Submitted:
21 March 2024
Posted:
22 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Genetic Material
2.2. Generation of Crosses
2.3. Field Evaluation
2.4. Data Collection
2.5. Data Analysis
3. Results
3.1. Analysis of Variance for Grain Yield and Other Traits under Managed Drought and Well-Watered Conditions
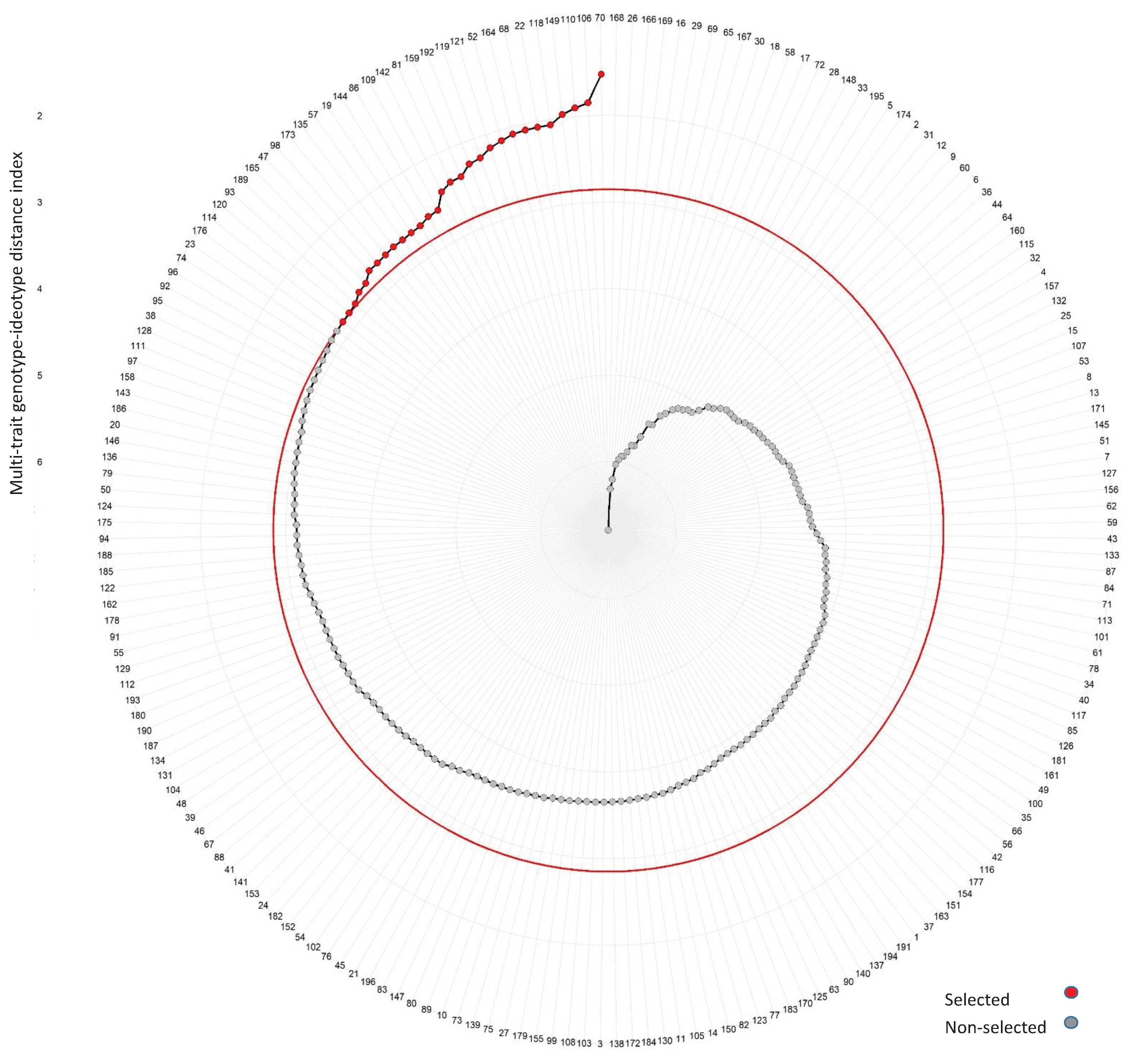
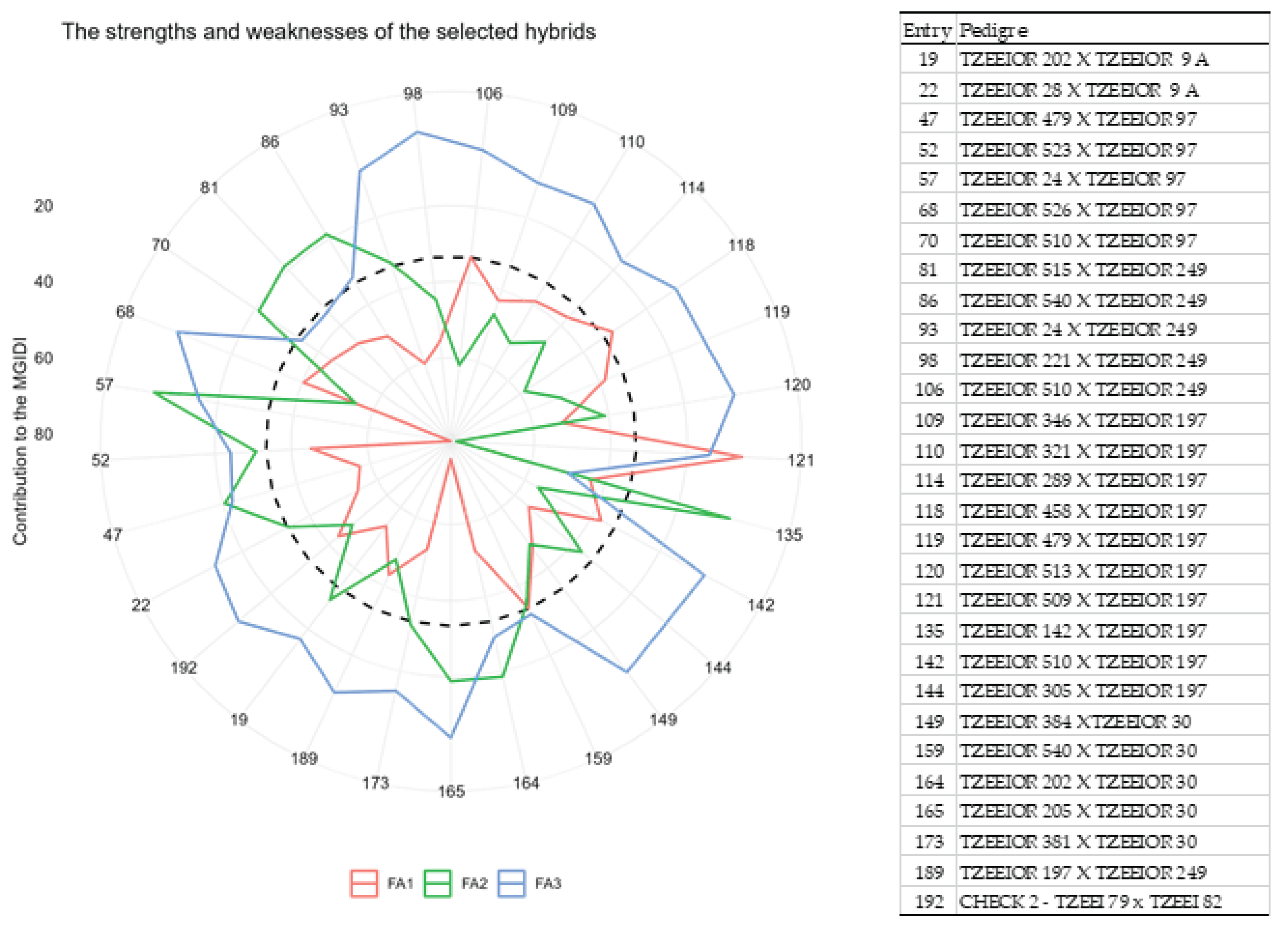
3.2. Selection of Outstanding PVA Hybrids with Drought Tolerance Using the MGIDI Selection Method
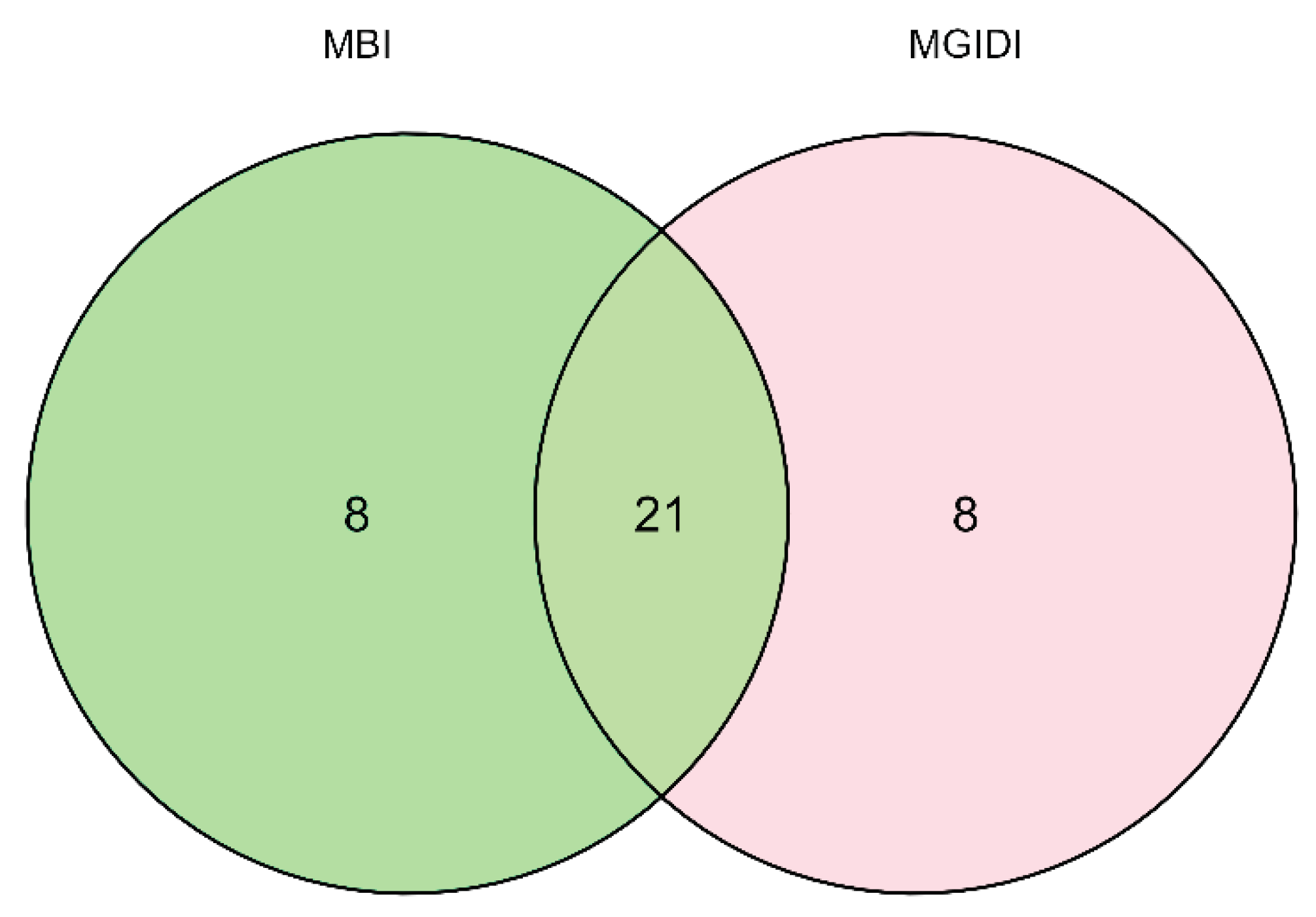
