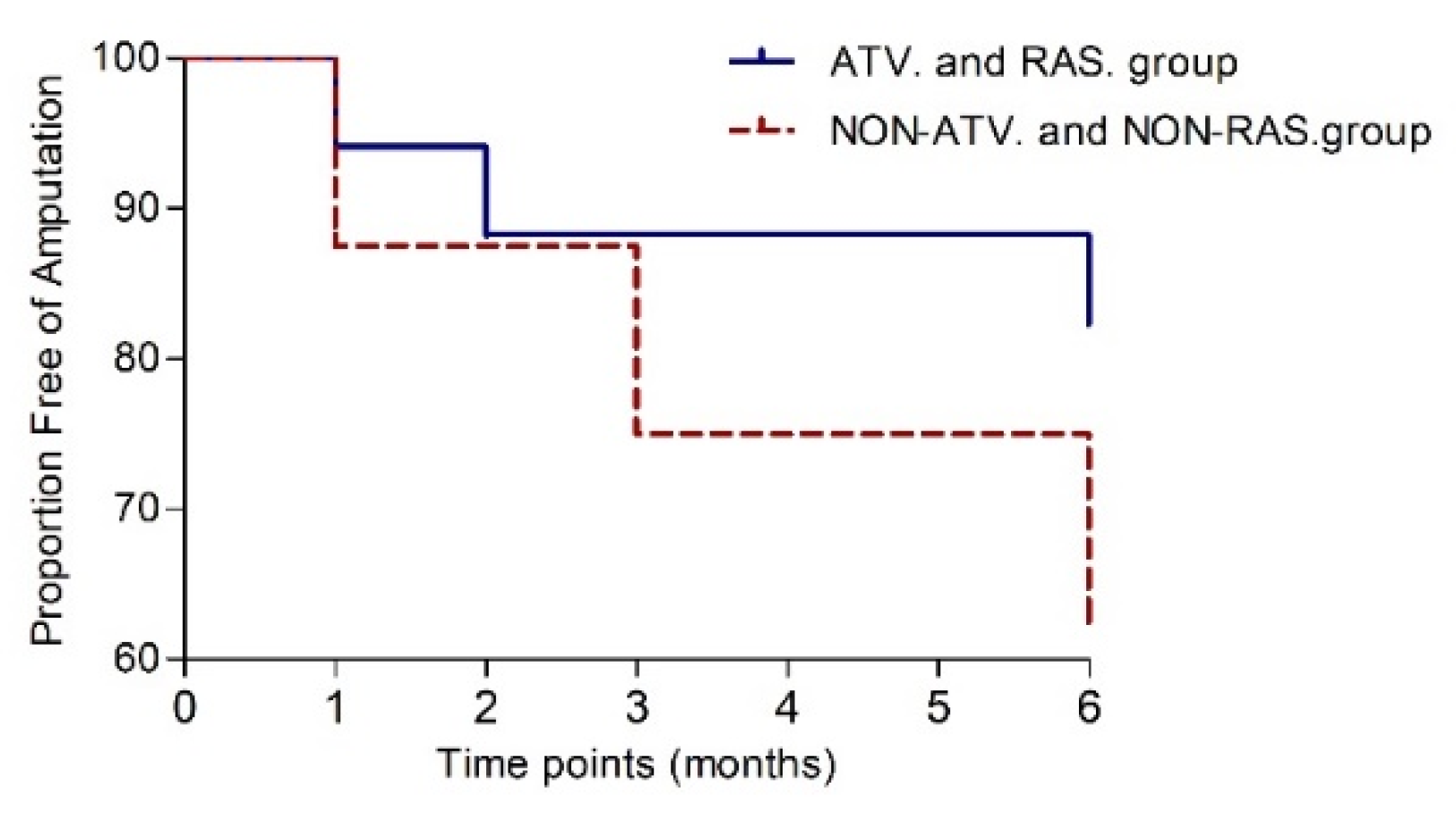
P=0.279). Amputation-free survival was achieved in 13 patients (76%) in the ATV and RAS group, while in NON-ATV and NON-RAS group was achieved in 5 patients (62.5%,
P=0.344). The risk of major amputation was decreased in those prescribed atorvastatin and RAS-acting agents (HR 0.39, 95% CI 0.07-2.3,
P=0.36) but did not differ significantly (
Figure 6).
Characteristics of Transplanted Bone Marrow Cells in ATV and RAS Group or NON-AT. and NON-RAS Group
Regarding the transplants, the transplanted BM-MNCs count appeared to be higher in the ATV and RAS group (3.56 ± 1.60 x 10
9) than in the NON-ATV and NON-RAS. group (2.60 ± 0.81 x 10
9). However, the difference was not statistically significant (
P=0.064) (
Table 14).
Results of Spearman Correlation Analysis for the Investigated Treatment Option
The Spearman correlation between investigated treatment options before BMCs transplantation and baseline characteristics is shown in
Table 15. Patients with CLI regression, patients considered as responders, showed a significant positive correlation with age (R=0.430,
P=0.012) and RAS treatment (R=0.351,
P=0.045). Relevantly, it was negatively correlated with CRP (R=-0.442,
P=0.011). Statin treatment before BMCs administration correlated with RAS treatment (R=0.482,
P=0.005). RAS-acting agents’ treatment was highly correlated with age of patients (R=0.566,
P=0.0006). Treatment with both investigated pharmacotherapies, statin and RAS treatment showed a significant positive correlation with CLI regression, responder (R=0.618,
P=0.001) and age of patients (R=0.454,
P=0.023).
Discussion
Summary of the Results
This study investigated the outcomes and possible predictive factors of autologous bone marrow cell transplantation in treating „no-option“ CLI patients. It was focused on exploring the clinical background and prior statin pharmacotherapy related to the therapeutic efficacy of BMCs treatment. Our results revealed that CRP and TcPO2 values positively correlated with the probability of being a responder. In contrast, CRP value, ABI, and BM-MNCs concentration were identified as prognostic factors of the super-responders. Based on the results of univariate logistic regression for responders, ROC analysis of CRP revealed a cut-off limit CRP > 8.1 mg/L before transplantation was predictive for negative clinical response after BMC transplantation. Linear regression analysis demonstrated a significant dependence between the levels of baseline CRP and the concentration of BM-MNCs in transplanted bone marrow.
Atorvastatin treatment prior to BMCs transplant revealed significant improvement of TcpO2 and reduction of pain scale after BMCs transplant compared to nonsignificant changes in the non-atorvastatin group. Our results showed that atorvastatin use was associated with decreased risk for major amputation. However, the difference was not statistically significant. Furthermore, our findings showed a significantly higher concentration of BM-MNCs in the transplanted bone marrow cells of patients in the atorvastatin group than in the non-atorvastatin group, which might contribute to the beneficial effect of cell therapy.
