Submitted:
22 March 2024
Posted:
22 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Regulation and Influence of Tumor Microenvironment
2.1. Characteristics of Tumor Microenvironment and Immunosuppressive Mechanism
2.1.1. Cell Interaction with Tumor Stroma
2.1.2. Immune Escape and Tumor Suppressor Cells
2.2. Role of Nanoparticles in the Immune System
2.2.1. Structure and Function of Lipid Calcium Phosphate Nanoparticles
2.2.2. Immune System Interaction with Nanoparticles
3. Design and Preparation of Mannose Modified Lipid Calcium Phosphate Nanoparticle Vaccine
3.1. Techniques and Principles of Mannose Modification
3.1.1. Mannose Modification and Immune Response
3.1.2. Effect of Mannose Modification on Vaccines
3.2. Design and Preparation of Lipid Calcium Phosphate Nanoparticles
3.2.1. Preparation Method and Structural Advantages
3.2.2. Stability and Biocompatibility of Nanoparticles
4. Immunomodulatory Mechanism of Mannose Modified Lipid Calcium Phosphate Nanoparticle Vaccine
4.1. Tumor Antigen Presentation and T Cell Activation
4.1.1.
4.1.2. Activation of T Cells by Mannose Modified Lipid Calcium Phosphate Nanoparticle Vaccine
4.2. Enhancement of Tumor Immune Response and Establishment of Immune Memory
4.3. Analysis of Immune Cell Infiltration in Tumor Tissue
5. Enlightenment and Research Prospect of Preclinical Research
6. Research Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J. L., Chen, H. Z., & Gao, X. L. Lipid-coated calcium phosphate nanoparticle and beyond: a versatile platform for drug delivery. Journal of drug targeting 2018, 26(5-6), 398–406. [CrossRef]
- Haynes, M. T., & Huang, L. Lipid-coated calcium phosphate nanoparticles for nonviral gene therapy. Advances in genetics 2014, 88, 205–229. [Google Scholar] [CrossRef]
- Shen, Y., & Ma, H. Oridonin-loaded lipid-coated calcium phosphate nanoparticles: preparation, characterization, and application in A549 lung cancer. Pharmaceutical development and technology 2022, 27(5), 598–605. [Google Scholar] [CrossRef]
- Favarin, B. Z., Bolean, M., Ramos, A. P., Magrini, A., Rosato, N., Millán, J. L., Bottini, M., Costa-Filho, A. J., & Ciancaglini, P. (2020). Lipid composition modulates ATP hydrolysis and calcium phosphate mineral propagation by TNAP-harboring proteoliposomes. Archives of biochemistry and biophysics, 691, 108482. [CrossRef]
- Satterlee, A. B., & Huang, L. (2016). Current and Future Theranostic Applications of the Lipid-Calcium-Phosphate Nanoparticle Platform. Theranostics, 6(7), 918–929. [CrossRef]
- Zhou, C., Yu, B., Yang, X., Huo, T., Lee, L. J., Barth, R. F., & Lee, R. J. (2010). Lipid-coated nano-calcium-phosphate (LNCP) for gene delivery. International journal of pharmaceutics, 392(1-2), 201–208. [CrossRef]
- Dong, K., Zhang, Y., Ji, H. R., Guan, Z. L., Wang, D. Y., Guo, Z. Y., Deng, S. J., He, B. Y., Xing, J. F., & You, C. Y. (2024). Dexamethasone-Loaded Lipid Calcium Phosphate Nanoparticles Treat Experimental Colitis by Regulating Macrophage Polarization in Inflammatory Sites. International journal of nanomedicine, 19, 993–1016. [CrossRef]
- Cruz, M. A. E., Ferreira, C. R., Tovani, C. B., de Oliveira, F. A., Bolean, M., Caseli, L., Mebarek, S., Millán, J. L., Buchet, R., Bottini, M., Ciancaglini, P., & Paula Ramos, A. (2020). Phosphatidylserine controls calcium phosphate nucleation and growth on lipid monolayers: A physicochemical understanding of matrix vesicle-driven biomineralization. Journal of structural biology, 212(2), 107607. [CrossRef]
- Tang, J., Li, B., Howard, C. B., Mahler, S. M., Thurecht, K. J., Wu, Y., Huang, L., & Xu, Z. P. (2019). Multifunctional lipid-coated calcium phosphate nanoplatforms for complete inhibition of large triple negative breast cancer via targeted combined therapy. Biomaterials, 216, 119232. [CrossRef]
- Li, J., Chen, Y. C., Tseng, Y. C., Mozumdar, S., & Huang, L. (2010). Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. Journal of controlled release : official journal of the Controlled Release Society, 142(3), 416–421. [CrossRef]
- Zhang, J., Zhang, H., Jiang, J., Cui, N., Xue, X., Wang, T., Wang, X., He, Y., & Wang, D. (2020). Doxorubicin-Loaded Carbon Dots Lipid-Coated Calcium Phosphate Nanoparticles for Visual Targeted Delivery and Therapy of Tumor. International journal of nanomedicine, 15, 433–444. [CrossRef]
- Wu, C., Xu, J., Hao, Y., Zhao, Y., Qiu, Y., Jiang, J., Yu, T., Ji, P., & Liu, Y. (2017). Application of a lipid-coated hollow calcium phosphate nanoparticle in synergistic co-delivery of doxorubicin and paclitaxel for the treatment of human lung cancer A549 cells. International journal of nanomedicine, 12, 7979–7992. [CrossRef]
- Oyane, A., Wang, X., Sogo, Y., Ito, A., & Tsurushima, H. (2012). Calcium phosphate composite layers for surface-mediated gene transfer. Acta biomaterialia, 8(6), 2034–2046. [CrossRef]
- Liu, H.,, Zhang, H.,, Yin, N.,, Zhang, Y.,, Gou, J.,, Yin, T.,, He, H.,, Ding, H.,, Zhang, Y.,, & Tang, X., (2020). Sialic acid-modified dexamethasone lipid calcium phosphate gel core nanoparticles for target treatment of kidney injury. Biomaterials science, 8(14), 3871–3884. [CrossRef]
- Li, J., Yang, Y., & Huang, L. (2012). Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. Journal of controlled release : official journal of the Controlled Release Society, 158(1), 108–114. [CrossRef]
- Tseng, Y. C., Xu, Z., Guley, K., Yuan, H., & Huang, L. (2014). Lipid-calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials, 35(16), 4688–4698. [CrossRef]
- Sethuraman, V., Janakiraman, K., Krishnaswami, V., Natesan, S., & Kandasamy, R. (2021). In vivo synergistic anti-tumor effect of lumefantrine combined with pH responsive behavior of nano calcium phosphate based lipid nanoparticles on lung cancer. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 158, 105657. [CrossRef]
- Dolci, L. S., Panzavolta, S., Albertini, B., Campisi, B., Gandolfi, M., Bigi, A., & Passerini, N. (2018). Spray-congealed solid lipid microparticles as a new tool for the controlled release of bisphosphonates from a calcium phosphate bone cement. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 122, 6–16. [CrossRef]
- Tanaka, Y., & Schroit, A. J. (1986). Calcium/phosphate-induced immobilization of fluorescent phosphatidylserine in synthetic bilayer membranes: inhibition of lipid transfer between vesicles. Biochemistry, 25(8), 2141–2148. [CrossRef]
- Wang, X., Zhang, M., Zhang, L., Li, L., Li, S., Wang, C., Su, Z., Yuan, Y., & Pan, W. (2017). Designed Synthesis of Lipid-Coated Polyacrylic Acid/Calcium Phosphate Nanoparticles as Dual pH-Responsive Drug-Delivery Vehicles for Cancer Chemotherapy. Chemistry (Weinheim an der Bergstrasse, Germany), 23(27), 6586–6595. [CrossRef]
- Genge, B. R., Wu, L. N., & Wuthier, R. E. (2008). Mineralization of annexin-5-containing lipid-calcium-phosphate complexes: modulation by varying lipid composition and incubation with cartilage collagens. The Journal of biological chemistry, 283(15), 9737–9748. [CrossRef]
- Guo, W., Morrisett, J. D., Lawrie, G. M., DeBakey, M. E., & Hamilton, J. A. (1998). Identification of different lipid phases and calcium phosphate deposits in human carotid artery plaques by MAS NMR spectroscopy. Magnetic resonance in medicine, 39(2), 184–189. [CrossRef]
- Liu, Y., Hu, Y., & Huang, L. (2014). Influence of polyethylene glycol density and surface lipid on pharmacokinetics and biodistribution of lipid-calcium-phosphate nanoparticles. Biomaterials, 35(9), 3027–3034. [CrossRef]
- Tang, J.,, Howard, C. B.,, Mahler, S. M.,, Thurecht, K. J.,, Huang, L.,, & Xu, Z. P., (2018). Enhanced delivery of siRNA to triple negative breast cancer cells in vitro and in vivo through functionalizing lipid-coated calcium phosphate nanoparticles with dual target ligands. Nanoscale, 10(9), 4258–4266. [CrossRef]
- Skrtic, D., & Eanes, E. D. (1992). Membrane-mediated precipitation of calcium phosphate in model liposomes with matrix vesicle-like lipid composition. Bone and mineral, 16(2), 109–119. [CrossRef]
- Claudio T. (1992). Stable expression of heterologous multisubunit protein complexes established by calcium phosphate- or lipid-mediated cotransfection. Methods in enzymology, 207, 391–408. [CrossRef]
- Dolci, L. S., Panzavolta, S., Torricelli, P., Albertini, B., Sicuro, L., Fini, M., Bigi, A., & Passerini, N. (2019). Modulation of Alendronate release from a calcium phosphate bone cement: An in vitro osteoblast-osteoclast co-culture study. International journal of pharmaceutics, 554, 245–255. [CrossRef]
- Chen, J., Gao, P., Yuan, S., Li, R., Ni, A., Chu, L., Ding, L., Sun, Y., Liu, X. Y., & Duan, Y. (2016). Oncolytic Adenovirus Complexes Coated with Lipids and Calcium Phosphate for Cancer Gene Therapy. ACS nano, 10(12), 11548–11560. [CrossRef]
- Kashiwa, H. K., & Mukai, C. D. (1971). Lipid-calcium-phosphate spherules in chondrocytes of developing long bones. Clinical orthopaedics and related research, 78, 223–229. [CrossRef]
- Sethuraman, V., Janakiraman, K., Krishnaswami, V., Natesan, S., & Kandasamy, R. (2019). pH responsive delivery of lumefantrine with calcium phosphate nanoparticles loaded lipidic cubosomes for the site specific treatment of lung cancer. Chemistry and physics of lipids, 224, 104763. [CrossRef]
- Ke, C. H., Chiu, Y. H., Huang, K. C., & Lin, C. S. (2022). Exposure of Immunogenic Tumor Antigens in Surrendered Immunity and the Significance of Autologous Tumor Cell-Based Vaccination in Precision Medicine. International journal of molecular sciences, 24(1), 147. [CrossRef]
- Ramirez-Valdez, R. A., Baharom, F., Khalilnezhad, A., Fussell, S. C., Hermans, D. J., Schrager, A. M., Tobin, K. K. S., Lynn, G. M., Khalilnezhad, S., Ginhoux, F., Van den Eynde, B. J., Leung, C. S. K., Ishizuka, A. S., & Seder, R. A. (2023). Intravenous heterologous prime-boost vaccination activates innate and adaptive immunity to promote tumor regression. Cell reports, 42(6), 112599. [CrossRef]
- Jeong, M., Kim, H., Yoon, J., Kim, D. H., & Park, J. H. (2022). Coimmunomodulation of tumor and tumor-draining lymph nodes during in situ vaccination promotes antitumor immunity. JCI insight, 7(12), e146608. [CrossRef]
- Mehdizadeh, R., Shariatpanahi, S. P., Goliaei, B., & Rüegg, C. (2023). Targeting myeloid-derived suppressor cells in combination with tumor cell vaccination predicts anti-tumor immunity and breast cancer dormancy: an in silico experiment. Scientific reports, 13(1), 5875. [CrossRef]
- Medina-Echeverz, J., Hinterberger, M., Testori, M., Geiger, M., Giessel, R., Bathke, B., Kassub, R., Gräbnitz, F., Fiore, G., Wennier, S. T., Chaplin, P., Suter, M., Hochrein, H., & Lauterbach, H. (2019). Synergistic cancer immunotherapy combines MVA-CD40L induced innate and adaptive immunity with tumor targeting antibodies. Nature communications, 10(1), 5041. [CrossRef]
