1. Introduction
Jujubes are the most important fruit producing
Ziziphus species in the Rhamnaceae family [
1]. They are rich in various secondary metabolites associated to nutrition and biological activities [
2]. Free amino acids, phenolics, antioxidant activities and flavonoids from jujube have health promoting effects that varies but may be dependent on the cultivar and growing conditions [
3]. Cultivation of jujubes include conventional orchards, intensive orchards, intercropped cultivation and greenhouse cultivation which were usually applied with farmyard manure and other types of fertilization [
4]. Most of the current research in jujube concentrates on germplasm study, variety and breeding improvement, propagation systems, cultivation systems, pest and disease management and postharvest studies [
5]. Although fertilizer application and management are not the main research priority in jujube research, it is an essential component for environmentally friendly and high-quality food safety.
In most cases, application of organic fertilizers in agroecosystems usually entails application of manure, plant residues, or combinations of both either fresh or composted [
6]. Also, the adoption of organic management system should incorporate not just their perceived beneficial effects, but also their potential contribution to global environmental issues like greenhouse gas emissions as exemplified by rice agroecosystems [
7]. To evaluate soil quality, we need to also include biological and biochemical components of the soil as essential indicators of sustainable agriculture with the goal of mitigating environmental issues [
8,
9]. This is in stark contrast with conventional evaluation of soil quality based mainly on crop productivity and economic value. The application of organic fertilizers especially long-term application has led to changes in physicochemical components of the soil concurrently changing its microbial components leading to overall changes that distinctly separates soils with and without fertilizer application. These changes ultimately change biochemical features of the soil affecting their overall function and services [
10,
11].
In Korea, organic fertilizer-based research is promoted for eco-friendly agriculture and oil cake is an unconventional organic fertilizer that is used across Korean agricultural fields including its input in jujube orchards [
12]. Oil cake can be easily mineralized by soil microbes and the nutrients can be utilized by the crops improving nutrient, crop growth and productivity [
13,
14]. Oil cake has 1~3 times higher nitrogen content compared to livestock compost, and its nitrogen is mainly organic in nature [
15].
Microbiomes are affected by various factors including fertilization [
16], irrigation [
17] and tillage [
18]. On the other hand, soil microbial enzymes play critical roles in agroecosystems catalyzing important reactions necessary for nutrient cycling and decomposition which in turn supports various biological processes important for soil microorganisms [
19]. There are various studies that concentrated on unraveling the soil microbial community profiles in different agricultural fields treated with conventional organic fertilizers [
20,
21,
22,
23,
24]. The application of organic fertilizer could alter soil properties [
10,
11,
15], soil microbial communities [
20,
21,
22,
23], soil function [
20,
21,
22,
23] and even crop yield and quality [
24]. Although there are numerous studies on the effect of organic fertilization on agroecosystems, there have been less studies focusing on the effect of oil cake-based fertilization on the communities of microbes in jujube orchards.
Hence, this study characterized the bacterial community composition of jujube orchards treated with oil cake and to compare them with conventional NPK fertilized treatments. Furthermore, this study also predicted the abundance of soil enzymes resulting from compositional alternation of bacterial community. This study helps understand dynamic shifts in bacterial community structure, composition and diversity due to the unconventional application of an oil cake-based fertilization supporting its beneficial impact and evaluation on jujube orchard soils.
2. Materials and Methods
2.1. Research Sites, Fertilizer Application and Sampling
The Jujube Research Institute, Boeun, South Korea (36°34'39.6"N 127°44'54.1"E) allocated study sites for the research. The jujube variety used in this study was Bokjo (
Zizyphus jujuba var. inermis (Bunge) Rehder), and the distance between two trees were maintained at 4 m x 2 m. The fertilizer treatments were as follows: (1) NPK: chemical fertilizer and (2) OC: oil cake fertilization. The fertilization was carried out according to the nitrogen content of the soil, and irrigation was performed periodically. Urea was used as nitrogen fertilizer, fused phosphate was used as phosphate fertilizer, potassium sulfate was used to provide potassium, and oil cake was used as an organic fertilizer. The oil cake fertilizer contained a mixed ratio of 30% rape seed cake, 58% caster oil cake, and 12% rice bran cake. Topsoil (0~30 cm deep) samples were collected from the plastic rain shelter in August 2020. Individual samples from three trees were pooled together and were considered as one composite sample. Each treatment has three composite replicates. Roots and stones were removed and the soils were sieved (2.0-mm mesh). Samples were packed on ice for laboratory transport to conduct soil chemical analyses and DNA isolation. Soil samples were separated into two portions. One was directly used for DNA isolation and the other portion of the soil is for soil chemical analyses. Soil samples were air-dried under shade for soil chemical analyses following standard protocols [
25].
2.2. Extracting Soil DNA
The soil DNA extraction kit developed by QIAGEN (DNeasy PowerSoil Pro Kit; Hilden, Germany) was used for isolating DNA. NanoDrop spectrophotometer and fluorometer (Thermo Fisher; Qubit®, Life Technologies Corporation) were used to check DNA quality and quantity. Final samples were always stored − 80 °C.
2.3. DNA Sequencing and Analyses
Sample preparation for sequencing were done following the Illumina 16S Metagenomics sequencing library protocols (Nexbio, Daejeon, Korea). For the library, primers targeting the V3-V4 region PCR were used with sequences 341F (5'-ACTCCTACGGGAGGCAGCAG-3') and 806R (5'-GGACTACHVGGTWTCTAAT-3'). Amplicons were analyzed using Bioanalyzer DNA 1000 (Agilent Technologies). After filtering raw sequences, overlapping paired-end reads were merged with the tag, and the tag was clustered in a 97% sequence similarity OTU (Operational Taxonomic Unit). Greengenes reference database was used for bacterial classification with Mothur pipeline v. 1.39.5 as described previously [
26]. Diversity indices were analyzed based on taxonomic ranks and OTU.
2.4. Statistical Analysis and Visualization of Results
Statistical differences between treatment means were performed through T test at p < 0.05 using SPSS (V25, IBM, USA). The built-in PCA function in Mothur was used to perform Principal Coordinate Analysis (PoCA) and visualized differences in treatments using bacterial community structure. Biomarker discovery of the bacterial community was determined using LEfSe (
http://huttenhower.sph.harvard.edu/lefse/). Prediction of the functional profiles was conducted using the PICRUST tool [
27]. Metagenome predictions used reference genomes and marker gene in KEGG databases (Kyoto Encyclopedia of Genes and Genomes).