Patients treated with RAS-acting agents before BMCs transplant had significantly reduced pain scale after six months, compared to the NON-RAS group. Similar results, reduced pain scale as well as improved TcPO2, were achieved in a group of patients treated with atorvastatin and RAS-acting agents before BMCs treatment. Results of Spearman correlation showed a significant positive correlation between CLI regression, patients considered as responders, and previous treatment before BMCs transplant with RAS-acting agents alone or together with atorvastatin.
Prognostic Factors of the Therapeutic Responses to Autologous BMCs Treatment
As the terminal stage of peripheral arterial disease, CLI is characterised by an extremely high risk of amputation and other vascular issues, causing severe morbidity and mortality in affected patients. Therapeutic angiogenesis with cell-based therapies aims to increase blood flow to ischemic regions. One of the most encouraging cells used as an alternative for the surgical treatment of CLI is mesenchymal stem cells appearing in the bone marrow-derived mononuclear stem cells population [
19]. Although stem cell therapies are studied clinically for their benefit in the treatment of cardiac repair or patients with CLI, their effects are still controversial and considered experimental [
13,
20,
21]. The published data show that treatment with bone marrow stem cells is associated with limb salvage, increased TcPO
2, ABI or blood flow perfusion [
22]. The results of our study present the beneficial effects of autologous BMCs transplantation in TcPO
2, pain scoring and Rutherford category, but not ABI. ABI did not change after six months, similar to the results of other authors [
23,
24], but it was inconsistent with the report of Gupta et al. 2013 and Benoit et al. 2013 [
25,
26]. The prevalence of amputation-free survival at 82% was similar to the results of Zafarhandi et al. 2010, who also reported the effects of cell therapy after six months [
27].
TcPO
2 represents the tension of oxygen disseminated from subcutaneous microcirculation and can reflect the distal peripheral perfusion [
28]. TcPO
2 measurement is a metabolic test and is considered a helpful predictor for chronic ischemic ulcer healing, with a threshold value of 20-40 mmHg [
29]. On the contrary, the unfavourable course for spontaneous healing is less than 10 mmHg [
30,
31]. In our study, the evaluation of the baseline characteristics has shown that responders had higher baseline TcPO
2 than non-responders, but it was not statistically significant. The univariate regression analysis showed that the baseline TcPO
2 value was a prognostic factor for being a responder to BMCs treatment.
Moreover, according to the longitudinal data, we found significant improvement of TcPO
2 at six months post-transplantation in the responder group, while the non-responders did not. Moreover, the TcPO
2 at baseline correlated significantly with values at six months post-transplantation. Based on these data, we assume that baseline TcPO
2 represents an important criterion for the patients undergoing transplantation of bone marrow stem cells, consistent with several previous studies [
29,
32,
33]. These results suggest that the restoration of peripheral perfusion after BMCs therapy is characterised by a favourable baseline condition of local microcirculation, which might explain the predictive role of baseline TcPO
2.
Inflammatory markers such as C-reactive protein (CRP), fibrinogen and serum amyloid A are associated with an increased risk of cardiovascular events. They are considered critical risk factors for the development and progression of PAD [
34,
35]. Our study demonstrates that responders to the BMCs therapy are characterised by significantly lower baseline CRP levels than non-responders. The univariate regression analysis showed that the baseline CRP value was a prognostic factor for being a responder to BMC treatment. CRP belongs to acute-phase reactant protein and is produced during inflammation, which negatively modulates the local inflammatory reaction in the transplantation site and, thus, the entire process of therapeutic angiogenesis after BM-MNCs transplantation [
36]. Moreover, CRP influences the systemic inflammatory reaction and can negatively regulate bone marrow cells' characteristics. Indeed, we found that depressive numbers of BM-MNCs in the bone marrow transplant were associated with elevated levels of CRP in patients with critical limb ischaemia. We found that these two characteristics are significantly dependent upon each other. The receiver operating characteristics analysis showed a cut-off limit of baseline CRP > 8.1 mg/L before transplantation was predictive for negative clinical response after BMCs transplantation.
In addition to the patient’s clinical background, the characteristic of the bone marrow transplant has a critical role in the healing prognosis. An important predictor of the therapeutic response is the number of administrated nucleated cells, bone marrow-derived mononuclear cells, which strongly relate to clinical benefit in the PROVASA trial and are considered an independent predictor of improved ulcer healing [
12]. On the other hand, in the study of Yusoff et al. 2020, the authors found that the difference in the number of BM-MNCs derived from bone marrow did not alter the major amputation-free survival rate or mortality rate in atherosclerotic PAD patients with CLI [
37]. Our results demonstrate a significant difference in the concentration of BM-MNCs; however, in the subgroup analysis of super-responders, patients with limb salvage and complete ischemic wound healing, compared to super non-responders, patients with major limb amputation. Specifically, a significantly higher number of BM-MNCs was present in the BMCs transplant of the super-responder group compared to the super-non-responder group.
Moreover, the univariate regression analysis showed that the concentration of BM-MNCs was a prognostic factor for being a super-responder to BMCs treatment. Accordingly, we believe that the concentration of BM-MNCs in transplant might be feasible for NO-CLI patients. A longer-term follow-up study will be needed to compare the outcomes and advantages of used transplantation types.
Subgroup Analysis of Treatment Approach before BMCs Transplant
Many studies demonstrated the poor survival and retention of transplanted bone marrow cells
in vivo, caused by stem cells' properties, the extremely hostile microenvironment of implantation or a combination of both [
13]. For these reasons, the effort has been focused on improving the tolerance of transplanted stem cells to the microenvironment. This would lead to developing a clinical approach with enhanced stem cell survival and angiogenesis in the implantation site. Recent pre-clinical and clinical studies describe the use of statin drugs to augment the function of MSCs or endothelial progenitor cells (EPC) for regenerative cell-based therapy. Promising results have been reported in treating AMI in animal models but have yet to be written in human studies. Pre-clinical and clinical studies reported a beneficial effect of statin therapy that improves MSCs or EPC function and their numbers. However, statin use in enhancing cell-based vascular repair warrants further study [
38,
39,
40]. Systematic review and meta-analysis of nineteen studies with 26,985 patients with CLI showed that statin use was associated with decreased risk of major amputation, mortality, and more remarkable amputation-free survival and overall patency rates [
16].
In this study, we examined the efficacy of atorvastatin treatment before BMCs transplant on outcomes of cell-based therapy. Our data confirmed a significant improvement of TcPO2 and a reduction of pain scoring in the atorvastatin group, compared to a nonsignificant improvement in the non-atorvastatin group. Our results showed that statin use was associated with a decreased risk of major amputation. However, the difference was not statistically significant. Furthermore, our findings showed a significantly higher concentration of BM-MNCs in transplanted bone marrow cells in patients with prior atorvastatin treatment compared to the non-atorvastatin group. We suggest that these beneficial effects of atorvastatin contribute to the valuable effect of cell therapy in CLI patients and are related to the clinical benefit of stem cell treatment.