- Clark, P. A., Sriramaneni, R. N., Bates, A. M., Jin, W. J., Jagodinsky, J. C., Hernandez, R., Le, T., Jeffery, J. J., Marsh, I. R., Grudzinski, J. J., Aluicio-Sarduy, E., Barnhart, T. E., Anderson, B. R., Chakravarty, I., Arthur, I. S., Kim, K., Engle, J. W., Bednarz, B. P., Weichert, J. P., & Morris, Z. S. (2021). Low-Dose Radiation Potentiates the Propagation of Anti-Tumor Immunity against Melanoma Tumor in the Brain after In Situ Vaccination at a Tumor outside the Brain. Radiation research, 195(6), 522–540. [CrossRef]
- Kim, N. J., Yoon, J. H., Tuomi, A. C., Lee, J., & Kim, D. (2023). In-situ tumor vaccination by percutaneous ablative therapy and its synergy with immunotherapeutics: An update on combination therapy. Frontiers in immunology, 14, 1118845. [CrossRef]
- Varypataki, E. M., Hasler, F., Waeckerle-Men, Y., Vogel-Kindgen, S., Høgset, A., Kündig, T. M., Gander, B., Halin, C., & Johansen, P. (2019). Combined Photosensitization and Vaccination Enable CD8 T-Cell Immunity and Tumor Suppression Independent of CD4 T-Cell Help. Frontiers in immunology, 10, 1548. [CrossRef]
- Dong, W., Du, J., Shen, H., Gao, D., Li, Z., Wang, G., Mu, X., & Liu, Q. (2010). Administration of embryonic stem cells generates effective antitumor immunity in mice with minor and heavy tumor load. Cancer immunology, immunotherapy : CII, 59(11), 1697–1705. [CrossRef]
- Koido, S., Ito, M., Sagawa, Y., Okamoto, M., Hayashi, K., Nagasaki, E., Kan, S., Komita, H., Kamata, Y., & Homma, S. (2014). Vaccination with vascular progenitor cells derived from induced pluripotent stem cells elicits antitumor immunity targeting vascular and tumor cells. Cancer immunology, immunotherapy : CII, 63(5), 459–468. [CrossRef]
- Accolla, R. S., & Tosi, G. (2013). Adequate antigen availability: a key issue for novel approaches to tumor vaccination and tumor immunotherapy. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology, 8(1), 28–36. [CrossRef]
- Tong, W., Maira, M., Roychoudhury, R., Galan, A., Brahimi, F., Gilbert, M., Cunningham, A. M., Josephy, S., Pirvulescu, I., Moffett, S., & Saragovi, H. U. (2019). Vaccination with Tumor-Ganglioside Glycomimetics Activates a Selective Immunity that Affords Cancer Therapy. Cell chemical biology, 26(7), 1013–1026.e4. [CrossRef]
- Park, J., Hsueh, P. C., Li, Z., & Ho, P. C. (2023). Microenvironment-driven metabolic adaptations guiding CD8+ T cell anti-tumor immunity. Immunity, 56(1), 32–42. [CrossRef]
- Castro Dopico, X., Ols, S., Loré, K., & Karlsson Hedestam, G. B. (2022). Immunity to SARS-CoV-2 induced by infection or vaccination. Journal of internal medicine, 291(1), 32–50. [CrossRef]
- Bevers, S., Kooijmans, S. A. A., Van de Velde, E., Evers, M. J. W., Seghers, S., Gitz-Francois, J. J. J. M., van Kronenburg, N. C. H., Fens, M. H. A. M., Mastrobattista, E., Hassler, L., Sork, H., Lehto, T., Ahmed, K. E., El Andaloussi, S., Fiedler, K., Breckpot, K., Maes, M., Van Hoorick, D., Bastogne, T., Schiffelers, R. M., … De Koker, S. (2022). mRNA-LNP vaccines tuned for systemic immunization induce strong antitumor immunity by engaging splenic immune cells. Molecular therapy : the journal of the American Society of Gene Therapy, 30(9), 3078–3094. [CrossRef]
- Schetters, S. T. T., Li, R. J. E., Kruijssen, L. J. W., Engels, S., Ambrosini, M., Garcia-Vallejo, J. J., Kalay, H., Unger, W. W. J., & van Kooyk, Y. (2020). Adaptable antigen matrix platforms for peptide vaccination strategies and T cell-mediated anti-tumor immunity. Biomaterials, 262, 120342. [CrossRef]
- Bruni, L., Saura-Lázaro, A., Montoliu, A., Brotons, M., Alemany, L., Diallo, M. S., Afsar, O. Z., LaMontagne, D. S., Mosina, L., Contreras, M., Velandia-González, M., Pastore, R., Gacic-Dobo, M., & Bloem, P. (2021). HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Preventive medicine, 144, 106399. [CrossRef]
- Li, S., Zhang, Q., Bai, H., Huang, W., Shu, C., Ye, C., Sun, W., & Ma, Y. (2019). Self-Assembled Nanofibers Elicit Potent HPV16 E7-Specific Cellular Immunity And Abolish Established TC-1 Graft Tumor. International journal of nanomedicine, 14, 8209–8219. [CrossRef]
- Dolina, J. S., Lee, J., Brightman, S. E., McArdle, S., Hall, S. M., Thota, R. R., Zavala, K. S., Lanka, M., Ramamoorthy Premlal, A. L., Greenbaum, J. A., Cohen, E. E. W., Peters, B., & Schoenberger, S. P. (2023). Linked CD4+/CD8+ T cell neoantigen vaccination overcomes immune checkpoint blockade resistance and enables tumor regression. The Journal of clinical investigation, 133(17), e164258. [CrossRef]
- Kim, Y., Lee, S., & Jon, S. (2024). Liposomal Delivery of an Immunostimulatory CpG Induces Robust Antitumor Immunity and Long-Term Immune Memory by Reprogramming Tumor-Associated Macrophages. Advanced healthcare materials, 13(6), e2300549. [CrossRef]
- Carter, J. A., Matta, B., Battaglia, J., Somerville, C., Harris, B. D., LaPan, M., Atwal, G. S., & Barnes, B. J. (2023). Identification of pan-cancer/testis genes and validation of therapeutic targeting in triple-negative breast cancer: Lin28a-based and Siglece-based vaccination induces antitumor immunity and inhibits metastasis. Journal for immunotherapy of cancer, 11(12), e007935. [CrossRef]
- Liu, Y., Li, H., Zhao, H., Hao, Y., Van Herck, S., Xu, Z., Wang, G., Wang, X., Zhang, X., Ge, X., Li, X., Yang, A., Chen, H., Zou, J., Wang, W., De Geest, B. G., & Zhang, Z. (2022). In Situ Tumor Vaccination with Calcium-Linked Degradable Coacervate Nanocomplex Co-Delivering Photosensitizer and TLR7/8 Agonist to Trigger Effective Anti-Tumor Immune Responses. Advanced healthcare materials, 11(12), e2102781. [CrossRef]
- Wang, H., Gan, M., Wu, B., Zeng, R., Wang, Z., Xu, J., Li, J., Zhang, Y., Cao, J., Chen, L., Di, D., Peng, S., Lei, J., Zhao, Y., Song, X., Yuan, T., Zhou, T., Liu, Q., Yi, J., Wang, X., … Liu, L. (2023). Humoral and cellular immunity of two-dose inactivated COVID-19 vaccination in Chinese children: A prospective cohort study. Journal of medical virology, 95(1), e28380. [CrossRef]
- Zhao, X., Zhang, J., Chen, B., Ding, X., Zhao, N., & Xu, F. J. (2023). Rough Nanovaccines Boost Antitumor Immunity Through the Enhancement of Vaccination Cascade and Immunogenic Cell Death Induction. Small methods, 7(5), e2201595. [CrossRef]
- Luo, X., Qiu, Y., Dinesh, P., Gong, W., Jiang, L., Feng, X., Li, J., Jiang, Y., Lei, Y. L., & Chen, Q. (2021). The functions of autophagy at the tumour-immune interface. Journal of cellular and molecular medicine, 25(5), 2333–2341. [CrossRef]
- Perciani, C. T., Liu, L. Y., Wood, L., & MacParland, S. A. (2021). Enhancing Immunity with Nanomedicine: Employing Nanoparticles to Harness the Immune System. ACS nano, 15(1), 7–20. [CrossRef]
- Abascal, J., Oh, M. S., Liclican, E. L., Dubinett, S. M., Salehi-Rad, R., & Liu, B. (2023). Dendritic Cell Vaccination in Non-Small Cell Lung Cancer: Remodeling the Tumor Immune Microenvironment. Cells, 12(19), 2404. [CrossRef]
- McAuliffe, J., Chan, H. F., Noblecourt, L., Ramirez-Valdez, R. A., Pereira-Almeida, V., Zhou, Y., Pollock, E., Cappuccini, F., Redchenko, I., Hill, A. V., Leung, C. S. K., & Van den Eynde, B. J. (2021). Heterologous prime-boost vaccination targeting MAGE-type antigens promotes tumor T-cell infiltration and improves checkpoint blockade therapy. Journal for immunotherapy of cancer, 9(9), e003218. [CrossRef]
- Morera-Díaz, Y., Canaán-Haden, C., Sánchez-Ramírez, J., Bequet-Romero, M., Gonzalez-Moya, I., Martínez, R., Falcón, V., Palenzuela, D., Ayala-Ávila, M., & Gavilondo, J. V. (2023). Active immunization with a structurally aggregated PD-L1 antigen breaks T and B immune tolerance in non-human primates and exhibits in vivo anti-tumoral effects in immunocompetent mouse tumor models. Cancer letters, 561, 216156. [CrossRef]
- Femel, J., van Hooren, L., Herre, M., Cedervall, J., Saupe, F., Huijbers, E. J. M., Verboogen, D. R. J., Reichel, M., Thijssen, V. L., Griffioen, A. W., Hellman, L., Dimberg, A., & Olsson, A. K. (2022). Vaccination against galectin-1 promotes cytotoxic T-cell infiltration in melanoma and reduces tumor burden. Cancer immunology, immunotherapy : CII, 71(8), 2029–2040. Ishio, T., Tsukamoto, S., Yokoyama, E., Izumiyama, K., Saito, M., Muraki, H., Kobayashi, M., Mori, A., Morioka, M., & Kondo, T. (2023). Anti-CD20 antibodies and bendamustine attenuate humoral immunity to COVID-19 vaccination in patients with B-cell non-Hodgkin lymphoma. Annals of hematology, 102(6), 1421-1431. [CrossRef]
- Kershner, L. J., Choi, K., Wu, J., Zhang, X., Perrino, M., Salomonis, N., Shern, J. F., & Ratner, N. (2022). Multiple Nf1 Schwann cell populations reprogram the plexiform neurofibroma tumor microenvironment. JCI insight, 7(18), e154513. [CrossRef]
- Top, K. A., Vaudry, W., Morris, S. K., Pham-Huy, A., Pernica, J. M., Tapiéro, B., Gantt, S., Price, V. E., Rassekh, S. R., Sung, L., McConnell, A., Rubin, E., Chawla, R., & Halperin, S. A. (2020). Waning Vaccine Immunity and Vaccination Responses in Children Treated for Acute Lymphoblastic Leukemia: A Canadian Immunization Research Network Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 71(9), e439–e448. [CrossRef]
- Scheer, V., Goldammer, M., Flindt, S., van Zandbergen, G., & Hinz, T. (2020). Therapeutische Immunisierungen gegen Tumore und neurodegenerative Erkrankungen [Therapeutic vaccination for tumors and neurodegenerative diseases]. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz, 63(11), 1373–1379. [CrossRef]
- Chen, P. M., Pan, W. Y., Wu, C. Y., Yeh, C. Y., Korupalli, C., Luo, P. K., Chou, C. J., Chia, W. T., & Sung, H. W. (2020). Modulation of tumor microenvironment using a TLR-7/8 agonist-loaded nanoparticle system that exerts low-temperature hyperthermia and immunotherapy for in situ cancer vaccination. Biomaterials, 230, 119629. [CrossRef]
- Li, E., Butkovich, N., Tucker, J. A., Nelson, E. L., & Wang, S. W. (2023). Evaluating Anti-tumor Immune Responses of Protein Nanoparticle-Based Cancer Vaccines. Methods in molecular biology (Clifton, N.J.), 2671, 321–333. [CrossRef]
- Zhao, B., Kilian, M., Bunse, T., Platten, M., & Bunse, L. (2023). Tumor-reactive T helper cells in the context of vaccination against glioma. Cancer cell, 41(11), 1829–1834. [CrossRef]
- Liu, G., Zhu, M., Zhao, X., & Nie, G. (2021). Nanotechnology-empowered vaccine delivery for enhancing CD8+ T cells-mediated cellular immunity. Advanced drug delivery reviews, 176, 113889. [CrossRef]