3. Results
Organic amendments in agroecosystems can alter chemical properties of soils. In this study, both the NPK and OC amended soils were acidic in nature (
Table 1) with pH ranging from 4.5-5.2. The OM content was 23.9~28.7g kg
-1, showing no significant difference between organic and NPK fertilized soils. Calcium and magnesium contents increased in oil cake treated area compared to the NPK fertilized soil. Potassium (K), electric conductivity (EC) and NO
3-N showed significant differences between NPK and OC amended soils.
Rarefaction curves indicated that the number of reads retrieved from the fertilized soils satisfactorily reveal bacterial identities of the communities in those soil samples (
Figure S1). Numbers of OTUs were significantly greater in oil cake treated orchard lands than the NPK treated soils at the 97% similarity level with more than 200 distinct OTUs detected (
Table 2). Oil cake amended soil show significantly greater Shannon and inv-Simpson indices than in the NPK amended soil. Similar patterns were observed for richness indices (Chao and Ace).
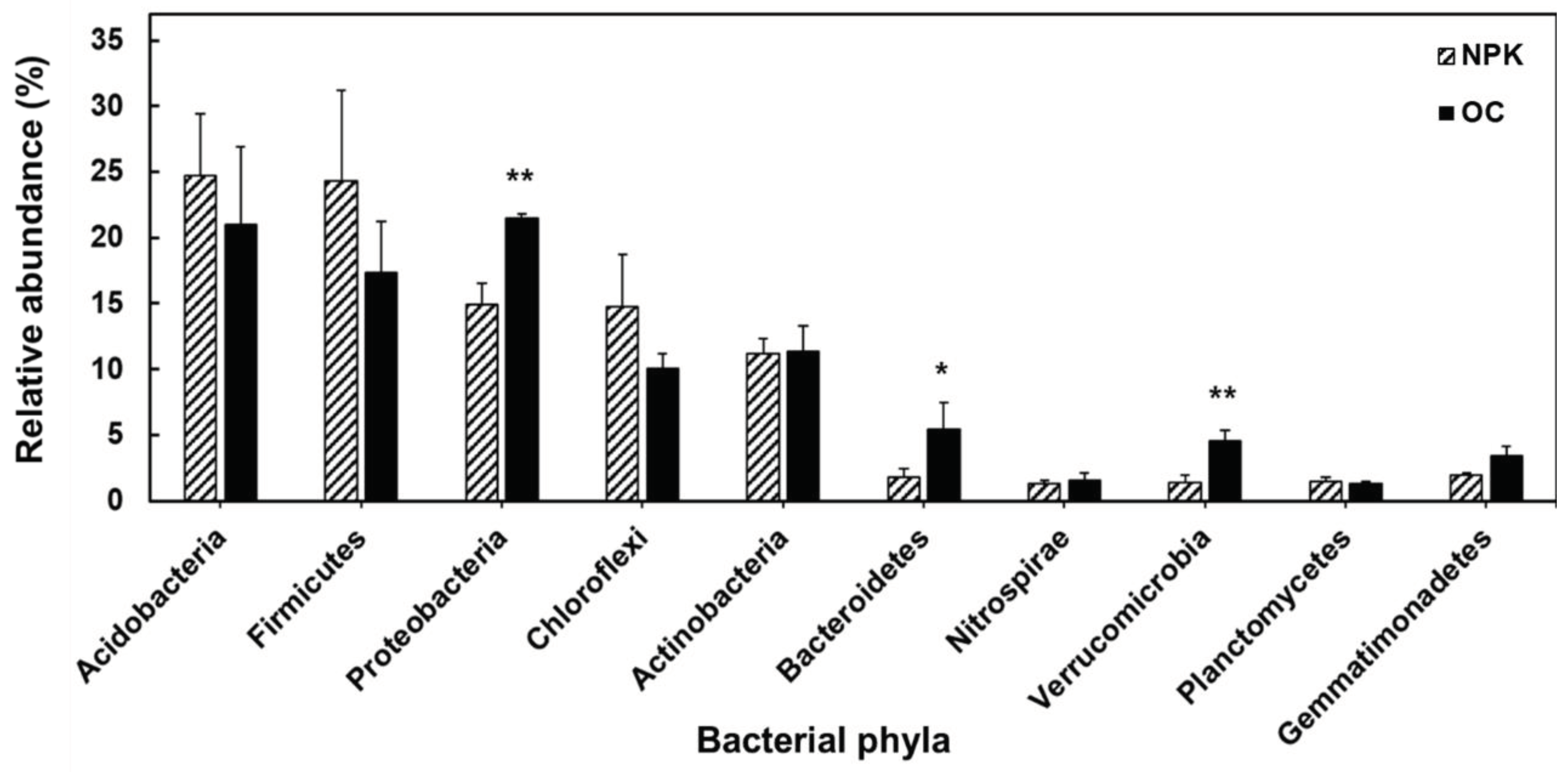
The bacterial community profile showed that the soil samples have 42 distinct and 34 common phyla. There were five dominant phyla with relative abundances of 10% or higher observed in both oil cake fertilized soils and NPK fertilized soils (
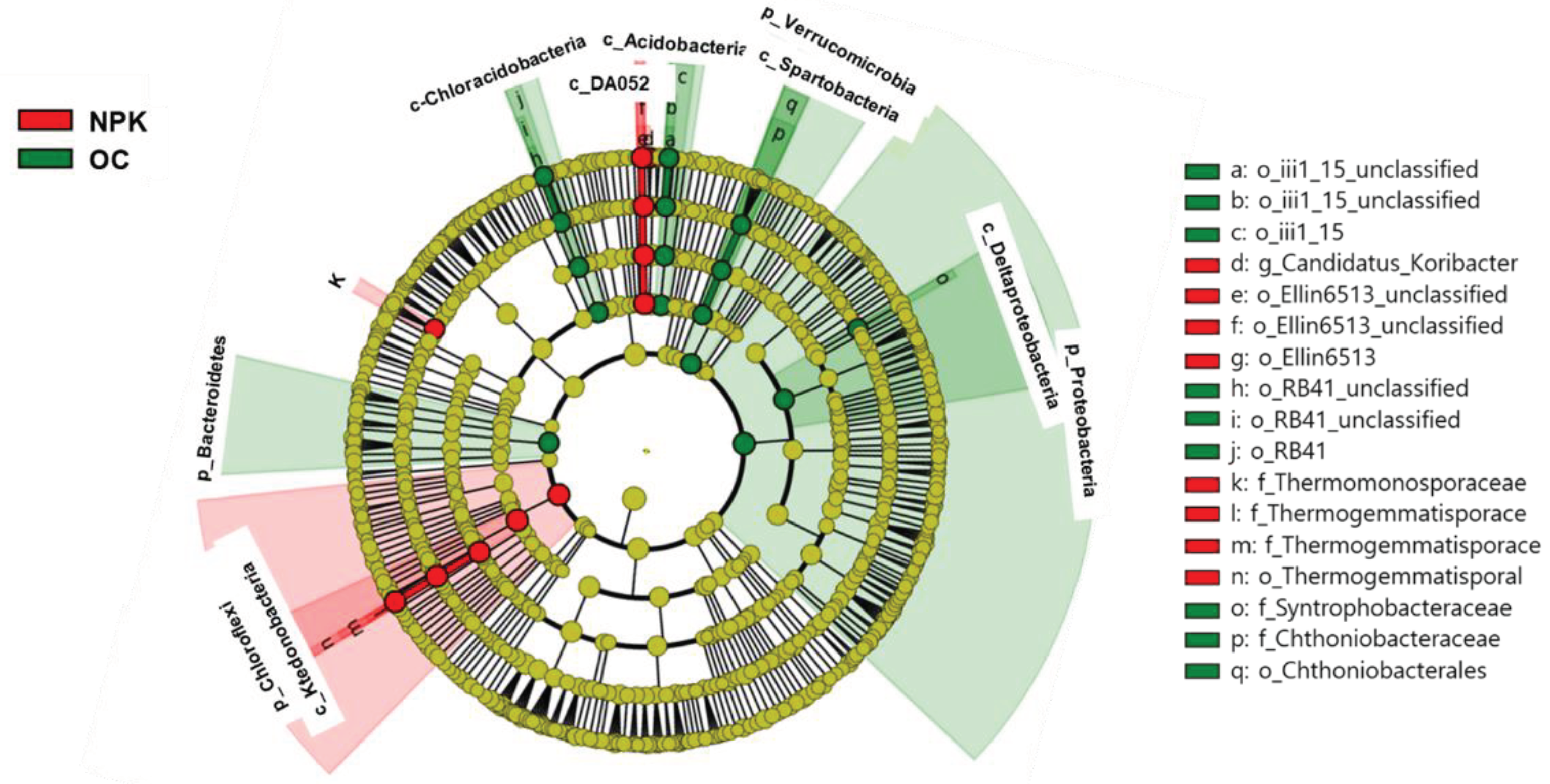
Figure 1). While comparing the distribution of bacteria at the phylum level, the soil treated with oil cake possessed Proteobacteria (21.5%), Acidobacteria (20.9%), Firmicutes (17.4%), Actinobacteria (11.4%), and Chloroflexi 10.1%) as its most dominant bacterial groups, whereas NPK fertilized soils were found to have Acidobacteria (24.7%), Firmicutes (24.3%), Proteobacteria (14.9%), Chloroflexi (14.8%) and Actinobacteria (11.2%) as its most abundant bacterial phyla. Proteobacteria, Bacteroidetes and Verrucomicrobia in oil cake fertilized soil were significantly higher compared to NPK fertilized soil. Furthermore, LEfSe showed the increase in abundances of Chloroflexi (Ktedonobacteria) and Acidobacteria in NPK fertilized soil compared to oil cake fertilized soils (