Moreover, we focused on the effects of RAS-acting agents’ treatment before BMCs transplant on the outcomes of cell-based therapy. RAS-acting agents (angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs)) should be considered as first-line therapy in patients with PAD and hypertension [
14]. Bodewes et al. 2018 revealed the long-term mortality benefit of RAS inhibitors in chronic limb-threatening ischaemia patients, whereas limb events were unaffected [
41]. Our study has demonstrated that patients treated with RAS-acting agents before BMCs transplant had significantly reduced pain scale after six months, compared to the NON-RAS group. Similar results, reduced pain scale and improved TcPO
2, were achieved in patients treated with atorvastatin and RAS-acting agents before BMCs treatment.
Results of the Spearman correlation showed a significant positive correlation between CLI regression, responders, and previous treatment before BMCs transplant with RAS-acting agents alone or with atorvastatin.
4. Limitations
There were several limitations to this study. First, no parallel placebo control group is present due to ethical concerns. Additionally, the sample size was insufficient to evaluate more specific variables. Moreover, our study focused on short-term responders (6 months) to BMCs therapy rather than long-term remission from CLI, which is also the target of therapeutic angiogenesis. Therefore, a prolonged cohort follow-up with larger numbers of patients is needed in the future.
Conclusion
Our data confirm the potential of detailed therapeutic management of patients with CLI before BMCs transplantation, which can increase the benefits of cell therapy. Our results revealed that CRP and TcPO2 values positively correlated with the probability of being a responder. In contrast, CRP value, ABI and BM-MNCs concentration were identified as prognostic factors of the super-responders. A baseline CRP level > 8.1 mg/L predicted a negative clinical response after BMCs transplantation.
In agreement with the recommended guidelines for treating patients with CLI, including cholesterol-lowering agents statins, we found that long-term statin treatment improved outcomes after BMCs therapy. Atorvastatin treatment was associated with significant improvement of TcpO2 and reduction of pain scale after BMCs transplant compared to nonsignificant changes in the non-atorvastatin group. Moreover, it significantly increased the number of BM-MNCs in BMCs transplant, contributing to cell therapy's beneficial effect. Furthermore, treatment of CLI patients with RAS-acting agents significantly reduced pain scale after BMCs transplant, compared to nonsignificant changes in the NON-RAS group. Treatment with atorvastatin and RAS-acting agents was associated with reduced pain scale and improved TcPO2 values in CLI patients after the procedure. Results of the Spearman correlation revealed a significant positive correlation between such therapies and CLI regression.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Ministry of the Health of the Slovak Republic 2016/13-FAUK-1.
Availability of data and materials
The data that support the findings of this study are available upon request.
Ethics approval and consent to participate
The local ethical committee of the National Institute of Cardiovascular Diseases, Bratislava, approved the study design. This study was carried out by the Code of Ethics of the World Medical Association, Declaration of Helsinki (WMA Declaration of Helsinki, 2013).
Patient consent for publication
All patients in the study were informed about the nature of the study and gave their written informed consent.
Acknowledgements
Not applicable
Conflicts of Interest
The authors declare no conflict of interest.
Competing interest statement
All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare no support from any organisation for the submitted work.
Transparency declaration
The manuscript is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted, and any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Abbreviations
| ABI |
ankle-brachial index |
| ACEIs |
angiotensin-converting enzyme inhibitors |
| ARB's |
angiotensin receptor blockers |
| ATV |
atorvastatin |
| AUC |
area under the ROC curve |
| BMCs |
bone marrow cells |
| BM-MNCs |
bone marrow-derived mononuclear cells |
| CI |
confidence interval |
| CLI |
critical limb ischaemia |
| CRP |
C-reactive protein |
| EPC |
endothelial progenitor cells |
| HR |
hazard ratio |
| MI |
myocardial infarct |
| MSCs |
mesenchymal stem cells |
| NO-CLI |
“no-option” critical limb ischaemia |
| OR |
odds ration |
| PAD |
peripheral arterial disease |
| RAS |
renin-angiotensin system |
| ROC |
receiver operating characteristics |
| SD |
standard deviation |
| SEM |
standard error |
| TASC |
Transatlantic Inter-Society Consensus |
| TcPO2
|
transcutaneous oxygen pressure |
| VAS |
Visual analogue scale |
References
- Dalla Paola, L.; Cimaglia, P.; Carone, A.; Scavone, G.; Boscarino, G.; Bernucci, D.; et al. Limb salvage in diabetic patients with no-option critical limb ischaemia: outcomes of a specialised centre experience. Diabet Foot Ankle 2019, 10, 1696012. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007, 45, S5–67. [Google Scholar] [CrossRef] [PubMed]
- Abdul Wahid, S.F.; Ismail, N.A.; Wan Jamaludin, W.F.; Muhamad, N.A.; Abdul Hamid, M.K.A.; Harunarashid, H.; et al. Autologous cells derived from different sources and administered using different regimens for “no-option” critical lower limb ischaemia patients. Cochrane Database Syst Rev 2018. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, M.; Khabbazi, S.; Peymanfar, Y.; Hassanshahi, A.; Hosseini-Khah, Z.; Su, Y.; et al. Critical limb ischaemia: Current and novel therapeutic strategies. J Cell Physiol 2019, 234, 14445–59. [Google Scholar] [CrossRef]