- Carlson, P. M., Patel, R. B., Birstler, J., Rodriquez, M., Sun, C., Erbe, A. K., Bates, A. M., Marsh, I., Grudzinski, J., Hernandez, R., Pieper, A. A., Feils, A. S., Rakhmilevich, A. L., Weichert, J. P., Bednarz, B. P., Sondel, P. M., & Morris, Z. S. (2023). Radiation to all macroscopic sites of tumor permits greater systemic antitumor response to in situ vaccination. Journal for immunotherapy of cancer, 11(1), e005463. [CrossRef]
- Vajari, M. K., Sanaei, M. J., Salari, S., Rezvani, A., Ravari, M. S., & Bashash, D. (2023). Breast cancer vaccination: Latest advances with an analytical focus on clinical trials. International immunopharmacology, 123, 110696. [CrossRef]
- Vieira, J. F., Peixoto, A. P., Murta, E. F. C., & Michelin, M. A. (2021). Prophylactic Dendritic Cell Vaccination in Experimental Breast Cancer Controls Immunity and Hepatic Metastases. Anticancer research, 41(7), 3419–3427. [CrossRef]
- Ngamcherdtrakul, W., Reda, M., Nelson, M. A., Wang, R., Zaidan, H. Y., Bejan, D. S., Hoang, N. H., Lane, R. S., Luoh, S. W., Leachman, S. A., Mills, G. B., Gray, J. W., Lund, A. W., & Yantasee, W. (2021). In Situ Tumor Vaccination with Nanoparticle Co-Delivering CpG and STAT3 siRNA to Effectively Induce Whole-Body Antitumor Immune Response. Advanced materials (Deerfield Beach, Fla.), 33(31), e2100628. [CrossRef]
- Koeken, V. A. C. M., Qi, C., Mourits, V. P., de Bree, L. C. J., Moorlag, S. J. C. F. M., Sonawane, V., Lemmers, H., Dijkstra, H., Joosten, L. A. B., van Laarhoven, A., Xu, C. J., van Crevel, R., Netea, M. G., & Li, Y. (2022). Plasma metabolome predicts trained immunity responses after antituberculosis BCG vaccination. PLoS biology, 20(9), e3001765. [CrossRef]
- Rahdan, S., Razavi, S. A., Shojaeian, S., Shokri, F., Amiri, M. M., & Zarnani, A. H. (2022). Immunization with placenta-specific 1 (plac1) induces potent anti-tumor responses and prolongs survival in a mouse model of melanoma. Advances in medical sciences, 67(2), 338–345. [CrossRef]
- Ammons, D. T., Guth, A., Rozental, A. J., Kurihara, J., Marolf, A. J., Chow, L., Griffin, J. F., 4th, Makii, R., MacQuiddy, B., Boss, M. K., Regan, D. P., Frank, C., McGrath, S., Packer, R. A., & Dow, S. (2022). Reprogramming the Canine Glioma Microenvironment with Tumor Vaccination plus Oral Losartan and Propranolol Induces Objective Responses. Cancer research communications, 2(12), 1657–1667. [CrossRef]
- Kostinov, M. P., Akhmatova, N. K., Karpocheva, S. V., Vlasenko, A. E., Polishchuk, V. B., & Kostinov, A. M. (2021). Vaccination Against Diphtheria and Tetanus as a Way to Activate Adaptive Immunity in Children with Solid Tumors. Frontiers in immunology, 12, 696816. [CrossRef]
- Corradini, P., Agrati, C., Apolone, G., Mantovani, A., Giannarelli, D., Marasco, V., Bordoni, V., Sacchi, A., Matusali, G., Salvarani, C., Zinzani, P. L., Mantegazza, R., Tagliavini, F., Lupo-Stanghellini, M. T., Ciceri, F., Damian, S., Uccelli, A., Fenoglio, D., Silvestris, N., Baldanti, F., … VAX4FRAIL Study Group (2023). Humoral and T-Cell Immune Response After 3 Doses of Messenger RNA Severe Acute Respiratory Syndrome Coronavirus 2 Vaccines in Fragile Patients: The Italian VAX4FRAIL Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 76(3), e426–e438. [CrossRef]
- Perrino, M. R., Ahmari, N., Hall, A., Jackson, M., Na, Y., Pundavela, J., Szabo, S., Woodruff, T. M., Dombi, E., Kim, M. O., Köhl, J., Wu, J., & Ratner, N. (2024). C5aR plus MEK inhibition durably targets the tumor milieu and reveals tumor cell phagocytosis. Life science alliance, 7(5), e202302229. [CrossRef]
- Schrezenmeier, E., Rincon-Arevalo, H., Jens, A., Stefanski, A. L., Hammett, C., Osmanodja, B., Koch, N., Zukunft, B., Beck, J., Oellerich, M., Proß, V., Stahl, C., Choi, M., Bachmann, F., Liefeldt, L., Glander, P., Schütz, E., Bornemann-Kolatzki, K., López Del Moral, C., Schrezenmeier, H., … Budde, K. (2022). Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI insight, 7(9), e157836. [CrossRef]
- Jiang, C., Kumar, A., Yu, Z., Shipman, T., Wang, Y., McKay, R. M., Xing, C., & Le, L. Q. (2023). Basement membrane proteins in extracellular matrix characterize NF1 neurofibroma development and response to MEK inhibitor. The Journal of clinical investigation, 133(12), e168227. [CrossRef]
- Xu, Y. Y., Chen, Q. H., Liu, Y., Ji, C., Du, J., Li, M. Y., Shen, H. P., Zhang, X. C., Che, X. R., & Zhao, G. (2024). Zhonghua yu fang yi xue za zhi [Chinese journal of preventive medicine], 58(1), 87–91. [CrossRef]
- Cho, H., Binder, J., Weeratna, R., Dermyer, M., Dai, S., Boccia, A., Li, W., Li, S., Jooss, K., Merson, J., & Hollingsworth, R. E. (2023). Preclinical development of a vaccine-based immunotherapy regimen (VBIR) that induces potent and durable T cell responses to tumor-associated self-antigens. Cancer immunology, immunotherapy : CII, 72(2), 287–300. [CrossRef]
- Liu, H. Y., Altman, A., Canonigo-Balancio, A. J., & Croft, M. (2023). Experimental Melanoma Immunotherapy Model Using Tumor Vaccination with a Hematopoietic Cytokine. Journal of visualized experiments : JoVE, (192), 10.3791/64082. [CrossRef]
- Flies, A. S., Flies, E. J., Fox, S., Gilbert, A., Johnson, S. R., Liu, G. S., Lyons, A. B., Patchett, A. L., Pemberton, D., & Pye, R. J. (2020). An oral bait vaccination approach for the Tasmanian devil facial tumor diseases. Expert review of vaccines, 19(1), 1–10. [CrossRef]
- Huang, X., Zhang, G., Bai, X., & Liang, T. (2020). Reviving the role of MET in liver cancer therapy and vaccination: an autophagic perspective. Oncoimmunology, 9(1), 1818438. [CrossRef]
- Wan, J., Ren, L., Li, X., He, S., Fu, Y., Xu, P., Meng, F., Xian, S., Pu, K., & Wang, H. (2023). Photoactivatable nanoagonists chemically programmed for pharmacokinetic tuning and in situ cancer vaccination. Proceedings of the National Academy of Sciences of the United States of America, 120(8), e2210385120. [CrossRef]
- Hosseinalizadeh, H., Rahmati, M., Ebrahimi, A., & O'Connor, R. S. (2023). Current Status and Challenges of Vaccination Therapy for Glioblastoma. Molecular cancer therapeutics, 22(4), 435–446. [CrossRef]
- van Dam, K. P. J., Volkers, A. G., Wieske, L., Stalman, E. W., Kummer, L. Y. L., van Kempen, Z. L. E., Killestein, J., Tas, S. W., Boekel, L., Wolbink, G. J., van der Kooi, A. J., Raaphorst, J., Takkenberg, R. B., D'Haens, G. R. A. M., Spuls, P. I., Bekkenk, M. W., Musters, A. H., Post, N. F., Bosma, A. L., Hilhorst, M. L., … T2B! Immunity against SARS-CoV-2 study group (2023). Primary SARS-CoV-2 infection in patients with immune-mediated inflammatory diseases: long-term humoral immune responses and effects on disease activity. BMC infectious diseases, 23(1), 332. [CrossRef]
- Hall, V. G., & Teh, B. W. (2023). COVID-19 Vaccination in Patients With Cancer and Patients Receiving HSCT or CAR-T Therapy: Immune Response, Real-World Effectiveness, and Implications for the Future. The Journal of infectious diseases, 228(Suppl 1), S55–S69. [CrossRef]
- Doukas, P. G., St Pierre, F., Karmali, R., Mi, X., Boyer, J., Nieves, M., Ison, M. G., Winter, J. N., Gordon, L. I., & Ma, S. (2023). Humoral Immunity After COVID-19 Vaccination in Chronic Lymphocytic Leukemia and Other Indolent Lymphomas: A Single-Center Observational Study. The oncologist, 28(10), e930–e941. [CrossRef]
- He, Y., Cheng, C., Liu, Y., Chen, F. M., Chen, Y., Yang, C., Zhao, Z., Dawulieti, J., Shen, Z., Zhang, Y., Du, J. Z., Guan, S., & Shao, D. (2024). Intravenous Senescent Erythrocyte Vaccination Modulates Adaptive Immunity and Splenic Complement Production. ACS nano, 18(1), 470–482. [CrossRef]
- Hartmann, A. K., Bartneck, J., Pielenhofer, J., Meiser, S. L., Arnold-Schild, D., Klein, M., Stassen, M., Schild, H., Muth, S., Probst, H. C., Langguth, P., Grabbe, S., & Radsak, M. P. (2023). Optimized dithranol-imiquimod-based transcutaneous immunization enables tumor rejection. Frontiers in immunology, 14, 1238861. [CrossRef]
- Del Poeta, G., Laureana, R., Bomben, R., Rossi, F. M., Pozzo, F., Zaina, E., Cattarossi, I., Varaschin, P., Nanni, P., Boschian Boschin, R., Nunzi, A., Postorino, M., Pasqualone, G., Brisotto, G., Steffan, A., Muraro, E., Zucchetto, A., Del Principe, M. I., & Gattei, V. (2023). COVID-19 vaccination: Evaluation of humoral and cellular immunity after the booster dose in chronic lymphocytic leukemia patients. Hematological oncology, 41(3), 559–562. [CrossRef]
- Andorko, J. I., Tsai, S. J., Gammon, J. M., Carey, S. T., Zeng, X., Gosselin, E. A., Edwards, C., Shah, S. A., Hess, K. L., & Jewell, C. M. (2022). Spatial delivery of immune cues to lymph nodes to define therapeutic outcomes in cancer vaccination. Biomaterials science, 10(16), 4612–4626. [CrossRef]
- Zhang, Y., Sriramaneni, R. N., Clark, P. A., Jagodinsky, J. C., Ye, M., Jin, W., Wang, Y., Bates, A., Kerr, C. P., Le, T., Allawi, R., Wang, X., Xie, R., Havighurst, T. C., Chakravarty, I., Rakhmilevich, A. L., O'Leary, K. A., Schuler, L. A., Sondel, P. M., Kim, K., … Morris, Z. S. (2022). Multifunctional nanoparticle potentiates the in situ vaccination effect of radiation therapy and enhances response to immune checkpoint blockade. Nature communications, 13(1), 4948. [CrossRef]
- Bukhari, S. I., Jehan, F., & Belgaumi, A. (2024). Global Immunization Crisis Amid the COVID-19 Pandemic: Implications for Pediatric Oncology. JCO global oncology, 10, e2300477. [CrossRef]
- Alonso-Miguel, D., Valdivia, G., Guerrera, D., Perez-Alenza, M. D., Pantelyushin, S., Alonso-Diez, A., Beiss, V., Fiering, S., Steinmetz, N. F., Suarez-Redondo, M., Vom Berg, J., Peña, L., & Arias-Pulido, H. (2022). Neoadjuvant in situ vaccination with cowpea mosaic virus as a novel therapy against canine inflammatory mammary cancer. Journal for immunotherapy of cancer, 10(3), e004044. [CrossRef]
- Lam, B., Kung, Y. J., Lin, J., Tseng, S. H., Tu, H. F., Huang, C., Lee, B., Velarde, E., Tsai, Y. C., Villasmil, R., Park, S. T., Xing, D., Hung, C. F., & Wu, T. C. (2024). In situ vaccination via tissue-targeted cDC1 expansion enhances the immunogenicity of chemoradiation and immunotherapy. The Journal of clinical investigation, 134(1), e171621. [CrossRef]
- Eini, L., Naseri, M., Karimi-Busheri, F., Bozorgmehr, M., Ghods, R., & Madjd, Z. (2023). Preventive cancer stem cell-based vaccination modulates tumor development in syngeneic colon adenocarcinoma murine model. Journal of cancer research and clinical oncology, 149(7), 4101–4116. [CrossRef]
- Peng, S., Chen, S., Hu, W., Mei, J., Zeng, X., Su, T., Wang, W., Chen, Z., Xiao, H., Zhou, Q., Li, B., Xie, Y., Hu, H., He, M., Han, Y., Tang, L., Ma, Y., Li, X., Zhou, X., Dai, Z., … Kuang, M. (2022). Combination Neoantigen-Based Dendritic Cell Vaccination and Adoptive T-Cell Transfer Induces Antitumor Responses Against Recurrence of Hepatocellular Carcinoma. Cancer immunology research, 10(6), 728–744. [CrossRef]