3.3. Selection of Drought-Tolerant Hybrids Using the Base Index derived from Multiple Traits
3.4. Yield Performance and Grain Yield Reduction under Drought
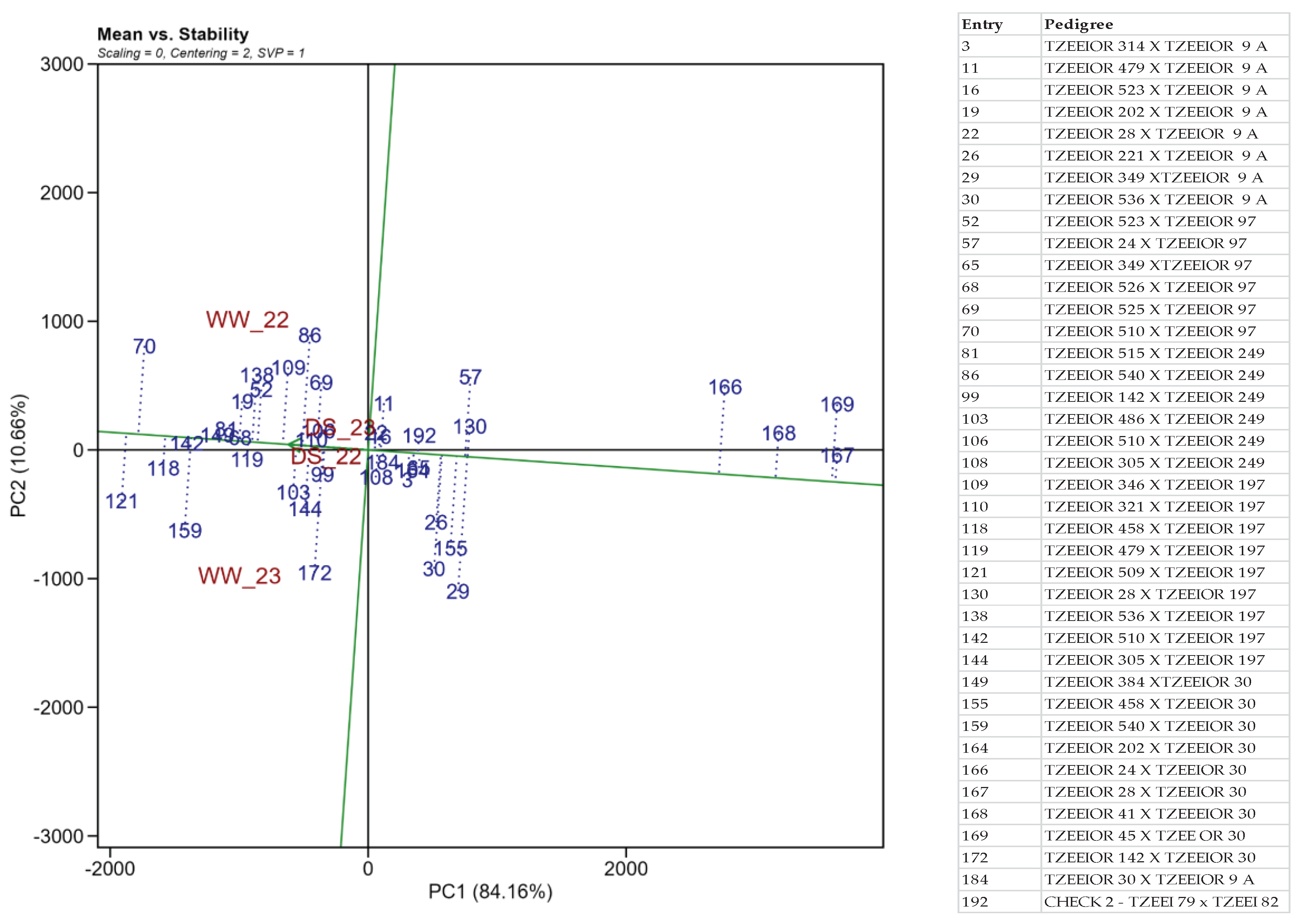
3.5. Stability Analysis of Forty PVA Hybrids Comprising Best, Average, and Worst Performing Genotypes across all Environments