- Uccioli, L.; Meloni, M.; Izzo, V.; Giurato, L.; Merolla, S.; Gandini, R. Critical limb ischaemia: current challenges and prospects. Vasc Health Risk Manag 2018, 14, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Jiang, X.; Fang, Y.; Pan, T.; Liu, H.; Ren, B.; et al. Autologous peripheral blood-derived stem cells transplantation for treatment of no-option angiitis-induced critical limb ischaemia: 10-year management experience. Stem Cell Res Ther 2020, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Jiang, Y.; Deng, W.; Zhang, Y.; Liang, Z.; Wu, Q.; et al. Long-Term Outcomes of BMMSC Compared with BMMNC for Treatment of Critical Limb Ischaemia and Foot Ulcer in Patients with Diabetes. Cell Transplant 2019, 28, 645–52. [Google Scholar] [CrossRef]
- Procházka, V.; Gumulec, J.; Jalůvka, F.; Šalounová, D.; Jonszta, T.; Czerný, D.; et al. Cell Therapy, a New Standard in Management of Chronic Critical Limb Ischaemia and Foot Ulcer. Cell Transplant 2010, 19, 1413–24. [Google Scholar] [CrossRef]
- Szabó, G.V.; Kövesd, Z.; Cserepes, J.; Daróczy, J.; Belkin, M.; Acsády, G. Peripheral blood-derived autologous stem cell therapy for the treatment of patients with late-stage peripheral artery disease—results of the short- and long-term follow-up. Cytotherapy 2013, 15, 1245–52. [Google Scholar] [CrossRef]
- Ozturk, A.; Kucukardali, Y.; Tangi, F.; Erikci, A.; Uzun, G.; Bashekim, C.; et al. The therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischaemia. J Diabetes Complications 2012, 26, 29–33. [Google Scholar] [CrossRef]
- Teraa, M.; Sprengers, R.W.; Schutgens, R.E.G.; Slaper-Cortenbach, I.C.M.; van der Graaf, Y.; Algra, A.; et al. Effect of Repetitive Intra-Arterial Infusion of Bone Marrow Mononuclear Cells in Patients With No-Option Limb Ischaemia: The Randomized, Double-Blind, Placebo-Controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) Trial. Circulation 2015, 131, 851–60. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.H.; Krankenberg, H.; Balzer, J.O.; Kalka, C.; Baumgartner, I.; Schlüter, M.; et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischaemia: a randomized-start, placebo-controlled pilot trial (PROVASA). Circ Cardiovasc Interv 2011, 4, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Xu, Q.; Luo, R.; Gao, J.; Chen, H.; Deng, Y.; et al. Atorvastatin treatment improves effects of implanted mesenchymal stem cells: meta-analysis of animal models with acute myocardial infarction. BMC Cardiovasc Disord 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J 2017. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Bhatt, D.L.; Pradhan, A.D.; Glynn, R.J.; MacFadyen, J.G.; Nissen, S.E. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials, The Lancet 2023, 401, 1293–1301. ISSN 2023, 0140–6736. [Google Scholar] [CrossRef]
- Kokkinidis, D.G.; Arfaras-Melainis, A.; Giannopoulos, S.; Katsaros, I.; Jawaid, O.; Jonnalagadda, A.K.; et al. Statin therapy for reduction of cardiovascular and limb-related events in critical limb ischaemia: A systematic review and meta-analysis. Vasc Med 2020, 25, 106–17. [Google Scholar] [CrossRef] [PubMed]
- Samakova, A.; Gazova, A.; Sabova, N.; Valaskova, S.; Jurikova, M.; Kyselovic, J. The pi3k/Akt Pathway Is Associated With Angiogenesis, Oxidative Stress and Survival of Mesenchymal Stem Cells in Pathophysiologic Condition in Ischaemia 2019, 68, 8. 68.
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; et al. Recommended standards for reports dealing with lower extremity ischaemia: Revised version. J Vasc Surg 1997, 26, 517–38. [Google Scholar] [CrossRef]
- Shirbaghaee, Z.; Hassani, M.; Heidari Keshel, S.; Soleimani, M. Emerging roles of mesenchymal stem cell therapy in patients with critical limb ischaemia. Stem Cell Res Ther 2022, 13, 462. [Google Scholar] [CrossRef]
- Peeters Weem, S.M.O.; Teraa, M.; de Borst, G.J.; Verhaar, M.C.; Moll, F.L. Bone Marrow-derived Cell Therapy in Critical Limb Ischaemia: A Meta-analysis of Randomized Placebo Controlled Trials. Eur J Vasc Endovasc Surg 2015, 50, 775–83. [Google Scholar] [CrossRef]
- Jaluvka, F.; Ihnat, P.; Madaric, J.; Vrtkova, A.; Janosek, J.; Prochazka, V. Current Status of Cell-Based Therapy in Patients with Critical Limb Ischaemia. Int J Mol Sci 2020, 21, 8999. [Google Scholar] [CrossRef]
- Compagna, R.; Amato, B.; Massa, S.; Amato, M.; Grande, R.; Butrico, L.; et al. Cell Therapy in Patients with Critical Limb Ischaemia. Stem Cells Int 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Klepanec, A.; Mistrik, M.; Altaner, C.; Valachovicova, M.; Olejarova, I.; Slysko, R.; et al. No Difference in Intra-Arterial and Intramuscular Delivery of Autologous Bone Marrow Cells in Patients with Advanced Critical Limb Ischaemia. Cell Transplant 2012, 21, 1909–18. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Tatsumi, T.; Murohara, T.; Imaizumi, T.; Katsuda, Y.; Ito, M.; et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischaemia. Am Heart J 2008, 156, 1010–8. [Google Scholar] [CrossRef] [PubMed]
- Benoit, E.; O’donnell, T.F.; Patel, A.N. Safety and Efficacy of Autologous Cell Therapy in Critical Limb Ischaemia: A Systematic Review. Cell Transplant 2013, 22, 545–62. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chullikana, A.; Parakh, R.; Desai, S.; Das, A.; Gottipamula, S.; et al. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow-derived mesenchymal stem cell in critical limb ischaemia 2013, 11.