- Zandvakili, R., Basirjafar, P., Masoumi, J., Zainodini, N., Taghipour, Z., Khorramdelazad, H., Yousefi, S., Tavakoli, T., Safdel, S., Gheitasi, M., Ayoobi, F., & Jafarzadeh, A. (2023). Vaccination with celecoxib-treated dendritic cells improved cellular immune responses in an animal breast cancer model. Advances in medical sciences, 68(1), 157–168. [CrossRef]
- Shou, J., Mo, F., Zhang, S., Lu, L., Han, N., Liu, L., Qiu, M., Li, H., Han, W., Ma, D., Guo, X., Guo, Q., Huang, Q., Zhang, X., Ye, S., Pan, H., Chen, S., & Fang, Y. (2022). Combination treatment of radiofrequency ablation and peptide neoantigen vaccination: Promising modality for future cancer immunotherapy. Frontiers in immunology, 13, 1000681. [CrossRef]
- Niavarani, S. R., St-Cyr, G., Daniel, L., Lawson, C., Giguère, H., Alkayyal, A. A., & Tai, L. H. (2023). Heterologous prime-boost cellular vaccination induces potent antitumor immunity against triple negative breast cancer. Frontiers in immunology, 14, 1098344. [CrossRef]
- Domingos-Pereira, S., Roh, V., Hiou-Feige, A., Galliverti, G., Simon, C., Tolstonog, G. V., & Nardelli-Haefliger, D. (2021). Vaccination with a nanoparticle E7 vaccine can prevent tumor recurrence following surgery in a human papillomavirus head and neck cancer model. Oncoimmunology, 10(1), 1912473. [CrossRef]
- Sunil, V., Mozhi, A., Zhan, W., Teoh, J. H., Ghode, P. B., Thakor, N. V., & Wang, C. H. (2022). In-situ vaccination using dual responsive organelle targeted nanoreactors. Biomaterials, 290, 121843. [CrossRef]
- Ellingsen, E. B., O'Day, S., Mezheyeuski, A., Gromadka, A., Clancy, T., Kristedja, T. S., Milhem, M., & Zakharia, Y. (2023). Clinical Activity of Combined Telomerase Vaccination and Pembrolizumab in Advanced Melanoma: Results from a Phase I Trial. Clinical cancer research : an official journal of the American Association for Cancer Research, 29(16), 3026–3036. [CrossRef]
- Repáraz, D., Ruiz, M., Silva, L., Aparicio, B., Egea, J., Guruceaga, E., Ajona, D., Senent, Y., Conde, E., Navarro, F., Barace, S., Alignani, D., Hervás-Stubbs, S., Lasarte, J. J., Llopiz, D., & Sarobe, P. (2022). Gemcitabine-mediated depletion of immunosuppressive dendritic cells enhances the efficacy of therapeutic vaccination. Frontiers in immunology, 13, 991311. [CrossRef]
- Ghanaat, M., Kaboosi, H., Negahdari, B., Fattahi, E., & Malekshahi, Z. V. (2023). Heterologous Prime-boost Vaccination Using Adenovirus and Albumin Nanoparticles as Carriers for Human Papillomavirus 16 E7 Epitope. Current pharmaceutical biotechnology, 24(9), 1195–1203. [CrossRef]
- Clark, P. A., Sriramaneni, R. N., Jin, W. J., Jagodinsky, J. C., Bates, A. M., Jaquish, A. A., Anderson, B. R., Le, T., Lubin, J. A., Chakravarty, I., Arthur, I. S., Heinze, C. M., Guy, E. I., Kler, J., Klar, K. A., Carlson, P. M., Kim, K. M., Kuo, J. S., & Morris, Z. S. (2020). In situ vaccination at a peripheral tumor site augments response against melanoma brain metastases. Journal for immunotherapy of cancer, 8(2), e000809. [CrossRef]
- Mair, M. J., Berger, J. M., Berghoff, A. S., Starzer, A. M., Ortmayr, G., Puhr, H. C., Steindl, A., Perkmann, T., Haslacher, H., Strassl, R., Tobudic, S., Lamm, W. W., Raderer, M., Mitterer, M., Fuereder, T., Fong, D., & Preusser, M. (2022). Humoral Immune Response in Hematooncological Patients and Health Care Workers Who Received SARS-CoV-2 Vaccinations. JAMA oncology, 8(1), 106–113. [CrossRef]
- Son, H. Y., Jeong, H. K., Apostolopoulos, V., & Kim, C. W. (2022). MUC1 expressing tumor growth was retarded after human mucin 1 (MUC1) plasmid DNA immunization. International journal of immunopathology and pharmacology, 36, 3946320221112358. [CrossRef]
- Shin, H., & Na, K. (2020). Cancer-Targetable pH-Sensitive Zinc-Based Immunomodulators Combined with Photodynamic Therapy for in Situ Vaccination. ACS biomaterials science & engineering, 6(6), 3430–3439. [CrossRef]
- Pol, J. G., Bridle, B. W., & Lichty, B. D. (2020). Detection of Tumor Antigen-Specific T-Cell Responses After Oncolytic Vaccination. Methods in molecular biology (Clifton, N.J.), 2058, 191–211. [CrossRef]
- Friedrich, R. E., Nörnberg, L. K. N., & Hagel, C. (2022). Peripheral Nerve Sheath Tumors in Patients With Neurofibromatosis Type 1: Morphological and Immunohistochemical Study. Anticancer research, 42(3), 1247–1261. [CrossRef]
- He, T., Shi, Y., Kou, X., Shen, M., Liang, X., Li, X., Wu, R., You, Y., Wu, Q., & Gong, C. (2023). Antigenicity and adjuvanticity co-reinforced personalized cell vaccines based on self-adjuvanted hydrogel for post-surgical cancer vaccination. Biomaterials, 301, 122218. [CrossRef]
- Figueiredo, J. C., Merin, N. M., Hamid, O., Choi, S. Y., Lemos, T., Cozen, W., Nguyen, N., Finster, L. J., Foley, J., Darrah, J., Gong, J., Paquette, R., Mita, A. C., Vescio, R., Mehmi, I., Basho, R., Tourtellotte, W. G., Huynh, C. A., Melmed, G. Y., Braun, J., … Merchant, A. (2021). Longitudinal SARS-CoV-2 mRNA Vaccine-Induced Humoral Immune Responses in Patients with Cancer. Cancer research, 81(24), 6273–6280. [CrossRef]
- Bakhtadze, S., Lim, M., Craiu, D., & Cazacu, C. (2021). Vaccination in acute immune-mediated/inflammatory disorders of the central nervous system. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society, 34, 118–122. [CrossRef]
- Radbruch, A., & Melchers, F. (2024). Warum die Regeneration von immunologischer Toleranz durch Impfen schwierig ist [Why the regeneration of immunological tolerance by vaccination is difficult]. Zeitschrift fur Rheumatologie, 83(2), 105–111. [CrossRef]
- Li, Y., Luo, Y., Hou, L., Huang, Z., Wang, Y., & Zhou, S. (2023). Antigen-Capturing Dendritic-Cell-Targeting Nanoparticles for Enhanced Tumor Immunotherapy Based on Photothermal-Therapy-Induced In Situ Vaccination. Advanced healthcare materials, 12(22), e2202871. [CrossRef]
- Liu, X., Su, Q., Song, H., Shi, X., Zhang, Y., Zhang, C., Huang, P., Dong, A., Kong, D., & Wang, W. (2021). PolyTLR7/8a-conjugated, antigen-trapping gold nanorods elicit anticancer immunity against abscopal tumors by photothermal therapy-induced in situ vaccination. Biomaterials, 275, 120921. [CrossRef]
- Patenaude, R., Yasmin-Karim, S., Peng, Y., Wucherpfennig, K. W., Ngwa, W., Kheir, J. N., & Polizzotti, B. D. (2023). Injectable Oxygen Microparticles Boost Radiation-Mediated In Situ Vaccination and Systemic Antitumor Immune Responses. International journal of radiation oncology, biology, physics, 116(4), 906–915. [CrossRef]
- Salewski, I., Gladbach, Y. S., Kuntoff, S., Irmscher, N., Hahn, O., Junghanss, C., & Maletzki, C. (2020). In vivo vaccination with cell line-derived whole tumor lysates: neoantigen quality, not quantity matters. Journal of translational medicine, 18(1), 402. [CrossRef]
- Stegmann, T., Wiekmeijer, A. S., Kwappenberg, K., van Duikeren, S., Bhoelan, F., Bemelman, D., Beenakker, T. J. M., Krebber, W. J., Arens, R., & Melief, C. J. M. (2023). Enhanced HPV16 E6/E7+ tumor eradication via induction of tumor-specific T cells by therapeutic vaccination with virosomes presenting synthetic long peptides. Cancer immunology, immunotherapy : CII, 72(8), 2851–2864. [CrossRef]
- Jackson, K., Samaddar, S., Markiewicz, M. A., & Bansal, A. (2023). Vaccination-Based Immunoprevention of Colorectal Tumors: A Primer for the Clinician. Journal of clinical gastroenterology, 57(3), 246–252. [CrossRef]
- Trabbic, K. R., Whalen, K., Abarca-Heideman, K., Xia, L., Temme, J. S., Edmondson, E. F., Gildersleeve, J. C., & Barchi, J. J., Jr (2019). A Tumor-Selective Monoclonal Antibody from Immunization with a Tumor-Associated Mucin Glycopeptide. Scientific reports, 9(1), 5662. [CrossRef]
- Preusser, M., & van den Bent, M. J. (2023). Autologous tumor lysate-loaded dendritic cell vaccination (DCVax-L) in glioblastoma: Breakthrough or fata morgana?. Neuro-oncology, 25(4), 631–634. [CrossRef]
- Szallasi, Z., Prosz, A., Sztupinszki, Z., & Moldvay, J. (2024). Are tumor-associated carbohydrates the missing link between the gut microbiome and response to immune checkpoint inhibitor treatment in cancer?. Oncoimmunology, 13(1), 2324493. [CrossRef]
- Fan, Q., Ma, Q., Bai, J., Xu, J., Fei, Z., Dong, Z., Maruyama, A., Leong, K. W., Liu, Z., & Wang, C. (2020). An implantable blood clot-based immune niche for enhanced cancer vaccination. Science advances, 6(39), eabb4639. [CrossRef]
- Caldera, F., Farraye, F. A., Necela, B. M., Cogen, D., Saha, S., Wald, A., Daoud, N. D., Chun, K., Grimes, I., Lutz, M., Van Helden, S. R., Swift, M. D., Virk, A., Bharucha, A. E., Patel, T. C., Gores, G. J., Chumsri, S., Hayney, M. S., & Knutson, K. L. (2023). Higher Cell-Mediated Immune Responses in Patients With Inflammatory Bowel Disease on Anti-TNF Therapy After COVID-19 Vaccination. Inflammatory bowel diseases, 29(8), 1202–1209. [CrossRef]
- Shahgolzari, M., Pazhouhandeh, M., Milani, M., Fiering, S., & Khosroushahi, A. Y. (2021). Alfalfa mosaic virus nanoparticles-based in situ vaccination induces antitumor immune responses in breast cancer model. Nanomedicine (London, England), 16(2), 97–107. [CrossRef]
- Zhao, Z., Ukidve, A., Krishnan, V., Fehnel, A., Pan, D. C., Gao, Y., Kim, J., Evans, M. A., Mandal, A., Guo, J., Muzykantov, V. R., & Mitragotri, S. (2021). Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases. Nature biomedical engineering, 5(5), 441–454. [CrossRef]
- Nosan, G., Paro-Panjan, D., Ihan, A., Kopitar, A. N., Čučnik, S., & Avčin, T. (2019). Vaccine immune response, autoimmunity and morbidity after neonatal blood exchange transfusion. Vaccine, 37(30), 4076–4080. [CrossRef]
- Cerna, K., Duricova, D., Hindos, M., Hindos Hrebackova, J., Lukas, M., Machkova, N., Hruba, V., Mitrova, K., Kubickova, K., Kastylova, K., Teplan, V., & Lukas, M. (2022). Cellular and Humoral Immune Responses to SARS-CoV-2 Vaccination in Inflammatory Bowel Disease Patients. Journal of Crohn's & colitis, 16(9), 1347–1353. [CrossRef]
- Osborne, N., Sundseth, R., Burks, J., Cao, H., Liu, X., Kroemer, A. H., Sutton, L., Cato, A., & Smith, J. P. (2019). Gastrin vaccine improves response to immune checkpoint antibody in murine pancreatic cancer by altering the tumor microenvironment. Cancer immunology, immunotherapy : CII, 68(10), 1635–1648. [CrossRef]
- Elizondo, C. R., Bright, J. D., & Bright, R. K. (2022). Vaccination with a shared oncogenic tumor-self antigen elicits a population of CD8+ T cells with a regulatory phenotype. Human vaccines & immunotherapeutics, 18(6), 2108656. [CrossRef]