Figure 2). While bacterial clades Deltaproteobacteria, Bacteroidetes, Chloracidobacteria, and Spartobacteria are more dominant in OC amended soils.
The distinct community profiles of the studied soil groups are shaped by the chemical properties of the soil. The PCoA showed that soil pH, Ca and Mg were bacterial community composition determinants in oil cake amended soils, and the other parameters determined the community profiles of NPK amended soils (
Figure 3). Distinct clustering of the treatment replicates dictated by soil chemical properties indicate clear variabilities between the OC and NPK treated jujube orchards. Principal component 1 (PC1) explained 68.5% of the overall variability in community structure while PC2 explained 12.4% with an overall cumulative variability of 80.9% indicating considerable inclusion of main soil physico-chemical factors explaining overall variabilities observed. Furthermore, correlations of the microbial abundance and soil chemical parameters indicate potential relationship between the abundances of specific microbial group as affected by specific soil chemical parameter. Spearman correlation had shown that the Verrucomicrobia population was positively correlated with soil pH and Ca content. Similarly, EC and NO
3-N were positively correlated with Firmicutes, Chloroflexi and Actinobacteria (
Table 3). Additionally, Verrumicrobia and Nitrospirae showed a negative correlation with EC and NO
3-N.
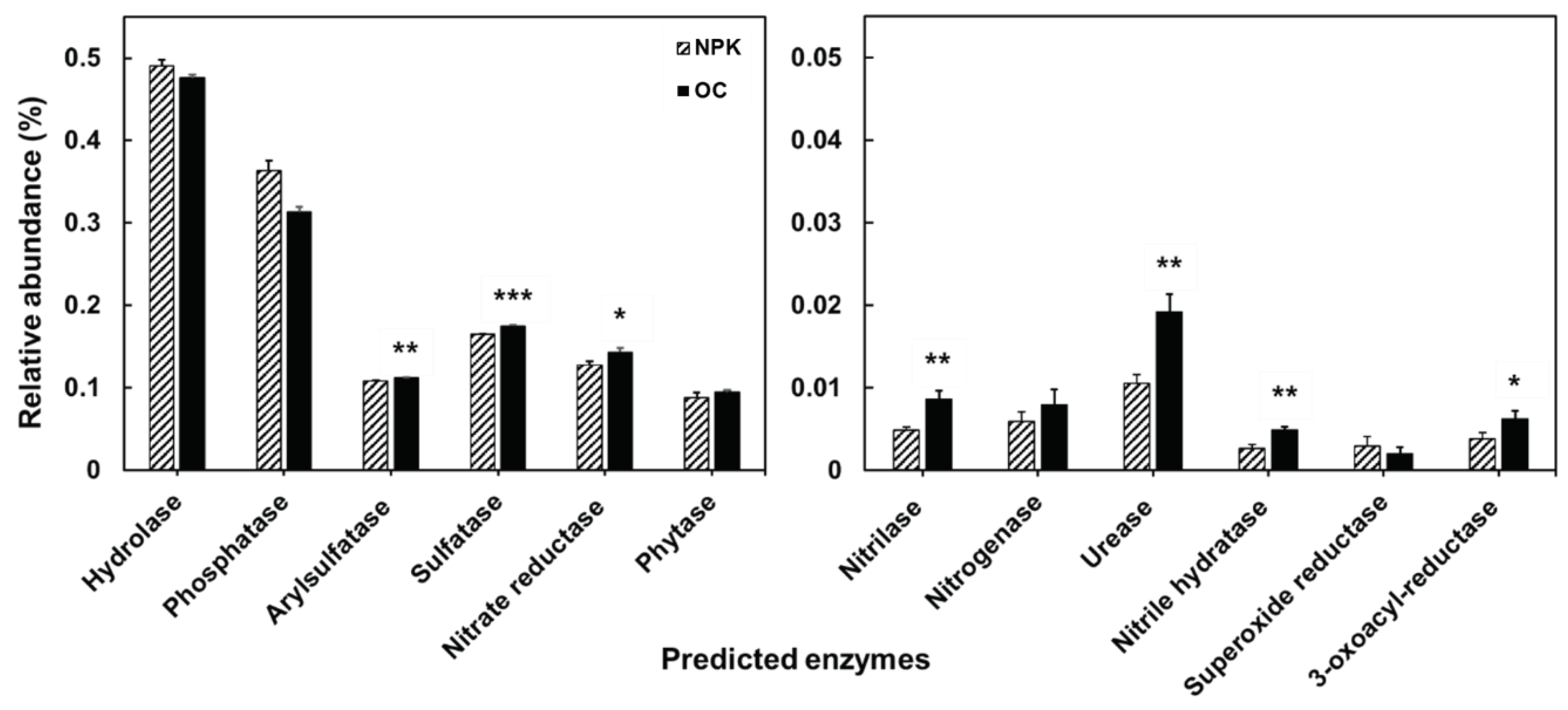
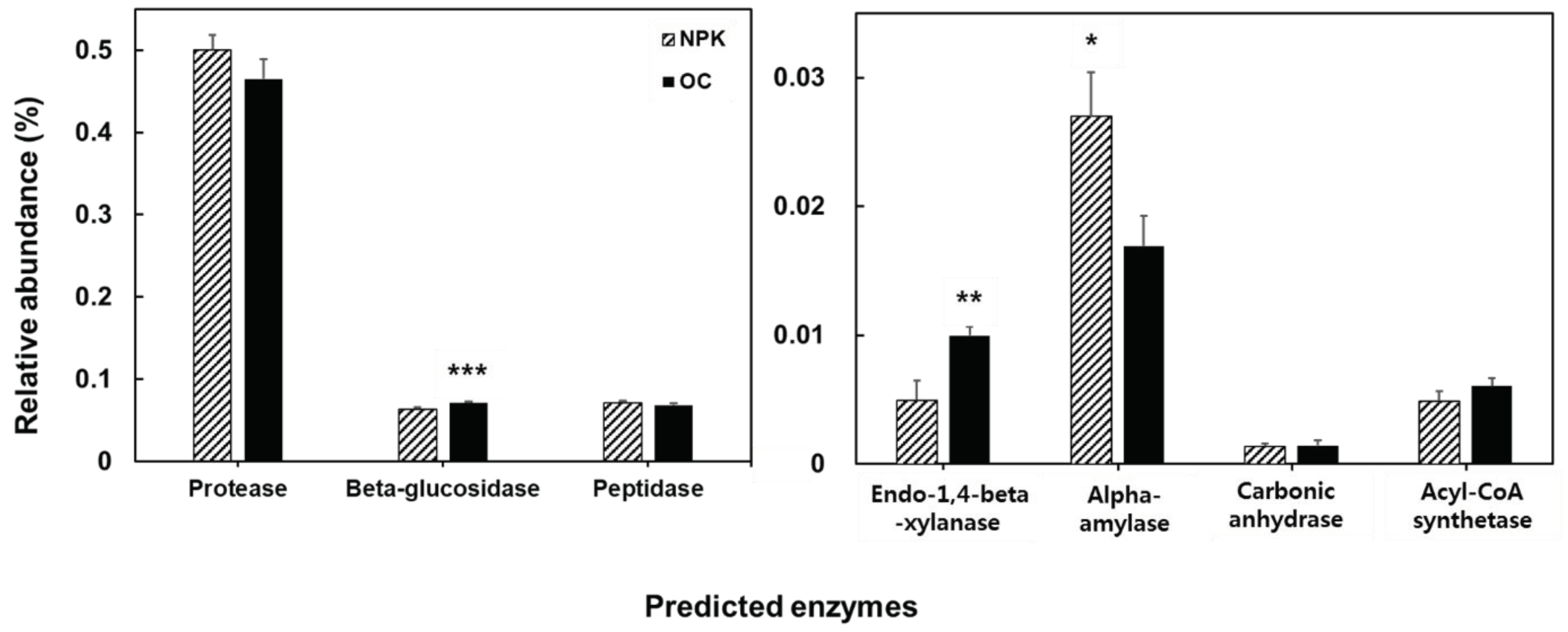
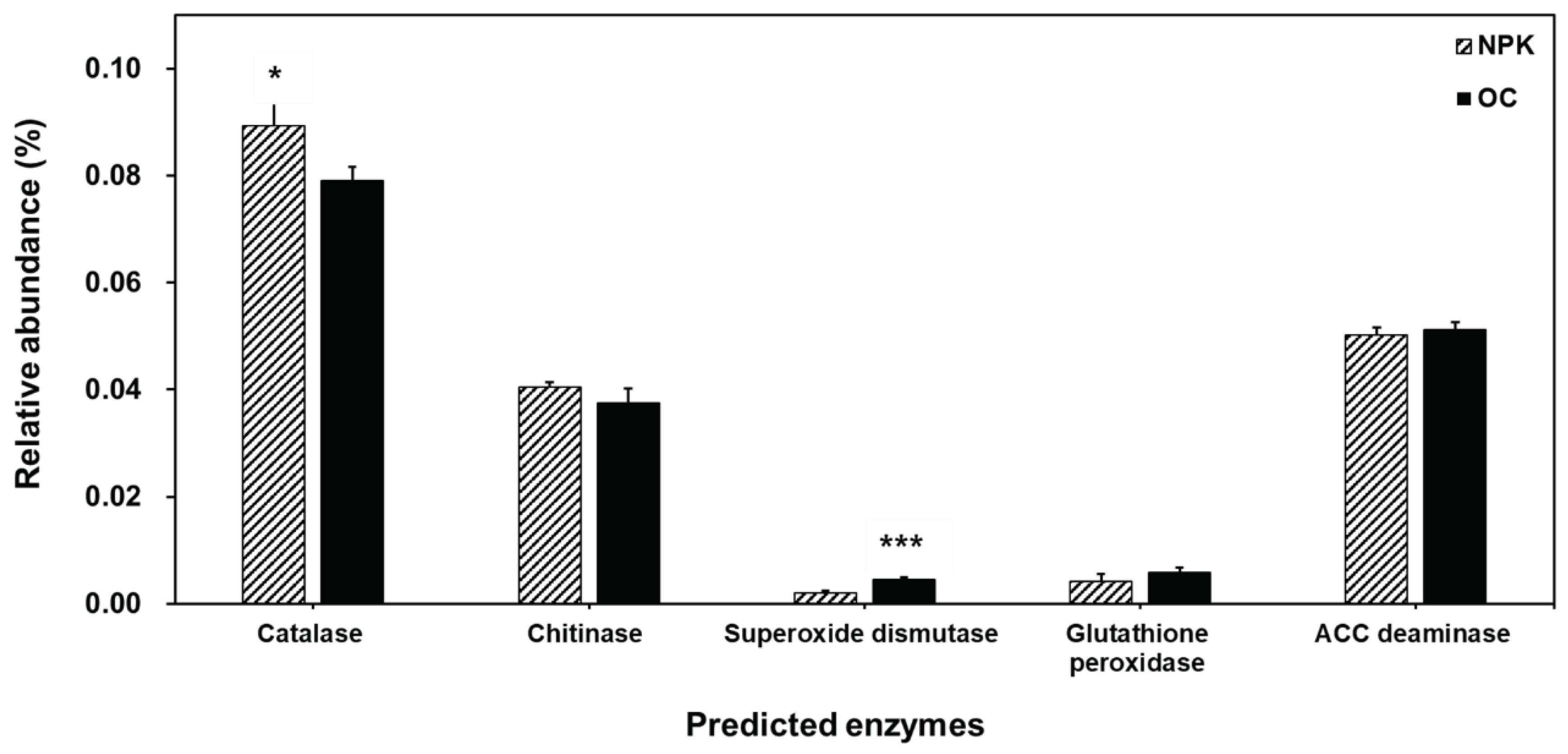
The functional profiles of the bacterial community were determined using PICRUSt. We divided the functional traits based on soil enzyme encoding genes (
Figure 4), decomposition related genes (
Figure 5) and plant growth-promotion related genes (
Figure 6). Among the typical soil enzyme encoding genes, majority of the genes were significantly higher in oil cake amended soils. These include arylsulfatase, sulfatase, urease, nitrate reductase, nitrilase, nitrile hydratase, and 3-oxoacyl-reductase. Significantly abundant decomposition related enzyme-encoding genes observed in OC amended soils include beta-glucosidase and endo-1,4-beta-xylanase while alpha-amylase is significantly abundant in the NPK amended soils. Superoxide dismutase is significantly more abundant in the OC amended soils related to plant growth promotion and stress responses while the NPK treated soil has a significantly more abundant catalase enzyme-encoding gene.