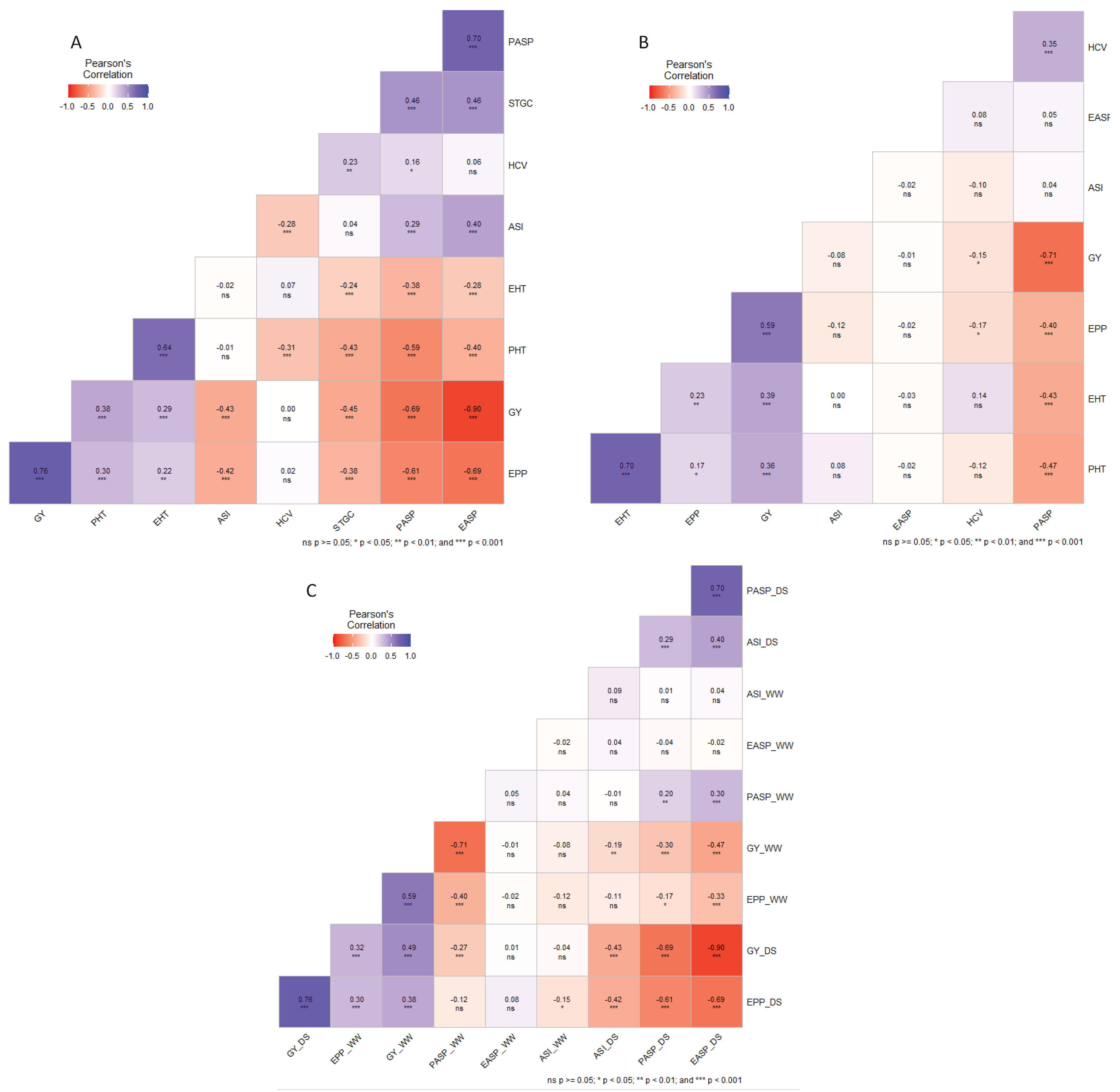
3.6. Phenotypic Correlations among Measured Traits under Managed Drought, Well-Watered, and across Research Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- FAOSTAT. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL.