- Chung, D. J., Shah, G. L., Devlin, S. M., Ramanathan, L. V., Doddi, S., Pessin, M. S., Hoover, E., Marcello, L. T., Young, J. C., Boutemine, S. R., Serrano, E., Sharan, S., Momotaj, S., Margetich, L., Bravo, C. D., Papanicolaou, G. A., Kamboj, M., Mato, A. R., Roeker, L. E., Hultcrantz, M., … Knorr, D. A. (2021). Disease- and Therapy-Specific Impact on Humoral Immune Responses to COVID-19 Vaccination in Hematologic Malignancies. Blood cancer discovery, 2(6), 568–576. [CrossRef]
- Toret, E., Yel, S. E., Suman, M., Duzenli Kar, Y., Ozdemir, Z. C., Dinleyici, M., & Bor, O. (2021). Immunization status and re-immunization of childhood acute lymphoblastic leukemia survivors. Human vaccines & immunotherapeutics, 17(4), 1132–1135. [CrossRef]
- Oketch, S. Y., Ochomo, E. O., Orwa, J. A., Mayieka, L. M., & Abdullahi, L. H. (2023). Communication strategies to improve human papillomavirus (HPV) immunisation uptake among adolescents in sub-Saharan Africa: a systematic review and meta-analysis. BMJ open, 13(4), e067164. [CrossRef]
- Ellingsen, E. B., Aamdal, E., Guren, T., Lilleby, W., Brunsvig, P. F., Mangsbo, S. M., Aamdal, S., Hovig, E., Mensali, N., Gaudernack, G., & Inderberg, E. M. (2022). Durable and dynamic hTERT immune responses following vaccination with the long-peptide cancer vaccine UV1: long-term follow-up of three phase I clinical trials. Journal for immunotherapy of cancer, 10(5), e004345. [CrossRef]
- Wagner, A., Garner-Spitzer, E., Schötta, A. M., Orola, M., Wessely, A., Zwazl, I., Ohradanova-Repic, A., Weseslindtner, L., Tajti, G., Gebetsberger, L., Kratzer, B., Tomosel, E., Kutschera, M., Tobudic, S., Pickl, W. F., Kundi, M., Stockinger, H., Novacek, G., Reinisch, W., Zielinski, C., … Wiedermann, U. (2022). SARS-CoV-2-mRNA Booster Vaccination Reverses Non-Responsiveness and Early Antibody Waning in Immunocompromised Patients - A Phase Four Study Comparing Immune Responses in Patients With Solid Cancers, Multiple Myeloma and Inflammatory Bowel Disease. Frontiers in immunology, 13, 889138. [CrossRef]
- Wieske, L., Stalman, E. W., van Dam, P. J. K., Kummer, L. Y., Steenhuis, M., van Kempen, Z. L. E., Killestein, J., Volkers, A. G., Tas, S. W., Boekel, L., Wolbink, G., Van der Kooi, A., Raaphorst, J., Löwenberg, M., Takkenberg, B., D'Haens, G. R. A. M., Spuls, P. I., Bekkenk, M. W., Musters, A. H., Post, N. F., … T2B! immunity against SARS-CoV-2 study group (2023). Persistence of seroconversion at 6 months following primary immunisation in patients with immune-mediated inflammatory diseases. Annals of the rheumatic diseases, 82(6), 883–885. [CrossRef]
- Melssen, M. M., Pollack, K. E., Meneveau, M. O., Smolkin, M. E., Pinczewski, J., Koeppel, A. F., Turner, S. D., Sol-Church, K., Hickman, A., Deacon, D. H., Petroni, G. R., & Slingluff, C. L., Jr (2021). Characterization and comparison of innate and adaptive immune responses at vaccine sites in melanoma vaccine clinical trials. Cancer immunology, immunotherapy : CII, 70(8), 2151–2164. [CrossRef]
- Ogasawara, M., Miyashita, M., Yamagishi, Y., & Ota, S. (2022). Wilms' tumor 1 peptide-loaded dendritic cell vaccination in patients with relapsed or refractory acute leukemia. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy, 26(3), 537–547. [CrossRef]
- Xi, X., Ye, T., Wang, S., Na, X., Wang, J., Qing, S., Gao, X., Wang, C., Li, F., Wei, W., & Ma, G. (2020). Self-healing microcapsules synergetically modulate immunization microenvironments for potent cancer vaccination. Science advances, 6(21), eaay7735. [CrossRef]
- Shi, Y., Zhu, C., Liu, Y., Lu, Y., Li, X., Qin, B., Luo, Z., Luo, L., Jiang, M., Zhang, J., Guan, G., Zheng, C., & You, J. (2021). A Vaccination with Boosted Cross Presentation by ER-Targeted Antigen Delivery for Anti-Tumor Immunotherapy. Advanced healthcare materials, 10(8), e2001934. [CrossRef]
- Aleman, A., van Kesteren, M., Zajdman, A. K., Srivastava, K., Cognigni, C., Mischka, J., Chen, L. Y., Upadhyaya, B., Serebryakova, K., Nardulli, J. R., Lyttle, N., Kappes, K., Jackson, H., Gleason, C. R., Oostenink, A., Cai, G. Y., Van Oekelen, O., PVI/MM/Seronet Study Group, van Bakel, H., Sordillo, E. M., … Parekh, S. (2023). Cellular mechanisms associated with sub-optimal immune responses to SARS-CoV-2 bivalent booster vaccination in patients with Multiple Myeloma. EBioMedicine, 98, 104886. [CrossRef]
- Pasqualetti, F., & Zanotti, S. (2023). Nonrandomised controlled trial in recurrent glioblastoma patients: the promise of autologous tumour lysate-loaded dendritic cell vaccination. British journal of cancer, 129(6), 895–896. [CrossRef]
- Goradel, N. H., Negahdari, B., Mohajel, N., Malekshahi, Z. V., Shirazi, M. M. A., & Arashkia, A. (2021). Heterologous administration of HPV16 E7 epitope-loaded nanocomplexes inhibits tumor growth in mouse model. International immunopharmacology, 101(Pt B), 108298. [CrossRef]
- Holm-Yildiz, S., Dysgaard, T., Krag, T., Pedersen, B. S., Hamm, S. R., Pérez-Alós, L., Hansen, C. B., Pries-Heje, M. M., Heftdal, L. D., Hasselbalch, R. B., Fogh, K., Madsen, J. R., Frikke-Schmidt, R., Hilsted, L. M., Sørensen, E., Ostrowski, S. R., Bundgaard, H., Garred, P., Iversen, K., Nielsen, S. D., … Vissing, J. (2023). Humoral immune response to COVID-19 vaccine in patients with myasthenia gravis. Journal of neuroimmunology, 384, 578215. [CrossRef]
- Bersanelli, M., Buti, S., De Giorgi, U., Di Maio, M., Giannarelli, D., Pignata, S., & Banna, G. L. (2019). State of the art about influenza vaccination for advanced cancer patients receiving immune checkpoint inhibitors: When common sense is not enough. Critical reviews in oncology/hematology, 139, 87–90. [CrossRef]
- Dykman, L. A., Staroverov, S. A., Kozlov, S. V., Fomin, A. S., Chumakov, D. S., Gabalov, K. P., Kozlov, Y. S., Soldatov, D. A., & Khlebtsov, N. G. (2022). Immunization of Mice with Gold Nanoparticles Conjugated to Thermostable Cancer Antigens Prevents the Development of Xenografted Tumors. International journal of molecular sciences, 23(22), 14313. [CrossRef]
- Valanparambil, R. M., Carlisle, J., Linderman, S. L., Akthar, A., Millett, R. L., Lai, L., Chang, A., McCook-Veal, A. A., Switchenko, J., Nasti, T. H., Saini, M., Wieland, A., Manning, K. E., Ellis, M., Moore, K. M., Foster, S. L., Floyd, K., Davis-Gardner, M. E., Edara, V. V., Patel, M., … Ahmed, R. (2022). Antibody Response to COVID-19 mRNA Vaccine in Patients With Lung Cancer After Primary Immunization and Booster: Reactivity to the SARS-CoV-2 WT Virus and Omicron Variant. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 40(33), 3808–3816. [CrossRef]
- Mair, M. J., Berger, J. M., Mitterer, M., Gansterer, M., Bathke, A. C., Trutschnig, W., Berghoff, A. S., Perkmann, T., Haslacher, H., Lamm, W. W., Raderer, M., Tobudic, S., Fuereder, T., Buratti, T., Fong, D., & Preusser, M. (2022). Third dose of SARS-CoV-2 vaccination in hemato-oncological patients and health care workers: immune responses and adverse events - a retrospective cohort study. European journal of cancer (Oxford, England : 1990), 165, 184–194. [CrossRef]
- Meneveau, M. O., Kumar, P., Lynch, K. T., Patel, S. P., & Slingluff, C. L. (2022). The vaccine-site microenvironment: impacts of antigen, adjuvant, and same-site vaccination on antigen presentation and immune signaling. Journal for immunotherapy of cancer, 10(3), e003533. [CrossRef]
- Wankhede, D., Grover, S., & Hofman, P. (2023). Determinants of humoral immune response to SARS-CoV-2 vaccines in solid cancer patients: A systematic review and meta-analysis. Vaccine, 41(11), 1791–1798. [CrossRef]
- Faustini, S. E., Hall, A., Brown, S., Roberts, S., Hill, H., Stamataki, Z., (PITCH) consortium, Jenner, M. W., Owen, R. G., Pratt, G., Cook, G., Richter, A., Drayson, M. T., Kaiser, M. F., & Heaney, J. L. J. (2023). Immune responses to COVID-19 booster vaccinations in intensively anti-CD38 antibody treated patients with ultra-high-risk multiple myeloma: results from the Myeloma UK (MUK) nine OPTIMUM trial. British journal of haematology, 201(5), 845–850. [CrossRef]
- Meza, L., Zengin, Z., Salgia, S., Malhotra, J., Karczewska, E., Dorff, T., Tripathi, A., Ely, J., Kelley, E., Mead, H., Hsu, J., Dizman, N., Salgia, N., Chawla, N., Chehrazi-Raffle, A., Muddasani, R., Govindarajan, A., Rock, A., Liu, S., Salgia, R., … Pal, S. K. (2023). Twelve-Month Follow-up of the Immune Response After COVID-19 Vaccination in Patients with Genitourinary Cancers: A Prospective Cohort Analysis. The oncologist, 28(9), e748–e755. [CrossRef]
- Deng, M. Y., Debus, J., & König, L. (2023). Verlängerung des Gesamtüberlebens durch die Impfung von autologen tumorlysatbeladenen dendritischen Zellen (DCVax-L) bei Patienten mit neu diagnostiziertem und rezidivierendem Glioblastom [Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma]. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft... [et al], 199(3), 327–329. [CrossRef]
- Souan, L., Abdel-Razeq, H., Al Zughbieh, M., Al Badr, S., & Sughayer, M. A. (2023). Comparative Assessment of the Kinetics of Cellular and Humoral Immune Responses to COVID-19 Vaccination in Cancer Patients. Viruses, 15(7), 1439. [CrossRef]
- Yang, J., Eresen, A., Shangguan, J., Ma, Q., Yaghmai, V., & Zhang, Z. (2021). Irreversible electroporation ablation overcomes tumor-associated immunosuppression to improve the efficacy of DC vaccination in a mice model of pancreatic cancer. Oncoimmunology, 10(1), 1875638. [CrossRef]
- Pedrazzoli, P., Lasagna, A., Cassaniti, I., Ferrari, A., Bergami, F., Silvestris, N., Sapuppo, E., Di Maio, M., Cinieri, S., & Baldanti, F. (2022). Vaccination for herpes zoster in patients with solid tumors: a position paper on the behalf of the Associazione Italiana di Oncologia Medica (AIOM). ESMO open, 7(4), 100548. [CrossRef]
- MacKerracher, A., Sommershof, A., & Groettrup, M. (2022). PLGA particle vaccination elicits resident memory CD8 T cells protecting from tumors and infection. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 175, 106209. [CrossRef]
- Barrière, J., Re, D., Peyrade, F., & Carles, M. (2021). Current perspectives for SARS-CoV-2 vaccination efficacy improvement in patients with active treatment against cancer. European journal of cancer (Oxford, England : 1990), 154, 66–72. [CrossRef]
- Zhuang, W. H., & Wang, Y. P. (2020). Analysis of the immunity effects after enhanced hepatitis B vaccination on patients with lymphoma. Leukemia & lymphoma, 61(2), 357–363. [CrossRef]
- Storti, P., Marchica, V., Vescovini, R., Franceschi, V., Russo, L., Notarfranchi, L., Raimondi, V., Toscani, D., Burroughs Garcia, J., Costa, F., Dalla Palma, B., Iannozzi, N. T., Sammarelli, G., Donofrio, G., & Giuliani, N. (2022). Immune response to SARS-CoV-2 mRNA vaccination and booster dose in patients with multiple myeloma and monoclonal gammopathies: impact of Omicron variant on the humoral response. Oncoimmunology, 11(1), 2120275. [CrossRef]