4. Discussion
This study evaluates the impact of amending jujube orchards soils with an unconventional fertilizer made up of composted oil cake and comparing it with chemical fertilizer application. The effects of organic compost and NPK fertilization were evaluated with regards to the alterations in chemical properties of soils then changes in the bacterial community structure and diversity then their potential impact on predicted soil functions and activity.
4.1. Impact of Oil Cake Amendment and Chemical Fertilization on Jujube Orchard Soils Chemistry
The application of organic fertilizers in agroecosystems can bring significant alterations in soils including both chemical and physical properties [
28,
29]. This is exemplified in long-term continuous applications either only as organic fertilizer amendment or mixing of organic and inorganic chemical fertilizers changing soil chemistry and ultimately affecting functional and structural soil microbiomes [
10,
11]. The most commonly used organic fertilizer amendment at least in paddy fields are fresh or composted animal manure singly, composted crop residues such as rice straw or a combination of both types [
6]. The oil cake used in the study contained a combination of rapeseed cake, castor oil cake, and rice bran cake which will become the source of nutrient input into the soils of jujube orchards. Oil cakes were observed to have higher nitrogen content compared to livestock compost [
15] and to undergo rapid mineralization of organic nitrogen [
12,
15] potentially contributing to the nitrogen contents of the soil. The OM content showed no significant difference between organic and NPK fertilized soils. Kim et al. [
30] also suggested that the organic content of the oil cake fertilizer is more than 70% of the raw material, but it is easily decomposed due to the nature of the raw material and is not effective in increasing soil organic matter. In this study, both the NPK and OC amended soils were acidic. The soil pH affects soil nutrient availability directly correlated with productivity [