- Kurilich, A.C.; Juvik, J.A. Quantification of carotenoid and tocopherol antioxidants in Zea mays. J Agric Food Chem. 1999, 47, 1948–1955. [Google Scholar] [CrossRef]
- Tien, L.D.; Duc, C.H.; Quynh, L.N. Improving nutritional quality of plant proteins through genetic engineering. Curr Genomics. 2016, 17, 220–229. [Google Scholar] [CrossRef]
- Villamor, E.; Fawzi, W.W. Vitamin A supplementation: Implications for morbidity and mortality in children. Journal of Infectious Diseases. 2000, 182, S122–S133. [Google Scholar] [CrossRef]
- Sommer, A. Vitamin A deficiency and clinical disease: An historical overview. J Nutr. 2008, 138, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Semagn, K.; Beyene, Y.; Babu, R.; Nair, S.; Gowda, M.; Das, B.; et al. Quantitative trait loci mapping and molecular breeding for developing stress resilient maize for Sub-Saharan Africa. Crop Sci. 2015, 55, 1449–1459. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fakorede, M.A.B. Advances in Genetic Enhancement of Early and Extra-Early Maize for Sub-Saharan Africa; Springer, 2017; 230p. [Google Scholar]
- NeSmith, D.S.; Ritchie, J.T. Effects of soil water-deficits during tassel emergence on development and yield component of maize (Zea mays). F Crop Res. 1992, 28, 251–256. [Google Scholar] [CrossRef]
- Menkir, A.; Akintunde, A.O. Evaluation of the performance of maize hybrids, improved open-pollinated and farmers’ local varieties under well-watered and drought stress conditions. Maydica 2001, 46, 227–238. [Google Scholar]
- Badu-Apraku, B.; Fontem, L.A.; Akinwale, R.O.; Oyekunle, M. Biplot analysis of diallel crosses of early maturing tropical yellow maize inbreds in stress and nonstress environments. Crop Sci. 2011, 51, 173–188. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Oyekunle, M. Genetic analysis of grain yield and other traits of extra-early yellow maize inbreds and hybrid performance under contrasting environments. F Crop Res. 2012, 129, 99–110. [Google Scholar] [CrossRef]
- Jazayeri, S.M.; Torres, R.V. Genomic and transcriptomic approaches toward plant selection. J Sci Res Rev Cienc e Investig. 2017, 2, 54. [Google Scholar] [CrossRef]
- Campos, H.; Cooper, M.; Edmeades, G.O.; Löffler, C.; Schussler, J.R.; Ibañez, M. Changes in drought tolerance in maize associated with fifty years of breeding for yield in the U.S. corn belt. Maydica. 2006, 51, 369–381. [Google Scholar]
- Olivoto, T.; Nardino, M. MGIDI: Toward an effective multivariate selection in biological experiments. Bioinformatics. 2021, 37, 1383–1389. [Google Scholar] [CrossRef]
- Bizari, E.H.; Val, B.H.P.; Pereira, E.D.M.; Mauro, A.O.D.; Unêda-Trevisoli, S.H. Selection indices for agronomic traits in segregating populations of soybean. Rev Cienc Agron. 2017, 48, 110–117. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J Stat Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C. metan: An R package for multi-environment trial analysis. Methods Ecol Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fakorede, M.A.B.; Oyekunle, M.; Akinwale, R.O. Selection of extra-early maize inbreds under low N and drought at flowering and grain-filling for hybrid production. Maydica. 2011, 56, 29–42. [Google Scholar]