- Mitchell, D. K., Burgess, B., White, E. E., Smith, A. E., Sierra Potchanant, E. A., Mang, H., Hickey, B. E., Lu, Q., Qian, S., Bessler, W., Li, X., Jiang, L., Brewster, K., Temm, C., Horvai, A., Albright, E. A., Fishel, M. L., Pratilas, C. A., Angus, S. P., Clapp, D. W., … Rhodes, S. D. (2024). Spatial Gene-Expression Profiling Unveils Immuno-oncogenic Programs of NF1-Associated Peripheral Nerve Sheath Tumor Progression. Clinical cancer research : an official journal of the American Association for Cancer Research, 30(5), 1038–1053. [CrossRef]
- Martin, S. D., Nziza, N., Miozzo, P., Bartsch, Y., Farkas, E. J., Kane, A. S., Boal, L. H., Friedmann, A., Alter, G., & Yonker, L. M. (2023). Humoral profiling of pediatric patients with cancer reveals robust immunity following anti-SARS-CoV-2 vaccination superior to natural infection. Pediatric blood & cancer, 70(8), e30473. [CrossRef]
- Rensink, M. J., van Laarhoven, H. W. M., & Holleman, F. (2021). Cocoon vaccination for influenza in patients with a solid tumor: a retrospective study. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer, 29(7), 3657–3666. [CrossRef]
- Iavarone, M., Tosetti, G., Facchetti, F., Topa, M., Er, J. M., Hang, S. K., Licari, D., Lombardi, A., D'Ambrosio, R., Degasperi, E., Loglio, A., Oggioni, C., Perbellini, R., Caccia, R., Bandera, A., Gori, A., Ceriotti, F., Scudeller, L., Bertoletti, A., & Lampertico, P. (2023). Spike-specific humoral and cellular immune responses after COVID-19 mRNA vaccination in patients with cirrhosis: A prospective single center study. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver, 55(2), 160–168. [CrossRef]
- Shakibapour, M., Kefayat, A., Reza Mofid, M., Shojaie, B., Mohamadi, F., Maryam Sharafi, S., Mahmoudzadeh, M., & Yousofi Darani, H. (2021). Anti-cancer immunoprotective effects of immunization with hydatid cyst wall antigens in a non-immunogenic and metastatic triple-negative murine mammary carcinoma model. International immunopharmacology, 99, 107955. [CrossRef]
- Oltmanns, F., Vieira Antão, A., Irrgang, P., Viherlehto, V., Jörg, L., Schmidt, A., Wagner, J. T., Rückert, M., Flohr, A. S., Geppert, C. I., Frey, B., Bayer, W., Gravekamp, C., Tenbusch, M., Gaipl, U., & Lapuente, D. (2024). Mucosal tumor vaccination delivering endogenous tumor antigens protects against pulmonary breast cancer metastases. Journal for immunotherapy of cancer, 12(3), e008652. [CrossRef]
- Patchett, A. L., Tovar, C., Blackburn, N. B., Woods, G. M., & Lyons, A. B. (2021). Mesenchymal plasticity of devil facial tumour cells during in vivo vaccine and immunotherapy trials. Immunology and cell biology, 99(7), 711–723. [CrossRef]
- Masoumi, J., Jafarzadeh, A., Tavakoli, T., Basirjafar, P., Zandvakili, R., Javan, M. R., Taghipour, Z., & Moazzeni, S. M. (2022). Inhibition of apelin/APJ axis enhances the potential of dendritic cell-based vaccination to modulate TH1 and TH2 cell-related immune responses in an animal model of metastatic breast cancer. Advances in medical sciences, 67(1), 170–178. [CrossRef]
- Kim, J., Jeong, J., Lee, C. M., Lee, D. W., Kang, C. K., Choe, P. G., Kim, N. J., Oh, M. D., Lee, C. H., Park, W. B., Lee, K. H., & Im, S. A. (2022). Prospective longitudinal analysis of antibody response after standard and booster doses of SARS-COV2 vaccination in patients with early breast cancer. Frontiers in immunology, 13, 1028102. [CrossRef]
- Lyski, Z. L., Kim, M. S., Xthona Lee, D., Raué, H. P., Raghunathan, V., Griffin, J., Ryan, D., Brunton, A. E., Curlin, M. E., Slifka, M. K., Messer, W. B., & Spurgeon, S. E. (2022). Cellular and humoral immune response to mRNA COVID-19 vaccination in subjects with chronic lymphocytic leukemia. Blood advances, 6(4), 1207–1211. [CrossRef]
- Jung, E., Mao, C., Bhatia, M., Koellhoffer, E. C., Fiering, S. N., & Steinmetz, N. F. (2023). Inactivated Cowpea Mosaic Virus for In Situ Vaccination: Differential Efficacy of Formalin vs UV-Inactivated Formulations. Molecular pharmaceutics, 20(1), 500–507. [CrossRef]
- Cecil, D. L., Liao, J. B., Dang, Y., Coveler, A. L., Kask, A., Yang, Y., Childs, J. S., Higgins, D. M., & Disis, M. L. (2021). Immunization with a Plasmid DNA Vaccine Encoding the N-Terminus of Insulin-like Growth Factor Binding Protein-2 in Advanced Ovarian Cancer Leads to High-level Type I Immune Responses. Clinical cancer research : an official journal of the American Association for Cancer Research, 27(23), 6405–6412. [CrossRef]
- Taylor, A. C., Hopkins, L. W., & Moore, G. (2021). Increasing human papillomavirus immunization in the primary care setting. The Nurse practitioner, 46(10), 37–42. [CrossRef]
- Stumpf, J., Anders, L., Siepmann, T., Schwöbel, J., Karger, C., Lindner, T., Faulhaber-Walter, R., Langer, T., Escher, K., Anding-Rost, K., Seidel, H., Hüther, J., Pistrosch, F., Martin, H., Schewe, J., Stehr, T., Meistring, F., Paliege, A., Schneider, D., Bast, I., … Hugo, C. (2024). 9-Month observational Dia-Vacc study of vaccine type influence on SARS-CoV-2 immunity in dialysis and kidney transplant patients. Vaccine, 42(2), 120–128. [CrossRef]
- Purshouse, K., Thomson, J. P., Vallet, M., Alexander, L., Bonisteel, I., Brennan, M., Cameron, D. A., Figueroa, J. D., Furrie, E., Haig, P., Heck, M., McCaughan, H., Mitchell, P., McVicars, H., Primrose, L., Silva, I., Templeton, K., Wilson, N., & Hall, P. S. (2023). The Scottish COVID Cancer Immunity Prevalence Study: A Longitudinal Study of SARS-CoV-2 Immune Response in Patients Receiving Anti-Cancer Treatment. The oncologist, 28(3), e145–e155. [CrossRef]
- Bacova, B., Kohutova, Z., Zubata, I., Gaherova, L., Kucera, P., Heizer, T., Mikesova, M., Karel, T., & Novak, J. (2023). Cellular and humoral immune response to SARS-CoV-2 mRNA vaccines in patients treated with either Ibrutinib or Rituximab. Clinical and experimental medicine, 23(2), 371–379. [CrossRef]
- Ukidve, A., Zhao, Z., Fehnel, A., Krishnan, V., Pan, D. C., Gao, Y., Mandal, A., Muzykantov, V., & Mitragotri, S. (2020). Erythrocyte-driven immunization via biomimicry of their natural antigen-presenting function. Proceedings of the National Academy of Sciences of the United States of America, 117(30), 17727–17736. [CrossRef]
- Müller, K. E., Dohos, D., Sipos, Z., Kiss, S., Dembrovszky, F., Kovács, N., Solymár, M., Erőss, B., Hegyi, P., & Sarlós, P. (2022). Immune response to influenza and pneumococcal vaccines in adults with inflammatory bowel disease: A systematic review and meta-analysis of 1429 patients. Vaccine, 40(13), 2076–2086. [CrossRef]
- Debie, Y., Van Audenaerde, J. R. M., Vandamme, T., Croes, L., Teuwen, L. A., Verbruggen, L., Vanhoutte, G., Marcq, E., Verheggen, L., Le Blon, D., Peeters, B., Goossens, M. E., Pannus, P., Ariën, K. K., Anguille, S., Janssens, A., Prenen, H., Smits, E. L. J., Vulsteke, C., Lion, E., … van Dam, P. A. (2023). Humoral and Cellular Immune Responses against SARS-CoV-2 after Third Dose BNT162b2 following Double-Dose Vaccination with BNT162b2 versus ChAdOx1 in Patients with Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research, 29(3), 635–646. [CrossRef]
- Salmon, C., Conus, F., Parent, M. É., Benedetti, A., & Rousseau, M. C. (2020). Association between Bacillus Calmette-Guerin (BCG) vaccination and lymphoma risk: A systematic review and meta-analysis. Cancer epidemiology, 65, 101696. [CrossRef]
- Gharibi, Z., Rahdar, M., Pirestani, M., Tavalla, M., & Tabandeh, M. R. (2021). The Immunization of Protoscolices P29 DNA Vaccine on Experimental Cystic Echinococosis in Balb/c Mice. Acta parasitologica, 66(4), 1114–1121. [CrossRef]
- Ishida, E., Lee, J., Campbell, J. S., Chakravarty, P. D., Katori, Y., Ogawa, T., Johnson, L., Mukhopadhyay, A., Faquin, W. C., Lin, D. T., Wirth, L. J., Pierce, R. H., & Pai, S. I. (2019). Intratumoral delivery of an HPV vaccine elicits a broad anti-tumor immune response that translates into a potent anti-tumor effect in a preclinical murine HPV model. Cancer immunology, immunotherapy : CII, 68(8), 1273–1286. [CrossRef]
- Aleman, A., Van Oekelen, O., Upadhyaya, B., Beach, K., Kogan Zajdman, A., Alshammary, H., Serebryakova, K., Agte, S., Kappes, K., Gleason, C. R., Srivastava, K., PVI/MM/Seronet Study Group, Almo, S., Cordon-Cardo, C., Krammer, F., Merad, M., Jagannath, S., Wajnberg, A., Simon, V., & Parekh, S. (2022). Augmentation of humoral and cellular immune responses after third-dose SARS-CoV-2 vaccination and viral neutralization in myeloma patients. Cancer cell, 40(5), 441–443. [CrossRef]
- Hou, X., Shi, Y., Kang, X., Rousu, Z., Li, D., Wang, M., Ainiwaer, A., Zheng, X., Wang, M., Jiensihan, B., Li, L., Li, J., Wang, H., & Zhang, C. (2022). Echinococcus granulosus: The establishment of the metacestode in the liver is associated with control of the CD4+ T-cell-mediated immune response in patients with cystic echinococcosis and a mouse model. Frontiers in cellular and infection microbiology, 12, 983119. [CrossRef]
- Campal-Espinosa, A. C., Junco-Barranco, J. A., Fuentes-Aguilar, F., Calzada-Aguilera, L., Rivacoba-Betancourt, A., Rodríguez-Bueno, R. H., Bover-Campal, A. C., Bover-Fuentes, E. E., González, L., de Quesada, L., Alvarez, A., & Garay-Pérez, H. E. (2023). Influence of Humoral Response Against GnRH, Generated by Immunization with a Therapeutic Vaccine Candidate on the Evolution of Patients with Castration-Sensitive Prostate Adenocarcinoma. Technology in cancer research & treatment, 22, 15330338231207318. [CrossRef]
- Behrendt, D., Burger, D., Gremmes, S., Szunyog, K., Röthemeier, S., & Sieme, H. (2021). Active immunisation against GnRH as treatment for unilateral granulosa theca cell tumour in mares. Equine veterinary journal, 53(4), 740–745. [CrossRef]
- Lehrnbecher, T., Sack, U., Speckmann, C., Groll, A. H., Boldt, A., Siebald, B., Hettmer, S., Demmerath, E. M., Reemtsma, J., Schenk, B., Ciesek, S., Klusmann, J. H., Jassoy, C., & Hoehl, S. (2023). Longitudinal Immune Response to 3 Doses of Messenger RNA Vaccine Against Coronavirus Disease 2019 (COVID-19) in Pediatric Patients Receiving Chemotherapy for Cancer. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 76(3), e510–e513. [CrossRef]
- Cobanoglu, O., Delval, L., Ferrari, D., Deruyter, L., Heumel, S., Wolowczuk, I., Hussein, A., Menevse, A. N., Bernard, D., Beckhove, P., Alves, F., & Trottein, F. (2023). Depletion of preexisting B-cell lymphoma 2-expressing senescent cells before vaccination impacts antigen-specific antitumor immune responses in old mice. Aging cell, 22(12), e14007. [CrossRef]