31]. Calcium and magnesium contents increased in oil cake treated area compared to the NPK fertilized soil. Since calcium and magnesium content is low, it is estimated that the pH of the NPK fertilized soil is more acidic than that of the oil cake amended soil [
32].
4.2. Impact of Oil Cake and Chemical Fertilization on the Communities of Bacteria in Jujube Orchard Soils
Rarefaction curves showed that the reads from the fertilized soils satisfactorily reveal bacterial identities of the communities. The present study also showed that OC amended orchards have significantly higher number of OTUs along with significantly higher diversity indices in contrast to the NPK amended jujube orchards. This indicates that the application of oil cake amendments resulted to the increase in members of other bacterial clades magnifying the bacterial richness of OC amended soils leading to the observed increased in diversity indices. The amendment of organic matter has been shown to enhance the population, diversity and heterogeneity of microbial communities in soil [
33,
34]. In a long-term organic amendment, microbial populations of heterotrophic bacterial population along with specific microbial guilds have increased mainly attributed to the long-term fertilization altering chemical characteristics of the soils [
35].
The application of different types of organic fertilizers as well as different types of nitrogen sources and other kinds of fertilizer amendment impact microbial guilds differently. For instance, the application of cattle versus swine manure have led to enrichment of methanogens belonging to Methanomicrobiaceae not observed in soils with swine manure application [
36]. There is also a clade-specific changes related to nitrogen cycling microbial communities as influenced by nitrogen fertilization [
37]. The differing responses of diverse microbial guilds may show common trends as well as taxon-specific or clade-specific microbial responses to different types of fertilization input in agricultural lands which could be better elucidated under long-term fertilization studies [
6,
10,
11].