- Yue, H.; Wei, J.; Xie, J.; Chen, S.; Peng, H.; Cao, H.; et al. A study on genotype-by-environment interaction analysis for agronomic traits of maize genotypes across huang-huai-hai region in china. Phyton-International J Exp Bot. 2022, 91, 57–81. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Betrán, F.J.; Rooney, L.W. Combining abilities of quality protein maize inbreds. Crop Sci. 2004, 44, 1997–2005. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Annor, B.; Oyekunle, M.; Akinwale, R.O.; Fakorede, M.A.B.; Talabi, A.O.; et al. Grouping of early maturing quality protein maize inbreds based on SNP markers and combining ability under multiple environments. F Crop Res. 2015, 183, 169–183. [Google Scholar] [CrossRef]
- Bhadmus, O.A.; Badu-apraku, B.; Adeyemo, O.A.; Ogunkanmi, A.L. Genetic analysis of early white quality protein maize inbreds and derived hybrids under low-nitrogen and combined drought and heat stress environments. Plants. 2021, 10, 2596. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Olivoto, T. Multicollinearity in Path Analysis: A Simple Method to Reduce Its Effects. Agron. J. 2017, 109, 131–142. [Google Scholar] [CrossRef]
- Singamsetti, A.; Zaidi, P.H.; Seetharam, K.; Vinayan, M.T.; Olivoto, T.; Mahato, A.; et al. Genetic gains in tropical maize hybrids across moisture regimes with multi-trait-based index selection. Front Plant Sci. 2023, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pour-Aboughadareh, A.; Sanjani, S.; Nikkhah-Chamanabad, H.; Mehrvar, M.R.; Asadi, A.; Amini, A. Identification of salt-tolerant barley genotypes using multiple-traits index and yield performance at the early growth and maturity stages. Bull Natl Res Cent. 2021, 45, 117. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fakorede, M.A.B.; Talabi, A.O.; Oyekunle, M.; Aderounmu, M.; Lum, A.F.; et al. Genetic studies of extra-early provitamin-A maize inbred lines and their hybrids in multiple environments. Crop Sci. 2020, 60, 1325–1345. [Google Scholar] [CrossRef] [PubMed]
- Edmeades, G.O.; Bolanos, J.; Hernandez, M.; Bello, S. Causes for silk delay in a lowland tropical maize population. Crop Sci. 1993, 33, 1029–1035. [Google Scholar] [CrossRef]
- Oyekunle, M. Genetic analysis and molecular characterisation of early maturing maize (Zea mays L.) inbred lines for drought tolerance; University of Ibadan, 2014. [Google Scholar]
- Badu-Apraku, B.; Menkir, A.; Ajala, S.O.; Akinwale, R.O.; Oyekunle, M.; Obeng-Antwi, K. Performance of tropical early-maturing maize cultivars in multiple stress environments. Can J Plant Sci. 2010, 90, 831–852. [Google Scholar] [CrossRef]
- Széles, A.; Horváth, É.; Simon, K.; Zagyi, P.; Huzsvai, L. Maize production under drought stress: Nutrient supply, yield prediction. Plants. 2023, 12, 3301. [Google Scholar] [CrossRef]
- Sah, R.P.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; et al. Impact of water deficit stress in maize: Phenology and yield components. Sci Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Meseka, S.; Menkir, A.; Bossey, B.; Mengesha, W. Performance assessment of drought tolerant maize hybrids under combined drought and heat stress. Agronomy. 2018, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Nelimor, C.; Badu-Apraku, B.; Tetteh, A.Y.; N’guetta, A.S.P. Assessment of genetic diversity for drought, heat and combined drought and heat stress tolerance in early maturing maize landraces. Plants. 2019, 8, 1–19. [Google Scholar] [CrossRef]