- Zafarghandi, M.R.; Ravari, H.; Aghdami, N.; Namiri, M.; Moazzami, K.; Taghiabadi, E.; et al. Safety and efficacy of granulocyte–colony-stimulating factor administration following autologous intramuscular implantation of bone marrow mononuclear cells: a randomized controlled trial in patients with advanced lower limb ischaemia. Cytotherapy 2010, 12, 783–91. [Google Scholar] [CrossRef] [PubMed]
- Leenstra, B.; Wijnand, J.; Verhoeven, B.; Koning, O.; Teraa, M.; Verhaar, M.C.; et al. Applicability of Transcutaneous Oxygen Tension Measurement in the Assessment of Chronic Limb-Threatening Ischaemia. Angiology 2020, 71, 208–16. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Liu, H.; Fang, Y.; Wei, Z.; Gu, S.; Fang, G.; et al. Predictors of responders to mononuclear stem cell-based therapeutic angiogenesis for no-option critical limb ischaemia. Stem Cell Res Ther 2019, 10, 15. [Google Scholar] [CrossRef]
- Got, I. [Transcutaneous oxygen pressure (TcPO2): advantages and limitations]. Diabetes Metab 1998, 24, 379–84. [Google Scholar]
- Yang, C.; Weng, H.; Chen, L.; Yang, H.; Luo, G.; Mai, L.; et al. Transcutaneous oxygen pressure measurement in diabetic foot ulcers: mean values and cut-point for wound healing. J Wound Ostomy Cont Nurs Off Publ Wound Ostomy Cont Nurses Soc 2013, 40, 585–9. [Google Scholar] [CrossRef]
- Madaric, J.; Klepanec, A.; Valachovicova, M.; Mistrik, M.; Bucova, M.; Olejarova, I.; et al. Characteristics of responders to autologous bone marrow cell therapy for no-option critical limb ischaemia. Stem Cell Res Ther 2016, 7. [Google Scholar] [CrossRef]
- Aoyama, N.; Nishinari, M.; Ohtani, S.; Kanai, A.; Noda, C.; Hirata, M.; et al. Clinical features and predictors of patients with critical limb ischaemia who responded to autologous mononuclear cell transplantation for therapeutic angiogenesis. Heart Vessels 2017, 32, 1099–108. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, S.; Annex, B.H. Biomarkers and Genetics in Peripheral Artery Disease. Clin Chem 2017, 63, 236–44. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Ridker, P.M.; Belkin, M.; Hamdan, A.D.; Pomposelli, F.; Logerfo, F.; et al. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg 2007, 45, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kuliszewski, M.A.; Li, S.-H.; Szmitko, P.E.; Zucco, L.; Wang, C.-H.; et al. C-Reactive Protein Attenuates Endothelial Progenitor Cell Survival, Differentiation, and Function: Further Evidence of a Mechanistic Link Between C-Reactive Protein and Cardiovascular Disease. Circulation 2004, 109, 2058–67. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, F.M.; Kajikawa, M.; Takaeko, Y.; Kishimoto, S.; Hashimoto, H.; Maruhashi, T.; et al. Relationship between cell number and clinical outcomes of autologous bone-marrow mononuclear cell implantation in critical limb ischaemia. Sci Rep 2020, 10, 19891. [Google Scholar] [CrossRef]
- Park, A.; Barrera-Ramirez, J.; Ranasinghe, I.; Pilon, S.; Sy, R.; Fergusson, D.; et al. Use of Statins to Augment Progenitor Cell Function in Pre-clinical and Clinical Studies of Regenerative Therapy: a Systematic Review. Stem Cell Rev Rep 2016, 12, 327–39. [Google Scholar] [CrossRef]
- Jia, W.; Zhao, Y.; Yang, J.; Wang, W.; Wang, X.; Ling, L.; et al. Simvastatin Promotes Dental Pulp Stem Cell–induced Coronal Pulp Regeneration in Pulpotomized Teeth. J Endod 2016, 42, 1049–54. [Google Scholar] [CrossRef]
- Tahamtan, S.; Shirban, F.; Bagherniya, M.; Johnston, T.P.; Sahebkar, A. The effects of statins on dental and oral health: a review of pre-clinical and clinical studies. J Transl Med 2020, 18, 155. [Google Scholar] [CrossRef]
- Bodewes, T.C.F.; Darling, J.D.; O’Donnell, T.F.X.; Deery, S.E.; Shean, K.E.; Mittleman, M.A.; et al. Long-term mortality benefit of renin-angiotensin system inhibitors in patients with chronic limb-threatening ischaemia undergoing vascular intervention. J Vasc Surg 2018, 67, 800–808. [Google Scholar] [CrossRef]
Figure 2.
Comparison of longitudinal TcPO2 changes (mean with SEM) in the ischemic limbs of the responders and non-responders (a) and linear regression between TcPO2 at baseline and at six months post-transplantation, depicted with a solid fitting line, dotted 95% confidential interval bars and lined 95% prediction intervals (b). *The difference between 6 months and baseline in responders was statistically significant (P=0.008). TcP02, transcutaneous oxygen pressure.
Figure 2.
Comparison of longitudinal TcPO2 changes (mean with SEM) in the ischemic limbs of the responders and non-responders (a) and linear regression between TcPO2 at baseline and at six months post-transplantation, depicted with a solid fitting line, dotted 95% confidential interval bars and lined 95% prediction intervals (b). *The difference between 6 months and baseline in responders was statistically significant (P=0.008). TcP02, transcutaneous oxygen pressure.
Figure 3.
Receiver operating characteristics of CRP levels for predicting the BMCs therapeutic response. Area under the receiver operating characteristic (ROC) curve: CRP=0.768 (CI 0.572-0.96, P=0.014) (a) and linear regression between CRP and BM-MNCs, with lined 95% confidential interval bars and lined 95% prediction intervals (b). CRP, C-reaction protein; BMCs, bone marrow cells; BM-MNCs, bone marrow-derived mononuclear cells.
Figure 3.
Receiver operating characteristics of CRP levels for predicting the BMCs therapeutic response. Area under the receiver operating characteristic (ROC) curve: CRP=0.768 (CI 0.572-0.96, P=0.014) (a) and linear regression between CRP and BM-MNCs, with lined 95% confidential interval bars and lined 95% prediction intervals (b). CRP, C-reaction protein; BMCs, bone marrow cells; BM-MNCs, bone marrow-derived mononuclear cells.
Figure 4.
Comparison of longitudinal TcPO2 changes (mean with SEM) in the ischemic limbs of patients treated with or without atorvastatin prior BMCs treatment (a) and comparison of longitudinal pain scale changes (mean with SEM) in the ischemic limbs of patients treated with or without atorvastatin prior BMCs treatment (b) and Kaplan-Meier curve to 6 months post-transplantation showing the proportion free of amputation (c). TcP02, transcutaneous oxygen pressure.
Figure 4.
Comparison of longitudinal TcPO2 changes (mean with SEM) in the ischemic limbs of patients treated with or without atorvastatin prior BMCs treatment (a) and comparison of longitudinal pain scale changes (mean with SEM) in the ischemic limbs of patients treated with or without atorvastatin prior BMCs treatment (b) and Kaplan-Meier curve to 6 months post-transplantation showing the proportion free of amputation (c). TcP02, transcutaneous oxygen pressure.
Figure 5.
Kaplan-Meier curve to 6 months post-transplantation showing the proportion free of amputation.
Figure 5.
Kaplan-Meier curve to 6 months post-transplantation showing the proportion free of amputation.
Figure 6.
Kaplan-Meier curve to 6 months post-transplantation showing the proportion free of amputation.
Figure 6.
Kaplan-Meier curve to 6 months post-transplantation showing the proportion free of amputation.
Table 1.
Characteristics of patients before stem cell treatment.
Table 1.