In terms of differences in bacterial clades between OC and NPK amended soils, clear significant differences occur in the dominance of bacterial taxa from phylum to class and lower taxonomic groups. At the phylum level, Proteobacteria, Bacteroidetes and Verrucomicrobia in oil cake fertilized soil were significantly higher compared to NPK fertilized soil. These trends are also observed in other studies showing inorganic fertilization regimes decrease the population of Proteobacteria and Verrucomicrobia [
38,
39]. Additionally, Proteobacteria contains bacterial populations that are important for carbon, nitrogen and sulfur cycling in soil [
40]. Proteobacteria is also the most dominant bacterial group where most plant growth-promoting bacteria belong to mainly associated to different crop plants [
41,
42,
43,
44]. Bacteroides contain several polysaccharide-degrading genes encoding enzymes [
45,
46], which are believed to play an important role in lignocellulosic degradation which is present in high abundance in oil cake fertilized soils [
47]. Furthermore, LEfSe showed the increase in abundances of Chloroflexi and Acidobacteria in NPK fertilized soil compared to oil cake fertilized soils. This observation is similarly observed in a previous study showing increased abundances of Acidobacteria in soil amended with chemical fertilizers [
48]. On the other hand, Proteobacteria were abundant in oil cake treated soil which corroborates to a previous study where sugarcane fields amended with organic fertilizer had shown higher abundances of Proteobacteria [
49].
Principal component analysis showed the distinct community profiles of the studied groups mainly attributed to the soil chemical properties. The OC amended soils were mainly affected by soil pH, Ca and Mg while the variabilities of NPK amended soils were determined by other soil parameters. These observations are supported by positive and negative correlations of specific soil chemical parameters and different bacterial populations found in each treatment. A previous study supports our observations, where nitrogen treatment augmented the ratio of Actinobacteria and Firmicutes and diminished the ratio of Acidobacteria and Verrucomicrobia in soils [
50]. The differential impact of fertilizer application on soil chemical parameters also lead to changes in the community structure, diversity, and abundance of microbial groups in agricultural lands. As observed in our study, the clear clustering of the OC and NPK amended soils mainly influenced by soil chemical parameter and different microbial guilds indicates significant variabilities that exist between the two jujube orchard soils treated with different fertilizer regimes. These differences in terms of soils become prominent particularly in long-term application of compost, NPK, and compost plus NPK treatments compared to the control creating characteristic soils with different soil physico-chemical properties and varying microbial community composition, diversity and abundances which eventually lead to changes in functional activities and services occurring in the soils [
6,
10,
11]
4.3. Impact of Oil Cake and Chemical Fertilization on Microbial Functional Diversity of Jujube Orchard Soils
The functional profiles mainly based on the community composition and abundance also shows significant differences between OC and NPK amended soils. Arylsulfatase, sulfatase, urease, nitrate reductase, and nitrilase are involved in the sulfur and nitrogen cycle, and 3-oxoacyl-reductase is metabolically related in the biosynthesis of fatty acids and polyunsaturated fatty acids. Arylsulphatase hydrolyzes soil sulfate esters [
51,
52], while sulfatase is induced by sulfate restriction and affects plant growth promotion of some bacteria [
51]. Soil sulfatase activity indicates soil health after fertilizations as it reflects soil microbial activity [
53]. The higher urease activity in OC treated soils were similarly observed in farmyard manure-treated paddy fields and they attribute this increase due to the production of the end product, ammonium ions [
35]. All these enzymes can be typically attributed to various plant-based organic fertilization amendments in agricultural soils.
Organic decomposition related functional genes encoding beta-glucosidase and endo-1,4-beta-xylanase were significantly higher in oil cake fertilized soil. Beta-glucosidase hydrolyzes various beta-glucosides present in plant debris [
54]. Increase in beta-glucosidase was also observed in long-term organically fertilized paddy fields using composted farmyard manure. Endo-β-1,4-xylanase hydrolyzes β-1,4 bonds in plant xylan found in hemicellulose [
55]. Also, superoxide dismutase among plant growth promoting enzymes was high in oil cake amended soil. Superoxide dismutase is important in catalyzing superoxide anions to hydrogen peroxide (H
2O
2) and O
2 and protecting against oxidative stress [
56]. It is thought to exhibit resistance to environmental stresses that may be exposed during the period of growing jujubes treated with oil cake.
In general, the changes in the functional profile or microbial functional diversity observed in different soils are due to the combination of alterations in chemical properties of the soil concurrent to the shifts in bacterial community structure, diversity and abundance. The functions provided by soils are mainly dependent on microbial actions attributed to particular microbial clades capable of these functions [
8,
9]. For example, methane emission dictated by methane production and methane and methanol oxidation is dependent on methanogenic archaea and methanotrophic and methylotrophic bacterial communities [
6,
10]. This is also true for nitrogen transformative processes in agricultural soils where many processes are linked to the action of nitrogen-cycling related microbial guilds [
11].
Our study supports that the application of oil cake compost into jujube orchards have enhanced the soil quality increasing richness and diversity of bacterial communities leading to enriched functional microbial diversity. It is interesting to investigate if long-term input of oil cake amendments will lead to similar observations in long-term fertilized lands with increased nutrient content such as soil labile carbon and nitrogen pools enhancing soil enzymatic activities as well as higher microbial population and diversity [
10,
11,
35].
5. Conclusions
The use of oil cake fertilization as an organic amendment in jujube orchard resulted in the alteration of chemical properties of fertilized soils and eventually changes bacterial community profiles. Changes in soil pH, exchangeable cations, electrical conductivity and nitrogen contents were the major determinants in defining the subsequent modifications in bacterial community profiles of both the oil cake and NPK fertilized soils with predictive changes in enzyme abundances enhancing soil health and fertility.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Conceptualization, H.P. and T.S.; methodology, H.P. and K.K.; software, H.P. and K.K.; validation, H.P., R.Z. and T.S.; formal analysis, H.P. and K.K.; investigation, H.P.; resources, T.S.; data curation, H.P.; writing—original draft preparation, H.P. and D.I.W.; writing—review and editing, D.I.W; visualization, H.P. and K.K.; supervision, T.S. and R.Z.; project administration, T.S. and R.Z.; funding acquisition, T.S. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research was funded by Basic Science Research Program, National Research Foundation of Korea (NRF), Ministry of Education, Science and Technology [2021R1A2C1006608], Republic of Korea. The APC was waived for publication.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Aritra Roy Choudhury for his guidance and suggestions on the improvement of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chiou, C.Y.; Shih, H.C.; Tsai, C.C.; Jin, X.L.; Ko, Y.Z.; Mantiquilla, J.A.; Weng, I.S.; Chiang, Y.C. The genetic relationships of Indian jujube (Ziziphus mauritiana) cultivars using SSR markers. Heliyon 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Gao, Z.; Jiao, X.; Shi, J.; Wang, R. Widely targeted metabolomic analysis of active compounds at different maturity stages of ‘Hupingzao’jujube. J. Food Compos. Anal. 2020, 88, 103417. [Google Scholar] [CrossRef]
- Choi, S.H.; Ahn, J.B.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J. Agric. Food Chem. 2011, 59, 6594–6604. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Zhao, Z.H. Germplasm resources and production of jujube in China. In International Jujube Symposium 2008, 840 (pp. 25-32).