- Cairns, J.E.; Crossa, J.; Zaidi, P.H.; Grudloyma, P.; Sanchez, C.; Luis, A.J.; et al. Identification of drought, heat, and combined drought and heat tolerant donors in maize. Crop Sci. 2013, 53, 1335–1346. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fakorede, M.A.B.; Talabi, A.O.; Oyekunle, M.; Akaogu, I.C.; Akinwale, R.O.; et al. Gene action and heterotic groups of early white quality protein maize inbreds under multiple stress environments. Crop Sci. 2016, 56, 183–199. [Google Scholar] [CrossRef]
- Nasser, L.M.; Badu-Apraku, B.; Gracen, V.E.; Mafouasson, H.N.A. Combining ability of early-maturing yellow maize inbreds under combined drought and heat stress and well-watered environments. Agronomy. 2020, 10, 19–22. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G.O. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. F Crop Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
| Source of variation | DF | GY | ASI | PHT | EHT | PASP | EASP | EPP | HCV |
| Environment (Env) | 1 | 37593481* | 46.76* | 6.65ns | 50.26ns | 37.12* | 3.46ns | 1.25* | 3.71ns |
| Genotype (Gen) | 195 | 4330349*** | 0.71** | 422.55*** | 192.61*** | 2.07*** | 0.85ns | 0.02*** | 7.19*** |
| Env × Gen | 195 | 1174838ns | 0.42ns | 67.95ns | 54.75ns | 0.65ns | 1.00ns | 0.01ns | 0.82ns |
| Residual | 1108155 | 0.40 | 69.28 | 52.12 | 0.89 | 0.75 | 0.01 | 0.84 | |
| Min | 590.5 | 0.00 | 129.2 | 57 | 2 | 2 | 0.38 | 1 | |
| Max | 10064 | 4.00 | 213.8 | 180 | 8 | 8 | 1.27 | 8 | |
| Mean | 5568 | 0.62 | 179.3 | 83.56 | 4.44 | 4.68 | 0.92 | 3.92 |
| Source of variation | DF | GY | ASI | PHT | EHT | PASP | EASP | EPP | HCV | STGC |
| Environment (Env) | 1 | 1560132ns | 35.57* | 673.19ns | 2.05ns | 8.51ns | 2.45ns | 0.19ns | 81.67*** | 0.10ns |
| Genotype (Gen) | 195 | 1464750*** | 3.085*** | 485.84*** | 193.81*** | 1.55*** | 1.61*** | 0.05*** | 3.90*** | 1.93*** |
| Env × Gen | 195 | 979359.6ns | 2.16* | 258.2** | 70.25ns | 1.08ns | 1.15ns | 0.04** | 0.95ns | 1.07*** |
| Residual | 835346 | 1.57ns | 191.5 | 65.2 | 0.98 | 1 | 0.03 | 0.78 | 0.99 | |
| Min | 0 | 0 | 65 | 34 | 2 | 1 | 0 | 1 | 2 | |
| Max | 8407 | 9 | 205.8 | 128.4 | 9 | 9 | 1.17 | 8 | 9 | |
| Mean | 2138.74 | 1.5 | 147.9 | 76.27 | 5.42 | 5.13 | 0.71 | 3.97 | 5.02 |
| traits | FA1 | FA2 | FA3 | Xo | Xs | Predicted gain | sense | goal |
| ASI | -0.62 | 0.49 | -0.13 | 1.51 | 1.49 | -1.03 | decrease | 100 |
| PHT | -0.28 | -0.33 | 0.80 | 147.87 | 148.24 | 0.25 | increase | 100 |
| EHT | -0.11 | 0.13 | 0.93 | 76.36 | 78.69 | 3.04 | increase | 100 |
| HCV | 0.00 | -0.90 | -0.02 | 3.97 | 3.57 | -10.13 | decrease | 100 |
| PASP | -0.73 | -0.20 | 0.41 | 5.42 | 5.41 | -0.17 | decrease | 100 |
| EASP | -0.89 | -0.08 | 0.19 | 5.13 | 5.06 | -1.24 | decrease | 100 |
| EPP | -0.85 | 0.02 | 0.11 | 0.71 | 0.73 | 2.63 | increase | 100 |
| GY | -0.91 | -0.02 | 0.19 | 2138.74 | 2267.90 | 6.04 | increase | 100 |
| STGC | -0.48 | -0.47 | 0.27 | 5.02 | 4.82 | -4.08 | decrease | 100 |
| Traits | FA1 | FA2 | FA3 | Communality | Uniqueness |
| ASI | 0.13 | 0.26 | 0.87 | 0.84 | 0.16 |
| PHT | 0.15 | -0.84 | -0.05 | 0.73 | 0.27 |
| EHT | -0.27 | -0.71 | 0.10 | 0.59 | 0.41 |
| HCV | -0.02 | 0.36 | -0.74 | 0.69 | 0.31 |