Characteristics of patients before stem cell treatment.
| |
All patients (n=33) |
Responders (n=22) |
Non-responders (n=11) |
P value |
| Age (years) |
64.9 ± 10 |
67.4 ± 9.3 |
59.8 ± 10.7 |
0.014 |
| Sex (males) |
31 (94%) |
21 (95%) |
10 (91%) |
1.000 |
| Rutherford class (1-6) |
5.0 ± 0.30 |
5.0 |
4.9 ± 0.54 |
0.208 |
| Body mass index (kg/m2) |
27 ± 4.1 |
27.2 ± 3.8 |
27.6 ± 4.9 |
0.756 |
| |
Risk factors of limb ischaemia |
| Diabetes mellitus |
11 (33%) |
7 (32%) |
4 (36%) |
1.000 |
| Arterial hypertension |
27 (82%) |
18 (82%) |
9 (82%) |
1.000 |
| Hyperlipidaemia |
23 (70%) |
15 (68%) |
8 (73%) |
1.000 |
| Smoker |
10 (30%) |
6 (27%) |
4 (36%) |
0.696 |
| |
Blood examination |
| CRP (mg/L) |
13.3 ± 21.3 |
8.1 ± 12.3 |
23.3 ± 30.5 |
0.013 |
| Creatinine (µmol/L) |
95.9 ± 34.6 |
95.4 ± 35.7 |
96.7 ± 34.2 |
0.946 |
| |
Treatment history |
| Statins |
22 (67%) |
16 (73%) (NNT 5.5) |
6 (55%) |
0.255 |
| Antiplatelet drugs |
24 (73%) |
17 (77%) |
7 (64%) |
0.438 |
| RAS-acting agents |
20 (61%) |
16 (73%) (NNT 2.75) |
4 (36%) |
0.065 |
| Statins and RAS-acting agents |
17 (52%) |
13 (59%) (NNT4.4) |
4 (36%) |
0.282 |
| Naftidrofuryl |
28 (85%) |
18 (82%) |
10 (91%) |
0.643 |
| Post-MI |
31 (94%) |
22 (100%) |
9 (82%) |
0.104 |
| |
Parameters of limb ischaemia |
| TcPO2<10 mmHg |
19 (58%) |
11 (50%) |
8 (73%) |
0.278 |
| ABI |
0.51 ± 0.38 |
0.57 ± 0.35 |
0.37 ± 0.43 |
0.183 |
| Pain scale (0-10) |
5.75 ± 1.6 |
5.62 ± 1.75 |
6.14 ± 1.07 |
0.366 |
Table 2.
Outcomes of BMCs treatment after six months in responders and non-responders.
Table 2.
Outcomes of BMCs treatment after six months in responders and non-responders.
| Parameters of limb ischaemia |
responders |
non-responders |
| |
Baseline (n=22) |
Six months (n=17) |
P value |
Baseline (n=11) |
Six months (n=11) |
P value |
| Rutherford class (1-6) |
5.0 ± 0.30 |
4.2 ± 1.0 |
0.008 |
4.9 ± 0.54 |
5.55 ± 0.52 |
0.059 |
| TcPO2 (mmHg) |
12.5 ± 14.0 |
22.7 ± 15.2 |
0.008 |
2.4 ± 1.77 |
3.18 ± 8.4 |
0.573 |
| ABI |
0.57 ± 0.35 |
0.60 ± 0.34 |
0.480 |
0.37 ± 0.43 |
0.029 ± 0.08 |
0.043 |
| Pain scale (0-10) |
5.62 ± 1.75 |
1.78 ± 1.22 |
<0.001 |
6.14 ± 1.07 |
6.86 ± 3.76 |
0.445 |
Table 3.
Characteristics of super responders and super non-responders before stem cell treatment.
Table 3.
Characteristics of super responders and super non-responders before stem cell treatment.
| |
All patients
(n=33) |
super-responders
(n=13) |
super-non-responders (n=6) |
P value |
| Age (years) (mean ± SD) |
64.9 ± 10 |
64.8 ± 10.4 |
66.8 ± 4.0 |
0.690 |
| Sex (males) |
31 (94%) |
12 (92%) |
6 (100%) |
1.000 |
| Rutherford class (1-6) |
5.0 ± 0.30 |
5.0 |
4.83 ± 0.75 |
0.175 |
| Body mass index (kg/m2) |
27 ± 4.1 |
27.1 ± 4.04 |
25.6 ± 3.76 |
0.450 |
| |
Risk factors of limb ischaemia |
| Diabetes mellitus (n, %) |
11 (33%) |
5 (38%) |
3 (50%) |
1.000 |
| Arterial hypertension |
27 (82%) |
11 (85%) |
6 (100%) |
1.000 |
| Hyperlipidemia |
23 (70%) |
10 (77%) |
5 (83%) |
1.000 |
| Smoker |
10 (30%) |
3 (23%) |
2 (33%) |
1.000 |
| |
Blood examination |
| CRP (mg/L) |
13.3 ± 21.3 |
5.2 ± 3.45 |
36.6 ± 37.01 |
<0.001 |
| Creatinine (µmol/l) |
95.9 ± 34.6 |
98.7 ± 41.02 |
102.5 ± 39.78 |
0.914 |
| |
Treatment history |
| Statins (n, %) |
22 (67%) |
11 (85%) |
3 (50%) |
0.262 |
| Antiplatelet drugs |
24 (72%) |
12 (92%) |
3 (50%) |
0.071 |
| RAS-acting agents |
20 (61%) |
9 (69%) |
3 (50%) |
0.617 |
| Statins and RAS-acting agents |
17 (52%) |
9 (69%) |
2 (33%) |
0.319 |
| Naftidrofuryl |
28 (85%) |
12 (92%) |
6 (100%) |
1.000 |
| Post-MI |
31 (94%) |
12 (92%) |
5 (83%) |
1.000 |
| |
Parameters of limb ischaemia |
| TcPO2<10 mmHg |
19 (58%) |
6 (46%) |
4 (67%) |
0.629 |
| ABI |
0.51 ± 0.38 |
0.57 ± 0.37 |
0.18 ±0.36 |
0.101 |
| Pain scale (0-10) |
5.75 ± 1.6 |
5.61 ± 1.71 |
6.33 ± 0.58 |
0.907 |
Table 4.
Characteristics of transplanted BMCs.
Table 4.
Characteristics of transplanted BMCs.
| Group analysis |
responders (n=22) |
non-responders (n=11) |
P value |
| BM-MNCs (109 cells/ml) |
3.53 ± 1.5 |
2.86 ± 1.13 |
0.213 |
| Viability of BM-MNCs (%) |
99.70 ± 0.3 |
99.27 ± 0.86 |
0.464 |
| MSCs (104 cells/ml) |
0.79 ± 0.61 |
1.46 ± 1.56 |
0.123 |
| Subgroup analysis |
super-responders (limb salvage and complete ischemic wound healing, n=13) |
super-non-responders (major limb amputation, n=6) |
P value |
| BM-MNCs (109 cells/ml) |
3.68 ± 1.51 |
2.26 ± 0.74 |
0.049 |
| Viability of BM-MNCs (%) |
99.71 ± 0.32 |
99.05 ± 0.94 |
0.263 |
| MSCs (104 cells/ml) |
0.83 ± 0.52 |
1.68 ± 2.14 |
0.639 |
Table 5.
Univariate logistic regression analysis of prognostic factors.
Table 5.