- Liu, M.; Wang, J.; Wang, L.; Liu, P.; Zhao, J.; Zhao, Z.; Yao, S.; Stănică, F.; Liu, Z.; Wang, L.; et al. The historical and current research progress on jujube–a superfruit for the future. Hort. Res. 2020., 7. [CrossRef] [PubMed]
- Kim, C.; Walitang, D.I.; Sa, T. Methanogenesis and methane oxidation in paddy fields under organic fertilization. Korean J. Environ. Agric. 2021, 40, 295–312. [Google Scholar] [CrossRef]
- Pandey, A.; Dou, F.; Morgan, C.L.; Guo, J.; Deng, J.; Schwab, P. Modeling organically fertilized flooded rice systems and its long term effects on grain yield and methane emissions. Sci. Total Environ. 2021, 755, 142578. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.G.; Nannipieri, P.; Benedetti, A.; Hopkins, D.W. Defining soil quality. In Microbiological Methods for Assessing Soil Quality; Bloem, J., Hopkins, D.W., Benedetti, A., Eds.; CABI Publishing: Cambridge, UK, 2006; pp. 15–22. [Google Scholar]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils. 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Kim, C.; Walitang, D.I.; Roy Choudhury, A.; Lee, Y.; Lee, S.; Chun, H.; Heo, T.Y.; Park, K.; Sa, T. Changes in soil chemical properties due to long-term compost fertilization regulate methane turnover related gene abundances in rice paddy. Appl. Sci. 2022, 12, 2652. [Google Scholar] [CrossRef]
- Walitang, D.I.; Kim, K.; Lee, Y.; Heo, T.; Sa, T. Nitrification and denitrification gene abundances under stable soil chemical properties established by long-term compost fertilization. Appl. Sci. 2023, 13, 11146. [Google Scholar] [CrossRef]
- Im, J.U.; Kim, S.Y.; Yoon, Y.E.; Kim, J.H.; Lee, S.B.; Lee, Y.B. Nitrogen mineralization in soil amended with oil-cake and amino acid fertilizer under upland condition. Korean J. Org. Agric. 2015, 23, 867–873. [Google Scholar] [CrossRef]
- Das, A.; Prasad, M.; Shivay, Y.S.; Subha, K.M. Productivity and sustainability of cotton (Gossypium hirsutum)–wheat (Triticum aestivum) cropping system as influenced by prilled urea, farmyard manure and Azotobacter. J. Agron. Crop Sci. 2004, 190, 298–304. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Iwuafor, E.N.O.; Ladan, Z.; Agbaji, A.S.; Abdusalam, Z.; Yusuf, H.A. Evaluation of neem-based compound fertilizer for crop production in Samaru, moist savanna of Nigeria. J. Agric. Sci. Technol. 2011, 1, 235–245. [Google Scholar]
- Cho, S.H.; Chang, K.W. Nitrogen mineralization of oil cakes according to changes in temperature, moisture, soil depth and soil texture. J. Korea Org. Resour. Recycl. Assoc. 2007, 15, 149–158. [Google Scholar]
- Ma, W.; Jiang, S.; Assemien, F.; Qin, M.; Ma, B.; Xie, Z.; Liu, Y.; Feng, H.; Du, G.; Ma, X.; et al. Response of microbial functional groups involved in soil N cycle to N, P and NP fertilization in Tibetan alpine meadows. Soil Biol. Biochem. 2016, 101, 195–206. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, B.; Zheng, X.; Liu, G. Plant biomass, soil water content and soil N: P ratio regulating soil microbial functional diversity in a temperate steppe: A regional scale study. Soil Biol. Biochem. 2010, 42, 445–450. [Google Scholar] [CrossRef]
- Yang, A.; Hu, J.; Lin, X.; Zhu, A.; Wang, J.; Dai, J.; Wong, M.H. Arbuscular mycorrhizal fungal community structure and diversity in response to 3-year conservation tillage management in a sandy loam soil in North China. J. Soils Sediments. 2012, 12, 835–843. [Google Scholar] [CrossRef]
- Pozzo, T.; Pasten, J.L.; Karlsson, E.N.; Logan, D.T. Structural and functional analyses of β-glucosidase 3B from Thermotoga neapolitana: A thermostable three-domain representative of glycoside hydrolase 3. J. Mol. Biol. 2010, 397, 724–739. [Google Scholar] [CrossRef] [PubMed]
- van der Bom, F.; Nunes, I.; Raymond, N.S.; Hansen, V.; Bonnichsen, L.; Magid, J.; Nybroe, O.; Jensen, L.S. Long-term fertilisation form, level and duration affect the diversity, structure and functioning of soil microbial communities in the field. Soil Biol. Biochem. 2018, 122, 91–103. [Google Scholar] [CrossRef]
- Holík, L.; Hlisnikovský, L.; Honzík, R.; Trögl, J.; Burdová, H.; Popelka, J. Soil microbial communities and enzyme activities after long-term application of inorganic and organic fertilizers at different depths of the soil profile. Sustainability, 2019, 11, 3251. [Google Scholar] [CrossRef]
- Armalytė, J.; Skerniškytė, J.; Bakienė, E.; Krasauskas, R.; Šiugždinienė, R.; Kareivienė, V.; Kerzienė, S.; Klimienė, I.; Sužiedėlienė, E.; Ružauskas, M. Microbial diversity and antimicrobial resistance profile in microbiota from soils of conventional and organic farming systems. Frontiers Microbiol. 2019, 10, 892. [Google Scholar] [CrossRef]
- Gu, S.; Hu, Q.; Cheng, Y.; Bai, L.; Liu, Z.; Xiao, W.; Gong, Z.; Wu, Y.; Feng, K.; Deng, Y.; et al. Application of organic fertilizer improves microbial community diversity and alters microbial network structure in tea (Camellia sinensis) plantation soils. Soil and Tillage Research, 2019; 195, 104356. [Google Scholar]
- Jin, N.; Jin, L.; Wang, S.; Li, J.; Liu, F.; Liu, Z.; Luo, S.; Wu, Y.; Lyu, J.; Yu, J. Reduced chemical fertilizer combined with bio-organic fertilizer affects the soil microbial community and yield and quality of lettuce. Frontiers Microbiol. 2022, 13, 863325. [Google Scholar] [CrossRef] [PubMed]
- NIAST, R. Methods of soil and plant analysis. National Institute of Agricultural Sciences and Technology. 2000.