- Kallionpää, R. A., Peltonen, S., Le, K. M., Martikkala, E., Jääskeläinen, M., Fazeli, E., Riihilä, P., Haapaniemi, P., Rokka, A., Salmi, M., Leivo, I., & Peltonen, J. (2024). Characterization of Immune Cell Populations of Cutaneous Neurofibromas in Neurofibromatosis 1. Laboratory investigation; a journal of technical methods and pathology, 104(1), 100285. [CrossRef]
- Borgogna, C., Bruna, R., Griffante, G., Martuscelli, L., De Andrea, M., Ferrante, D., Patriarca, A., Mahmoud, A. M., Ucciero, M. A. M., Gaidano, V., Marchetti, M., Rapezzi, D., Lai, M., Pistello, M., Ladetto, M., Massaia, M., Gaidano, G., & Gariglio, M. (2022). Induction of robust humoral immunity against SARS-CoV-2 after vaccine administration in previously infected haematological cancer patients. British journal of haematology, 199(3), 463–467. [CrossRef]
- Kang, C. K., Kim, H. R., Song, K. H., Keam, B., Choi, S. J., Choe, P. G., Kim, E. S., Kim, N. J., Kim, Y. J., Park, W. B., Kim, H. B., & Oh, M. D. (2020). Cell-Mediated Immunogenicity of Influenza Vaccination in Patients With Cancer Receiving Immune Checkpoint Inhibitors. The Journal of infectious diseases, 222(11), 1902–1909. [CrossRef]
- Viana, J. H. M., Pereira, N. E. S., Faria, O. A. C., Dias, L. R. O., Oliveira, E. R., Fernandes, C. A. C., & Siqueira, L. G. B. (2021). Active immunization against GnRH as an alternative therapeutic approach for the management of Bos indicus oocyte donors diagnosed with chronic cystic ovarian disease. Theriogenology, 172, 133–141. [CrossRef]
- Martins-Branco, D., Nader-Marta, G., Tecic Vuger, A., Debien, V., Ameye, L., Brandão, M., Punie, K., Loizidou, A., Willard-Gallo, K., Spilleboudt, C., Awada, A., Piccart, M., & de Azambuja, E. (2023). Immune response to anti-SARS-CoV-2 prime-vaccination in patients with cancer: a systematic review and meta-analysis. Journal of cancer research and clinical oncology, 149(7), 3075–3080. [CrossRef]
- Vanni, A., Salvati, L., Mazzoni, A., Lamacchia, G., Capone, M., Francalanci, S., Kiros, S. T., Cosmi, L., Puccini, B., Ciceri, M., Sordi, B., Rossolini, G. M., Annunziato, F., Maggi, L., & Liotta, F. (2023). Bendamustine impairs humoral but not cellular immunity to SARS-CoV-2 vaccination in rituximab-treated B-cell lymphoma-affected patients. Frontiers in immunology, 14, 1322594. [CrossRef]
- Titova, E., Kan, V. W., Lozy, T., Ip, A., Shier, K., Prakash, V. P., Starolis, M., Ansari, S., Goldgirsh, K., Kim, S., Pelliccia, M. C., Mccutchen, A., Megalla, M., Gunning, T. S., Kaufman, H. W., Meyer, W. A., 3rd, & Perlin, D. S. (2024). Humoral and cellular immune responses against SARS-CoV-2 post-vaccination in immunocompetent and immunocompromised cancer populations. Microbiology spectrum, 12(3), e0205023. [CrossRef]
- Weitgasser, L., Mahrhofer, M., & Schoeller, T. (2021). Potential immune response to breast implants after immunization with COVID-19 vaccines. Breast (Edinburgh, Scotland), 59, 76–78. [CrossRef]
- Aurisicchio, L., Fridman, A., Mauro, D., Sheloditna, R., Chiappori, A., Bagchi, A., & Ciliberto, G. (2020). Safety, tolerability and immunogenicity of V934/V935 hTERT vaccination in cancer patients with selected solid tumors: a phase I study. Journal of translational medicine, 18(1), 39. [CrossRef]
- Xu, P., Ma, J., Zhou, Y., Gu, Y., Cheng, X., Wang, Y., Wang, Y., & Gao, M. (2024). Radiotherapy-Triggered In Situ Tumor Vaccination Boosts Checkpoint Blockaded Immune Response via Antigen-Capturing Nanoadjuvants. ACS nano, 18(1), 1022–1040. [CrossRef]
- Peeters, M., Verbruggen, L., Teuwen, L., Vanhoutte, G., Vande Kerckhove, S., Peeters, B., Raats, S., Van der Massen, I., De Keersmaecker, S., Debie, Y., Huizing, M., Pannus, P., Neven, K., Ariën, K. K., Martens, G. A., Van Den Bulcke, M., Roelant, E., Desombere, I., Anguille, S., Goossens, M., … van Dam, P. (2021). Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO open, 6(5), 100274. [CrossRef]
- Lövgren, T., Wolodarski, M., Wickström, S., Edbäck, U., Wallin, M., Martell, E., Markland, K., Blomberg, P., Nyström, M., Lundqvist, A., Jacobsson, H., Ullenhag, G., Ljungman, P., Hansson, J., Masucci, G., Tell, R., Poschke, I., Adamson, L., Mattsson, J., & Kiessling, R. (2020). Complete and long-lasting clinical responses in immune checkpoint inhibitor-resistant, metastasized melanoma treated with adoptive T cell transfer combined with DC vaccination. Oncoimmunology, 9(1), 1792058. [CrossRef]
- Enssle, J. C., Campe, J., Büchel, S., Moter, A., See, F., Grießbaum, K., Rieger, M. A., Wolf, S., Ballo, O., Steffen, B., Serve, H., Rabenau, H. F., Widera, M., Bremm, M., Huenecke, S., Ciesek, S., von Metzler, I., & Ullrich, E. (2022). Enhanced but variant-dependent serological and cellular immune responses to third-dose BNT162b2 vaccination in patients with multiple myeloma. Cancer cell, 40(6), 587–589. [CrossRef]
- Enssle, J. C., Campe, J., Büchel, S., Moter, A., See, F., Grießbaum, K., Rieger, M. A., Wolf, S., Ballo, O., Steffen, B., Serve, H., Rabenau, H. F., Widera, M., Bremm, M., Huenecke, S., Ciesek, S., von Metzler, I., & Ullrich, E. (2022). Enhanced but variant-dependent serological and cellular immune responses to third-dose BNT162b2 vaccination in patients with multiple myeloma. Cancer cell, 40(6), 587–589. [CrossRef]
- Alimam, S., Ann Timms, J., Harrison, C. N., Dillon, R., Mare, T., DeLavallade, H., Radia, D., Woodley, C., Francis, Y., Sanchez, K., Kordasti, S., & McLornan, D. P. (2021). Altered immune response to the annual influenza A vaccine in patients with myeloproliferative neoplasms. British journal of haematology, 193(1), 150–154. [CrossRef]
- Zou, Z., Guo, L., Mautner, V., Smeets, R., Kiuwe, L., & Friedrich, R. E. (2020). Propranolol Specifically Suppresses the Viability of Tumorous Schwann Cells Derived from Plexiform Neurofibromas In Vitro. In vivo (Athens, Greece), 34(3), 1031–1036. [CrossRef]
- Mohan, M., Nagavally, S., Shah N, N. N., Michaelis, L., Chhabra, S., Souza, A. D., Abedin, S., Runaas, L., Guru Murthy, G. S., Longo, W., Hamadani, M., Dhakal, B., Hari, P., & Fenske, T. S. (2022). Shorter Interval between Treatment and COVID Immunization Is Associated With Poor Seroconversion in Patients with Hematological Malignancies. Clinical lymphoma, myeloma & leukemia, 22(7), e495–e497. [CrossRef]
- Meena, J., Kumar, R., Singh, M., Ahmed, A., & Panda, A. K. (2020). Modulation of immune response and enhanced clearance of Salmonella typhi by delivery of Vi polysaccharide conjugate using PLA nanoparticles. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 152, 270–281. [CrossRef]
- Suzuki, T., Kusumoto, S., Kamezaki, Y., Hashimoto, H., Nishitarumizu, N., Nakanishi, Y., Kato, Y., Kawai, A., Matsunaga, N., Ebina, T., Nakamura, T., Marumo, Y., Oiwa, K., Kinoshita, S., Narita, T., Ito, A., Inagaki, A., Ri, M., Komatsu, H., Aritsu, T., … Iida, S. (2023). A comprehensive evaluation of humoral immune response to second and third SARS-CoV-2 mRNA vaccination in patients with malignant lymphoma. International journal of hematology, 117(6), 900–909. [CrossRef]
- Cerda, C., Martínez-Valdebenito, C., Barriga, F., Contreras, M., Vidal, M., Moreno, R., Claverie, X., Contreras, P., Huenuman, L., García, T., Rathnasighe, R., Medina, R., Ferrés, M., & Le Corre, N. (2020). Respuesta inmune humoral inducida por la vacuna influenza en niños con diagnóstico de leucemia linfoblástica aguda [Humoral immune response induced by influenza vaccine in children with acute lymphoblastic leukemia]. Revista chilena de infectologia : organo oficial de la Sociedad Chilena de Infectologia, 37(2), 138–146. [CrossRef]
- He, Y., Chen, D., Fu, Y., Huo, X., Zhao, F., Yao, L., Zhou, X., Qi, P., Yin, H., Cao, L., Ling, H., & Zeng, T. (2023). Immunization with Tp0954, an adhesin of Treponema pallidum, provides protective efficacy in the rabbit model of experimental syphilis. Frontiers in immunology, 14, 1130593. [CrossRef]
- Dahiya, S., Luetkens, T., Lutfi, F., Avila, S., Iraguha, T., Margiotta, P., Hankey, K. G., Lesho, P., Law, J. Y., Lee, S. T., Baddley, J., Kocoglu, M., Yared, J. A., Hardy, N. M., Rapoport, A. P., & Atanackovic, D. (2022). Impaired immune response to COVID-19 vaccination in patients with B-cell malignancies after CD19 CAR T-cell therapy. Blood advances, 6(2), 686–689. [CrossRef]
- Barber, V. S., Peckham, N., Duley, L., Francis, A., Abhishek, A., Moss, P., Cook, J. A., & Parry, H. M. (2023). Protocol for a multicentre randomised controlled trial examining the effects of temporarily pausing Bruton tyrosine kinase inhibitor therapy to coincide with SARS-CoV-2 vaccination and its impact on immune responses in patients with chronic lymphocytic leukaemia. BMJ open, 13(9), e077946. [CrossRef]
- Kanjanapan, Y., Blinman, P., Underhill, C., Karikios, D., Segelov, E., & Yip, D. (2021). Medical Oncology Group of Australia position statement: COVID-19 vaccination in patients with solid tumours. Internal medicine journal, 51(6), 955–959. [CrossRef]
- Fang, S., Agostinis, P., Salven, P., & Garg, A. D. (2020). Decoding cancer cell death-driven immune cell recruitment: An in vivo method for site-of-vaccination analyses. Methods in enzymology, 636, 185–207. [CrossRef]
- Stumpf, J., Klimova, A., Mauer, R., Steglich, A., Gembardt, F., Martin, H., Glombig, G., Frank, K., Tonn, T., & Hugo, C. (2022). Equivalent humoral and cellular immune response but different side effect rates following SARS-CoV-2 vaccination in peritoneal and haemodialysis patients using messenger RNA vaccines. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 37(4), 796–798. [CrossRef]
- Lozano-Rodríguez, R., Terrón-Arcos, V., Montalbán-Hernández, K., Casalvilla-Dueñas, J. C., Bergón-Gutierrez, M., Pascual-Iglesias, A., Quiroga, J. V., Aguirre, L. A., Pérez de Diego, R., Vela-Olmo, C., López-Morejón, L., Martín-Quirós, A., Del Balzo-Castillo, Á., Peinado-Quesada, M. A., García-Garrido, M. A., Gómez-Lage, L., Herrero-Benito, C., Llorente-Fernández, I., Martín-Miguel, G., Torrejón, M., … López-Collazo, E. (2022). Prior SARS-CoV-2 infection balances immune responses triggered by four EMA-approved COVID-19 vaccines: An observational study. Clinical and translational medicine, 12(5), e869. [CrossRef]
- Boerenkamp, L. S., Pothast, C. R., Dijkland, R. C., van Dijk, K., van Gorkom, G. N. Y., van Loo, I. H. M., Wieten, L., Halkes, C. J. M., Heemskerk, M. H. M., & Van Elssen, C. H. M. J. (2022). Increased CD8 T-cell immunity after COVID-19 vaccination in lymphoid malignancy patients lacking adequate humoral response: An immune compensation mechanism?. American journal of hematology, 97(12), E457–E461. [CrossRef]