| PASP | 0.72 | 0.21 | 0.01 | 0.57 | 0.43 |
| EASP | 0.91 | -0.06 | 0.03 | 0.82 | 0.18 |
| EPP | 0.74 | 0.00 | 0.14 | 0.57 | 0.43 |
| GY | 0.81 | -0.08 | 0.19 | 0.69 | 0.31 |
| STGC | 0.38 | 0.09 | -0.18 | 0.18 | 0.82 |
| Entries | Genotypes | GY (kg/ha) under managed drought conditions | GY (kg/ha) under well-watered conditions | YR% | MBI | MGIDI |
| 19 | TZEEIOR 202 × TZEEIOR 9 A | 2098.17 | 6714.28 | 68.75 | 5.39* | 2.65 |
| 22 | TZEEIOR 28 × TZEEIOR 9 A | 2501.02 | 5341.02 | 53.17 | 7.79 | 2.06 |
| 39 | TZEEIOR 314 × TZEEIOR 97 | 3604.17 | 6993.1 | 48.46 | 8.38 | 3.37* |
| 47 | TZEEIOR 479 × TZEEIOR 97 | 2857.75 | 5247.55 | 45.54 | 7.70 | 2.69 |
| 52 | TZEEIOR 523 × TZEEIOR 97 | 3054.03 | 6385.56 | 52.17 | 10.5 | 2.12 |
| 57 | TZEEIOR 24 × TZEEIOR 97 | 2502.56 | 4392.24 | 43.02 | 6.41 | 2.66 |
| 68 | TZEEIOR 526 × TZEEIOR 97 | 3318.75 | 6435.87 | 48.43 | 9.69 | 2.07 |
| 70 | TZEEIOR 510 × TZEEIOR 97 | 3568.91 | 7379.62 | 51.64 | 15.07 | 1.53 |
| 74 | TZEEIOR 321 × TZEEIOR 249 | 3207.81 | 4918.39 | 34.78 | 9.31 | 2.89* |
| 81 | TZEEIOR 515 × TZEEIOR 249 | 2917.19 | 6722.3 | 56.6 | 9.81 | 2.36 |
| 86 | TZEEIOR 540 × TZEEIOR 249 | 2922.97 | 5818.24 | 49.76 | 9.02 | 2.60 |
| 92 | TZEEIOR 205 × TZEEIOR 249 | 2558.98 | 5240.41 | 51.17 | 8.46 | 2.91* |
| 93 | TZEEIOR 24 × TZEEIOR 249 | 1988.74 | 4367.42 | 54.46 | 2.29* | 2.83 |
| 98 | TZEEIOR 221 × TZEEIOR 249 | 2156.96 | 5186.18 | 58.41 | 4.19* | 2.68 |
| 106 | TZEEIOR 510 × TZEEIOR 249 | 3294.78 | 5845.61 | 43.64 | 12.15 | 1.85 |
| 109 | TZEEIOR 346 × TZEEIOR 197 | 2438.1 | 6397.55 | 61.89 | 6.58 | 2.59 |
| 110 | TZEEIOR 321 × TZEEIOR 197 | 3116.3 | 5714.04 | 45.46 | 11.24 | 1.90 |
| 114 | TZEEIOR 289 × TZEEIOR 197 | 2873.28 | 7341.28 | 60.86 | 7.61 | 2.85 |
| 118 | TZEEIOR 458 × TZEEIOR 197 | 3642.91 | 7062.23 | 48.42 | 15.91 | 2.06 |
| 119 | TZEEIOR 479 × TZEEIOR 197 | 3073.8 | 6428.36 | 52.18 | 10.41 | 2.24 |
| 120 | TZEEIOR 513 × TZEEIOR 197 | 2799.11 | 6953.46 | 59.75 | 6.03* | 2.85 |
| 121 | TZEEIOR 509 × TZEEIOR 197 | 4044.13 | 7454.93 | 45.75 | 15.94 | 2.17 |
| 128 | TZEEIOR 205 × TZEEIOR 197 | 2308.17 | 5989.75 | 61.46 | 6.71 | 2.95* |
| 135 | TZEEIOR 142 × TZEEIOR 197 | 2578.45 | 5953.68 | 56.69 | 6.03* | 2.66 |
| 142 | TZEEIOR 510 × TZEEIOR 197 | 2628.79 | 7050.98 | 62.72 | 8.90 | 2.42 |
| 144 | TZEEIOR 305 × TZEEIOR 197 | 2648.3 | 5885.05 | 55 | 6.08* | 2.64 |
| 149 | TZEEIOR 384 ×TZEEIOR 30 | 3414.92 | 6752.58 | 49.43 | 10.71 | 1.96 |
| 158 | TZEEIOR 509 × TZEEIOR 30 | 3096.97 | 5469.03 | 43.37 | 6.84 | 3.01* |
| 159 | TZEEIOR 540 × TZEEIOR 30 | 3261.14 | 7086.18 | 53.98 | 10.69 | 2.36 |
| 164 | TZEEIOR 202 × TZEEIOR 30 | 2297.08 | 4917.55 | 53.29 | 8.97 | 2.08 |
| 165 | TZEEIOR 205 × TZEEIOR 30 | 2359.71 | 6279.68 | 62.42 | 4.80* | 2.76 |
| 173 | TZEEIOR 381 × TZEEIOR 30 | 2999.96 | 5897.69 | 49.13 | 6.97 | 2.67 |
| 175 | TZEEIOR 536 × TZEEIOR 30 | 3498.09 | 7390.22 | 52.67 | 6.34 | 3.13* |
| 178 | TZEEIOR 525 × TZEEIOR 30 | 3217.1 | 5617.52 | 42.73 | 8.85 | 3.17* |
| 179 | TZEEIOR 510 × TZEEIOR 30 | 3805.47 | 6392.13 | 40.47 | 15.18 | 3.63* |
| 189 | TZEEIOR 197 × TZEEIOR 249 | 2299.81 | 5464.1 | 57.91 | 4.53 | 2.78 |
| 192 | CHECK 2 - TZEEI 79 × TZEEI 82 | 3015.16 | 4720.41 | 36.13 | 7.91 | 2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