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Bulluck Iii, L.R.; Brosius, M.; Evanylo, G.K.; Ristaino, J.B. Organic and synthetic fertility amendments influence soil microbial, physical and chemical properties on organic and conventional farms. Appl. Soil Ecol. 2002, 19, 147–160. [Google Scholar] [CrossRef]
- Lee, J. Effect of application methods of organic fertilizer on growth, soil chemical properties and microbial densities in organic bulb onion production. Sci. Hortic. 2010, 124, 299–305. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.J.; Kim, S.H.; Hwang, H.Y.; Shim, J.H.; Lee, Y.H. Effects of application amount of organic compound fertilizer on lettuce growth and soil chemical properties under plastic film house. J. Korea Org. Res. Recyc. Assoc. 2020, 28, 37–44. [Google Scholar]
- McCauley, A.; Jones, C.; Jacobsen, J. Soil pH and organic matter. Nutrient management module, 2009, 8, 1–12. [Google Scholar]
- Xu, J.M.; Tang, C.; Chen, Z.L. The role of plant residues in pH change of acid soils differing in initial pH. Soil Biol. Biochem. 2006, 38, 709–719. [Google Scholar] [CrossRef]
- Lupatini, M.; Korthals, G.W.; De Hollander, M.; Janssens, T.K.; Kuramae, E.E. Soil microbiome is more heterogeneous in organic than in conventional farming system. Frontiers Microbiol. 2017, 7, 2064. [Google Scholar] [CrossRef]
- Kim, S.; Samaddar, S.; Chatterjee, P.; Roy Choudhury, A.; Choi, J.; Sa, T. Structural and functional shift in soil bacterial community in response to long-term compost amendment in paddy field. Appl. Sci. 2021, 11, 2183. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Nayak, A.K.; Mohanty, S.; Tripathi, R.; Shahid, M.; Kumar, A.; Raja, R.; Panda, B.B.; Roy, K.S.; Neogi, S.; et al. Greenhouse gas emission in relation to labile soil C, N pools and functional microbial diversity as influenced by 39 years long term fertilizer management in tropical rice. Soil Tillage Res. 2013, 129, 93–105. [Google Scholar] [CrossRef]
- Kim, S.Y.; Pramanik, P.; Bodelier, P.L.; Kim, P.J. Cattle manure enhances methanogens diversity and methane emissions compared to swine manure under rice paddy. PLoS ONE. 2014, 9, e113593. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, F.; Hu, C.; Liu, B. Metagenomics reveals taxon-specific responses of the nitrogen-cycling microbial community to long-term nitrogen fertilization. Soil Biol. Biochem. 2021, 156, 108214. [Google Scholar] [CrossRef]
- Hartmann, M.; Widmer, F. Community structure analyses are more sensitive to differences in soil bacterial communities than anonymous diversity indices. Appl. Environ. Microbiol. 2006, 72, 7804–7812. [Google Scholar] [CrossRef]
- Matsushita, Y.; Egami, K.; Sawada, A.; Saito, M.; Sano, T.; Tsushima, S.; Yoshida, S. , Analyses of soil bacterial community diversity in naturally and conventionally farmed apple orchards using 16S rRNA gene sequencing. Appl. Soil Ecol. 2019, 141, 26–29. [Google Scholar] [CrossRef]
- Kersters, K.; De Vos, P.; Gillis, M.; Swings, J.; Vandamme, P. Introduction to the Proteobacteria. In: Dwarkin M.; Falkow, S.; Rosenberg, E.; Schleifer, K.H. Stackebrandt (eds). The Prokaryotes 3rd edn 2006, 5, 3-37.
- Sessitsch, A.; Hardoim, P.; Döring, J.; Weilharter, A.; Krause, A.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrich, F.; Rahalkar, M.; et al. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant-Microbe Interact. 2012, 25, 28–36. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Walitang, D.I.; Kim, K.; Madhaiyan, M.; Kim, Y.K.; Kang, Y.; Sa, T. Characterizing endophytic competence and plant growth promotion of bacterial endophytes inhabiting the seed endosphere of Rice. BMC Microbiol. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nature Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Jiménez, D.J.; Chaves-Moreno, D.; van Elsas, J.D. Unveiling the metabolic potential of two soil-derived microbial consortia selected on wheat straw. Sci. Rep. 2015, 5, 13845. [Google Scholar] [CrossRef]
- Larsbrink, J.; McKee, L.S. Bacteroidetes bacteria in the soil: Glycan acquisition, enzyme secretion, and gliding motility. Adv. Appl. Microbiol. 2020, 110, 63–98. [Google Scholar] [PubMed]
- Kougias, P.G.; Campanaro, S.; Treu, L.; Tsapekos, P.; Armani, A.; Angelidaki, I. Spatial distribution and diverse metabolic functions of lignocellulose-degrading uncultured bacteria as revealed by genome-centric metagenomics. Appl. Environ. Microbiol. 2018, 84, e01244-18. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Lin, M.; Zhou, H.; Wu, H.; Li, Z.; Lin, W. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE, 2019, 14, e0217018. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pang, Z.; Zhou, Y.; Fallah, N.; Hu, C.; Lin, W.; Yuan, Z. Metagenomic analysis exploring taxonomic and functional diversity of soil microbial communities in sugarcane fields applied with organic fertilizer. BioMed Res. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.C.; Cockell, C.S.; Summers, S. Diverse microbial species survive high ammonia concentrations. Int. J. Astrobiol 2012, 11, 125–131. [Google Scholar] [CrossRef]
- Kertesz, M.A.; Mirleau, P. The role of soil microbes in plant sulphur nutrition. J. Exp. Bot. 2004, 55, 1939–1945. [Google Scholar] [CrossRef]
- Makoi, J.H.; Ndakidemi, P.A. Selected soil enzymes: Examples of their potential roles in the ecosystem. African J. Biotechnol. 2008, 7. [Google Scholar]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Biochemical and microbiological activity of soil contaminated with o-cresol and biostimulated with Perna canaliculus mussel meal. Environ. Monit. Assess. 2018, 190, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.E.; Tabatabai, M.A. Decomposition of biotechnology by-products in soils (Vol. 26, No. 3, 625-632). American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America. 1997.
- Rodrigues, A.G. Endo-β-1, 4-xylanase: An overview of recent developments. Microbial Enzymes in Bioconversions of Biomass 2016, 125–149. [Google Scholar]
- Boguszewska, D.; Grudkowska, M.; Zagdańska, B. Drought-responsive antioxidant enzymes in potato (Solanum tuberosum). Pot. Res. 2010, 53, 373–382. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).