Univariate logistic regression analysis of prognostic factors.
| Candidate variable |
responder |
|
super-responder |
| |
OR (95% CI) |
P value |
|
OR (95% CI) |
P value |
| Age ≥ 50 years |
1.097 (0.993-1.212) |
0.056 |
|
0.972 (0.863-1.095) |
0.633 |
| Rutherford class |
2.803 (0.221-35.552) |
0.411 |
|
3.026 (0.222-41.289) |
0.389 |
| Body mass index (kg/m2) |
0.977 (0.819-1.166) |
0.800 |
|
1.118 (0.847-1.476) |
0.412 |
| Smoker |
0.700 (0.148-3.301) |
0.653 |
|
0.667 (0.078-5.678) |
0.712 |
| CRP (mg/L) |
0.955 (0.901-1.012) |
0.044 |
|
0.544 (0.221-1.341) |
<0.0001 |
| Creatinine (µmol/L) |
0.999 (0.978-1.020) |
0.919 |
|
0.997 (0.973-1.023) |
0.843 |
| TcPO2 (mmHg) |
1.149 (0.949-1.392) |
0.021 |
|
1.119 (0.945-1.325) |
0.071 |
| ABI |
5.084 (0.346-74.61) |
0.199 |
|
56.140 (0.531-5932.9) |
0.035 |
| BM-MNCs (109 cells/ml) |
1.560 (0.896-3.138) |
0.151 |
|
1.0 (0.999-1.0) |
0.035 |
Table 6.
Characteristics of patients before stem cell treatment.
Table 6.
Characteristics of patients before stem cell treatment.
| |
All patients (n=33) |
ATV group (n=22) |
NON-ATV group (n=11) |
P value |
| Age (years) (mean ± SD) |
64.9 ± 10 |
64.3 ± 11 |
65.9 ± 9.2 |
0.983 |
| Sex (males) |
31 (94%) |
21 (95%) |
10 (91%) |
1.000 |
| Rutherford class (1-6) |
5 ± 0.30 |
5 ± 0.21 |
5 ± 0.45 |
0.829 |
| Body mass index (kg/m2) |
27 ± 4.1 |
27.5 ± 3.8 |
26.9 ± 4.9 |
0.080 |
| |
Risk factors of limb ischaemia |
| Diabetes mellitus (n, %) |
11 (33%) |
8 (22%) |
3 (27%) |
0.709 |
| Arterial hypertension |
27 (82%) |
19 (86%) |
8 (73%) |
0.375 |
| Hyperlipidemia |
23 (70%) |
17 (77%) |
6 (55%) |
0.240 |
| Smoker |
10 (30%) |
5 (23%) |
5 (45%) |
0.240 |
| |
Blood examination |
| CRP (mg/L) |
13.3 ± 21.3 |
12.8 ± 24.6 |
14.2 ± 14 |
0.341 |
| Creatinine (µmol/L) |
95.9 ± 34.6 |
97 ± 37.6 |
94 ± 29.7 |
0.977 |
| |
Parameters of limb ischaemia |
| TcPO2<10 mmHg |
19 (58%) |
11 (50%) |
8 (73%) |
0.278 |
| ABI |
0.51 ± 0.38 |
0.51 ± 0.36 |
0.52 ± 0.47 |
0.951 |
| Pain scale (0-10) |
5.75 ± 1.6 |
5.74 ± 1.52 |
5.78 ± 1.86 |
0.951 |
Table 7.
Outcomes of BMCs treatment after 3 and 6 months in all patients with or without atorvastatin treatment prior to BMCs transplant.
Table 7.
Outcomes of BMCs treatment after 3 and 6 months in all patients with or without atorvastatin treatment prior to BMCs transplant.
| |
ATV group (n=22) |
NON-ATV group (n=11) |
| |
Before BMCs |
Three months |
Six months |
P value |
Before BMCs |
Three months |
Six months |
P value |
| Rutherford category |
4.95 ± 0.21 |
4.95 ± 0.60 |
4.55 ± 1.1 |
0.092 |
5.0 ± 0.45 |
5.0 ± 0.78 |
5.0 ± 0.93 |
0.655 |
| TcPO2 (mmHg) |
10.72 ± 13.01 |
14.4 ± 19.9 |
17.3 ± 16.5 |
0.015 |
7.5 ± 12.46 |
10.7 ± 14.9 |
10.2 ± 14.7 |
0.611 |
| ABI |
0.51 ± 0.36 |
0.44 ± 0.41 |
0.42 ± 0.34 |
0.532 |
0.52 ± 0.47 |
0.47 ± 0.47 |
0.38 ± 0.51 |
0.655 |
Pain scale
(0-10) |
5.74 ± 1.52 |
4.45 ± 2.7 |
3.6 ± 3.35 |
0.004 |
5.78 ± 1.86 |
5.2 ± 3.42 |
4.8 ± 4.07 |
0.202 |
Table 8.
Characteristics of transplanted BMCs.
Table 8.
Characteristics of transplanted BMCs.
| |
ATV group (n=22) |
NON-ATV group (n=11) |
P value |
| BM-MNCs (109 cells/ml) |
3.64 ± 1.53 |
2.58 ± 0.73 |
0.038 |
| Viability of BM-MNCs (%) |
99.5 ± 0.57 |
99.6 ± 0.67 |
0.537 |
| MSCs (104 cells/ml) |
0.79 ± 0.52 |
1.47 ± 1.62 |
0.281 |
Table 9.
Characteristics of patients before stem cell treatment.
Table 9.
Characteristics of patients before stem cell treatment.
| |
All patients (n=33) |
RAS group (n=20) |
NON-RAS group (n=13) |
P value |
| Age (years) (mean ± SD) |
64.9 ± 10 |
68.65 ± 8.82 |
59.0 ± 9.80 |
0.006 |
| Sex (males) |
31 (94%) |
19 (95%) |
12 (92%) |
1.000 |
| Rutherford class (1-6) |
5 ± 0.30 |
4.95 ± 0.22 |
5.0 ± 0.41 |
0.652 |
| Body mass index (kg/m2) |
27 ± 4.1 |
26.56 ± 2.85 |
28.47 ± 5.57 |
0.385 |
| |
Risk factors of limb ischaemia |
| Diabetes mellitus (n, %) |
11 (33%) |
8 (40%) |
3 (23%) |
0.277 |
| Arterial hypertension |
27 (82%) |
19 (95%) |
8 (62%) |
0.025 |
| Hyperlipidemia |
23 (70%) |
14 (70%) |
9 (69%) |
1.000 |
| Smoker |
10 (30%) |
4 (21%) |
6 (46%) |
0.244 |
| |
Blood examination |
| CRP (mg/L) |
13.3 ± 21.3 |
16.09 ± 26.1 |
9.26 ± 10.77 |
0.367 |
| Creatinine (µmol/L) |
95.9 ± 34.6 |
100.1 ± 37.77 |
89.69 ± 29.8 |
0.258 |
| |
Parameters of limb ischaemia |
| TcPO2<10 mmHg |
19 (58%) |
10 (50%) |
9 (69%) |
0.310 |
| ABI |
0.51 ± 0.38 |
0.59 ± 0.37 |
0.38 ± 0.37 |
0.165 |
| Pain scale (0-10) |
5.75 ± 1.6 |
5.56 ± 1.42 |
6.10 ± 1.91 |
0.426 |
Table 10.