- Choi, D. K., Strzepka, J. T., Hunt, S. R., Tannenbaum, V. L., & Jang, I. E. (2020). Vaccination in pediatric cancer survivors: Vaccination rates, immune status, and knowledge regarding compliance. Pediatric blood & cancer, 67(10), e28565. [CrossRef]
- Xing, Y., Yang, J., Yao, P., Xie, L., Liu, M., & Cai, Y. (2024). Comparison of the immune response and protection against the experimental Toxoplasma gondii infection elicited by immunization with the recombinant proteins BAG1, ROP8, and BAG1-ROP8. Parasite immunology, 46(2), e13023. [CrossRef]
- Sesques, P., Bachy, E., Ferrant, E., Safar, V., Gossez, M., Morfin-Sherpa, F., Venet, F., & Ader, F. (2022). Immune response to three doses of mRNA SARS-CoV-2 vaccines in CD19-targeted chimeric antigen receptor T cell immunotherapy recipients. Cancer cell, 40(3), 236–237. [CrossRef]
- Seban, R. D., Champion, L., Yeh, R., Schwartz, L. H., & Dercle, L. (2021). Assessing immune response upon systemic RNA vaccination on [18F]-FDG PET/CT for COVID-19 vaccine and then for immuno-oncology?. European journal of nuclear medicine and molecular imaging, 48(11), 3351–3352. [CrossRef]
- Moulik, N. R., Mandal, P., Chandra, J., Bansal, S., Jog, P., Sanjay, S., Shah, N., & Arora, R. S. (2019). Immunization of Children with Cancer in India Treated with Chemotherapy - Consensus Guideline from the Pediatric Hematology-Oncology Chapter and the Advisory Committee on Vaccination and Immunization Practices of the Indian Academy of Pediatrics. Indian pediatrics, 56(12), 1041–1048.
- Motwani, K. K., Hashash, J. G., Farraye, F. A., Kappelman, M. D., Weaver, K. N., Zhang, X., Long, M. D., & Cross, R. K. (2023). Impact of Holding Immunosuppressive Therapy in Patients with Inflammatory Bowel Disease Around mRNA COVID-19 Vaccine Administration on Humoral Immune Response and Development of COVID-19 Infection. Journal of Crohn's & colitis, 17(10), 1681–1688. [CrossRef]
- Safavi, A., Kefayat, A., Ghahremani, F., Mahdevar, E., & Moshtaghian, J. (2019). Immunization using male germ cells and gametes as rich sources of cancer/testis antigens for inhibition of 4T1 breast tumors' growth and metastasis in BALB/c mice. International immunopharmacology, 74, 105719. [CrossRef]
- Lundstrom K. (2021). Immune Responses of Alphavirus Vaccination in Patients with HPV-Induced Cancers. Molecular therapy : the journal of the American Society of Gene Therapy, 29(2), 415–416. [CrossRef]
- Oosting, S. F., van der Veldt, A. A. M., Fehrmann, R. S. N., Bhattacharya, A., van Binnendijk, R. S., GeurtsvanKessel, C. H., Dingemans, A. C., Smit, E. F., Hiltermann, T. J. N., den Hartog, G., Jalving, M., Westphal, T. T., de Wilt, F., Ernst, S. M., Boerma, A., van Zijl, L., Rimmelzwaan, G. F., Kvistborg, P., van Els, C. A. C. M., Rots, N. Y., … de Vries, E. G. E. (2023). Factors associated with long-term antibody response after COVID-19 vaccination in patients treated with systemic treatment for solid tumors. ESMO open, 8(4), 101599. [CrossRef]
- Óskarsson, Ý., Thors, V., Vias, R. D., Lúðvíksson, B. R., Brynjólfsson, S. F., Gianchecchi, E., Razzano, I., Montomoli, E., Gísli Jónsson, Ó., & Haraldsson, Á. (2024). Adequate immune responses to vaccines after chemotherapy for leukaemia diagnosed in childhood. Acta paediatrica (Oslo, Norway : 1992), 113(3), 606–614. [CrossRef]
- Kleebayoon, A., & Wiwanitkit, V. (2023). Comment on: Humoral profiling of pediatric patients with cancer reveals robust immunity following anti-SARS-CoV-2 vaccination superior to natural infection. Pediatric blood & cancer, 70(10), e30509. [CrossRef]
- Woodfield, M. C., Carpenter, P. A., & Pergam, S. A. (2020). Shots, Not Moonshots-The Importance of Broad Population Immunization to Patients Who Undergo Cancer Treatment. JAMA oncology, 6(1), 23–24. [CrossRef]
- Veinalde R. (2020). Evaluation of Oncolytic Virus-Induced Therapeutic Tumor Vaccination Effects in Murine Tumor Models. Methods in molecular biology (Clifton, N.J.), 2058, 213–227. [CrossRef]
- Ryu, H. H., Chang, K., Kim, N., Lee, H. S., Hwang, S. W., Park, S. H., Yang, D. H., Byeon, J. S., Myung, S. J., Yang, S. K., & Ye, B. D. (2021). Insufficient vaccination and inadequate immunization rates among Korean patients with inflammatory bowel diseases. Medicine, 100(45), e27714. [CrossRef]
- Wang, W., Li, X., Qin, X., Miao, Y., Zhang, Y., Li, S., Yao, R., Yang, Y., Yu, L., Zhu, H., Song, L., Mao, S., Wang, X., Chen, J., Feng, H., & Li, Y. (2023). Germline Neurofibromin 1 mutation enhances the anti-tumour immune response and decreases juvenile myelomonocytic leukaemia tumourigenicity. British journal of haematology, 202(2), 328–343. [CrossRef]
- Ginefra, P., Lorusso, G., & Vannini, N. (2020). Innate Immune Cells and Their Contribution to T-Cell-Based Immunotherapy. International journal of molecular sciences, 21(12), 4441. [CrossRef]
- Alicke, B., Totpal, K., Schartner, J. M., Berkley, A. M., Lehar, S. M., Capietto, A. H., Cubas, R. A., & Gould, S. E. (2020). Immunization associated with primary tumor growth leads to rejection of commonly used syngeneic tumors upon tumor rechallenge. Journal for immunotherapy of cancer, 8(2), e000532. [CrossRef]
- Song, X., Jiang, Y., Zhang, W., Elfawal, G., Wang, K., Jiang, D., Hong, H., Wu, J., He, C., Mo, X., & Wang, H. (2022). Transcutaneous tumor vaccination combined with anti-programmed death-1 monoclonal antibody treatment produces a synergistic antitumor effect. Acta biomaterialia, 140, 247–260. [CrossRef]
- Muhammad, Q., Jang, Y., Kang, S. H., Moon, J., Kim, W. J., & Park, H. (2020). Modulation of immune responses with nanoparticles and reduction of their immunotoxicity. Biomaterials science, 8(6), 1490–1501. [CrossRef]
- Fujii, S. I., & Shimizu, K. (2019). Immune Networks and Therapeutic Targeting of iNKT Cells in Cancer. Trends in immunology, 40(11), 984–997. [CrossRef]
- Ollila, T. A., Masel, R. H., Reagan, J. L., Lu, S., Rogers, R. D., Paiva, K. J., Taher, R., Burguera-Couce, E., Zayac, A. S., Yakirevich, I., Niroula, R., Barth, P., & Olszewski, A. J. (2022). Seroconversion and outcomes after initial and booster COVID-19 vaccination in adults with hematologic malignancies. Cancer, 128(18), 3319–3329. [CrossRef]
- Mao, C., Beiss, V., Ho, G. W., Fields, J., Steinmetz, N. F., & Fiering, S. (2022). In situ vaccination with cowpea mosaic virus elicits systemic antitumor immunity and potentiates immune checkpoint blockade. Journal for immunotherapy of cancer, 10(12), e005834. [CrossRef]
- You, W., Ouyang, J., Cai, Z., Chen, Y., & Wu, X. (2022). Comprehensive Analyses of Immune Subtypes of Stomach Adenocarcinoma for mRNA Vaccination. Frontiers in immunology, 13, 827506. [CrossRef]
- Elizondo, C. R., Bright, J. D., Byrne, J. A., & Bright, R. K. (2020). Analysis of the CD8+ IL-10+ T cell response elicited by vaccination with the oncogenic tumor-self protein D52. Human vaccines & immunotherapeutics, 16(6), 1413–1423. [CrossRef]
- Sangeeta, K., & Yenugu, S. (2022). Ablation of the sperm-associated antigen 11A (SPAG11A) protein by active immunization promotes epididymal oncogenesis in the rat. Cell and tissue research, 389(1), 115–128. [CrossRef]
- Rakshit, S., Adiga, V., Ahmed, A., Parthiban, C., Chetan Kumar, N., Dwarkanath, P., Shivalingaiah, S., Rao, S., D'Souza, G., Dias, M., Maguire, T. J. A., Doores, K. J., Zoodsma, M., Geckin, B., Dasgupta, P., Babji, S., van Meijgaarden, K. E., Joosten, S. A., Ottenhoff, T. H. M., Li, Y., … Vyakarnam, A. (2022). Evidence for the heterologous benefits of prior BCG vaccination on COVISHIELD™ vaccine-induced immune responses in SARS-CoV-2 seronegative young Indian adults. Frontiers in immunology, 13, 985938. [CrossRef]
- Xu, H., Zhao, F., Wu, D., Zhang, Y., Bao, X., Shi, F., Cai, Y., & Dou, J. (2023). Eliciting effective tumor immunity against ovarian cancer by cancer stem cell vaccination. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 161, 114547. [CrossRef]
- Takeshita, K., Ishiwada, N., Takeuchi, N., Ohkusu, M., Ohata, M., Hino, M., Hishiki, H., Takeda, Y., Sakaida, E., Takahashi, Y., Shimojo, N., & Hamada, H. (2022). Immunogenicity and safety of routine 13-valent pneumococcal conjugate vaccination outside recommended age range in patients with hematological malignancies and solid tumors. Vaccine, 40(9), 1238–1245. [CrossRef]
- Fitzpatrick, T., Alsager, K., Sadarangani, M., Pham-Huy, A., Murguía-Favela, L., Morris, S. K., Seow, C. H., Piché-Renaud, P. P., Jadavji, T., Vanderkooi, O. G., Top, K. A., Constantinescu, C., & Special Immunization Clinic Network investigators (2023). Immunological effects and safety of live rotavirus vaccination after antenatal exposure to immunomodulatory biologic agents: a prospective cohort study from the Canadian Immunization Research Network. The Lancet. Child & adolescent health, 7(9), 648–656. [CrossRef]
- Mezzapelle, R., De Marchis, F., Passera, C., Leo, M., Brambilla, F., Colombo, F., Casalgrandi, M., Preti, A., Zambrano, S., Castellani, P., Ertassi, R., Silingardi, M., Caprioglio, F., Basso, V., Boldorini, R., Carretta, A., Sanvito, F., Rena, O., Rubartelli, A., Sabatino, L., … Bianchi, M. E. (2021). CXCR4 engagement triggers CD47 internalization and antitumor immunization in a mouse model of mesothelioma. EMBO molecular medicine, 13(6), e12344. [CrossRef]
- Jindra, C., Hainisch, E. K., Rümmele, A., Wolschek, M., Muster, T., & Brandt, S. (2021). Influenza virus vector iNS1 expressing bovine papillomavirus 1 (BPV1) antigens efficiently induces tumour regression in equine sarcoid patients. PloS one, 16(11), e0260155. [CrossRef]
- Huang, M., Xiong, D., Pan, J., Zhang, Q., Wang, Y., Myers, C. R., Johnson, B. D., Hardy, M., Kalyanaraman, B., & You, M. (2022). Prevention of Tumor Growth and Dissemination by In Situ Vaccination with Mitochondria-Targeted Atovaquone. Advanced science (Weinheim, Baden-Wurttemberg, Germany), 9(12), e2101267. [CrossRef]
- Abdolkarimi, B., Amanati, A., Molavi Vardanjani, H., Jamshidi, S., & Tabaeian, S. A. P. (2022). Antibody waning after immunosuppressive chemotherapy and immunomodulators, re-immunization considerations in pediatric patients with malignancy and chronic immune thrombocytopenic purpura. BMC infectious diseases, 22(1), 657. [CrossRef]
- Ota, S., Miyashita, M., Yamagishi, Y., & Ogasawara, M. (2021). Baseline immunity predicts prognosis of pancreatic cancer patients treated with WT1 and/or MUC1 peptide-loaded dendritic cell vaccination and a standard chemotherapy. Human vaccines & immunotherapeutics, 17(12), 5563–5572. [CrossRef]
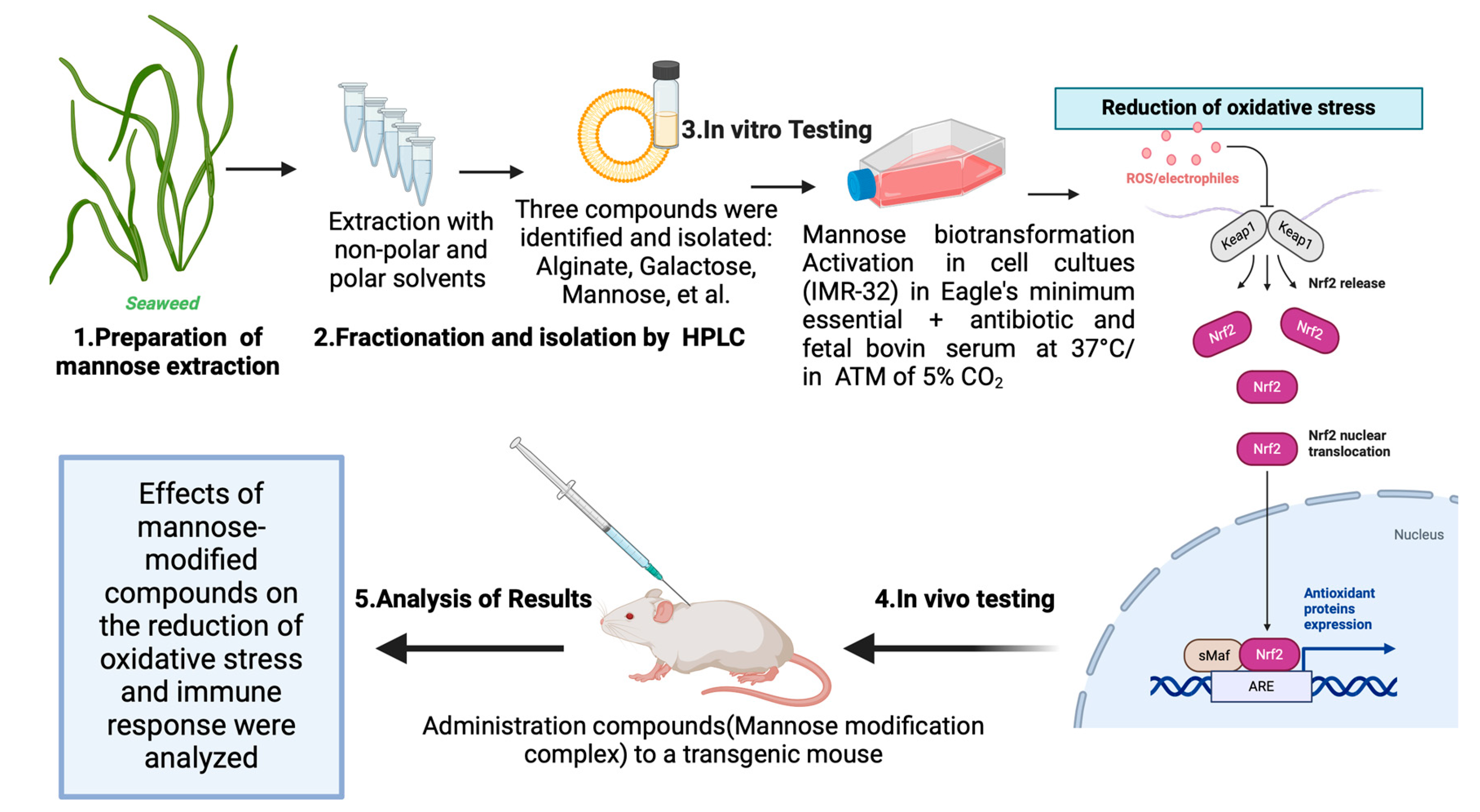
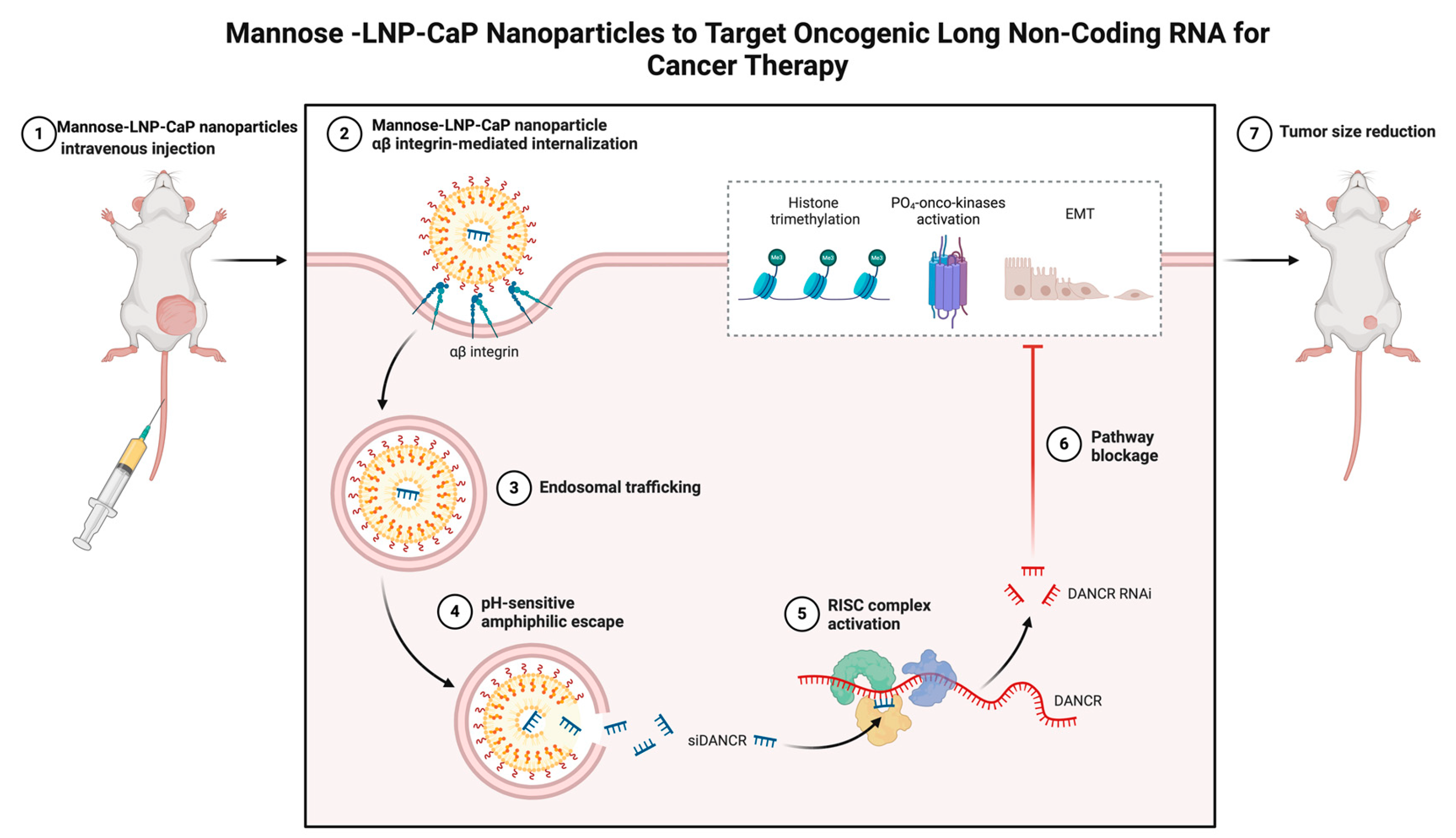
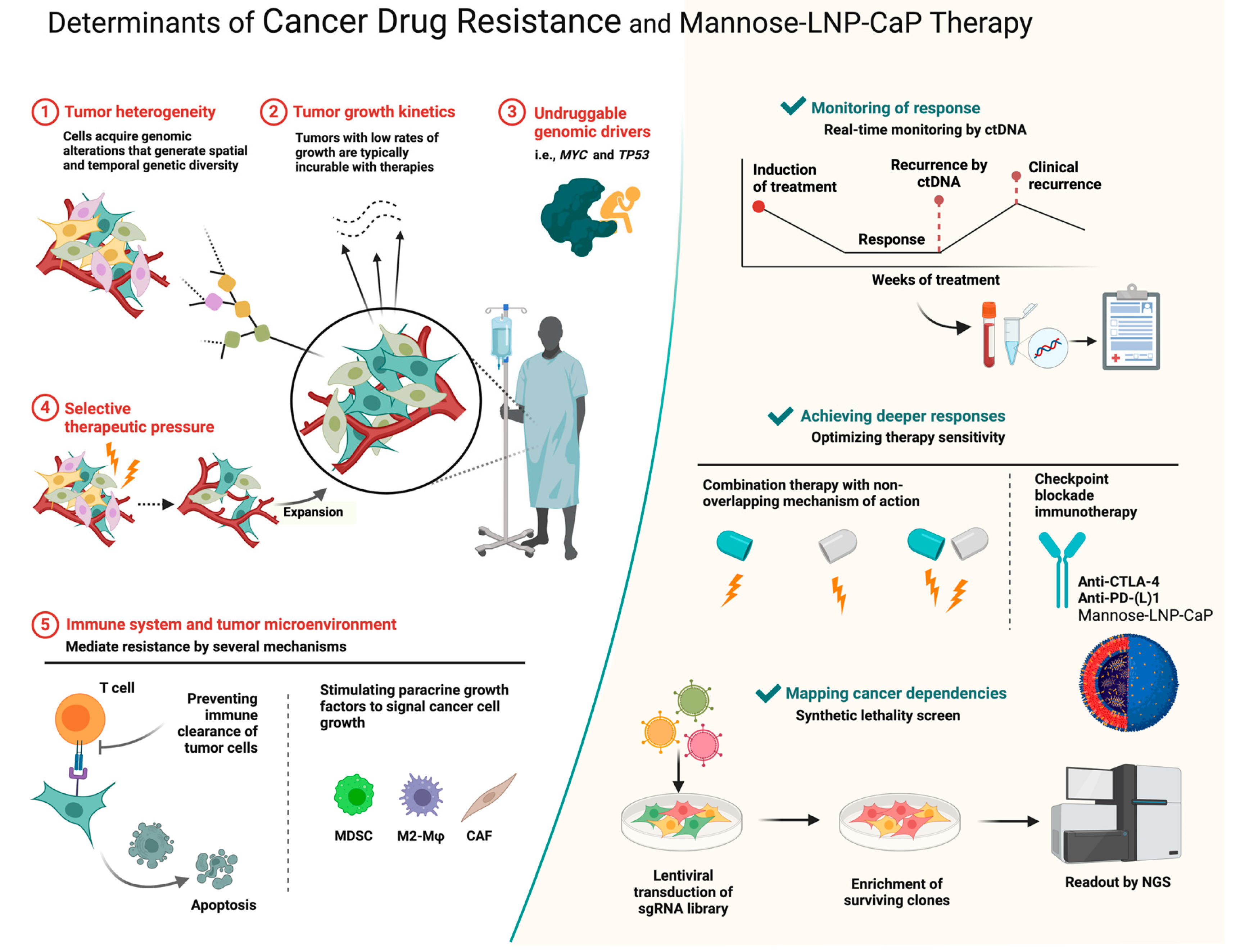
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