Outcomes of BMCs treatment after six months in all patients with or without RAS treatment prior to BMCs transplant.
Table 10.
Outcomes of BMCs treatment after six months in all patients with or without RAS treatment prior to BMCs transplant.
| Parameters of limb ischaemia |
RAS group |
NON-RAS group |
| |
Baseline (n=20) |
Six months (n=18) |
P value |
Baseline (n=13) |
Six months (n=10) |
P value |
| Rutherford class (1-6) |
4.95 ± 0.22 |
4.61 ± 0.41 |
0.164 |
5.0 ± 0.41 |
4.8 ± 1.03 |
0.276 |
| TcPO2 (mmHg) |
11.4 ± 13.5 |
15.5 ± 14.5 |
0.077 |
6.7 ± 11.4 |
14.3 ± 18.8 |
0.201 |
| ABI |
0.59 ± 0.37 |
0.53 ± 0.41 |
0.408 |
0.38 ± 0.37 |
0.38 ± 0.26 |
0.500 |
| Pain scale (0-10) |
5.56 ± 1.42 |
3.33 ± 3.25 |
0.005 |
6.10 ± 1.91 |
4.22 ± 3.83 |
0.139 |
Table 11.
Characteristics of transplanted BMCs.
Table 11.
Characteristics of transplanted BMCs.
| |
RAS group
(n=18) |
NON-RAS group
(n=12) |
P value |
| BM-MNCs (109 cells/ml) |
3.44 ± 1.55 |
3.05 ± 1.61 |
0.462 |
| Viability of BM-MNCs (%) |
99.6 ± 0.49 |
99.5 ± 0.75 |
0.859 |
| MSCs (104 cells/ml) |
0.86 ± 0.60 |
1.23 ± 1.50 |
0.730 |
Table 12.
Characteristics of patients before stem cell treatment.
Table 12.
Characteristics of patients before stem cell treatment.
| |
All patients
(n=33) |
ATV and RAS group
(n=17) |
NON-ATV and NON-RAS group (n=8) |
P value |
| Age (years) (mean ± SD) |
64.9 ± 10 |
67.41 ± 9 |
62.25 ± 7.94 |
0.026 |
| Sex (males) |
31 (94%) |
16 (94%) |
7 (88%) |
1.000 |
| Rutherford class (1-6) |
5 ± 0.30 |
4.94 ± 0.24 |
5 ± 0.54 |
0.817 |
| Body mass index (kg/m2) |
27 ± 4.1 |
26.37 ± 2.73 |
26.87 ± 5.50 |
0.763 |
| |
Risk factors of limb ischaemia |
| Diabetes mellitus (n, %) |
11 (33%) |
7 (41%) |
2 (25%) |
0.661 |
| Arterial hypertension |
27 (82%) |
16 (94%) |
5 (63%) |
0.081 |
| Hyperlipidemia |
23 (70%) |
13 (76%) |
5 (63%) |
0.640 |
| Smoker |
10 (30%) |
4 (24%) |
5 (63%) |
0.087 |
| |
Blood examination |
| CRP (mg/L) |
13.3 ± 21.3 |
16.06 ± 27.51 |
13.43 ± 12.05 |
0.653 |
| Creatinine (µmol/L) |
95.9 ± 34.6 |
100.7 ± 41.31 |
93.06 ± 35.29 |
0.425 |
| |
Parameters of limb ischaemia |
| TcPO2 < 10 mmHg |
9.6 ± 12.7 |
8 (47%) |
6 (75%) |
0.234 |
| ABI |
0.51 ± 0.38 |
0.54 ± 0.33 |
0.34 ± 0.32 |
0.540 |
| Pain scale (0-10) |
5.75 ± 1.6 |
5.80 ± 1.42 |
6.50 ± 1.87 |
0.063 |
Table 13.
Outcomes of BMCs treatment after six months in all patients with or without ATV-RAS treatment prior to BMCs transplant.
Table 13.
Outcomes of BMCs treatment after six months in all patients with or without ATV-RAS treatment prior to BMCs transplant.
| PARAMETERS OF LIMB ISCHAEMIA |
ATV and RAS group |
NON-ATV and NON-RAS group |
| |
Baseline (n=17) |
Six months (n=15) |
P value |
Baseline (n=8) |
Six months (n=7) |
P value |
| Rutherford class (1-6) |
4.94 ± 0.24 |
4.56 ± 1.15 |
0.164 |
5.0 ± 0.54 |
5.0 ± 1.10 |
0.655 |
| TcPO2 (mmHg) |
11.6 ± 13.5 |
16.5 ± 15.0 |
0.033 |
6.3 ± 11.8 |
10.9 ± 16.4 |
0.344 |
| ABI |
0.54 ± 0.33 |
0.47 ± 0.36 |
0.409 |
0.34 ± 0.32 |
0.18 ± 0.32 |
0.650 |
| Pain scale (0-10) |
5.80 ± 1.42 |
3.50 ± 3.43 |
0.009 |
6.50 ± 1.87 |
5.17 ± 4.45 |
0.279 |
Table 14.
Characteristics of transplanted BMCs.
Table 14.
Characteristics of transplanted BMCs.
| |
ATV and RAS group (n=17) |
NON-ATV and NON-RAS group (n=8) |
P value |
| BM-MNCs (109 cells/ml) |
3.56 ± 1.60 |
2.60 ± 0.81 |
0.064 |
| Viability of BM-MNCs (%) |
99.6 ± 0.51 |
99.6 ± 0.75 |
0.642 |
| MSCs (104 cells/ml) |
0.78 ± 0.56 |
1.53 ± 1.89 |
0.549 |
Table 15.
Results of Spearman correlation analysis (R) for the investigated treatment option and baseline characteristics of patients.
Table 15.
Results of Spearman correlation analysis (R) for the investigated treatment option and baseline characteristics of patients.
| |
|
Responder |
Age |
CRP |
RAS-acting agents’ treatment |
| Responder |
Spearman’s r |
- |
0.430 |
-0.442 |
0.351 |
| P value |
- |
0.012 |
0.011 |
0.045 |
| Statin treatment |
Spearman’s r |
- |
- |
- |
0.482 |
| P value |
- |
- |
- |
0.005 |
| RAS-acting agents’ treatment |
Spearman’s r |
0.351 |
0.566 |
- |
- |
| P value |
0.045 |
0.0006 |
- |
- |
| Statin and RAS. Treatment |
Spearman’s r |
0.618 |
0.454 |
- |
1 |
| A P value |
0.001 |
0.023 |
- |
<0.0001 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).